Keywords: female, hyperthermia, hormones, heat illness, exercise
Abstract
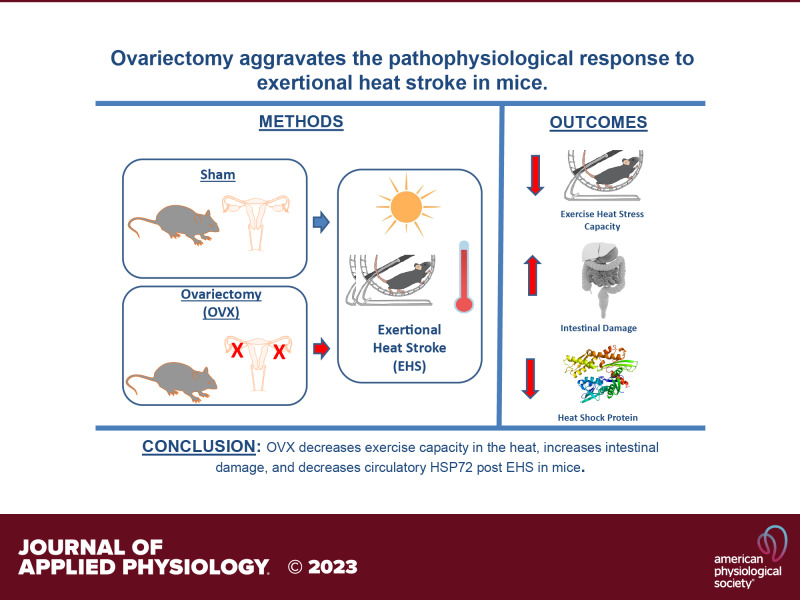
Female mice have a greater capacity for exercising in the heat than male mice, reaching greater power output and longer times of heat exposure before succumbing to exertional heat stroke (EHS). Differences in body mass, size, or testosterone do not explain these distinct sex responses. Whether the ovaries could account for the superior exercise capacity in the heat in females remains unknown. Here, we determined the influence of ovariectomy (OVX) on exercise capacity in the heat, thermoregulation, intestinal damage, and heat shock response in a mouse EHS model. We performed bilateral OVX (n = 10) or sham (n = 8) surgeries in young adult (4 mo) female C57/BL6J mice. Upon recovery from surgeries, mice exercised on a forced wheel placed inside an environmental chamber set at 37.5 °C and 40% relative humidity until experiencing loss of consciousness (LOC). Terminal experiments were performed 3 h after LOC. OVX increased body mass by the time of EHS (sham = 3.8 ± 1.1, OVX = 8.3 ± 3.2 g, P < 0.05), resulted in shorter running distance (sham = 753 ± 189, OVX = 490 ± 87 m, P < 0.05), and shorter time to LOC (sham = 126.3 ± 21, OVX = 99.1 ± 19.8 min, P < 0.05). Histopathological assessment of the intestines revealed damage in the jejunum (sham = 0.2 ± 0.7, OVX = 2.1 ± 1.7 AU, P < 0.05) and ileum (sham = 0.3 ± 0.5, OVX = 1.8 ± 1.4 AU, P < 0.05). OVX increased mesenteric microvascular density (sham = 101 ± 25, OVX = 156 ± 66 10−2 mm/mm2, P < 0.05) and decreased concentration of circulatory heat shock protein 72 (HSP72) (sham = 26.7 ± 15.8, OVX = 10.3 ± 4.6 ng/mL, P < 0.05). No differences were observed in cytokines or chemokines between groups. Our findings indicate that OVX aggravates the pathophysiological response to EHS in mice.
NEW & NOTEWORTHY Females outperform males in a mouse model of exertional heat stroke (EHS). Here, we show for the first time the impact of ovariectomy (OVX) on EHS pathophysiology. OVX resulted in a shorter exercise capacity in the heat, greater intestinal damage, and lower heat shock response following EHS.
INTRODUCTION
Exertional heat stroke (EHS) is the most severe manifestation of exertional heat illnesses and one of the main causes of death in athletes during all types of physical activity (1, 2). Environmental aspects such as climate change and increases in heat wave’s frequency and duration are additional contributing factors to the increased risk of EHS occurrence in the general population (3). The rate at which women will experience EHS is expected to increase markedly in the upcoming years. This prediction is supported by the increasing participation of women in multiple competitive sports modalities as well as military specialty roles previously accessible only to men (4). Indeed, increased rates of EHS in females can already be observed in military populations. The US armed forces reported an increase of nearly 12% in EHS cases among female warfighters (5, 6). The risk is comparable to males, whereas, in the past, the rate in males exceeded that of females (5–7). The lack of studies addressing the physiological responses of females to EHS has generated a gap in knowledge regarding their susceptibility to heat-related illnesses (8). Understanding the fundamental factors associated with the EHS response in females is necessary to develop therapeutic strategies tailored specifically to this population (9–11).
Preclinical evidence suggests some relevant physiological sex differences in response to EHS (12). Previously, we observed that female mice exercise ∼40% longer than male mice in the heat before loss of consciousness (LOC) due to EHS (12). This greater exercise capacity could not be explained by differences in body mass and size between biological sexes. A recent study reported that castration in male mice did not interfere with males’ exercise capacity during the EHS trial (13), suggesting that testosterone does not explain the physiological sex differences. Given that physical differences (12) and testosterone (13) do not explain these distinct responses to EHS between female and male mice, we hypothesized that the ovaries could play a role in female’s unique physiological response to EHS.
The ovaries are a key component of the female reproductive system as they produce and secrete estrogens [e.g., estrone, estradiol (17β-estradiol), and estriol] and progesterone (14). These hormones have been shown to influence body temperature through effects on neuronal and cardiovascular components (4). In mammals (e.g., humans and rodents), the thermoregulatory set point is increased in the presence of high levels of progesterone and decreased in the presence of high levels of estradiol (15, 16). When examining the autonomic influences of estrogens and progesterone, estradiol promotes vasodilation, whereas progesterone promotes heat conservation (17). To date, no one has examined whether ovary-related hormones can explain the higher exercise capacity in the heat observed in female mice and whether they influence organ damage in response to EHS. The intestines are among the main organs damaged by EHS (18). The intestines are highly vascularized with microvessels and undergo ischemia-reperfusion during exercise heat stress, which leads to increased permeability often allowing bacteria and toxins to leak through the intestinal wall (19–21). Therefore, it remains unknown whether the ovaries play a protective role against gut damage and/or preserve intestinal microvascular function after EHS.
In the present study, we set out to determine the impact of ovariectomy (OVX) on the physiological and pathophysiological response of mice exposed to EHS. We measured exercise capacity and thermoregulation leading to EHS as well as intestinal damage, mesenteric microvascular function, heat shock response, and inflammatory markers at 3 h after LOC. We hypothesized that due to the influence of ovary-related hormones on thermoregulation and potentially organ damage (15), OVX would increase EHS severity in mice.
