Abstract
The stable incorporation of transgenes and recombinant DNA material into the host genome is a bottleneck in many bioengineering applications. Due to the low efficiency, identifying the transgenic animals is often a needle in the haystack. Thus, optimal conditions require efficient screening procedures, but also known and safe landing sites that do not interfere with host expression, low input material and strong expression from the new locus. Here, we leverage an existing library of 300 different loci coding for fluorescent markers that are distributed over all 6 chromosomes in Caenorhabditis elegans as safe harbors for versatile transgene integration sites using CRISPR/Cas9. We demonstrated that a single crRNA was sufficient for cleavage of the target region and integration of the transgene of interest, which can be easily followed by loss of the fluorescent marker. The same loci can also be used for extrachromosomal landing sites and as co-CRISPR markers without affecting body morphology or animal behavior. Thus, our method overcomes the uncertainty of transgene location during random mutagenesis, facilitates easy screening through fluorescence interference and can be used as co-CRISPR markers without further influence in phenotypes.
Keywords: C. elegans, CRISPR, transgenesis, safe landing sites
Introduction
The ability to engineer transgenic and mutant animals has afforded one of the biggest revolutions in life sciences. Caenorhabditis elegans is a popular laboratory animal, with ten thousand strains carrying exogenous, recombinant DNA available. The first transgenic C. elegans animals were generated by microinjection into the worm’s gonad to establish extrachromosomal arrays (Stinchcomb et al. 1985). These arrays are, however, unstable, do not follow Mendelian inheritance and get lost mitotically, leading to mosaic animals in which not all somatic cell express the transgene. Classical approaches rely on the use of genetic selection markers (Mello et al. 1991), however, when the ectopic DNA is not accompanied by a visible marker, this effect can be misinterpreted as a lack of phenotype. Several strategies have been proposed to circumvent this phenomenon, from the enrichment of the transgenic animals using antibiotic selection (Giordano-Santini et al. 2010; Semple et al. 2010; Radman et al. 2013) to rescue from strong phenotypes such as temperature-sensitive lethality (pha-1(ts)) (Granato et al. 1994) or paralysis (unc-119) (Maduro 2015), however, none of them succeeded in eliminating the mosaic expression. Furthermore, extrachromosomal arrays contain large copy numbers of the injected DNA, which often causes overexpression artifacts, but have the advantage that transgenes become visible even beyond their native levels. For example, many fluorescent tags to endogenous proteins are poorly visible due to their low expression levels and promoter activity (Walker 2000; Das et al. 2021). The problem of unstable inheritance can be mitigated by integrating the transgenic array. Traditional integration methods are based on random mutagenesis, either using a gene gun (Praitis et al. 2001), that allows integration at low frequencies, or chemicals like UV/TMP, X-ray irradiation (Mariol et al. 2013) or singlet oxygen generators (miniSOG) (Noma and Jin 2018). However, cumbersome and time-consuming screening efforts are necessary to identify the integrants, and the locus of integration remains unknown unless subsequent mapping experiments are conducted. In addition, the mutagenesis causes extensive DNA double-strand breaks, and thus, the resultant animals needs to be backcrossed several times and verified to ensure minimal genetic variability. Even though targeted, MOS-transposase directed, single copy integrations (Frøkjær-Jensen et al. 2008, 2012), recombination-mediated cassette exchange (Nonet 2020, 2021), and CRISPR transgenesis (Friedland et al. 2013; Dickinson et al. 2015; Paix et al. 2017) are available, extrachromosomal arrays were and still are the standard in many laboratories for fast and efficient generation and screening of transgenic phenotypes.
Over the last few years, many different methods have been proposed and demonstrated for site-directed CRISPR/Cas9 mediated locus-specific integration of ectopic DNA such as extrachromosomal arrays (Yoshina et al. 2016; El Mouridi et al. 2022) or single copy transgenes (Silva-García et al. 2019; El Mouridi et al. 2022) into safe habor integration sites. These methods rely on a crRNA that recognizes a single site in the genome and facilitates Cas9-mediated double-strand DNA breaks. The subsequent nonhomologous end joining (NHEJ) or homology-directed repair probabilistically integrates the co-delivered ectopic DNA. Even though these methods overcome many of the above-mentioned shortages of unstable transgenesis and variable expression, so far, there are only a limited number of target sites available (e.g. ben-1, dpy-3, MosSCI) (Frøkjær-Jensen et al. 2008; Yoshina et al. 2016; El Mouridi et al. 2022). Recently, Frokjaer-Jensen and colleagues generated a library containing 147 strains carrying single copy loci expressing the red fluorophore tdTomato in somatic nuclei, in addition to 142 nuclearly localized GFP strains (Frøkjær-Jensen et al. 2014), which have aided mapping and genetic experiments (Fay 2006; LaBella et al. 2020; Noble et al. 2020; Das et al. 2021). Originally, these strains were generated as dominant genetic markers and can also be used as landmarks to map genetic position of mutants and transgenes. Because the integrated transgenes of many of these strains locate to intergenic regions and are transcriptionally active, we reasoned that these loci would satisfy many if not all conditions as further safe-harbor integration sites.
Here, we leverage these strains and demonstrate that a single crRNA can cut the tdTomato (or GFP) DNA sequence at high efficiency, affording a selection of 147 (142 for GFP) possible integration sites, 121 of which are intergenic (Frøkjær-Jensen et al. 2014). Moreover, the loss of tdTomato fluorescence during the integration not only facilitates screening purposes, but can also be used as co-CRISPR marker during gene-editing at distant loci. Importantly, we show that the integration of a model transgene per se does not affect worm physiology, and even intragenic insertions appear to be phenotypically silent. This method has considerable advantages in multiplexed genome engineering, when the co-CRISPR locus cannot be unlinked easily from the editing site. Lastly, we propose future extensions of FLInt for the use of single copy GFP sequences as a dominant marker for homology-directed repair through genetic conversion of the GFP to BFP chromophore with a single nucleotide change.
Materials and methods
Animal maintenance
Nematodes were cultivated on NGM plates seeded with E. coli OP50 bacteria using standard protocols (Stiernagle 2006; Porta-de-la Riva et al. 2012). The integration efficiency of all tested target strains is listed in Supplementary Table S1. All transgenic strains in this study are listed in Supplementary Table S2. The parental strains carrying eft-3p::tdTomato::H2B and eft-3p::gfp::NLS used as the identified landing sites from miniMos (Frøkjær-Jensen et al. 2014) were maintained and cultured at 20°C prior to injection.
Molecular biology
Gibson assembly was regularly used for plasmid construction. Briefly, specific primers were designed, and PCR was performed using KOD DNA polymerase (Sigma Aldrich). The amplification of DNA fragments was done following manufacturer’s instructions into a Bioer GeneExplorer thermal Cycler. The visualization of DNA fragments was done using an Azure c600 (Azure Biosystems) gel imaging device. Gibson assembly was performed by mixing fragments of the different DNAs at a 3:1 ratio (insert:vector) and a 2X homemade Gibson Assembly Master Mix. The bacterial transformation was done using either NEB 5-alpha or 5-alpha F’Iq Competent E. coli.
The plasmids (see Supplementary Table S3) used as the co-injection markers are pCFJ90 (myo-2p::mCherry), pCFJ68 (unc-122p::gfp), and pCFJ104 (myo-3p::mCherry). The plasmids used as the transgene for integration are pNM5 (nlp-12p::ChRmine), pNM10 (cct-2p::mtagBFP2::myosin::spectrin::cryolig2::wrmScarlet(1-10), pNM11 (mec-4p::trp-4::wrmScarlet), pNM12 (mec-4p::RGECO1 syntron), pNM13 (ges-1p::CRE), pNM14 (rab-3p::CRE), pNMSB91 (15xUAS::delta pes-10p::ACR1), and pHW393 (rab-3p::GAL4). pHW393 (Prab-3::GAL4-SK(DBD)::VP64::let-858 3'UTR) was a gift from Paul Sternberg (Addgene plasmid # 85583; http://n2t.net/addgene:85583; RRID:Addgene_85583, Wang et al. 2017). The injection mix was prepared by mixing the plasmid of interest, the co-injection markers, and DNA ladder (1 kb Plus DNA Ladder, Invitrogen) at varying ratios. All primer sequences are available in Supplementary Table S4.
crRNA design and selection of the target sequence
All crRNAs were designed using Benchling’s DNA editor with single guide option, 20-nt length, PAM sequence (NGG) and were purchased from Integrated DNA Technologies (IDT, Supplementary Table S5). The crRNA against tdTomato (5′-GTGATGAACTTCGAGGACGG—CGG-3′) recognizes 2 sites in the tdTomato gene due to the tandem repeat (Fig. 1). The recognition sites are at the 306th and the 1032th nucleotides. Off and on-target specificity has been compiled with CRISPOR (Concordet and Haeussler 2018). Off-target sites that are recognized with 4 mismatches include ubc-3, gcy-11, Y73F8A.5, C55B7.3, and F10G8.1. The crRNAs against gfp excise DNA at the middle of the gene (5′-CTTGTCACTACTTTCTGTTA-3′) and 3′ downstream region (5′-TGAACTATACAAATGCCCGG-3′). All HR template sequences are shown in Supplementary Table S6.
