Abstract
Background
Liver diseases are a spectrum of diseases that include hepatic steatosis, nonalcoholic fatty liver disease, hepatitis, liver fibrosis, cirrhosis, and hepatic cancer. These diseases not only severely decrease the quality of life for patients, but also cause financial burden. Although apigenin (APG) has recently become the primary treatment for liver injuries and diseases (LIADs), there has been no systematic review of its use.
Purpose
To review the existing literature and put forward novel strategies for future APG research on LIADs.
Methods
A search was conducted in PubMed, Science Direct, Research Gate, Web of Science, VIP, Wanfang, and CNKI, and 809 articles were obtained. After applying inclusion and exclusion criteria, 135 articles were included.
Results
APG is promising in treating LIADs via various mechanisms arising from its anti-inflammation, anti-proliferation, anti-infection, anti-oxidation, and anti-cancer properties.
Conclusion
This review summarizes the evidence supporting the use of APG as a treatment for LIADs and provides an insight into the intestinal microbiota, which may have important implications in its future clinical use.
Keywords: Apigenin, Liver injuries and diseases, Phytochemistry, Pharmacological effects, Toxicology
1. Introduction
Liver injuries and diseases (LIADs) have a great impact on human health. Liver injuries have multiple etiologies, and mainly include alcohol consumption, viral infection, and drug poisoning [1,2]. Stimulation of these factors can result in inflammation and oxidative stress in the liver [3]. Chronic liver injury leads to the overproduction of extracellular matrix and the progression of hepatic fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [4]. The most effective way to reduce the incidence of liver diseases is to prevent them by targeting their causes, but medical intervention is the commonly used method in practice. Available treatments for LIADs include the use of transforming growth factor-β (TGF-β) inhibitors [5], antivirals [6], cell-based therapies and nanoparticles [7], chemotherapeutics, and traditional Chinese medicine. Although liver transplantation is also considered an effective treatment for liver disease, obtaining transplantable livers is difficult and organ rejection is an important possible complication [8]. Therefore, it is necessary to explore new strategies to prevent and treat LIADs.
Fortunately, evidence has shown that natural plant extracts have better bioactivity in terms of their hepatoprotection, anti-inflammation, anti-oxidation, and anti-cancer effects; they are also considered to have high safety [[9], [10], [11], [12]]. For instance, the flavonoid quercetin exhibits anti-inflammatory [13], antioxidant [14], and anti-cancer [15] effects in LIADs. Apigenin (APG), another flavonoid compound, has also attracted increasing attention. Evidence has shown that APG possesses strong biological properties, such as anti-inflammation, anti-proliferation, antibacterial, anti-oxidation, and anti-cancer effects [16]. In preclinical and clinical studies, APG has been demonstrated to be a possible treatment for rheumatoid arthritis, autoimmune disorders, Parkinson’s disease, Alzheimer’s disease, and various cancers [17,18]. APG has shown protective effects in the liver, but there has been no systematic review of the literature on APG in LIADs. Therefore, we summarize the properties of APG in LIADs considering the aspects of botany, chemical composition, pharmacological effects, analytical methods and quality control, toxicology, and safety, with the aim of further understanding APG and uncovering its pharmacological activities and effects on human health.
2. Methodology
Studies published before November 2022 were searched from the PubMed, Web of Science CNKI, VIP, and Wanfang databases. The keywords included “Apigenin” + “liver injury”/“liver disease”, “phytochemistry”, “viral hepatitis”, “liver cancer”/“liver fibrosis and cirrhosis”/“pharmacological effects” + “toxicology” in both English and Chinese. We deleted and removed all duplicates. One author assessed the availability of the literature. All experiments were included, and studies that were not focused on the therapeutic effects of APG on LIADs were excluded.
3. Source, structure, absorption, and metabolism of APG
APG, with the molecular formula C15H10O5, belongs to a subclass of flavones [19]. APG is a yellow crystalline powder, and is the aglycone of several natural occurring glycosides [20]. It is acquired from various fruits, vegetables, and herbs in the form of O-glycosides and C-glycosides such as celery, grapes, apples and vervain [21]; parsley and celery are important food sources of APG (Fig. 1).
Fig. 1.
Plant source and processed products of APG (A and B). Chemical structure of APG (C).
APG is an extremely insoluble substance, indicating its poor bioavailability through oral administration. Many factors can influence the bioavailability of APG, such as the manner of intake and diet. Diet is a common factor that influences the absorption of both food and medicine by the digestive system. Indeed, yogurt has been shown to delay the plasma Tmax of APG-4′-glucuronide (APG-4′-GlcUA) and APG-7-sulfate by influencing the pharmacokinetics of APG-7-O-(2′-O-apiosyl) glucoside [22]. Chrysanthemum morifolium extract is a compound that can prevent cardiovascular diseases and cancers, and it mediates a relatively rapid absorption and slow elimination of APG [23]. Different intake forms also cause different results, with modern studies reporting that ingestion of parsley leaves with yogurt extended the maximum concentration (Cmax) of APG-4′-GlcUA. Moreover, consumption of chamomile tea containing APG-7′-O-glucoside has been shown to extend the Cmax of APG-4′-GlcUA [22]. Furthermore, recent studies have shown that mesoporous silica nanoparticles enhance the solubility, dissolution, and oral bioavailability of APG [24]. Therefore, bioavailability can be increased by diet intervention, by changing the intake form, and through chemical means, which provide practical significance for clinical application.
In food, APG is present almost exclusively as a glycoside, but it is influenced by methylation, sulfation, and glucuronidation in the body [25]. In tissues, the conversion of APG to larger molecules influences its circulating levels and subsequent bio-distribution. APG is distributed in the plasma, liver, and intestinal mucosa plasma, but at lower levels than quercetin, aspergillin, and tricin [26]. The main conjugation reaction of APG in rats is glucuronidation via uridine diphosphate-glucuronosyltransferases [27].
In terms of biosynthesis, APG is naturally produced by the phenylpropanoid metabolic pathway, in which 4-coumaroyl-CoA is produced from the phenylalanine amino acid through the shikimate pathway. The 4-coumaroyl-CoA combines with malonylCoA to yield the main backbone of flavonoids, called chalconaringenin, through the action of chalcone synthase [28]. Chalconaringenin, an intermediate chalconoid, undergoes stereo-specific and spontaneous cyclization into naringenin through the action of chalcone isomerase [29]. Chemically, APG synthesis includes the formation of chalcone and demethylation. The cyclization of chalcone resulted in the methylated derivative through the action of iodine with dimethyl sulfoxide followed by demethylation using pyridine hydrochloride, which further led to the aglycone form.
4. Protective effects of APG on liver injuries
4.1. Chemical pollutant-induced liver injury
Chemical pollutant exposure is inevitable in human life, while long-term or excessive exposure can cause liver injury. Di 2-ethylhexyl phthalate (DEHP) is a ubiquitous pollutant in water, food, air, soil, and sediments, and is commonly used as a plasticizer [30]. Han et al. found that APG can protect against DEHP-induced ferroptosis-like damage by activating glutathione (GSH) peroxidase 4 and restraining intracellular iron accumulation [31]. Nickel oxide nanoparticles (NiONPs) are regularly applied medically, including as sensors of urea and glucose levels in the blood [32]. Ali et al. proved that APG pretreatment could relieve the effects of NiONPs, which prevented oxidative stress, fibrosis, and inflammation [33]. Numerous reports have demonstrated that CCl4-induced liver injury is primarily ascribed to the products of CCl4 (CCl3·and CCl3OO·). The accumulated free radicals cause oxidative damage and activate liver macrophages to secret inflammatory cytokines, resulting in liver injury [34,35]. APG also has protective functions against liver oxidative injury induced by CCl4 because of its antioxidant effects [36].
4.2. Drug-induced liver injury (DILI)
Although DILI is uncommon, it has a significant influence in contemporary hepatology [37]. The physicochemical properties of various drugs are responsible for the different mechanisms of DILI. Drugs are metabolized in the liver and their metabolites may cause liver damage. Typical liver damage drugs include acetaminophen (APAP), isoniazid, and antineoplastic drugs. The following sections mainly introduce the related studies on the protective effects of APG on DILI.
4.2.1. APAP
APAP is a commonly used drug that is often used to treat pain and fever, and overdose can cause severe liver damage [38]. The pathogenesis of APAP-induced liver injury (AILI) is related to the oxidized product of APAP. N-acetyl-p-benzoquinone imine (NAPQI), a toxic intermediate, is correlated with GSH. An overdose of APAP will lead NAPQI to deplete GSH, trigger oxidative stress, and result in apoptosis and liver injury. Fortunately, some studies have shown that APG can protect against AILI through different mechanisms. Indeed, Yang et al. found that APG could protect the liver by enhancing GSH reductase activity and reducing the content of GSH and malondialdehyde [39]. Zhao et al. showed that APG promotes autophagy and improves inflammatory responses and oxidative stress to prevent AILI [40,41].
