Abstract
Background
Immune checkpoint inhibitor (ICI) monotherapy and neoadjuvant immunochemotherapy have shown promising results in esophageal carcinoma. However, it is still unclear whether more courses of immunochemotherapy are therapeutically better. We aimed to investigate the safety and efficacy of three courses of neoadjuvant treatment for patients with locally advanced esophageal squamous cell carcinoma (ESCC).
Methods
Patients with locally advanced ESCC received three courses of camrelizumab plus nab‐paclitaxel and capecitabine before undergoing surgery. Additionally, patients received safety, computed tomography (CT), and endoscopy (with endoscopic ultrasonography and mucosal biopsy) assessments before and in the second and third courses of treatment. We used the CT and endoscopic assessment results from the second and third courses for comparison.
Results
From May 2020 to December 2021, 47 patients were enrolled at Sun Yat‐sen University Cancer Center. In our study, 43 patients completed three courses of preoperative chemotherapy combined with anti‐Programmed cell death‐1 (PD‐1) therapy and radical surgical resection. The toxicity of the third course of immunochemotherapy was mild and well tolerated without increased treatment‐related adverse events (TRAEs) and mortality compared with that of the second course of treatment. In terms of efficacy, an additional course of treatment after the second course of treatment was effective, with increased CT and endoscopy T (clinical T stage) downstaging rates by 16.3% and 25.9%, N (clincial N stage) downstaging rates by 7.0% and 11.1%, and objective response rates (ORRs) by 13.6% and 22.0%, respectively.
Conclusions
Regardless of downstaging or ORR, three courses of immunochemotherapy appear to be superior to two courses of treatment without increasing TRAEs.
Keywords: esophageal cancer, immunochemotherapy, treatment courses
The workflow and outcomes of this study.
Neoadjuvant immunochemotherapy has excellent potential for treating locally advanced esophageal cancer.
Three courses of immunochemotherapy showed better efficacy than two courses of treatment and did not increase the rate of treatment‐related adverse events.

INTRODUCTION
Esophageal cancer (EC) is the seventh most common malignancy and the sixth leading cause of cancer‐related death worldwide. 1 China is a high‐incidence area of EC, and more than 90% of EC cases are esophageal squamous cell carcinoma (ESCC). 2
As a new treatment for EC, anti‐Programmed cell death‐(Ligand)1 (PD‐(L)1) therapy can specifically block the combination of Programmed cell death‐1 (PD‐1) or Programmed cell death ligand 1 (PD‐L1) through the application of PD‐(L)1 inhibitors and restore T‐cell antitumor immune activities. 3 Camrelizumab is a humanized high‐affinity Immunoglobulin G4 (IgG4)‐kappa anti‐PD‐1 monoclonal antibody whose efficacy and safety have been verified in advanced ESCC. In the recent randomized phase III ESCORT‐1st study, 4 the additional use of camrelizumab with chemotherapy improved the objective response rate (ORR) compared to chemotherapy alone and thus it has been approved as a first‐line treatment for unresectable advanced ESCC. In addition, neoadjuvant administration of PD‐1 blockade combined with chemotherapy has also been shown to encourage antitumor activity in multiple malignancies, such as lung and colorectal cancer. 5 , 6 However, its application in locally advanced ESCC has not yet been established.
To date, several clinical trials have reported that neoadjuvant immunochemotherapy induced favorable clinical pathological responses and had tolerable toxicity in patients with locally advanced ESCC. 7 , 8 However, most of these studies only focused on two courses of treatment. According to a recent report, about 25% of locally advanced ESCC patients recur within 1 year after two courses of immunochemotherapy. 9 Another randomized controlled non‐small‐cell lung cancer clinical study 10 suggested that the major pathological response (MPR) rate of three courses of neoadjuvant immunochemotherapy was higher than that of two courses without increasing treatment‐related adverse events (TRAEs). Those reports indicated that a two‐course regimen might not be sufficient to eliminate minimal residual disease, which may lead to eventual postoperative recurrence.
In our pilot study, 11 three courses of neoadjuvant camrelizumab combined with chemotherapy showed promising efficacy in locally advanced ESCC without increased complications. We therefore conducted this prospective phase II clinical trial to initially explore whether three courses of immunochemotherapy are better for locally advanced ESCC patients.
METHODS
Patients and study design
We conducted a single‐arm, single‐center, phase II trial investigating camrelizumab combined with chemotherapy followed by surgery in locally advanced ESCC. This study was a secondary analysis of this clinical trial data. Overall, the main inclusion criteria were as follows: (1) a diagnosis of clinical stage II to III locally advanced ESCC that was deemed to be resectable before enrollment; (2) no cervical lymph node metastasis or distant organ metastasis; (3) no secondary primary tumors; (4) an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1; and (5) no prior exposure to anticancer treatment, including radiotherapy, chemotherapy, immunotherapy, and targeted therapy.
