Abstract
The genome sequence of the diploid and highly homozygous Vitis vinifera genotype PN40024 serves as the reference for many grapevine studies. Despite several improvements to the PN40024 genome assembly, its current version PN12X.v2 is quite fragmented and only represents the haploid state of the genome with mixed haplotypes. In fact, being nearly homozygous, this genome contains several heterozygous regions that are yet to be resolved. Taking the opportunity of improvements that long-read sequencing technologies offer to fully discriminate haplotype sequences, an improved version of the reference, called PN40024.v4, was generated. Through incorporating long genomic sequencing reads to the assembly, the continuity of the 12X.v2 scaffolds was highly increased with a total number decreasing from 2,059 to 640 and a reduction in N bases of 88%. Additionally, the full alternative haplotype sequence was built for the first time, the chromosome anchoring was improved and the number of unplaced scaffolds was reduced by half. To obtain a high-quality gene annotation that outperforms previous versions, a liftover approach was complemented with an optimized annotation workflow for Vitis. Integration of the gene reference catalogue and its manual curation have also assisted in improving the annotation, while defining the most reliable estimation of 35,230 genes to date. Finally, we demonstrated that PN40024 resulted from 9 selfings of cv. “Helfensteiner” (cross of cv. “Pinot noir” and “Schiava grossa”) instead of a single “Pinot noir”. These advances will help maintain the PN40024 genome as a gold-standard reference, also contributing toward the eventual elaboration of the grapevine pangenome.
Keywords: Vitis vinifera, genotype PN40024, reference genome, long reads, improved annotation
Introduction
Cultivated grapevine (Vitis vinifera ssp. vinifera) was the fourth plant whose genome was sequenced and assembled (Jaillon et al. 2007). Because of the grapevine's high level of heterozygosity [one Single Nucleotide Polymorphism (SNP) per 100 bp and one Indel per 450 bp, Velasco et al. 2007], the genotype selected for sequencing was PN40024, whose ∼475 Mb genome (Lodhi and Reisch 1995) is nearly homozygous (estimated at ∼93%). PN40024 was indeed generated through 9 rounds of selfing and supposedly originated from “Pinot noir”, hence its identification as “PN”. This unique genome characteristic allowed a high-quality whole-genome shotgun assembly based on 8X coverage Sanger reads (Jaillon et al. 2007). In 2009, a 4X coverage was added, which improved the overall coverage of the genome (from 68.9% for the 8X version to 91.2% for the 12X.v0) (http://urgi.versailles.inra.fr/Species/Vitis/Data-Sequences/Genome-sequences; FN597015-FN597047 at EMBL, release 102; Supplementary File 1 and Supplementary Fig. 1). In 2017, a third assembly version, named 12X.v2, was published as the result of a large anchoring effort using 6 dense parental genetic maps (Canaguier et al. 2017). Despite these advances, no additional sequencing efforts have been made and although it is of very high quality, the 12X.v0 Sanger contigs are numerous (14,642), the 12X.v2 scaffolds are composed of large N gaps (3.1% of the cumulative scaffold size) and the 19 pseudomolecules are quite fragmented (19.3 scaffolds on average per pseudomolecule).
In recent years, the advent of third generation sequencing technologies, especially those from the Pacific Biosciences (PacBio) platform, have allowed the assembly of grapevine diploid genomes with a higher level of contiguity compared to the 12X.v2 version of the PN40024 genome (e.g., cv. “Cabernet Sauvignon” genome assembly, Massonnet et al. 2020).
Along with the versions of each genome assembly, several versions of gene annotations were made available (Supplementary File 1 and Supplementary Fig. 1). The first version of the grapevine genome assembly, 8X, was published along with the prediction of 30,434 gene models based on the GAZE software (Howe et al. 2002; Jaillon et al. 2007). For the 12X.v0, 3 different versions of gene predictions were made available: the v0 version (26,346 gene models), based on the GAZE software (Howe et al. 2002), the CRIBIv1 version (29,971 gene models), based on the JIGSAW software (Allen and Salzberg 2005), and the CRIBIv2 version (31,845 gene models), with an effort made on the discovery of splicing variants (Vitulo et al. 2014). For the 12X.v2, the International Grapevine Genome Program (IGGP) led the initiative of merging annotations from NCBI Refseq, CRIBIv1, and VCost, which was based on the Eugene software (Sallet et al. 2019) and was generated in the frame of the COST Action FA1106. This version, called VCost.v3, resulted in an exhaustive view of the PN40024 grapevine gene content with its 42,413 gene models (Canaguier et al. 2017). However, after several years as the reference annotation by the grapevine scientific community, it appeared that the great increase in number of gene models for VCost.v3 compared to all the previous annotation versions was caused by many small and fragmented predictions that were probably erroneous.
