Abstract
Background
To investigate the clinical significance of preoperative systemic immune‐inflammation index (SII) in patients with thymoma who underwent radical resection.
Methods
This retrospective study involved 425 patients with thymoma who underwent radical resection at the First Affiliated Hospital of Nanjing Medical University between September 1, 2008 and December 30, 2019. Data regarding routine preoperative blood tests and clinical features were collected to calculate and analyze the SII, platelet‐to‐lymphocyte ratio (PLR), and neutrophil‐to‐lymphocyte ratio (NLR).
Results
Univariate analysis indicated that age (p = 0.021), tumor size (p = 0.003), extended resection (p < 0.001), Masaoka‐Koga stage (p < 0.001), PLR (p = 0.012), NLR (p = 0.041), and SII (p = 0.003) were related to patient prognosis. A higher SII (>345.83) was a significant independent prognostic factor in this cohort (p = 0.001, HR = 5.756, 95% CI: 2.144–15.457). Multivariate analysis showed that a high PLR was significantly associated with overall survival (OS) (p = 0.008, HR = 3.29, 95% CI: 1.371–7.896), while a high NLR was a significant independent prognostic factor for shorter OS (p = 0.024, HR = 2.654, 95% CI: 1.138–6.19). SII had an area under the curve (AUC) of 70.6% (AUC = 0.706) exceeding the predictive value for PLR (AUC = 0.678) and NLR (AUC = 0.654).
Conclusion
Preoperative SII can predict the prognosis of thymoma patients who have undergone radical resection but further multicenter prospective studies are needed to investigate the role of SII in thymoma.
Keywords: NLR, PLR, prognosis, SII, thymoma
PLR platelet‐to‐lymphocyte ratio, NLR neutrophil‐to‐lymphocyte ratio, SII systemic immune‐inflammation index.
INTRODUCTION
Thymoma is a rare tumor with an annual incidence of 1 2.73 per 1 million person‐years in China, 1 which is higher than in Europe 2 and with a 5‐year survival rate of approximately 78%. 3 The tumor is usually a well‐defined round or lobulated mass in the thymus 4 typically graded using the Masaoka‐Koga staging system. 5 Completeness of resection is important for the prognosis; 6 however, simple thymomectomy (ST) or thymothymectomy (TT) for stage I remains controversial. 7 , 8 , 9 , 10
The WHO histological classification, 11 , 12 radical resection, 13 Masaoka‐Koga stage and The American Joint Committee on Cancer's AJCC Staging Manual, eighth edition (AJCC 8E) TNM staging system 12 , 14 are independent prognostic factors for disease‐free survival (DFS) and overall survival (OS). Intense efforts have been devoted to investigating the utility of novel markers in risk prediction. For example, SUV max and SUV avg based on 2‐ [18 F] FDG PET/CT are independent factors for OS prediction. The overexpression of programmed cell death protein 1 (PD‐1) and programmed death ligand 1 (PD‐L1) in patients with thymoma is associated with a poor prognosis. 15 , 16
Considering the cost and convenience, attention has turned to blood tests with numerous routine blood parameters investigated as risk factor indicators for various cancers, such as absolute lymphocyte count (ALC), circulating white blood cells (WBC), absolute neutrophil count, circulating C‐reactive protein (CRP), and lactate dehydrogenase (LDH) level. Preoperative platelet‐to‐lymphocyte ratio (PLR) and neutrophil‐to‐lymphocyte ratio (NLR) performed well for the survival prediction of solid tumors. 17 , 18 , 19 These two biomarkers have also been applied to the study of OS in patients with lung cancer. 20 , 21 , 22 Indeed, a high NLR and PLR are associated with a poor prognosis in thymic epithelial tumors. 23 , 24 , 25 However, NLR or PLR had a significant effect on OS in univariable Cox models but not in variable Cox models, therefore it remains to be confirmed whether NLR or PLR are independent risk factors for thymoma.
To date, systemic inflammation indicators, based on neutrophil, platelet, and lymphocyte counts, have rarely been used for indolent tumors but the SII is a novel promising inflammation‐based prognostic marker for thoracic neoplasms. 26 Therefore, this study evaluated the prognostic significance of NLR, PLR and SII for the OS of patients with thymoma.
METHODS
Patients
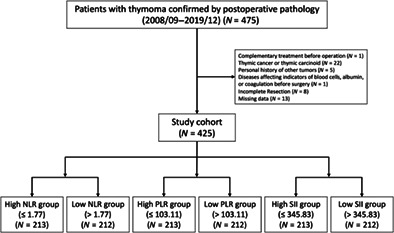
We retrospectively reviewed 475 patients treated at the Department of Thoracic Surgery, the First Affiliated Hospital of Nanjing Medical University between September 1, 2008 and December 30, 2019. Participants were followed up for at least 1 year by telephone or outpatient clinic visits. Inclusion criteria were: (1) thymoma confirmed by postoperative pathology; (2) free of chemotherapy, radiotherapy, immunotherapy, or other complementary treatment before operation; (3) no personal history of other tumors; (4) free of liver or kidney injury, acute or chronic infection, hematological diseases, or other diseases affecting indicators of blood cells, albumin, or coagulation before surgery; (5) successful radical resection and (6) complete data (including clinical information, laboratory examination and follow‐up) (Figure 1).