4.2.2. Chemotherapeutic agents
Methotrexate (MTX) is a folate antagonist that can be used to treat cancer and autoimmune diseases, such as rheumatoid arthritis and Crohn’s disease, in the clinic [42]. However, MTX contributes to oxidative stress, resulting in apoptosis, tissue injury, and inflammation, ultimately causing hepatotoxicity [43]. Pretreatment with APG has been shown to reduce the toxicity of MTX to the liver via enhancing the antioxidant defense ability and attenuating caspase-3, C-reaction protein, granulocyte colony stimulating factor, and inducible nitric oxide synthase expression [44]. Cyclophosphamide (CP) is an alkylating chemotherapeutic agent that is commonly used to treat cancer, minimal change disease in children, and autoimmune disorders, and is also used in the transplantation of blood and marrow [45]. CP is known to restrain hepatic nuclear factor erythroid 2/heme oxygenase1 (Nrf2/HO-1) and NADH quinone dehydrogenase-1 (NQO-1) signaling, resulting in liver injury. APG reduces hepatic Nrf2/HO-1 signaling by upregulating Nrf2, NQO-1, and HO-1, which enhance antioxidant enzymes [46]. Sorafenib, an FDA-approved chemotherapeutic drug, has been shown to generate oxidative stress and liver injury via the activation of CYP34A, which leads to the production of reactive oxygen species (ROS). However, APG could protect against this liver injury of CYP34A by enhancing antioxidation and inhibiting the production of ROS [47]. The above-mentioned studies infer that although APG could bring the hepatoprotective activity into play through different mechanisms, the core and final link of these mechanisms is attenuating oxidative stress, apoptosis, and inflammation.
4.3. Alcohol-induced liver injury
Long-term excessive consumption of alcohol constantly contributes to alcoholic liver disease (ALD) [48]. ALD is a form of hepatitis ranging from asymptomatic fatty liver to fibrosis, cirrhosis, and alcoholic hepatitis [49]. Zhao et al. suggested that APG was important in enhancing hepatic function via decreasing the alanine aminotransferase activity, serum dyslipidemia such as LDL cholesterol and total cholesterol (TC) levels, lipid peroxidation, and oxidative stress capacity while enhancing superoxide dismutase (SOD) and GSH peroxidase activities [50]. Wang et al. showed that APG might protect against alcohol-induced liver injury via regulating hepatic cytochrome P450 2E1-mediated oxidative stress and peroxisome proliferator-activated receptor alpha (PPARα)-mediated lipogenic gene expression [51].
4.4. Other factors
Bacterial infection commonly induces liver injury, depending on the invading endotoxin or exotoxin. Lipopolysaccharides (LPSs) are the endotoxins of Gram-negative bacteria [52]. APG reduces oxidative stress and inflammatory events, and it has a protective effect on LPS-induced acute liver injury [53]. Zhou et al. showed that APG can protect against d-Galactosamine/LPS-induced hepatocellular injury by increasing Nrf-2 nuclear translocation, which increases the levels of SOD, CAT, and PPARγ protein expression, thus inhibiting the inflammatory reaction [54]. Ischemia/reperfusion (I/R) injury is common in liver transplantation and hepatectomies [55]. APG may have protective effects on hepatic I/R injury in the Fas/FasL-mediated pathway of apoptosis [56]. Additionally, the suppression of inflammation and oxidative stress has important effects in hepatic I/R injury with APG [57] (Table 1).
Table 1.
Protective effects of APG on liver injuries.
| Factors | Mechanisms | References | |
|---|---|---|---|
| Chemical pollutant | DEHP | Activate GPX4 and suppress intracellular iron accumulation | [31] |
| NiONPs | Alleviate the NiONP-induced deleterious effects on all the studied parameters in rats. | [33] | |
| CCl4 | Antioxidant properties, scavenge ROS | [36] | |
| Drug | APAP | Increase the hepatic GR activity and reduce GSH content, and decrease the hepatic malondialdehyde content | [39] |
| Regulate the sirtuin 1-p53 axis, promote autophagy and ameliorate inflammatory responses and oxidative stress | [40,41] | ||
| MTX | Restore the antioxidant defenses system and downregulation in caspase-3, CRP, G-CSF and iNOS expressions | [44] | |
| CP | Upregulate hepatic Nrf2/HO-1 signaling, and upregulate Nrf2, NQO-1, and HO-1 | [46] | |
| Sorafenib | Enhance anti-oxidant properties and inhibite ROS production | [47] | |
| Alcohol | Decrease the alanine aminotransferase activity, serum dyslipidemia such as LDL cholesterol and TC levels in serum, lipid peroxidation and oxidative stress, and enhancing SOD and GSH-Px activities | [50] | |
| Regulate hepatic CYP2E1-mediated oxidative stress and PPARa-mediated lipogenic gene expression | [51] | ||
| Other factors | LPS | Increase the SOD and CAT levels and PPARγ protein expression | [54] |
| Hepatic I/R | Decrease Fas receptor levels and inhibite apoptosis | [57] | |
Acetaminophen (APAP); Chloramphenical acetyltransferase (CAT); Cyclophosphamide (CP); C-reactive protein (CRP); Di(2-ethylhexyl) phthalate (DEHP); Granulocyte colony stimulating factor (G-CSF); Glutathione peroxidase 4 (GPX4); Glutathione reductase (GR); Glutathione (GSH); Glutathione peroxidase (GSH-Px); Human recombinant-1 (HO-1); Inducible nitric oxide synthase (iNOS); Ischemia/reperfusion (I/R); Low density lipids (LDL); Lipopolysaccharide (LPS); Methotrexate (MTX); Nanoparticles (NiONPs); NAD(P)H quinone oxidoreductase-1 (NQO-1); Nuclear factor erythroid 2 (Nrf2); Peroxisome proliferator-activated receptor (PPARa); Reactive oxygen species (ROS); Superoxide dismutase (SOD); Total cholesterol (TC).
5. Protective effects of APG on hepatitis, liver fibrosis, and cirrhosis
5.1. Hepatic steatosis
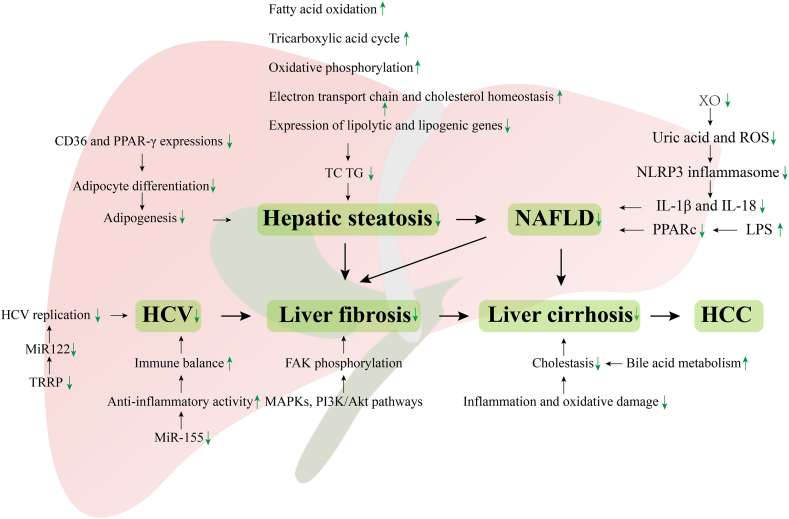
Increasing attention has been paid to extracts from natural products for the treatment of liver lipid accumulation diseases, which has provided new insights into traditional treatment methods. Liu et al. demonstrated that APG decreased the palmitic acid-induced elevation of TC, triglyceride (TG), and intracellular lipid levels [58]. Recent studies have shown that low-dose APG can ameliorate high-fat-diet (HFD)-induced obesity by accelerating lipid catabolism, thermogenesis, and browning [59]. The effects of APG on the improvement of hepatic steatosis and hepatomegaly were partly on account of the upregulation of genes responsible for regulating fatty acid oxidation, the tricarboxylic acid cycle, oxidative phosphorylation, the electron transport chain, cholesterol homeostasis, downregulation of lipids, and lipid-derived gene expression, as well as the reduction of enzymes responsible for the synthesis of TG and hepatic cholesterol ester [60].
Furthermore, autophagy, which is closely related to lipid droplet metabolism, is one of the breakdown pathways of lipids. Studies have shown that APG can induce autophagy to stimulate the degradation of autophagic lipids [61], can suppress the expression of PPAR-γ and CD36, and can inhibit adipocyte differentiation [62]. Another study showed that APG could attenuate HFD-induced hypercholesterolemia by suppressing the biosynthesis of cholesterol [63]. APG can fully interact with the intestine and become a potential substrate there due to its low bioavailability. Qiao et al. found that APG can obviously regulate gut microbiota and restore the gut barrier by reducing metabolic endotoxemia [64].
