Abstract
Preclinical large animal models of chronic heart failure (HF) are crucial to both understanding pathological remodeling and translating fundamental discoveries into novel therapeutics for HF. Canine models of ischemic cardiomyopathy are historically limited by either high early mortality or failure to develop chronic heart failure. Twenty-nine healthy adult dogs (30 ± 4 kg, 15/29 male) underwent thoracotomy followed by one of three types of left anterior descending (LAD) coronary artery ligation procedures: group 1 (n = 4) (simple LAD: proximal and distal LAD ligation); group 2 (n = 14) (simple LAD plus lateral wall including ligation of the distal first diagonal and proximal first obtuse marginal); and group 3 (n = 11) (total LAD devascularization or TLD: simple LAD plus ligation of proximal LAD branches to both the right and left ventricles). Dogs were followed until chronic severe HF developed defined as left ventricular ejection fraction (LVEF) < 40% and NH2-terminal-prohormone B-type natriuretic peptide (NT-proBNP) > 900 pmol/L. Overall early survival (48-h postligation) in 29 dogs was 83% and the survival rate at postligation 5 wk was 69%. Groups 1 and 2 had 100% and 71% early survival, respectively, yet only a 50% success rate of developing chronic HF. Group 3 had excellent survival at postligation 48 h (91%) and a 100% success in the development of chronic ischemic HF. The TLD approach, which limits full LAD and collateral flow to its perfusion bed, provides excellent early survival and reliable development of chronic ischemic HF in canine hearts.
NEW & NOTEWORTHY The novel total left anterior descending devascularization (TLD) approach in a canine ischemic heart failure model limits collateral flow in the ischemic zone and provides excellent early survival and repeatable development of chronic ischemic heart failure in the canine heart. This work provides a consistent large animal model for investigating heart failure mechanisms and testing novel therapeutics.
Keywords: canine, chronic heart failure, heart failure with reduced ejection fraction, ischemic cardiomyopathy
INTRODUCTION
Preclinical large animal heart failure (HF) models have a critical and expanding role in translating basic science findings for developing and testing novel cardiovascular therapeutics (1). Although small animal models are generally less expensive, allow for larger sample sizes, and offer a greater variety of transgenic models (2), they have significant limitations in HF research, including small organ and body size, faster heart rates, different action potential characteristics, and different myocardial metabolism (3). In vivo studies using large mammalian hearts are also advantageous because they are more physiologically and clinically relevant (4) and permit realistic testing of clinically significant interventions (5).
Canines and swine are the most frequently used large animal species for chronic HF studies because of comparable cardiac anatomy to humans (6). Coronary anatomy of pigs has minimal collateral circulation (7), whereas the canine model has greater epicardial collateralization than man. Canine hearts also more closely replicate humans with ischemic heart disease (which promotes collateral growth), especially in older individuals. Furthermore, the social nature of dogs often eliminates the need for sedation during minimally invasive procedures such as transport, echocardiography, electrocardiogram monitoring, and phlebotomy. A limitation of dog use in chronic infarction models is that extensive collateral circulation makes it difficult to induce consistent myocardial infarctions of sufficient size for HF to develop. If too much coronary circulation is impaired, postligation mortality of canines is unacceptably high, between 30 and 60% (8–12), similar to that of the porcine infarction models (13).
An optimized approach for developing chronic ischemic HF in a canine model will minimize early mortality and consistently result in chronic dilated cardiomyopathy. In this work, we explore three different coronary ligation surgical procedures in canines and successfully test the hypothesis that the elimination of the left anterior descending (LAD, also termed paraconal interventricular branch of the left main coronary in dogs) artery and its full collateral circulation will both minimize acute mortality while improving the consistency of HF progression. We present a total LAD devascularization (TLD) approach, which provides a reliable model of chronic ischemic HF in canines. This model should be a valuable tool for the preclinical testing of therapeutics and medical devices. It will also be of great importance for basic research into heart failure with reduced ejection fraction (HFrEF) and ischemic HF mechanisms.
METHODS
All studies followed the guidelines from the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (14) and the standards of United States regulatory agencies. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of The University of Utah.
Study Design and Size
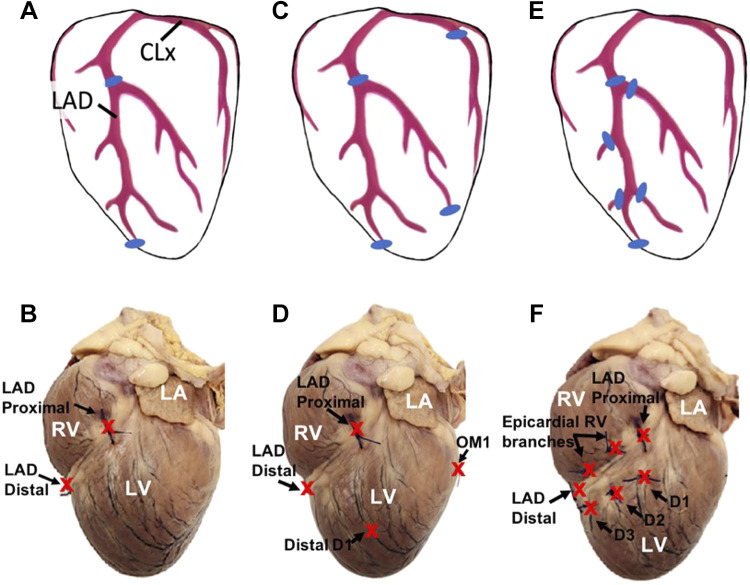
Surgical lateral thoracotomy procedures were performed on 29 healthy adult dogs (Canis lupus, purchased from Oak Hill Genetics, Ewing, IL), weighing 30 ± 4 kg (range, 25.4–39.6 kg). Twenty-nine dogs (female, n = 14) were divided into three groups based on the surgical approach (Fig. 1): group 1 (simple LAD, n = 4), LAD ligation proximal to the first diagonal and a second ligation at the junction of the distal LAD and the collateral connection to the posterior descending artery (also called the subsinousal interventricular branch in dogs) near the apex of the LV; group 2 (LAD and lateral, n = 14); ligations at the proximal and distal LAD, the distal first diagonal, and the proximal first obtuse marginal artery; and group 3 (total LAD devascularization, TLD, n = 11), ligations at the proximal and distal LAD, proximal aspects of visible diagonal arteries of the LAD, and proximal aspects of visible right ventricular branches of the LAD below the first diagonal artery.
Figure 1.
Left anterior descending (LAD) coronary artery ligation positions of the three groups. A and B: group 1: simple LAD (proximal and distal LAD ligation). C and D: group 2: LAD and lateral (proximal and distal LAD, distal first diagonal, and proximal first obtuse marginal). E and F: group 3: total LAD devascularization (proximal and distal LAD, proximal aspects of visible diagonals, and proximal aspects of epicardial right ventricular branches of the LAD). Top: cartoon images show ligation positions marked as “blue” for three groups. Bottom: representative images show ligation positions marked as “red-colored X” for three groups. LA, left atrium; RV, right ventricle; LV, left ventricle; OM1, first obtuse marginal artery off of the circumflex artery; D1–D3, epicardial diagonal branches 1, 2, and 3, off of the LAD toward the LV.