By combining the top-quality Sanger contigs from the 12X version and long reads generated here by Single-Molecule Real-Time (SMRT) sequencing (PacBio), we provide an improved version of the PN40024 genome sequence assembly, referred to as PN40024.v4. Along with this new assembly, we also provide a new version of the gene annotation, PN40024.v4.2, based on a newly developed annotation workflow, RNA-Seq datasets and an exhaustive manual curation of a set of catalogued genes of functional interest to the community. Finally, we demonstrate that PN40024 originates from selfings of the “Helfensteiner” cultivar instead of “Pinot noir”.
Methods
Plant material, DNA extractions and sequencing
DNA extractions of young leaves of cv. “Pinot noir” clone 162 (ID code FRA038-193.Col.162), cv. “Schiava grossa” (synonymous “Trollinger”, ID code FRA038-2525.Col.1), and cv. “Helfensteiner” (ID code FRA038-2744.Col.1) were performed as described by Merdinoglu et al. (2005). Illumina DNA PCR-Free Prep kit was used to prepare the resequencing libraries according to provider procedures. Paired-end Illumina HiSeq 4000 sequencing at about 15× coverage was performed for “Pinot noir” and “Schiava grossa”, respectively. Paired-end Illumina NovaSeq 6000 sequencing at about 15× coverage was performed for “Helfensteiner”.
One gram of young leaves (1 cm2) of PN40024 (ID code FRA038-40024.Col.1) was collected and DNA was extracted using QIAGEN Genomic-tips 100/G kit. SMRT sequencing on a Sequel I machine (3 SMRTCells; PacBio) and dedicated library preparation were performed according to provider procedures.
Genotyping-by-sequencing (GBS) was performed on the population “Riesling” × “Gewurztraminer” [exhaustively described by Duchêne et al. (2020) using the procedure described by Girollet et al. (2019)].
All data generated in the frame of this study were submitted under the ENA Study Accession PRJEB45423.
Genome assembly
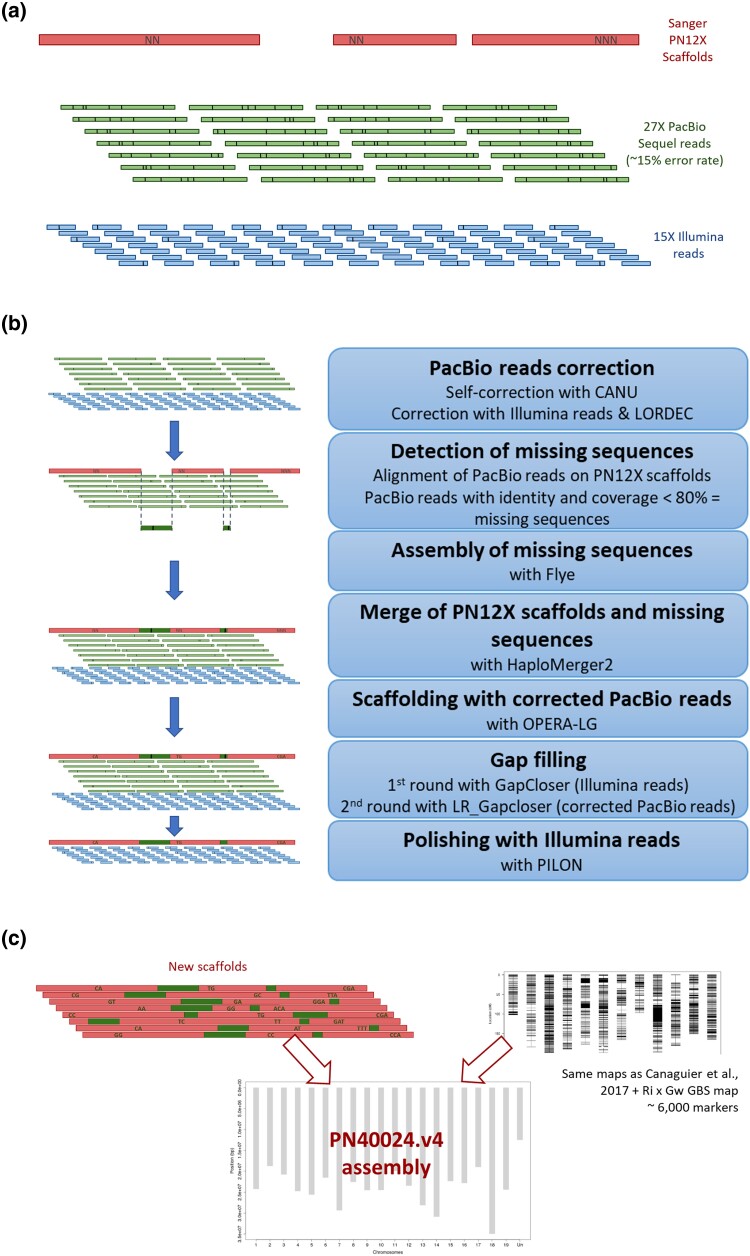
Raw SMRT reads (ERR7997743) were self-corrected using CANU (v.1.6) (Koren et al. 2017), followed by a correction with PN40024 Illumina reads (SRR8835144) using LORDEC (v.0.5.3) (Salmela and Rivals 2014). The corrected reads were mapped on PN12X scaffolds (https://urgi.versailles.inra.fr/download/vitis/VV_12X_embl_102_Scaffolds.fsa.zip) using minimap2 (v2.17-r954-dirty) (Li 2018). A total of 163,446 reads (15%) were aligned on <80% of their length and/or with <80% identity and were thus considered as missing from PN12X scaffolds. These unmapped reads were assembled using Flye (v2.4-gc9db046) (Kolmogorov et al. 2019). We aligned these new contigs on the Uniprot Arabidopsis database (release 2019_01) using blastx (Altschul et al. 1990). Contigs longer than 5 kb and having hit(s) with Arabidopsis proteins with >60% identity and >60% length coverage, were selected for the next step. The fasta files of these new contigs and the PN12X scaffolds were concatenated to generate the new assembly. Firstly, the repeats were masked using Red (v05/22/2015) (Girgis 2015). Then, Haplomerger2 (v20180603) (Huang et al. 2017) was used following 3 steps according to developer procedures: (1) break