The current study was approved by the ethics committee of the Sun Yat‐sen University Cancer Center and registered with www.chictr.org.cn, ChiCTR2000029807. All patients signed written informed consent forms.
Procedure
The patients enrolled in the study received three courses of PD‐1 blockade combined with chemotherapy. For each course of treatment, all participants received a flat dose of camrelizumab (200 mg, i.v. drip) plus a single dose of nab‐paclitaxel (260 mg/m2, i.v. drip) on day 1, and capecitabine was administered twice daily (1250 mg/m2) on days 1–14. The regimen was repeated every 3 weeks. Granulocyte colony‐stimulating factor (G‐CSF) was applied prophylactically on day 4 of each course. At baseline and after the second and third neoadjuvant treatment course, contrast‐enhanced thoracic/abdominal computed tomography (CT), endoscopy (with endoscopic ultrasonography and mucosal biopsy), and cervical/supraclavicular ultrasonography were performed. Subsequently, we used the CT and endoscopic assessment results of the second and third courses for comparison (Figure 1).
FIGURE 1.
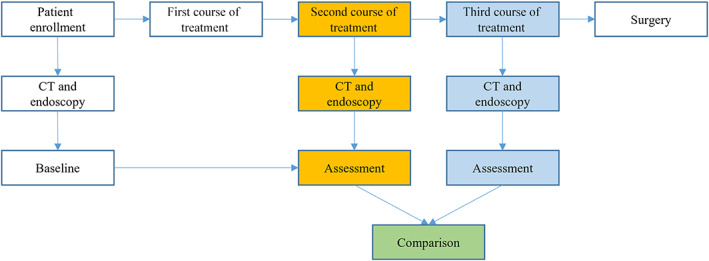
The workflow of this study. CT, computed tomography
Thoracoscopic esophagectomy with cervical esophagogastric anastomosis and modern two‐field lymph node dissection was performed approximately 4–6 weeks after the last course of neoadjuvant therapy.
Assessment of safety
Safety and TRAEs were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. 12 Safety was defined as no treatment‐related death or serious TRAEs caused by neoadjuvant treatment.
Assessment of response
CT evaluation: According to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, 13 the assessment criteria for the CT assessment were as follows:
Complete response (CR): All target lesions disappeared and no new lesions were found.
Partial response (PR): At least a 30% decrease in the sum of the greatest dimensions of the target lesions, taking the sum of the greatest dimensions at baseline as the reference.
Stable disease (SD): Neither PR nor progressive disease.
Progressive disease (PD): At least a 20% increase in the sum of the greatest dimensions of the target lesions, taking the smallest sum of the greatest dimensions recorded after the start of treatment as the reference.
The ORR was defined as the sum of CR and PR.
Endoscopic assessment: We noticed that endoscopic ultrasonography (EUS) was not used to evaluate tumors in the endoscopic evaluation criteria of the Japan Esophageal Society. 14 Based on this, we added the changes in EUS T (clinical T stage) staging combined with the endoscopic evaluation criteria of the Japan Esophageal Society to evaluate the efficacy of treatment. The criteria details for the endoscopic assessment are presented as follows:
Endoscopic complete response (eCR): Disappearance of endoscopic findings suggesting the presence of a tumor; the entire esophagus could be observed using endoscopy; negative endoscopic biopsy findings from the area of the primary lesion; no endoscopic findings of active esophagitis; and endoscopy showed no hypoechoic nodules at all anatomical levels of the esophagus.
Endoscopic partial response (ePR): The tumor or surrounding bulge shrank or became flattened, the ulcer shrank, and endoscopy showed a decrease in the T stage from baseline.
Endoscopic stable disease (eSD): The tumor mass showed no significant change from baseline, and endoscopy showed a T stage after treatment equal to that at baseline.
Endoscopic progressive disease (ePD): The tumor grew significantly larger or progressed, and endoscopy indicated an increase in the T stage from baseline.
The endoscopic objective response rate (eORR) was defined as the sum of eCR and ePR.
Two senior imaging specialists and endoscopists reviewed all CT and endoscopy imaging data independently, and clinical staging was performed according to the TNM classification (8th edition). 15
Pathological assessment
Tissue sections were stained with hematoxylin and eosin (H&E), and pathological regression was assessed by two independent pathologists. Pathological complete remission (pCR) was defined as the absence of residual invasion disease. Tumors with ≤10% residual viable tumor cells were considered as obtaining an MPR.
Statistical analysis
The Mann–Whitney U test was used to compare continuous distributed variables, and the Wilcoxon signed‐rank test was used to compare two related categorical variables among groups. All reported p‐values were two‐tailed. A p value of <0.05 was considered statistically significant. All analyses were performed using the SPSS 26.0 software package and graphs were generated with GraphPad Prism version 9.0.3 and R 4.0.3.
