Abstract
The behavioural variant of Alzheimer’s disease (bvAD) is characterized by early predominant behavioural changes, mimicking the behavioural variant of frontotemporal dementia (bvFTD), which is characterized by social cognition deficits and altered biometric responses to socioemotional cues. These functions remain understudied in bvAD.
We investigated multiple social cognition components (i.e. emotion recognition, empathy, social norms and moral reasoning), using the Ekman 60 faces test, Interpersonal Reactivity Index, empathy eliciting videos, Social Norms Questionnaire and moral dilemmas, while measuring eye movements and galvanic skin response. We compared 12 patients with bvAD with patients with bvFTD (n = 14), typical Alzheimer’s disease (tAD, n = 13) and individuals with subjective cognitive decline (SCD, n = 13), using ANCOVAs and age- and sex-adjusted post hoc testing.
Patients with bvAD (40.1 ± 8.6) showed lower scores on the Ekman 60 faces test compared to individuals with SCD (49.7 ± 5.0, P < 0.001), and patients with tAD (46.2 ± 5.3, P = 0.05) and higher scores compared to patients with bvFTD (32.4 ± 7.3, P = 0.002). Eye-tracking during the Ekman 60 faces test revealed no differences in dwell time on the eyes (all P > 0.05), but patients with bvAD (18.7 ± 9.5%) and bvFTD (19.4 ± 14.3%) spent significantly less dwell time on the mouth than individuals with SCD (30.7 ± 11.6%, P < 0.01) and patients with tAD (32.7 ± 12.1%, P < 0.01). Patients with bvAD (11.3 ± 4.6) exhibited lower scores on the Interpersonal Reactivity Index compared with individuals with SCD (15.6 ± 3.1, P = 0.05) and similar scores to patients with bvFTD (8.7 ± 5.6, P = 0.19) and tAD (13.0 ± 3.2, P = 0.43). The galvanic skin response to empathy eliciting videos did not differ between groups (all P > 0.05). Patients with bvAD (16.0 ± 1.6) and bvFTD (15.2 ± 2.2) showed lower scores on the Social Norms Questionnaire than patients with tAD (17.8 ± 2.1, P < 0.05) and individuals with SCD (18.3 ± 1.4, P < 0.05). No group differences were observed in scores on moral dilemmas (all P > 0.05), while only patients with bvFTD (0.9 ± 1.1) showed a lower galvanic skin response during personal dilemmas compared with SCD (3.4 ± 3.3 peaks per min, P = 0.01).
Concluding, patients with bvAD showed a similar although milder social cognition profile and a similar eye-tracking signature to patients with bvFTD and greater social cognition impairments and divergent eye movement patterns compared with patients with tAD. Our results suggest reduced attention to salient facial features in these phenotypes, potentially contributing to their emotion recognition deficits.
Keywords: Alzheimer’s disease, behaviour, frontotemporal dementia, social cognition, biometrics
Singleton et al. show that individuals with the behavioural variant of Alzheimer’s disease have similar but milder social cognition impairments versus individuals with behavioural variant frontotemporal dementia, as well as greater social cognition impairments and distinct eye-tracking patterns compared with individuals with typical Alzheimer’s disease.
Introduction
The behavioural variant of Alzheimer’s disease (bvAD) is a rare atypical variant of Alzheimer’s disease, characterized by early and predominant behavioural and personality changes with underlying Alzheimer’s disease pathology.1–3 The clinical phenotype of bvAD overlaps substantially with that of the behavioural variant of frontotemporal dementia (bvFTD),3 which is characterized by social cognition deficits that are thought to underlie its behavioural disturbances.4 Social cognition refers to all processes necessary for adequate social behaviour, i.e. the identification, perception and interpretation of socially relevant stimuli.5 Although currently a lack of consensus exists on the theoretical framework regarding subdomains of social cognition,6 several functions have been described as central social cognitive abilities, including emotion recognition, empathy, theory of mind and social and moral reasoning.7 These subcomponents rely on multiple sensory and cognitive processes and may be interdependent. For instance, a prerequisite for adequate social behaviour is that social cues such as facial expressions are sufficiently and accurately perceived (perception and attribution of salience).7,8 Next, these elementary perceptions are interpreted to extract the affective states of the other person and form the basis for adequate emotion recognition, empathy and ‘theory of mind’ (interpretation).7,8 Furthermore, this perspective taking serves as necessary input to guide social decision making, as well as higher-order social reasoning based on knowledge of social norms and moral reasoning.8
Deficits along all components of social cognition have been described in bvFTD4 and may contribute substantially to the behavioural and personality changes. Social cognition deficits have been suggested as a possible underlying mechanism in the bvAD phenotype as well.9–12 However, these reports are mainly based on case studies. Moreover, social cognition tests are prone to confounding by deficits in other cognitive domains such as memory or executive functioning that are likely to be impaired in dementia. To overcome this hurdle, biometric measures have been used to capture experiential aspects of social cognition in the context of frontotemporal dementia. For example, eye tracking and galvanic skin response (GSR) may yield more direct and sensitive measurements of elementary, bottom-up, processes of emotions and social behaviour,13 as previous studies have captured emotional blunting using GSR14,15 and deficits in emotion recognition using eye tracking16 in frontotemporal dementia. As such, these tools may help unveil primary physiological processes contributing to social cognitive functioning, in addition to potentially providing earlier and objective measures to capture decline in social cognition. As adequate social cognition relies on the complex interplay between elementary sensory (bottom-up) processing, as well as semantic cognitive appraisal (top-down) processing,17 it is important to capture both sensory and cognitive processing underlying social cognition.