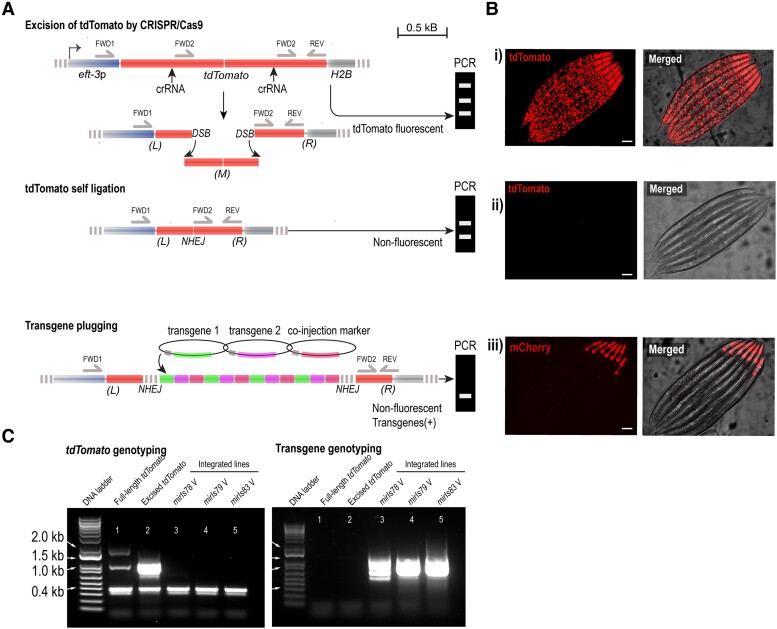
Fig. 1.
Principle and expected outcomes of FLInt. a, b: a) Sketch of the different genetic interventions and B) expected phenotypic outcome in tdTomato or transgene fluorescence. (i) Red nuclear fluorescence indicating parental tdTomato fluorescence; (ii) loss of red indicates successful gene editing; (iii) target transgene fluorescence and loss of red nuclear fluorescence as candidates for stable transgenesis. c: A 3-primer PCR genotyping strategy can be used to follow landing site disruption and successful integration. Primers are designed to reveal expected bands for an unedited, edited and integrated tdTomato locus (a).
Off-target assessment of the crRNA
We assessed off-target gene editing of the loci mentioned in the previous section (see Supplemental Data File 1). With the off-target analysis using CRISPOR (Concordet and Haeussler 2018), we selected a candidate gene, C55B7.3 (I:1.17 / 0.000 cM), for verifying whether it could be recognized and edited while integrating the transgenes on the tdTomato locus. The C55B7.3 gene was amplified from the integrated strains generated by tdTomato excision. Ten animals were pooled from 15 strains (MSB1110, MSB1111, MSB1112, MSB1113, MSB1115, MSB1116, MSB1117, MSB1118, MSB1119, MSB1120, MSB1121, MSB1122, MSB1123, MSB1124, and MSB1125). The lysates were prepared using a variation of the single worm DNA extraction described in Williams et al. (1992). Briefly, 10 PCR buffer from BIOTAQ DNA Polymerase (Bioline, Cat. No. BIO-21040) was diluted to 1 and supplemented with proteinase K (Fisher Scientific, Cat. No. 10181030) at g/L final concentration. Each worm was lysed in L lysis buffer and incubated at 65°C for 10 min and 95°C for 2 min in a thermal cycler. L of milliQ water were added to the lysis reaction and L used as template for PCR. The PCR primers were designed by CRISPOR; forward primer (5′-TCGTCGGCAGCGTCCTTCCCGAGCAAGAAGGGTG-3′) and reverse primer (5′-GTCTCGTGGGCTCGGTGGAACTTACCGTCACCGAAG-3′). The PCR amplicons were sequenced using the 5′-CTTCCCGAGCAAGAAGGGTG-3′ primer. The off-target effect was assessed by comparing the sequencing data to the wild-type nucleotide sequence.
Microinjection
Similar to the preparation of the conventional injection mix (transgene DNA co-injection markers) (Rieckher and Tavernarakis 2017), this method requires an additional portion of CRISPR reagents. The CRISPR mix was prepared by mixing M of crRNA, M of Alt-R CRISPR-Cas9 tracrRNA (IDT), and milliQ water. The crRNA-tracrRNA dimer was induced by incubating the mix at 95°C for 5 min and RT for 5 min. Then, Streptococus pyogenes Cas9 nuclease (IDT) was added to form the ribonucleoprotein complex. The CRISPR mix was aliquoted into PCR tubes (L each) and stored at C for further use. The injection mix was prepared by mixing the purified plasmid DNA (Zymo D4016 PLASMID MINIPREP-CLASSIC) with DNA ladder (1 kb Plus DNA Ladder, Invitrogen), 100 ng/L DNA in total (see Supplementary Table S7). We added the L of CRISPR mix (mentioned above) into the L injection solution to make a total of L. The mix was centrifuged at the highest speed for 8–10 min before injecting. The transgenic strains used as the P0 animals were established by miniMos technique (Frøkjær-Jensen et al. 2014) expressing tdTomato and GFP in all cellular nuclei. We selected the following transgenic landing sites (see also Supplementary Table S1; note, all oxTi transgenes carry the Cbr-unc-119(+) rescue construct, in the unc-119(ed3) mutant background, in addition to the landing site):
EG7835 [oxTi556 I (eft-3p::tdTomato::H2B)],
EG7846 [oxTi700 I (eft-3p::tdTomato::H2B)],
EG7860 [oxTi677 II (eft-3p::tdTomato::H2B)],
EG7866 [oxTi564 II (eft-3p::tdTomato::H2B)],
EG7898 [oxTi619 III (eft-3p::tdTomato::H2B)],
EG7900 [oxTi546 III (eft-3p::tdTomato::H2B)],
EG7905 [oxTi390 IV (eft-3p::tdTomato::H2B)],
EG7911 [oxTi705 IV (eft-3p::tdTomato::H2B)],
EG7944 [oxTi553 V (eft-3p::tdTomato::H2B)],
EG7945 [oxTi543 V (eft-3p::tdTomato::H2B)],
EG7985 [oxTi566 X (eft-3p::tdTomato::H2B)],
EG7989 [oxTi668 X (eft-3p::tdTomato::H2B)],
EG8958 [oxTi1022 I (eft-3p::gfp::NLS)],
EG8888 [oxTi936 X (eft-3p::gfp::NLS)], and
MSB1247 [unc-119(ed3) III; oxTi553 (eft-3p::tdTomato::H2b) V; oxSi1091(mex-5p::Cas9(smu-2 introns) + unc-119(+)) II].
All transgenic animals that we used as background strains are available in CGC.
Using FLInt with an germline competent Cas9: Besides the use of recombinant, purified Cas9 protein, we leveraged an integrated, germline expressing Cas9 to perform ectopic transgene integration with the FLInt method. To do so, we used the transgenic strain EG9615 carrying the optimized Cas9 gene which is expressed in (Schwartz et al. 2021). We generated MSB1247 carrying the integrated Cas9 and tdTomato landing site by crossing EG9615 (other strains with fluorescently labeled Cas9 locus are available to guide transgene selection) with EG7944 (oxTi553 V). Then, we followed the method for FLInt integration (described earlier), without the addition of Cas9 protein in CRISPR mix. We injected 30 P0 animals with myo-3p::mCherry marker, isolated 80 positive F1(s), and eventually obtained 5 integrated lines (integration efficiency = 6.25%). We noticed that the animals carried extrachromosomal array mostly showed tdTomato excision, indicative of RNP formation from the co-injected crRNA-tracrRNA. We found that the integration efficiency was not different from the previous trial using RNP, suggesting the similar probability of arrays to be integrated. This result demonstrated that the integrated Cas9 can be an alternative option for gene editing in C. elegans at reduced cost. To further optimize this technique, it might be possible to co-integrated sgRNA (tdTomato) with Cas9 gene in order to reduce the FLInt reagents. However, the only concern of this technique is the landing site of Cas9 on chromosome II that need to be outcrossed.