the misjoins and output the new diploid assembly; (2) separate/merge 2 haplotypes and output haploid assemblies (REF and ALT); and (3) remove tandem errors from haploid assemblies. Some scaffolds/contigs were deleted by Haplomerger2 during the assembly process but sequences longer than 10 kb were retrieved and added to the REF scaffolds. The 2 haploid assemblies (REF/ALT) were then scaffolded with the OPERA-LG tool (v2.0.6) (Gao et al. 2016), which uses both, corrected SMRT reads and Illumina reads. A first gap-filling step (2 rounds) was carried out with Illumina reads using GapCloser (v1.12) (Luo et al. 2012) and a second gap-filling step (3 rounds) was carried out with corrected SMRT reads using LR_Gapcloser (v1.0) (Xu et al. 2019). A final polishing step was performed with the Illumina reads using PILON (v1.23-1-g41e0b8e) (Walker et al. 2014) (Fig. 1, a and b).
Fig. 1.
Assembly process for the PN40024.v4 genome sequence assembly. a) Initial datasets: Sanger-based scaffolds of PN12X.v2 with unknown bases (“N's”), genomic PacBio SMRT reads, and genomic Illumina short reads. Erroneous bases are represented by vertical lines. b) Scaffold assembly steps. Dark regions represent newly incorporated PacBio SMRT assembled regions. c) Pseudomolecule construction using the new scaffolds and genetic maps. The new scaffolds are a mosaic of 12X.v2 scaffolds and newly incorporated PacBio SMRT assembled regions.
The anchoring of the new haploid scaffolds was performed using the 6 genetic maps used for the same purpose by Canaguier et al. (2017) and 2 new genetic maps from cv. “Riesling” and cv. “Gewurztraminer” derived from GBS. To transfer the markers from Canaguier et al. (2017) from PN12X.v2 to the scaffolds of PN40024.v4, BLAST (v2.2.28) (Altschul et al. 1990) or ipcress (ipcress from exonerate v2.2.0) (Slater and Birney 2005) was used to align the markers and find the position of each on the scaffolds of PN40024.v4 REF and ALT. A total of 2,333 markers for REF and 2,326 markers for ALT were used from these 6 maps to anchor the scaffolds. For the 2 new genetic maps from “Riesling” and “Gewurztraminer”, 5,884 (“Riesling”) and 5,840 (“Gewurztraminer”) SNP markers were available for REF and 5,866 (“Riesling”) and 5,832 (“Gewurztraminer”) for ALT. The SNP markers were derived from GBS data (ERR8657388 to ERR8657647) and were analyzed with Fast-GBS (Torkamaneh et al. 2017) with modifications to allow paired-end read analysis (https://forgemia.inra.fr/sophie.blanc/gbs). The 2 genetic maps were built using R ASMap package with the “kosambi” parameter (Taylor and Butler 2017). A first run of Allmaps (v0.9.13) (Tang et al. 2015) was performed with the “merge” command to merge all genetic maps and then “split”, “gaps”, “refine” and “build” commands to create breakpoints (58 for REF scaffolds and 47 for ALT scaffolds), with default parameters. Subsequently, all maps were recreated for new scaffolds and then orientation and anchoring of new haploid scaffolds on the 19 pseudochromosomes were performed using Allmaps with the “merge” command to merge all maps and “path” command to anchor, with default parameters (Fig. 1c).