FIGURE 1.
Flow chart. PLR, platelet‐to‐lymphocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; SII, systemic immune‐inflammation index.
Clinical information collection and calculation
Detailed case information (including pathology reports) was captured using an electronic case report form in the First Affiliated Hospital of Nanjing Medical University. Participants were followed up for at least 1 year by telephone or outpatient clinic visits until death, May 11, 2021, or lost to follow‐up. Blood routine examinations before surgery included platelet counts (PLT), absolute neutrophil count (ANC) and absolute lymphocyte count (ALC). NLR, PLR and SII were calculated according to the following equations: NLR = ANC/ALC; PLR = PLT/ALC; SII = NLR × PLT = ANC × PLT/ALC.
Statistical analysis
Descriptive analysis was performed using the median for continuous variables. The NLR, PLR and SII were analyzed using the median value as the cutoff. The Mann–Whitney U test and chi‐squared test were used to compare groups. OS was defined as the time between diagnosis and/or biopsy examination until the date of death. All patients were followed up until death, loss of follow‐up, or until December 31, 2019 (10 years follow‐up). Survival analyses were performed using the Kaplan–Meier technique and Cox regression was used to predict survival probabilities. A p‐value < 0.05 was considered statistically significant. The analyses were performed using SPSS (version 26, https://www.ibm.com/analytics/spss-statistics-software) or R software (version 4.1.0, http://www.r-project.org).
RESULTS
Clinical characteristics
The data of 475 potentially eligible participants treated at the Department of Thoracic Surgery in the First Affiliated Hospital of Nanjing Medical University between September 1, 2008 and December 30, 2019 were reviewed, of which 425 patients met the inclusion criteria and were included in the final analysis. The patient characteristics are detailed in Table 1 showing that the cohort included 200 (47.06%) men and 225 (52.94%) women with a median age of 56 years (range 17–79 years).
TABLE 1.
Patient characteristics I.
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 200 (47.06%) |
| Female | 225 (52.94%) |
| Age (years) | |
| Median (range) | 56 (17–79) |
| ≤ | 212 (49.88%) |
| > | 213 (50.11%) |
| WHO type | |
| A | 38 (8.94%) |
| AB | 153 (36%) |
| B1 | 63 (14.82%) |
| B2 | 93 (21.88%) |
| B3 | 78 (18.35%) |
| Myasthenia | |
| Yes | 50 (11.76%) |
| No | 375 (88.23%) |
| Extended resection | |
| Yes | 33 (07.76%) |
| No | 392 (92.24%) |
| Masaoka‐Koga stage | |
| I | 241 (56.71%) |
| II | 127 (29.88%) |
| III | 46 (10.82%) |
| IV | 11 (02.59%) |
| Tumor size (cm) | |
| Median (range) | 6 (1–18) |
| ≤ | 251 (59.01%) |
| > | 174 (40.94%) |
| PLR | |
| Median (range) | 103.11 (12.64–376.47) |
| Low | 213 (50.12%) |
| High | 212 (49.88%) |
| NLR | |
| Median (range) | 1.77 (0.24–21.54) |
| Low | 213 (50.12%) |
| High | 212 (49.88%) |
| SII | |
| Median (range) | 345.83 (33.49–6893.18) |
| Low | 213 (50.12%) |
| High | 212 (49.88%) |
Abbreviations: NLR neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; SII, systemic immune‐inflammation index; WHO World Health Organization.
Preoperative PLR, NLR and SII in patients with thymomas
The patients were stratified according to the median NLR, PLR and SII (Table 2). There were no statistically significant differences in the histological classification of the WHO, Masaoka‐Koga stage and tumor size. However, there was a significant difference concerning age (p = 0.029) observed in the SII group. There was a statistically significant difference between the PLR (p = 0.013) and NLR (p = 0.018) groups regarding gender, as well as in myasthenia between the two PLR groups (p = 0.037).
TABLE 2.
Patient characteristics II.
| Characteristics | NLR | p‐value | PLR | p‐value | SII | p‐value | |||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | ||||
| Gender | 0.018 | 0.013 | 0.16 | ||||||
| Male | 89 | 111 | 113 | 87 | 93 | 107 | |||
| Female | 126 | 99 | 100 | 125 | 120 | 105 | |||
| Age (years) | 0.16 | 0.961 | 0.029 | ||||||
| ≤ | 100 | 112 | 106 | 106 | 95 | 117 | |||
| > | 115 | 98 | 107 | 106 | 118 | 95 | |||
| WHO type | 0.783 | 0.286 | 0.576 | ||||||
| A | 21 | 19 | 21 | 19 | 21 | 19 | |||
| AB | 75 | 78 | 69 | 84 | 72 | 81 | |||
| B (B1, B2, B3) | 119 | 113 | 123 | 109 | 120 | 112 | |||
| Tumor size | 0.69 | 0.812 | 0.155 | ||||||
| ≤ | 129 | 122 | 127 | 124 | 133 | 118 | |||
| > | 86 | 88 | 86 | 88 | 80 | 94 | |||
| Myasthenia | 0.929 | 0.037 | 0.376 | ||||||
| Yes | 25 | 26 | 32 | 19 | 28 | 23 | |||
| No | 190 | 184 | 181 | 193 | 185 | 189 | |||
| Extended resection | 0.540 | 0.2 | 0.845 | ||||||
| Yes | 15 | 18 | 13 | 20 | 16 | 17 | |||
| No | 200 | 192 | 200 | 192 | 197 | 195 | |||
| Masaoka‐Koga stage | 0.145 | 0.437 | 0.44 | ||||||
| I | 137 | 123 | 131 | 129 | 132 | 128 | |||
| II | 57 | 62 | 61 | 58 | 59 | 60 | |||
| III | 19 | 18 | 17 | 20 | 19 | 18 | |||
| IV | 2 | 7 | 4 | 5 | 3 | 6 | |||
Note: Bold values represent statistically significant results.
