Abstract
Background and Objectives
Disparities in esophageal cancer are well-established. The standard treatment for locally advanced esophageal cancer is chemoradiation followed by surgery. We sought to evaluate the association between socioeconomic factors, time to surgery, and patient outcomes.
Methods
All patients ≥18 years old diagnosed with T2/3/4 or node-positive esophageal cancer between 2004-2016 and who underwent chemoradiation and esophagectomy in the National Cancer Database were included. Multivariable regression was used to assess the association between socioeconomic variables and time to surgery (grouped into <56 days, 56-84 days, and 85-112 days).
Results
12,157 patients were included. 5-year overall survival was 39%, 35% and 35% for the three groups examined. Post-operative 30 and 90-day mortality was increased in both the 56-84 days to surgery group (OR 1.30 and 1.20, respectively) and the 85-112 days group (OR 1.37 and 1.56, respectively) when compared to <56 days. Patients of minority race, public insurance, or lower income were more likely to have a longer time to surgery.
Conclusions
Longer time to surgery is associated with increased postoperative mortality and is more common in patients with lower socioeconomic status. Further research exploring reasons for delays to esophagectomy among disadvantaged patients could help target interventions to reduce disparities.
Keywords: Esophagectomy, Postoperative Complications, Healthcare Disparities, Combined Modality Therapy
Introduction
Neo-adjuvant chemoradiation followed by esophagectomy is the standard of care for locally advanced, non-metastatic esophageal cancer.[1-4] Randomized controlled trials have demonstrated an improved survival with neoadjuvant chemoradiation (nCRT) plus surgery compared with surgery alone, but have used a variable time interval to surgery between 2-8 weeks [1,3,5]. Despite multiple studies, the optimal timing of esophagectomy after neo-adjuvant therapy remains controversial [6-12]. However, the largest studies and meta-analyses suggest that surgery delayed beyond 8 weeks is associated with an increased rate of pathologic complete response (pCR), but a decreased rate of overall survival [9,10,13].
Disparities in outcomes for esophageal cancer are well-established. Black and Hispanic patients with esophageal cancer have increased all-cause mortality [14]. The causes of these disparities are less clear, but are related to increased incidence, lower rates of chemotherapy and/or radiation, and lower rates of surgery [15-17]. For patients who receive recommended therapy, disparities are significantly improved, but race remains a predictor of failure to rescue after major complications [18,19].
We sought to evaluate the association between socioeconomic factors and time to surgery among adults who received nCRT and surgery, as well as the impact of timing of surgery on patient outcomes. We hypothesized that low socioeconomic status would be associated with longer time to surgery and that longer time to surgery would be associated with negative outcomes.
Material and methods
This study was determined to be exempt by the University of North Carolina Institutional Review Board (IRB# 20-2225). A retrospective review was conducted using the National Cancer Database (NCDB) esophageal cancer site-specific 2016 Participant User File (PUF). The NCDB is a joint endeavor by the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society and is the largest hospital-based cancer registry program in the United States [20]. The NCDB includes more than 1,500 facilities and captures more than 70% of newly diagnosed cancer cases annually.
All adult patients (≥18 years old) diagnosed with esophageal cancer (cancer site: C151, C152, C154, C155, C158, and C159), who had adjusted AJCC 6th or 7th edition clinical T2-4N0-1 or T1N1 between 2004-2016 eligible for inclusion (n=59,574). Cancer histology was classified as esophageal adenocarcinoma (histology 8020, 8140, 8143, 8145, 8147, 8255, 8260 – 8263, 8480, 8481, 8560, 8562, 8570 – 8574), squamous cell carcinoma (8070-8076, 8078); patients with other histologies were not included. Patients with metastatic disease (adjusted AJCC 6th or 7th edition M1, n=9,644) or who were undergoing palliative treatment (n=3,473), were excluded. Patients were also required to undergo partial or total esophagectomy without laryngectomy (surgery codes 30, 40, 50, 52-54 and 80) (n=18,138, 41%) and nCRT (n=13,913, 77% of those undergoing esophagectomy). To calculate the time interval between nCRT and esophagectomy, we used a previously described method of (E – (RS + RD)), where E is days between diagnosis and surgery, RS is days between diagnosis and start of radiation, and RD is duration of radiation therapy [9].