Visual screening of transgenic animals
The screening of the fluorescent progenies from P0 was performed using a fluorescent stereomicroscope (SMZ25, Nikon Instruments) equipped with a white-light LED light source (Lumencor, Sola S2). We searched for the nonred animals with co-injection marker expression, called positive F1, 3-day postinjection. Then, we singled them out into new NGM/OP50 plates. The individual positive F1 were cultured for 3 days at 25°C, and plates were searched for F2 progenies with high transmission frequency (approx. 75%). Six F2(s) of each of those plates were singled out. After 3 days, the F3 progenies were checked for homozygous expression of the co-injection marker and, if integration had taken place, the integrated lines were characterized. The F3 progenies from the same F1 are determined as identical transgenic line. We calculated the integration efficiency by (no. of integrated line/no. of positive F1) 100.
Determination of the integration efficiency on different loci
Six different tdTomato landing sites in different chromosomes were used for assessing integration effeciency: EG7835 (oxTi556 I:1.23), EG7846 (oxTi700, I:22.30), EG7866 (oxTi564, II:-0.38), EG7860 (oxTi677, II:-12.17), EG7898 (oxTi619, III:1.23), EG7900 (oxTi546, III:11.80), , EG7905 (oxTi390, IV:-26.93), EG7911 (oxTi705, IV:0.09), EG7944 (oxTi553, V:0.29), EG7985 (oxTi566, X:-4.88), and EG7989 (oxTi668, X:0.19) (Fig. 3a). Animals were injected with 2 ng/L myo-2p::mCherry, 98 ng/L DNA ladder (Invitrogen), and tdTomato CRISPR mix. Unless otherwise specified, the P0 animals were cultured at 25°C after injection, as well as the F1, F2, and F3. The integration efficiency was then calculated from 3 experimental replicates (see Supplementary Table S1).
Fig. 3.
Integration efficiency correlates with chromosomal position. a: Summary schematic of the different landing sites and their chromosomal position used and their integration/tdTomato cutting efficiencies in black. The cutting efficiencies to the proximal sites are shown in green. b: Plot of integration efficiency vs genetic position irrespective of the linkage group. A strong drop in efficiency is observed for sites close to the chromosomal periphery. Grey are data points from individual experiments, green dots show meanstandard deviation for each landing site. See also Supplementary Table S1. c: Fluorescence intensity for the single copy tdTomato transgenes at the indicated sites. d: Plot of the tdTomato fluorescence intensity vs chromosomal position.
Integrated copy number analysis with qPCR
qPCR was used for detecting and measuring the copy number of the integrated pCFJ90 (myo-2p::mCherry) of 9 integrated strains (MSB884, MSB886, MSB898, MSB905, MSB911, MSB912, MSB913, MSB914, and MSB915). Sample preparation was done by culturing worms in peptone-enriched plates with NA22 as food source. When plates were full of adult worms, they were washed off the plates with M9 buffer, excess bacteria eliminated by successive washes and lysed in L lysis buffer supplemented with proteinase K (see Off-target assessment of the crRNA section above). The genomic DNA was purified using the Zymoclean Gel DNA Recovery Kit (Zymo Research). qPCR analyses were carried out by AllGenetics & Biology SL (www.allgenetics.eu). Briefly, absolute qPCR was performed with primers indicated in Supplementary Table S4. The qPCR experiment was performed in triplicate for each sample and controls. The qPCRs reactions were carried out in a final volume of L, containing L of NZY qPCR Green Master Mix ROX plus (NZYTech), M of the amplification primers, L of template cDNA, and ultrapure water up to L. The reaction mixture was incubated as follows: an initial incubation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing/extension at 65°C for 1 min. A 5 point 10-fold serial dilution of a known number of copies of the genes under study was used to establish the standard curve and evaluate the reaction efficiency. These dilutions were also performed in triplicate. The Y-intercept and slope were also obtained from the standard curve. Copy number was calculated by the formula: copy number = 10(Cq - Yintercept)/(slope). Copy number of integrated transgenes was obtained by normalizing with rps-25.
Screening for loss of tdTomato fluorescence as a ‘co-injection’ marker
Having multiple transgenes or multicolor phenotype could negatively affect animal health as it constitutes a metabolic burden and limits the degrees of experimental freedom during microscopy experiments (e.g. multicolor imaging acquisitions). Importantly, the above-mentioned integration protocol and simplicity of the screening procedure also facilitates the integration of transgenes without the use of visible markers, e.g. such as the myo-2p::mCherry. To demonstrate this, we generated a dual-fluorescence CRE/lox reporter strain (based on SV2049) with constitutive BFP expression and conditional, CRE-dependent mCherry expression, with the ubiquitous tdTomato expression from the landing site in the background (MSB934). After injecting this strain with a plasmid encoding for an intestinal CRE (ges-1p::CRE) together with tdTomato CRISPR mix, we confirmed loss of tdTomato and a BFP/mCherry color switch in intestinal nuclei in the F1. Importantly, the intestinal red fluorescence is indicative for the tissue specific CRE-recombination, that would otherwise be obscured had the tdTomato cleavage not taken place. To isolate homozygous integrants, we followed the CRE-dependent BFP/mCherry color switch during the F3 (Supplementary Figure S3). We also demonstrated the co-injection marker free integration using the binary UAS/GAL4 expression system (Wang et al. 2017), and integrated a panneuronal rab-3p::GAL4 driver construct in the background of a silent UAS::GFP effector strain carrying the tdTomato landing site. Following our experimental pipeline, we obtained positive F1 that panneuronally expressed GFP signal with the loss of tdTomato (Supplementary Figure S3). Our results demonstrate that the negative selection due to fluorescence interference of the tdTomato landing site facilitates the screening step in C. elegans transgenesis and serves as a safe harbor for transgene expression.
Integration of extrachromosomal array using FLInt
The integration of the existing extrachromosomal array was done first by crossing the strain of interest to the desired tdTomato marker strain. A CRISPR injection mix containing M of crRNA against tdTomato, M crRNA against Ampicilin resistance gene (AmpR), M of tracrRNA and Cas9 endonuclease was injected in the resulting strain and the progeny scored for loss of tdTomato expression. One hundred percent transmission of the extrachromosomal marker was used as an indicator for integration.
Screening integrations with PCR
To follow the double-strand break, excision and integration efficiency at the tdTomato site, we designed PCR primers that bind to several regions along the tdTomato gene (Fig. 1a, Supplementary Table S4); (1) A forward primer that binds to the region upstream the tdTomato gene, in the eft-3 promoter (FWD1: 5′-TTTATAATGAGGTCAAACATTCAGTCCCAGCGTTTT-3′) (2) another forward primer that binds to the middle of the gene in both tandem repeats, downstream the excision sites (FWD2: 5′-GACCCAGGACTCCTCCCT-3′), (3) the reverse primer, that binds at the end of tdTomato ORF (REV: 5′-TTACTTGTACAGCTCGTCCATGC-3′). This strategy gives rise to 3 bands when genotyping tdTomato (Fig. 1a,c). We utilized this technique for investigating the tdTomato gene before and after being excised by CRISPR/Cas9. The full-length tdTomato is recognized by the 4 binding sites of the 3 primers amplifying 3 different band sizes: 1.7 kb, 1.1 kb, and 0.4 kb (Fig. 1c, lane 1). The excised tdTomato splits the middle chunk of gene, losing one primer binding site. Only 2 PCR bands (1.1 kb and 0.4 kb) were detected (Fig. 1c, lane 2). Lastly, in integrated strains only the smallest band (0.4 kb), outside of the integration region is amplified (Fig. 1c, lanes 3–5). To avoid competition between the 2 different FWD primers, the following PCR conditions proved optimal: FWD primer mM; FWD primer mM; REV mM; C; extension min.
Screening for lat-1::loxP::ΔmCherry insertions using tdTomato as Co-CRISPR
The insertion of a loxP site into lat-1 locus was done using CRISPR/Cas9. To excise lat-1 gene, we introduced the crRNA (5′-ATGTACACGCATCAAAGATA-3′) (IDT), tracrRNA (IDT), and Cas9 (IDT). The loxP site and additional sequence (mCherry) insertion and PAM mutation was induced by the HR template (Supplementary Table S6) with 35-nt homology arms (IDT). The CRISPR mix was prepared followed the details above and injected into the gonad of the background strain EG7944 (oxTi553 V [eft-3p::tdTomato::H2B]). The concentration of the homology repair template was 167 ng/L. The screening of F1 was done after 3 days using the fluorescent microscope. The candidate F1(s) were selected from the jackpot plates based on the loss of tdTomato fluorescent signals among the F1 population. The candidates were singled out onto new NGM/OP50 plates before genotyping. To genotype the loxP insertion, worms were lysed and genotyped as detailed in the Off-target assessment of the crRNA section with primers 5′-CGATGTTGACAACTGAAGTGA-3′ and 5′-GGTAATTTCTGACATGGCTCA-3′. The edits were observed in an electrophoresis gel by the shift of the edited DNA band (417 bp) compared to the wild-type (291 bp). The efficiency of lat-1::loxP::mCherry insertion from each jackpot plate was calculated by (no. of edits/no. of candidate F1) 100.