Abbreviations: NLR neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; SII, systemic immune‐inflammation index; WHO World Health Organization.
Survival analysis
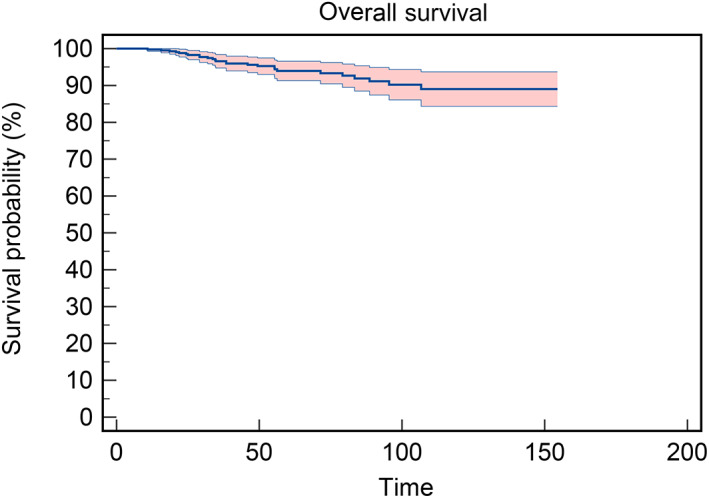
Patient follow‐up ranged from 17 months to 154 months postoperatively and 6.35% of patients died during follow‐up. The 5‐year OS rate was 94.0% and the 10‐year OS rate was 89.2% (Figure 2). The Kaplan–Meier overall survival analysis is presented in Figure 3, showing that gender, myasthenia and WHO type were not significantly associated with patient survival. However, age, tumor size, expanded resection and Masaoka‐Koga stage significantly affected patient survival, with patients with a high PLR (p = 0.008), NLR (p = 0.035), and SII (p = 0.001) having shorter survival times.
FIGURE 2.
Overall survival (OS). The 5‐year OS rate was 94.0% and the 10‐year OS rate was 89.2%.
FIGURE 3.
Kaplan–Meier overall survival (OS) analysis. (a) Kaplan–Meier OS analysis of gender (p = 0.321). (b) Kaplan–Meier OS analysis of age (p = 0.016). (c) Kaplan–Meier OS analysis of WHO type (p = 0.174). (d) Kaplan–Meier OS analysis of tumor size (p = 0.001). (e) Kaplan–Meier OS analysis of myasthenia (p = 0.122). (f) Kaplan–Meier OS analysis of extended resection (p < 0.001). (g) Kaplan–Meier OS analysis of Masaoka‐Koga stage (p < 0.001). (h) Kaplan–Meier OS analysis of PLR (p = 0.008). (i) Kaplan–Meier OS analysis of NLR (p = 0.035). (j) Kaplan–Meier OS analysis of SII (p = 0.001).
Correlations between the immune‐inflammation index and clinicopathological factors in thymoma
Correlation analysis was performed to identify independent factors in Cox regression models, revealing that PLR, NLR and SII were strongly correlated (Figure 4). Masaoka‐Koga stage and the WHO histological classification had a weak but significant correlation (r = 0.226 and p < 0.001), as had WHO and extended resection (r = 0.153 and p = 0.002). In the univariate Cox regression analysis, these factors except gender and WHO stage were significantly associated with OS (Table 3).
FIGURE 4.
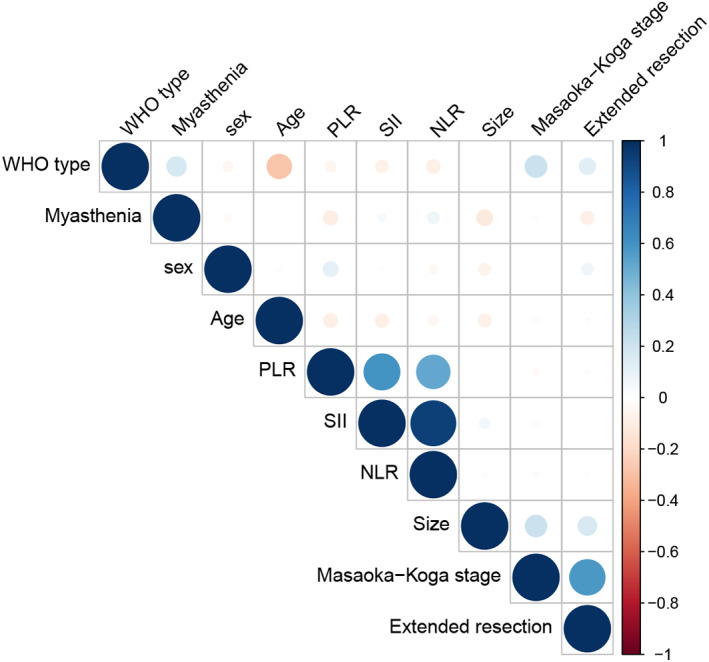
Correlation analysis and selection of predictors. Platelet‐to‐lymphocyte ratio (PLR), neutrophil‐to‐lymphocyte ratio (NLR) and systemic immune‐inflammation index (SII) were strongly correlated. The Masaoka‐Koga stage and the World Health Organization (WHO) histological classification had a weak but significant correlation (r = 0.226 and p < 0.001). WHO histological classification and extended resection had a weak but significant correlation (r = 0.153 and p = 0.002).