Quality assessment of the PN40024.v4 genome sequence assembly
A quality analysis of the genome assembly was done with Merqury v1.3 (Rhie et al. 2020). Since PN40024 is a “Helfensteiner” selfing (demonstrated below) and since “Helfensteiner” originated from a cross between “Pinot noir precoce” and “Schiava grossa”, “Schiava grossa” was used as the maternal parent. The run was carried out on the scaffolds using genomic paired-end short reads of PN40024 as the child data (SRR8835144), short reads of “Pinot noir” as the paternal data (ERR8014965) and short reads of cv. “Schiava grossa” as the maternal data (ERR8014964). A k-mer database was built for the 3 read datasets with k = 19, the Merqury hap-mer databases were computed and the PN40024.v4 genome assembly was evaluated using “num_switch 100” and “short_range 20,000”. For comparison reasons, the Merqury quality analysis was carried out on PN12X.v2 using the same k-mer databases.
The “Flowering locus T” (FT) and the “Adenine phosphoribosyltransferase 3” (APRT3) genes are absent and truncated in PN12X.v2, respectively. To check whether these genes could be retrieved in the new genome assembly, cDNA sequences of FT (NM_001280978.1) and APRT3 (GSVIVT00007310001, PN8X version) were used to perform blastn (Altschul et al. 1990) against PN8X, PN12X, PN12X.v2, and PN40024.v4 genome assemblies. High scoring pairs were then accumulated for each analysis and the mean percentage identity, query overlap, hit query start and end were calculated.
PN40024 (SRR8835144), and the cultivars “Silvaner Gruen” (SRR5891620), “Cabernet Franc” (SRR5891774), “Cabernet Sauvignon” (SRR5891776), “Chardonnay” (SRR5891778), “Muscat Hamburg” (SRR5891787), “Semillon” (SRR5891866), “Pinot noir” (SRR5891886), “Merlot” (SRR5891890), “Sauvignon Blanc” (SRR5891893), “Muscat of Alexandria” (SRR5891985), and “Riesling” (SRR5891989) genomic paired-end resequencing datasets were aligned against PN40024.v4 REF, PN12X.v2 and “Cabernet Sauvignon” haplotype 1 (Massonnet et al. 2020) pseudomolecule assemblies (without chrUn or unplaced contigs/scaffolds) using bwa-mem2 (v2.0) (Vasimuddin et al. 2019) “mem” command with default parameters. “Samtools” (v1.9) (Li et al. 2009) “flagstat” command was used with default parameters to compute alignment statistics.
PN12X scaffolds were mapped against PN40024.v4 REF pseudomolecules using NUCmer (MUMmer v3.1) (Kurtz et al. 2004) with “-maxmatch -l 100 -c 500” parameters. The output file was filtered using MUMmer show-coords command with “-l -g -I 99.5” parameters. The resulting file was formatted into BED format and merged with the bed file corresponding to N gap regions in the PN40024.v4 assembly. Pseudomolecule regions over 100 bp that did not correspond to either PN12X scaffolds or N gap regions were identified as “newly assembled” PacBio long read-based regions.
The identification of variants between PN40024 paired-end Illumina resequencing (SRR8835144) and PN40024.v4 REF and ALT pseudomolecules was performed as described in the section “Origin of PN40024”. The homozygous calls “1/1” were considered as assembly errors. The densities of the heterozygous calls “0/1” along the REF and ALT pseudomolecules were used to define 7 heterozygous regions of the PN40024 genome.
Origin of PN40024
PN40024 (SRR8835144), “Pinot noir” (ERR8014965), “Schiava grossa” (ERR8014964), “Helfensteiner” (ERR8014963), and “Araklinos” (SRR8835172) paired-end resequencing datasets were all analyzed using the same pipeline. Datasets were aligned against PN40024.v4 REF assembly using bwa-mem2 (v2.0) (Vasimuddin et al. 2019) “mem” command with default parameters. Samtools (v1.9) (Li et al. 2009) “view” and “sort” commands with default parameters were used to convert and sort the output BAM files. GATK (v4.1.4.0) (McKenna et al. 2010) “MarkDuplicatesSpark”, “HaplotypeCaller”, and “GenotypeGVCFs” commands with default parameters were used to generate variant files in VCF format. The GATK “VariantFiltration” command was used to filter out variants meeting at least one of the following criteria: QD < 8.0, QUAL < 100.0, FS > 60.0, SOR > 3.0, DP < 3, DP > 30, AD < 2. The final variant files were obtained using GATK “SelectVariants” command with “–exclude-filtered –exclude-non-variants” parameters. The homozygous SNP calls “1/1” were selected for each analyzed genotype. All SNPs corresponding to a homozygous call in PN40024 genotypes were excluded from the analysis as they represent assembly errors. The remaining homozygous SNPs were used to draw density plots on the PN40024.v4 pseudomolecules. The regions that are rich in homozygous SNPs for a given genotype correspond to regions for which this genotype does not share a haplotype with PN40024.