Screening of GFP color switch as the HDR-mediated co-CRISPR marker
The HDR-mediated fluorescent conversion from GFP to BFP (P4) was done with the eft-3p::GFP::NLS background strains, EG8888 [oxTi936 X] and EG8958 [oxTi1022 I]. The single point mutation of gfp gene was triggered by DNA double-strand break via CRISPR/Cas9 approach followed by the HDR that introduces the change of amino acid from the background (Y66H). To do this, the crRNA against gfp (5′-CTTGTCACTACTTTCTGTTA-3′), tracrRNA, Cas9 nuclease, and the HR template (5′-TTAAATTTTCAGCCAACACTTGTCACTACTTTCTGTTATGGT GTTCAATGCTTCTCGAGATACCCAGATCATAT-3′; see Supplementary Table S6), purchased from IDT, were injected into the P0 animals. After 3-day post injection, the F1(s) progenies were screened for the loss of GFP single which replaced by the expression of BFP in the nuclei. The candidates were then singled out and screened for few generations to obtain the homozygous genotype.
Fluorescence microscopy
The fluorescence signal of the worms was observed using a confocal microscope (Andor DragonFly 502, Oxford Instruments) attached to a Nikon Eclipse Ti2 inverted microscope body through either a 20 0.75 oil or a 60 1.3 oil immersion lens and a back-illuminated sCMOS camera (Sona, Andor). The tdTomato fluorescence signal was excited with a 561 nm laser beam (power intensity 30%, exposure ms) and the emitted signal transmitted using a 594 nm filter. The GFP fluorescence signal was excited with a 488 nm laser beam (power intensity 60%, exposure ms) and transmitted using 521 nm filter. The mCherry fluorescence signal was excited with a 514 nm laser beam (power intensity 40%, exposure ms) and transmitted through a 594 nm filter. The P4 and BFP fluorescence signals were excited with a 405 nm laser beam (power intensity 40% and 20%, respectively, exposure ms and 200 ms, respectively) and transmitted using a 445 nm filter. The fluorescence signal was captured using Z-scan protocol (0.7 step size) through the confocal apparatus (Andor DragonFly).
Healthspan assessment
The wt (N2), full-length tdTomato (EG7944), excised tdTomato (MSB910), 3 myo-2p::mCherry integrated lines (MSB1115, MSB1118, and MSB1122) and myo-2p::mCherry (extrachromosomal array) animals were cultured and their development, locomotion, body length, and lifespan compared (supplementary Figure S2). The fluorescence intensity and development were done in N2, EG7944, MSB910, and MSB1115. Development was assessed based on the worms size over time from L1 to egg-laying adult stage. Synchronized L1 (Porta-de-la Riva et al. 2012) were seeded onto NGM/OP50 plates and incubated at 20°C. We captured the worms at L1 stage (prior to seeding), L3 stage (24 h after seeding), L4 stage (40–48 h after seeding), and egg-laying stage (72 h after seeding) based on the developmental timeline of N2 (Porta-de-la Riva et al. 2012). We imaged tdTomato fluorescence intensity in young adult worms using the Z scan protocol (step m) with 20 magnification (20/0.75 MImm objective lens). The maximum Z-projection was performed using ImageJ (Fiji). Then, the ROI was drawn using segmented line across the body edge. The average intensity was measured and collected from individual worms. On the last day of developmental assessment, the adult animals were placed on the new plate and the moving trace on bacterial lawn captured. The locomotion behavior was observed under the lab-built microscope (WormTracker Das et al. 2021). We took the sinusoidal wave appearing in the bacterial lawn after the worm passed as reference of the body angle during locomotion.
Body length was captured in a lab-built microscope (WormTracker Das et al. 2021) using 2x magnification and measured in ImageJ. By using the segmented line tool, the body length was measured from the nose tip to the tail tip.
The lifespan assay was conducted by counting number of dead and alive worms in FUDR plates until the whole population diminished. The decrease of viability of each strain were plotted as the survival curve. The mean of lifespan was calculated from the average of age from individual animals in each population. Then, the mean of lifespan was compared with wt strain.
Statistical analysis
No statistical method was applied to predetermine sample size based on data variability. All data sets were first tested for normality using KS test or Tukey adjusted ANOVA for multiple comparisons as indicated in the Figure legends.
Results and discussion
Single tdTomato transgenes as safe harbor landing pads for exogenous transgenes
To demonstrate that the single-copy tdTomato loci can function as versatile sites to integrate transgenes into the genome of C. elegans, we designed a single crRNA against the tdTomato ORF (Fig. 1a, see Methods) that is not predicted to have a full length off-target binding probability. Because tdTomato is a tandem-dimer gene of a single fluorophore, the successful Cas9 cleavage will cut the DNA twice, excising a large portion of the gene. The concomitant loss of fluorescence should, in principle, facilitate the screening process, and therefore speed up the identification of successful integrations. The tdTomato site serves a dual function: as a landing site and a visual marker for transgenesis. We therefore coined the method “FLInt”, for Fluorescent Landmark Interference. We first sought to test whether the selected crRNA cleaves the tdTomato sequence. We reasoned that successful double-strand DNA break results in a loss in tdTomato fluorescence in the filial generations. Indeed, many animals in the F1 of an injected P0 have already lost their tdTomato fluorescence, which is readily identifiable in a normal fluorescence stereomicroscope (Fig. 1b). Some animals, however, showed a considerably lower fluorescence, indicative for a single edit on one parental chromosome. We also frequently observed a mosaic pattern in the somatic cells of the F1s, possibly due to cleavage after the first cell division. These animals would eventually give rise to nonred animals in the F2 generation according to Mendelian segregation. In jackpot broods, we frequently observe 25% of nonred animals from a single injection. We benchmarked the DNA cleavage efficiency for the tdTomato against the widely used, highly efficient dpy-10 protospacer (Arribere et al. 2014) and coinjected M for both crRNAs together with recombinant Cas9 (Paix et al. 2017). We then screened for nonred and Dpy animals as a readout for simultaneous cleavage of both DNA strands at the dpy-10 and tdTomato locus. From the total 13 jackpot broods we screened, we found 34% red, wild-type animals, 56% nonred, Dpy animals and 0% red, Dpy worms. Since all Dpy animals had also lost the tdTomato fluorescence in the F1, we reasoned that the crRNA for tdTomato is, at least, as efficient as the highly efficient dpy-10 protospacer (Arribere et al. 2014; El Mouridi et al. 2017). In addition, we found 10% nonred, wild-type worms (Table 1), suggesting a slightly higher efficiency of the tdTomato protospacer and making FLInt an extremely well suited candidate method for transgene integration at many potential sites across the genome. Together, these results not only indicate that the selected crRNA for tdTomato efficiently guides Cas9 for subsequent DNA cutting, but also that it does so at a high efficiency, allowing identification of events already in the F1. As a last test for the suitability of FLInt as safe habor sites, we assessed if the tdTomato crRNA causes any unwanted off-target effects. The genotyping of 9 different strains at the most likely predicted off-target site (containing 4 mismatches), however, did not identify any further edits (Supplementary Data File 1). Likewise, we also did not detect gross defects in healthspan and locomotion or any other behavioral phenotypes compared with N2 wild-type animals (Supplementary Figure S2). We also observed that the strains generated on the oxTi553 allele reverted the parental phenotype at 25°C (Frøkjær-Jensen et al. 2014). This suggests that the edits are not interfering with the normal physiology of the animal and have nearly wild-type behavior (Supplementary Figure S2).