METHODS
Animal Care and Housing
Eighteen young adult (18-wk-old at time of purchase; sham n = 8; OVX n = 10) female C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were studied. All protocols were approved by the Institutional Animal Care and Use Committee (#20200061) reported information about the procedures conformed to the Animal Research Reporting of in vivo Experiments—ARRIVE guidelines (22). Mice were housed in standard 7.25” (W) × 11.75” (L) × 5” (H) polycarbonate cages containing corncob bedding. The housing room was maintained at controlled environmental conditions at 22.5°C with a relative humidity (RH) of 30%–45% and 12-h light cycle (light 0700–1900). Water and food were provided ad libitum throughout the entire experiment. Mice were housed in groups until the day of surgery and then were housed individually until the terminal end point of the experiment.
Survival Surgeries
E-Mitter implantation.
For placement of the telemetry devices (miniature reusable battery-free radiotelemetry device; 16.5 × 6.5 mm, G2 E-Mitter; Starr Life Sciences, Oakmont, PA), animals were anesthetized with isoflurane (4%, 0.4–0.6 L/min of O2 flow) in an induction chamber. Upon induction, animals were placed under continuous anesthesia via a nose cone (1.5%, 0.6 L/min). Eye lube was used to protect the animal’s eyes from damage or injury during surgery. To prepare for the surgical site, the lower back was shaved with small animal hair clippers. A single dose of slow-release buprenorphine (Ethiqa, 1.3 mg/mL) was administered subcutaneously during this time. The skin was scrubbed with three washes of povidone-iodine followed by 70% isopropyl alcohol rinse. The animal was then transferred to the surgical area. An adhesive drape was used to isolate the surgical site on the mouse. Using sterile instruments and aseptic technique, a ∼1 cm incision was made on the midline along the lumbar spine, ∼0.5 cm from the costal margin. Then, the skin was separated from the muscle layer and a slightly smaller incision was carefully made on the muscle layer. Once the muscle layer was opened, the sterile telemeter was placed into the intraperitoneal cavity in front of the caudal arteries and veins and dorsal to the digestive organs to allow it to float freely. All telemeters were cleaned with soap and water, followed by immersion in sterilization solutions (following the manufacturer’s recommendation for dilution and immersion time) to sterilize the telemeters. The same skin and muscle incision was used for the ovariectomy and sham procedures.
Ovariectomy and sham procedures.
Bilateral OVX was performed as demonstrated by Ström et al. (23). In brief, sterile tweezers were used to gently pull out the adipose tissue that surrounds the ovaries. Upon visual identification of the ovaries, a cauterizer was used at the uterine horn to remove the ovary. This procedure was repeated on the other side of the body for bilateral OVX. Animals in the sham group underwent a control surgery consisting of incision of the skin and muscle layers, but without removing the ovaries. After the completion of the procedure, the muscle layer was suture closed with a sterile 5-0 absorbable suture and the skin was closed using simple interrupted stitches with 5-0 proline suture. All animals were monitored twice a day to verify wound healing and clinical signs of pain and distress. After 4 wk of recovery, animals performed the familiarization and EHS trial.
Familiarization to the Running Wheels
Running wheels (Model 0297-0521; Columbus Instruments, Columbus, OH) were placed into the cages 4 wk post surgery to allow for voluntary wheel running. After 3 wk, mice were familiarized in a rodent environmental chamber (Powers Scientific Doylestown, PA). During this week of familiarization, mice underwent four consecutive days of forced wheel training on a wheel running bed (Model 80800 A-10; Lafayette Instruments, Lafayette, IN) mimicking the exercise component of the EHS protocol (described in detail below) to familiarize mice. Wheel speed started at 3 m/min and increased by 0.5 m/min every 10 min (Scurry Activity Monitoring Software; Lafayette Instruments, Lafayette, IN) for 60 min once a day. After the familiarization days, mice were allowed up to four rest days before the EHS experimental day.
Exertional Heat Stroke Protocol
We used our preclinical model of EHS as previously described (24). There are two versions of this EHS protocol. A severe version, in which animals are kept in the heat post loss of consciousness (LOC), and a rapid cooling version in which animals are immediately removed out of the chamber after LOC. In brief, mice were weighed and placed in a forced running wheel (Model 80840; Lafayette, Lafayette, IN) in the chamber set to 37.5°C, 40% RH. Mice were allowed to rest in the wheels to allow the chamber to reach target temperature. When the chamber reached 37.5°C, the EHS protocol started. Running wheel speed began at 3.0 m/min and increased 0.5 m/min every 10 min until it reached 9.5 m/min, at which point that speed was maintained until LOC. Core temperature was monitored (VitalView; STARR Life Sciences Corp., Oakmont, PA) throughout the entirety of the EHS protocol. The EHS trial was halted once mice achieved LOC as determined by three consecutive wheel revolutions without any response from the animal. Mice were immediately removed from wheels, weighed, and returned to their original cages at room temperature (22.5°C, 45% RH). Core temperature was monitored for 3 h after loss of consciousness (LOC), when tissue harvesting began. We initially intended to use the severe version of the EHS protocol. However, two OVX mice died following LOC. We then switched to the rapid cooling version of the protocol. Therefore, only body weight, run time, distance, LOC temperature, and ascending heat load data were usable in these mice. These mice were replaced by two additional mice in the OVX group. All data collected from the two fatalities were included in the analysis. This justifies the n = 10 for some OVX parameters. The body surface area (BSA in cm2) was calculated using Meeh’s equation, i.e., BSA = k × mass2/3 (25). This equation predicts BSA from mass across all species of animals, with the k constant changing with different species and even between different strains of mice. Cheung et al. (26) reported k = 9.822 ± 0.09, applicable to nonobese, C57BL/6J mice of both sexes, and this was the equation used in this experiment. External mechanical power output was calculated in milliwatts (mW) by the equation: [mass (kg) × 9.806 m/s2 × running speed (m/s) × 1,000]. Ascending thermal area was used as an index of thermal load and calculated as the area under the curve with the core temperature baseline set at 39.5°C.
Mesenteric Microvascular Function Assessment and Whole Blood Collection
We measured the mesenteric microvascular function using intravital microscopy 3 h after LOC. The mesentery was chosen because of its proximity to the intestines, where damage is known to occur in response to EHS (18), and due to its high vascularization. Mice were anesthetized with isoflurane (3%) in room air at 100 mL/min flow rate and placed in the supine position on a heated platform (37°C). Subsequently, an incision was made along the linea alba, and the intestines were mobilized, gently exteriorized, and placed into a warm isotonic 0.9% saline bath (37°C). The cecum was identified and positioned for intravital microscopy. Intravital microscopy was performed with a handheld video capillary microscope (CapiScope HVCS, KK Technology, Honiton, UK) to view the mesenteric microcirculation. The intravital microscope is equipped with a side stream dark-field (SDF) camera that uses green light-emitting stroboscopic diodes (540 nm). The green light is primarily absorbed by hemoglobin in red blood cells (RBCs), which allows RBCs to be viewed in contrast to the background as they flow through the microcirculation, as described previously (27). Video of the mesenteric microcirculation was recorded and analyzed using an automated capture and analysis system (GlycoCheck, MicroVascular Health Solutions LLC, Alpine, UT). For each mouse, 24 trials were acquired with each trial consisting of several 2-s video recordings that capture 5,000 microvessel segments that are 10 μm in length and range from 4 to 25 μm in perfused diameter. After two to three trials are obtained in an area, the microscope is moved to a different area of the cecum to counterbalance the spatial heterogeneity within the cecal mesenteric microcirculation. After 24 trials are completed, the automated analysis system determines functional outcomes in perfused microvessel segments. The measurements lasted ∼15 min per mouse. Immediately after microvascular function assessment, a heparinized blood sample was collected through transthoracic cardiac puncture and placed on ice.