Group studies of social cognition test scores in conjunction with biometric measures in bvAD are currently lacking. In this exploratory study, we examined social cognition across multiple components, including emotion recognition, empathy, knowledge of social norms and moral reasoning in patients with bvAD compared to bvFTD, typical Alzheimer’s disease (tAD) and subjective cognitive decline (SCD) in conjunction with eye tracking and GSR. We hypothesized that bvAD participants would show intermediate social cognition performance compared with bvFTD and tAD (i.e. worse than tAD and better than bvFTD), based on the similar yet milder behavioural profile in bvAD compared with bvFTD.3
Materials and methods
Participants
Between February 2020 and October 2021, we included 12 patients clinically diagnosed with bvAD from the Amsterdam Dementia Cohort, the Netherlands (Table 1).18 In accordance with inclusion procedures in our previous work,19,20 we included cases if they showed at least two of six bvFTD features21 in conjunction with positive amyloid-β biomarkers based on CSF or PET examinations (Table 2). Owing to the absence of formal diagnostic or research criteria for bvAD, we recently proposed research criteria for this phenotype, defining ‘clinical bvAD’ as a combined behavioural and cognitive syndrome including two of five bvFTD behavioural features21 in conjunction with either memory or executive impairments, and defining additional levels (i.e. ‘possible’, ‘probable’ and ‘definite’ bvAD) based on different levels of biomarker, genetic and/or histological confirmation.3 As all bvAD cases showed memory and/or executive impairments on neuropsychological assessment as concluded by a trained neuropsychologist during clinical workup, in addition to two of five bvFTD behavioural features (Tables 1 and 2), all bvAD cases retrospectively met criteria for at least ‘possible bvAD’ (i.e. clinical bvAD in combination with positive amyloid-β biomarkers3; Table 2). In the same period, we consecutively included patients with ‘tAD’ (defined as amyloid-β-positive patients with mild cognitive impairment or Alzheimer’s disease dementia with an amnestic-predominant presentation) and patients with probable bvFTD behavioural variant of frontotemporal dementia according to the Rascovsky criteria21 and with a negative amyloid status. All tAD and bvFTD cases showed amyloid and tau positivity based on CSF assessment or amyloid positivity on PET assessment. Furthermore, all cases with bvFTD were amyloid negative, except for one amyloid-positive case who had a strong family history and a clinical profile suspect for bvFTD. Importantly, this bvFTD case was p-tau negative based on CSF, whereas all bvAD cases were p-tau positive (10/12) or amyloid PET positive (2/12). The cognitively normal control group consisted of individuals who presented to our clinic with SCD in whom objective cognitive impairment was ruled out by neuropsychological assessments during screening.22 The majority of SCD cases were amyloid negative (76.9%) on CSF assessment, the mean Mini-Mental State Examination (MMSE) was 28.1 ± 1.4, domain Z-scores for memory (−0.14 ± 0.73), attention (0.17 ± 0.60), language (0.05 ± 0.50) and executive functioning (0.19 ± 0.60) were within the normative range (compared to a reference group of cognitively normal amyloid-β-negative individuals; n = 583)23 and neuropsychiatric symptoms were limited as indicated by a mean Geriatric Depression Scale score of 1.58 ± 1.68 and a mean Neuropsychiatric Inventory score of 2.43 ± 4.16. In addition, no significant neurodegeneration was observed by trained neuroradiologists during clinical workup, as indicated by median Fazekas, Medial Temporal Atrophy and Global Cortical Atrophy scores of 0.00 (0, 1.00) (Supplementary Table 1). Supplementary Fig. 1 provides an overview of the inclusion flow for each diagnostic group. Age, sex and level of education were ascertained for all participants. Level of education was classified using the Verhage system ranging from 1 (no or little education) to 7 (highest academic degree).24 All participants provided written consent according to the Declaration of Helsinki. This study was approved by the ethical review board of the Amsterdam University Medical Center.
Table 1.
Demographic characteristics across diagnostic groups
| bvAD | tAD | bvFTD | SCD | P-value | |
|---|---|---|---|---|---|
| n | 12 | 12 | 14 | 13 | |
| Age | 66.6 (5.7) | 64.6 (6.7) | 66.4 (7.0) | 57.5 (5.8) | 0.09 |
| Sex, % male | 75.0 | 38.5 | 64.3 | 38.5 | 0.38 |
| MMSE | 24.8 (2.5) | 24.7 (4.6) | 26.2 (2.3) | 28.1 (1.5) | 0.05 |
| Education (Verhage score 1–7) | 5.1 (1.4) | 5.8 (1.3) | 5.4 (0.9) | 5.8 (0.7) | 0.008 |
| APOE ε4, % carrier | 6/10 (60.0%) | 10/12 (83.3%) | 1/6 (17.0%) | 5/12 (38.5%) | 0.04 |
| Attention domain Z-score | −1.04 (1.12) | −0.94 (1.30) | −0.67 (0.84) | 0.17 (0.60) | bvAD < SCD, P = 0.05, tAD < SCD, P = 0.04 |
| Language domain Z-score | −1.22 (1.85) | −0.59 (0.44) | −1.50 (1.23) | 0.05 (0.50) | bvAD < SCD, P = 0.002, bvFTD < SCD, P = 0.003 |
| Memory domain Z-score | −2.34 (1.55) | −3.38 (2.60) | −1.36 (0.77) | −0.14 (0.73) | bvAD < SCD, P = 0.009, tAD < SCD, P < 0.0001, |
| Executive domain Z-score | −1.49 (1.36) | −0.77 (1.16) | −1.11 (0.73) | 0.19 (0.60) | bvAD < SCD, P = 0.002, bvFTD < SCD, P = 0.03 |
Mean (SD) are reported unless stated otherwise. Domain Z-scores were calculated in reference to a cognitively normal amyloid-negative control group (n = 583).23 Cognition scores were age- and sex-adjusted.
Table 2.
Overview of behavioural, cognitive and biomarker features of bvAD cases
| bvAD1 | bvAD2 | bvAD3 | bvAD4 | bvAD5 | bvAD6 | bvAD7 | bvAD8 | bvAD9 | bvAD10 | bvAD11 | bvAD12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Male | Male | Male | Male | Male | Female | Female | Male | Female | Male | Male |
| Age | 62 | 67 | 76 | 60 | 71 | 57 | 73 | 68 | 61 | 66 | 62 | 64 |
| MMSE | 26 | 28 | 25 | 26 | 27 | 23 | 22 | 27 | 26 | 19 | 27 | 25 |
| bvFTD criteria | ||||||||||||
| Disinhibition | Y | Y | Y | Y | Y | Y | Y | Y | Y | |||
| Loss of empathy | Y | Y | Y | Y | Y | |||||||
| Compulsivity | Y | Y | Y | Y | Y | Y | Y | Y | ||||
| Hyperorality | Y | Y | Y | Y | ||||||||
| Apathy | Y | Y | Y | Y | Y | Y | ||||||
| Other | Y, delusions | Y, depression, delusions | Y, restlessness | Y, anxiety, depression | Y, delusions | Y, delusions | ||||||
| Total bvFTD symptoms | 4 | 3 | 2 | 2 | 4 | 2 | 2 | 3 | 4 | 2 | 2 | 2 |
| Memory domain Z-score | −0.41 | - | −1.43 | −1.52 | −2.22 | −2.86 | −1.09 | −1.28 | −4.98 | −5.30 | −2.61 | −2.05 |
| Executive domain Z-score | −0.11 | - | −1.39 | −0.08 | −1.47 | −2.70 | −3.54 | −0.96 | −1.31 | −3.82 | 0.30 | −1.32 |
| Memory impairment on NPAa | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Executive impairment on NPAa | Y | Y | Y | Y | Y | Y | Y | |||||
| Amyloid confirmation (CSF/amyloid PET) | PiB-PET+ | CSF A+/T+ | CSF A+/T+ | CSF A+/T+ | CSF A+/T+ | CSF A+/T+ | CSF A+/T+ | CSF A+/T+ | PiB-PET+ | CSF A+/T+ | CSF A+/T+ | PiB-PET+ |
Mean (SD) are reported unless stated otherwise. Y = present.
NPA = neuropsychological assessment at baseline by a trained neuropsychologist.
Experimental procedures
Participants underwent social cognition testing at the Alzheimer Center Amsterdam in a room with consistent lighting conditions. The test protocol included all tasks and biometric measures described below and had a total duration of approximately 1 h. While participants were tested, their caregiver filled out questionnaires in the waiting room. In the case of the SCD group, relatives filled out the questionnaires at home and returned them by mail.
Cognitive measures
Domain Z-scores were normalized to a reference group of 583 cognitively normal individuals from the Amsterdam Dementia Cohort18 with negative amyloid-β biomarker status.23 Attention domain Z-scores were based on the Digit Span forward, Trail Making Test A and Stroop 1 and 2 cards. Memory domain Z-scores were based on the Rey Verbal Auditory Memory Test immediate and delayed recall conditions and the Visual Association Test. Language domain Z-scores were based on the Visual Association Test naming condition and Animal Fluency. Executive domain Z-scores were based on the Digit Span backward, Trail Making Test B, Stroop card 3 and Letter Fluency.23 In addition, for all behavioural variant of Alzheimer’s disease patients, clinical charts were examined for results of formal neuropsychological assessment in order to ascertain memory and/or executive impairments.
