Keywords: ETA receptors, menopause, vascular function
Abstract
Endothelin-1 (ET-1) contributes to vascular dysfunction in postmenopausal women (PMW). Although aerobic exercise is beneficial in reducing ET-1-mediated vasoconstrictor tone in men, it is unknown whether this favorable vascular effect occurs in women. We tested the hypothesis that aerobic exercise training reduces ET-1-mediated vasoconstriction in PMW. We further hypothesized that reductions in ET-1 vasoconstrictor tone underly exercise-induced improvements in endothelium-dependent vasodilatation in PMW. Forearm blood flow (FBF) responses to intra-arterial infusion of selective ETA receptor blockade (BQ-123, 100 nmol/min for 60 min) and acetylcholine (4.0, 8.0, and 16.0 μg/100 mL tissue/min) in the absence and presence of ETA receptor blockade were determined before and after a 12-wk aerobic exercise training intervention in 18 healthy, sedentary PMW (58 ± 4 yr). Women exercised an average of 4.9 ± 0.7 day/wk for 51 ± 7 min/day at 71 ± 3% of maximal heart rate. Before exercise, BQ-123 significantly increased FBF (∼25%) in sedentary PMW; however, this effect was abolished following the exercise intervention. FBF responses to acetylcholine were also significantly higher after exercise training (from 4.2 ± 1.2 to 14.0 ± 3.8 mL/100 mL tissue/min) versus before (from 4.1 ± 1.0 to 11.4 ± 3.3 mL/100 mL tissue/min; ∼25% increase; P < 0.05). Before exercise training, coinfusion of BQ-123 with acetylcholine enhanced (∼25%; P < 0.05) the vasodilator response (from 4.4 ± 1.1 to 13.9 ± 4.2 mL/100 mL tissue/min) compared with acetylcholine alone; after exercise training, the presence of BQ-123 did not significantly affect the vasodilator response to acetylcholine. Aerobic exercise training reduces ET-1-mediated vasoconstriction in PMW. Furthermore, decreased ET-1-mediated vasoconstriction is an important mechanism underlying aerobic exercise-induced improvement in endothelium-dependent vasodilation in PMW.
NEW & NOTEWORTHY Endothelin-1 (ET-1) contributes to declines in endothelial function in postmenopausal women. To our knowledge, we show for the first time that aerobic exercise reduces ET-1-mediated vasoconstriction in previously sedentary postmenopausal women. Moreover, aerobic exercise improved endothelial-dependent dilation due in part to the reductions in ET-1-mediated vasoconstriction.
INTRODUCTION
Cardiovascular disease (CVD) remains the leading cause of death in women, and prevalence rates for CVD dramatically increase in women after menopause (1). Vascular endothelial dysfunction precedes the development of atherosclerosis and CVD. Although declines in endothelial function begin around the time of menopause, the rate of decline is accelerated in postmenopausal women (PMW) (2–4) compared with men. This reduced endothelial function may contribute to the higher rates of CVD observed in women after menopause (1). Therefore, understanding the underlying mechanism(s) as well as effective intervention strategies to offset the decline in endothelial function, and, in turn, improve overall cardiovascular health in women, is paramount.
Endothelin-1 (ET-1) is a potent vasoconstrictor that contributes to age-related declines in vascular endothelial function in both men and women (5, 6). The constricting effects of ET-1 are mediated by both ETA and ETB receptors located on vascular smooth muscle cells. However, ETB receptors are also located on endothelial cells, contributing to endothelial-dependent dilation via nitric oxide production, as well as facilitating the clearance of ET-1 (7, 8). We have previously demonstrated that blockade of the ETA receptors improves endothelial function in older men (9) as well as PMW (5). In older men, heightened ETA-mediated vasoconstriction can be attenuated with aerobic exercise training (10) and is one mechanism by which aerobic exercise training improves endothelial function and reduces cardiovascular risk in older men (10). However, it is currently unknown whether aerobic exercise training reduces ETA-mediated vasoconstriction in PMW.
With this background in mind, the purpose of this study was to investigate the effects of aerobic exercise training on ETA-mediated vasoconstriction in sedentary PWM. We hypothesized that aerobic exercise training would reduce ETA-mediated vasoconstriction in previously sedentary postmenopausal women and, if so, that the reductions in ETA-mediated vasoconstrictor tone would contribute to exercise-induced improvements in endothelium-dependent vasodilation in PMW.
METHODS
Subjects
Eighteen healthy sedentary postmenopausal women ranging in age from 51 to 65 yr were studied (mean age, 58 ± 4 yr). All women were at least 12 mo postmenopausal (range, 13–156 mo) and had never taken hormone replacement therapy. Postmenopausal status was verified by follicle-stimulating hormone levels > 25.8 mIU/mL (range, 32.3–108.9 mIU/mL). All women were non-Hispanic White. None of the women smoked, was taking medications (including vitamins or other dietary supplements), presented with a body mass index (BMI) ≥ 35 kg/m2, or performed regular aerobic physical activity for ≥1 yr before study participation. All women were normotensive and free of overt disease as assessed by medical history, physical examination, fasting blood chemistries, and resting and exercise electrocardiograms. Before participation, all the subjects had the research study and its potential risks and benefits explained fully before providing written, informed consent according to the guidelines of the University of Colorado Boulder. The study protocol was approved by the University of Colorado Boulder Institutional Review Board.