Surgical timing was classified as <56 days (0-7 weeks), 56-84 days (8-12 weeks), and 85-112 days (13-15 weeks). Patients who underwent surgery >112 days after completing nCRT (n=573, ≥16 weeks after diagnosis) were excluded. These time points were based on prior studies and clinical experience. First, 56 days (8 weeks) has been previously described by Ranney et al. (2017) as an inflection point in modeling the association between timing of esophagectomy and clinical outcomes [21]. Several previous studies, including a meta-analysis, have also used this as an important time variable [7,8,13,22]. The second time point (84 days or 12 weeks) is similar to those described in other studies evaluating rates of pathologic complete response in delayed esophagectomy [11,12]. The third group (85-112 days) was included as it is intended to represent patients who may have had some issue with timeliness of surgery but for whom surgery in planned fashion was still being undertaken with curative intent. Beyond 112 days, it is unlikely surgery is being performed as part of planned trimodality therapy. Patients who received both neoadjuvant and adjuvant therapy (n=567) or were missing information on surgical timing were excluded (n=328); patients missing information on clinical M or N status were also excluded (n=288).
Chi-square and Wilcoxon-Mann-Whitney tests were used to compare patient and cancer characteristics across surgical timing and patient outcomes, where appropriate. Multivariable generalized logistic regression was used to assess whether socioeconomic variables (race/ethnicity, primary insurance type, median residential income in the patients’ ZIP code, and the proportion of adults with less than a high school education in the patients’ ZIP code) were associated with time to surgery, after adjusting for year of diagnosis, patient age, gender, Charlson-Deyo comorbidity score, cancer histology, clinical T, and clinical N status. Facility characteristics were not included as these variables are restricted in patients <40 years old. Age was treated as a restricted quadratic spline. Interaction terms and Wald Chi square tests were used to assess effect measure modification between race/ethnicity, primary insurance type, and median residential income.
Multivariable linear, logistic, and Cox proportional hazards regression were used to estimate association between surgical timing and patient outcomes. Outcomes of interest included length of stay (LOS) after surgery, 30-day unplanned readmission after surgery, 30-day mortality after surgery, 90-day mortality after surgery, and 5-year, all-cause mortality. As before, all models were adjusted for year of diagnosis, patient age, gender, race/ethnicity, primary insurance type, Charlson-Deyo comorbidity score, median residential income in the patients’ ZIP code, residential education level in the patients’ ZIP code, cancer histology, clinical T, and clinical N status. Patients diagnosed in 2016 were excluded from these analyses, as survival data is suppressed for these patients in the 2016 PUF. For 5-year all-cause mortality, follow-up began 4 months after diagnosis in order to account for potential immortal person-time [23]. Patient follow-up was administratively censored 5 years after diagnosis. We also performed a sensitivity analysis where the cohort was restricted to patients with esophageal adenocarcinoma.
All analyses were performed using SAS 9.4 (SAS Inc., Cary, NC).
Results
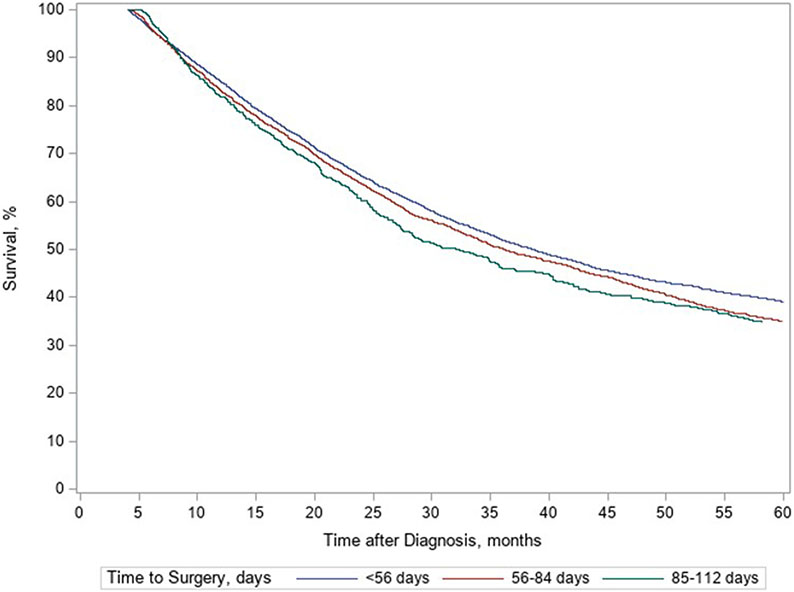
Overall, 12,157 patients were included; 61% underwent surgery in <56 days, 32% between 56-84 days, and 8% between 85-112 days. Patient sex, pre-operative co-morbidities, and cancer staging were similar across time to surgery groups (Table 1). 5-year survival when analyzed by the three time to surgery groups was 39% in patients who underwent surgery <56 days after nCRT, 35% in patients at 56-84 days, and 35% in patients treated 85-112 days (Figure 1). Comparison of survival between groups is shown in Table 2. A slight decrease in mortality was seen in the 56-84 days to surgery group when compared to the <56 days to surgery group (OR 1.08; p=0.01), but there was no difference in mortality between the 85-112 days to surgery group and <56 days group (OR 1.10; p=0.06). Comparison between the 56-84 days and 85-112 days to surgery groups showed no difference in 5-year survival (OR 0.99, 95% CI 0.94, 1.04, p=0.74).