TABLE 3.
Predictors of the overall survival of patients with thymoma according to the univariate Cox proportional hazard model.
| Factors | Hazard ratio | 95% CI | p‐value |
|---|---|---|---|
| Gender | 0.679 | 0.314–1.466 | 0.324 |
| Age | 0.377 | 0.165–0.862 | 0.021 |
| WHO type | 1.932 | 0.933–4.003 | 0.076 |
| Tumor size | 3.757 | 1.586–8.901 | 0.003 |
| Extended resection | 5.792 | 2.601–12.9 | <0.001 |
| Masaoka‐Koga stage | 3.402 | 2.3–5.03 | <0.001 |
| PLR | 3.037 | 1.283–7.189 | 0.012 |
| NLR | 2.367 | 1.036–5.411 | 0.041 |
| SII | 4.318 | 1.634–11.409 | 0.003 |
Note: Bold values represent statistically significant results.
Abbreviations: NLR neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; SII, systemic immune‐inflammation index; WHO World Health Organization.
As shown in previous studies, the Masaoka‐Koga stage and the WHO histological classification are well‐established independent prognostic factors. The WHO histological classification was included in the multivariate Cox regression model and an SII greater than 345.83 was a significant independent prognostic factor after adjusting for common factors (p = 0.001, HR = 5.756, 95% CI: 2.144–15.457) (Table 4). Multivariate analysis also revealed that the PLR was significantly associated with OS (p = 0.008, HR = 3.29, 95% CI: 1.371–7.896) (Table 5). A high NLR was a significant independent prognostic factor for shorter OS (p = 0.024, HR = 2.654, 95% CI: 1.138–6.19) (Table 6). The survival curve based on the mean of the covariates in Figure 5 shows that SII had an AUC of 70.6% (AUC = 0.706) thus exceeding the predictive value for PLR (AUC = 0.678) and NLR (AUC = 0.654).
TABLE 4.
Predictors of the overall survival of patients with thymoma according to the multivariate Cox proportional hazard model (SII).
| Factors | Hazard ratio | 95% CI | p‐value |
|---|---|---|---|
| Age | 3.34 | 1.45–7.693 | 0.005 |
| Tumor size | 2.786 | 1.155–6.72 | 0.023 |
| Masaoka‐Koga stage | 3.124 | 2.111–4.625 | <0.001 |
| SII | 5.756 | 2.144–15.457 | 0.001 |
Abbreviations: SII, systemic immune‐inflammation index; WHO, World Health Organization.
TABLE 5.
Predictors of the overall survival of patients with thymoma according to the multivariate Cox proportional hazard model (PLR).
| Factors | Hazard ratio | 95% CI | p‐value |
|---|---|---|---|
| Age | 3.021 | 1.317–6.93 | 0.009 |
| Tumor size | 2.885 | 1.182–7.042 | 0.02 |
| Masaoka‐Koga stage | 2.958 | 1.989–4.4 | <0.001 |
| PLR | 3.29 | 1.371–7.896 | 0.008 |
Abbreviation: PLR platelet‐to‐lymphocyte ratio.
TABLE 6.
Predictors of the overall survival of patients with thymoma according to the multivariate Cox proportional hazard model (NLR).
| Factors | Hazard ratio | 95% CI | p‐value |
|---|---|---|---|
| Age | 3.412 | 1.465–7.947 | 0.004 |
| Tumor size | 2.752 | 1.125–6.733 | 0.027 |
| Masaoka‐Koga stage | 2.92 | 1.967–4.335 | <0.001 |
| NLR | 2.654 | 1.138–6.19 | 0.024 |
Abbreviation: NLR, neutrophil‐to‐lymphocyte ratio.
FIGURE 5.