Body Composition
Body mass was measured to the nearest 0.1 kg using a medical beam balance (Detecto, Webb City, MO). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Percentage of body fat was determined by dual-energy X-ray absorptiometry (Lunar, Madison, WI). Minimal waist circumference was measured according to published guidelines (11).
Maximal Oxygen Consumption
Aerobic fitness was assessed by incremental treadmill exercise using a modified Balke Protocol. Maximal oxygen consumption (V̇o2max) was measured with online computer-assisted open-circuit spirometry, as previously described in detail (12). All tests were performed using Ultima CardiO2 from MGC Diagnostics (St. Paul, MN). This system is calibrated weekly by the Clinical Translational Research Center (CTRC) exercise physiologist. Heart rate was measured by 12-lead electrocardiography throughout the protocol, and the rate of perceived exertion and total exercise time to exhaustion was recorded. To ensure that a valid maximal oxygen consumption has been obtained, the following criteria must be satisfied: 1) a plateau in oxygen consumption with increasing workload, 2) a respiratory exchange ratio at maximal exercise > 1.10, 3) achievement of age-predicted maximal heart rate, and 4) a rating of perceived exertion > 19 on the Borg Scale (13).
Metabolic Measurements
Fasting plasma lipid and lipoprotein, glucose, and insulin concentrations were determined using standard techniques by the clinical laboratory affiliated with the Clinical Translational Research Center (CTRC) at the University of Colorado (Boulder, CO).
Protocol
Forearm blood flow (FBF) was measured via strain-gauge venous occlusion plethysmography (D. E. Hokanson) at rest and in response to each pharmacological agent in all women before and after the 12-wk aerobic exercise intervention. This technique is well established in the literature (14–16); additional details on this procedure have been previously described by our laboratory (9). Briefly, a cuff placed around the wrist was inflated to suprasystolic levels. A separate cuff placed around the upper arm was then inflated to 50 mmHg to occlude venous flow for 10 s, allowing for arterial inflow but no venous drainage. This is followed by a period of deflation for 5 s to allow venous drainage. This pattern is repeated four times each minute (9, 14–16). All measurements were performed in a temperature-controlled room between 7:00 am and 10:00 am after an overnight fast, as previously described by our laboratory (17).
Intra-arterial Infusions
Briefly, a 5-cm, 20-gauge catheter was inserted into the brachial artery of the nondominant arm under local anesthesia (1% lidocaine). Heart rate and mean arterial pressure were continuously measured throughout the infusion protocol. Drug infusion rates were normalized per 100 mL forearm tissue and infused at 4 mL/min by a syringe pump. Forearm volume was determined by water displacement. Following the measurement of resting blood flow for 5 min, acetylcholine (IOLAB Pharmaceuticals, Duluth, GA) was infused intra-arterially at rates of 4.0, 8.0, and 16.0 µg/100 mL tissue/min and sodium nitroprusside (Nitropress, Abbott Laboratories) at 1.0, 2.0, and 4.0 µg/100 mL tissue/min for 5 min at each dose to assess endothelium-dependent and endothelium-independent vasodilation, respectively. Acetylcholine binds to muscarinic receptors on endothelial cells, which are G protein-coupled receptors; this causes an increase in intracellular calcium, which binds to calmodulin, and stimulates the enzyme endothelial nitric oxide synthase (eNOS), which drives the production of nitric oxide, and therefore elicits endothelial-dependent dilation. In contrast, sodium nitroprusside is a nitric oxide donor and therefore not dependent on nitric oxide being produced by the endothelium; this readily available nitric oxide has direct effects on the vascular smooth muscle to cause vasodilation, therefore eliciting endothelial-independent dilation. Flow was recorded four times each minute at rest and throughout each drug infusion protocol. Flows during the last minute of rest and each drug dose were measured and the mean value was reported. The total amount of blood flow across the forearm in response to acetylcholine (with and without the coinfusion of BQ-123) was calculated as the area under the curve above baseline using a trapezoidal model. Following the initial infusions of acetylcholine and sodium nitroprusside, BQ-123 (Clinalfa), a selective ETA receptor antagonist, was infused at a rate of 100 nmol/min for 60 min and FBF was measured every 10 min for at least 1 min and the mean value reported. After 60 min, infusion of BQ-123 was continued at the same dose and FBF was measured during coadministration of acetylcholine as performed earlier.