Table 1.
Patient demographics and cancer characteristics, stratified by surgical timing.
| <56 days 7,366 (61%) |
56-84 days 3,844 (32%) |
85-112 days 947 (8%) |
|
|---|---|---|---|
| Age, median (IQR) | 62 (56 – 68) | 63 (57 – 69) | 64 (57 – 70) |
| Male, n (%) | 6,306 (86) | 3,187 (83) | 772 (82) |
| Race/ethnicity, n (%) | |||
| Non-Hispanic white | 6794 (93) | 3529 (92) | 832 (88) |
| Non-Hispanic black | 243 (3) | 153 (4) | 58 (6) |
| Hispanic | 145 (2) | 81 (2) | 33 (4) |
| Non-Hispanic other | |||
| Charlson score, n (%) | |||
| 0 | 5534 (75) | 2742(71) | 679 (72) |
| 1 | 1470 (20) | 846 (22) | 199 (21) |
| 2 | 280 (4) | 191 (5) | 48 (5) |
| 3+ | 82 (1) | 65 (2) | 21 (2) |
| Primary insurance type, n (%) | |||
| Uninsured | 135 (2) | 91 (2) | 17 (2) |
| Private insurance/managed care | 3866 (53) | 1812 (48) | 371 (40) |
| Medicaid | 343 (5) | 185 (5) | 81 (9) |
| Medicare | 2801 (39) | 1625 (43) | 440 (47) |
| Other government | 98 (1) | 82 (2) | 24 (3) |
| Median residential income a , n (%) | |||
| <$40,227 | 1019 (14) | 611 (16) | 188 (20) |
| $40,227 – $50,353 | 1603 (22) | 905 (24) | 229 (25) |
| $50,354 – $63,332 | 1800 (25) | 961 (25) | 225 (24) |
| ≥$63,333 | 2843 (39) | 1319 (35) | 297 (32) |
| Residential education b , n (%) | |||
| ≥17.6% | 984 (14) | 569 (15) | 178 (19) |
| 10.9% – 17.5% | 1816 (25) | 1081 (28) | 263 (28) |
| 6.3% – 10.8% | 2414 (33) | 1118 (31) | 290 (31) |
| <6.3% | 2067 (28) | 963 (25) | 210 (22) |
| Histologic type, n (%) | |||
| Esophageal adenocarcinoma | 6209 (84) | 3212 (83) | 763 (79) |
| Squamous cell carcinoma | 1157 (16) | 632 (16) | 184 (19) |
| Clinical T, n (%) | |||
| T1 | 178 (2) | 88 (2) | 28 (3) |
| T2 | 1679 (23) | 824 (21) | 215 (23) |
| T3 | 5295 (72) | 2824 (73) | 662 (70) |
| T4 | 214 (3) | 108 (3) | 42 (4) |
| Clinical N, n (%) | |||
| N0 | 2746 (37) | 1487 (39) | 381 (40) |
| N1 | 4620 (63) | 2357 (61) | 566 (60) |
| Clinical stage, n (%) | |||
| II | 3736 (51) | 1953 (51) | 492 (52) |
| III | 3630 (49) | 1891 (49) | 455 (48) |
| Facility program c , n (%) | |||
| Community cancer program | 361 (5) | 188 (5) | 65 (7) |
| Comprehensive community program | 2180 (30) | 1164 (31) | 305 (32) |
| Academic/Research | 3637 (50) | 1960 (52) | 472 (50) |
| Integrated network cancer program | 1058 (15) | 485 (13) | 97 (10) |
Abbreviations: IQR, interquartile range
Median residential household income of each patient’s ZIP code was estimated using the 2016 American Community Survey
Proportion of adults in patient’s ZIP code who did not complete high school, measured in the 2016 American Community Survey
Facility type is suppressed for patients <40 years old, n=188
Figure 1.
5-year survival, stratified by surgical timing.
Table 2.