RESULTS
Baseline characteristics
A total of 47 patients were enrolled in this study from May 2020 to December 2021 at Sun Yat‐sen University Cancer Center. Four patients (8.5%) discontinued treatment due to economic reasons. A total of 43 patients who had finished three courses of PD‐1‐based neoadjuvant immunochemotherapy and radical surgery resection were finally enrolled for analysis. Of these, Thirty‐four patients were male and nine patients were female. The median age of the entire cohort was 57.0 years (range 44–70 years). Eleven (25.6%) patients had stage II disease and 32 (74.4%) patients had stage III disease. More details of the clinical baseline characteristics of the patients are shown in Table 1.
TABLE 1.
Baseline characteristics of the patients
| Characteristic | N = 43 |
|---|---|
| Age, median (IQR) (years) | 57 (44–70) |
| Sex, n (%) | |
| Male | 34 (79.1) |
| Female | 9 (20.9) |
| Tumor site, n (%) | |
| Upper third | 2 (4.7) |
| Middle third | 17 (39.5) |
| Lower third | 24 (55.8) |
| Tumor differentiation, n (%) | |
| Well | 5 (11.6) |
| Moderately | 27 (62.8) |
| Poorly | 11 (25.6) |
| Clinical stage, n (%) | |
| I | 0 (0) |
| II | 11 (25.6) |
| III | 32 (74.4) |
| IV | 0 (0) |
| Clinical T stage, n (%) | |
| cT1 | 0 (0) |
| cT2 | 12 (27.9) |
| cT3 | 31 (72.1) |
| cT4 | 0 (0) |
| Clinical N stage, n (%) | |
| cN0 | 2 (4.7) |
| cN1 | 18 (41.9) |
| cN2 | 23 (53.4) |
| cN3 | 0 (0) |
| ECOG score, n (%) | |
| 0 | 38 (88.4) |
| 1 | 5 (11.6) |
Note: The clinical stage was assessed using endoscopy (combined with endoscopic ultrasonography [EUS] and mucosa biopsy) or computed tomography (CT) and was classified according to the Union for International Cancer Control (UICC) tumor–node–metastasis (TNM) classification, 8th edition.
Abbreviation: IQR, interquartile range.
Safety
Three courses of treatment did not increase TRAEs compared to two courses of treatment (Table 2). Neoadjuvant use of camrelizumab in combination with nab‐paclitaxel and capecitabine did not cause any previously unreported toxicities. All patients reported at least one adverse event during the neoadjuvant treatment, and most of the TRAEs were grade 1–2. The most common grade 1–2 TRAEs of the second and third courses of treatment were alopecia (30/69.7% vs. 31/72.0%), reactive cutaneous capillary endothelial proliferation (RCCEP) (24/55.8% vs. 28/65.1%), fatigue (25/58.1% vs. 25/58.1%), anemia (23/53.5% vs. 22/51.2%), muscle soreness (18/41.8% vs. 18/41.8%), numbness of limbs (20/46.5% vs. 20/46.5%), and increased alanine transaminase (9/20.9% vs. 10/23.3%). Leukopenia occurred in only three (6.4%) patients in the second and third courses of treatment. Two (4.6%) patients in the second course developed grade 3–4 adverse events during treatment, including fatigue and limb numbness. Three (7.0%) patients in the third course of treatment developed grade 3–4 adverse events, including one with fatigue, one with limb numbness, and one with anemia. No grade 5 events or treatment‐related mortality were observed.
TABLE 2.