5.2. Nonalcoholic fatty liver disease (NAFLD)
NAFLD, a leading cause of morbidity and mortality worldwide, is a benign lesion that can develop into more serious liver damage through a process that is partly mediated by redox balance [65]. NAFLD can cause a variety of pathological consequences caused by changes in lipids, glucose, and lipoproteins, such as type II diabetes, liver fibrosis, and even liver cancer. Research has suggested that dietary interventions aimed at inducing weight loss may have a protective effect on NAFLD because they can reduce oxidative stress and lipid accumulation in the liver, thus preventing the disease from progressing to a more severe condition [66].
The beneficial effects of APG against NAFLD may be partly due to its inhibition of NLRP3 inflammasome assembly, which is activated by downregulating xanthine oxidase (XO) and inhibiting uric acid and ROS production. These effects further decrease the excessive production of pro-inflammatory cytokines IL-1β and IL-18, thus preventing hepatitis virus and lipid accumulation in NAFLD [67]. Recent studies have shown that lipid accumulation and inflammation in the liver were reduced in NAFLD-induced mice treated with APG. Meanwhile, APG restrained the NLRP3/NF-κB signaling pathway stimulated by LPS [58].
Feng et al. found that APG inhibited the activation of PPARc via Nrf2 activation to attenuate HFD-induced NAFLD [68,69]. Evidence has also shown that inhibiting autophagy leads to an acceleration of lipid accumulation and NAFLD pathogenesis [70], while the decrease in autophagic function is correlated with the progression of steatosis to steatohepatitis. One previous study showed that APG could degrade autophagic lipids via restoring blocked autophagic flow [71].
5.3. Viral hepatitis
Viral hepatitis, particularly the Hepatitis C virus (HCV), is the main pathogenesis of chronic hepatitis, cirrhosis, and HCC. Although rapid progress has been made in recent years in the development of direct-acting antiviral drugs to treat HCV infection, there is still a need to develop more affordable antiviral drugs. Fortunately, studies have shown that APG could inhibit HCV replication without affecting cell viability [72], which may be explained by the decrease in mature miR122 levels inhibiting the phosphorylation of trans-activating response RNA-binding protein (TRRP), a component of miRNA complexes generated by impaired mitogen-activated protein kinase [73,74]. Notably, APG has powerful anti-inflammatory properties, as it can reduce the expression of miR-155 by LPSs, thus restoring immune balance [75]. APG can also decrease pro-inflammatory cytokine and mediator levels and increase anti-inflammatory cytokine levels, thereby alleviating hepatitis [[76], [77], [78]]. Oxidative stress is closely related to inflammation, and many ROS or reactive nitrogen species can enhance the expression of pro-inflammatory genes by stimulating the intracellular signal cascade [79]. Studies have demonstrated that APG has protective effects against oxidative stress, which may be related to its anti-inflammatory properties [36]. Furthermore, APG regulates the activation of NLRP3 inflammatory corpuscles and the release of inflammatory cytokines IL-1β and IL-18 [67]. Therefore, APG may be an effective alternative therapy for HCV [80].
5.4. L iver fibrosis and cirrhosis
HCV, hepatitis B virus, ALD, and NAFLD are the main causative factors of cirrhosis [[81], [82], [83], [84]]. Fibrosis is the precursor of cirrhosis and hepatocarcinoma, while cirrhosis is the final pathological outcome of many chronic liver diseases [85]. However, there remains no effective treatment for these diseases. Many studies have shown that APG could be used to treat liver fibrosis by inhibiting the activation of hematopoietic stem cells, thus promoting the accumulation of ECM and the secretion of α-SMA, collagen 1, and other fibrotic factors [[86], [87], [88]]. APG has also been shown to improve CCl4-induced liver fibrosis through phosphorylation of the MAPK, PI3K/Akt, HIF-1, ROS, and eNOS pathways, and it is expected to become a natural product with anti-liver fibrosis activity [89].
Cholestasis is another important cause of liver fibrosis and cirrhosis [90]. Studies have shown that APG could improve dihydroxyphenylalanine decarboxylase-induced cholestasis by alleviating inflammation and oxidative damage and improving bile acid metabolism, suggesting that APG has potential application in the treatment of cholestasis (Fig. 2).
Fig. 2.
Protective effects of APG on hepatic steatosis, NAFLD, HCV, liver fibrosis, and cirrhosis. Hepatic steatosis can develop into cirrhosis, which ultimately leads to HCC. HCV can also progress to liver fibrosis and cirrhosis, eventually leading to HCC. APG could be used to treat these liver diseases through its anti-inflammation and anti-oxidation properties, as well as its regulation of lipid and bile acid metabolism and inhibition of HCV replication. HCC: Hepatocellular carcinoma, HCV: Hepatitis C virus, NAFLD: Nonalcoholic fatty liver disease, PPARα: Proliferator-activated receptor alpha, ROS: Reactive oxygen species, TC: Total cholesterol, TG: Triglyceride, TRRP: Trans-activating response RNA-binding protein, XO: Xanthine oxidase.
6. Protective effects of APG on liver cancer
HCC is the most common type of liver cancer worldwide, and its morbidity and mortality are increasing annually, threatening human health [91]. Although the development of chemotherapeutic drugs has played an important role in the treatment of liver cancer, drug resistance still hinders its therapeutic effect. In line with this, the role of plant-derived components against drug resistance of liver cancer has recently gained attention, with the study of the plant flavonoid APG being particularly notable.
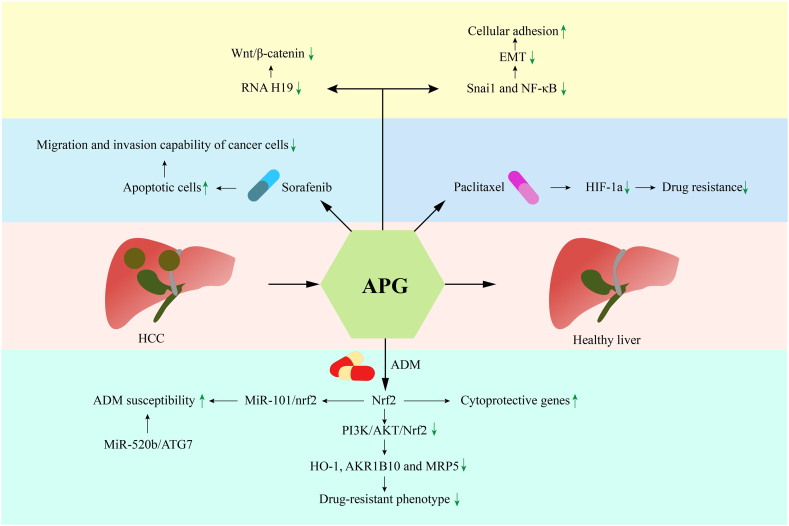
Doxorubicin, also called Adriamycin (ADM), is a widely applied effective anti-cancer drug, although HCC shows resistance to ADM [92]. In the process of ADM acting on hepatoma cells, nrf2 regulates the expression of cytoprotective genes by binding with antioxidant-response elements in the corresponding gene promoters. However, overexpression of nrf2 can lead to ADM resistance in hepatoma cells. The combination of APG and ADM appears to represent a way to improve efficacy against ADM-resistant cells. Indeed, Gao et al. showed that APG could reverse the drug-resistant phenotype by inhibiting the PI3K/AKT/Nrf2 signaling pathway and downregulating HO-1, AKR1B10, and MRP5 [93]. Recently, Gao et al. also showed that APG increased susceptibility to ADM by regulating the miR-101/nrf2-related apoptotic pathway [94]. The results of both studies suggested that combined application of APG and ADM may increase the chemotherapeutic reaction of ADM-resistant patients, although further clinical experiments are needed to verify the feasibility. Coincidentally, another study showed that APG sensitizes ADM-resistant cells to the miR-520b/ATG7 signaling pathway, which supports APG as a potential HCC chemosensitizer [95].
Sorafenib is another common chemotherapeutic agent used for HCC treatment [96]. However, sorafenib has a cytotoxic effect on healthy cells, and patients can develop sorafenib resistance over a short period of time, which reduces the success rate of the treatment and limits the therapeutic application [97]. A previous study showed that combination of sorafenib and APG could significantly reduce cell viability, induce apoptosis, and reduce the migration and invasion abilities of cancer cells [98]. APG is a natural hypoxia-inducible factor 1a (HIF-1a) inhibitor, which can also inhibit the expression of HIF-1a in various ways and reverse hypoxia-induced drug resistance. For example, paclitaxel combined with APG can be used to overcome the hypoxia-induced drug resistance of cancer cells to paclitaxel. This significantly enhanced the anticancer activity of paclitaxel.
Moreover, APG could induce G1 block in HepG2. APG showed high cytotoxicity in HepG2, SMMC-7721, and Huh-7, possibly through activation of the p38 MAPK-p21 signaling pathway [99].