Intestines Collection, Microscopy, and Grading
We collected and prepared the intestines for histopathological analyses according to the protocol described by Williams et al. (28). In brief, intestines were flushed with a phosphate buffer solution (1× PBS) and suspended in formalin for histological analysis. Intestines were sectioned into regions of interest (duodenum, jejunum, and ileum) before being delivered to the Molecular Core Pathology Laboratory at the University of Florida. Intestines were then paraffin processed and embedded before being sectioned onto microscopy slides for histochemistry using hematoxylin and eosin stain to quantify intestinal villi damage and damage to duodenum, jejunum, and ileum.
Histological villi grading was based on the methodology developed by Chiu et al. (29). All slides had regions marked but no indication of treatment. Two independent graders, blinded to the experimental conditions to increase rigor, measured villi height, villi width, and scored villi integrity on a scale of 0–5. Zero signified an intact villus with no damage, whereas five signified a villus with a disintegration of lamina propria, ulceration, or hemorrhage. Each grader chose a villus at random and went in a counterclockwise motion grading one villus before moving to the next two villi and grading again. To minimize the influence of lost villus on intestinal slides, any intestinal section missing villi had a grade of zero added to the averaging of the score. This was done to be more conservative and if a difference in damage occurred, it would not be due to a lack of grading of villus integrity or averaging the scores by a lower number.
Circulatory HSP72, Cytokines, and Chemokines Analyses
Blood samples were spun at 4°C, 2,000 relative centrifugal field (RCF) for 10 min. Plasma was aliquoted and stored at −80°C. Plasma heat shock protein 72 (HSP72) was analyzed using a commercially available ELISA kit (Mouse HSP72 ELISA kit, MyBioSource, CA). Plasma cytokines and chemokines were analyzed using a multiplex panel (Cytokine/chemokine Magnetic Bead Panel, MCYTOMAG-70K, Millipore Sigma, Burlington, MA). The protocols were followed per the manufacturer’s directions. The sample size was six to eight mice per group. All samples were run in duplicate.
Statistical Analysis
Data were analyzed with SAS JMP Pro 15 (Cary, NC). Shapiro–Wilk test was used to determine data distribution. G*Power 3.1 was used to calculate the effect size and the minimum sample size needed for the experiment’s primary outcome variables of “exercise time” in the heat and “ascending thermal area” obtained from previous research (12). Using effect size of d = 1.3 for both exercise time and ascending thermal area and β–1 = 0.8, α = 0.05, for two-tailed tests, a minimum sample size of 8 in each group was determined to be necessary. Run time, distance, body weight, HSP72, absolute and relative heat load, and sex hormones were analyzed with nonpaired t test. For body weight changes over time between groups, ANOVA was used followed by limited orthogonal t test comparisons, restricted to differences in body weight between groups at three specific time points, with corrections for false discovery rate using Bonferroni’s test.
RESULTS
Changes in Body Mass, Performance, and Thermoregulation
To monitor the impact of OVX on physical characteristics of the mice, we recorded body mass throughout the experimental period (Table 1). Baseline body mass was similar between the two experimental groups before surgeries (sham = 21.6 ± 0.7 g, OVX = 19.9 ± 3.2 g). Four weeks after surgeries, we observed a gain of 3.6 ± 1.1 g in the sham group and of 8.2 ± 2.9 g in the OVX group (P < 0.05). BSA was greater in OVX mice (sham = 85 ± 2 cm2, OVX = 92 ± 3 cm2, P < 0.05). On the other hand, BSA-to-mass ratio was smaller in OVX mice (sham = 3.3 ± 0.04 cm2/g, OVX = 3.2 ± 0.05 cm2/g, P < 0.05) After the EHS trial, both sham and OVX groups dehydrated similarly (sham = 7.4 ± 1.7%, OVX = 6.5 ± 0.3%).
Table 1.
Responses of sham and ovariectomized mice before surgery, during heat exposure, and recovery
| Parameter | Sham | OVX | P Value |
|---|---|---|---|
| Surgery | |||
| Body mass pre surgery, g | 21.6 ± 0.7 | 19.9 ± 3.2 | 0.138 |
| Heat exposure | |||
| Body mass pre EHS, g | 25.4 ± 1.1 (n = 8) | 28.2 ± 1.5 (n = 10) | <0.001* |
| Body surface area, cm2 | 84.8 ± 2.3 (n = 8) | 91.8 ± 3.0 (n = 10) | <0.001* |
| BSA/mass, cm2/g | 3.3 ± 0.04 (n = 8) | 3.2 ± 0.05 (n = 10) | <0.001* |
| Tc,max, °C | 42.2 ± 0.5 (n = 8) | 42.2 ± 0.3 (n = 10) | 0.895 |
| Time to Tc,max, min | 126.3 ± 21 (n = 8) | 99.1 ± 19.8 (n = 10) | 0.013* |
| Thermal load, AUC | 138.4 ± 47.9 (n = 8) | 117.5 ± 25.6 (n = 10) | 0.253 |
| Distance ran, m | 753 ± 188 (n = 8) | 528 ± 163 (n = 10) | 0.015* |
| Recovery | |||
| Body mass post EHS, g | 23.5 ± 0.8 (n = 8) | 26.7 ± 1.6 (n = 8) | <0.001* |
| Dehydration, % | 7.4% ± 1.7% (n = 8) | 6.5% ± 1.4% (n = 8) | 0.276 |
| Time to hypothermia, min | 25.4 ± 4.1 (n = 8) | 33.2 ± 9.72 (n = 8) | 0.052 |
| Depth of hypothermia, °C | 31.45 ± 1.0 (n = 8) | 30.8 ± 3.2 (n = 8) | 0.606 |
Maximal core temperature at loss of consciousness (Tc, max). BSA, body surface area; EHS, exertional heat stroke; OVX, ovariectomy. *P < 0.05.