Summary of treatment‐related adverse events (TRAEs)
| All events | Second course of treatment | Third course of treatment | p value* | ||||
|---|---|---|---|---|---|---|---|
| Total | Grade 1–2 | Grade 3–4 | Total | Grade 1–2 | Grade 3–4 | ||
| Alopecia | 31 (72.1) | 31 (72.1) | 0 (0) | 31 (72.1) | 31 (72.1) | 0 (0) | 0.157 |
| Reactive cutaneous capillary endothelial proliferation | 24 (55.8) | 24 (55.8) | 0 (0) | 28 (65.1) | 28 (65.1) | 0 (0) | 0.046 |
| Fatigue | 24 (55.8) | 23 (53.5) | 1 (2.3) | 25 (58.1) | 24 (55.8) | 1 (2.3) | 0.317 |
| Anemia | 23 (53.5) | 23 (53.5) | 0 (0) | 23 (53.5) | 22 (51.2) | 1 (2.3) | 0.317 |
| Muscle soreness | 18 (41.7) | 18 (41.7) | 0 (0) | 18 (41.7) | 18 (41.7) | 0 (0) | 0.317 |
| Limb numbness | 20 (46.5) | 19 (44.2) | 1 (2.3) | 20 (46.5) | 19 (44.2) | 1 (2.3) | 0.564 |
| Increased alanine transaminase | 9 (20.9) | 9 (20.9) | 0 (0) | 10 (23.3) | 10 (23.3) | 0 (0) | 0.083 |
| Constipation | 7 (16.3) | 7 (16.3) | 0 (0) | 7 (16.3) | 7 (16.3) | 0 (0) | 1.000 |
| Diarrhea | 4 (9.3) | 4 (9.3) | 0 (0) | 4 (9.3) | 4 (9.3) | 0 (0) | 0.317 |
| Immune‐related hyperthyroidism | 4 (9.3) | 4 (9.3) | 0 (0) | 4 (9.3) | 4 (9.3) | 0 (0) | 0.317 |
| Leukopenia | 2 (4.7) | 2 (4.7) | 0 (0) | 2 (4.7) | 2 (4.7) | 0 (0) | 1.000 |
| Vomiting | 2 (4.7) | 2 (4.7) | 0 (0) | 2 (4.7) | 2 (4.7) | 0 (0) | 1.000 |
| Nausea | 2 (4.7) | 2 (4.7) | 0 (0) | 2 (4.7) | 2 (4.7) | 0 (0) | 1.000 |
| Immune‐related hypothyroidism | 2 (4.7) | 2 (4.7) | 0 (0) | 2 (4.7) | 2 (4.7) | 0 (0) | 1.000 |
| Thrombocytopenia | 2 (4.7) | 2 (4.7) | 0 (0) | 2 (4.7) | 2 (4.7) | 0 (0) | 0.317 |
| Immune‐related myocarditis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Cough | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Immune‐related pneumonia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Immune‐related hepatitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Immune‐related nephritis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
Note: All adverse events were reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
The p value represents the difference between the total TRAEs from the second course treatment and the third course treatment.
Downstaging analysis
After the second and third courses of neoadjuvant immunochemotherapy, CT assessment showed that 28/65.1% versus 35/81.4% of patients had T (clincial T stage) downstaging, and 23/53.5% versus 26/60.5% of patients had N (clinical N stage) downstaging. Similarly, the endoscopic assessment showed that 17/63.0% versus 24/88.9% of patients had T downstaging, and 18/66.7% versus 21/77.8% of patients had N downstaging (Figure 2). We found that the third course of treatment had higher rates of T downstaging and N downstaging than the second course of treatment. In addition, one course of neoadjuvant therapy based on the second course increased the CT and endoscopy T downstaging rates by 16.3% and 25.9%, and the N downstaging rates by 7.0% and 11.1%, respectively.
FIGURE 2.
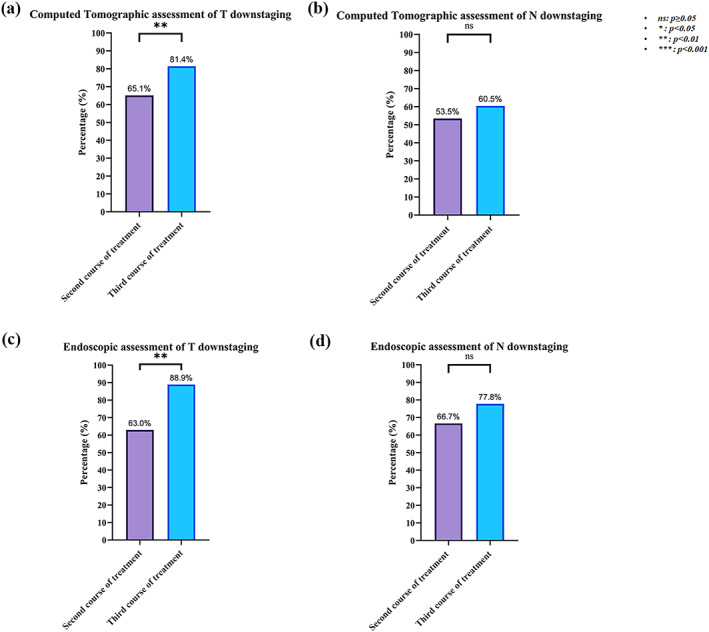
Clinical T&N downstaging assessment of Computed tomography (CT) and endoscopy
Clinical and pathological responses
CT assessment
All 43 patients completed the CT assessment before treatment and the second and third courses after treatment. According to the RECIST 1.1 criteria, the second and third courses of neoadjuvant therapy resulted in a CR of 1/2.3% versus 3/7.0%, a PR of 28/65.1% versus 32/74.4%, and an SD of 14/32.6% versus 8/18.6%, respectively. We found that no patients developed as PD in either the second or third course of treatment (Figure 3a). The ORR was 67.4% versus 81.0% (Figure 3c). Compared with the second course of treatment, the third course of treatment could further convert 4 patients/14.3% of PR patients into CR and 6 patients/42.9% of SD patients into PR. The ORR of the third course of treatment was further increased by 13.6% compared with the second course of treatment, and statistically significant differences were found (Figure 3c).