Qin et al. found that APG can reduce the expression of Snai1 and NF-κB, reverse the epithelial–mesenchymal transition (EMT), increase cell adhesion, regulate actin polymerization and cell migration, and inhibit the invasion and migration of HCC cells [100]. One study suggested that APG-induced apoptosis of HepG2 cells may be mediated by a H2O2-dependent pathway by reducing antioxidant defenses. Moreover, APG has been shown to inhibit cell proliferation and induce autophagy by inhibiting the PI3K/Akt/mTOR pathway. Therefore, the combination of APG and autophagy inhibitors may represent an effective chemotherapeutic method for HCC [101].
In addition to APG combinational treatment, there are also separate studies on the therapeutic effects of APG on liver cancer. Long noncoding RNA H19 mediates tumorigenesis and cancer progression, and it has been shown to be frequently elevated in HCC [102]. A previous study showed that APG downregulated H19 expression to inhibit the growth of liver cancer cells and induce inactivation of the canonical Wnt/β-catenin signaling pathway [36]. Recent studies have shown that APG nanoparticles can be used to increase the solubility and bioavailability of APG, thus improving the effect of APG on HCC [103]. Taken together, these findings indicate that APG holds promise for developing new therapies for liver cancer (Fig. 3).
Fig. 3.
Protective effects of APG on HCC. The combination of APG and ADM reverses the drug-resistant phenotype through various pathways. The combination of sorafenib and APG can reduce cell viability, induce apoptosis, and reduce the ability of cancer cells to migrate and invade. The combination of APG and paclitaxel inhibits the expression of HIF-1a and reverses the drug resistance induced by hypoxia. As a signal, APG can reduce the expression of Snai1 and NF-κB, reverse the elevation of EMT, increase cell adhesion, and inhibit the invasion and migration of HCC cells. APG downregulates H19 expression to inhibit the growth of liver cancer cells, inducing inactivation of the canonical Wnt/β-catenin signaling pathway. ADM: Adriamycin, APG: Apigenin, EMT: Epithelial-mesenchymal transition, HCC: Hepatocellular carcinoma.
7. Health improving effects of APG by altering the intestinal microbiota
The intestinal microbiota is a symbiont that contributes to the health of the host and is a biological barrier that contributes to nutrient absorption, immune regulation, and energy metabolism [104]. Patients with cancer who present with intestinal microbiota disorders may gain tumor-ameliorating benefits through modulation of the gut flora [105]. Although APG can be degraded by intestinal microbiota, its metabolites can in turn regulate the structure and function of the intestinal microbiota. To date, these regulatory effects on microbiota have not been fully determined [106]. Bian’s study demonstrated that the effect of APG on intestinal microbiota composition can inhibit tumors. However, the exact mechanism by which APG affects the intestinal microbiota and exerts its anti-tumor effects has not yet been determined [107]. Additionally, APG affects the growth and gene expression of Enterococcus by upregulating genes involved in DNA repair, stress responses, cell wall synthesis, and protein folding. APG has also been shown to promote the growth and diversity of bacteria, reduce the ratio of Firmicutes to Bacteroidetes, and motivate the production of short-chain fatty acids, including butyrate, which is related to good health [108].
8. Therapeutic application
Although APG shows an excellent treatment effect in liver cancer, its low oral bioavailability owing to its low lipid and water solubility limits its clinical development [109]. Fortunately, nanotechnology can improve APG bioavailability. A novel carbon nano-powder (CNP) drug carrier was developed to improve the oral bioavailability of APG. The CNP-APG system improved the APG dissolution, heightened the APG area under the curve by 1.83 times, raised the peak, and shortened the time to peak compared to pure APG.
Micelles are assembled by water-soluble amphiphilic surfactants and can dissolve hydrophobic drug molecules that dissolve poorly in water, playing a key role in drug delivery [110]. Zhang et al. developed a novel mixed micelle system and prepared APG-loaded mixed micelles (APG-M) using an ethanol thin-film hydration method in which the APG-M showed higher oral bioavailability than free APG [111]. Moreover, Zhai et al. fabricated APG-loaded micelles by a thin-film dispersion method and showed that compared to free drug, the micelles increased the absorption of APG in the intestinal tract [112]. Ganguly et al. developed galactose-tailored PLGA NPs loaded with APG (APG-GAL-NPs) for the liver-targeted therapy of HCC [113]. Compared to APG and APG-NPs, APG-GAL-NPs had higher cytotoxicity and apoptotic potential against HepG2 and could be used to treat HCC efficiently. These findings highlight micelles as being an excellent nanocarrier for APG.
Nanoparticles of metals such as zinc, silver, and silica are called metal or metallic NPs, and they are suitable for combination with APG due to their various health-related properties [103]. Zarei et al. used APG as a reducing bioactive compound to synthesize silver nanoparticles, thus enhancing anticancer, antibacterial, and antioxidant properties [114]. Another study showed that the release of prepared APG mesoporous silica nanoparticles was higher than that of raw APG [115]. These studies provide sufficient evidence that nanotechnology may be a novel way to enhance the oral bioavailability of APG. In the future, the establishment of effective therapeutic drugs requires more clinical trials to determine the mode of administration and the exact dose.
APG plays a role in inflammation by inhibiting pro-inflammatory cytokines through multiple intracellular pathways in macrophages and by acting as modulators of pro-inflammatory genes, which are potential inhibitors of COX-2 [116]. Furthermore, APG inhibits oxidative stress within cells while also inhibiting the activities of DNA-binding proteins such as DNA polymerase, cAMP-response element binding proteins, DNA topoisomerase, and histone deacetylases. Therefore, compared with other treatments for liver injury and disease, APG may be more targeted and have fewer side effects than chemotherapy.
9. Toxicology
APG has lower intrinsic toxicity to normal cells and cancer cells than other related flavonoids [117,118]. Indeed, no reports of APG toxicity have been reported to date, which may be related to the low solubility and low biological metabolism of APG [119]. Compared to MCF-7 cells, MDAMB-231 cells were found to be more sensitive to the genotoxic effects of APG in breast cancer. APG induced significant DNA damage in MDAMB-231 cells [120]. The cytotoxicity of APG in rat hepatoma and glioma cells demonstrated that C8 prenylation of a flavone enhanced its cytotoxicity by inducing the apoptosis and death of H4IIE cells without affecting antioxidation [121]. These cytotoxic effects of APG were aimed to induce apoptosis in cancer cells. In general, APG has low toxicity and high safety; thus, it can be presumed that APG is safe for normal cells in terms of cytogenotoxicity.
10. Conclusion
Numerous studies have suggested that APG may hold considerable value for various LIADs. Its potential therapeutic effects may involve various mechanisms, including anti-inflammation, anti-proliferation, anti-bacteria, anti-oxidation, and anti-cancer properties. Considering the variety of interactive APG pathways, it is also worth expanding its application range and exploring the corresponding mechanisms.
Acknowledgments
This work was funded by Yue Di Element General Curriculum Project of Shaoxing Higher Education Institutions (No. 20225306) and the Science and Technology Planning Project of Shaoxing City (No. 2020A13061). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Contributor Information
Guiying Xing, Email: xingguiying@usx.edu.cn.
Zheng Liu, Email: liuzheng1202@usx.edu.cn.