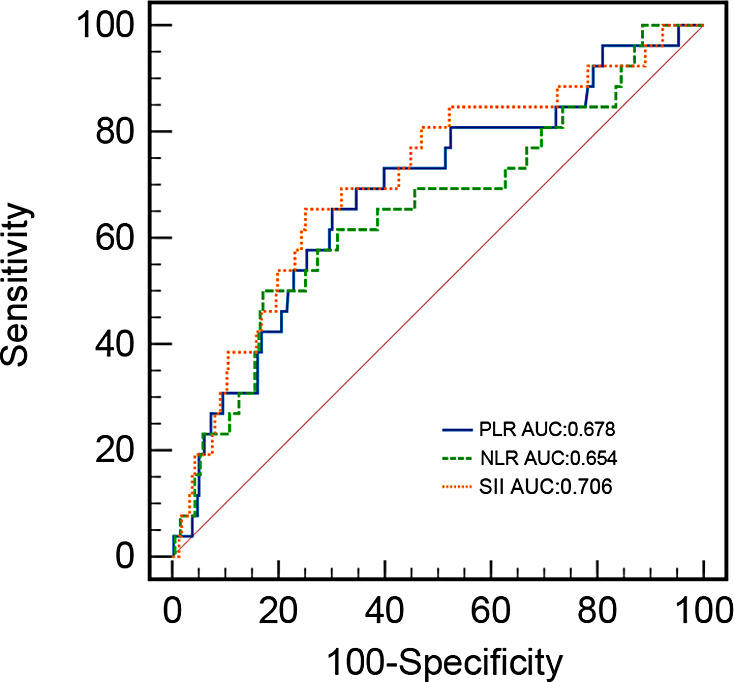
Receiver operating characteristic (ROC) curve. Systemic immune‐inflammation index (SII) had an area under the curve (AUC) of 70.6% (AUC = 0.706), thus exceeding the predictive value for platelet‐to‐lymphocyte ratio (PLR) (AUC = 0.678) and neutrophil‐to‐lymphocyte ratio (NLR) (AUC = 0.654).
DISCUSSION
This is the first study to investigate the predictive effect of SII on the OS of patients who had undergone complete resection of thymoma with radical resection, showing that a high preoperative SII was independently prognostic of an increased risk of short OS in patients with radical resection.
Many studies have demonstrated that the infection site, chronic stimulation and inflammation are the origins of tumors 27 and tumors alter the inflammatory milieu in the tumor microenvironment (TME). 28 Moreover, systemic inflammation disrupts the dynamic equilibrium in TME, inducing a supportive environment for tumor growth. 29 It has been shown that inflammation may play an important role in cancer development, especially in dictating metastatic progression rather than in early tumorigenesis. 30 Therefore, inflammation is implicated in tumorigenesis and tumor development. 31 , 32 , 33
TME is fundamentally characterized by persistent inflammation 34 , 35 , 36 , 37 and an array of cell types cooperate to foster tumor cell migration and invasion such as tumor cells, stromal cells and extracellular matrix (ECM). 38 , 39 Neutrophils can promote tumorigenesis and metastasis in TME through complex mechanisms. 40 It has been shown that neutrophils can release granules containing neutrophil elastase (NE), neutrophil collagenase (MMP8), and gelatinase B (MMP9) to remodel the ECM and accelerate cancer progression. 37 , 41 Although the role of platelets in tumor metastasis remains to be investigated, there is evidence of their involvement in tumor development. 42 It is currently considered that tumor cells may directly induce clustering and activation of platelets, which in turn, release multiple growth factors to promote cell proliferation in tumor cells. 43 , 44 However, various lymphocytes traffic into the tumor microenvironment and inhibit the proliferation of tumor cells. 45 These derived ratios calculated in this study are considered to reflect the strength of peripheral immunity and inflammatory status, 46 and the three parameters PLR, NLR and SII were strongly correlated.
Recent clinical studies have shown that the NLR and PLR are prognostic factors for survival in many solid tumor types. 44 , 47 , 48 According to a meta‐analysis in 2014, a high NLR was a poor predictive factor for OS among various disease subgroups and across disease stages of 100 studies comprising 40 559 patients and a high NLR was significantly associated with OS, similar to the present study. Although the role of PLR requires a meta‐analysis of randomized trials, previous studies showed that a high PLR was an independent predictor of poor OS for epithelial ovarian cancers. 49 To date, several studies focused on the role of NLR, PLR, and SII in thymic epithelial tumors. 23 , 24 , 25 , 50 , 51 , 52 , 53 In 2021, Huang et al. established a nomogram to predict outcomes of thymomas by combining clinical features, NLR, PLR and other blood assays but PLR was not an independent prognostic factor, rather the primary outcome indicator was relapse‐free survival rather than OS. 51 Furthermore, Wang et al. demonstrated that NLR, as a tumor glycolysis‐related inflammatory marker, enhanced tumor glycolytic activity in TETs metastasis but was not associated with systemic inflammatory indicators and OS. 52 Our study focused on the patients who underwent radical resection of thymic epithelial tumors (TETs) and the study results are consistent with two studies that showed that a high NLR correlated with poor prognosis in patients with radical resection of thymic carcinoma. 24 , 50 Moreover, NLR was an important independent risk factor for disease‐related survival (DRS) and cumulative incidence of recurrence (CIR) but not for OS and disease‐free survival (DFS). Veraar et al. showed that SII was not statistically significantly different for OS and DFS. 53 Considering that our study involved 425 patients, more cases than in these studies, we are confident that our results are more meaningful.