FIGURE 3.
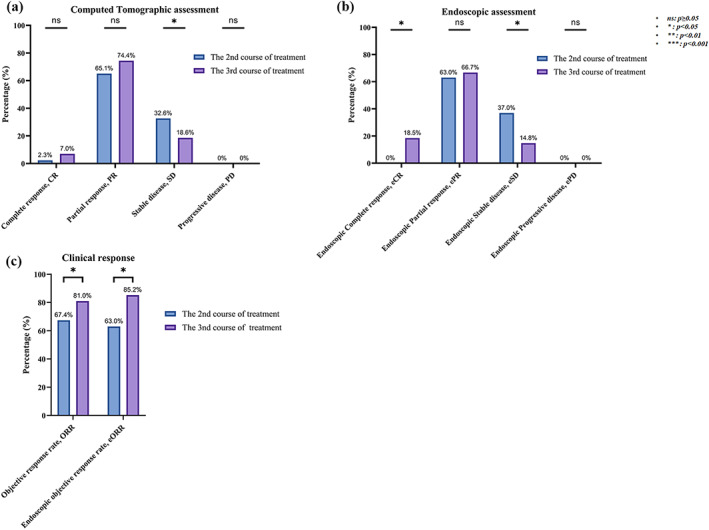
Clinical responses to neoadjuvant camrelizumab combined with chemotherapy. CR, complete response; CT, computed tomography; eORR, endoscopic objective response rate; ePD, endoscopic Progressive disease; eSD, endoscopic stable disease; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease
Endoscopic assessment
A total of 27 patients completed the endoscopic assessment before and after the second and third courses of treatment. According to the endoscopic diagnostic criteria of the Japanese Esophageal Society, the second and third courses of neoadjuvant therapy resulted in 0 patients/0% versus 5 patients/18.5% eCR, 17 patients/63.0% versus 18 patients/66.7% ePR, and 10 patients/37.0% versus 4 patients/14.8% eSD, respectively. None of the patients had ePD in either the second or third course of treatment (Figure 3b). The eORR was 63.0% versus 85.2% (Figure 3c). Compared with the second course of treatment, the third course of treatment further converted 5 patients/29.4% of ePR patients to eCR and 6 patients/33.3% of eSD patients to ePR. The eORR of the third course was further increased by 22.0% compared with that of the second course of treatment, and there was a statistically significant difference (Figure 3c).
Pathology assessment
Among the 43 patients who underwent surgery, postoperative pathological results showed median tumor regression of 90% (range 10–100%). A total of 27 (62.8%) patients had an MPR in the primary tumor. pCR was achieved in 14 (32.6%) cases (Figure 4).
FIGURE 4.
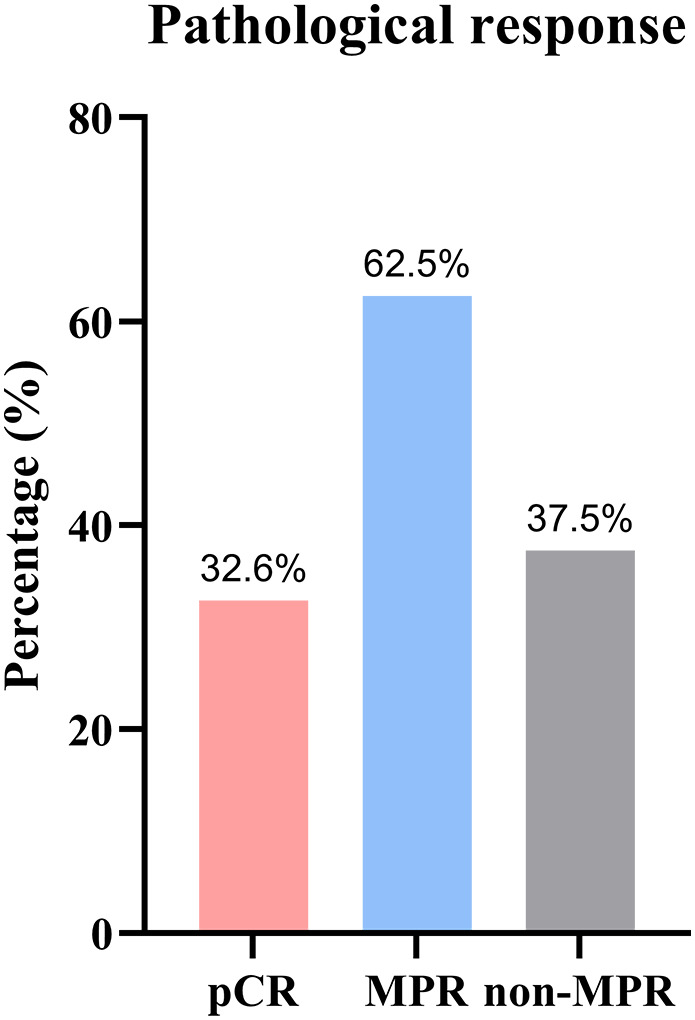
Pathological responses to neoadjuvant camrelizumab combined with chemotherapy. (MPR, major pathological response; pCR, pathological complete remission)
DISCUSSION
Neoadjuvant therapy combined with surgery is the standard treatment regimen for patients with locally advanced ESCC. Two courses of neoadjuvant chemoradiotherapy can benefit patient survival. 16 However, a recent study reported that there is still a 23.9% distant metastasis rate remaining after two courses of treatment. 17 For neoadjuvant immunochemotherapy, most clinical trials currently also focus on the effect of a two‐course regimen, which may lead to the limited efficacy of immunotherapy. In our previous pilot study 11 we found that three courses of neoadjuvant immunochemotherapy were safe, feasible, and effective. These results were further confirmed in this phase II trial.