References
- 1.Hernández-Aquino E., Muriel P. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J. Gastroenterol. 2018;24(16):1679–1707. doi: 10.3748/wjg.v24.i16.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin E., Jeong S. Natural history, clinical manifestations, and pathogenesis of hepatitis A. CSH Perspect. Med. 2018;8(9):a31708–a31720. doi: 10.1101/cshperspect.a031708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louvet A., Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment, Nature reviews. Gastroenterol. Hepatol. 2015;12(4):231–242. doi: 10.1038/nrgastro.2015.35. [DOI] [PubMed] [Google Scholar]
- 4.Czech B., Dettmer K., Valletta D., Saugspier M., Koch A., Stevens A.P., Thasler W.E., Muller M., Oefner P.J., Bosserhoff A.K., Hellerbrand C. Expression and function of methylthioadenosine phosphorylase in chronic liver disease. PLoS One. 2013;8(12):e80703–e80717. doi: 10.1371/journal.pone.0080703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehal W.Z., Schuppan D. Antifibrotic therapies in the liver. Semin. Liver Dis. 2015;35(2):184–198. doi: 10.1055/s-0035-1550055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuppan D. Liver fibrosis: common mechanisms and antifibrotic therapies. Clin. Res. Hepatol. Gas. 2015;39:S51–S59. doi: 10.1016/j.clinre.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Poilil Surendran S., George Thomas R., Moon M.J., Jeong Y.Y. Nanoparticles for the treatment of liver fibrosis. Int. J. Nanomed. 2017;12:6997–7006. doi: 10.2147/IJN.S145951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlegel A., van Reeven M., Croome K., Parente A., Dolcet A., Widmer J., Meurisse N., De Carlis R., Hessheimer A., Jochmans I., Mueller M., van Leeuwen O.B., Nair A., Tomiyama K., Sherif A., Elsharif M., Kron P., van der Helm D., Borja-Cacho D., Bohorquez H., Germanova D., Dondossola D., Olivieri T., Camagni S., Gorgen A., Patrono D., Cescon M., Croome S., Panconesi R., Carvalho M.F., Ravaioli M., Caicedo J.C., Loss G., Lucidi V., Sapisochin G., Romagnoli R., Jassem W., Colledan M., De Carlis L., Rossi G., Di Benedetto F., Miller C.M., van Hoek B., Attia M., Lodge P., Hernandez-Alejandro R., Detry O., Quintini C., Oniscu G.C., Fondevila C., Malagó M., Pirenne J., Ijzermans J., Porte R.J., Dutkowski P., Taner C.B., Heaton N., Clavien P.A., Polak W.G., Muiesan P. A multicentre outcome analysis to define global benchmarks for donation after circulatory death liver transplantation. J. Hepatol. 2022;76(2):371–382. doi: 10.1016/j.jhep.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Cordell G.A., Colvard M.D. Natural products and traditional medicine: turning on a paradigm. J. Nat. Prod. 2012;75(3):514–525. doi: 10.1021/np200803m. [DOI] [PubMed] [Google Scholar]
- 10.Madrigal-Santillán E. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014;20(40):14787–14804. doi: 10.3748/wjg.v20.i40.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulsen N.B., Lambert M.N.T., Jeppesen P.B. The effect of plant derived bioactive compounds on inflammation: a systematic review and meta‐analysis. Mol. Nutr. Food Res. 2020;64(18):2000473–2000486. doi: 10.1002/mnfr.202000473. [DOI] [PubMed] [Google Scholar]
- 12.Shin S., Moon S.Y., Kim W., Paek S., Park H.H., Lee C.S. Structure-based classification and anti-cancer effects of plant metabolites. Int. J. Mol. Sci. 2018;19(9):2651–2683. doi: 10.3390/ijms19092651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa A.C.D.F., de Sousa L.M., Dos Santos Alves J.M., Goes P., Pereira K.M.A., Alves A.P.N.N., Vale M.L., Gondim D.V. Anti-inflammatory and hepatoprotective effects of quercetin in an experimental model of rheumatoid arthritis. Inflammation. 2021;44(5):2033–2043. doi: 10.1007/s10753-021-01479-y. [DOI] [PubMed] [Google Scholar]
- 14.Yang H., Yang T., Heng C., Zhou Y., Jiang Z., Qian X., Du L., Mao S., Yin X., Lu Q. Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice. Phytother Res. 2019;33(12):3140–3152. doi: 10.1002/ptr.6486. [DOI] [PubMed] [Google Scholar]
- 15.Guan X., Gao M., Xu H., Zhang C., Liu H., Lv L., Deng S., Gao D., Tian Y. Quercetin-loaded poly (lactic-co-glycolic acid)-d-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles for the targeted treatment of liver cancer. Drug Deliv. 2016;23(9):3307–3318. doi: 10.1080/10717544.2016.1176087. [DOI] [PubMed] [Google Scholar]
- 16.Kowalczyk A., Bodalska A., Miranowicz M., Karłowicz-Bodalska K. Insights into novel anticancer applications for apigenin. Adv. Clin. Exp. Med. 2017;26(7):1143–1146. doi: 10.17219/acem/41978. [DOI] [PubMed] [Google Scholar]
- 17.Salehi B., Venditti A., Sharifi-Rad M., Kręgiel D., Sharifi-Rad J., Durazzo A., Lucarini M., Santini A., Souto E.B., Novellino E., Antolak H., Azzini E., Setzer W.N., Martins N. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019;20(6):118814–118855. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang D., Chen K., Huang L., Li J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expet Opin. Drug Metabol. Toxicol. 2017;13(3):323–330. doi: 10.1080/17425255.2017.1251903. [DOI] [PubMed] [Google Scholar]
- 19.Chao S., Huang S., Hu D., Lin H. Subtoxic levels of apigenin inhibit expression and secretion of VEGF by uveal melanoma cells via suppression of ERK1/2 and PI3K/Akt pathways. Evid.-Based Compl. Alt. 2013;2013:1–9. doi: 10.1155/2013/817674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pourzand C., Albieri-Borges A., Raczek N.N. Shedding a new light on skin aging, iron- and redox-homeostasis and emerging natural antioxidants. Antioxidants. 2022;11(3):471–506. doi: 10.3390/antiox11030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alshehri S.M., Shakeel F., Ibrahim M.A., Elzayat E.M., Altamimi M., Mohsin K., Almeanazel O.T., Alkholief M., Alshetaili A., Alsulays B., Alanazi F.K., Alsarra I.A. Dissolution and bioavailability improvement of bioactive apigenin using solid dispersions prepared by different techniques. Saudi Pharmaceut. J.: SPJ. 2019;27(2):264–273. doi: 10.1016/j.jsps.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borges G., Fong R.Y., Ensunsa J.L., Kimball J., Medici V., Ottaviani J.I., Crozier A. Absorption, distribution, metabolism and excretion of apigenin and its glycosides in healthy male adults. Free Radical Bio Med. 2022;185:90–96. doi: 10.1016/j.freeradbiomed.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Chen T., Li L.P., Lu X.Y., Jiang H.D., Zeng S. Absorption and excretion of luteolin and apigenin in rats after oral administration of Chrysanthemum morifolium extract. J. Agric. Food Chem. 2007;55(2):273–277. doi: 10.1021/jf062088r. [DOI] [PubMed] [Google Scholar]
- 24.He X., Song Z., Jiang C., Zhang C. Absorption properties of luteolin and apigenin in Genkwa Flos Using In situ single-pass intestinal perfusion system in the rat. Am. J. Chin. Med. 2017;45(8):1745–1759. doi: 10.1142/S0192415X1750094X. [DOI] [PubMed] [Google Scholar]
- 25.Sharma A., Ghani A., Sak K., Tuli H.S., Sharma A.K., Setzer W.N., Sharma S., Das A.K. Probing into therapeutic anti-cancer potential of apigenin: recent trends and future directions. Recent Pat. Inflamm. Allergy Drug Discov. 2019;13(2):124–133. doi: 10.2174/1872213X13666190816160240. [DOI] [PubMed] [Google Scholar]
- 26.Cai H., Boocock D.J., Steward W.P., Gescher A.J. Tissue distribution in mice and metabolism in murine and human liver of apigenin and tricin, flavones with putative cancer chemopreventive properties. Cancer Chemoth. Pharm. 2007;60(2):257–266. doi: 10.1007/s00280-006-0368-5. [DOI] [PubMed] [Google Scholar]
- 27.Wang S.W.J., Kulkarni K.H., Tang L., Wang J.R., Yin T., Daidoji T., Yokota H., Hu M. Disposition of flavonoids via enteric recycling: UDP-glucuronosyltransferase (UGT) 1As deficiency in Gunn rats is compensated by increases in UGT2Bs activities. J. Pharmacol. Exp. Ther. 2009;329(3):1023–1031. doi: 10.1124/jpet.108.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikulic-Petkovsek M., Ivancic A., Gacnik S., Veberic R., Hudina M., Marinovic S., Molitor C., Halbwirth H. Biochemical characterization of black and green mutant elderberry during fruit ripening. Plants (Basel, Switzerland) 2023;12(3):504–519. doi: 10.3390/plants12030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Q., Zhu J., Zhao S., Hou Y., Li F., Tai Y., Wan X., Wei C. Camellia sinensis Transcriptome profiling using single-molecule direct RNA sequencing approach for in-depth understanding of genes in secondary metabolism pathways of. Front. Plant Sci. 2017;8:1205–1215. doi: 10.3389/fpls.2017.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan W., Yang H., Zhang J., Chen Q. Association between co-exposure to phenols and phthalates mixture and infertility risk in women. Environ. Res. 2022:114244–114252. doi: 10.1016/j.envres.2022.114244. [DOI] [PubMed] [Google Scholar]