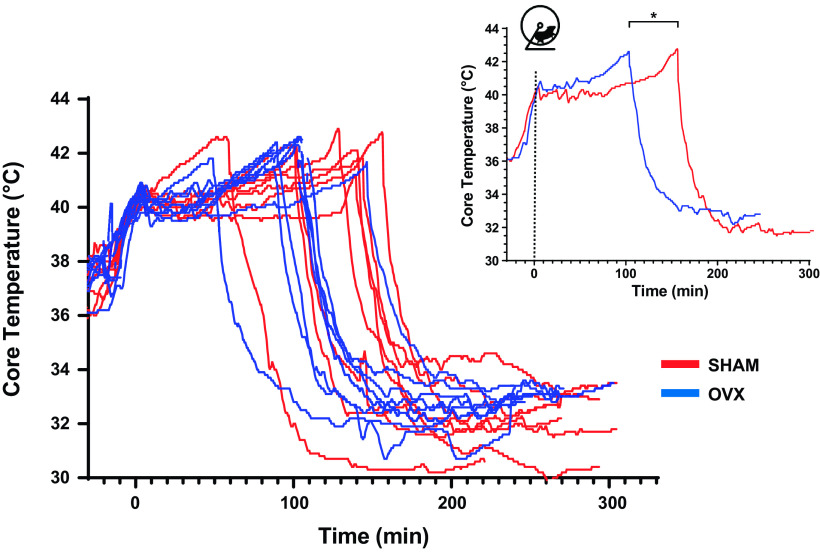
The individual core temperature profiles of each mouse are presented in Fig. 1. Both groups achieved similar maximal core temperatures at LOC (Table 1). We monitored the time taken for animals to achieve LOC as well as the total distance covered during the EHS trial. OVX mice collapsed 25% sooner (sham = 126 ± $11 min vs. OVX = 99 ± 19 min, P < 0.05, Table 1) and ran a 30% shorter distance than sham mice (sham = 753 ± 189 m vs. 528 ± 163 m, P < 0.05, Table 1). In terms of heat balance and thermoregulation, OVX mice were subjected to a similar thermal load than their sham counterparts (Table 1).
Figure 1.
Individual core temperature profiles during exercise in the heat and recovery. Smaller panel represents the average core temperature profile for each group. Red lines denote sham (n = 8) and blue lines denote ovariectomized (OVX) (n = 10) animals. *P < 0.05, ANOVA.
Intestinal Damage
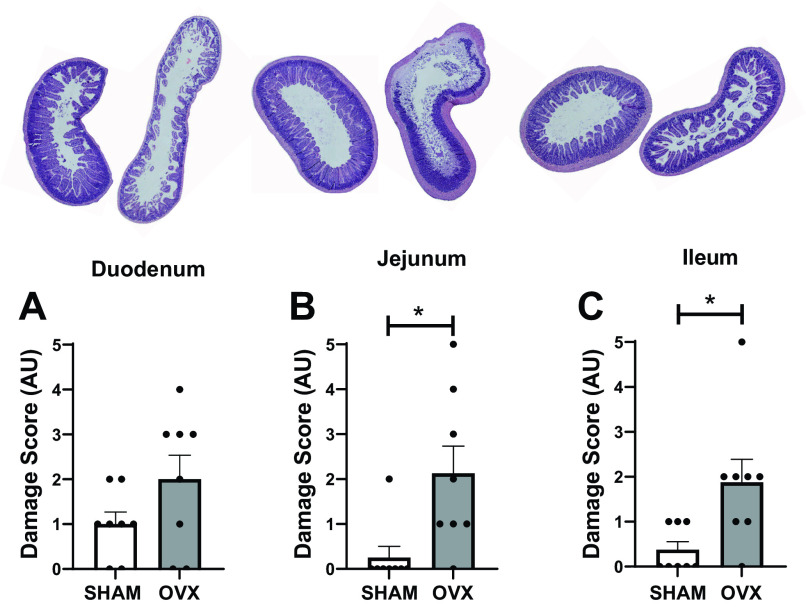
We did not observe differences in injury to the duodenum of the intestine (sham = 1.0 ± 0.7 AU, OVX = 2.0 ± 1.5 AU, Fig. 2A). However, the extent of damage to the jejunum (sham = 0.2 ± 0.7 AU, OVX = 2.1 ± 1.7 AU, P < 0.05, Fig. 2B) and ileum (sham = 0.3 ± 0.5 AU, OVX = 1.8 ± 1.4 AU, P < 0.05, Fig. 2C) was greater in the OVX mice after EHS.
Figure 2.
Intestinal damage scores for duodenum (A), jejunum (B), and ileum (C) of sham (n = 8) and ovariectomized (OVX) (n = 8) mice. *P < 0.05, t test.
Mesenteric Microvascular Function
When examining total microvasculature density (Fig. 3A), OVX mice displayed higher density than sham mice (sham = 101 ± 25 10−2 mm/mm2, OVX = 156 ± 66 10−2 mm/mm2, P < 0.05). Microvascular density at each microvessel segment diameter is shown in Fig. 3B. Microvascular density summed across 10- to 25-μm microvessel segment diameters was higher in OVX than in sham mice (P < 0.05).
Figure 3.
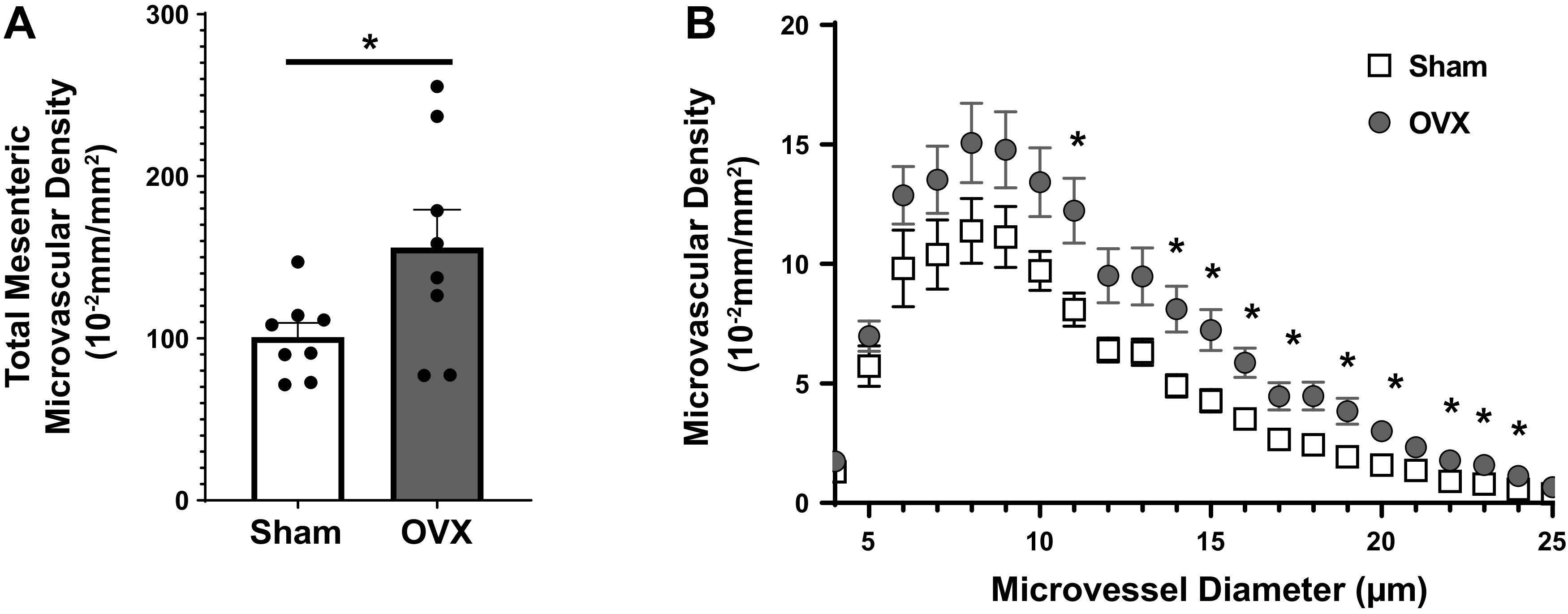
A: total mesenteric microvascular density in sham (n = 8) and ovariectomized (OVX) (n = 8) mice after EHS. Dots represent individual values and bars represent mean and standard deviation. B: microvascular density as a function of microvessel diameter ranging from 5 to 25 µm. *P < 0.05, ANOVA.