Table 1.
crRNA efficiency of tdTomato and dpy-10. Table with the crRNA cleavage efficiency at the tdTomato locus at oxTi553 compared with the well characterized dpy-10 locus. Similar efficiencies have been found for tdTomato sites distributed over all 6 linkage groups (see also Fig. 3).
| Phenotypes | No. of worms | Percentage |
|---|---|---|
| Red nuclei, wt | ||
| Nonfluorescent, wt | ||
| Red nuclei, Dpy | ||
| Nonfluorescent, Dpy |
Having established highly efficient DNA cleavage using the tdTomato crRNA, we proceeded to inject 20 P0 animals with the CRISPR mix and a myo-2p::mCherry plasmid as transgene-of-interest (TOI) into the eft-3p::tdTomato::H2B V strain (EG7944) (Supplementary Figure S1a), following loss of red nuclear fluorescence from the tdTomato and gain of mCherry expression in the pharynx during the filial generations (Supplementary Figure S1a). Consistent with our prior observations, we found that some F1 had already lost the strong tdTomato nuclear fluorescence displayed by the P0, an indication of the successful disruption of both homologous chromosomes in the first generation after injection. We singled out animals positive for red pharynx (Supplementary Figure S1b), noticing that most of the transgenic animals that expressed mCherry had also lost nuclear tdTomato expression. To distinguish between expression from the extrachromosomal array and integrants, we selected 6 F2 animals from high transmission plates (myo-2p::mCherry, loss of nuclear tdTomato) and eventually obtained 1–3 integrated lines based on 100% transmission frequency in the F3 from one injection (see also Supplementary Table S1).
Often, the TOI does not lead to a visible phenotype, for example effector or driver strains in bipartite expression systems (Wang et al. 2017; Das et al. 2021; Porta-de-la Riva et al. 2021). To follow the integration of such transgenes, we developed a PCR genotyping strategy (Fig. 1a, d, see Methods) using 3 primers that target the region around tdTomato for amplification, with different amplicon sizes according to the genetic recombination occurred. We selected animals from the 3 different populations: tdTomato::H2B (no excision), nonfluorescent (loss of tdTomato) and nonfluorescent/myo-2p::mCherry (expectedly tdTomato inserted) to isolate their DNA for genotyping. In the parental strain with tdTomato expression, the 3 primers would anneal (one of them twice) and 3 bands of different sizes would be amplified. Expectedly, we found that loss of tdTomato signal in absence of the transgenic marker was genomically accompanied by the loss of the longest DNA band, indicative for a successful Cas9 activity, and repair through NHEJ. However, in myo-2p::mCherry homozygous animals carrying the successful integration, we were unable to amplify the region flanked by the 2 crRNA target sites. We reasoned that the region with inserted transgene could not be amplified due to the large size of the multicopy transgene, which could be up to millions bases in length (El Mouridi et al. 2022). However, the small band corresponding to the end of the tdTomato gene and downstream the expected integration site (0.4 kb) was amplified, serving as a positive control for PCR (Fig. 1c, lane 3–5). Taken together, these results established that ectopic transgenes can be integrated by CRISPR using site-specific crRNAs into the tdTomato landing sites as multicopy transgenes with very high efficiency and reliability.
During the expansion of the injected animals, we consistently observed different integration efficiency based on the culturing conditions. Similarly to what had been previously described for integrations through the miniMos technique (Frøkjær-Jensen et al. 2014), we hypothesized that the temperature at which the P0 is grown after injection might affect the integration efficiency. To investigate this, we reared the injected P0 at 16°C or 25°C for 2 days until we screened F1 for positive transgenesis events. The F2 and F3 progenies, however, were invariably raised at 25°C. After obtaining the integrated lines, we found that culturing the P0 at 25°C promoted higher integration efficiency compared with 16°C (Table 2). At this temperature, we obtained 100% success rate, with integrated lines from every injection round (3/3). Conversely, only one integrated line was obtained of the P0 incubated at 16°C (1/4, Table 2). This result is in agreement with previous reports in vertebrates and plants showing that Streptococus Pyogenes Cas9 efficiency is higher at elevated temperatures (Moreno-Mateos et al. 2017; LeBlanc et al. 2018) and suggests that transgene integration is temperature-dependent.
Table 2.
The effect of temperature on FLInt mediated integration. EG7944 animals carrying oxTi553 were injected with myo-2p::mCherry and DNA ladder and followed for integration.
| Experiment | Incubating Temp. | No. of positive F1 | No. of high transmission F2 | No. of integrated line | Integration frequency |
|---|---|---|---|---|---|
| 1 | 16°C | 13 | 3 | 1 | 7.69 |
| 2 | 16°C | 57 | 13 | 0 | 0 |
| 3 | 16°C | 64 | 12 | 0 | 0 |
| 4 | 16°C | 25 | 12 | 0 | 0 |
| 1 | 25°C | 34 | 11 | 2 | 5.88 |
| 3 | 25°C | 45 | 9 | 1 | 2.22 |
| 4 | 25°C | 63 | 25 | 4 | 6.34 |
We were then curious to understand how many copies of the coinjected plasmid were integrated into the safe harbor locus and how this related to the relative amount of DNA injected. Previous integration methods suggested a large variability of integrated copies, ranging from few copies (derived from biolistic transformations (Sarov et al. 2012)) to hundreds after integrating traditional extrachromosomal arrays with random mutagenesis (Noma and Jin 2018). We thus injected varying ratio of coinjection marker/transgene together with the tdTomato CRISPR mix into EG7944 oxTi553 V or EG7846 oxTi700 I and quantified their integrated copy number using quantitative PCR. We found that a higher plasmid ratio led to a higher copy number, which in turn led to a higher transgene expression from the co-injection marker (myo-2p:mCherry) (Fig. 2). Thus, a careful titration of injected plasmid would thus facilitate a balanced expression (in our hands ranging from 20 to 150 copies of the transgene) of the desired transgenes in a known safe harbor locus.
Fig. 2.
Transgene copy number for 9 different transgenes. Different relative concentrations of myo-2p::mCherry were injected together with the CRISPR mix and a target plasmid (ratio, left bars) and integrated into the same tdTomato locus (oxTi). The resulting homozygous transgene copy numbers were quantified by qPCR (right bars). The middle plot shows the pharynx fluorescence and the inset shows the integrated copy number as a function of the injected plasmid ratio.
Taken together, highly efficient integration methods reduce the time consuming screening required in traditional transgene integration procedures. Compared with the conventional method using UV/TMP, in which worms are propagated for several generations during 3–5 weeks before the screening (Mariol et al. 2013) and require posterior outcross to nonmutagenized worms, our method establishes integrated lines within 9 days post injection, essentially bypassing the formation of an extrachromosomal array. Besides, the loss of fluorescence provides a visual, dominant marker for screening, allowing fast identification of positive F1 among the background phenotype. In addition, since the loss of fluorescence only takes place after cleavage of both chromosomes, rapid screening of homozygous edits is facilitated, even granting the omission of another injection marker than the loss of the same tdTomato. For example, we successfully integrated ges-1p::CRE and rab-3p:GAL4 into their effector strain and recovered transgenic after a single injection (Supplementary Figure S3 and Methods). Thanks to previous work in the generation of the many miniMos strains, (Frøkjær-Jensen et al. 2014), a single crRNA can be used on the tdTomato present in single copy in 147 different loci from strains that are available in the CGC, providing high flexibility in designing transgenic animals and downstream experiments.
GFP as an alternative FLInt landing site
Often, the choice of the co-injection marker is guided by the TOI. Thus, the tdTomato sites are incompatible if the TOI already contains a tdTomato fluorophore. Likewise, if the transgene encodes for a nuclear localized mCherry, downstream analysis can be confusing. With the aim of posing an alternative in those cases, we approached the single copy GFP marker strains described in (Frøkjær-Jensen et al. 2014) to assess if they could serve as a convenient alternative. We designed a pair of crRNAs to disrupt the GFP ORF (Supplementary Figure S4b) generating a deletion and verified that this event led both to a potent loss of GFP expression and to the generation of integrants (Supplementary Figure S4b). We then compared the GFP protospacer against the tdTomato protospacer by means of injecting a CRISPR mix that contained these crRNAs into a transgenic animal that had both landing sites. In the screening, we found around 34% of nonred/nongreen animals in the F1 and similar frequencies of animals with either loss of green or loss of red (Supplementary Figure S4b,c), suggesting that both crRNAs have comparable cutting efficiencies if their cognate loci. Together, these demonstrate that the single copy GFP loci serve as good alternative targets for FLInt. However, due to abundant gut autofluorescence and the generally weaker fluorescent signal, transgene screening is more difficult than in the tdTomato strains.