Social cognition measures
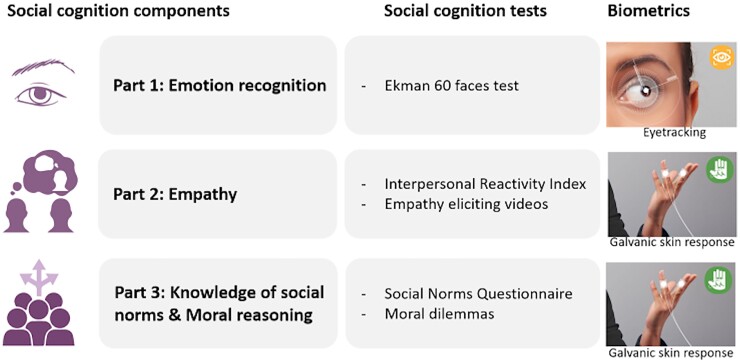
All tasks were presented in the iMotions platform (iMotions 8.0, iMotions A/S) that integrates several biometric measures, enabling the investigation of eye movements and GSR simultaneously in each participant, while performing a social cognition test (Fig. 1). For part one, emotion recognition, the Ekman 60 faces test was used.25 Subjects were asked to identify which of the six basic emotions (i.e. angry, sad, happy, surprise, fear or disgust) is shown by the facial expression on each of the 60 images. The test consists of 60 items, with 10 different faces of men and women each expressing the six basic emotions. Each correct item is awarded one point, with scores between 0 and 60 for the total score and 0 and 10 for individual emotions. During the Ekman test, eye movement patterns were recorded using the iMotions platform (see the next section). For each item, a 5-s window was presented within the platform with the face only, followed by the same face with the answer options below. This 5-s window was presented without the answer options in order to capture conscious face processing and to avoid capturing search behaviour involved in matching the face to the answer option. The 5-s window was preceded by a fixation cross for 2 s. There was no time restriction for participants to provide answers. For the eye-tracking analyses, only data from the first 5-s window were included. Missing data were imputed using the R package ‘mice’, with 5% missing values for the Ekman (Supplementary Fig. 2). Cases with missing data were distributed evenly across diagnostic groups (SCD, four; bvAD, three; tAD, five; and bvFTD, four). For part two, empathy, two empathy-eliciting videos were shown according to previously reported procedures.26 Briefly, an ‘uplifting’ empathy-eliciting video about a surf project for children with autism and Down syndrome and a ‘distressing’ empathy-eliciting video about a charity foundation for severely malnourished children were shown. The duration of each video was between 60 and 80 s. Immediately after watching the video, participants were asked to rate on a 5-point Likert-scale (1 = not at all, 5 = extremely) the degree to which they felt ‘sympathetic’, ‘moved’, ‘compassionate’, ‘disturbed’, ‘upset’ and ‘worried’. The three former phrases were averaged as a measure of empathetic concern, while the average of the three latter phrases was used as a measure of personal distress.27,28 During this task, the GSR was administered using the iMotions platform. As a baseline condition, nature videos (i.e. scenes showing a desert landscape or forest views) were shown of approximately the same duration as the empathy videos. In addition, the Dutch version of the Interpersonal Reactivity Index was administered in informants.29 This is a questionnaire consisting of 28 items with a 5-point Likert scale, measuring four subscales: Perspective Taking (the tendency to adopt another’s psychological perspective), Empathetic Concern (the tendency to experience feelings of warmth, sympathy and concern toward others), Fantasy (the tendency to identify strongly with fictitious characters) and Personal Distress (the tendency to have feelings of discomfort of concern when witnessing other’s negative experiences). For part three, higher-order social reasoning functions were assessed, including knowledge of social norms and moral reasoning. For knowledge of social norms, the Dutch version of the Social Norms Questionnaire was administered in participants, consisting of 22 questions assessing the ability to understand and identify social boundaries,30 each marked as correct or incorrect, with a total score between 0 and 22. A break score indicated a tendency to break social norms (for example, indicating whether it is socially acceptable to tell a stranger you think he or she is overweight) and an over-adherence score indicated a tendency to over-adhere to a social norm (i.e. applying a social rule too rigidly; for example, indicating that it is not socially acceptable to laugh when you trip and fall yourself). Missing data were imputed using the R package ‘mice’ with 4% missing values (Supplementary Fig. 2). No data were imputed for bvAD cases and the cases for whom data were imputed were evenly distributed across the remaining groups (SCD: three; tAD: two; bvFTD: two). For moral reasoning, two classic moral dilemmas were presented to participants, consisting of the trolley (impersonal) and footbridge (personal) dilemmas.31 In the trolley dilemma participants were asked whether they would hit a switch that would redirect a trolley that is on its way to kill five individuals on a train rail to a train rail heading for one individual (yes/no). In the footbridge dilemma, participants are asked whether they would push a man off a bridge in order to stop the train from killing the five individuals. These stories were presented using prerecorded audio fragments with an average duration of approximately 1 min per dilemma and were additionally presented in text on the screen. The percentage of rational answers was calculated per condition. During this task, GSR was administered using the iMotions platform.
Figure 1.
Social cognition framework and biometrics implemented in the current study. This figure shows the different social components measured in this study (first column), with the corresponding social cognition tests and biometric measures (second and third columns). Images under the biometrics column were provided by iMotions (iMotions 8.0, iMotions A/S, Frederiksberg, Denmark).
Biometric measures
Eye movements were recorded with a Tobii Pro X2-60 screen-based eye tracker with a sampling rate of 60 Hz. The tasks were presented on a 24′′ monitor with a screen resolution of 1920 × 1200 pixels. Patients were positioned between 55 and 75 cm from the screen. Before the task, participants completed a 9-point calibration procedure to ensure optimal eye tracking accuracy. The eye tracker uses near-infrared technologies to track and calculate gaze points. The dwell time is recorded as the percentage of time that the gaze was directed in a specific (manually defined) area of interest during presentation of the stimulus.32 Areas of interest of the same size were drawn on the eyes and mouth of each Ekman face (Supplementary Fig. 3) as these form the most salient features of the face to extract emotions33 and form the central regions of interest in the psychiatry literature on eye-tracking patterns in emotion recognition.34 GSR was measured using the Shimmer 3 GSR+ system (Shimmer, Consensys, https://shimmersensing.com/) attached to the plantar side of two fingers of participants’ non-dominant hand. Signals were sampled at 128 Hz. Data were online band-pass filtered between 0.01 and 1 Hz and were subsequently analysed through a standardized R notebook (see Supplementary Table 3 for details) according to previously reported procedures,35 resulting in GSR peaks per minute per stimulus per respondent. For sensitivity analyses, mean GSR within nature and empathy-eliciting videos were calculated in addition to peaks per minute.
Statistics
Differences in demographic variables were assessed using χ2 tests for dichotomous data and ANOVAs for continuous variables. Differences among groups were assessed using ANCOVAs and emmeans post hoc tests, adjusting for age and sex, in R version 4.0.2. The Supplementary material additionally shows the results without adjusting for age and sex (Supplementary Tables 4–11) and when excluding the three individuals with SCD with unknown amyloid status (Supplementary Tables 12–22).
Data availability
The data that support these findings are available from the author upon reasonable request.