Circulatory HSP72, Cytokines, and Chemokines
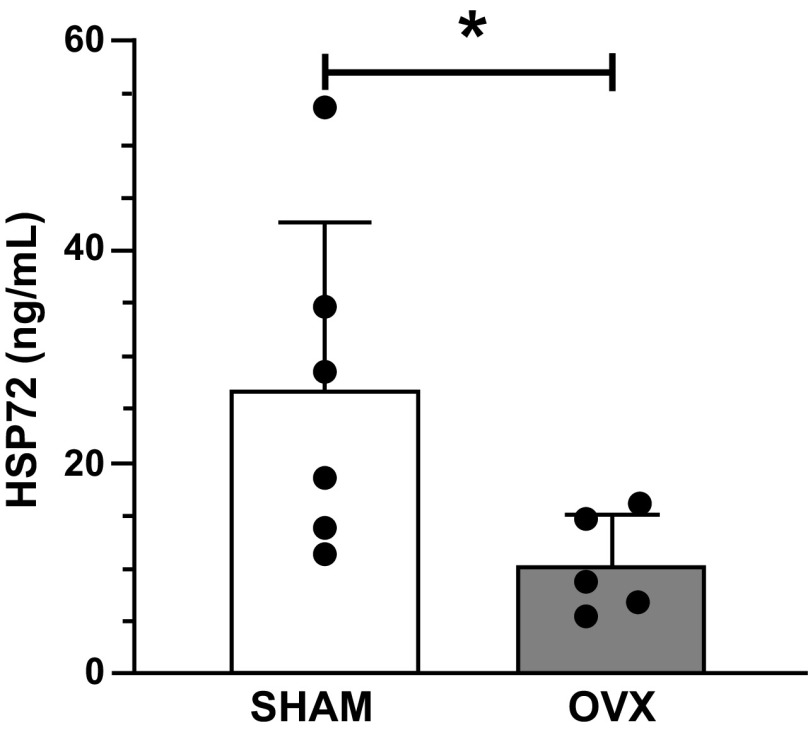
To determine the influence of OVX on circulatory factors after EHS, we analyzed plasma samples for changes in HSP72, cytokines, and chemokines. OVX diminished the levels of plasma HSP72 (sham = 27 ± 16 ng/mL, OVX = 10 ± 5 ng/mL, P < 0.05, Fig. 4). Plasma levels of cytokines and chemokines were not different between sham and OVX, at the time point studied (Table 2).
Figure 4.
Plasma heat shock protein (HSP72) for sham (n = 6) and ovariectomy (OVX) (n = 6) mice. Dots represent individual values and bars represent mean and standard deviation. *P < 0.05.
Table 2.
Circulatory levels of cytokines and chemokines in sham and OVX mice
| Cytokine/Chemokine | Sham | OVX | P Value |
|---|---|---|---|
| G-CSF | 873 ± 495 | 682 ± 322 | 0.2792 |
| IL-6 | 99 ± 57 | 81 ± 49 | 0.7392 |
| CXCL1 (KC) | 459 ± 380 | 454 ± 243 | 0.2698 |
| CXCL2 (MIP2a) | 460 ± 371 | 193 ± 131 | 0.0943 |
| CXCL10 (IP10) | 80 ± 34 | 82 ± 70 | 0.0743 |
| CCL2 (MCP1) | 54 ± 30 | 50 ± 29 | 0.9499 |
| CCL3 (MIP1a) | 18 ± 17 | 13 ± 10 | 0.2678 |
| CCL4 (MIP1b) | 17 ± 7 | 16 ± 8 | 0.9305 |
| RANTES | 1.2 ± 0.7 | 1.1 ± 0.6 | 0.7748 |
Values are means ± SD (in pg/ml); n = 8 mice per group. OVX, ovariectomy.
DISCUSSION
The main finding of our study was that OVX worsened the EHS pathophysiology in female mice despite the lower total time of heat exposure and shorter time to LOC. We observed a reduced exercise capacity that was characterized by a shorter time and distance ran to elicit the EHS end point in our model (i.e., LOC). Despite the shorter time and distance covered, OVX mice demonstrated greater intestinal damage and higher microvascular density at the mesentery 3 h post collapse. In addition, lower circulatory HSP72, a molecular indicative of lower thermal tolerance, was also observed in OVX mice. These findings reveal that the ovaries, and likely their related hormones, may play a key role in prolonging the exercise capacity in the heat and delaying the occurrence and severity of EHS in female mice.
Previous preclinical studies have shown that females exercise for longer periods of time than males in both temperate (30–34) and hot environments (12). In a temperate environment, Oydanich et al. (30) compared the exercise capacity of male and female C57BL/6J mice. They compared age-matched groups as well as weight-matched groups. In addition, they also tested the impact of OVX in females and estrogen supplementation in males. Females exhibited greater exercise capacity for running distance and work to exhaustion compared with age-matched males. These differences persisted when comparing weight-matched females and males. OVX reduced the enhanced exercise capacity in females, and estrogen administered to males increased their exercise capacity (30). In a hot environment, Garcia et al. (12) compared males and females using the same model of EHS used in our present study. In agreement with findings from Oydanich et al., females in the Garcia et al. study also outperformed males by running ∼40% longer in the heat. In our present study, OVX mice ran ∼30% shorter than sham mice in the heat. In a study designed to understand the role of testosterone on the EHS pathophysiology, Garcia et al castrated C57BL/6J mice before exposing them to the EHS model. Castrated mice ran similar distances as sham control mice in the heat (13), indicating that testosterone does not explain the lower exercise capacity of males in comparison with females in the EHS model. In the present study, OVX mice lost their superior exercise capacity and displayed running times comparable with males from the previous Garcia et al. studies (12, 13). Altogether, these findings, in temperate and hot environments, indicate that ovarian hormones are necessary for the superior exercise capacity of female mice both in temperate and hot environments. In addition, ovarian hormones appear to influence females’ superior capacity to exercise in the heat before EHS collapse.