Inclusion and Exclusion Criteria
Healthy dogs with baseline left ventricular ejection fraction (LVEF) > 40% and NH2-terminal prohormone B-type natriuretic peptide (NT-proBNP) < 900 pmol/L were included in the study. Dogs were classified as having “chronic ischemic HF” when LVEF < 40% and NT-proBNP > 900 pmol/L. Data were acquired and characterized at different time points including at baseline (BL) in awake animals before the surgical thoracotomy procedure and during chronic ischemic HF development (weeks 1 to 16). In our study, the final end point refers to time point when either the dogs were determined to be in the chronic ischemic HF condition with LVEF < 40% and NT-proBNP > 900 pmol/L or the dogs were determined to have LVEF > 40% by week 16. Among 29 dogs, 9 did not survive to postligation week 5 and were excluded from the group analysis. For the surviving dogs (n = 20), data were gathered and compared between baseline and final end point.
Animal Procedures
Dogs were fasted for 12–18 h before surgery and a transdermal fentanyl patch (50–100 µg/h) was placed 3–12 h. before the infarct surgical procedure. Dogs were anesthetized with an injection of fentanyl (2–10 µg/kg iv) followed by propofol (5–8 mg/kg iv) to effect. Dogs were then intubated, and anesthesia was maintained with a mixture of 1.0–3.0% isoflurane in 100% oxygen through a vaporizer and maintained with mechanical ventilation. Heart rate (HR), core body temperature, oxygen saturation, and electrocardiogram were monitored. End-tidal CO2 was monitored and titrated to 35–45 mmHg by adjusting tidal volume and respiratory rate. Once the animal was hemodynamically stable under general anesthesia, fentanyl was administered by constant rate infusion at 2–10 µg/(kg × h) IV, and cefazolin (20 mg/kg iv) was given every 90–120 min. Intraoperative hypotension (mean arterial pressure < 60 mmHg) was treated with a bolus of lactated Ringer’s solution (2–3 mL/kg iv) followed by an infusion of dopamine [2–10 µg/(kg·min)], if needed. Intravenous atropine (0.01–0.05 mg/kg iv bolus) was also administered to treat sinus bradycardia. A femoral artery catheter (5-Fr) was used for invasive blood pressure monitoring, and the other femoral artery was cannulated for pressure-volume (PV) loop transducer access. Before the lateral thoracotomy, bupivacaine (1–2 mg/kg) was injected subcutaneously as an intercostal nerve block two ribs spaces above and below the thoracotomy site. Next, the heart was exposed by incising the skin over the fifth intercostal space, followed by transecting and/or sparing the underlying parallel muscle and fascia. The pericardium was opened medially and parallel to the left phrenic nerve, exposing the heart and coronary vessels. As shown in Fig. 1, the LAD artery and collateral vessels were isolated and ligated as per assigned group procedure. Once the surgical ligation procedure was completed, a chest tube was inserted before closing the thoracotomy site and used to evacuate air and fluid from the thorax after thoracotomy closure. The tube was removed before anesthetic recovery, and the dog was returned to the recovery kennel. During the closure, bupivacaine (1 mg/kg im/sc) or bupivacaine liposome (Nocita 5.3 mg/kg, maximum dose, im/sc) was administered at the surgical site. During recovery from anesthesia, hydromorphone (0.05–0.3 mg/kg iv, im, or sc) was administered to control postoperative pain as the fentanyl transdermal patch reached therapeutic levels. The time point “baseline” indicates data recorded before the surgical thoracotomy procedure, and the “end point” refers to final time point when either the dogs were determined to be in the chronic ischemic HF condition with LVEF < 40% and NT-proBNP > 900 pmol/L or the dogs were determined not to be in chronic HF if LVEF was > 40% by week 16. The animals were euthanized at the completion of their final data collection study.
Antiarrhythmic Drugs and Protocols
Cardiac arrhythmic suppression trials–CAST data (15–17), did not demonstrate a mortality benefit when antiarrhythmic drugs are used following myocardial infarction. However, because of increased ectopy following the coronary ligation procedure, mexiletine (3–8 mg/kg PO TID) began the evening before the ligation procedure and continued for 3 days postligation in groups 2 and 3 dogs. For acute arrhythmias, a bolus of lidocaine (0.8–2.0 mg/kg iv) was also given in response to frequent premature ventricular contractions and nonsustained ventricular tachycardia during the procedure and during recovery for up to 4 days postligation.
Left Ventricular End-Diastolic Pressure
For left ventricular end-diastolic pressure (LVEDP) measurements, a 7F combined pressure and conductance catheter (CD Leycom, Zoetermeer, the Netherlands) was introduced from the femoral artery into the LV via the aortic valve at baseline and at the end point. The catheter was connected to a Cardiac Function Laboratory (CD Leycom) to acquire pressure data. All recordings were made during sinus beats with and without mechanical ventilation. Data were exported and analyzed in a custom-built MATLAB program to evaluate LVEDP.
Echocardiography Recordings and Analysis
LV function was assessed by echocardiography (Acuson SC2000, Siemens, Munich, Germany) in awake dogs before the baseline coronary ligation procedure and throughout HF development. Data were acquired weekly (BL to 4 wk postligation) and then biweekly (>4 wk postligation) to evaluate both systolic and diastolic dysfunction over time. Data were gathered with the four-chamber apical, parasternal long-axis, and midpapillary muscle short-axis views. E and e′ peaks were recorded using pulse wave (PW) with tissue-Doppler imagining (TDI) and PW alone, respectively, from a four-chamber apical view. Left ventricular ejection fraction (LVEF) was evaluated from a four-chamber apical view. Left ventricular internal diameter end diastole (LVIDd) was evaluated from the four-chamber apical view. The end-point septal separation (EPSS) was obtained by placing the M-mode tracer over the mitral valve’s anterior leaflet’s free edge (distal). The image displayed the movement of structures over several cardiac cycles along a specific plane. Data spanning at least three consecutive heartbeats were acquired and analyzed.
Statistical Analysis
Continuous variables were expressed as means ± SD. Changes in heart function and dimension between baseline and end point in each group were evaluated using paired t tests. A two-way mixed analysis of variance (ANOVA) model with Bonferroni post hoc tests were used to examine the time effect (baseline to end point), groups effect (groups 1–3), and interaction between time and group effect on LVEF, NT-proBNP, LVIDd, and LVEDP. Post hoc tests with Bonferroni correction were conducted to check for differences between group 1 versus group 2, group 1 versus group 3, and group 2 versus group 3 at the final end point. For EPSS and E/e′, only groups 2 and 3 were included in the two-way ANOVA mixed model analysis. All analyses were conducted in GraphPad PRISM 9.0. Furthermore, categorical variables such as sex (Male/Female), survival at 48-h postligation (yes/no), survival at postligation week 5 (yes/no), and development chronic ischemic HF (yes/no) at the end point were expressed as percentages. These data were compared using the Fisher exact tests. A P value of < 0.05 was considered statistically significant.