In this study, camrelizumab combined with nab‐paclitaxel and capecitabine was used as neoadjuvant therapy. The NCCN guidelines for EC recommend neoadjuvant regimens such as paclitaxel combined with platinum 18 , 19 or paclitaxel combined with fluorouracil. 20 Capecitabine, a new generation of oral fluorouracil compounds, has lower hematological toxicity than platinum‐based compounds 21 and is effective against a variety of gastrointestinal tumors. The ORR in the treatment of gastric cancer 22 , 23 is 34–50%, and that of colorectal cancer 24 is 58.6%, with no serious adverse events. Sandor Schokker et al. 25 combined nab‐paclitaxel and capecitabine for EC, with an ORR of 54%, median PFS and OS of 8 and 12.8 months, respectively, and tolerable adverse events. We therefore selected camrelizumab combined with nab‐paclitaxel and capecitabine as the neoadjuvant regimen in this study to reduce the toxicity of neoadjuvant therapy.
In terms of safety, the toxicity of the third course of camrelizumab combined with chemotherapy when compared with the second course was tolerable. Most TRAEs were grade 1–2, similar to other two‐course regimens. RCCEP induced by camrelizumab was found in 28 patients/65.1% of patients after the third course of treatment compared with 24 patients/55.8% of patients in the second course of treatment. Similarly, the overall incidence of RCCEP after the third course of treatment was higher than that reported in other two‐cycle regimen studies (26.1–39.1%). 7 , 9 This difference may be due to the addition of one course of camrelizumab. Notably, a previous study 9 showed that more than half of patients developed leukopenia during neoadjuvant immunochemotherapy or chemoradiotherapy. Severe leukopenia may even lead to dose reduction or treatment discontinuation. In our cohort, the incidence of leukopenia was relatively low. Only 6.4% of patients had leukopenia during the second and third cycles. This difference can be attributed to two reasons. First, we used G‐CSF prophylactically after each immunochemotherapy course. Second, we replaced platinum with capecitabine in our immunochemotherapy regimen. Capecitabine, an oral drug that can be converted to fluorouracil, 26 in combination with paclitaxel, has shown comparable efficacy and lower toxicity than platinum‐based regimens in head and neck squamous cell carcinomas and breast cancer. 27 , 28 , 29 The drug's low toxicity makes it suitable for use in combination with PD‐1 blockers. Overall, the toxicity of the three‐course treatment regimen was manageable and worthy of promotion.
In terms of efficacy, this study compared the CT and endoscopic assessment results of the second and third courses of treatment. We found that an additional course of treatment after the second course was effective, increasing the CT and endoscopy T downstaging rates by 16.3% and 25.9%, N downstaging rates by 7.0% and 11.1%, and ORRs by 13.6% and 22.0%, respectively. We observed that the T downstaging, N downstaging, ORRs, CR rates, and PR rates assessed by CT or endoscopy after the third course of treatment were higher than those after the second course of therapy, and the SD rate was lower than that after the second course of treatment. No patients (0%) developed as PD in either the second or the third course of treatment, which implies that three courses of treatment may not increase the risk of PD. On the other hand, after three courses of treatment, the surgical pathology showed that the MPR of this study reached 62.8%. Similarly, a recent randomized controlled non‐small‐cell lung cancer clinical study 10 showed that after neoadjuvant immunochemotherapy in patients with lung squamous cell carcinoma, the MPR induced by three courses of neoadjuvant therapy was 60%, compared with 43.8% after two courses of treatment. Both groups tolerated the treatment well; only 5% (3/60) had grade 3 immune‐related adverse events. In 2021, a randomized controlled study 30 in Japan showed that three courses of neoadjuvant chemotherapy resulted in better clinical responses without increased TRAEs or complications than two courses of neoadjuvant chemotherapy.