Even though OVX mice were heavier than sham controls in our study, we do not credit the differences in exercise capacity and thermoregulation solely to differences in body mass between the two experimental groups. Body mass has multiple influences in thermodynamics as recently reviewed by Cramer et al. (35). In brief, absolute metabolic rate at rest and during fixed-speed weight-bearing locomotion increases with body mass, resulting in a greater absolute rate of metabolic heat production in those carrying greater body mass (36–38). However, mass also represents a heat sink and the rise in core body temperature for a given change in heat load is inversely related to body mass (35). Thus, for a given absolute rate of metabolic heat production, a heavier mass should attenuate the rise in core body temperature, provided similar regulatory control of thermoeffector responses. In our study, we observed the opposite response where the heavier OVX had a faster increase in core temperature than the lighter sham counterparts. This response reinforces the notion that factors other than body mass are involved in the effects of OVX in our EHS model.
Despite the shorter distance ran and the shorter time to achieve the EHS end point in our model, OVX mice displayed greater intestinal damage after EHS. Intestinal damage is among the main pathophysiological features of EHS (3, 18). King et al. (18) observed intestinal damage as early as 0.5 h post collapse in the same EHS model used in our study. Intestinal damage has important implications to EHS pathophysiology as the leaky gut has been proposed as a trigger for the systemic inflammatory response syndrome (SIRS) in EHS victims, which is characterized by increased circulating levels of HSP72, cytokines, and chemokines (2, 8). OVX has been shown by others to provoke gut microbiome dysbiosis (39) and estrogen administration has been shown to protect rodents against intestinal ischemia-reperfusion injury via inducible nitric oxide synthase expression (40). Another interesting observation from our study was that OVX resulted in greater perfused microvascular density at the mesentery. The mesentery is a highly vascularized part of the intestines. We interpret that this higher perfused microvascular density following EHS in OVX mice could be explained by the higher injury observed in the jejunum and ileum. Overall, our findings in the intestines of OVX mice after EHS indicate that thermal protection may be compromised in the absence of the ovaries and their related hormones. Whether the greater microvascular density observed in the OVX group is associated with thermoregulatory factors requires further investigation.
Heat shock proteins (HSPs) represent one of the main molecular pathways for thermal protection (41). The expression of HSP72 provides cytoprotection and anti-inflammatory effects by inhibiting proinflammatory pathways such as JNK and NF-κB. Increase in HSP is required for most cellular adaptation to stress, whereas blocking HSP72 expression increases the susceptibility of tissues such as the skeletal muscles to stress (42). We observed a decreased concentration of HSP72 in the circulation of OVX mice post EHS. Circulatory HSP72 can be secreted from a variety of cells in free form and in membrane-bounded particles. In addition, they can be released from cells undergoing necrotic death when membranes are disrupted, and the HSP can leak passively out of the cells (43). HSP72 has been shown to be strongly immunostimulatory (44). In the context of thermoregulation, HSP72 can be secreted due to the hyperthermic stimulus and damage associated with EHS. Since HSPs are associated with multiple stressors by inducing refolding of damaged proteins, they are necessary for thermal tolerance. It has been shown in rodents that OVX decreases HSP72 resulting in a reduced capability to handle stress from muscular contraction (45). In rodent cardiac muscle, HSP72 is two-fold higher in females than males; however, this effect is abolished when females underwent OVX and rescued when OVX rodents received estrogen (46). Although speculative, it seems that there is a relationship between estrogen levels and HSPs which may indicate why females are more resistant than male mice in preclinical models of EHS. Though we did not observe significant effects of OVX on cytokines and chemokines in response to EHS, the results are within range with previous studies using the same model (12, 47) and are consistent with a typical inflammatory response to EHS. Thus, despite lower heat tolerance (shorter time to LOC) and greater intestinal damage, OVX mice showed a similar SIRS response during recovery as sham mice. Whether this similarity would persist throughout recovery (i.e., >3 h) warrants further investigation.
Although our study is the first to show the impact of OVX on EHS as an attempt to establish the mechanisms behind the unique female response to EHS, we are aware that our study is not without limitations. First, although the bilateral removal of the ovaries offers a great model to establish the potential relevance of ovary-related hormones on the physiological response of female mice, it does not allow us to understand what exact hormones are behind these responses. The ovaries produce and release two groups of sex hormones—progesterone and estrogens. There are three major estrogens, known as estradiol, estrone, and estriol, which work together to promote the development of female sex characteristics during puberty and to ensure fertility through the lifespan (48). The study of ovary-related hormone effects on body systems is challenging because of the complex and multifaceted influences of these hormones, both individually and in combination. Studies are warranted to determine whether estrogens, progesterone, or the interaction of both with other gonadotropins (e.g., luteinizing and follicle stimulant hormones), are required to explain the beneficial role of the ovaries in the context of EHS. Second, we studied a single time point following LOC (i.e., 3 h after LOC). This time point was chosen because of previous observations indicating that most physiological alterations peak at this time point (12, 18). We know that some abnormalities, particularly in females, only emerge after 9–14 days post EHS (49). Thus, studies addressing more prolonged time points during recovery are warranted to shed light into the mechanisms underlying the role of OVX in the EHS pathophysiology. Third, we did not monitor the estrous cycle in the sham group. In mice, the estrous cycle has four phases, namely, proestrus, estrus, metestrus, and diestrus, and lasts for 4 to 5 days. Monitoring the estrous cycle would be important to account for hormonal variations in the sham group. However, it would also have required a greater number of mice for each group of estrous cycle to power the study accordingly. Therefore, studies addressing whether female mice at different stages of the estrous cycle would respond differently to EHS are still warranted.
In conclusion, our results demonstrate that the OVX aggravates the pathophysiological response to exertional heat stroke in mice. Whether these responses are due to estrogens or progesterone remains unknown and warrants further investigation.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This project was supported by the United States Army Research Institute of Environmental Medicine (USARIEM) and funded by a DOD contract #W81XWH21R0020 to O.L. D.R.M. was funded by the NIH R00 (R00AT010017-05) award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.R.L. and O.L. conceived and designed research; L.I.R., X.Z., I.V., D.R.M., and O.L. performed experiments; L.I.R., X.Z., D.R.M., and O.L. analyzed data; L.I.R., X.Z., D.R.M., L.R.L., and O.L. interpreted results of experiments; L.I.R., X.Z., and O.L. prepared figures; L.I.R. and O.L. drafted manuscript; L.I.R., X.Z., I.V., D.R.M., L.R.L., and O.L. edited and revised manuscript; L.I.R., X.Z., I.V., D.R.M., L.R.L., and O.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Christian Garcia, Gabriel Leite-Santos, Jillian Carta, and Corrine Hickman for their technical contributions.