RESULTS
Early Mortality and Overall Survival
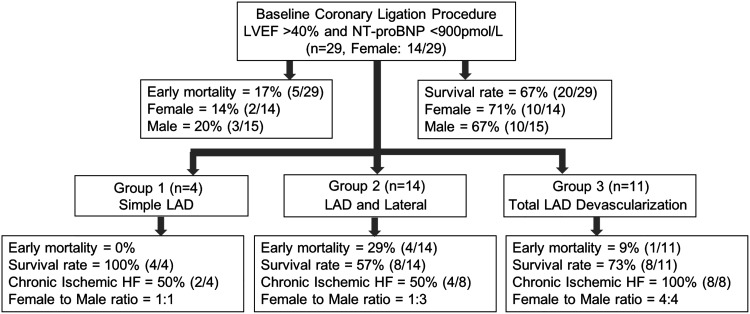
Twenty-nine dogs (female, n = 14) were subjected to coronary ligation surgical procedures as explained in Fig. 2. The troponin level was typically > 50 ng/mL the day following coronary ligation. The overall early mortality rate was 17% (n = 5/29), with 20% male (n = 3/15) and 14% female (n = 2/14). These five dogs experienced apparent sudden cardiac death within 48 h of coronary ligation. From postligation 48 h to week 5, four dogs were lost, and the overall survival rate at postligation week 5 was 67% (n = 20/29), with males 67% (n = 10/15) and females 71% (n = 10/14). Of these four animals, one dog experienced acute pulmonary hemorrhage on postligation day 8; the second developed a pneumothorax on postligation day 4, and the third had intractable sustained VT on postligation day 5. The fourth animal experienced apparent sudden death in the kennel at week 5 postligation.
Figure 2.
Flow chart description (sample size, sex, early mortality, and survival rate) of 29 healthy adult dogs enrolled in the study. Dogs were studied into three groups based on different left anterior descending (LAD) coronary artery ligation positions. “Early mortality” refers to 48-h postligation mortality. “Survival rate” refers to 5-wk postligation survival. Chronic ischemic HF refers to dogs with LVEF < 40% and NT-proBNP > 900 pmol/L. HF, heart failure; LVEF, left ventricular ejection fraction; NT-proBNP, NH2-terminal-prohormone B-type natriuretic peptide.
The effect of sex on survival at 48 h and 5 wk postligation was not statistically significant (Fisher exact test: P = 0.6841 and P = 0.7822, respectively). From postligation week 5 to the final end point, 70% of animals (n = 14/20) developed similar levels of chronic ischemic HF, with female 60% (n = 6/10) and male 80% (n = 8/10) (P = 0.3291).
The 29 dogs were categorized into three groups based on their different coronary ligation surgical procedures. As seen in Fig. 2, the differences among the three groups at 48-h and 5-wk postligation survival rate were statistically not significant (P = 0.4016 and P = 0.3001, respectively). As outlined in Table 1, in groups 1 and 2, the postligation week 5 survival rate was 100% (n = 4/4) and 57% (n = 8/14), respectively. Furthermore, in each group, the success rate of developing chronic ischemic HF in surviving dogs was only 50% (group 1- n = 2/4 and group 2- n = 4/8). The other 50% of the surviving 5-wk postligation dogs (n = 6) developed compromised LVEF at postligation week 1 but failed to achieve LVEF < 40% by postligation week 16. Group 3 (TLD approach) had a 100% success rate of developing chronic ischemic HF (n = 8/8) with an early mortality rate of 9% (n = 1/11) and postligation week 5 survival rate of 73% (n = 8/11). Overall, by postligation week 16, the incidence of chronic ischemic HF developed between groups was statistically significant with Freeman–Halton extension of the Fisher exact probability test (P = 0.0493).
Table 1.
Early mortality, survival rate, and cardiac performance of three groups of dogs in developing chronic ischemic HF
| Canine Model | n | Coronary Ligation Positions (Ischemia Territory) | Early Mortality (Postligation 48 h), % (n) | Postligation Survival Week 5, % (n) | Chronic Ischemic HF Developed by week 16, % (n) | Time to Develop Ischemic HF, wk (n) | LV Functional Data and NT-proBNP Outcome in All Survived Canines at End Point |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | LVEF, % | NT-proBNP, pmol/L | LVEDV, mL | LVIDd, cm | LVEDP, mmHg | E/e′ | EPSS, mm | |||||||
| Group 1 | 4 | Proximal and distal LAD | 0 (0/4) | 100 (4/4) | 50 (2/4) | 9 ± 1 (2/4) | 4 | 45 ± 7 | 676 ± 248 | 56 ± 7 | 5.61 ± 0.17 | 8.11 ± 1.76 | NA | NA |
| Group 2 | 14 | Proximal and distal LAD, and OM1/distal D1 | 29 (4/14) | 57 (8/14) | 50 (4/8) | 13 ± 3 (4/8) | 8 | 42 ± 5 | 1,299 ± 1,009 | 63 ± 21 | 6.02 ± 0.29 | 9.34 ± 1.65 | 8.19 ± 0.62 | 8.31 ± 1.01 |
| Group 3 | 11 | Total LAD devascularization | 9 (1/11) | 73 (8/11) | 100 (8/8) | 11 ± 2 (8/8) | 8 | 35 ± 2 | 1,416 ± 496 | 67 ± 27 | 6.36 ± 0.37 | 12.96 ± 2.63 | 9.92 ± 0.72 | 9.48 ± 0.35 |
n = number of canines. LVEF, left ventricular (LV) ejection fraction; NT-proBNP, NH2-terminal-prohormone B-type natriuretic peptide; LVEDV, LV end-diastolic volume; LVEPD; LV diastolic pressure; LVIDd, LV internal diastolic diameter; E, early mitral inflow velocity; e′, mitral annular early diastolic velocity; EPSS, end-point septal separation.
Left Ventricular Systolic Function
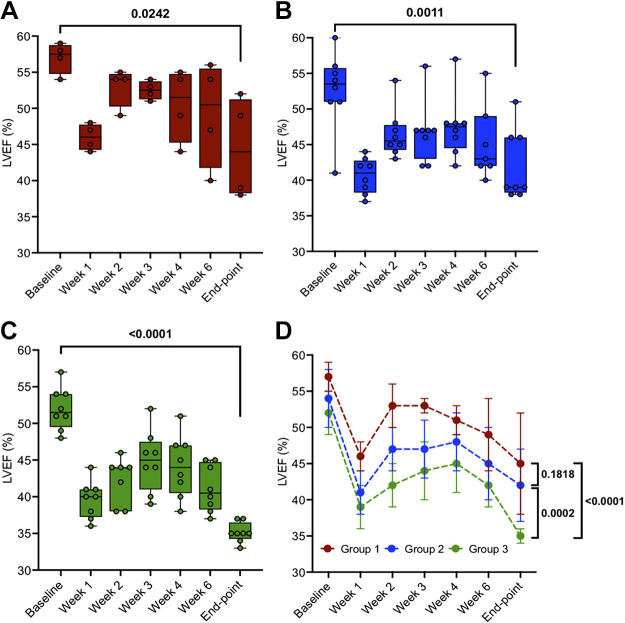
As seen in Fig. 3, from baseline to postligation week 1, LVEF decreased from 57 ± 2 to 46 ± 2% (P < 0.0001), 54 ± 4 to 41 ± 3% (P < 0.0001), and 52 ± 3 to 39 ± 3% (P < 0.0001), in groups 1 (Fig. 3A), 2 (Fig. 3B), and 3 (Fig. 3C), respectively. Beyond week 1, the systolic function partially recovered over 3–4 wk in all groups, followed by a subsequent progressive decline in LVEF. Two-tailed paired t tests indicated a significant decline in LVEF from baseline to end point for groups 1 (P = 0.0242), 2 (P = 0.0011), and 3 (P < 0.0001). The two-way mixed ANOVA model was used to evaluate LVEF outcome between the three groups from baseline to the final end point. Post hoc tests with Bonferroni correction are shown in Fig. 3D at end point between group 1 versus group 2 (P = 0.1818), group 1 versus group 3 (P < 0.0001), and group 2 versus group 3 (P = 0.0002).