Results
Demographic characteristics
Demographic characteristics are shown in Table 1. Patients with bvAD had an average age of 66.6 ± 5.7 versus 64.6 ± 6.7 in tAD, 66.4 ± 7.0 in bvFTD and 57.5 ± 5.8 in SCD. Seventy-five per cent of the bvAD cases were male versus 38.5% in tAD, 64.3% in bvFTD and 38.5% in SCD. No significant differences were found in MMSE scores or education between groups (all P > 0.05) and bvAD (60.0%) and tAD (83.3%) showed higher proportions of APOE ε4 carriers than the bvFTD (17.0%) and SCD (38.5%, P = 0.02). Cognition scores showed no significant differences in all domains between bvAD and tAD or bvFTD (all P > 0.05; Table 1 and Supplementary Fig. 4).
Emotion recognition
Ekman 60 faces test scores and eye tracking
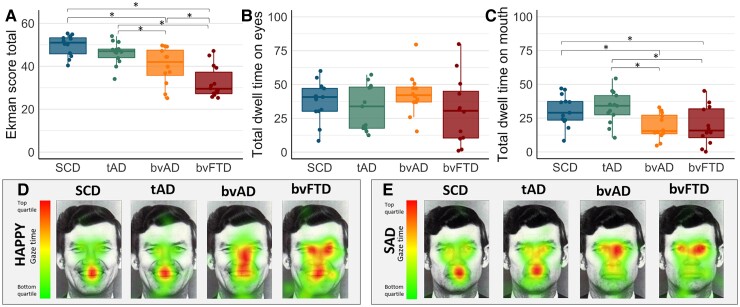
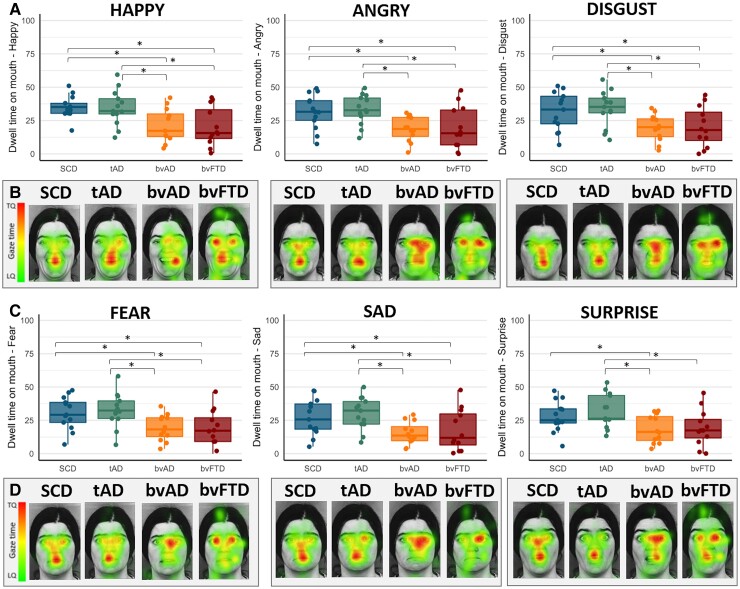
Participants with bvAD (40.1 ± 8.6) showed lower scores on the Ekman 60 faces test compared with SCD (49.67 ± 5.02, P < 0.003) and tAD (46.2 ± 5.3, P = 0.05) and higher scores compared to bvFTD (32.4 ± 7.3, P = 0.003, Fig. 2 and Supplementary Table 4). Compared with tAD, bvAD showed lower scores on the angry faces (6.2 ± 2.4 versus 8.4 ± 1.3, P = 0.02) and did not differ on the other emotions (all P > 0.05). The bvAD and the bvFTD groups did not differ on any emotion (all P > 0.05). Compared to SCD, bvAD showed significantly lower scores on the angry (6.2 ± 2.4 versus 8.8 ± 1.5, P = 0.005), disgusted (5.4 ± 2.5 versus 8.3 ± 1.6, P = 0.001) and surprised (8.1 ± 1.7 versus 9.0 ± 0.7, P = 0.05) faces and did not differ on the other emotions (all P > 0.05). Despite comparable dwell time in the eyes across groups (all P > 0.05; Fig. 3 and Supplementary Table 5), bvAD (18.7 ± 9.5) showed lower dwell time percentages in the mouth area of interest compared to SCD (30.7 ± 11.6, P = 0.01) and tAD (32.7 ± 12.1, P = 0.001), and did not differ from the bvFTD (19.4 ± 14.3, P = 0.78). This pattern was observed across all six emotions (Fig. 3 and Supplementary Table 6).
Figure 2.
Emotion recognition measured by the Ekman 60 faces test scores and total eye tracking dwell time across groups. (A) Scores on the Ekman test for emotion recognition across groups. (B) Total dwell time on the eyes as measured by eye tracking across groups. (C) Total dwell time on the mouth as measured by eye tracking across groups. (D) Examples of eye-tracking heat maps averaged across participants in each group while viewing a happy face. (E) Examples of eye-tracking heat maps averaged across participants in each group while viewing a sad face. The dwell time is a percentage of time that participants’ gaze was upon certain features of the image of the total time the stimulus was presented. Pixels with the highest amount of time spent by gaze are represented by red colours while pixels with the lowest amount of time spent by gaze are represented by green colours. For visualization purposes, one of the 10 persons is depicted in this figure as an example condition. Significant differences are age- and sex-adjusted at P < 0.05.
Figure 3.
Heat maps of eye-tracking dwell time on the mouth in Ekman 60 faces test images across diagnostic groups. (A) The dwell time on the mouth across groups while viewing happy, angry and disgusted faces. (B) Examples of eye-tracking heat maps averaged across participants per group while viewing happy, angry and disgusted faces. (C) The dwell time on the mouth while viewing fearful, sad and surprised faces. (D) Examples of eye-tracking heat maps averaged across participants per group while viewing fearful, sad and surprised faces. The dwell time is a percentage of time that participants’ gaze was upon certain features of the image of the total time the stimulus was presented. Pixels with the highest amount of time spent by gaze are represented by red colours while pixels with the lowest amount of time spent by gaze are represented by green colours. For visualization purposes, one of the 10 faces is depicted here as an example condition. Significant differences are age- and sex-adjusted at P < 0.05. TQ = top quartile; LQ = lower quartile.
Empathy
Interpersonal Reactivity Index
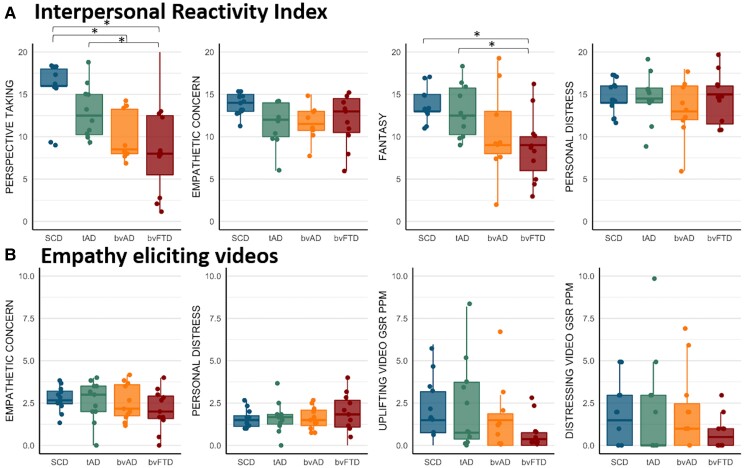
On this informant-rated questionnaire measuring empathy, the bvAD participants showed lower scores on the Perspective Taking subscale of the Interpersonal Reactivity Index (11.3 ± 4.6 versus 15.6 ± 3.1, P = 0.05) compared to SCD (Fig. 4 and Supplementary Table 7), while showing no significant differences with tAD (Perspective Taking: 13.0 ± 3.2) and bvFTD (Perspective Taking: 8.7 ± 5.6, all P > 0.05). No significant differences were observed between groups on the Empathetic Concern and Personal Distress subscales (all P > 0.05) of the Interpersonal Reactivity Index and bvFTD showed lower scores on the Fantasy subscale compared to SCD (8.6 ± 4.0 versus 13.6 ± 1.9, P = 0.01) and tAD (13.0 ± 3.1, P = 0.02).