REFERENCES
- 1. Howe AS, Boden BP. Heat-related illness in athletes. Am J Sports Med 35: 1384–1395, 2007. doi: 10.1177/0363546507305013. [DOI] [PubMed] [Google Scholar]
- 2. Laitano O, Leon LR, Roberts WO, Sawka MN. Controversies in exertional heat stroke diagnosis, prevention, and treatment. J Appl Physiol (1985) 127: 1338–1348, 2019. doi: 10.1152/japplphysiol.00452.2019. [DOI] [PubMed] [Google Scholar]
- 3. Bouchama A, Abuyassin B, Lehe C, Laitano O, Jay O, O'Connor FG, Leon LR. Classic and exertional heatstroke. Nat Rev Dis Primers 8: 8, 2022. doi: 10.1038/s41572-021-00334-6. [DOI] [PubMed] [Google Scholar]
- 4. Giersch GEW, Garcia CK, Stachenfeld NS, Charkoudian N. Are there sex differences in risk for exertional heat stroke? A translational approach. Exp Physiol 107: 1136–1143, 2022. doi: 10.1113/EP090402. [DOI] [PubMed] [Google Scholar]
- 5.Update: Heat Illness, Active Component, U.S. Armed Forces, 2020 [Online]. Mil Health Syst, 2021. https://armymedicine.health.mil/MHSHome/News/Articles/2021/04/01/Update-Heat-MSMR-2021 [2021. Sep 5]. [PubMed]
- 6.Heat Illness, Active Component, U.S. Armed Forces, 2021 [Online]. Mil Health Syst, 2022. https://www.health.mil/News/Articles/2022/04/01/Update-Ht-MSMR [2022. Sep 6].
- 7. Giersch GEW, Taylor KM, Caldwell AR, Charkoudian N. Body mass index, but not sex, influences exertional heat stroke risk in young healthy men and women. Am J Physiol Regul Integr Comp Physiol 324: R15–R19, 2023. doi: 10.1152/ajpregu.00168.2022. [DOI] [PubMed] [Google Scholar]
- 8. Garcia CK, Renteria LI, Leite-Santos G, Leon LR, Laitano O. Exertional heat stroke: pathophysiology and risk factors. BMJ Med 1: e000239, 2022. doi: 10.1136/bmjmed-2022-000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Druyan A, Makranz C, Moran D, Yanovich R, Epstein Y, Heled Y. Heat tolerance in women—reconsidering the criteria. Aviat Space Environ Med 83: 58–60, 2012. [Erratum in Aviat Space Environ Med 83: 155, 2012]. doi: 10.3357/ASEM.3130.2012. [DOI] [PubMed] [Google Scholar]
- 10. Kazman JB, Purvis DL, Heled Y, Lisman P, Atias D, Van Arsdale S, Deuster PA. Women and exertional heat illness: identification of gender specific risk factors. US Army Med Dep J Apr-Jun: 58–66, 2015. [PubMed] [Google Scholar]
- 11. Iyoho AE, Ng LJ, MacFadden L. Modeling of gender differences in thermoregulation. Mil Med 182: 295–303, 2017. doi: 10.7205/MILMED-D-16-00213. [DOI] [PubMed] [Google Scholar]
- 12. Garcia CK, Mattingly AJ, Robinson GP, Laitano O, King MA, Dineen SM, Leon LR, Clanton TL. Sex-dependent responses to exertional heat stroke in mice. J Appl Physiol (1985) 125: 841–849, 2018. doi: 10.1152/japplphysiol.00220.2018. [DOI] [PubMed] [Google Scholar]
- 13. Garcia CK, Robinson GP, Gambino BJ, Rua MT, Laitano O, Clanton TL. The impact of castration on physiological responses to exertional heat stroke in mice. PloS One 17: e0275715, 2022. doi: 10.1371/journal.pone.0275715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeMayo FJ, Zhao B, Takamoto N, Tsai SY. Mechanisms of action of estrogen and progesterone. Ann N Y Acad Sci 955: 48–59, 2002. doi: 10.1111/j.1749-6632.2002.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 15. Charkoudian N, Stachenfeld NS. Reproductive hormone influences on thermoregulation in women. Compr Physiol 4: 793–804, 2014. doi: 10.1002/cphy.c130029. [DOI] [PubMed] [Google Scholar]
- 16. Liu X-Y, Li P, Li X-S, Simoncini T, Cheng Y. 17β-Estradiol nongenomically induces vasodilation is enhanced by promoting phosphorylation of endophilin A2. Gynecol Endocrinol 38: 644–650, 2022. doi: 10.1080/09513590.2022.2088731. [DOI] [PubMed] [Google Scholar]
- 17. Charkoudian N, Hart ECJ, Barnes JN, Joyner MJ. Autonomic control of body temperature and blood pressure: influences of female sex hormones. Clin Auton Res 27: 149–155, 2017. doi: 10.1007/s10286-017-0420-z. [DOI] [PubMed] [Google Scholar]
- 18. King MA, Leon LR, Mustico DL, Haines JM, Clanton TL. Biomarkers of multiorgan injury in a preclinical model of exertional heat stroke. J Appl Physiol (1985) 118: 1207–1220, 2015. doi: 10.1152/japplphysiol.01051.2014. [DOI] [PubMed] [Google Scholar]
- 19. Pires W, Veneroso CE, Wanner SP, Pacheco DAS, Vaz GC, Amorim FT, Tonoli C, Soares DD, Coimbra CC. Association between exercise-induced hyperthermia and intestinal permeability: a systematic review. Sports Med 47: 1389–1403, 2017. doi: 10.1007/s40279-016-0654-2. [DOI] [PubMed] [Google Scholar]
- 20. Soares ADN, Costa KA, Wanner SP, Santos RGC, Fernandes SOA, Martins FS, Nicoli JR, Coimbra CC, Cardoso VN. Dietary glutamine prevents the loss of intestinal barrier function and attenuates the increase in core body temperature induced by acute heat exposure. Br J Nutr 112: 1601–1610, 2014. doi: 10.1017/S0007114514002608. [DOI] [PubMed] [Google Scholar]
- 21. Walter E, W Watt P, Gibson OR, Wilmott AGB, Mitchell D, Moreton R, Maxwell NS. Exercise hyperthermia induces greater changes in gastrointestinal permeability than equivalent passive hyperthermia. Physiol Rep 9: e14945, 2021. doi: 10.14814/phy2.14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8: e1000412, 2010. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ström JO, Theodorsson A, Ingberg E, Isaksson I-M, Theodorsson E. Ovariectomy and 17β-estradiol replacement in rats and mice: a visual demonstration. J Vis Exp 64: e4013, 2012. doi: 10.3791/4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. King MA, Alzahrani JM, Clanton TL, Laitano O. A Preclinical Model of Exertional Heat Stroke in Mice. J Vis Exp (173), 2021. doi: 10.3791/62738. [DOI] [PubMed] [Google Scholar]
- 25. Meeh K. Oberflächenmessungen des menschlichen Körpers. Z Biol 15: 425–485, 1879. [Google Scholar]