Exercise Intervention
The women participated in a 3-mo home-based aerobic exercise training program that has been previously described in detail elsewhere (10). Briefly, the women were instructed to exercise 5–7 days/wk for 45–60 min/day at 65%–75% of their individual maximum heart rate, as determined during the maximal exercise test. Women wore heart rate monitors (Polar Electro, Kempele, Finland) for the full duration of each exercise training session over the 12 wk (i.e., they put the monitor on before exercise, recorded their exercise session, and then took the monitor off after the exercise session). Most subjects walked, but some incorporated jogging as their fitness improved to maintain their heart rate within the prescribed range. Compliance with the exercise program was documented every 2 wk with data downloaded from heart rate monitors and a review of exercise logs with the CTRC exercise physiologist. All women were studied within 20–24 h after their last exercise session of the 12 wk intervention. This timing was to avoid the immediate (acute) effects of exercise while still representing their normal physiological state (i.e., habitually exercising).
Statistical Analysis
Changes in physical and metabolic characteristics, as well as FBF responses to acetylcholine, sodium nitroprusside, BQ-123, and acetylcholine + BQ-123 resulting from the exercise intervention were assessed by repeated-measures ANOVA. Relations between variables of interest were assessed by linear regression analysis. There were no outliers in the data as determined by Rosner’s test and verified by visualization using STATISTICA 10 software (StatSoft). All data are expressed as means ± SD. Mean value is denoted in whisker plots. Statistical significance was set a priori at P < 0.05.
RESULTS
All 18 postmenopausal women completed the 3-mo home-based exercise intervention. The women exercised an average of 4.9 ± 0.7 day/wk for 51 ± 7 min/day at an intensity of 71 ± 3% of maximal heart rate. There were no significant changes in body mass, BMI, body fat percentage, blood pressure, HDL cholesterol, LDL cholesterol, triglycerides, glucose, or insulin concentrations in response to exercise training (Table 1). Although levels were within clinically normal values total cholesterol was marginally, albeit significantly, lower after the exercise intervention. Although V̇o2max was unchanged, aerobic exercise training increased exercise time to exhaustion by ∼20% (P = 0.007) and significantly decreased heart rate and rating of perceived exertion (RPE) at the same absolute submaximal level of exercise (∼70% of baseline V̇o2max).
Table 1.
Participant characteristics before and after the exercise intervention
| Training |
||
|---|---|---|
| Variable | Before | After |
| n | 18 | 18 |
| Body mass, kg | 80.2 ± 11.3 | 79.7 ± 12.0 |
| BMI, kg/m2 | 29.4 ± 3.6 | 29.2 ± 3.9 |
| Body fat, % | 44.0 ± 5.3 | 43.5 ± 5.9 |
| Waist circumference, cm | 91.1 ± 9.2 | 89.5 ± 9.6 |
| Systolic BP, mmHg | 118 ± 10 | 116 ± 10 |
| Diastolic BP, mmHg | 73 ± 7 | 71 ± 8 |
| Total cholesterol, mg/dL | 219.2 ± 20.1 | 198.3 ± 28.5* |
| LDL cholesterol, mg/dL | 131.6 ± 22.4 | 120.1 ± 36.5 |
| HDL cholesterol, mg/dL | 62.0 ± 13.3 | 57.6 ± 10.1 |
| Trigylcerides, mg/dL | 129.1 ± 51.9 | 110.0 ± 58.2 |
| Glucose, mg/dL | 85.0 ± 10.7 | 88.7 ± 10.5 |
| Insulin, µU/mL | 8.1 ± 2.3 | 7.8 ± 2.4 |
| V̇o2max, mL/kg/min | 24.7 ± 0.9 | 25.9 ± 0.9 |
| Treadmill exercise time, min | 9.5 ± 1.0 | 10.6 ± 1.3* |
| Submaximal heart rate, beats/min | 139 ± 12 | 130 ± 13* |
| Submaximal RPE | 15 ± 2 | 13 ± 2* |
Values are means ± SD. *P < 0.05 vs. before training. BMI, body mass index; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; RPE, rating of perceived exertion.