Figure 3.
Left ventricular ejection fraction (LVEF) from “baseline” to “end point” in three groups: group 1, n = 4 (A), group 2, n = 8 (B), and group 3, n = 8 (C). D: means ± SD data of individual groups. Two-tailed paired t tests were used to compare data between two time points (baseline vs. end point) within the same group (A, B, and C). Two-way mixed ANOVA model for D was conducted with a post hoc test using Bonferroni correction to analyze the time effect (P < 0.0001), groups effect (P < 0.0001), and time/groups interaction effect (P = 0.6508) on LVEF. Post hoc tests with Bonferroni correction are shown at end points between the three groups.
Plasma NT-proBNP was studied along with LVEF to help assess chronic HF progression. In the 20 dogs that developed chronic HF, the NT-proBNP baseline level before coronary ligation was 300 ± 111 pmol/L. As shown in Fig. 4, compared with baseline measures, plasma NT-proBNP was elevated in all groups at week 2 (253 ± 6 vs. 1,052 ± 469 pmol/L, 356 ± 164 vs. 1,100 ± 830 pmol/L, and 266 ± 21 vs. 969 ± 420 pmol/L in group 1 (Fig. 4A), group 2 (Fig. 4B) and group 3 (Fig. 4C), respectively). Two-tailed pair t tests revealed that at final end point, groups 1– 3 exhibited a significant increase in NT-proBNP of 656 ± 248 pmol/L (P = 0.04310), 1,299 ± 1,009 pmol/L (P = 0.0217), and 1,416 ± 496 pmol/L (P = 0.0003), respectively. The two-way mixed ANOVA model was used to determine NT-proBNP outcome between groups from baseline to the final end point. As shown in Fig. 4D, post hoc tests with Bonferroni correction at the end point between the three groups were not conducted because the group effect during HF development was not statistically significant.
Figure 4.
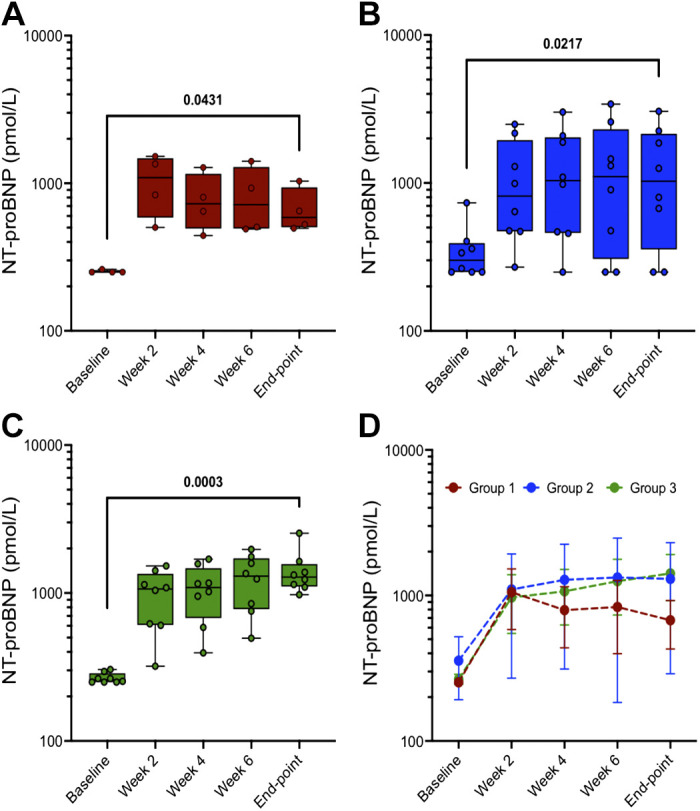
NT-proBNP from “baseline” to “end point” in three groups: group 1, n = 4 (A), group 2, n = 8 (B), and group 3, n = 8 (C). D: means ± SD data of individual groups. Two-tailed paired t tests were used to compare data between two time points (baseline vs. end point) within the same group (A, B, and C). A two-way mixed ANOVA model for D was conducted to analyze the time effect (P = 0.0005), groups effect (0.1423), and time/groups interaction effect (P = 0.9243) on NT-proBNP. The effect of groups was not statistically significant on NT-proBNP, and thus post hoc tests with Bonferroni correction are not shown at end points between the three groups. NT-proBNP, NH2-terminal-prohormone B-type natriuretic peptide.
Diastolic Dimensions and Function
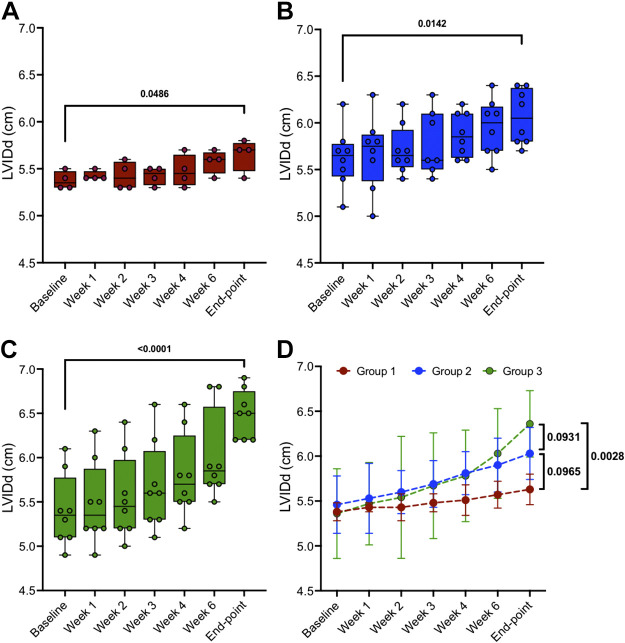
Groups 1–3 exhibited a significant change in left ventricular internal diameter at diastole (LVIDd) of 4.6, 7.5, and 19.1% from baseline to end point, respectively. For the same duration, as seen in Fig. 5, two-tailed paired t tests compared the significance of LVIDd change in group 1 (5.38 ± 0.10 to 5.63 ± 0.17 cm, P = 0.0486), group 2 (5.61 ± 0.32 to 6.03 ± 0.29 cm, P = 0.0142), and group 3 (5.34 ± 0.44 to 6.36 ± 0.37 cm, P < 0.0001). The two-way mixed ANOVA model was used to evaluate LVIDd between the three groups from baseline to the final end point. Post hoc tests with Bonferroni correction are shown in Fig. 5D at end points between group 1 versus group 2 (P = 0.0965), group 1 versus group 3 (P = 0.0028), and group 2 versus group 3 (P = 0.0931).