Figure 4.
Empathy measured by the Interpersonal Reactivity Index scores and responses to empathy-eliciting videos across diagnostic groups. (A) Scores on the four subscales of the Interpersonal Reactivity Index across groups. (B) Scores on ratings of empathetic concern and personal distress after watching empathy-eliciting videos and GSR while watching these videos. Significant differences are age- and sex-adjusted at P < 0.05. PPM = peaks per minute.
Empathy-eliciting videos
No significant differences were found among groups in empathetic concern and personal distress scores after watching empathy-eliciting videos, or in their GSR while watching those videos (all P > 0.05; Fig. 4 and Supplementary Table 8). In order to capture a change from baseline in the GSR, sensitivity analyses were performed comparing the mean GSR in a nature baseline video condition versus the empathy-eliciting videos. There were no differences between the nature and empathy video conditions, nor did the groups differ in the mean difference between these conditions (all P > 0.05; Supplementary Fig. 5 and Supplementary Table 9).
Social norms and moral dilemmas
Knowledge of social norms
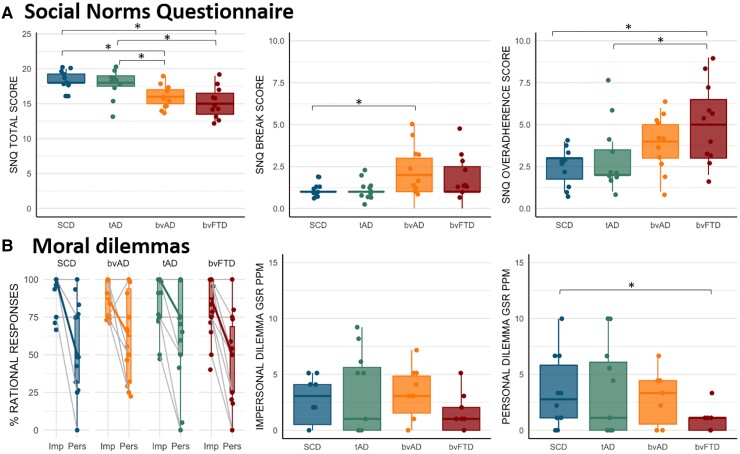
The bvAD participants (16.0 ± 1.6) showed lower scores on the Social Norms Questionnaire total score compared to SCD (18.3 ± 1.4, P = 0.01), and tAD (17.8 ± 2.1, P = 0.05; Fig. 5 and Supplementary Table 10). The bvAD participants showed a higher break score compared to SCD (2.1 ± 1.5 versus 1.1 ± 0.5, P = 0.05), indicating a tendency to break social rules, while the bvFTD showed a higher over-adherence score compared to SCD (5.1 ± 2.3 versus 2.6 ± 1.1, P = 0.004) and tAD (3.1 ± 2.1, P = 0.02), indicating a tendency to apply social rules too rigidly.
Figure 5.
Knowledge of social norms and (GSR to) moral reasoning across diagnostic groups. (A) Scores on the Social Norms Questionnaire across groups. (B) Scores on the Moral Dilemmas, depicted as percentage rational responses in the impersonal and personal conditions, across groups, and GSR in the impersonal and personal conditions. Significant differences are age- and sex-adjusted at P < 0.05. PPM = peaks per minute; Imp = impersonal; Pers = personal.
Moral dilemmas
No significant differences were found among groups in the percentage rational responses provided to moral dilemmas (all P > 0.05). Except for lower GSR peaks per minute in the personal dilemma condition in the bvFTD compared to SCD (0.9 ± 1.1 versus 3.4 ± 3.3, P = 0.01; Fig. 5 and Supplementary Table 11), there were no differences in GSR peaks per minute to moral dilemmas across groups (2.9 ± 2.3 in bvAD versus 3.4 ± 4.1 in tAD and 3.4 ± 3.3 in SCD (all P > 0.05)).
Sensitivity analyses
Sensitivity analyses were performed excluding three cases in the SCD group with unknown amyloid status and are presented in Supplementary Tables 12–22. The only significant change was the disappearance of the difference between bvAD and SCD in the Perspective Taking subscale of the Interpersonal Reactivity Index. However, because the effect size was comparable between the n = 10 and n = 13 groups (i.e. t = −1.99 versus t = −1.89), we argue this can largely be attributed to the reduced statistical power.
Discussion
In this exploratory study, we found social cognition deficits in multiple components of social cognition in bvAD and an eye-tracking signature that overlapped with bvFTD. The impairments on emotion recognition in patients with bvAD were observed in parallel with lower dwell time on the mouth while viewing emotional facial expressions compared to SCD and tAD. Regarding empathy, deficits were reported by informants of patients with bvAD while no differences were observed on subjective and biometric responses to empathy-eliciting videos. In addition, patients with bvAD showed impairments in knowledge of social norms. Interestingly, these patients showed a higher break score than SCD, indicating a potential tendency to break social norms. No differences were observed in the subjective and biometric responses to moral dilemmas across diagnostic groups. This multidimensional study of social cognition in bvAD, combining a wide range of social cognition tests with biometric measures, points towards deficits across different components of social cognition, showing a similar yet milder profile compared to bvFTD, and distinct elementary perceptual processes in bvAD compared to tAD.
A main finding in the present study was that eye movement patterns showed lower dwell time on the mouth and similar dwell times on the eyes in bvAD and bvFTD compared to tAD and SCD. This differs from eye movement patterns observed in other conditions where social cognition is impaired. For example, reduced social cognition in autism is observed in parallel with a lack of gaze on the eyes,36 while Williams syndrome (a hypersocial developmental disorder) is characterized by hyperfixation on the eyes.37 Our results also differ from recent work in bvFTD showing increased fixations on the eyes while spending the same amount of time on the mouth as SCD.33,38 A commonality between all studies is the suggestion of a mechanism by which patients with bvFTD ‘look but don’t recognize’, as they do spend most time on the salient features of the face. The question whether this represents a deficit in encoding or interpretation of emotionally salient stimuli is a topic of debate. The fact that the previous study showed a hyperfixation on the eyes in bvFTD may point towards interpretation deficits or compensatory mechanisms, while our findings of lower dwell time on the mouth in bvAD and the bvFTD may suggest that the elementary perceptual process of identification of salient features (i.e. encoding) is altered in these diseases, as they may not utilize all relevant facial features to extract emotional meaning. In addition, patients may spend more time on other features of the face than the eyes and mouth that hold less relevance for accurate detection of emotions. Either way, our exploratory results suggest that the analysis of eye-tracking patterns may hold potential for the differentiation of bvAD and bvFTD from tAD and SCD in addition to traditional social cognition scores. However, as our current results do not suggest that biometrics show superior differential diagnostic value to conventional social cognition test scores, biometrics may serve as a tool to better understand behavioural phenotypes in dementia. It is, for instance, important to determine to what degree sensory mechanisms versus more cognitive appraisal processes such as deficits in categorizing emotions contribute to deficits in emotion recognition in bvFTD and bvAD.