The efficiency of transgene integration varies with chromosomal position
Having shown that the single copy tdTomato can be used as FLInt landing sites, we wondered if the entire zoo of 147 possible landing sites would accept exogenously delivered transgenes with similar efficiencies. We thus selected a random set of landing sites distributed over all linkage groups, tested the integration potential of myo-2p::mCherry into 6 different background strains, one in each chromosome and calculated the integration efficiency from a standardized experiment (same injection mix, different landing sites, see Methods). We found that the most successful and efficient were on chromosomes I and II (oxTi556 I, 6.49%; oxTi564 II, 6.46%), followed by the landing sites on chromosomes X and III (oxTi668 X, oxTi619 III), 6.2% and 5.8%, respectively (Fig. 3a, Supplementary Table S1). These results showcase that, even when integration is possible on all linkage groups, it may be more probable in some than others, based on causes that are external to the transgene but internal to the landing site of the TOI. In addition to being on different chromosomes, landing sites are located at diverse genetic positions within each linkage group (LG1:1.23 and 22.30, LG2: 0.38 and 12.17, LG3:1.23 and 11.8, LG4:0.09 and 26.93, LG5:0.29, and LGX:1.29 and 4.88). In general, we found that the tdTomato landing sites in the center of the chromosome have higher efficiency compared with the farther tdTomato landing sites (Fig. 3b). This higher tendency for integrations at central sites also reflects the fact that more tdTomato landing sites are found closer to the center in the genetic map of each chromosome (compare Supplementary Figure S4b in Frøkjær-Jensen et al. (2014). In our hands, only one landing site (LG4:26.93) was not accepting any transgene after many trials, even though cutting efficiency was comparable to other loci. One explanation for the difference in integration efficiency at the chromosome arms may be the accessibility of the cellular machinery to the locus of interest. It has been previously described that C. elegans chromosome arms are regions rich in chromatin silencing marks (4 Mb from chromosome ends (Liu et al. 2011) and anchored to the nuclear envelope, being part of the peripheral heterochromatin, and with reduced accessibility of the DNA repair machinery (Ikegami et al. 2010). Along the same lines, the safe-harbor insertion sites described in the recently published MosTI work (El Mouridi et al. 2022) are also located in the permissive chromatin environment provided by the chromosome centers (Ho et al. 2014).
Even though we developed the integration procedure with a standardized injection mix to ensure that the results are independent of the DNA content, we also noted that the integration efficiency was superficially unchanged with the different target plasmids we used (see Supplementary Table S3). When the linkage group does not matter, we would recommend getting started with LG5, for which we obtained up to 23% integration efficiency in one of our trials. We also observed a high efficiency of integration (6.25% on oxTi553) with the use of animals that constitutively express Cas9 in the germline (Schwartz et al. 2021), but here the Cas9 needs to be crossed into the different strains carrying the desired landing site before the injection. Lastly, we asked if the different locations of the insertion sites could possibly lead to differences in downstream transgene expression. Because the integrated DNA exist as multicopy transgene with varying copy number (e.g. Fig. 2), we compared the fluorescence intensity of the original tdTomato loci (eft-3p::tdTomato::H2B) among the 6 linkage groups used in our experiments (EG7846 I, EG7860 II, EG7900 III, EG7905 IV, EG7944 V, and EG7985 X) and found that the tdTomato intensity from each strain is different (Fig. 3c) but uncorrelated with the genetic position based on their insertion sites. It should be noted that in this work we only assessed a small data set with the aim to gather more information that can help us infer the expression levels of our TOI in our candidate sites. However, a previous and exhaustive study of more than a hundred strains found that somatic transgene silencing is encountered with a higher probability at sites closer to the chromosome arms than in the center (Frøkjær-Jensen et al. 2016). Together, even though transgene integration at different loci varied, we concluded that transgene expression was unaffected (Fig. 3d) for the loci tested.
Together, our experiment revealed that the integration efficiency varies among the tdTomato inserted sites. The landing sites closer to the center appear to have higher efficiencies compared with the chromosome arms. Thus, in order to obtain the optimal integration efficiency, we suggest to use target loci closer to the center and in intergenic regions.
Integrating existing extrachromosomal arrays into fluorescent safe habor loci
Lastly, we were interested in the targeted integration of existing extrachromosomal arrays into the tdTomato site without the use of mutagens such as UV or TMP that cause pleiotropic DNA defects and require subsequent outcrosses. To do so, we first crossed the target strain bearing the extrachromosomal array with the desired tdTomato marker strain, which we injected with the tdTomato CRISPR ingredients. We introduced, though, a slight variation following the observations in Yoshina et al. (2016) in which they saw a correlation between integration frequency and fragmentation of the extrachromosomal array. This variation consisted of adding a crRNA that would target the Ampicillin resistance gene (which is present on the integrated vector plasmid), thus cutting the array in several pieces. Using the standard screening procedure (loss of NLS::tdTomato), we were able to recover 1 integrated line from 28 P0 (19 nonred F1) within 2–3 generations. The difference in the need of DNA cleavage between existing and de novo arrays probably lies in the fact that during the formation of the array, it already undergoes cleavage and assembly processes (Mello et al. 1991) that allow integration in one step. However, preexisting arrays are circular chromosomes (Woglar et al. 2020) and thus have no free ends. Hence, for NHEJ with the chromosomal cut site, the existing arrays was linearized in situ with a coinjected gRNA targeting the Ampicilin resistance gene. With the present method, we demonstrated that a previously generated extrachromosomal array can be integrated into the tdTomato cleavage site without the drawbacks of random mutagenesis.
Cas9-mediated disruption of tdTomato serves as a Co-CRISPR marker
A common bottleneck in the generation of CRISPR mutants is the efficient identification of successful gene edits. Without visible markers, PCR-based genotyping remains the ultimate option—a lengthy, tedious and potentially expensive process. Often, CRISPR-mediated genome editing in C. elegans is guided by a phenotypic conversion of an easily screenable co-CRISPR marker (Kim et al. 2014) that is eliminated after successful edits are isolated. In a successful edit, the mutated co-CRISPR locus results in an obvious phenotype which can be easily screened and distinguished from wild-type animals that were not edited. The marker phenotype thus, provides a visual representation of CRISPR efficiency and potentially reduces the number of progeny that eventually need to be sequenced to identify the desired edit. A large number of co-CRISPR marked progeny is indicative of putative edits at the gene of interest (GOI), always depending on the efficiency of the crRNA used for such locus. In C. elegans many co-CRISPR genes have been proposed. Among those, pha-1, unc-22, sqt-1, unc-58, ben-1, zen-4, and dpy-10 (Arribere et al. 2014; Kim et al. 2014; Ward 2014; Dickinson et al. 2015; El Mouridi et al. 2017) are popular, but may be problematic if its associated phenotype interferes with the GOI or is close to the target locus. Segregating alleles of genes that are in close proximity (e.g. dpy-10 from other LGII genes) becomes problematic, since it depends on the genetic distance between the 2 genes. Likewise, co-CRISPR methods can result in subtle mutations at the co-CRISPR locus not phenotypically associated with it that can be confounded with the edit at the GOI (Rawsthorne-Manning et al. 2022).
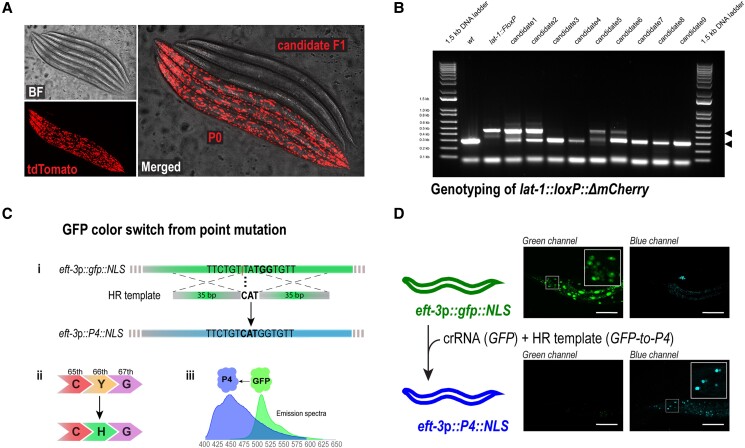
We have already shown that the efficiency to induce Cas9-mediated double-strand breaks of the crRNA for tdTomato is comparable, if not better, to the widely used dpy-10 crRNA (Table 1). Thus, tdTomato loci could pose an attractive alternative co-CRISPR locus, as its conversion does not result in any morphological or locomotion phenotype, and is “silent.” In addition, this could be beneficial when the co-CRISPR marker needs to be combined with a sublethal edit in essential genes that could lead to synthetic lethal phenotype (e.g. when combined with a dpy-10 or pha-1 co-CRISPR). Moreover, some phenotypic conversions (to a roller or a paralyzed animal), often preclude other phenotypic effects or can, in the worst cases, have a synthetic adverse effect with the desired gene modification. We specifically run into that problem when we designed a CRISPR edit for the GPCR lat-1, located physically close to dpy-10. We thus inserted a loxP::mCherry site at the lat-1 3′ end and used the tdTomato in oxTi390 IV as a co-CRISPR marker. After injection into P0 animals, the jackpot plates contained nonred worms (Fig. 4a) as well as some dimmer/mosaic red F1 progenies, which we interpreted as excised in only one chromosome (see above). We only selected the nonred F1(s) for PCR screening of the loxP::mCherry insertion at the lat-1 locus (Fig. 4b) and successfully identified several candidates (edit , ). Together, this result demonstrates that tdTomato can be used as a co-CRISPR locus that can be easily screened without causing a confounding phenotype and can be chosen such that it is not linked to the gene of interest and thus can be efficiently eliminated by outcrossing.