Figure 5.
Left ventricular internal diastolic diameter (LVIDd) from “baseline” to “end point” in three groups: group 1, n = 4 (A); group 2, n = 8 (B); and group 3, n = 8 (C). D: means ± SD data of individual groups. Two-tailed paired t tests were used to compare data between 2 time points (baseline vs. end point) within the same group (A, B, and C). A two-way mixed ANOVA model for D was conducted to analyze the time effect (P < 0.0001), groups effect (P = 0.0152), and time/groups interaction effect (P = 0.7635) on LVIDd. Post hoc tests with Bonferroni correction are shown at end point between the 3 groups.
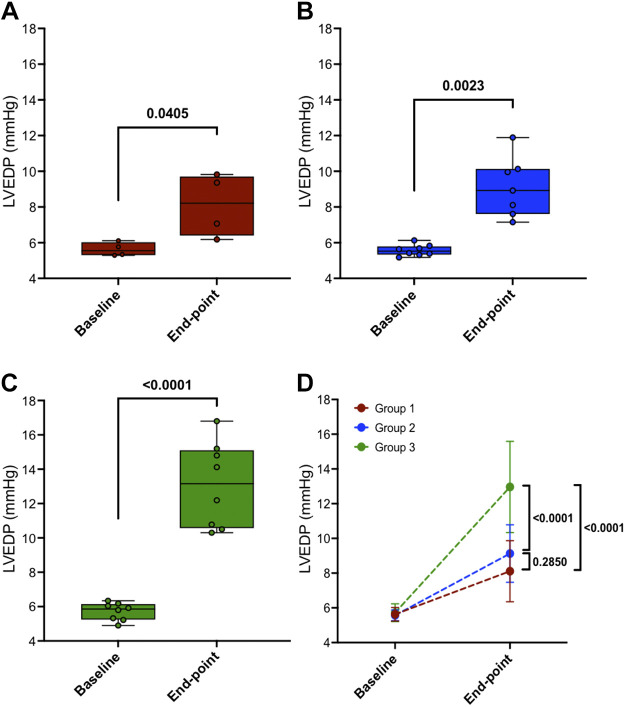
Before thoracotomy and at the final end-point surgical procedures, left ventricular end-diastolic pressure (LVEDP) was recorded in anesthetized dogs and the results are shown in Fig. 6 Two-tailed paired t tests revealed that LVEDP increased in all groups at the final end point from their baseline measures as 5.63 ± 0.39 to 8.11 ± 1.76 mmHg (P = 0.0405) in group 1, 5.57 ± 0.31 to 9.13 ± 1.655 mmHg (P = 0.0023) in group 2, and 5.72 ± 0.51 to 12.96 ± 2.63 mmHg (P < 0.0001) in group 3, as shown in Fig. 6, A–C, respectively. The two-way mixed ANOVA model was used to determine LVEDP between the three groups from baseline to the final end point. Post hoc tests with Bonferroni correction are shown in Fig. 6D at end points between group 1 versus group 2 (P = 0.2850), group 1 versus group 3 (P < 0.0001), and group 2 versus group 3 (P < 0.0001).
Figure 6.
Left ventricular end-diastolic pressure (LVEDP) from “baseline” to “end point” in three groups: group 1, n = 4 (A); group 2, n = 8 (B); and group 3, n = 8 (C). D: means ± SD data of individual groups. Two-tailed paired t tests were used to compare data between 2 time points (baseline vs. end point) within the same group (A, B, and C). A two-way mixed ANOVA model for D was conducted to analyze the time effect (P < 0.0001), groups effect (P = 0.0005), and time/groups interaction effect (P = 0.0009) on LVEDP. Post hoc tests with Bonferroni correction are shown at end points between the three groups.
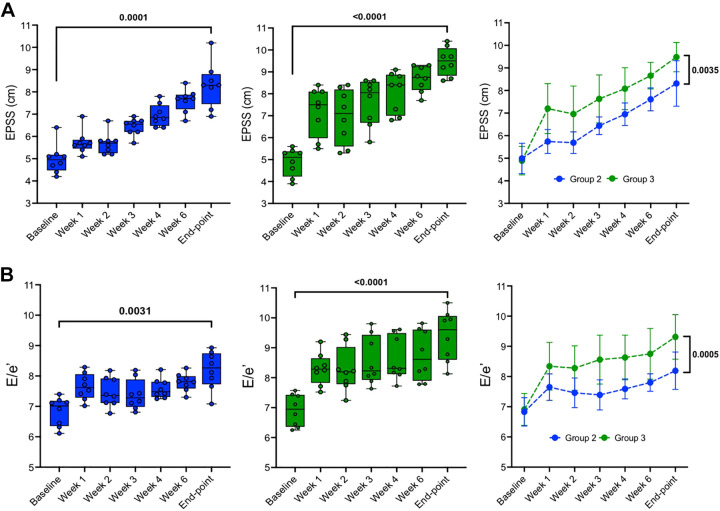
Ratio of early mitral inflow velocity to mitral annular early diastolic velocity (E/e′) and end-point septal separation (EPSS) were measured to assess LV diastolic dysfunction in groups 2 and 3, as shown in Fig. 7, A and B, respectively. From baseline to final end-point, two-tailed paired t tests showed that both groups experienced a significant increase in EPSS: group 1 went from 4.99 ± 0.67 to 8.31 ± 1.01 mm (P < 0.0001) and group 2 went from 4.89 ± 0.63 to 9.48 ± 0.65 mm (P < 0.0001), and E/e′ in group 1 went from 6.83 ± 0.47 to 8.19 ± 0.62 (P = 0.0031) and group 2 went from 6.91 ± 0.53 to 9.31 ± 0.74 (P < 0.0001). The two-way ANOVA mixed model was used to evaluate the change of EPSS and E/e′ from baseline to the final end point between groups. Post hoc tests with Bonferroni correction are shown in Fig. 7, A and B, right, and differences between two groups at end point are significant for both EPSS and E/e′, respectively.
Figure 7.
Left ventricular function data during ischemic heart failure (HF) progression. A: end-point septal separation (EPSS) data acquired in M-mode from a right parasternal long-axis view. B: E/e′ data acquired and quantified through pulse wave (PW) [tissue-Doppler imagining (TDI)] images from a 4-chamber apical view. Means ± SD of each group at same time point: group 2 (n = 8; left), group 3 (n = 8; middle), and two-tailed paired t tests (right) were used to compare data between two time points (baseline vs. end point) within the same group (left and middle). Two-way ANOVA mixed model (right) was conducted to analyze time effect, groups effect and time/groups interaction effect on EPSS (P < 0.0001, P < 0.0001, and P = 0.1253, respectively), and E/e′ (P < 0.0001, P < 0.0001, and P = 0.2021, respectively). Post hoc tests with Bonferroni correction are shown at end points between the two groups.