- 31.Han D., Yao Y., Chen L., Miao Z., Xu S. Apigenin ameliorates di(2-ethylhexyl) phthalate-induced ferroptosis: the activation of glutathione peroxidase 4 and suppression of iron intake. Food Chem. Toxicol. 2022;164:113089–113098. doi: 10.1016/j.fct.2022.113089. [DOI] [PubMed] [Google Scholar]
- 32.Sharifi E., Salimi A., Shams E., Noorbakhsh A., Amini M.K. Shape-dependent electron transfer kinetics and catalytic activity of NiO nanoparticles immobilized onto DNA modified electrode: fabrication of highly sensitive enzymeless glucose sensor. Biosens. Bioelectron. 2014;56:313–319. doi: 10.1016/j.bios.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Ali A.A., Mansour A.B., Attia S.A. The potential protective role of apigenin against oxidative damage induced by nickel oxide nanoparticles in liver and kidney of male Wistar rat, Rattus norvegicus. Environ. Sci. Pollut. R. 2021;28(22):27577–27592. doi: 10.1007/s11356-021-12632-3. [DOI] [PubMed] [Google Scholar]
- 34.Czekaj P., Król M., Limanówka Ł., Skubis-Sikora A., Kolanko E., Bogunia E., Hermyt M., Michalik M., Sikora B., Prusek A., Grajoszek A., Pająk J. Dynamics of acute liver injury in experimental models of hepatotoxicity in the context of their implementation in preclinical studies on stem cell therapy. Front. Biosci. (Landmark edition) 2022;27(8):237–251. doi: 10.31083/j.fbl2708237. [DOI] [PubMed] [Google Scholar]
- 35.Liu J., Fu Y., Zhang H., Wang J., Zhu J., Wang Y., Guo Y., Wang G., Xu T., Chu M., Wang F. The hepatoprotective effect of the probiotic Clostridium butyricum against carbon tetrachloride-induced acute liver damage in mice. Food Funct. 2017;8(11):4042–4052. doi: 10.1039/c7fo00355b. [DOI] [PubMed] [Google Scholar]
- 36.Pan X., Shao Y., Wang F., Cai Z., Liu S., Xi J., He R., Zhao Y., Zhuang R. Protective effect of apigenin magnesium complex on H2 O2-induced oxidative stress and inflammatory responses in rat hepatic stellate cells. Pharm. Biol. 2020;58(1):553–560. doi: 10.1080/13880209.2020.1772840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasmi B., Kleiner D.E. Liver histology: diagnostic and prognostic features. Clin. Liver Dis. 2020;24(1):61–74. doi: 10.1016/j.cld.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee W.M. Acetaminophen (APAP) hepatotoxicity—isn’t it time for APAP to go away? J. Hepatol. 2017;67(6):1324–1331. doi: 10.1016/j.jhep.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J., Wang X., Xue J., Gu Z., Xie M. Protective effect of apigenin on mouse acute liver injury induced by acetaminophen is associated with increment of hepatic glutathione reductase activity. Food Funct. 2013;4(6):939–943. doi: 10.1039/c3fo60071h. [DOI] [PubMed] [Google Scholar]
- 40.Mohamed W.R., Kotb A.S., Abd El Raouf O.M., Mohammad Fikry E. Apigenin alleviated acetaminophen‐induced hepatotoxicity in low protein‐fed rats: targeting oxidative stress, STAT3, and apoptosis signals. J. Biochem. Mol. Toxic. 2020;34(5) doi: 10.1002/jbt.22472. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L., Zhang J., Hu C., Wang T., Lu J., Wu C., Chen L., Jin M., Ji G., Cao Q., Jiang Y. Apigenin prevents acetaminophen-induced liver injury by activating the SIRT1 pathway. Front. Pharmacol. 2020;11:514–526. doi: 10.3389/fphar.2020.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan Z.A., Tripathi R., Mishra B. Methotrexate: a detailed review on drug delivery and clinical aspects. Expert. Opin. Drug Del. 2012;9(2):151–169. doi: 10.1517/17425247.2012.642362. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed Z.S.O., Hussein S., Ghandour R.A., Azouz A.A., El-Sakhawy M.A. Evaluation of the effect of methotrexate on the hippocampus, cerebellum, liver, and kidneys of adult male albino rat. Acta Histochem. 2021;123(2):151682–151694. doi: 10.1016/j.acthis.2021.151682. [DOI] [PubMed] [Google Scholar]
- 44.Sahindokuyucu-Kocasari F., Akyol Y., Ozmen O., Erdemli-Kose S.B., Garli S. Apigenin alleviates methotrexate-induced liver and kidney injury in mice. Hum. Exp. Toxicol. 2021;40(10):1721–1731. doi: 10.1177/09603271211009964. [DOI] [PubMed] [Google Scholar]
- 45.Emadi A., Jones R.J., Brodsky R.A. Cyclophosphamide and cancer: golden anniversary. Nat. Rev. Clin. Oncol. 2009;6(11):638–647. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- 46.Al-Amarat W., Abukhalil M.H., Alruhaimi R.S., Alqhtani H.A., Aldawood N., Alfwuaires M.A., Althunibat O.Y., Aladaileh S.H., Algefare A.I., Alanezi A.A., AbouEl-ezz A.M., Ahmeda A.F., Mahmoud A.M. Upregulation of Nrf2/HO-1 signaling and attenuation of oxidative stress, inflammation, and cell death mediate the protective effect of apigenin against cyclophosphamide hepatotoxicity. Metabolites. 2022;12(7):648–661. doi: 10.3390/metabo12070648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh D., Khan M.A., Akhtar K., Arjmand F., Siddique H.R. Apigenin alleviates cancer drug Sorafenib induced multiple toxic effects in Swiss albino mice via anti-oxidative stress. Toxicol. Appl. Pharm. 2022;447:116072–116084. doi: 10.1016/j.taap.2022.116072. [DOI] [PubMed] [Google Scholar]
- 48.Szabo G. Gut–liver Axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L., Zhu Y., Hou X., Yang L., Chu H. The role of gut bacteria and fungi in alcohol-associated liver disease. Front. Med. 2022;9:840752–840762. doi: 10.3389/fmed.2022.840752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao L., Zhang N., Yang D., Yang M., Guo X., He J., Wu W., Ji B., Cheng Q., Zhou F. Protective effects of five structurally diverse flavonoid subgroups against chronic alcohol-induced hepatic damage in a mouse model. Nutrients. 2018;10(11):1754–1767. doi: 10.3390/nu10111754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang F., Liu J., Zhou R., Zhao X., Liu M., Ye H., Xie M. Apigenin protects against alcohol-induced liver injury in mice by regulating hepatic CYP2E1-mediated oxidative stress and PPARα-mediated lipogenic gene expression. Chem. Biol. Interact. 2017;275:171–177. doi: 10.1016/j.cbi.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Szentirmai É., Massie A.R., Kapás L. Lipoteichoic acid, a cell wall component of Gram-positive bacteria, induces sleep and fever and suppresses feeding. Brain Behav. Immun. 2021;92:184–192. doi: 10.1016/j.bbi.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berköz M., Ünal S., Karayakar F., Yunusoğlu O., Özkan-Yılmaz F., Özlüer-Hunt A., Aslan A. Prophylactic effect of myricetin and apigenin against lipopolysaccharide-induced acute liver injury. Mol. Biol. Rep. 2021;48(9):6363–6373. doi: 10.1007/s11033-021-06637-x. [DOI] [PubMed] [Google Scholar]
- 54.Zhou R., Zhao Y., Fan K., Xie M. Protective effect of apigenin on d-galactosamine/LPS-induced hepatocellular injury by increment of Nrf-2 nucleus translocation. N. Schmied. Arch. Pharmacol. 2020;393(6):929–936. doi: 10.1007/s00210-019-01760-w. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z., Liu D., Yan Q., Liu F., Zhan M., Qi S., Fang Q., Yao L., Wang W., Zhang R., Du J., Chen L. Activated AXL protects against hepatic ischemia-reperfusion injury by upregulating SOCS-1 expression. Transplantation. 2022;106(7):1351–1364. doi: 10.1097/TP.0000000000004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsalkidou E.G., Tsaroucha A.K., Chatzaki E., Lambropoulou M., Papachristou F., Trypsianis G., Pitiakoudis M., Vaos G., Simopoulos C. The effects of apigenin on the expression of fas/FasL apoptotic pathway in warm liver ischemia-reperfusion injury in rats. BioMed Res. Int. 2014;2014:1–7. doi: 10.1155/2014/157216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsaroucha A., Tsiaousidou A., Ouzounidis N., Tsalkidou E., Lambropoulou M., Giakoustidis D., Chatzaki E., Simopoulos C. Intraperitoneal administration of apigenin in liver ischemia/reperfusion injury protective effects. Saudi J. Gastroentero. 2016;22(6):415–422. doi: 10.4103/1319-3767.195556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu J., Meng Z., Cheng B., Liu M., Tao S., Guan S. Apigenin reduces the excessive accumulation of lipids induced by palmitic acid via the AMPK signaling pathway in HepG2 cells. Exp. Ther. Med. 2019;18(4):2965–2971. doi: 10.3892/etm.2019.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y., Qu W. Dietary Apigenin promotes lipid catabolism, thermogenesis, and browning in adipose tissues of HFD-Fed mice. Food Chem. Toxicol. 2019;133:110780–110789. doi: 10.1016/j.fct.2019.110780. [DOI] [PubMed] [Google Scholar]