It is worth noting that previous studies reported that the degree of lymphocyte infiltration in tumor tissue increased with more treatment courses. 31 Another bladder cancer study also suggested that after three courses of anti‐PD‐1 therapy, the density of CD8+ T lymphocytes infiltrating the tumor stroma was significantly higher than that before treatment. 32 These studies imply that more courses of immunotherapy may improve efficacy by fully activating the immune system to remove minimal residual tumor lesions, therefore immune system activation appeared to be more thorough after three courses of therapy than after two courses, which may explain why three courses of treatment are more effective than two courses.
Our findings suggested that three courses of treatment showed better clinical responses without increased TRAEs compared with two courses of treatment. However, short‐term efficacy may affect long‐term survival, therefore it is necessary to perform studies with large sample sizes in the future for further verification. Nonetheless, our study has some limitations. First, the sample size of our study was small. Second, this study is a single‐center study. However, the single‐center study design and self‐controlled study approach made our data more consistent.
CONCLUSION
In summary, the downstaging rate and ORR of three courses of immunochemotherapy were better than those of two courses of treatment and did not increase the rate of TRAEs.
AUTHOR CONTRIBUTIONS
Yuanheng Huang: Ideas and writing the initial draft. Xiaodong Su: Formulation of overarching research aims. Qiyu Guo: Data collection and writing the initial draft. Guangyu Luo: Endoscopic assessment. Haoqiang He: CT assessment. Peiqiang Cai: CT assessment and Data visualization. Muyan Cai: Data verification. Haodong Yue: Initial draft review and statistical Analysis. Zhiqiang Wang: Management and coordination responsibility for the research activity planning and execution. Guozhen Yang: Organize research data. Peng Lin: Project administration. Xu Zhang: Oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team.
FUNDING INFORMATION
This study was supported by the Science and Technology Program of Guangzhou, China (202103000064) and the Science and Technology Project of Guangdong Esophageal Cancer Research Institute (M202017).
CONFLICT OF INTEREST STATEMENT
The author declares no conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to acknowledge all patients participating in the study.
Huang Y, Su X, Guo Q, Luo G, He H, Cai P, et al. Are more courses of immunochemotherapy beneficial for the short‐term outcome of locally advanced esophageal squamous cell carcinoma? Thorac Cancer. 2023;14(13):1153–1161. 10.1111/1759-7714.14843
Yuanheng Huang, Xiaodong Su, Qiyu Guo and Haodong Yue contributed equally to this work.
Contributor Information
Peng Lin, Email: linpeng@sysucc.org.cn.
Xu Zhang, Email: zhangxu@sysucc.org.cn.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Yang Z, Zeng H, Xia R, Liu Q, Sun K, Zheng R, et al. Annual cost of illness of stomach and esophageal cancer patients in urban and rural areas in China: a multi‐center study. Chin J Cancer Res. 2018;30(4):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD‐1 and PD‐L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of Camrelizumab vs placebo added to chemotherapy on survival and progression‐free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT‐1st randomized clinical trial. JAMA. 2021;326(10):916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD‐1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, et al. Neoadjuvant PD‐1 blockade with toripalimab, with or without celecoxib, in mismatch repair‐deficient or microsatellite instability‐high, locally advanced, colorectal cancer (PICC): a single‐centre, parallel‐group, non‐comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7(1):38–48. [DOI] [PubMed] [Google Scholar]
- 7. Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H, et al. Multicenter, single‐arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10(3):e004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gu Y, Chen X, Wang D, Ding M, Xue L, Zhen F, et al. 175P A study of neoadjuvant sintilimab combined with triplet chemotherapy of lipo‐paclitaxel, cisplatin, and S‐1 for resectable esophageal squamous cell carcinoma (ESCC). Ann Oncol. 2020;31:S1307–S8. [Google Scholar]
- 9. Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10(1):e003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qiu F, Fan J, Shao M, Yao J, Zhao L, Zhu L, et al. Two cycles versus three cycles of neoadjuvant sintilimab plus platinum‐doublet chemotherapy in patients with resectable non‐small‐cell lung cancer (neoSCORE): a randomized, single center, two‐arm phase II trial. J Clin Oncol. 2022;40(16_suppl):8500. [Google Scholar]