Despite evident differences in perceptual processes during the emotion recognition subcomponent of social cognition in bvAD, these patients did not exhibit lower scores on all tests across all subcomponents of social cognition compared with SCD and tAD. For example, they did not show differences in their own emotional valuations after watching empathy-eliciting videos or the amount of rational responses to moral dilemmas, or in GSR to those tasks. Regarding moral dilemmas, this may be due to the high cognitive demand of the tasks, hampering patients’ understanding of the dilemmas, which may influence bvAD and tAD disease groups to a greater extent than bvFTD. The empathy-eliciting videos may draw patients’ attention more exogenously and require less cognitive engagement. Indeed, in the uplifting empathy-eliciting video, a trend towards lower GSR was observed in bvAD and bvFTD compared to SCD, and in the personal condition of the moral dilemmas, bvFTD showed a significantly lower GSR compared to SCD (Figs 3 and 4 and Supplementary Tables 7 and 9). In addition, while a clinically well-validated test was used for emotion recognition (i.e. the Ekman 60 faces test), more experimental tests were used on other components (i.e. empathy-eliciting videos and moral dilemmas), with lower variance in scores. This may have hampered finding differences in these small samples.
Our results expand upon the scarce existing literature on social cognition deficits in bvAD, which is mainly based on case studies. Emotion recognition deficits were reported in a sample of eight behavioural variant of Alzheimer’s disease cases using the mini-SEA (Social cognition and Emotional Assessment)9 and two case studies using the Facial Emotion Recognition Test and the emotion recognition subtests of the TASIT (The Awareness of Social Inference Test).10,12 These studies involved patients with an initial bvFTD diagnosis, who had an Alzheimer’s disease biomarker profile. Impairments of Theory of Mind (ToM) were reported in the sample of eight behavioural variant of Alzheimer’s disease cases using the mini-SEA9 and one case study describing bvAD using the Reading the Mind in the Eyes Test and the ToM-15.11 Impairments in knowledge of social norms were reported in one clinical bvFTD case with Alzheimer’s disease biomarkers previously, based on the Social Norm Knowledge Questionnaire.11 The limited studies on social cognition in bvAD showed a lack of inclusion of tests along different components of social cognition and a lack of measurements capturing the experiential, non-cognitive processes of social cognition. Our findings in a group study including tests along multiple components of social cognition firmly established deficits in emotion recognition, empathy and knowledge of social norms in bvAD. In addition, the combination of social cognition tests and biometric measurements yielded insights into the elementary perception of emotional facial expressions. The elementary processing of social cues may contribute to higher-order social cognition deficits in bvAD and FTD.
The neurobiological origins of the observed social cognition deficits in bvAD are poorly understood. Neuroimaging studies have shown either a mix of anterior and posterior predominant patterns or a predominant temporoparietal pattern of neurodegeneration based on atrophy or hypometabolism,2,39–41 with a relative lack of involvement of anterior brain regions. Compared with bvFTD, bvAD participants showed less involvement of the salience network, which is one of the networks that regulate socioemotional processing and social cognition,42 alongside the semantic appraisal network43 and components of the default mode network.44,45 Moreover, neuropathological small samples of patients with bvAD suggested that they may not show a selective loss of Von Economo neurons in the anterior cingulate cortex,20,46 which are specialized neurons located within key regions of the salience network that serve social functioning in humans and highly intelligent mammals.47 Therefore, traditional regions implicated in social cognition in bvFTD may not underlie social cognition deficits in bvAD. In typical (amnestic-predominant) Alzheimer’s disease, different regions have been proposed to underlie social cognition, including the precuneus, posterior cingulate cortex, temporoparietal junction and hippocampus, either directly or indirectly.48 Alternatively, unique ‘bvAD’ features such as anterior default mode network involvement or altered amygdalar volumes41 compared to tAD may contribute to the observed deficits in bvAD, in addition to mild involvement of ‘bvFTD’-specific regions, such as subtle frontoinsular involvement.41
The strength of this study is that we applied a multidimensional social cognition test battery spanning multiple central subcomponents of social cognition and combined it with biometric measurements in biomarker-confirmed bvAD patients. There are also limitations. First, although this is the largest study of its kind to date, the sample size of this exploratory study was modest. Because GSR can show substantial variation across participants, as exemplified by large heterogeneity across individuals with SCD, the small samples sizes may have hampered the detection of group differences. Second, the groups differed substantially in proportions of males versus females due to inherent overrepresentation of males in both bvAD2 and bvFTD.49 As females are known to show better performance on social cognition tests,50 this may have influenced results in favour of the tAD and SCD groups. However, as all results were corrected for age and sex, the effects in the current work are deemed minimal. Third, due to the exploratory nature of this study and the low sample sizes, no correction for multiple testing was applied. Future, hypothesis-driven, work with larger groups should incorporate adequate correction. Fourth, the inclusion of cognitively unimpaired individuals with SCD as control group may be suboptimal, given the potential for these individuals to be in early stages of the Alzheimer’s disease continuum.51 However, as the majority of cases were amyloid negative, showed no cognitive impairments on extensive neuropsychological testing and showed limited neuropsychiatric symptoms and neurodegeneration (Supplementary Table 2), we minimized the potential confounding effect of early Alzheimer’s disease on the performance on social cognition tests. Fifth, although all bvAD cases met our recently proposed research criteria for ‘possible bvAD’,3 it is important to note that establishing consensus on the clinical criteria for this phenotype remains an active research area. Sixth, while the previous two studies38,52 on eye tracking in bvFTD assessed the number of fixations, the present work assessed dwell time as the eye-tracking metric, potentially hampering comparisons between studies. Dwell time represents the time that gaze coordinates were directed towards a specific area of interest,32 while fixation counts represent the amount of fixations made within the area of interest and as such do not contain a measure of time.32 In addition, because increased fixation counts have been interpreted both as heightened attention (beneficial)53 and hyperscanning (detrimental),54 the exact interpretation of fixation count remains poorly understood. Sixth, while the present exploratory work focused on crude regions of interests cardinal for emotion recognition, i.e. the eyes and mouth,33,34 more detailed work should focus on other facial areas as well, such as the nose, as differences in this region of interest have been observed in bvFTD compared to SCD.52 For the present study, we aimed to include a selective number of outcomes to minimize potential type one errors. Seventh, although differences in general cognitive functioning may hamper adequate comparison between bvAD and bvFTD on social cognition measures, this bias is deemed minimal, as the former showed better social cognition levels compared to the latter and the analyses of biometric responses showed similar patterns in these groups. Lastly, it should be noted that the social cognition tests for empathy and moral reasoning have not been clinically validated and these results should be interpreted with caution.