- 60.Jung U.J., Cho Y., Choi M. Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients. 2016;8(5):305–320. doi: 10.3390/nu8050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu J., Meng Z., Chen Y., Yu L., Gao B., Zheng Y., Guan S. Apigenin induced autophagy and stimulated autophagic lipid degradation. Food Funct. 2020;11(10):9208–9215. doi: 10.1039/d0fo00949k. [DOI] [PubMed] [Google Scholar]
- 62.Su T., Huang C., Yang C., Jiang T., Su J., Chen M., Fatima S., Gong R., Hu X., Bian Z., Liu Z., Kwan H.Y. Apigenin inhibits STAT3/CD36 signaling axis and reduces visceral obesity. Pharmacol. Res. 2020;152:104586–104596. doi: 10.1016/j.phrs.2019.104586. [DOI] [PubMed] [Google Scholar]
- 63.Wong T.Y., Tan Y.Q., Lin S., Leung L.K. Apigenin and luteolin display differential hypocholesterolemic mechanisms in mice fed a high-fat diet. Biomed. Pharmacother. 2017;96:1000–1007. doi: 10.1016/j.biopha.2017.11.131. [DOI] [PubMed] [Google Scholar]
- 64.Qiao Y., Zhang Z., Zhai Y., Yan X., Zhou W., Liu H., Guan L., Peng L. Apigenin alleviates obesity-associated metabolic syndrome by regulating the composition of the gut microbiome. Front. Microbiol. 2021;12:805827–805841. doi: 10.3389/fmicb.2021.805827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferro Y., Montalcini T., Mazza E., Foti D., Angotti E., Gliozzi M., Nucera S., Paone S., Bombardelli E., Aversa I., Musolino V., Mollace V., Pujia A. Randomized clinical trial: bergamot citrus and wild cardoon reduce liver steatosis and body weight in non-diabetic individuals aged over 50 years. Front. Endocrinol. 2020;11:494–503. doi: 10.3389/fendo.2020.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendes I.K.S., Matsuura C., Aguila M.B., Daleprane J.B., Martins M.A., Mury W.V., Brunini T.M.C. Weight loss enhances hepatic antioxidant status in a NAFLD model induced by high-fat diet. Appl. Physiol. Nutr. Metabol. 2018;43(1):23–29. doi: 10.1139/apnm-2017-0317. [DOI] [PubMed] [Google Scholar]
- 67.Lv Y., Gao X., Luo Y., Fan W., Shen T., Ding C., Yao M., Song S., Yan L. Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. J. Nutr. Biochem. 2019;71:110–121. doi: 10.1016/j.jnutbio.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 68.Feng X., Weng D., Zhou F., Owen Y.D., Qin H., Zhao J., Huang Y., Chen J., Fu H., Yang N. Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. EBioMedicine. 2016;9:61–76. doi: 10.1016/j.ebiom.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feng X., Yu W., Li X., Zhou F., Zhang W., Shen Q., Li J., Zhang C., Shen P. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem. Pharmacol. 2017;136:136–149. doi: 10.1016/j.bcp.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka S., Hikita H., Tatsumi T., Sakamori R., Nozaki Y., Sakane S., Shiode Y., Nakabori T., Saito Y., Hiramatsu N., Tabata K., Kawabata T., Hamasaki M., Eguchi H., Nagano H., Yoshimori T., Takehara T. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology. 2016;64(6):1994–2014. doi: 10.1002/hep.28820. [DOI] [PubMed] [Google Scholar]
- 71.Gonzalez-Rodriguez A., Mayoral R., Agra N., Valdecantos M.P., Pardo V., Miquilena-Colina M.E., Vargas-Castrillón J., Lo Iacono O., Corazzari M., Fimia G.M. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis. 2014;5(4):e1179–e1191. doi: 10.1038/cddis.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ginwala R., Bhavsar R., Chigbu D.I., Jain P., Khan Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants. 2019;8(2):35–62. doi: 10.3390/antiox8020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El-Tantawy W.H., Temraz A. Natural products for the management of the hepatitis C virus: a biochemical review. Arch. Physiol. Biochem. 2020;126(2):116–128. doi: 10.1080/13813455.2018.1498902. [DOI] [PubMed] [Google Scholar]
- 74.Shibata C., Ohno M., Otsuka M., Kishikawa T., Goto K., Muroyama R., Kato N., Yoshikawa T., Takata A., Koike K. The flavonoid apigenin inhibits hepatitis C virus replication by decreasing mature microRNA122 levels. Virology. 2014;462–463:42–48. doi: 10.1016/j.virol.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 75.Arango D., Diosa Toro M., Rojas Hernandez L.S., Cooperstone J.L., Schwartz S.J., Mo X., Jiang J., Schmittgen T.D., Doseff A.I. Dietary apigenin reduces LPS‐induced expression of miR‐155 restoring immune balance during inflammation. Mol. Nutr. Food Res. 2015;59(4):763–772. doi: 10.1002/mnfr.201400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berköz M., Yalın S., Özkan-Yılmaz F., Özlüer-Hunt A., Krośniak M., Francik R., Yunusoğlu O., Adıyaman A., Gezici H., Yiğit A., Ünal S., Volkan D., Yıldırım M. Protective effect of myricetin, apigenin, and hesperidin pretreatments on cyclophosphamide-induced immunosuppression. Immunopharm. Immun. 2021;43(3):353–369. doi: 10.1080/08923973.2021.1916525. [DOI] [PubMed] [Google Scholar]
- 77.Kumar K.S., Sabu V., Sindhu G., Rauf A.A., Helen A. Isolation, identification and characterization of apigenin from Justicia gendarussa and its anti-inflammatory activity. Int. Immunopharmacol. 2018;59:157–167. doi: 10.1016/j.intimp.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X., Wang G., Gurley E.C., Zhou H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS One. 2014;9(9):e107072–e107089. doi: 10.1371/journal.pone.0107072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biswas S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016;2016:1–9. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manvar D., Mishra M., Kumar S., Pandey V.N. Identification and evaluation of anti Hepatitis C virus phytochemicals from Eclipta alba. J. Ethnopharmacol. 2012;144(3):545–554. doi: 10.1016/j.jep.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chu C., Liaw Y. Hepatitis B virus-related cirrhosis: natural history and treatment. Semin. Liver Dis. 2006;26(2):142–152. doi: 10.1055/s-2006-939752. [DOI] [PubMed] [Google Scholar]
- 82.Conde I., Vinaixa C., Berenguer M. Cirrosis por hepatitis C. Estado actual. Med. Clínica. 2017;148(2):78–85. doi: 10.1016/j.medcli.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 83.Rivera Esteban J., Armandi A., Augustin S., Bugianesi E. Outcomes and potential surrogate markers for future clinical trials of non‐alcoholic steatohepatitis cirrhosis. Liver Int. 2021;41(9):1999–2008. doi: 10.1111/liv.15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stickel F., Datz C., Hampe J., Bataller R. Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver. 2017;11(2):173–188. doi: 10.5009/gnl16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou W. Pathogenesis of liver cirrhosis. World J. Gastroenterol. 2014;20(23):7312–7324. doi: 10.3748/wjg.v20.i23.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hicks D.F., Goossens N., Blas-Garcia A., Tsuchida T., Wooden B., Wallace M.C., Nieto N., Lade A., Redhead B., Cederbaum A.I., Dudley J.T., Fuchs B.C., Lee Y.A., Hoshida Y., Friedman S.L. Transcriptome-based repurposing of apigenin as a potential anti-fibrotic agent targeting hepatic stellate cells. Sci. Rep. 2017;7:42563–42570. doi: 10.1038/srep42563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malik S., Suchal K., Khan S.I., Bhatia J., Kishore K., Dinda A.K., Arya D.S. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-κB-TNF-α and TGF-β1-MAPK-fibronectin pathways. Am. J. Physiol.-Renal. 2017;313(2):F414–F422. doi: 10.1152/ajprenal.00393.2016. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J., Chao L., Liu X., Shi Y., Zhang C., Kong L., Li R. The potential application of strategic released apigenin from polymeric carrier in pulmonary fibrosis. Exp. Lung Res. 2017;43(9–10):359–369. doi: 10.1080/01902148.2017.1380086. [DOI] [PubMed] [Google Scholar]
- 89.Qiao M., Yang J., Zhu Y., Zhao Y., Hu J. Transcriptomics and proteomics analysis of system-level mechanisms in the liver of apigenin-treated fibrotic rats. Life Sci. 2020;248:117475–117486. doi: 10.1016/j.lfs.2020.117475. [DOI] [PubMed] [Google Scholar]