DISCUSSION
As outlined in Table 2, a canine model of chronic ischemic HF is often produced through single or multiple coronary microembolization. The early mortality rate of ischemia by microembolization varies between 30 and 60% (8, 10, 11, 18). Ameroid constrictors, another method to achieve ischemic cardiomyopathy, consists of the placement of constrictors on proximal portions of the LAD and left circumflex (LCx) coronary arteries. The ameroid approach results in an early mortality rate of ∼45% (13). In our chronic ischemic HF canine model (n = 29), we had an early mortality by postligation 48 h of 17% (n = 5/29) and overall mortality by postligation week 5 of 31% (n = 20/29). For group 3, as shown in Fig. 1, the TLD technique produced an early mortality of 9% with a 100% success rate of developing chronic ischemic HF.
Table 2.
Previously reported chronic ischemic HF canine models
| Canine Model, n | Procedure Type to Induce MI | Criteria to Evaluate HF, %LVEF | Mortality Rate, % | Time to Develop HF, wk | Reported Parameters in Developed Chronic Ischemic HF Models |
Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| EF, % | LVEDP, mmHg | LV infarct, % | Others | ||||||
| 20 | I/R–proximal and distal LAD and major diagonal branches | <40 | NA | 4–5 | <40 | 15 ± 3.5 | 16.3 ± 2.6 | NT-proBNP: 4,081 ± 1,123 pmol/L | 34 |
| 20 | Single coronary microembolization into LCx | <35 | 55% | 44 | 23 | 14.9 ± 2.5 | 23.1 ± 2.6 | NA | 10 |
| 17 | LAD coronary artery occlusion | <35 | NA | 4–5 | 31.7 ± 4.6 | NA | 11 ± 0.4 | PCWP: 18 ± 1.2 mmHg | 35 |
| 22 | Ameroid constrictors placement on proximal LAD, LCx and their major branches | NA | 45% | 6 | NA | NA | NA | ΔLVEDA: 36%↑ | 13 |
| ΔLVESA: 86%↑ | |||||||||
| ΔLV wall thickening: 57↓ | |||||||||
| 7 | Proximal and distal LAD and major diagonal branches | <35 | 0% | 8–10 | 28.7 ± 5.4 | NA | NA | ΔLVEDV: 14%↑ | 36 |
| ΔLVESV: 25%↑ | |||||||||
| 21 | Proximal LAD | >45 | 0% | 3 | >45 | ≥18 | NA | NA | 37 |
| 7 | I/R–proximal and distal LAD, LCx and D1 LAD | NA | NA | 4 | 48 ± 6 | 16.8 ± 6.4 | 13.3 ± 2.5 | NT-proBNP: 1,778 ± 195 pmol/L | 38 |
| 20 | Sequential coronary microembolization into LCx | <35 | 30% | 13 | 21 ± 1 | 22 ± 3 | 21 ± 3 (anterior) | ΔLVEDV: 40%↑ | 12 |
| 17 ± 2 (posterior) | PNE: 791 ± 131 pg/mL | ||||||||
| 32 | LAD coronary artery balloon occlusion | NA | 25% | 6 | NA | NA | 37.1 ± 8.2 | NA | 18 |
| 12 | Sequential coronary microembolization into LCx | <35 | NA | 17–18 | 23 ± 2 | 22 ± 3 | 22 ± 3 | ΔLVEDV: 35%↑ | 39 |
| ΔLVESV: 144%↑ | |||||||||
| 7 | Sequential coronary microembolization into LCx | 30–40 | NA | 18 | 26 ± 1 | 20 ± 5 | NA | ΔLVEDV: 28%↑ | 40 |
| PNE: 569 ± 44 pg/mL | |||||||||
| 15 | Sequential coronary microembolization into LCx | <40 | 60% | 8 | 35 ± 3 | NA | NA | ΔLVEDV: 22%↑ | 8 |
| PNE: 715 ± 276 pg/mL | |||||||||
| 8 | Sequential coronary microembolization into LCx | ≤35 | NA | 16 | 33 | NA | NA | ΔLVEDV: 11%↑ | 41 |
| ΔLVESV: 17%↑ | |||||||||
n = number of canines. LVEF, left ventricular (LV) ejection fraction; NT-proBNP, NH2-terminal prohormone B-type natriuretic peptide; LVEPD; LV diastolic pressure, LVEDV, LV end-diastolic volume; LVESV, LV end-systolic volume; Δ, change from the baseline to the final end point once the model reached chronic ischemic heart failure (HF) condition; PNE, plasma norepinephrine; LCx, left circumflex artery.
In all groups, in the first-month postligation, LVEF rapidly declined, followed by partial recovery as surviving animals transitioned from acute ischemic HF to physiologically chronic ischemic HF. A partial recovery of LV function after acute coronary occlusion has been reported (19). It may be due to subsequent recovery of stunned myocardium with the increased collateral flow, i.e., transient rather than permanent injury to cardiomyocytes (20). Beyond week 1, systolic function recovered over 2–3 wk and then slowly declined as the LV dilated and both systolic and diastolic function, worsened as indicated by cardiac biomarker and echocardiogram measurements. The slower secondary decline is consistent with cardiac remodeling and the slow development of chronic HF.
NT-proBNP is a cardiac-specific clinical biomarker secreted when stretch occurs within the ventricular myocardium (21). It is a derivative of BNP and in dogs, NT-proBNP is correlated with heart size and systolic function (22). Previously, NT-proBNP level > 900 pmol/L has been used as a threshold for evaluating dilated cardiomyopathy in canine model (23). Also, the concentration of the NT-proBNP was increased in dogs with advanced mitral valve disease (MVD) and dilated cardiomyopathy (DCM) (24–26). Oyama et al. (22) and Schober et al. (26) reported median serum NT-proBNP of >445 and 848 pmol/L, respectively, as a significant value to discriminate dogs with MVD but without congestive HF from control dogs. Furthermore, MVD dogs with congestive HF and DCM have elevated NT-proBNP levels of 2,750 and 3,830 pmol/L, respectively. Our results are in close agreement with these clinical studies (27, 28). Note, we presented the log-transformed levels of plasma NT-proBNP, consistent with the log-normal distribution of this biomarker (29, 30).
As reported in previous clinical studies (31, 32), patients with end-stage renal function (chronic hemodialysis) may have increased NT-proBNP, coinciding with worsening LVEF (<35%). In our study, from baseline to final end point, subjects (n = 20) exhibited a significant increase in NT-proBNP level from 300 ± 111 to 1,221 ± 746 pmol/L, P < 0.0001, despite unchanged creatine levels (0.82 ± 0.11 vs. 0.92 ± 0.14 mg/dL, P = 0.3422), and thus NT-proBNP data are not likely to be confounded by renal dysfunction.
As shown in Table 1, the approximate timeline to develop chronic ischemic HF was 13 ± 3 wk for group 2 and 11 ± 2 wk for group 3, but this difference between the two groups was statistically not significant (P = 0.2119). Still, group 3 exhibited a 100% success rate of ischemic HF development with a higher magnitude of LV systolic and diastolic dysfunction compared with group 2. Furthermore, the TLD surgical approach (group 3) resulted in a reduced mortality rate compared with groups 1 and 2. This form of coronary ligation resulted in more extensive loss of LV function at week 1 (Fig. 3) and increased development of chronic HF. Given these results, we believe that TLD is an optimized approach to achieving chronic ischemic cardiomyopathy and heart failure with reduced ejection fraction in the canine heart.