Association between undergoing surgery 56-84 days or 85-112 days, compared to <56 days, on post-operative and long-term outcomes.
| 56-84 days |
85-112 days |
|||
|---|---|---|---|---|
| Estimatea | p-value | Estimatea | p-value | |
| 5-year mortality, HR (95% CI) | 1.08 (1.02, 1.14) | 0.01 | 1.10 (1.00, 1.21) | 0.06 |
| Post-operative events, OR (95% CI) | ||||
| 30-day unplanned readmission | 0.90 (0.74, 1.10) | 0.31 | 0.86 (0.61, 1.22) | 0.41 |
| 30-day mortality | 1.30 (1.02, 1.65) | 0.03 | 1.37 (0.94, 2.00) | 0.10 |
| 90-day mortality | 1.20 (1.02, 1.41) | 0.03 | 1.56 (1.22, 2.00) | 0.0004 |
| LOS after surgery, CIE (95% CI) | 0.56 (0.07, 1.06) | 0.03 | 0.81 (−0.06, 1.68) | 0.07 |
Abbreviations: LOS, length of stay; CIE, change in estimate (linear regression); CI, confidence interval; OR, odds ratio (logistic regression); HR, hazard ratio (Cox proportional hazards regression)
Compared to patients who underwent surgery <56 days after nCRT; adjusted for year of diagnosis, patient age, gender, race/ethnicity, primary insurance type, Charlson-Deyo comorbidity score, median residential income, residential education level, cancer histology, clinical T, and clinical N status
Follow-up started 4 months after diagnosis to account for treatment duration
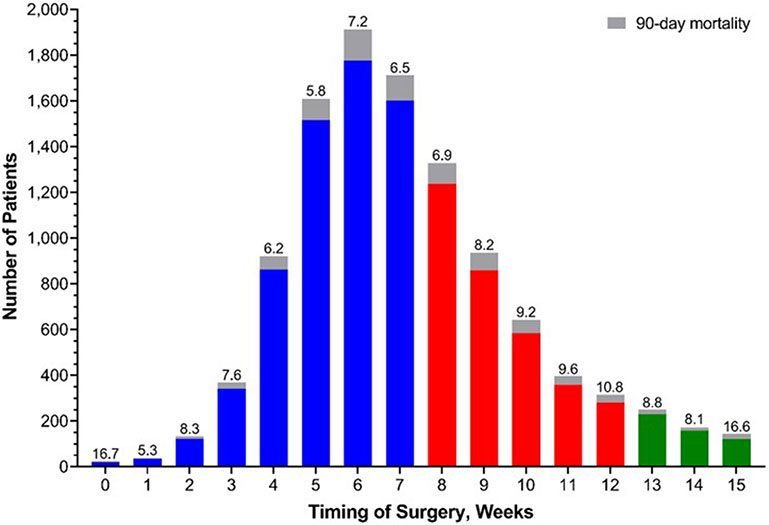
5% of patients had an unplanned readmission, 3% died within 30 days of surgery, and 7% died within 90 days (Table 2). For the group who underwent surgery between 56-84 days, rates of 30-day (OR 1.30, 95% CI 1.02, 1.65), 90-day (OR 1.20, 95% CI 1.02, 1.41), and overall mortality (HR 1.08, 95% CI 1.02, 1.14) were increased compared with those who underwent surgery before 56 days, Table 3. For the group with longer time to surgery (85-112 days), rates of 30-day (OR 1.37, 95% CI 0.94, 2.00), 90-day (OR 1.56, 95% CI 1.22, 2.00), and overall mortality (HR 1.10, 95% CI 1.00, 1.21) were also increased, although 30-day mortality did not show a significant difference, compared with patients undergoing surgery before 56 days. When examined separately, there were minimal differences between outcomes for patients with adenoncarcinoma and squamous carcinoma. Examined as a continuous variable by weeks after completion of neoadjuvant therapy, 90-day mortality across surgical timing is shown in Figure 2. Mortality by weeks after surgery ranged from 5.8% (5 weeks after completion) to 16.6% (15 weeks after completion), with the highest incidence at the extremes of the time range examined. Time to surgery was not associated with meaningful differences in 30-day readmission or length of stay.
Table 3.
Surgical and post-operative outcomes, stratified by surgical timing, among patients diagnosed between 2004 and 2015.
| <56 days 6,721 (62%) |
56-84 days 3,358 (31%) |
85-112 days 829 (8%) |
|
|---|---|---|---|
| Surgical margins, n (%) | |||
| No residual tumor | 6191 (95) | 3091 (95) | 761 (95) |
| Residual tumor, microscopic | 191 (3) | 75 (2) | 20 (2) |
| Residual tumor, macroscopic | 125 (2) | 83 (3) | 24 (3) |
| LOS after surgery, median (IQR) | 9 (7-14) | 10 (8-14) | 10 (8-15) |
| Post-operative events, n (%) | |||
| 30-day unplanned readmission | 336 (5) | 163 (5) | 40 (5) |
| 30-day mortality | 184 (3) | 124 (4) | 36 (4) |
| 90-day mortality | 443 (7) | 268 (8) | 91 (11) |
Abbreviations: IQR, interquartile range; LOS, length of stay
Figure 2.