Fig. 4.
FLInt as co-CRISPR marker a: Representative images of a cohort of co-CRISPR’ed animals showing animals with the P0 phenotype and candidate F1. b: Screening PCR for lat-1::loxP. c: GFP to P4/BFP conversion as a homology-directed CRISPR marker. i) PAM sequence highlighted in bold, vertical line indicates Cas9 cutsite. ii) A single amino acid change in the GFP protein switches the absorption and emission wavelength to the blue. iii) Change in the emission spectrum from GFP to P4/BFP. X-axis indicates emission wavelength. d: Representative images of the co-converted GFP locus imaged for the GFP and BFP filtersets.
Compared with dpy-10(cn64), in which potentially successful edits can be identified as heterozygous repairs in dpy-10 and segregate the GOI from the dpy-10 locus, the excised tdTomato site is identified as homozygous. If elimination of the remains of tdTomato from the background is desired, the only possibility is out-crossing. However, the use of FLInt as co-CRISPR marker may involve more possibilities than for integration. Because only excision and not repair with the exogenous array is needed to successfully identify the candidates, the possible strains to be used increase with respect to the integration, in which we also need to account for higher probability of NHEJ repair with the array. In addition, there is the possibility of using a tdTomato close to the GOI if the edit is difficult to screen in subsequent steps (e.g. a point mutation w/o visible phenotype in crosses). In those cases, PCR for the remaining tdTomato could be used in the screening processes. An important issue to consider is the choice of the tdTomato strain to use. Even though some of the 147 tdTomato target sites are mapped to genes, they do not result in visible phenotypes at 20°C. However, whenever possible, intergenic safe habor sites should be used before starting an integration to avoid possible synthetic effects in downstream analyses.
Future extensions of FLInt: single nucleotide conversion of GFP to BFP as a marker for HR-directed repair
Dominant co-CRISPR markers, as the widely employed dpy-10(cn64), have the advantage that homology-directed repair can be visualized and distiguished from non homologous end joining repair directly in the F1 (Arribere et al. 2014). The use of tdTomato as a co-CRISPR marker, however, does not allow for such distinction in repair. To generate a co-CRISPR alternative for those cases, we propose the possibility of changing the emission spectra of GFP from green to blue through a single nucleotide change (P4, Fig. 4c) (Heim et al. 1994). Towards this goal, we designed a crRNA that cleaves the GFP sequence at the presumptive chromophore region together with an HR template that introduces the single point mutation to convert a tyrosine to an histidine at position 66. This genetic intervention switched the green emission spectrum of the GFP (508 nm) into the blue emission spectrum (448 nm). This simple modification can be made visible on a standard fluorescence microscope with an appropriate filter set (Fig. 4c (i, ii, iii)). After confirming that the crRNA efficiently cleaved the GFP sequence and led to a loss of GFP fluorescence in F1 animals, we added the HR template for the conversion and the corresponding mix for the GOI. We selected those F1 animals that showed a loss of GFP and emergence of blue fluorescence (Fig. 4d) which we used to screen for the edit at the GOI. However, because the P4/BFP fluorescence is rather weak as a heterozygous and might be difficult to see on standard epifluorescence stereoscope, similar strategies might provide larger contrast and easier screening. For example, the opposite conversion (from P4/BFP to GFP) yielded bright green fluorescence. Alternatively, a set of mutations centered around the tdTomato chromophore could be potentially mutated and the bright red turned into a bright green signal (Wiens et al. 2016). Together, these improvements might facilitate the use of GFP as a co-CRISPR marker, also when the GOI is linked closely to the traditional co-CRISPR locus and thus phenotypically interferes with the edits and/or cannot easily be unlinked through genetic breeding.
Conclusion
In summary, we leveraged a library consisting of 147 marker strains that carry a single copy of a histone-tagged tdTomato and 142 strains with a nuclear localized GFP (Frøkjær-Jensen et al. 2014) as safe harbor landing sites for ectopic transgene integration. Importantly, all of these integrations reside at different locations on the 6 chromosomes in C. elegans, providing previously unmatched flexibility in the genetic design and follow-up experiments. We demonstrated that these synthetic landing sites, encoding an ubiquitously expressed fluorescent protein, aided the identification of successful edits during transgene integration with several significant advantages: first, the integration site is known and precisely mapped, second, screening is facilitated through interference with the bright fluorescent signal indicating successful integration, third, a single crRNA can be used for all tdTomato landing sites, and, finally, because the intergenic landing site is known, the transgene integration does not cause any inadvertent phenotypes and defects. Together, these improvements in single shot transgenesis greatly reduce the time needed to screen for stable mutants, is flexible and cost effective, and has the potential to greatly accelerate research in C. elegans. In principle, this method can be extended to other invertebrate, vertebrate and mammalian model systems in which a single copy fluorescent gene is available as gene editing target sites.
Supplementary Material
Acknowledgments
We would like to thank the NMSB lab for trouble shooting and beta-testing the procedure, Ravi Das for discussions and Julian Ceron for comments on the procedures and the manuscript, and the CGC (National Institutes of Health - Office of Research Infrastructure Programs (P40 OD010440)) for providing research reagents.
Data availability
All strains and plasmids generated during this study are available upon request to the corresponding author. Strains harboring the safe landing site, including MSB1247 are available through CGC and their information is accessible on wormbuilder.org. Supplementary material are available at G3 online.
Funding
MK acknowledges financial support from the ERC (MechanoSystems, 715243), the PID2021-123812OB-I00 project funded by MCIN/AEI/10.13039/501100011033/FEDER, UE, “Severo Ochoa” program for Centres of Excellence in R&D (CEX2019-000910-S; RYC-2016-21062), from Fundació Privada Cellex, Fundació Mir-Puig, and from Generalitat de Catalunya through the CERCA and Research program (2017 SGR 1012).
Author contributions
NM and MK conceived the project, NM and MP performed experiments and analyses, MK and MP supervised the project and MK contracted funding. All authors wrote the first draft.