- 26. Cheung MC, Spalding PB, Gutierrez JC, Balkan W, Namias N, Koniaris LG, Zimmers TA. Body surface area prediction in normal, hypermuscular, and obese mice. J Surg Res 153: 326–331, 2009. doi: 10.1016/j.jss.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 27. Zheng X, Deacon C, King AJ, Machin DR. Microcirculatory and glycocalyx properties are lowered by high-salt diet but augmented by Western diet in genetically heterogeneous mice. Am J Physiol Heart Circ Physiol 322: H328–H335, 2022. doi: 10.1152/ajpheart.00656.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams JM, Duckworth CA, Vowell K, Burkitt MD, Pritchard DM. Intestinal preparation techniques for histological analysis in the mouse. Curr Protoc Mouse Biol 6: 148–168, 2016. doi: 10.1002/cpmo.2. [DOI] [PubMed] [Google Scholar]
- 29. Oltean M, Olausson M. The Chiu/Park scale for grading intestinal ischemia–reperfusion: if it ain’t broke don’t fix it!. Intensive Care Med 36: 1095–1095, 2010. doi: 10.1007/s00134-010-1811-y. [DOI] [PubMed] [Google Scholar]
- 30. Oydanich M, Babici D, Zhang J, Rynecki N, Vatner DE, Vatner SF. Mechanisms of sex differences in exercise capacity. Am J Physiol Regul Integr Comp Physiol 316: R832–R838, 2019. doi: 10.1152/ajpregu.00394.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Achamrah N, Nobis S, Goichon A, Breton J, Legrand R, do Rego JL, do Rego JC, Déchelotte P, Fetissov SO, Belmonte L, Coëffier M. Sex differences in response to activity-based anorexia model in C57Bl/6 mice. Physiol Behav 170: 1–5, 2017. doi: 10.1016/j.physbeh.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 32. Bartling B, Al-Robaiy S, Lehnich H, Binder L, Hiebl B, Simm A. Sex-related differences in the wheel-running activity of mice decline with increasing age. Exp Gerontol 87: 139–147, 2017. doi: 10.1016/j.exger.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 33. Dworatzek E, Mahmoodzadeh S, Schubert C, Westphal C, Leber J, Kusch A, Kararigas G, Fliegner D, Moulin M, Ventura-Clapier R, Gustafsson J-A, Davidson MM, Dragun D, Regitz-Zagrosek V. Sex differences in exercise-induced physiological myocardial hypertrophy are modulated by oestrogen receptor beta. Cardiovasc Res 102: 418–428, 2014. doi: 10.1093/cvr/cvu065. [DOI] [PubMed] [Google Scholar]
- 34. Konhilas JP, Chen H, Luczak E, McKee LA, Regan J, Watson PA, Stauffer BL, Khalpey ZI, Mckinsey TA, Horn T, LaFleur B, Leinwand LA. Diet and sex modify exercise and cardiac adaptation in the mouse. Am J Physiol Heart Circ Physiol 308: H135–145, 2015. doi: 10.1152/ajpheart.00532.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cramer MN, Gagnon D, Laitano O, Crandall CG. Human temperature regulation under heat stress in health, disease, and injury. Physiol Rev 102: 1907–1989, 2022. doi: 10.1152/physrev.00047.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dennis SC, Noakes TD. Advantages of a smaller bodymass in humans when distance-running in warm, humid conditions. Eur J Appl Physiol Occup Physiol 79: 280–284, 1999. doi: 10.1007/s004210050507. [DOI] [PubMed] [Google Scholar]
- 37. Kleiber M. Body size and metabolic rate. Physiol Rev 27: 511–541, 1947. doi: 10.1152/physrev.1947.27.4.511. [DOI] [PubMed] [Google Scholar]
- 38. Miller AT, Blyth CS. Influence of body type and body fat content on the metabolic cost of work. J Appl Physiol 8: 139–141, 1955. doi: 10.1152/jappl.1955.8.2.139. [DOI] [PubMed] [Google Scholar]
- 39. Kosaka S, Nadatani Y, Higashimori A, Otani K, Fujimoto K, Nagata Y, Ominami M, Fukunaga S, Hosomi S, Kamata N, Tanaka F, Nagami Y, Taira K, Imoto S, Uematsu S, Watanabe T, Fujiwara Y. Ovariectomy-induced dysbiosis may have a minor effect on bone in mice. Microorganisms 9: 2563, 2021. doi: 10.3390/microorganisms9122563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiao X, Liu D, Zheng S, Fu J, Zhang H, Chen L. Protective effect of estrogen on intestinal ischemia-reperfusion injury in pubertal rats. J Pediatr Surg 39: 1828–1831, 2004. doi: 10.1016/j.jpedsurg.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 41. Lee EC-H, Muñoz CX, McDermott BP, Beasley KN, Yamamoto LM, Hom LL, Casa DJ, Armstrong LE, Kraemer WJ, Anderson JM, Maresh CM. Extracellular and cellular Hsp72 differ as biomarkers in acute exercise/environmental stress and recovery. Scand J Med Sci Sports 27: 66–74, 2017. doi: 10.1111/sms.12621. [DOI] [PubMed] [Google Scholar]
- 42. Laitano O, Oki K, Leon LR. The role of skeletal muscles in exertional heat stroke pathophysiology. Int J Sports Med 42: 673–681, 2021. doi: 10.1055/a-1400-9754. [DOI] [PubMed] [Google Scholar]
- 43. Mambula SS, Calderwood SK. Heat induced release of Hsp70 from prostate carcinoma cells involves both active secretion and passive release from necrotic cells. Int J Hyperthermia 22: 575–585, 2006. doi: 10.1080/02656730600976042. [DOI] [PubMed] [Google Scholar]
- 44. Asea A. Initiation of the immune response by extracellular Hsp72: chaperokine activity of Hsp72. Curr Immunol Rev 2: 209–215, 2006. doi: 10.2174/157339506778018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Locke M, Salerno SA. Ovariectomy alters lengthening contraction induced heat shock protein expression. Appl Physiol Nutr Metab Physiol Appl 45: 530–538, 2020. doi: 10.1139/apnm-2019-0212. [DOI] [PubMed] [Google Scholar]
- 46. Voss MR, Stallone JN, Li M, Cornelussen RNM, Knuefermann P, Knowlton AA. Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol 285: H687–H692, 2003. doi: 10.1152/ajpheart.01000.2002. [DOI] [PubMed] [Google Scholar]
- 47. King MA, Leon LR, Morse DA, Clanton TL. Unique cytokine and chemokine responses to exertional heat stroke in mice. J Appl Physiol (1985) 122: 296–306, 2017. doi: 10.1152/japplphysiol.00667.2016. [DOI] [PubMed] [Google Scholar]
- 48. Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson J-Å. Mechanisms of estrogen action. Physiol Rev 81: 1535–1565, 2001. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 49. Laitano O, Garcia CK, Mattingly AJ, Robinson GP, Murray KO, King MA, Ingram B, Ramamoorthy S, Leon LR, Clanton TL. Delayed metabolic dysfunction in myocardium following exertional heat stroke in mice. J Physiol 598: 967–985, 2020. doi: 10.1113/JP279310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.