Incidence of 90-day mortality across surgical timing, stratified by weeks.
Associations between socioeconomic status and surgical timing are described in Table 4. Non-Hispanic Black and Hispanic patients were more likely to have a longer time to surgery (85-112 days) (OR 1.53 95% CI (1.10, 2.13) and OR 1.63 (95% CI 1.09, 2.44), respectively), compared to non-Hispanic White patients; no difference was seen in time to surgery of 56-84 days. Medicaid and Medicare patients were also more likely to have a longer time to surgery compared with patients with private insurance (85-112 days) (OR 1.98, 95% CI 1.49, 2.62 and OR 1.45, 95% CI 1.17, 1.78, respectively). No significant difference was seen for patients with no insurance compared with patients with private insurance. Patients from lower income areas (<$48,000 median household income) were more likely to have longer time to surgery (85-112 days) compared with patients from higher income areas (≥$63,000) (OR 1.34, 95% CI 1.03, 1.74). Middle income groups of $38,000 - $47,999 and $48,000 - $62,999 showed no significant difference in time to surgery. Patient ZIP code education was not associated with time to surgery. Recognizing that squamous cancer may be associated with specific socioeconomic variables to a greater degree than adenocarcinoma, we also examined the impact of socioeconomic factors in adenocarcinoma alone. Similar disparities in surgical timing and outcomes were seen in this group of patients (Supplemental Tables 1 and 2).
Table 4.
Association between socioeconomic factors and undergoing surgery 56-84 days or 85-112 days, compared to <56 days.
| 56-84 days |
85-112 days |
|||
|---|---|---|---|---|
| OR (95% CI)a | p-value | OR (95% CI)a | p-value | |
| Race/ethnicity | ||||
| Non-Hispanic white | ref | – | ref | – |
| Non-Hispanic black | 1.14 (0.91, 1.43) | 0.24 | 1.53 (1.10, 2.13) | 0.01 |
| Hispanic | 0.98 (0.74, 1.31) | 0.91 | 1.63 (1.09, 2.44) | 0.02 |
| Non-Hispanic other | 0.92 (0.66, 1.27) | 0.60 | 1.06 (0.62, 1.80) | 0.84 |
| Primary insurance type | ||||
| Uninsured | 1.44 (1.09, 1.90) | 0.01 | 1.16 (0.69, 1.96) | 0.58 |
| Private insurance/managed care | ref | – | ref | – |
| Medicaid | 1.06 (0.87, 1.28) | 0.59 | 1.98 (1.49, 2.62) | <0.0001 |
| Medicare | 1.09 (0.96, 1.23) | 0.18 | 1.45 (1.17, 1.78) | 0.0005 |
| Other government | 1.68 (1.24, 2.29) | 0.001 | 2.23 (1.38, 3.62) | 0.001 |
| Median residential income b | ||||
| <$40,227 | 1.16 (0.99, 1.35) | 0.07 | 1.34 (1.03, 1.74) | 0.03 |
| $40,227 – $50,353 | 1.13 (1.00, 1.28) | 0.06 | 1.16 (0.93, 1.45) | 0.18 |
| $50,354 – $63,332 | 1.10 (0.98, 1.23) | 0.11 | 1.09 (0.89, 1.33) | 0.40 |
| ≥$63,333 | ref | – | ref | – |
| Residential education c | ||||
| ≥17.6% | 1.10 (0.93, 1.30) | 0.25 | 1.30 (0.98, 1.72) | 0.07 |
| 10.9% – 17.5% | 1.16 (1.02, 1.33) | 0.03 | 1.23 (0.97, 1.55) | 0.09 |
| 6.3% – 10.8% | 1.01 (0.90, 1.13) | 0.90 | 1.10 (0.90, 1.13) | 0.33 |
| <6.3% | ref | – | ref | – |
Abbreviations: OR, odds ratio; CI, confidence interval
Compared to patients who underwent surgery <56 days after nCRT; adjusted for all variables in table, year of diagnosis, patient age (treated as a restricted quadratic spline), sex, Charlson-Deyo comorbidity score, cancer histology, AJCC 6th or 7th edition clinical T and N
Median residential household income of each patient’s ZIP code was estimated using the 2016 American Community Survey
Proportion of adults in patient’s ZIP code who did not complete high school, measured in the 2016 American Community Survey
Discussion
The purpose of this study was to determine the association between socioeconomic factors and time to surgery after neo-adjuvant chemoradiation for esophageal cancer, as well as examine the association between timing of esophagectomy and outcomes. In doing so, we hoped to explore a possible source of disparities in esophageal cancer. We found that minority race, low household income, and public insurance is associated with a longer time to surgery and we re-demonstrated associations between longer time to surgery and increased peri-operative mortality.