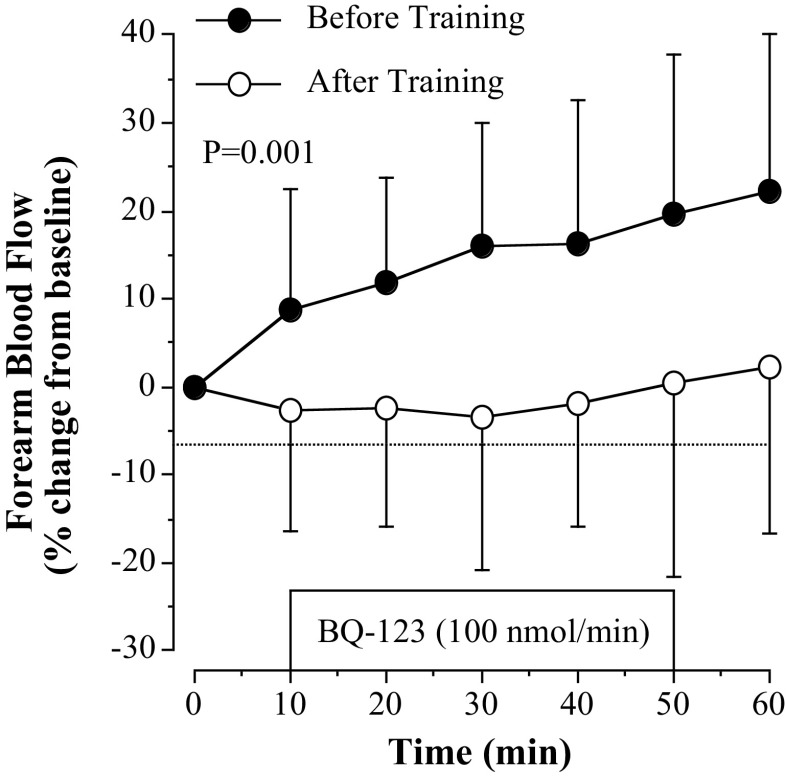
Regular aerobic exercise training significantly reduced ETA receptor-mediated vasoconstrictor tone (Fig. 1). After the exercise intervention, BQ-123 elicited a small nonsignificant increase in resting FBF in the previously sedentary postmenopausal women compared with significant vasodilation (∼25%; P = 0.001) before the exercise training program (Fig. 1). Figure 2 shows the vasodilator responses to acetylcholine (Fig. 2, A and B) and sodium nitroprusside (Fig. 2C). FBF responses to acetylcholine were significantly higher (∼25%; P = 0.03) after exercise training (from 4.2 ± 1.1 to 14.0 ± 3.8 mL/100 mL tissue/min) compared with before training (from 4.1 ± 1.0 to 11.4 ± 3.3 mL/100 mL tissue/min; Fig. 2A). Correspondingly, total FBF to acetylcholine was ∼40% higher (53.3 ± 21.1 vs. 73.2 ± 28.8 mL/100 mL tissue; P = 0.02) after the exercise intervention (Fig. 2B). FBF responses to sodium nitroprusside did not significantly change in response to the aerobic exercise intervention (Fig. 2C).
Figure 1.
Forearm blood flow (FBF) responses to BQ-123 before and after 3 mo of aerobic exercise training in postmenopausal women. Values are represented as means ± SD. The P value refers to the difference in the FBF response (main effect) to BQ-123 before vs. after the exercise training. Differences in FBF responses were determined by repeated-measures analysis of variance.
Figure 2.
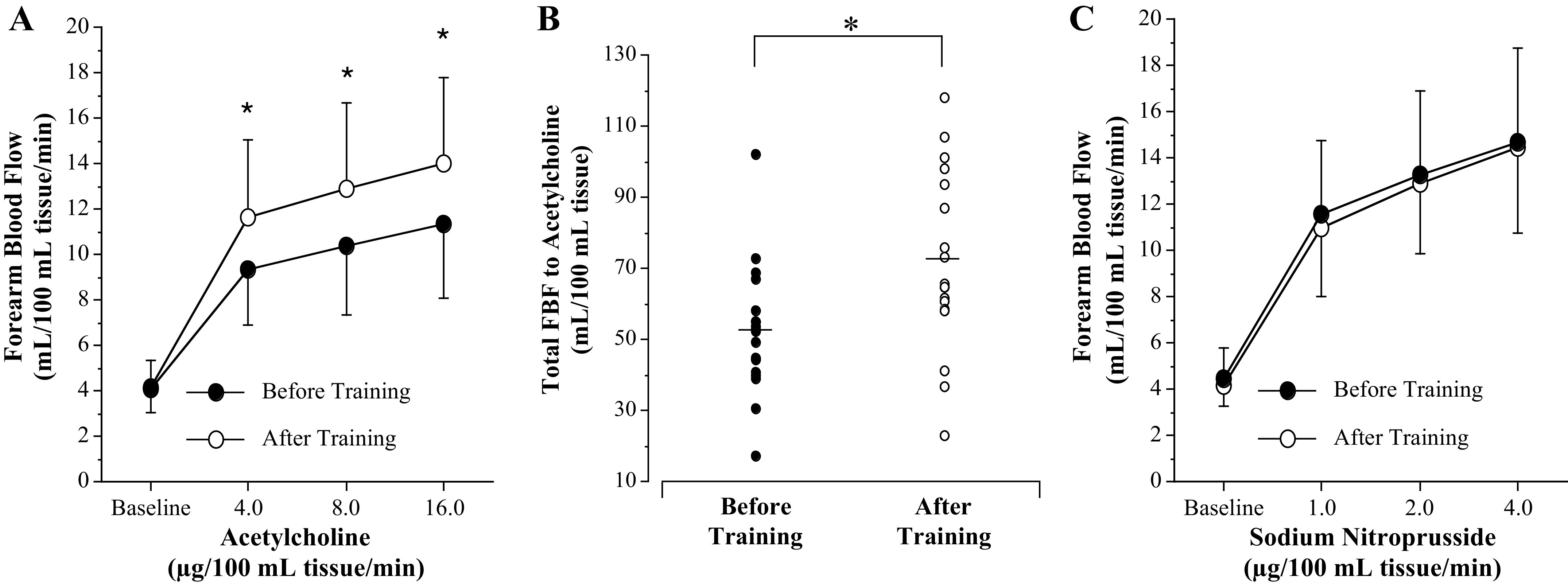
Forearm blood flow (FBF) responses (A) and total FBF (B) to acetylcholine and FBF responses to sodium nitroprusside (C) before and after 3 mo of aerobic exercise training in postmenopausal women. Values are represented as means ± SD. *P < 0.05 vs. before training. Differences in FBF responses and area under the curve were determined by repeated-measures analysis of variance.