The present study focuses on evaluating LV function, LV dilation, and evaluation of biomarkers indicative of the development of chronic ischemic HF. Future studies to quantify transmurality and size of infarct, arrhythmic burden, and stability of the HF over extended periods will be needed to further characterize these models of ischemic HF. We anticipate that this canine model of TLD-induced ischemic HF will be a useful resource for studying the development and progression of HF and testing of novel therapeutics.
In summary, our study indicates that a complete LAD coronary ligation, including ligation of collateral circulation branches, can create a robust canine model of chronic ischemic HF that is optimal for animal survival and the development of dilated cardiomyopathy. Furthermore, our study indicates that the TLD approach in canines is highly reproducible, with minimal early mortality and a 100% success in developing a robust preclinical model of ischemic HF.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01HL128752 and R21HL156039 (to D.J.D.), R21AG074593 (to R.M.S. and T.H.), R01HL152691 (to R.M.S.), and UL1TR002538 and KL2TR002539 (to G.L.H.) and Nora Eccles Treadwell Foundation research grants (to D.J.D. and R.M.S.). M.S.K. is supported through NIH Training Grant 5T32HL007576-035.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S., R.R., R.M.S., and D.J.D. conceived and designed research; M.S.K., D.S., Y.I., A.M.H., E.O., S.R.C., O.G., J.A.P., and L.K. performed experiments; M.S.K. and H.L. analyzed data; M.S.K., D.S., T.H., G.L.H., R.M.S., and D.J.D. interpreted results of experiments; M.S.K. and D.S. prepared figures; M.S.K. drafted manuscript; M.S.K., D.S., L.K., G.L.H., C.H.S., R.M.S., and D.J.D. edited and revised manuscript; M.S.K., D.S., Y.I., A.M.H., E.O., S.R.C., O.G., H.L., J.A.P., L.K., T.H., G.L.H., R.R., C.H.S., R.M.S., and D.J.D. approved final version of manuscript.
REFERENCES
- 1. Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314: H812–H838, 2018. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riehle C, Bauersachs J. Small animal models of heart failure. Cardiovasc Res 115: 1838–1849, 2019. doi: 10.1093/cvr/cvz161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS. Differences between human and mouse embryonic stem cells. Dev Biol 269: 360–380, 2004. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 4. Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail 2: 262–271, 2009. doi: 10.1161/CIRCHEARTFAILURE.108.814459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hearse DJ, Sutherland FJ. Experimental models for the study of cardiovascular function and disease. Pharmacol Res 41: 597–603, 2000. doi: 10.1006/phrs.1999.0651. [DOI] [PubMed] [Google Scholar]
- 6. Spannbauer A, Traxler D, Zlabinger K, Gugerell A, Winkler J, Mester-Tonczar J, Lukovic D, Müller C, Riesenhuber M, Pavo N, Gyöngyösi M. Large animal models of heart failure with reduced ejection fraction (HFrEF). Front Cardiovasc Med 6: 117, 2019. doi: 10.3389/fcvm.2019.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Recchia FA, Lionetti V. Animal models of dilated cardiomyopathy for translational research. Vet Res Commun 31,Suppl1: 35–41, 2007. doi: 10.1007/s11259-007-0005-8. [DOI] [PubMed] [Google Scholar]
- 8. Adamson PB, Vanoli E. Early autonomic and repolarization abnormalities contribute to lethal arrhythmias in chronic ischemic heart failure: characteristics of a novel heart failure model in dogs with postmyocardial infarction left ventricular dysfunction. J Am Coll Cardiol 37: 1741–1748, 2001. doi: 10.1016/s0735-1097(01)01185-8. [DOI] [PubMed] [Google Scholar]
- 9. White FC, Roth DM, Bloor CM. The pig as a model for myocardial ischemia and exercise. Lab Anim Sci 36: 351–356, 1986. [PubMed] [Google Scholar]
- 10. Franciosa JA, Heckel R, Limas C, Cohn JN. Progressive myocardial dysfunction associated with increased vascular resistance. Am J Physiol Heart Circ Physiol 239: H477–H482, 1980. doi: 10.1152/ajpheart.1980.239.4.H477. [DOI] [PubMed] [Google Scholar]
- 11. Smiseth OA, Lindal S, Mjøs OD, Vik-Mo H, Jørgensen L. Progression of myocardial damage following coronary microembolization in dogs. Acta Pathol Microbiol Immunol Scand A 91: 115–124, 1983. doi: 10.1111/j.1699-0463.1983.tb02735.x. [DOI] [PubMed] [Google Scholar]
- 12. Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol Heart Circ Physiol 260: H1379–H1384, 1991. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 13. Firoozan S, Wei K, Linka A, Skyba D, Goodman NC, Kaul S. A canine model of chronic ischemic cardiomyopathy: characterization of regional flow-function relations. Am J Physiol Heart Circ Physiol 276: H446–H455, 1999. doi: 10.1152/ajpheart.1999.276.2.H446. [DOI] [PubMed] [Google Scholar]
- 14.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 15. Greene HL, Roden DM, Katz RJ, Woosley RL, Salerno DM, Henthorn RW. The cardiac arrhythmia suppression trial: first CAST … then CAST-II. J Am Coll Cardiol 19: 894–898, 1992. doi: 10.1016/0735-1097(92)90267-q. [DOI] [PubMed] [Google Scholar]
- 16. Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 324: 781–788, 1991. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 17. Akhtar M, Breithardt G, Camm AJ, Coumel P, Janse MJ, Lazzara R, Myerburg RJ, Schwartz PJ, Waldo AL, Wellens HJ. CAST and beyond. Implications of the Cardiac Arrhythmia Suppression Trial. Task Force of the Working Group on Arrhythmias of the European Society of Cardiology. Circulation 81: 1123–1127, 1990. doi: 10.1161/01.cir.81.3.1123. [DOI] [PubMed] [Google Scholar]
- 18. Basso C, Thiene G, Della Barbera M, Angelini A, Kirchengast M, Iliceto S. Endothelin A-receptor antagonist administration immediately after experimental myocardial infarction with reperfusion does not affect scar healing in dogs. Cardiovasc Res 55: 113–121, 2002. doi: 10.1016/s0008-6363(02)00340-1. [DOI] [PubMed] [Google Scholar]
- 19. Theroux P, Ross J Jr, Franklin D, Covell JW, Bloor CM, Sasayama S. Regional myocardial function and dimensions early and late after myocardial infarction in the unanesthetized dog. Circ Res 40: 158–165, 1977. doi: 10.1161/01.res.40.2.158. [DOI] [PubMed] [Google Scholar]
- 20. Lindal S, Smiseth OA, Mjøs OD, Myklebust R, Jørgensen L. Reversible and irreversible changes in the dog heart during acute left ventricular failure due to experimental multifocal ischaemia. Acta Pathol Microbiol Immunol Scand A 94: 177–186, 1986. doi: 10.1111/j.1699-0463.1986.tb02983.x. [DOI] [PubMed] [Google Scholar]