Patients with esophageal cancer experience significant disparities based on socioeconomic factors such as race/ethnicity and income. Disparities appear to begin with incidence and diagnosis. In 2001, a case-control study on risk factors for esophageal squamous cell carcinoma involving 347 patients found that low income, alcohol, tobacco use, and infrequent consumption of raw fruits and vegetables contributed significantly to the excess incidence of esophageal cancer in Black patients compared with White patients [24]. A 2012 study of 1914 patients in the Netherlands found that low socio-economic status (SES) patients were diagnosed at an older age and with more advanced tumor stage [25].
Disparities also exist in the incidence of squamous cell carcinoma vs adenocarcinoma between socioeconomic groups. Our analysis of patient characteristics by histologic type does show a higher proportion of non-Hispanic Black race and lower income among those with squamous cell carcinoma compared with adenocarcinoma. However, subgroup analysis of patients with adenocarcinoma showed a consistent relationship between race/ethnicity, insurance type, and income with longer time to esophagectomy.
Disparities exist in treatment as well. An analysis of 6737 patients included in the Surveillance, Epidemiology, and End Results (SEER) database in 2013 found that Black and Hispanic patients were less likely to undergo esophagectomy compared with White patients [17]. In the VA system, 1521 patients were reviewed in 2002 and Black patients were found to undergo esophagectomy about half as frequently as White patients [14]. A more recent analysis published in 2019 confirmed that the disparity still exists, with Black patients, uninsured patients, and patients living in areas with lower median income and education levels receiving no treatment or no surgery at higher rates. These patients had correspondingly worse outcomes [26]. For patients who do receive recommended therapy, disparities in all-cause mortality appear to resolve [19,27]. However, among patients who experience a major complication after esophagectomy, Black race was associated with significantly increased in-hospital mortality [18].
Few studies have attempted to determine the causes of these disparities. One qualitative study, published in 2017, interviewed or surveyed 80 patients with esophageal cancer. They found that patients with low SES were offered surgery at lower rates, pursued 2nd opinions at a lower rate, and were more likely to lose their jobs. In addition, patients with low SES had difficulties communicating with their providers, understanding treatment, and maintaining their finances [15]. This study highlights the responsibility of providers to mobilize appropriate social services, explain therapies at an appropriate health literacy level, and recognize potential unconscious biases in treatment plans.
The current study adds to the literature on disparity in esophageal cancer by highlighting time to surgery, a pathway through which disadvantaged patients (non-White race, public insurance, and lower median household income) may experience worse outcomes. Although the optimal timing of esophagectomy remains unclear, previous studies have demonstrated an association between lower overall survival and longer time to esophagectomy. A 2017 retrospective review of 2444 patients showed that patients receiving surgery after 56 days had a higher rate of pathologic downstaging, but no difference in margin positivity and lower overall survival [21]. A similar 2018 retrospective review included 3102 patients and again demonstrated a higher rate of pathologic complete response, but higher rate of 30-day mortality [12]. A meta-analysis of 13 retrospective studies including 15,086 patients confirmed an association between time to surgery >56 days and improved pathologic complete response but higher 30-day mortality. It also demonstrated an association with lower 2 and 5 year survival [13]. Our study agrees with previously published work showing a higher rate of 30-day and 90-day mortality and a small difference in 5-year survival. Most recently, a 2019 retrospective review of the NCDB analyzed patients with esophageal adenocarcinoma based on a 90-day cutoff and found an increased rate of delayed surgery in Black patients and those insured with Medicare. It also showed a higher rate of pCR and increased 90-day mortality in the delayed group. Interestingly, this study showed no significant difference in overall survival [28]. There are key differences between this work and our analysis. First, Levinsky et al. focus on outcomes of delayed/salvage esophagectomy compared with timely esophagectomy with a cutoff of 90 days. Our study focuses on the relationship between socioeconomic factors and delayed esophagectomy with cutoffs of 56 days and 85 days. This difference in grouping is the largest difference in terms of statistical analysis and may explain the differences in overall survival. Other differences including the inclusion of squamous cell carcinoma and data from 2015-2016 are less likely to have affected the results, as our analysis showed no significant association between the covariates of cancer type and date of surgery and outcomes.
This study demonstrates a trend towards increased mortality in patients undergoing surgery 56-84 days after nCRT and a statistically significant increase in patients undergoing surgery after 85-112 days. Together with previously published data, this study confirms the association between longer time to surgery and worse outcomes. By separating the “late” surgery group into two groups, we demonstrate that delaying surgery beyond 85 days appears to increase the magnitude of its association with mortality.