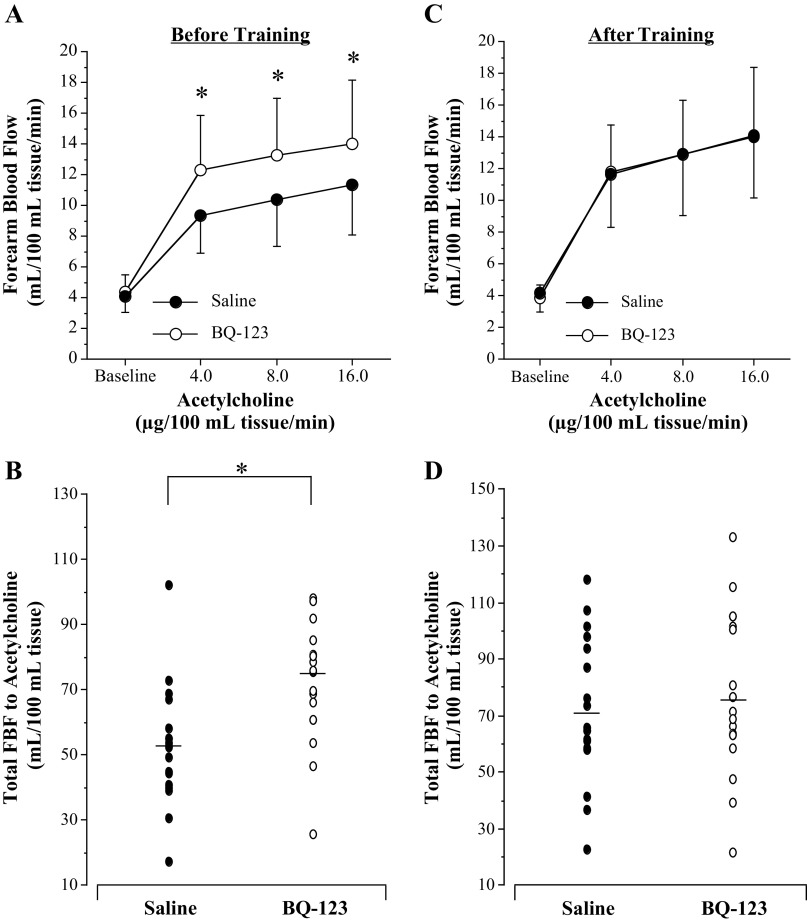
Before aerobic exercise training, the coinfusion of BQ-123 with acetylcholine resulted in an enhanced (∼25%; P = 0.02; Fig. 3A) vasodilator response (from 4.4 ± 1.1 to 13.9 ± 4.2 mL/100 mL tissue/min) compared with acetylcholine alone (from 4.1 ± 1.0 to 11.4 ± 3.3 mL/100 mL tissue/min). Indeed, total blood flow response to acetylcholine (area under the curve) was ∼40% greater (75.2 ± 23.6 vs. 53.4 ± 21.1 mL/100 mL tissue; P = 0.006, Fig. 3B) with the addition of BQ-123. After exercise training, the presence of BQ-123 did not significantly affect the vasodilator response to acetylcholine (Fig. 3, C and D). There was no significant difference (P = 0.97) in the FBF responses to acetylcholine in the presence (from 3.8 ± 0.8 to 14.1 ± 4.3 mL/100 mL tissue/min) or absence of BQ-123 (from 4.2 ± 1.2 to 14.0 ± 3.8 mL/100 mL tissue/min; Fig. 3C). Concordantly, there was no significant difference in the total blood flow response to acetylcholine with and without BQ-123 (77.5 ± 28.3 vs. 73.2 ± 28.8 mL/100 mL tissue; P = 0.65) after the exercise intervention (Fig. 3D).
Figure 3.
Forearm blood flow (FBF) responses (A and C) and total FBF (B and D) to acetylcholine in the absence and presence of selective ETA receptor blockade (BQ-123) before and after 3 mo of aerobic exercise training in postmenopausal women. Values are represented as means ± SD. *P < 0.05 vs. saline. Differences in FBF responses and area under the curve were determined by repeated-measures analysis of variance.
The modest change in plasma total cholesterol with the exercise intervention was not significantly correlated with the peak changes in FBF to either BQ-123 (r = −0.16; P = 0.51) or acetylcholine (r = 0.15; P = 0.59).