- 21. Borgarelli M, Ferasin L, Lamb K, Chiavegato D, Bussadori C, D'Agnolo G, et al. The predictive value of clinical, radiographic, echocardiographic variables and cardiac biomarkers for assessing risk of the onset of heart failure or cardiac death in dogs with preclinical myxomatous mitral valve disease enrolled in the DELAY study. J Vet Cardiol 36: 77–88, 2021. doi: 10.1016/j.jvc.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 22. Oyama MA, Fox PR, Rush JE, Rozanski EA, Lesser M. Clinical utility of serum N-terminal pro-B-type natriuretic peptide concentration for identifying cardiac disease in dogs and assessing disease severity. J Am Vet Med Assoc 232: 1496–1503, 2008. doi: 10.2460/javma.232.10.1496. [DOI] [PubMed] [Google Scholar]
- 23. Leto L, Testa M, Feola M. The predictive value of plasma biomarkers in discharged heart failure patients: role of plasma NT-proBNP. Minerva Cardioangiol 64: 157–164, 2016. [PubMed] [Google Scholar]
- 24. Xu L, Chen Y, Ji Y, Yang S. Influencing factors of NT-proBNP level inheart failure patients with different cardiacfunctions and correlation with prognosis. Exp Ther Med 15: 5275–5280, 2018. doi: 10.3892/etm.2018.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fine DM, DeClue AE, Reinero CR. Evaluation of circulating amino terminal-pro-B-type natriuretic peptide concentration in dogs with respiratory distress attributable to congestive heart failure or primary pulmonary disease. J Am Vet Med Assoc 232: 1674–1679, 2008. doi: 10.2460/javma.232.11.1674. [DOI] [PubMed] [Google Scholar]
- 26. Schober KE, Hart TM, Stern JA, Li X, Samii VF, Zekas LJ, Scansen BA, Bonagura JD. Detection of congestive heart failure in dogs by Doppler echocardiography. J Vet Intern Med 24: 1358–1368, 2010. doi: 10.1111/j.1939-1676.2010.0592.x. [DOI] [PubMed] [Google Scholar]
- 27. Lam CSP, Li YH, Bayes-Genis A, Ariyachaipanich A, Huan DQ, Sato N, Kahale P, Cuong TM, Dong Y, Li X, Zhou Y. The role of N-terminal pro-B-type natriuretic peptide in prognostic evaluation of heart failure. J Chin Med Assoc 82: 447–451, 2019. doi: 10.1097/JCMA.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 28. Rørth R, Jhund PS, Yilmaz MB, Kristensen SL, Welsh P, Desai AS, Køber L, Prescott MF, Rouleau JL, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV. Comparison of BNP and NT-proBNP in patients with heart failure and reduced ejection fraction. Circ Heart Fail 13: e006541, 2020. doi: 10.1161/CIRCHEARTFAILURE.119.006541. [DOI] [PubMed] [Google Scholar]
- 29. Chin KM, Rubin LJ, Channick R, Di Scala L, Gaine S, Galiè N, Ghofrani HA, Hoeper MM, Lang IM, McLaughlin VV, Preiss R, Simonneau G, Sitbon O, Tapson VF. Association of N-terminal pro brain natriuretic peptide and long-term outcome in patients with pulmonary arterial hypertension. Circulation 139: 2440–2450, 2019. doi: 10.1161/CIRCULATIONAHA.118.039360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schou M, Gustafsson F, Kjaer A, Hildebrandt PR. Long-term clinical variation of NT-proBNP in stable chronic heart failure patients. Eur Heart J 28: 177–182, 2007. doi: 10.1093/eurheartj/ehl449. [DOI] [PubMed] [Google Scholar]
- 31. Srisawasdi P, Vanavanan S, Charoenpanichkit C, Kroll MH. The effect of renal dysfunction on BNP, NT-proBNP, and their ratio. Am J Clin Pathol 133: 14–23, 2010. doi: 10.1309/AJCP60HTPGIGFCNK. [DOI] [PubMed] [Google Scholar]
- 32. Austin WJ, Bhalla V, Hernandez-Arce I, Isakson SR, Beede J, Clopton P, Maisel AS, Fitzgerald RL. Correlation and prognostic utility of B-type natriuretic peptide and its amino-terminal fragment in patients with chronic kidney disease. Am J Clin Pathol 126: 506–512, 2006. doi: 10.1309/M7AAXA0J1THMNCDF. [DOI] [PubMed] [Google Scholar]
- 34. Saku K, Kakino T, Arimura T, Sunagawa G, Nishikawa T, Sakamoto T, Kishi T, Tsutsui H, Sunagawa K. Left ventricular mechanical unloading by total support of impella in myocardial infarction reduces infarct size, preserves left ventricular function, and prevents subsequent heart failure in dogs. Circ Heart Fail 11: e004397, 2018. doi: 10.1161/CIRCHEARTFAILURE.117.004397. [DOI] [PubMed] [Google Scholar]
- 35. Suzuki M, Asano H, Tanaka H, Usuda S. Development and evaluation of a new canine myocardial infarction model using a closed-chest injection of thrombogenic material. Jpn Circ J 63: 900–905, 1999. doi: 10.1253/jcj.63.900. [DOI] [PubMed] [Google Scholar]
- 36. Kim WG, Shin YC, Hwang SW, Lee C, Na CY. Comparison of myocardial infarction with sequential ligation of the left anterior descending artery and its diagonal branch in dogs and sheep. Int J Artif Organs 26: 351–357, 2003. doi: 10.1177/039139880302600411. [DOI] [PubMed] [Google Scholar]
- 37. He KL, Dickstein M, Sabbah HN, Yi GH, Gu A, Maurer M, Wei CM, Wang J, Burkhoff D. Mechanisms of heart failure with well preserved ejection fraction in dogs following limited coronary microembolization. Cardiovasc Res 64: 72–83, 2004. doi: 10.1016/j.cardiores.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 38. Arimura T, Saku K, Kakino T, Nishikawa T, Tohyama T, Sakamoto T, Sakamoto K, Kishi T, Ide T, Sunagawa K. Intravenous electrical vagal nerve stimulation prior to coronary reperfusion in a canine ischemia-reperfusion model markedly reduces infarct size and prevents subsequent heart failure. Int J Cardiol 227: 704–710, 2017. doi: 10.1016/j.ijcard.2016.10.074. [DOI] [PubMed] [Google Scholar]
- 39. Gupta RC, Shimoyama H, Tanimura M, Nair R, Lesch M, Sabbah HN. SR Ca2+-ATPase activity and expression in ventricular myocardium of dogs with heart failure. Am J Physiol Heart Circ Physiol 273: H12–H18, 1997. doi: 10.1152/ajpheart.1997.273.1.H12. [DOI] [PubMed] [Google Scholar]
- 40. Sabbah HN, Shimoyama H, Kono T, Gupta RC, Sharov VG, Scicli G, Levine TB, Goldstein S. Effects of long-term monotherapy with enalapril, metoprolol, and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 89: 2852–2859, 1994. doi: 10.1161/01.cir.89.6.2852. [DOI] [PubMed] [Google Scholar]
- 41. Morita H, Khanal S, Rastogi S, Suzuki G, Imai M, Todor A, Sharov VG, Goldstein S, O'Neill TP, Sabbah HN. Selective matrix metalloproteinase inhibition attenuates progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Am J Physiol Heart Circ Physiol 290: H2522–7, 2006. doi: 10.1152/ajpheart.00932.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.