It remains unclear why a longer time to surgery is associated with worse outcomes. It appears that the ability of a patient to undergo surgery sooner after completion of neoadjuvant therapy is itself a proxy for improved outcomes. This may mean that patients who achieve early surgery have a better performance status and greater fitness for surgery, which would likely improve outcomes. Correspondingly, delays in time to surgery may be a marker of frailty, increased toxicity or complications of neo-adjuvant therapy, financial/social crisis, challenges in access to surgical care or some other residual confounder that results in worse outcomes. If longer time to surgery does cause worse outcomes, then this association represents a modifiable delay in care. If longer time to surgery is a marker of increased pre-operative risk due to some other clinical or social factor, then more intensive social services, “pre-habilitation,” and supportive care is warranted. Although examining this specifically is not feasible in the NCDB data examined in this study, further research exploring reasons for delays to surgery among disadvantaged groups could help clarify this question and target interventions to reduce disparities.
This study is not without limitations. First, we are only able to observe received treatment; if a patient had been planned to undergo nCRT and surgery but died before they could complete the treatment plan, they would not be included. However, this occurrence is rare, about 0.3%, in recent randomized controlled trials [1]. Second, while we chose our cut points using prior studies and clinical experience, there are no widely accepted and standard time interval groups. For example, while many studies use <56 days as the shortest time period, published studies have used <42 days [12] or <64 days [11]; however, regardless of the intervals these studies have all had similar results.
Additionally, while we adjusted for cancer characteristics, age, and Charlson-Deyo comorbidity score, residual confounding by patient frailty and illness severity may still exist. If surgical timing is also associated with frailty or illness severity, this could bias our results. We attempted to minimize the impact of unmeasured confounding by excluding patients who underwent surgery >112 days from nCRT, which is a clear deviation from standard of care. Additionally, data on education and income were at the ZIP code-level rather than individual level. Finally, there are specific limitations associated with data from the NCDB. First, this only includes facilities accredited by the American College of Surgeons CoC, so these results may not generalize to non-participating hospitals, especially community facilities. It is also limited with regard to interpretation of socioeconomic factors by the extrapolation of income and education status from zip code of residence instead of individual patient data. This makes precise interpretation of socioeconomic data more challenging.
Conclusion
Minority race (non-Hispanic Black and Hispanic), public insurance, and lower household income were associated with a longer time interval between completion of neoadjuvant chemoradiation and esophagectomy for esophageal cancer. Longer time intervals were also associated with increased post-operative mortality but minimal impact on long-term survival. This study demonstrates a potential source of socioeconomic disparities in outcomes among patients with esophageal cancer.
Supplementary Material
Synopsis:
Socioeconomic status (minority race, public insurance, low income) is associated with longer time interval between esophagectomy and neo-adjuvant chemoradiation for esophageal cancer. Longer time interval to surgery is associated with increased post-operative mortality, but not long-term survival.
Footnotes
Conflict of interest:
The authors have no conflicts of interest to declare.
Data sharing statement:
Data sharing not applicable to this article as no datasets were generated during the current study.
References
- 1.Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. [DOI] [PubMed] [Google Scholar]
- 2.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med. 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 3.Walsh TN, Grennell M, Mansoor S, et al. Neoadjuvant treatment of advanced stage esophageal adenocarcinoma increases survival*. Dis Esophagus. 2002;15:121–124. [DOI] [PubMed] [Google Scholar]
- 4.Urba SG, Orringer MB, Turrisi A, et al. Randomized Trial of Preoperative Chemoradiation Versus Surgery Alone in Patients With Locoregional Esophageal Carcinoma. J Clin Oncol. 2001;19:305–313. [DOI] [PubMed] [Google Scholar]