DISCUSSION
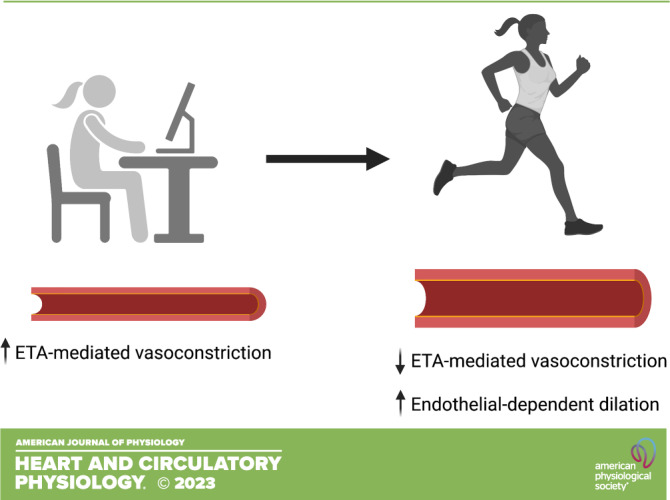
The primary novel finding of the current study was that 12 wk of aerobic exercise training reduced ET-1-mediated vasoconstriction in previously sedentary PMW. In addition, endothelium-dependent vasodilation improved in PMW following 12 wk of aerobic exercise training, in part because of reductions in ETA-mediated vasoconstriction. These findings demonstrate that ET-1 is a primary mechanistic pathway that can be targeted with aerobic exercise to improve endothelial function and possibly overall cardiovascular health in PMW. Given the increased rates of CVD in PMW, these findings have important clinical implications for women’s health.
Accumulating evidence shows an important role of ET-1 in contributing to vascular dysfunction in PMW (5). Although aerobic exercise has been shown to reduce plasma concentrations of ET-1 in PMW (18), to date, no studies have more thoroughly examined the impact of aerobic exercise on ET-1 system activity. Indeed, to our knowledge, this is the first study to assess the effects of chronic aerobic exercise training on ET-1-mediated vasoconstrictor tone in PMW. Although we have previously demonstrated a potent effect of aerobic exercise on reducing ET-1-mediated vasoconstriction in older men (10), the current study significantly extends these findings, for the first time, to women. Herein, we demonstrate that blockade of ETA receptors resulted in a significant increase in forearm blood flow in sedentary PMW, indicative of increased ET-1-mediated vasoconstrictor tone at baseline. Notably, this increase in forearm blood flow to ETA receptor blockade was completely abolished following aerobic exercise training, indicating exercise-induced reduction in ET-1-mediated vasoconstriction. Interestingly, the magnitude of reduction in ETA-mediated vasoconstriction from aerobic exercise training is comparable with what we previously observed in older men (10). This was somewhat surprising given our previous findings demonstrating greater ETA-mediated vasoconstrictor tone in older men compared with women (19). Of note, this sex difference was abolished with coinfusion of the BQ-788, an ETB receptor antagonist (19). The ETB receptor plays a critical role in regulating vascular function in women and is sensitive to changes in estradiol (5, 20, 21). Unfortunately, because of the lack of availability of the ETB receptor antagonist, BQ-788, we do not have data in the current study pertaining to the ETB receptor. Future studies are warranted to understand whether aerobic exercise alters ETB receptor function in PMW and how changing concentrations of estradiol modulate these responses before and after exercise training (22).
Our findings also demonstrate that ETA receptors contribute to impaired endothelium-dependent vasodilation in sedentary PMW. These findings are consistent with previous work from our group showing that both ETA and ETB receptors contribute to vascular dysfunction in PMW (5). However, a novel finding of the current study is that 12 wk of aerobic exercise enhanced endothelium-dependent vasodilation and that following the exercise intervention, coinfusion of the ETA receptor antagonist BQ-123 no longer enhanced forearm blood flow responses to acetylcholine. Thus, the reduction in ETA-mediated vasoconstrictor tone from aerobic exercise training is a prominent mechanism for improving endothelial function in PMW.
The results of our study provide novel additional insight given reported sex differences in the vascular responsiveness to aerobic exercise in middle-aged/older adults (22, 23). In sedentary older men, 12 wk of moderate-intensity aerobic exercise improved forearm blood flow responses to acetylcholine (9), essentially restoring endothelial function to that of younger men. These exercise-induced increases in endothelial function in older men have also been reported in measuring brachial artery flow-mediated dilation (24). However, aerobic exercise training of similar intensities (moderate intensity) was reported to have little effect on endothelial function as measured by brachial artery flow-mediated dilation in estrogen-deficient postmenopausal women (24, 25). Moreover, Santos-Parker et al. (26) examined both macro- and microvascular endothelial function in a cross-sectional study of sedentary and habitually active PMW. Both flow-mediated dilation and forearm blood flow responses to acetylcholine were not different between sedentary and habitually active postmenopausal women (26), suggesting that in contrast to men, the endothelial function may not be improved with chronic aerobic exercise in PMW. The discrepancy between our findings and those of Santos-Parker et al. (26) and others (9, 10) is not entirely clear, but central differences in study design and techniques used to assess endothelial vasomotor function are likely factors. Cross-sectional studies are inherently limited in their sensitivity to directly identify main effects, and results can be influenced by uncontrolled biological and environmental factors. In the present study, the direct effects of habitual aerobic exercise training on endothelial vasomotor function in PMW were determined. There were no significant changes in body mass, body composition, or blood pressure in response to the exercise intervention that could have influenced our findings. Although the absence of a nonexercise control group does not completely rule out the possibility that the results of the study were attributable to chance, experimental bias, or both, the 3-mo home-based aerobic exercise training program used in the present study has been repeatedly shown to improve endothelial vasomotor function, in adult humans varying in age and body composition independent of anthropometric or metabolic changes (9, 10, 27, 28). Thus, it is likely that the observed exercise-induced improvement in endothelial vasomotor function (reduced vasoconstriction and increased vasodilation) was a primary effect of exercise training.