- 11. Yang G, Su X, Yang H, Luo G, Gao C, Zheng Y, et al. Neoadjuvant programmed death‐1 blockade plus chemotherapy in locally advanced esophageal squamous cell carcinoma. Ann Transl Med. 2021;9(15):1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arrieta O, Barron F, Ramirez‐Tirado LA, Zatarain‐Barron ZL, Cardona AF, Diaz‐Garcia D, et al. Efficacy and safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non‐small cell lung cancer: the PROLUNG phase 2 randomized clinical trial. JAMA Oncol. 2020;6(6):856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 14. Japan ES. Japanese classification of esophageal cancer, 11th edition: part II and III. Esophagus. 2017;14(1):37–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long‐term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. 2021;156(8):721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu S, Wen J, Yang H, Li Q, Chen Y, Zhu C, et al. Recurrence patterns after neoadjuvant chemoradiotherapy compared with surgery alone in oesophageal squamous cell carcinoma: results from the multicenter phase III trial NEOCRTEC5010. Eur J Cancer. 2020;138:113–21. [DOI] [PubMed] [Google Scholar]
- 18. Tamtai A, Jiarpinitnun C, Hiranyatheb P, Unwanatham N, Sirachainun E, Supsamutchai C, et al. Tolerability and efficacy of concurrent chemoradiotherapy comparing carboplatin/paclitaxel versus platinum/5‐FU regimen for locally advanced esophageal and esophagogastric junction cancers. Med Oncol. 2017;34(9):157. [DOI] [PubMed] [Google Scholar]
- 19. Zheng Y, Li Y, Liu X, Zhang R, Wang Z, Sun H, et al. Neoadjuvant chemotherapy followed by minimally invasive esophagectomy versus primary surgery for management of esophageal carcinoma: a retrospective study. J Cancer. 2019;10(5):1097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao Y, Dai Z, Min W, Sui X, Kang H, Zhang Y, et al. Perioperative versus preoperative chemotherapy with surgery in patients with resectable squamous cell carcinoma of esophagus: a phase III randomized trial. J Thorac Oncol. 2015;10(9):1349–56. [DOI] [PubMed] [Google Scholar]
- 21. Mayer IA, Zhao F, Arteaga CL, Symmans WF, Park BH, Burnette BL, et al. Randomized phase III postoperative trial of platinum‐based chemotherapy versus capecitabine in patients with residual triple‐negative breast cancer following neoadjuvant chemotherapy: ECOG‐ACRIN EA1131. J Clin Oncol. 2021;39(23):2539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu Y, Fang Y, Shen Z, Wang Y, Yan M, Cao H, et al. Oxaliplatin plus capecitabine in the perioperative treatment of locally advanced gastric adenocarcinoma in combination with D2 gastrectomy: NEO‐CLASSIC study. Oncologist. 2019;24(10):1311–e989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hong YS, Song SY, Lee SI, Chung HC, Choi SH, Noh SH, et al. A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol. 2004;15(9):1344–7. [DOI] [PubMed] [Google Scholar]
- 24. Santini D, Vincenzi B, Schiavon G, Di Seri M, Virzí V, Spalletta B, et al. Chronomodulated administration of oxaliplatin plus capecitabine (XELOX) as first line chemotherapy in advanced colorectal cancer patients: phase II study. Cancer Chemother Pharmacol. 2007;59(5):613–20. [DOI] [PubMed] [Google Scholar]
- 25. Schokker S, van der Woude SO, van Kleef JJ, van Zoen DJ, van Oijen MGH, Mearadji B, et al. Phase I dose escalation study with expansion cohort of the addition of nab‐paclitaxel to capecitabine and oxaliplatin (CapOx) as first‐line treatment of metastatic esophagogastric adenocarcinoma (ACTION study). Cancers. 2019;11(6):827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27(1):23–44. [DOI] [PubMed] [Google Scholar]
- 27. Bentzen JKD, Kristensen CA, Overgaard M, Rytter C, Jensen K, Hansen HS. A non platinum regimen for the treatment of recurrent or metastatic squamous cell carcinoma of the head and neck region. Results from an extended phase II study with paclitaxel and capecitabine. Front Oncol. 2018;8:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bentzen JD, Hansen HS. Phase II analysis of paclitaxel and capecitabine in the treatment of recurrent or disseminated squamous cell carcinoma of the head and neck region. Head Neck. 2007;29(1):47–51. [DOI] [PubMed] [Google Scholar]
- 29. McDonald F, Miles D. Xeloda and taxotere: a review of the development of the combination for use in metastatic breast cancer. Int J Clin Pract. 2003;57(6):530–4. [PubMed] [Google Scholar]
- 30. Shiraishi O, Makino T, Yamasaki M, Tanaka K, Yamashita K, Ishida T, et al. Two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for treating locally advanced esophageal cancer: short‐term outcomes of a multicenter randomized phase II trial. Esophagus. 2021;18(4):825–34. [DOI] [PubMed] [Google Scholar]
- 31. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Lucianò R, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle‐invasive urothelial bladder carcinoma (PURE‐01): an open‐label, single‐arm, phase II study. J Clin Oncol. 2018;36(34):3353–60. [DOI] [PubMed] [Google Scholar]