The cutoff point for each marker is an essential aspect when comparing results from different studies. Investigators have selected different cutoffs for different tumors and these parameters often have a non‐normal distribution. The non‐normally distributed values are appropriately described by the median, which has been validated by previous studies. Some studies have reported that cutoffs were determined using receiver operating characteristic (ROC) curves (C‐index). In our study, the cutoffs for PLR, NLR, and SII were 103.11, 1.77, and 345.83, respectively, which were lower than the normal selections. Interestingly, previous studies have found that the association between NLR or SII cutoffs and OS was very small and unlikely to affect the interpretation of the results. 47
In this study, SII was used to predict OS and compared to PLR and NLR, SII effectively integrated blood‐based markers of inflammation instead of only calculating the ratio of the two types of immune cells, therefore has better predictive and prognostic capabilities. 54 SII has been used for various tumors such as lung adenocarcinoma, 55 hepatocellular carcinoma, 56 oesophagal squamous cell carcinoma (ESCC) and cervical cancer, and is superior to PLR and NLR as a predictive biomarker in ESCC and cervical cancer. 57 , 58 This was also the case in our study. Also, a previous meta‐analysis showed that a high SII was correlated with poor OS 59 and a higher SII implies higher platelet/neutrophil and/or lower lymphocyte counts. 54 , 60 Thus, a higher SII indirectly represents a deficiency of immune function and increased tumor aggressiveness in patients with thymoma.
It is worth mentioning that our study has some limitations, primarily stemming from the relatively small cohort and the short length of follow‐up. Thymomas are indolent tumors characterized by low proliferation, and patients with thymomas have longer survival compared to other solid tumors. In the present study, all participants underwent radical resection and the total number of deaths was too small to investigate OS, therefore larger, multicenter cohort studies are needed to further investigate and validate the use of systemic inflammatory indicators in patients during follow‐up. OS may not be the best assessment measure for surgical treatment as less than 50% of deaths are a result of tumors. Additionally, due to the difficulty of follow‐up, we were unable to track recurrence, so further research is needed to determine whether SII has excellent predictive performance for recurrence.
In conclusion, preoperative SII can predict the prognosis of thymoma patients who have undergone radical resection but further multicenter prospective studies are needed to investigate the role of SII in thymoma. Blood tests incorporating SII may be added to prognostic models to guide functional follow‐up.
AUTHOR CONTRIBUTIONS
Jun Zhao and Wei Wang conceived and designed the study; Qifan Li, Yiwei Pu, Zetian Gong, and Wei Sun performed the study; Qifan Li, Yiwei Pu, Zhike Chen, Zetian Gong and Wei Sun. analyzed the data; Qifan Li and Yiwei Pu drafted the manuscript.
FUNDING INFORMATION
This work was supported by the Natural Science Foundation for the Youth of Jiangsu Province (no. BK20200196), P112203521.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Li Q, Pu Y, Gong Z, Yu Y, Sun W, Cheng Z, et al. Preoperative systemic immune‐inflammation index for predicting the prognosis of thymoma with radical resection. Thorac Cancer. 2023;14(13):1192–1200. 10.1111/1759-7714.14854
Qifan Li, Yiwei Pu and Zetian Gong contributed equally to this work and share first authorship.
Contributor Information
Wei Wang, Email: wangwei-doctor@163.com.
Jun Zhao, Email: zhaojia0327@126.com.
DATA AVAILABILITY STATEMENT
Data for this study can be made available from the corresponding authors upon reasonable request.
REFERENCES
- 1. He J, Wei W. China cancer registration annual report 2019: People's Medical Publishing House (PMPH). 2020.
- 2. Scorsetti M, Leo F, Trama A, D'Angelillo R, Serpico D, Macerelli M, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol. 2016;99:332–50. [DOI] [PubMed] [Google Scholar]
- 3. Ettinger DS, Riely GJ, Akerley W, Borghaei H, Chang AC, Cheney RT, et al. Thymomas and thymic carcinomas: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2013;11(5):562–76. [DOI] [PubMed] [Google Scholar]
- 4. Marom EM. Imaging thymoma. J Thorac Oncol. 2010;5(10 Suppl 4):S296–303. [DOI] [PubMed] [Google Scholar]
- 5. Masaoka A. Staging system of thymoma. J Thorac Oncol. 2010;5(10 Suppl 4):S304–12. [DOI] [PubMed] [Google Scholar]
- 6. Hamaji M, Allen MS, Cassivi SD, Nichols FC 3rd, Wigle DA, Deschamps C, et al. The role of surgical management in recurrent thymic tumors. Ann Thorac Surg. 2012;94(1):247–54. [DOI] [PubMed] [Google Scholar]
- 7. Guerrera F, Falcoz PE, Moser B, van Raemdonck D, Bille A, Toker A, et al. Thymomectomy plus total thymectomy versus simple thymomectomy for early‐stage thymoma without myasthenia gravis: a European Society of Thoracic Surgeons Thymic working group study. Eur J Cardiothorac Surg. 2021;60(4):881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Narm KS, Lee CY, Do YW, Jung HS, Byun GE, Lee JG, et al. Limited thymectomy as a potential alternative treatment option for early‐stage thymoma: a multi‐institutional propensity‐matched study. Lung Cancer. 2016;101:22–7. [DOI] [PubMed] [Google Scholar]