Future research should focus on multiple issues. Our findings should be replicated in larger cohorts, both in terms of social cognition tests and biometrics. In addition, future, hypothesis-driven, work with larger groups should incorporate adequate multiple testing correction. Ideally, future work should include an age- and sex-matched cognitively unimpaired group that did not present with subjective cognitive complaints at a memory clinic, in order to provide a more representative reference group for social cognition. Regarding eye tracking, future work should especially investigate the role of perception of the mouth as a salient facial feature for emotion recognition in the context of normal ageing and disease, as well as investigating gaze patterns in more detail in bvAD and bvFTD. Moreover, future research should employ a similar study design with lower cognitive demands that sufficiently stimulates arousal to investigate the role of GSR to emotional stimuli. Future research should also incorporate more clinically validated tests for empathy and social and moral reasoning with larger statistical variety that are suitable for the acquisition of biometric measurements in order to capture experiential processing directly. Our current results suggest that validated social cognition tests and questionnaires, such as the Ekman 60 faces test, Interpersonal Reactivity Index and Social Norms Questionnaire, may support a clinical diagnosis of bvAD. Future work should investigate the added value of incorporating these tests into the recently proposed research criteria for bvAD.3 Furthermore, future work should investigate the relationship between biometric measures and social cognition scores and their potential additive diagnostic accuracy above social cognition tests alone. Lastly, given that the neurobiological underpinnings of social cognitive deficits likely differ between bvAD and bvFTD,48 extensive profiling of social cognitive deficits, their biometric and regional brain correlates in both disorders should be compared.
Conclusion
In conclusion, bvAD showed a similar although milder pattern of social cognition deficits compared to bvFTD, characterized by deficits in emotion recognition, empathy and knowledge of social norms compared to tAD and SCD, with a similar eye-tracking signature to bvFTD. Future research should focus on including larger sample sizes when assessing social cognition in conjunction with biometrics, incorporating multiple social cognition tasks with low cognitive demands and investigating brain correlates of social cognition. These social cognition and biometric measures provide important insights into the basis of the behavioural and personality changes in bvAD, and might serve as valuable tools for an accurate diagnosis of this atypical variant of Alzheimer’s disease in investigational and clinical settings in the future.
Supplementary Material
Acknowledgements
Research of the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. The Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds. W.F. holds the Pasman chair. The authors would furthermore like to acknowledge Chrissy Rijkers for the help in constructing the test protocol for the current manuscript, Dr Stefan van der Stigchel for advice regarding the setup of the eye-tracking part of the study, Dr Joke Spikman for advice on the construction of the social cognition test battery, Mardou Leeuwestijn-Koopmans for help in patient recruitment and Kiara Heide and Oscar Haven for their support in constructing and monitoring the study test platform and conduction.
Contributor Information
Ellen H Singleton, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
Jay L P Fieldhouse, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
Jochum J van ’t Hooft, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
Marta Scarioni, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
Marie-Paule E van Engelen, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
Sietske A M Sikkes, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands; Department of Clinical, Neuro and Developmental Psychology, Faculty of Movement and Behavioural Sciences, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Casper de Boer, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
Diana I Bocancea, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
Esther van den Berg, Department of Neurology and Alzheimer Center Rotterdam, Erasmus University Medical Center, Rotterdam, The Netherlands.
Philip Scheltens, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
Wiesje M van der Flier, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands; Department of Epidemiology and Data Science, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
Janne M Papma, Department of Neurology and Alzheimer Center Rotterdam, Erasmus University Medical Center, Rotterdam, The Netherlands.
Yolande A L Pijnenburg, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands.
Rik Ossenkoppele, Alzheimer Center Amsterdam, Department of Neurology, Amsterdam Neuroscience, Vrije Universiteit Amsterdam, Amsterdam UMC, Amsterdam, The Netherlands; Clinical Memory Research Unit, Department of Clinical Sciences Malmö, Lund University, Sweden.
Funding
Work at the Alzheimer Center Amsterdam was supported by the Netherlands Organization for Health Research and Development, ZonMw (70-73305-98-1214 to R.O., PI) under the BEAT-IT (BEhavioral symptoms in Alzheimer’s disease: Towards early Identification and Treatment) study (project #2004404).
Competing interests
E.H.S., J.L.P.F., J.J.H., M.S., M.P.E.E., S.A.M.S., C.d.B., D.B., E.v.d.B., W.v.d.F., J.M.P., Y.A.L.P. and R.O. report no competing interests. P.S. has received consultancy fees (paid to the university) from Alzheon, Brainstorm Cell and Green Valley. Within his university affiliation he is global PI of the phase 1b study of AC Immune, Phase 2b study with FUJI-film/Toyama and phase 2 study of UCB and phase 1 study with ImmunoBrain Checkpoint. He is chair of the EU steering committee of the phase 2b program of Vivoryon, the phase 2b study of Novartis Cardiology and co-chair of the phase 3 study with NOVO-Nordisk. He is also an employee of EQT Life Sciences (formerly LSP).
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: The IWG-2 criteria. Lancet Neurol. 2014;13:614–629. [DOI] [PubMed] [Google Scholar]
- 2. Ossenkoppele R, Pijnenburg YAL, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer's disease: Clinical, neuroimaging and pathological features. Brain. 2015;138:2732–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ossenkoppele R, Singleton EH, Groot C, et al. Research criteria for the behavioral variant of Alzheimer disease: A systematic review and meta-analysis. JAMA Neurol. 2022;79:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harciarek M, Cosentino S. Language, executive function and social cognition in the diagnosis of frontotemporal dementia syndromes. Int Rev Psychiatry. 2013;25:178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fiske ST, Taylor SE. Social cognition: From brains to culture. Sage; 2013. [Google Scholar]
- 6. Happé F, Cook JL, Bird G. The structure of social cognition: In (ter) dependence of sociocognitive processes. Annu Rev Psychol. 2017;68:243–267. [DOI] [PubMed] [Google Scholar]
- 7. Dickerson BC. Dysfunction of social cognition and behavior. Continuum (Minneapolis, Minn). 2015;21:660–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arioli M, Crespi C, Canessa N. Social cognition through the lens of cognitive and clinical neuroscience. Biomed Res Int. 2018;2018:4283427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Souza LC, Bertoux M, Funkiewiez A, et al. Frontal presentation of Alzheimer's disease: A series of patients with biological evidence by CSF biomarkers. Dement Neuropsychol. 2013;7:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Souza LC, Mariano LI, de Moraes RF, Caramelli P. Behavioral variant of frontotemporal dementia or frontal variant of Alzheimer's disease? A case study. Dement Neuropsychol. 2019;13:356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duclos H, de La Sayette V, Bonnet AL, et al. Social cognition in the frontal variant of Alzheimer's disease: A case study. J Alzheimers Dis. 2017;55:459–463. [DOI] [PubMed] [Google Scholar]