- 5.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III Trial of Trimodality Therapy With Cisplatin, Fluorouracil, Radiotherapy, and Surgery Compared With Surgery Alone for Esophageal Cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruol A, Rizzetto C, Castoro C, et al. Interval Between Neoadjuvant Chemoradiotherapy and Surgery for Squamous Cell Carcinoma of the Thoracic Esophagus. Ann Surg. Published online 2010. [DOI] [PubMed] [Google Scholar]
- 7.Kim JY, Correa AM, Vaporciyan AA, et al. Does the Timing of Esophagectomy After Chemoradiation Affect Outcome ? Ann Thorac Surg. 2012;93:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaikh T, Ruth K, Scott WJ, et al. Increased Time From Neoadjuvant Chemoradiation to Surgery Is Associated With Higher Pathologic Complete Response Rates in Esophageal Cancer. Ann Thorac Surg. 2015;99:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franko J, Mcavoy S. Timing of esophagectomy after neoadjuvant chemoradiation treatment in squamous cell carcinoma ✩. Surgery. 2018;164:455–459. [DOI] [PubMed] [Google Scholar]
- 10.Lee A, Wong AT, Schwartz D, et al. Is There a Benefit to Prolonging the Interval Between Neoadjuvant Chemoradiation and Esophagectomy in Esophageal Cancer? Ann Thorac Surg. 2016;102:433–438. [DOI] [PubMed] [Google Scholar]
- 11.Tsang JS, Tong DKH, Lam KO, et al. Appropriate timing for surgery after neoadjuvant chemoradiation for esophageal cancer. Dis Esophagus. Published online 2017:1–8. [DOI] [PubMed] [Google Scholar]
- 12.Van Der Werf LR, Dikken JL, Van Der Willik EM. Time interval between neoadjuvant chemoradiotherapy and surgery for oesophageal or junctional cancer : A nationwide study. Eur J Cancer. 2018;91:76–85. [DOI] [PubMed] [Google Scholar]
- 13.Qin Q, Xu H, Liu J, et al. Does timing of esophagectomy following neoadjuvant chemoradiation a ff ect outcomes ? A meta-analysis. Int J Surg. 2019;59:11–18. [DOI] [PubMed] [Google Scholar]
- 14.Dominitz JA, Maynard C, Billingsley KG, et al. Race, Treatment, and Survival of Veterans With Cancer of the Distal Esophagus and Gastric Cardia. Med Care. 2002;40:I-14–I-26. [DOI] [PubMed] [Google Scholar]
- 15.Lineback CM, Mervak CM, Revels SL, et al. Barriers to Accessing Optimal Esophageal Cancer Care for Socioeconomically Disadvantaged Patients. Ann Thorac Surg. 2017;103:416–421. [DOI] [PubMed] [Google Scholar]
- 16.Schlottmann F, Gaber C, Strassle PD, et al. Disparities in esophageal cancer: less treatment, less surgical resection, and poorer survival in disadvantaged patients. Dis Esophagus. Published online May 10, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Revels SL, Morris AM, Reddy RM, et al. Racial Disparities in Esophageal Cancer Outcomes. Ann Surg Oncol. 2013;20:1136–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liou DZ, Serna-Gallegos D, Mirocha J, et al. Predictors of Failure to Rescue After Esophagectomy. In: Annals of Thoracic Surgery. Vol 105. Elsevier; USA; 2018:871–878. [DOI] [PubMed] [Google Scholar]
- 19.Steyerberg EW, Earle CC, Neville BA, et al. Racial differences in surgical evaluation, treatment, and outcome of locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol. 2005;23:510–517. [DOI] [PubMed] [Google Scholar]
- 20.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: A powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranney DN, Mulvihill MS, Yerokun BA, et al. Surgical resection after neoadjuvant chemoradiation for oesophageal adenocarcinoma : what is the optimal timing ? Eur J Cardio-thoracic Surg. 2017;52:543–551. [DOI] [PubMed] [Google Scholar]
- 22.Tessier W, Gronnier C, Messager M, et al. Does Timing of Surgical Procedure After Neoadjuvant Chemoradiation Affect Outcomes in Esophageal Cancer ? Ann Thorac Surg. 2014;97:1181–1189. [DOI] [PubMed] [Google Scholar]
- 23.Hernán MA, Sauer BC, Hernández-Díaz S, et al. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris Brown L, Hoover R, Silverman D, et al. Excess Incidence of Squamous Cell Esophageal Cancer among US Black Men: Role of Social Class and Other Risk Factors. Am J Epidemiol. 2001;153:114–122. [DOI] [PubMed] [Google Scholar]
- 25.Bus P, Aarts MJ, Lemmens VEPP, et al. The Effect of Socioeconomic Status on Staging and Treatment Decisions in Esophageal Cancer. J Clin Gastroenterol. 2012;46:833–839. [DOI] [PubMed] [Google Scholar]
- 26.Cairns AL, Schlottmann F, Strassle PD, et al. Racial and Socioeconomic Disparities in the Surgical Management and Outcomes of Patients with Colorectal Carcinoma. World J Surg. 2019;43:1342–1350. [DOI] [PubMed] [Google Scholar]
- 27.Dhungel B, Diggs BS, Hunter JG, et al. Patient and Peri-operative Predictors of Morbidity and Mortality After Esophagectomy: American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), 2005-2008. J Gastrointest Surg. 2010;14:1492–1501. [DOI] [PubMed] [Google Scholar]
- 28.Levinsky NC, Wima K, Morris MC, et al. Outcome of delayed versus timely esophagectomy after chemoradiation for esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2020;159:2555–2566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated during the current study.