- 9. Gu Z, Fu J, Shen Y, Wei Y, Tan L, Zhang P, et al. Thymectomy versus tumor resection for early‐stage thymic malignancies: a Chinese alliance for research in thymomas (ChART) retrospective database analysis. Zhongguo Fei Ai Za Zhi. 2016;19(7):459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakagawa K, Yokoi K, Nakajima J, Tanaka F, Maniwa Y, Suzuki M, et al. Is Thymomectomy alone appropriate for stage I (T1N0M0) Thymoma? Results of a propensity‐score analysis. Ann Thorac Surg. 2016;101(2):520–6. [DOI] [PubMed] [Google Scholar]
- 11. Weis CA, Yao X, Deng Y, Detterbeck FC, Marino M, Nicholson AG, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol. 2015;10(2):367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu H, Gu Z, Qiu B, Detterbeck FC, Roden AC, Ruffini E, et al. A recurrence predictive model for Thymic tumors and its implication for postoperative management: a Chinese Alliance for research in Thymomas database study. J Thorac Oncol. 2020;15(3):448–56. [DOI] [PubMed] [Google Scholar]
- 13. Fang W, Filosso PL, Roden AC, Gu Z, Liu Y, Agzarian J, et al. Clinicopathological features and current treatment outcomes of neuroendocrine thymic tumours. Eur J Cardiothorac Surg. 2021;59(5):1004–13. [DOI] [PubMed] [Google Scholar]
- 14. Detterbeck FC, Stratton K, Giroux D, Asamura H, Crowley J, Falkson C, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence‐based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol. 2014;9(9 Suppl 2):S65–72. [DOI] [PubMed] [Google Scholar]
- 15. Berardi R, Goteri G, Brunelli A, Pagliaretta S, Paolucci V, Caramanti M, et al. Prognostic relevance of programmed cell death protein 1/programmed death‐ligand 1 pathway in thymic malignancies with combined immunohistochemical and biomolecular approach. Expert Opin Ther Targets. 2020;24(9):937–43. [DOI] [PubMed] [Google Scholar]
- 16. Yokoyama S, Miyoshi H, Nishi T, Hashiguchi T, Mitsuoka M, Takamori S, et al. Clinicopathologic and prognostic implications of programmed death ligand 1 expression in Thymoma. Ann Thorac Surg. 2016;101(4):1361–9. [DOI] [PubMed] [Google Scholar]
- 17. Yeap BY, De Rienzo A, Gill RR, Oster ME, Dao MN, Dao NT, et al. Mesothelioma risk score: a new prognostic pretreatment, clinical‐molecular algorithm for malignant pleural mesothelioma. J Thorac Oncol. 2021;16:1925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Giorgi U, Procopio G, Giannarelli D, Sabbatini R, Bearz A, Buti S, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with Nivolumab. Clin Cancer Res. 2019;25(13):3839–46. [DOI] [PubMed] [Google Scholar]
- 19. Yamashita S, Sheth RA, Niekamp AS, Aloia TA, Chun YS, Lee JE, et al. Comprehensive complication index predicts cancer‐specific survival after resection of colorectal metastases independent of RAS mutational status. Ann Surg. 2017;266(6):1045–54. [DOI] [PubMed] [Google Scholar]
- 20. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–81. [DOI] [PubMed] [Google Scholar]
- 21. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non‐small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8(6):886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peng L, Wang Y, Liu F, Qiu X, Zhang X, Fang C, et al. Peripheral blood markers predictive of outcome and immune‐related adverse events in advanced non‐small cell lung cancer treated with PD‐1 inhibitors. Cancer Immunol Immunother. 2020;69(9):1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janik S, Raunegger T, Hacker P, Ghanim B, Einwallner E, Mullauer L, et al. Prognostic and diagnostic impact of fibrinogen, neutrophil‐to‐lymphocyte ratio, and platelet‐to‐lymphocyte ratio on thymic epithelial tumors outcome. Oncotarget. 2018;9(31):21861–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muriana P, Carretta A, Ciriaco P, Bandiera A, Negri G. Assessment of the prognostic role of neutrophil‐to‐lymphocyte ratio following complete resection of thymoma. J Cardiothorac Surg. 2018;13(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuan ZY, Gao SG, Mu JW, Xue Q, Mao YS, Wang DL, et al. Prognostic value of preoperative neutrophil‐lymphocyte ratio is superior to platelet‐lymphocyte ratio for survival in patients who underwent complete resection of thymic carcinoma. J Thorac Dis. 2016;8(7):1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seban RD, Assie JB, Giroux‐Leprieur E, Massiani MA, Bonardel G, Chouaid C, et al. Prognostic value of inflammatory response biomarkers using peripheral blood and [18F]‐FDG PET/CT in advanced NSCLC patients treated with first‐line chemo‐ or immunotherapy. Lung Cancer. 2021;159:45–55. [DOI] [PubMed] [Google Scholar]
- 27. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Candido J, Hagemann T. Cancer‐related inflammation. J Clin Immunol. 2013;33(Suppl 1):S79–84. [DOI] [PubMed] [Google Scholar]
- 29. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379(9826):1591–601. [DOI] [PubMed] [Google Scholar]
- 31. Liu H, Liu Y, Liu W, Zhang W, Xu J. EZH2‐mediated loss of miR‐622 determines CXCR4 activation in hepatocellular carcinoma. Nat Commun. 2015;6:8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li J, Maruyama T, Zhang P, Konkel JE, Hoffman V, Zamarron B, et al. Mutation of inhibitory helix‐loop‐helix protein Id3 causes gammadelta T‐cell lymphoma in mice. Blood. 2010;116(25):5615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang S, Qu Y, Xia P, Chen Y, Zhu X, Zhang J, et al. Transdifferentiation of tumor infiltrating innate lymphoid cells during progression of colorectal cancer. Cell Res. 2020;30(7):610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dvorak HF. Tumors: wounds that do not heal. similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–9. [DOI] [PubMed] [Google Scholar]