The haplotypic blocks were defined after segmentation of homozygous SNP densities along the chromosomes using the R package changepoint (v2.2.2) (Killick and Eckley 2014) with command “cpt.mean” and the parameters method=“PELT” and penalty=“AIC”. Some manual curation of the segments was performed to join directly adjacent segments of the same origin (“Pinot noir” or “Schiava grossa”). The size of the segments was used to calculate the proportion of “Pinot noir”, “Schiava grossa”, and common haplotypes.
Gene prediction
Before performing gene prediction, the PN40024.v4 genome assembly was repeat masked with RepeatMasker v4.1.2 (Smit et al. 2013) using crossmatch as search engine. Predictions with a Smith–Waterman (SW)-Score <1,000 were filtered out and predictions with a SW-Score between 1,000 and 2,000 were only kept if the reported percentage of substitutions were <20%. The PN40024.v4 genome assembly was softmasked with BEDTool (v2.26.0) (Quinlan and Hall 2010).
To annotate the PN40024.v4 genome assembly, publicly available V. vinifera stranded (Supplementary File 2 and Supplementary Table 1) and unstranded (Supplementary File 2 and Supplementary Table 2) paired-end RNA-Seq datasets of different tissues and treatments were collected. RNA-Seq data were trimmed with Trimmomatic (v0.39) (Bolger et al. 2014). The annotation pipeline was first tested on the PN40024 12X.v0 genome assembly using VCost.v3 gene annotation as quality reference. The gene predictors SNAP (Korf 2004) and BRAKER2 (Hoff et al. 2016, 2019; Brůna et al. 2021) were trained and tested on the softmasked 12X.v0 genome assembly. The RNA-Seq data was mapped on 12X.v0 and on PN40024.v4 REF and ALT sequences with GMAP/GSNAP v2020-09-12 setting “-B 5 –novelsplicing 1” (Wu and Watanabe 2005). Primary mappings were extracted with SAMTools v1.9 (Li et al. 2009). Based on the primary mappings, stranded and unstranded reference-guided transcriptome assemblies were computed with PsiCLASS v1.0.1 using default parameters (Song et al. 2019).
Additionally, Arabidopsis thaliana protein sequences (UniProt/SwissProt release 2020_02), eudicotyledone protein sequences (UniProt/SwissProt release 2020_02, OrthoDB10 v1), and Viridiplantae and Vitales sequences (UniProt/SwissProt release 2020_02) were aligned on 12X.v0 and on PN40024.v4 REF and ALT with pBLAT v1.9 (Wang and Kong 2019), a parallel implementation of the original blat algorithm (Kent 2002). The genome regions on which the protein data mapped were extracted and the protein sequences were aligned to these regions with exonerate v2.4.0 (Slater and Birney 2005). Only the proteins that aligned on the reference genome with an identity of 25%, a similarity of 50% and with a sequence alignment coverage of at least 80%, were retained and included in the gene prediction.
The gene predictor GlimmerHMM v3.0.4 (Majoros et al. 2004) was trained on 12X.v0 and on PN40024.v4 REF and ALT using 7,500 (12X.v0) and 15,000 (PN40024.v4) random PsiCLASS transcripts of the 12X.v0 or PN40024.v4 REF or ALT stranded transcriptome assembly, respectively. The training was followed by gene prediction with GlimmerHMM with default settings.
Moreover, the gene predictor SNAP v2006-07-28 was trained on the 12X.v0 genome assembly. For this, the 12X.v0 genome assembly, the stranded transcriptome assembly, the Viridiplantae protein sequences, and the eudicotyledone protein sequences were given to MAKER2 v3.01.03 (Holt and Yandell 2011; Campbell et al. 2014) and initial data alignment with BLAST (ncbi-blast-2.10.1+) (Altschul et al. 1990; Camacho et al. 2009) and exonerate was performed followed by MAKER2 ab initio gene prediction. MAKER2 was run with “max_dna_len = 300000” and “split_hit = 20000”. A SNAP hmm file was generated with the MAKER2 gff file and a second MAKER2 run was performed with enabled SNAP gene prediction and the SNAP hmm file as input. Hmm file generation and SNAP gene prediction with MAKER2 and the new hmm file were repeated. The hmm file generated with the 12X.v0 assembly was used to run SNAP gene prediction on the PN40024.v4 REF and ALT genome sequences.