Differences in methods used to assess endothelial vasodilation can also be attributed to the apparent discrepancy in findings on the effect of exercise training on endothelial vasodilation in PMW. In contrast to the present study, previous studies evaluating the effects of aerobic exercise training on PMW used ultrasound measures of brachial artery dilation during reactive hyperemia. Although both brachial artery dilation during reactive hyperemia and blood flow responses measured by plethysmography to intrabrachial infusion of endothelium-dependent vasodilators are widely used, well-established techniques to assess peripheral vascular endothelial vasodilation, they can, and often do, provide divergent results. In fact, it has been suggested that they should not be viewed as surrogate measures of each other (29). Blood flow measured in the forearm by venous occlusion plethysmography reflects vasodilation in the arterioles, whereas ultrasound measures of forearm reactive hyperemia reflect vasodilation in the conduit brachial artery. The reactive hyperemia response with flow-mediated dilation is dependent on sheer stress (30) and may also be impacted by local ischemic events, which can increase sympathetic tone (31). Thus, the differences noted in the effects of habitual aerobic exercise on endothelial vasodilator capacity in PMW between studies may be related to the vascular bed in question. Although exercise-induced changes in peripheral endothelial vasodilation may not be apparent at the level of the conduit (i.e., brachial) artery in PMW, significant, favorable adaptations in vasomotor function do occur in the arteriole vasculature that, importantly, is associated with reduced cardiovascular risk.
Limitations
As mentioned, the lack of a nonexercising control group can be viewed as a limitation of our study. Nevertheless, this home-based exercise intervention has consistently demonstrated improvements in vascular function (9, 10, 27, 28). In addition, we were not able to analyze plasma ET-1 or additional sex hormones such as estradiol or assess the contribution of ETB receptors to exercise-induced improvements in vascular tone and endothelial function. These are important questions for future consideration. Finally, our sample lacked diversity, and these results are not generalizable to all women.
In summary, the results of the present study demonstrate that aerobic exercise training reduces ETA-mediated vasoconstrictor tone in sedentary estrogen-deficient PMW. Moreover, this reduction in ETA-mediated vasoconstriction is associated with improved endothelium-dependent vasodilation. From a public health perspective, it is important to emphasize that these exercise-induced adaptations were accomplished with a moderate-intensity home-based aerobic exercise training program that can be safely performed by most, if not all, generally healthy PMW. The mechanisms underlying these favorable aerobic exercise-related changes in vascular function in PMW are an area of current investigation. It cannot, and should not, be assumed that the mechanisms underlying these vascular adaptations would be the same as those reported in men (22). Regardless of the mechanisms involved, the data presented herein demonstrate the role of ET-1 in endothelial dysfunction in PMW and the beneficial effects of regular aerobic exercise on vascular function in PMW.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by National Institutes of Health Grants HL076434 and HL077450 (to C. A. DeSouza), HL146558 (to M.M.W.), UL1TR001082, and T32 HL007822.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C. A. DeSouza conceived and designed research; C. A. Dow, J.J.G., B.L.S., and C. A. DeSouza performed experiments; C. A. Dow, J.J.G., and C. A. DeSouza analyzed data; M.M.W., C. A. Dow, B.L.S., and C. A. DeSouza interpreted results of experiments; J.J.G. prepared figures; M.M.W., L.M.W., and C.A. DeSouza drafted manuscript; M.M.W. and C.A. DeSouza edited and revised manuscript; M.M.W., L.M.W., C. A. Dow, J.J.G., B.L.S., and C. A. DeSouza approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all the women who participated in the study and the staff of the University of Colorado Boulder Clinical and Translational Research Center.