- 35. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. [DOI] [PubMed] [Google Scholar]
- 36. Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, et al. SCF‐mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112(4):1269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol. 2016;37(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, et al. Deletion of p120‐catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell. 2011;19(4):470–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ghosh S, Ashcraft K, Jahid MJ, April C, Ghajar CM, Ruan J, et al. Regulation of adipose oestrogen output by mechanical stress. Nat Commun. 2013;4:1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ocana A, Nieto‐Jimenez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. 2017;16(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23(3):141–8. [DOI] [PubMed] [Google Scholar]
- 42. Hou C, Jiang F, Ma H, Zhu Q, Wang Z, Zhao B, et al. Prognostic role of preoperative platelet, fibrinogen, and D‐dimer levels in patients with non‐small cell lung cancer: a multicenter prospective study. Thorac Cancer. 2019;10(2):304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu X, Huang Z, He X, Zheng X, Jia Q, Tan J, et al. Blood prognostic predictors of treatment response for patients with papillary thyroid cancer. Biosci Rep. 2020;40(10):BSR20202544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang H, Chen WM, Zhou YH, Shi JP, Huang YQ, Wang WJ. Combined PLT and NE to predict the prognosis of patients with locally advanced cervical cancer. Sci Rep. 2020;10(1):11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yin H, Zhang X, Yang P, Zhang X, Peng Y, Li D, et al. RNA m6A methylation orchestrates cancer growth and metastasis via macrophage reprogramming. Nat Commun. 2021;12(1):1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nøst TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, et al. Systemic inflammation markers and cancer incidence in the UK biobank. Eur J Epidemiol. 2021;36(8):841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Templeton AJ, McNamara MG, Seruga B, Vera‐Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. J Natl Cancer Inst. 2014;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 48. Shaul ME, Fridlender ZG. Tumour‐associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16(10):601–20. [DOI] [PubMed] [Google Scholar]
- 49. El Bairi K, Al Jarroudi O, Afqir S. Inexpensive systemic inflammatory biomarkers in ovarian cancer: an umbrella systematic review of 17 prognostic meta‐analyses. Front Oncol. 2021;11:694821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yanagiya M, Nitadori JI, Nagayama K, Anraku M, Sato M, Nakajima J. Prognostic significance of the preoperative neutrophil‐to‐lymphocyte ratio for complete resection of thymoma. Surg Today. 2018;48(4):422–30. [DOI] [PubMed] [Google Scholar]
- 51. Huang YY, Wu LL, Liu X, Liang SH, Ma GW. Nomogram predict relapse‐free survival of patients with thymic epithelial tumors after surgery. BMC Cancer. 2021;21(1):847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang L, Ruan M, Yan H, Lei B, Sun X, Chang C, et al. Pretreatment serum neutrophil‐to‐lymphocyte and monocyte‐to‐lymphocyte ratios: two tumor‐related systemic inflammatory markers in patients with thymic epithelial tumors. Cytokine. 2020;133:155149. [DOI] [PubMed] [Google Scholar]
- 53. Veraar C, Janik S, Thanner J, Veraar C, Mouhieddine M, Schiefer AI, et al. Clinical prognostic scores for patients with thymic epithelial tumors. Sci Rep. 2019;9(1):18581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li J, Cao D, Huang Y, Xiong Q, Tan D, Liu L, et al. The prognostic and clinicopathological significance of systemic immune‐inflammation index in bladder cancer. Front Immunol. 2022;13:865643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Islas‐Vazquez L, Aguilar‐Cazares D, Galicia‐Velasco M, Rumbo‐Nava U, Meneses‐Flores M, Luna‐Rivero C, et al. IL‐6, NLR, and SII markers and their relation with alterations in CD8+ T‐lymphocyte subpopulations in patients treated for lung adenocarcinoma. Biology. 2020;9(11):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune‐inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–22. [DOI] [PubMed] [Google Scholar]
- 57. Zhang Y, Xiao G, Wang R. Clinical significance of systemic immune‐inflammation index (SII) and C‐reactive protein‐to‐albumin ratio (CAR) in patients with esophageal cancer: a meta‐analysis. Cancer Manag Res. 2019;11:4185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, et al. Prognostic value of preoperative systemic immune‐inflammation index in patients with cervical cancer. Sci Rep. 2019;9(1):3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of systemic immune‐inflammation index in cancer: a meta‐analysis. J Cancer. 2018;9(18):3295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li X, Gu L, Chen Y, Chong Y, Wang X, Guo P, et al. Systemic immune‐inflammation index is a promising non‐invasive biomarker for predicting the survival of urinary system cancers: a systematic review and meta‐analysis. Ann Med. 2021;53(1):1827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study can be made available from the corresponding authors upon reasonable request.