An AUGUSTUS species model was computed with BRAKER2 v2.1.5-master_20200915 and the 12X.v0 genome assembly. BRAKER2 was run with enabled softmasking and in etpmode calling GeneMark-ETP + v4.61 (Lomsadze et al. 2005, 2014; Brůna et al. 2020) for initial gene prediction followed by AUGUSTUS training and gene prediction (AUGUSTUS version master_v3.3.3_20200914) (Stanke et al. 2006, 2008). With BRAKER2, the programs DIAMOND v0.9.24.125 (Buchfink et al. 2015), SAMtools v1.9-180-gf9e1caf (Li et al. 2009), SPALN version 2.3.3f (Gotoh 2008; Iwata and Gotoh 2012), ProtHint version 2.5.0, and BamTools v2.5.1 (Barnett et al. 2011) were called. The stranded RNA-Seq primary mappings, the eudicotyledon protein sequences (OrthoDB10 v1), and the Viridiplantae protein sequences were used as input. The gene prediction on PN40024.v4 REF and ALT was performed with BRAKER2 v2.1.5-master_20210218, the generated AUGUSTUS species model, and AUGUSTUS version master_v3.4.0_20210218. Again, the stranded RNA-Seq mappings and the same protein sequences were used as input. The BRAKER2 parameter settings were left the same as above.
The last ab initio gene prediction was done on the PN40024.v4 genome assembly with GeneID v1.4.5-master-20200916 and the publicly available V. vinifera parameter set using default settings. To add the VCost.v3 gene annotation to the set of predictions, an annotation liftover was performed with liftoff v1.5.1 (Shumate and Salzberg 2021) with default parameters onto the PN40024.v4 genome assembly.
To combine the predictions and evidence data into an overall gene model set, the GlimmerHMM, SNAP, BRAKER2, and GeneID ab initio gene prediction as well as the lifted VCost.v3 annotation, the stranded and unstranded transcriptome assemblies, the GFF file with the aligned protein data, the repeat annotation GFF file, and the PN40024.v4 genome assembly were given to EvidenceModeler v1.1.1 (Haas et al. 2008). The used weights are listed in Supplementary File 2 and Supplementary Table 3.
Subsequently, the raw gene models were quality filtered. Gene models only supported by ab initio predictors were kept if at least 2 gene prediction programs predicted them, if the start and stop codon was present and if the gene length was equal or larger than 300 bp. However, ab initio supported gene models not matching these constraints were kept if they had a database hit with the UniProt/SwissProt or NCBI nonredundant database. To obtain that, a blastp search of the protein sequences against the 2 databases was run, allowing hits with an e-value <1e−6. Of the gene models only supported by evidence data or by VCost.v3 lifted annotation, those gene models with missing start and stop and a gene length <300 bp were discarded.
The gene models generated by EvidenceModeler were finally processed by PASA (v2.4.1, default parameters) using the stranded transcriptome assembly as a reference to add UTR regions and to calculate alternatively spliced models. Genes with overlapping UTRs were shortened. tRNAs were predicted with tRNAscan-SE-2.0 (Chan et al. 2021) on the PN40024.v4 genome assembly.
To retain gene naming of VCost.v3 gene models, a reciprocal best blast hit (RBH) search between protein sequences of PN40024.v4.1 gene models and protein sequences of VCost.v3 gene models was carried out. For the RBH search, only the longest protein sequence per gene was used, the e-value was set to 1e−4 and the query coverage and identity was set to 70%. Moreover, only RBHs with genes on the same pseudochromosome and showing collinearity with other genes were considered valid. Thus, genes with a valid RBH were named according to the VCost.v3 gene, novel genes received the prefix “04” at the start of the gene number and genes predicted for alternative heterozygous sequence regions received the suffix “_alt” (Supplementary File 2 and Supplementary Table 4).
The PN40024.v4.1 gene models were functionally annotated with Blast2GO (v1.5.1) (Conesa et al. 2005; Götz et al. 2008). For this, protein domains of the PN40024.v4.1 proteins were identified with InterProScan (v5.52-86.0) (Jones et al. 2014) with options “–goterms –pathways -dp” using the databases/tools CDD-3.18 (Lu et al. 2020), Coils-2.2.1 (Lupas et al. 1991), Gene3D-4.3.0 (Sillitoe et al. 2019), Hamap-2020_05 (Pedruzzi et al. 2013), MobiDBLite-2.0 (Necci et al. 2017), PANTHER-15.0 (Mi et al. 2021), Pfam-33.1 (Mistry et al. 2021), PIRSF-3.10 (Wu et al. 2004), PIRSR-2021_02, PRINTS-42.0 (Attwood et al. 2012), ProSitePatterns-2021_01, ProSiteProfiles-2021_01 (Sigrist et al. 2013), SFLD-4 (Akiva et al. 2014), SMART-7.1 (Letunic and Bork 2018), SUPERFAMILY-1.75 (Gough et al. 2001; Wilson et al. 2009), and TIGRFAM-15.0 (Haft et al. 2013). PN40024.v4.1 protein sequences were aligned with diamond “blastp” (v2.0.11) (Buchfink et al. 2015) to the NCBI nr database (nr.07_07_2021.fasta) with options “–sensitive –top 5 -e 1e-5 -f 5”. InterProScan and diamond results were used as input for Blast2GO.