- 90.Hirschfield G.M., Heathcote E.J., Gershwin M.E. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139(5):1481–1496. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 91.Venook A.P., Papandreou C., Furuse J., Ladrón De Guevara L. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncol. 2010;15(S4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 92.Yang Y., He J., Chen J., Lin L., Liu Y., Zhou C., Su Y., Wei H. Dihydroartemisinin sensitizes mutant p53 (R248Q)-Expressing hepatocellular carcinoma cells to doxorubicin by inhibiting P-gp expression. BioMed Res. Int. 2019;2019:8207056–8207065. doi: 10.1155/2019/8207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao A.M., Ke Z.P., Wang J.N., Yang J.Y., Chen S.Y., Chen H. Apigenin sensitizes doxorubicin-resistant hepatocellular carcinoma BEL-7402/ADM cells to doxorubicin via inhibiting PI3K/Akt/Nrf2 pathway. Carcinogenesis. 2013;34(8):1806–1814. doi: 10.1093/carcin/bgt108. [DOI] [PubMed] [Google Scholar]
- 94.Gao A., Zhang X., Ke Z. Apigenin sensitizes BEL-7402/ADM cells to doxorubicin through inhibiting miR-101/Nrf2 pathway. Oncotarget. 2017;8(47):82085–82091. doi: 10.18632/oncotarget.18294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao A., Zhang X., Hu J., Ke Z. Apigenin sensitizes hepatocellular carcinoma cells to doxorubic through regulating miR-520b/ATG7 axis. Chem. Biol. Interact. 2018;280:45–50. doi: 10.1016/j.cbi.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 96.Yu L., Wang Z., Mo Z., Zou B., Yang Y., Sun R., Ma W., Yu M., Zhang S., Yu Z. Synergetic delivery of triptolide and Ce6 with light-activatable liposomes for efficient hepatocellular carcinoma therapy. Acta Pharm. Sin. B. 2021;11(7):2004–2015. doi: 10.1016/j.apsb.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang W., Chen Z., Zhang W., Cheng Y., Zhang B., Wu F., Wang Q., Wang S., Rong D., Reiter F.P., De Toni E.N., Wang X. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct. Targeted Ther. 2020;5(1):87–109. doi: 10.1038/s41392-020-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Şirin N., Elmas L., Seçme M., Dodurga Y. Investigation of possible effects of apigenin, sorafenib and combined applications on apoptosis and cell cycle in hepatocellular cancer cells. Gene. 2020;737:144428–144435. doi: 10.1016/j.gene.2020.144428. [DOI] [PubMed] [Google Scholar]
- 99.Papachristou F., Anninou N., Koukoulis G., Paraskakis S., Sertaridou E., Tsalikidis C., Pitiakoudis M., Simopoulos C., Tsaroucha A. Differential effects of cisplatin combined with the flavonoid apigenin on HepG2, Hep3B, and Huh7 liver cancer cell lines. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021;866:503352–503364. doi: 10.1016/j.mrgentox.2021.503352. [DOI] [PubMed] [Google Scholar]
- 100.Qin Y., Zhao D., Zhou H., Wang X., Zhong W., Chen S., Gu W., Wang W., Zhang C., Liu Y., Liu H., Zhang Q., Guo Y., Sun T., Yang C. Apigenin inhibits NF-κB and snail signaling, EMT and metastasis in human hepatocellular carcinoma. Oncotarget. 2016;7(27):41421–41431. doi: 10.18632/oncotarget.9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang J., Pi C., Wang G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018;103:699–707. doi: 10.1016/j.biopha.2018.04.072. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y., Zhu R., Wang J., Cui Z., Wang Y., Zhao Y. Upregulation of lncRNA H19 promotes nasopharyngeal carcinoma proliferation and metastasis in let-7 dependent manner. Artif. Cell Nanomed. Biotechnol. 2019;47(1):3854–3861. doi: 10.1080/21691401.2019.1669618. [DOI] [PubMed] [Google Scholar]
- 103.Zhou Y., Yu Y., Lv H., Zhang H., Liang T., Zhou G., Huang L., Tian Y., Liang W. Apigenin in cancer therapy: from mechanism of action to nano-therapeutic agent. Food Chem. Toxicol. 2022;168:113385–113393. doi: 10.1016/j.fct.2022.113385. [DOI] [PubMed] [Google Scholar]
- 104.Kwon Y., Cho Y.S., Lee Y.M., Kim S.J., Bae J., Jeong S.J. Changes to gut microbiota following systemic antibiotic administration in infants. Antibiotics (Basel, Switzerland) 2022;11(4):470–481. doi: 10.3390/antibiotics11040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Popa C.M., Ianosi S.L., Dorobantu S.C., Saftoiu A. Gut microbiota imbalance in metastatic colorectal patients treated with EGFRI and long-term antibiotic therapy for cutaneous toxicity: a pilot study. Cureus. 2022;14(5):e25007–e25023. doi: 10.7759/cureus.25007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Candellone A., Cerquetella M., Girolami F., Badino P., Odore R. Acute diarrhea in dogs: current management and potential role of dietary polyphenols supplementation. Antioxidants (Basel, Switzerland) 2020;9(8):725–741. doi: 10.3390/antiox9080725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bian S., Wan H., Liao X., Wang W. Inhibitory effects of apigenin on tumor carcinogenesis by altering the gut microbiota. Mediat. Inflamm. 2020;2020:1–9. doi: 10.1155/2020/7141970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang M., Firrman J., Zhang L., Arango-Argoty G., Tomasula P., Liu L., Xiao W., Yam K. Apigenin impacts the growth of the gut microbiota and alters the gene expression of Enterococcus. Molecules. 2017;22(8):1292–1313. doi: 10.3390/molecules22081292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ding S., Zhang Z., Song J., Cheng X., Jiang J., Jia X. Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. Int. J. Nanomed. 2014:2327–2333. doi: 10.2147/IJN.S60938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu Y., Yue Z., Xie J., Wang W., Zhu H., Zhang E., Cao Z. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. 2018;2(5):318–325. doi: 10.1038/s41551-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Z., Cui C., Wei F., Lv H. Improved solubility and oral bioavailability of apigenin via Soluplus/Pluronic F127 binary mixed micelles system. Drug Dev. Ind. Pharm. 2017;43(8):1276–1282. doi: 10.1080/03639045.2017.1313857. [DOI] [PubMed] [Google Scholar]
- 112.Zhai Y., Guo S., Liu C., Yang C., Dou J., Li L., Zhai G. Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Colloids Surf. A Physicochem. Eng. Asp. 2013;429:24–30. [Google Scholar]
- 113.Ganguly S., Dewanjee S., Sen R., Chattopadhyay D., Ganguly S., Gaonkar R., Debnath M.C. Apigenin-loaded galactose tailored PLGA nanoparticles: a possible strategy for liver targeting to treat hepatocellular carcinoma. Colloids Surf. B Biointerfaces. 2021;204:111778–111789. doi: 10.1016/j.colsurfb.2021.111778. [DOI] [PubMed] [Google Scholar]
- 114.Zarei M., Karimi E., Oskoueian E., Es-Haghi A., Yazdi M. Comparative study on the biological effects of sodium citrate-based and apigenin-based synthesized silver nanoparticles. Nutr. Cancer. 2021;73(8):1511–1519. doi: 10.1080/01635581.2020.1801780. [DOI] [PubMed] [Google Scholar]
- 115.Huang Y., Zhao X., Zu Y., Wang L., Deng Y., Wu M., Wang H. Enhanced solubility and bioavailability of apigenin via preparation of solid dispersions of mesoporous silica nanoparticles. Iran. J. Pharm. Res. (IJPR) 2019;18(1):168–182. [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X., Wang G., Gurley E.C., Zhou H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS One. 2014;9(9):e107072–e107089. doi: 10.1371/journal.pone.0107072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ali F., Rahul, Naz F., Jyoti S., Siddique Y.H. Health functionality of apigenin: a review. Int. J. Food Prop. 2017;20(6):1197–1238. [Google Scholar]
- 118.Lotha R., Sivasubramanian A. Flavonoids nutraceuticals in prevention and treatment of cancer: a review. Asian J. Pharmaceut. Clin. Res. 2018;11:42–47. [Google Scholar]
- 119.Zhang J., Liu D., Huang Y., Gao Y., Qian S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharmaceut. 2012;436(1–2):311–317. doi: 10.1016/j.ijpharm.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 120.Vrhovac Madunić I., Madunić J., Antunović M., Paradžik M., Garaj-Vrhovac V., Breljak D., Marijanović I., Gajski G. Apigenin, a dietary flavonoid, induces apoptosis, DNA damage, and oxidative stress in human breast cancer MCF-7 and MDA MB-231 cells. N. Schmied. Arch. Pharmacol. 2018;391(5):537–550. doi: 10.1007/s00210-018-1486-4. [DOI] [PubMed] [Google Scholar]
- 121.Wätjen W., Weber N., Lou Y., Wang Z., Chovolou Y., Kampkötter A., Kahl R., Proksch P. Prenylation enhances cytotoxicity of apigenin and liquiritigenin in rat H4IIE hepatoma and C6 glioma cells. Food Chem. Toxicol. 2007;45(1):119–124. doi: 10.1016/j.fct.2006.08.008. [DOI] [PubMed] [Google Scholar]