- 12. Wong S, Strudwick J, Devenney E, Hodges JR, Piguet O, Kumfor F. Frontal variant of Alzheimer's disease masquerading as behavioural-variant frontotemporal dementia: A case study comparison. Neurocase. 2019;25:48–58. [DOI] [PubMed] [Google Scholar]
- 13. Mauss IB, Robinson MD. Measures of emotion: A review. Cogn Emot. 2009;23:209–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joshi A, Mendez MF, Kaiser N, Jimenez E, Mather M, Shapira JS. Skin conductance levels may reflect emotional blunting in behavioral variant frontotemporal dementia. J Neuropsychiatry Clin Neurosci. 2014;26:227–232. [DOI] [PubMed] [Google Scholar]
- 15. Kumfor F, Hazelton JL, Rushby JA, Hodges JR, Piguet O. Facial expressiveness and physiological arousal in frontotemporal dementia: Phenotypic clinical profiles and neural correlates. Cogn Affect Behav Neurosci. 2019;19:197–210. [DOI] [PubMed] [Google Scholar]
- 16. Russell LL, Greaves CV, Convery RS, et al. Novel instructionless eye tracking tasks identify emotion recognition deficits in frontotemporal dementia. Alzheimers Res Ther. 2021;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gendron M, Lindquist KA, Barsalou L, Barrett LF. Emotion words shape emotion percepts. Emotion. 2012;12:314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Flier WM, Scheltens P. Amsterdam Dementia Cohort: Performing research to optimize care. J Alzheimers Dis. 2018;62:1091–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singleton E, Hansson O, Pijnenburg YAL, et al. Heterogeneous distribution of tau pathology in the behavioural variant of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2021;92:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singleton EH, Pijnenburg YAL, Gami-Patel P, et al. The behavioral variant of Alzheimer’s disease does not show a selective loss of Von Economo and phylogenetically related neurons in the anterior cingulate cortex. Alzheimers Res Ther. 2022;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rascovsky K, Hodges JR, Knopman Det al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slot RER, Sikkes SAM, Berkhof J, et al. Subjective cognitive decline and rates of incident Alzheimer's disease and non–Alzheimer's disease dementia. Alzheimers Dement. 2019;15:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Groot C, van Loenhoud AC, Barkhof F, et al. Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology. 2018;90:e149–e156. [DOI] [PubMed] [Google Scholar]
- 24. Verhage F. Intelligentie en leeftijd: Onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar. van Gorcum; 1964. [Google Scholar]
- 25. Young AW, Perrett D, Calder A, Sprengelmeyer R, Ekman P. Facial expressions of emotion: Stimuli and tests (FEEST). Thames Valley Test Company; 2002. [Google Scholar]
- 26. Sze JA, Gyurak A, Goodkind MS, Levenson RW. Greater emotional empathy and prosocial behavior in late life. Emotion. 2012;12:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Batson CD. Prosocial motivation: Is it ever truly altruistic? Adv Exp Soc Psychol. 1987;20:65–122. [Google Scholar]
- 28. Eisenberg N, Schaller M, Fabes RA, et al. Differentiation of personal distress and sympathy in children and adults. Dev Psychol. 1988;24:766. [Google Scholar]
- 29. De Corte K, Buysse A, Verhofstadt LL, Roeyers H, Ponnet K, Davis MH. Measuring empathic tendencies: Reliability and validity of the Dutch version of the Interpersonal Reactivity Index. Psychol Belg. 2007;47:235–260. [Google Scholar]
- 30. van den Berg E, Poos J, Jiskoot L, et al. Impaired knowledge of social norms in dementia and psychiatric disorders: Validation of the Social Norms Questionnaire–Dutch Version (SNQ-NL). Assessment. 2022;29:1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–2108. [DOI] [PubMed] [Google Scholar]
- 32. Vansteenkiste P, Cardon G, Philippaerts R, Lenoir M. Measuring dwell time percentage from head-mounted eye-tracking data—Comparison of a frame-by-frame and a fixation-by-fixation analysis. Ergonomics. 2015;58:712–721. [DOI] [PubMed] [Google Scholar]
- 33. Oliver LD, Virani K, Finger EC, Mitchell DGV. Is the emotion recognition deficit associated with frontotemporal dementia caused by selective inattention to diagnostic facial features? Neuropsychologia. 2014;60:84–92. [DOI] [PubMed] [Google Scholar]
- 34. Schneider F, Gur RC, Koch K, et al. Impairment in the specificity of emotion processing in schizophrenia. Am J Psychiatry. 2006;163:442–447. [DOI] [PubMed] [Google Scholar]
- 35. Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. J Neurosci Methods. 2010;190:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cuve HC, Gao Y, Fuse A. Is it avoidance or hypoarousal? A systematic review of emotion recognition, eye-tracking, and psychophysiological studies in young adults with autism spectrum conditions. Res Autism Spectr Disord. 2018;55:1–13. [Google Scholar]
- 37. Riby D, Hancock PJ. Looking at movies and cartoons: Eye-tracking evidence from Williams syndrome and autism. J Intellect Disabil Res. 2009;53:169–181. [DOI] [PubMed] [Google Scholar]
- 38. Hutchings R, Palermo R, Bruggemann J, Hodges JR, Piguet O, Kumfor F. Looking but not seeing: Increased eye fixations in behavioural-variant frontotemporal dementia. Cortex. 2018;103:71–81. [DOI] [PubMed] [Google Scholar]
- 39. Woodward MC, Rowe CC, Jones G, Villemagne VL, Varos TA. Differentiating the frontal presentation of Alzheimer's disease with FDG-PET. J Alzheimers Dis. 2015;44:233–242. [DOI] [PubMed] [Google Scholar]
- 40. Phillips JS, Da Re F, Dratch L, et al. Neocortical origin and progression of gray matter atrophy in nonamnestic Alzheimer's disease. Neurobiol Aging. 2018;63:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singleton EH, Pijnenburg YAL, Sudre CH, et al. Investigating the clinico-anatomical dissociation in the behavioral variant of Alzheimer disease. Alzheimers Res Ther. 2020;12:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rankin KP. Brain networks supporting social cognition in dementia. Curr Behav Neurosci Rep. 2020;7:203–211. [Google Scholar]
- 44. Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Satpute AB, Lindquist KA. The default mode network's role in discrete emotion. Trends Cogn Sci (Regul Ed). 2019;23:851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tan RH, Yang Y, McCann H, Shepherd C, Halliday GM, Whitwell J. Von Economo neurons in behavioral variant frontotemporal dementia with underlying Alzheimer's disease. J Alzheimers Dis. 2019;69:963–967. [DOI] [PubMed] [Google Scholar]
- 47. Seeley WW, Carlin DA, Allman JM, et al. Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol. 2006;60:660–667. [DOI] [PubMed] [Google Scholar]
- 48. Strikwerda-Brown C, Ramanan S, Irish M. Neurocognitive mechanisms of theory of mind impairment in neurodegeneration: A transdiagnostic approach. Neuropsychiatr Dis Treat. 2019;15:557–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Boer SCM, Riedl L, van der Lee SJ, et al. Differences in sex distribution between genetic and sporadic frontotemporal dementia. J Alzheimers Dis. 2021;84:1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kessels RPC, Montagne B, Hendriks AW, Perrett DI, de Haan EHF. Assessment of perception of morphed facial expressions using the emotion recognition task: Normative data from healthy participants aged 8–75. J Neuropsychol. 2014;8:75–93. [DOI] [PubMed] [Google Scholar]
- 51. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Polet K, Hesse S, Morisot A, et al. Eye-gaze strategies during facial emotion recognition in neurodegenerative diseases and links with neuropsychiatric disorders. Cogn Behav Neurol. 2022; 35:14–31. [DOI] [PubMed] [Google Scholar]
- 53. Chau SA, Chung J, Herrmann N, Eizenman M, Lanctôt KL. Apathy and attentional biases in Alzheimer’s disease. J Alzheimers Dis. 2016;51:837–846. [DOI] [PubMed] [Google Scholar]
- 54. Phillipou A, Abel LA, Castle DJ, et al. Self perception and facial emotion perception of others in anorexia nervosa. Front Psychol. 2015;6:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support these findings are available from the author upon reasonable request.