Quality assessment of the PN40024.v4.1 gene annotation
To estimate completeness of the PN40024.v4.1 gene model set, plant core genes were predicted with BUSCO v5.1.2 (Simão et al. 2015; Waterhouse et al. 2018) using the database eudicots_odb10.
Samples previously analyzed by Palumbo et al. (2014) were used to perform differential gene expression analysis by using either PN12X.v2 assembly with VCost.v3 annotations or PN40024.v4 assembly with PN40024.v4.1 annotations. We analyzed cv. “Sangiovese” (SRR1631822; SRR1631823; SRR1631824), cv. “Barbera” (SRR1631834; SRR1631835; SRR1631836), and cv. “Refosco” samples (SRR1631858; SRR1631859; SRR1631860) for the stage “Berries beginning to touch” (∼EL35 according to Eichhorn and Lorenz phenological scale, Eichhorn and Lorenz 1977). The RNA-Seq data were downloaded from the SRA with SRA Toolkit (v2.10.8) (SRA Toolkit Development Team; https://trace.ncbi.nlm.nih.gov/Traces/sra/sra.cgi?view=software) and analyzed with an in-house pipeline using FASTQC (v0.11.5) (Andrews 2010), STAR (v2.5.3a) (Dobin et al. 2013), SAMtools (v1.4.1) (Li et al. 2009), Bamtools (v2.4.0) (Barnett et al. 2011), featureCounts (v1.5.3) (Liao et al. 2014), and SARTools (v1.7.3) (Varet et al. 2016).
Manual gene model curation
For manual gene model curation, an Apollo Webserver v2.6.4 (https://github.com/GMOD/Apollo/blob/master/README.md) (Dunn et al. 2019) was set up for the PN40024.v4 genome assembly and provided with different data tracks such as PN40024.v4.1 and previous gene annotations, RNA-Seq mappings and exonerate protein mappings (see Gene prediction). By these means, gene models were manually inspected and curated if needed or also new genes were added following dedicated guidelines offered to the community (https://integrape.eu/resources/data-management/). Using Apollo, the plant core genes classified as fragmented or missing by BUSCO were manually curated and adapted if necessary. In the frame of this study, we also began to manually curate genes present in the grape reference catalogue (Navarro-Payá et al. 2022; https://grapedia.org/genes/). A home-made python script was used to generate the PN40024.v4.2 version of gene annotations including those manually curated (https://gitlab.com/MSVteam/pn40024-visualization-tools/-/tree/master/update_gff3_script).
Results and discussion
Improved metrics for the genome assembly of PN40024
A hybrid strategy was developed to assemble the genome of PN40024 genotype using 27X of SMRT long reads along with the PN12X scaffolds and 15X PN40024 Illumina paired-end resequencing data (Fig. 1). This new assembly was named PN40024.v4. Six hundred and forty scaffolds were produced with a N50 size of 6.5 Mb for a cumulative size of 474.5 Mb for the PN40024.v4 REF haplotype (Table 1). Compared to the former PN12X.v2, the number of scaffolds was reduced by a factor of 3 and the N50 was doubled. Moreover, the number of unknown bases, marked as N in the new scaffold sequences, represents 1.8 Mb and 0.4% of the assembly size versus 15.0 Mb and 3.1% for PN12X.v2 scaffolds. Thus, PN40024.v4 REF is more contiguous and has more informative sequences than PN12X.v2. Also, the PN40024.v4 assembly size is closer to the grapevine genome size of 475 Mb, estimated using flow cytometry by Lodhi and Reisch (1995). Phasing efforts on the partially heterozygous genotype resulted in the reconstruction of the second PN40024 haplotype (PN40024.v4 ALT) with 485 scaffolds and a total genome assembly size of ∼463 Mb (Supplementary File 2 and Supplementary Table 5). Thus, the PN40024.v4 genome assembly now represents both haplotypes of the diploid PN40024 genome.
Table 1.