REFERENCES
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. . Heart Disease and Stroke Statistics-2019 update: a report from the American Heart Association. Circulation 139: e56–e528, 2019. [Erratum in Circulation 141: e33, 2020]. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 3. Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab 97: 4692–4700, 2012. doi: 10.1210/jc.2012-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996. doi: 10.1161/01.hyp.28.4.576. [DOI] [PubMed] [Google Scholar]
- 5. Wenner MM, Sebzda KN, Kuczmarski AV, Pohlig RT, Edwards DG. ETB receptor contribution to vascular dysfunction in postmenopausal women. Am J Physiol Regul Integr Comp Physiol 313: R51–R57, 2017. doi: 10.1152/ajpregu.00410.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Westby CM, Weil BR, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstriction and the age-related decline in endothelium-dependent vasodilatation in men. Clin Sci (Lond) 120: 485–491, 2011. doi: 10.1042/CS20100475. [DOI] [PubMed] [Google Scholar]
- 7. Ishikawa K, Ihara M, Noguchi K, Mase T, Mino N, Saeki T, Fukuroda T, Fukami T, Ozaki S, Nagase T. and Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc Natl Acad Sci USA 91: 4892–4896, 1994. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mazzuca MQ, Khalil RA. Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochem Pharmacol 84: 147–162, 2012. doi: 10.1016/j.bcp.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 10. Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50: 403–409, 2007. doi: 10.1161/HYPERTENSIONAHA.107.088294. [DOI] [PubMed] [Google Scholar]
- 11. Lohman TR, Mortorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics, 1988. [Google Scholar]
- 12. DeSouza CA, Jones PP, Seals DR. Physical activity status and adverse age-related differences in coagulation and fibrinolytic factors in women. Arterioscler Thromb Vasc Biol 18: 362–368, 1998. doi: 10.1161/01.atv.18.3.362. [DOI] [PubMed] [Google Scholar]
- 13. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2: 92–98, 1970. [PubMed] [Google Scholar]
- 14. Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol (1985) 91: 2431–2441, 2001. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- 15. Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol 52: 631–646, 2001. doi: 10.1046/j.0306-5251.2001.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hewlett AW, Van Zwaluwenburg JG. The rate of blood flow in the arm. Heart 1: 631–646, 1909. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/653175. [Google Scholar]
- 17. Van Guilder GP, Stauffer BL, Greiner JJ, Desouza CA. Impaired endothelium-dependent vasodilation in overweight and obese adult humans is not limited to muscarinic receptor agonists. Am J Physiol Heart Circ Physiol 294: H1685–H1692, 2008. doi: 10.1152/ajpheart.01281.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, Kuno S, Ajisaka R, Yamaguchi I, Matsuda M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol (1985) 95: 336–341, 2003. doi: 10.1152/japplphysiol.01016.2002. [DOI] [PubMed] [Google Scholar]
- 19. Stauffer BL, Westby CM, Greiner JJ, Van Guilder GP, Desouza CA. Sex differences in endothelin-1-mediated vasoconstrictor tone in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol 298: R261–R265, 2010. doi: 10.1152/ajpregu.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shoemaker LN, Haigh KM, Kuczmarski AV, McGinty SJ, Welti LM, Hobson JC, Edwards DG, Feinberg RF, Wenner MM. ETB receptor-mediated vasodilation is regulated by estradiol in young women. Am J Physiol Heart Circ Physiol 321: H592–H598, 2021. doi: 10.1152/ajpheart.00087.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stanhewicz AE, Wenner MM, Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol 315: H1569–H1588, 2018. doi: 10.1152/ajpheart.00396.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seals DR, Nagy EE, Moreau KL. Aerobic exercise training and vascular function with ageing in healthy men and women. J Physiol 597: 4901–4914, 2019. doi: 10.1113/JP277764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moreau KL, Ozemek C. Vascular adaptations to habitual exercise in older adults: time for the sex talk. Exerc Sport Sci Rev 45: 116–123, 2017. doi: 10.1249/JES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin Sci (Lond) 120: 13–23, 2011. doi: 10.1042/CS20100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moreau KL, Stauffer BL, Kohrt WM, Seals DR. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J Clin Endocrinol Metab 98: 4507–4515, 2013. doi: 10.1210/jc.2013-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Santos-Parker JR, Strahler TR, Vorwald VM, Pierce GL, Seals DR. Habitual aerobic exercise does not protect against micro- or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. J Appl Physiol (1985) 122: 11–19, 2017. doi: 10.1152/japplphysiol.00732.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dow CA, Stauffer BL, Brunjes DL, Greiner JJ, DeSouza CA. Regular aerobic exercise reduces endothelin-1-mediated vasoconstrictor tone in overweight and obese adults. Exp Physiol 102: 1133–1142, 2017. doi: 10.1113/EP086454. [DOI] [PubMed] [Google Scholar]
- 28. Mestek ML, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Regular aerobic exercise, without weight loss, improves endothelium-dependent vasodilation in overweight and obese adults. Obesity (Silver Spring) 18: 1667–1669, 2010. doi: 10.1038/oby.2009.467. [DOI] [PubMed] [Google Scholar]
- 29. Eskurza I, Seals DR, DeSouza CA, Tanaka H. Pharmacologic versus flow-mediated assessments of peripheral vascular endothelial vasodilatory function in humans. Am J Cardiol 88: 1067–1069, 2001. doi: 10.1016/s0002-9149(01)01997-x. [DOI] [PubMed] [Google Scholar]
- 30. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sinoway LI, Wilson JS, Zelis R, Shenberger J, McLaughlin DP, Morris DL, Day FP. Sympathetic tone affects human limb vascular resistance during a maximal metabolic stimulus. Am J Physiol 255: H937–H946, 1988. doi: 10.1152/ajpheart.1988.255.4.H937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.