Literature Cited
- Arribere Ja, Bell RT, Fu BXH, Artiles KL, Hartman PS, Fire AZ. Efficient marker-free recovery of custom genetic modifications with CRISPR/CAS9 in caenorhabditis elegans. Genetics. 2014;198:837–846. doi: 10.1534/genetics.114.169730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concordet JP, Haeussler M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018;46:W242–W245. doi: 10.1093/nar/gky354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Lin LC, Català-Castro F, Malaiwong N, Sanfeliu-Cerdán N, Porta-De-la Riva M, Pidde A, Krieg M. An asymmetric mechanical code ciphers curvature-dependent proprioceptor activity. Sci Adv. 2021;7:1–20. doi: 10.1126/sciadv.abg4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DJ, Pani AM, Heppert JK, Higgins CD. Streamlined genome engineering with a self-excising drug selection cassette. Genetics. 2015;200: 1035–1049. doi: 10.1534/genetics.115.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mouridi S, Alkhaldi F, Frøkjær-Jensen C. Modular safe-harbor transgene insertion for targeted single-copy and extrachromosomal array integration in Caenorhabditis elegans. G3: Genes, Genomes, Genetics. 2022;12:jkac184. doi: 10.1093/g3journal/jkac184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mouridi S, Lecroisey C, Tardy P, Mercier M, Leclercq-Blondel A, Zariohi N, Boulin T. Reliable CRISPR/Cas9 genome engineering in Caenorhabditis elegans using a single efficient sgRNA and an easily recognizable phenotype. G3: Genes, Genomes, Genetics. 2017;7:1429–1437. doi: 10.1534/g3.117.040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay D. Genetic mapping and manipulation: Chapter 1–Introduction and Basics. WormBook. 2006;1–4. doi: 10.1895/wormbook.1.96.2. [DOI] [PMC free article] [PubMed]
- Friedland AE, Tzur YB, Esvelt KM, Church GM, Calarco JA. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat Methods. 2013;10: 741–743. doi: 10.1038/nmeth.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Davis MW, Ailion M, Jorgensen EM. Improved Mos1-mediated transgenesis in C. elegans. Nat Methods. 2012;9:117–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Davis MW, Hopkins CE, Newman B, Thummel JM, Olesen Sp, Grunnet M, Jorgensen EM. Single copy insertion of transgenes in C. elegans. Nat Genet. 2008;40:1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Davis MW, Sarov M, Taylor J, Flibotte S, LaBella M, Pozniakovsky A, Moerman DG, Jorgensen EM. Random and targeted transgene insertion in Caenorhabditis elegans using a modified Mos1 transposon. Nat Methods. 2014;11:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Jain N, Hansen L, Davis MW, Li Y, Zhao D, Rebora K, Millet JRR, Liu X, Kim SK, et al. An abundant class of non-coding DNA can prevent stochastic gene silencing in the C. elegans germline. Cell. 2016;166:343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano-Santini R, Milstein S, Svrzikapa N, Tu D, Johnsen R, Baillie D, Vidal M, Dupuy D. An antibiotic selection marker for nematode transgenesis. Nat Methods. 2010;7:721–723. doi: 10.1038/nmeth.1494. [DOI] [PubMed] [Google Scholar]
- Granato M, Schnabel H, Schnabel R. pha-1, a selectable marker for gene transfer in C.elegans. Nucleic Acids Res. 1994;22:1762–1763. doi: 10.1093/nar/22.9.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim R, Prasher DC, Tsien RY. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci USA. 1994;91:12501–12504. doi: 10.1073/pnas.91.26.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JW, Jung YL, Liu T, Alver BH, Lee S, Ikegami K, Sohn KA, Minoda A, Tolstorukov MY, Appert A, et al. Comparative analysis of metazoan chromatin organization. Nature. 2014;512:449–452. doi: 10.1038/nature13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K, Egelhofer TA, Strome S, Lieb JD. Caenorhabditis elegans chromosome arms are anchored to the nuclear membrane via discontinuous association with LEM-2. Genome Biol. 2010;11: R120. doi: 10.1186/gb-2010-11-12-r120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Ishidate T, Ghanta KS, Seth M, Conte D, Shirayama M, Mello CC. A Co-CRISPR strategy for efficient genome editing in Caenorhabditis elegans. Genetics. 2014;197:1069–1080. doi: 10.1534/genetics.114.166389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBella ML, Hujber EJ, Moore KA, Rawson RL, Merrill SA, Allaire PD, Ailion M, Hollien J, Bastiani MJ, Jorgensen EM. Casein kinase 1 stabilizes mature axons by inhibiting transcription termination of ankyrin. Dev Cell. 2020;52:88–103.e6. doi: 10.1016/j.devcel.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc C, Zhang F, Mendez J, Lozano Y, Chatpar K, Irish VF, Jacob Y. Increased efficiency of targeted mutagenesis by CRISPR/Cas9 in plants using heat stress. Plant J. 2018;93:377–386. doi: 10.1111/tpj.13782. [DOI] [PubMed] [Google Scholar]
- Liu T, Rechtsteiner A, Egelhofer TA, Vielle A, Latorre I, Cheung MS, Ercan S, Ikegami K, Jensen M, Kolasinska-Zwierz P, et al. Broad chromosomal domains of histone modification patterns in C. elegans. Genome Res. 2011;21:227–236. doi: 10.1101/gr.115519.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro MF. 20 years of unc-119 as a transgene marker. Worm. 2015;4:e1046031. doi: 10.1080/21624054.2015.1046031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariol MC, Walter L, Bellemin S, Gieseler K. A rapid protocol for integrating extrachromosomal arrays with high transmission rate into the C. elegans genome. J Vis Exp. 2013;82: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Mateos MA, Fernandez JP, Rouet R, Vejnar CE, Lane MA, Mis E, Khokha MK, Doudna JA, Giraldez AJ. CRISPR-Cpf1 mediates efficient homology-directed repair and temperature-controlled genome editing. Nat Commun. 2017;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble LM, Miah A, Kaur T, Rockman MV. The ancestral caenorhabditis elegans cuticle suppresses rol-1. G3 (Bethesda). 2020;10:2385–2395. doi: 10.1534/g3.120.401336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K, Jin Y. Rapid integration of multi-copy transgenes using optogenetic mutagenesis in Caenorhabditis elegans. G3 (Bethesda). 2018;8:2091–2097. doi: 10.1534/g3.118.200158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M. Additional landing sites for recombination-mediated cassette exchange in C. elegans. microPubl. Biol. 2021;2021:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML. Efficient transgenesis in caenorhabditis elegans using flp recombinase-mediated cassette exchange. Genetics. 2020;215:903–921. doi: 10.1534/genetics.120.303388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix A, Folkmann A, Seydoux G. Precision genome editing using CRISPR-Cas9 and linear repair templates in C. elegans. Methods. 2017;121–122:86–93. doi: 10.1016/j.ymeth.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta-de-la Riva M, Fontrodona L, Villanueva A, Cerón J. Basic Caenorhabditis elegans methods: synchronization and observation. J Vis Exp. 2012;64: e4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta-de-la Riva M, Gonzalez AC, Sanfeliu-cerdan N, Karimi S, Gonzales S, Morales-curiel LF, Hurth C, Krieg M. Deploying photons for communication within neuronal networks. bioRxiv. 2021.
- Praitis V, Casey E, Collar D, Austin J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 2001;157:1217–1226. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman I, Greiss S, Chin JW. Efficient and rapid C. elegans transgenesis by bombardment and hygromycin B selection. PLoS ONE. 2013;8: e76019. doi: 10.1371/journal.pone.0076019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawsthorne-Manning H, Calahorro F, Izquierdo PG, Tardy P, Boulin T, Holden-Dye L, O’Connor V, Dillon J. Confounds of using the unc-58 selection marker highlights the importance of genotyping co-CRISPR genes. PLoS ONE. 2022;17:1–13. doi: 10.1371/journal.pone.0253351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckher M, Tavernarakis N. Caenorhabditis elegans microinjection. Bio Protoc. 2017;7:e2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov M, Murray JI, Schanze K, Pozniakovski A, Niu W, Angermann K, Hasse S, Rupprecht M, Vinis E, Tinney M, et al. A genome-scale resource for in vivo tag-based protein function exploration in C. elegans. Cell. 2012;150:855–866. doi: 10.1016/j.cell.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ML, Wayne Davis M, Rich MS, Jorgensen EM. High-efficiency CRISPR gene editing in C. elegans using Cas9 integrated into the genome. PLoS Genet. 2021;17:1–18. doi: 10.1371/journal.pgen.1009755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple JI, Garcia-Verdugo R, Lehner B. Rapid selection of transgenic C. elegans using antibiotic resistance. Nat Methods. 2010;7:725–727. doi: 10.1038/nmeth.1495. [DOI] [PubMed] [Google Scholar]
- Silva-García CG, Lanjuin A, Heintz C, Dutta S, Clark NM, Mair WB. Single-copy knock-in loci for defined gene expression in caenorhabditis elegans. G3 (Bethesda). 2019;9:2195–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. WormBook. 2006;1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed]
- Stinchcomb DT, Shaw JE, Carr SH, Hirsh D. Extrachromosomal DNA transformation of Caenorhabditis elegans. Mol Cell Biol. 1985;5:3484–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RG. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu J, Gharib S, Chai CM, Schwarz EM, Pokala N, Sternberg PW. CGAL, a temperature-robust GAL4-UAS system for Caenorhabditis elegans. Nat Methods. 2017;14:145–148. doi: 10.1038/nmeth.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JD. Rapid and precise engineering of the Caenorhabditis elegans genome with lethal mutation co-conversion and inactivation of NHEJ repair. Genetics. 2014;199:363–377. doi: 10.1534/genetics.114.172361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens MD, Shen Y, Li X, Salem MA, Smisdom N, Zhang W, Brown A, Campbell RE. A tandem green–red heterodimeric fluorescent protein with high FRET efficiency. Chembiochem. 2016;17:2361–2367. doi: 10.1002/cbic.201600492. [DOI] [PubMed] [Google Scholar]
- Williams BD, Schrank B, Huynh C, Shownkeen R, Waterston RH. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics. 1992;131:609–624. doi: 10.1093/genetics/131.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woglar A, Yamaya K, Roelens B, Boettiger A, Köhler S, Villeneuve AM. Quantitative cytogenetics reveals molecular stoichiometry and longitudinal organization of meiotic chromosome axes and loops. PLoS Biol. 2020;18:1–30. doi: 10.1371/journal.pbio.3000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshina S, Suehiro Y, Kage-Nakadai E, Mitani S. Locus-specific integration of extrachromosomal transgenes in C. elegans with the CRISPR/Cas9 system. Biochem Biophys Rep. 2016;5:70–76. doi: 10.1016/j.bbrep.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All strains and plasmids generated during this study are available upon request to the corresponding author. Strains harboring the safe landing site, including MSB1247 are available through CGC and their information is accessible on wormbuilder.org. Supplementary material are available at G3 online.