Assembly statistics of the PN40024.v4 REF and PN12X.v2 genome assembly. The table lists statistics of the PN40024.v4 REF and PN12X.v2 scaffolded and chromosome-anchored genome assemblies. N denotes the number (No.) of unknown bases.
| Scaffolds | No. Scaf. | Min. size [bp] | Avg. size [kb] | Median size [kb] | L50 | N50 [Mb] | Max. size [Mb] | Sum [Mb] | No. N | GC [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| PN12X.v2 | 2,059 | 2,001 | 236 | 5 | 41 | 3.43 | 13.10 | 485.19 | 14,976,411 | 33.5 |
| PN40024.v4 | 640 | 542 | 742 | 20 | 25 | 6.50 | 15.23 | 474.61 | 1,755,062 | 34.4 |
| Anchored PN12X.v2 | 367 | 2,010 | 1,250 | 277 | 37 | 3.57 | 13.10 | 465.64 | 11,921,253 | 33.6 |
| Anchored PN40024.v4 | 165 | 1,085 | 2,801 | 1,506 | 24 | 6.57 | 15.23 | 462.14 | 1,631,047 | 34.4 |
There are 7,640 newly assembled PacBio long read-based regions that were identified as missing from PN12X.v2 scaffolds. Their cumulative size is 24.1 Mb, that is 5.1% of the total PN40024.v4 genome assembly size (average = 3,152 bp; median = 558 bp; max = 32,650 bp).
A total of 2,333 markers were used from the 6 Canaguier's maps (Canaguier et al. 2017), in addition to 5,884 and 5,840 SNP markers from cv. “Riesling” and cv. “Gewurztraminer” GBS maps, respectively, to anchor these scaffolds. We were able to anchor 165 PN40024.v4 REF scaffolds to the 19 pseudochromosomes, for a cumulative size of ∼462 Mb (97.4%) (Table 1). The 19 PN40024.v4 REF pseudomolecules are composed of 8.7 scaffolds on average (min = 3; max = 26; median = 6) whereas 19.3 scaffolds on average composed the PN12X.v2 pseudomolecules (min = 5; max = 82; median = 13). The remaining unplaced scaffolds were ordered according to their size to generate “chrUn” sequence representing 12.5 Mb (−47% compared to PN12X.v2 unplaced scaffolds). Thus, PN40024.v4 anchoring was improved as the pseudomolecules are less fragmented and as the size of chrUn has almost been halved.
At the chromosome scale, 10 pseudomolecules became shorter in PN40024.v4 compared to PN12X.v2 (average loss = ∼448 kb; median = ∼255 kb; min = 2,961 bp; max = 1,133,439 bp). Chromosome 6 showed the biggest reduction as the location of a large fragment has been correctly assigned to chromosome 9 (Supplementary File 1 and Supplementary Fig. 2). Nine pseudomolecules became larger (average gain = ∼869 kb; median = ∼582 kb; min = 15,118 bp; max = 2,045,414 bp), notably chromosome 9, 7, and 15, which gained 1.5, 1.9, and 2.0 Mb, respectively.
By aligning PN40024 Illumina paired-end reads against PN40024.v4 genome assembly, we identified 101,778 heterozygous variations. Using their density along the chromosomes, we were able to identify 7 well-defined heterozygous regions in PN40024.v4 genome assembly as it was the first time that a software dedicated to diploid assembly (Haplomerger2) was used to assemble the PN40024 genome. These regions were located on chromosomes 2, 3, 4, 7, 10, 11, and 15 with the 2 largest regions being on chromosome 7 and 10 (11.4 and 5.5 Mb, respectively) (Fig. 3). Their overall cumulative size of 20.6 Mb represents 4.3% of PN40024.v4, which is less than the residual heterozygosity size of 7%, estimated by Jaillon et al. (2007) based on genetic markers. Using the same procedure, we identified 6 heterozygous regions in PN12X.v2 assembly on the same chromosomes as PN40024.v4 except for the one on chromosome 15. Their overall cumulative size of 16.6 Mb represents 3.4% of PN12X.v2 and 4 Mb less than the heterozygous regions anchored on the PN40024.v4 chromosomes. These sequences were badly resolved and mostly located in the unanchored fraction of PN12X.v2 assembly (Supplementary File 1 and Supplementary Fig. 2). Thus, we conclude that PN40024.v4 is a better diploid assembly compared to PN12X.v2.
Fig. 3.
Location of regions assembled using long reads, density of errors and of heterozygous SNPs in the PN40024.v4 genome sequence assembly. The x-axis shows the 19 main pseudochromosomes and the artificial chrUn (“Un”). The y-axis shows the base position in [bp]. “Pacbio regions” refers to sequences derived from genomic SMRT reads. The 7 heterozygous regions are squared.
Quality of the PN40024.v4 genome assembly
The BUSCO analysis performed on the PN40024.v4 genome assembly confirmed that the gene space was more complete with 98.1% of the 2,326 total searched Eudicots BUSCO genes being complete, compared to PN12X.v2 with 97.6% (Fig. 6). The FT gene is conserved among all flowering plants as it promotes transition from vegetative growth to flowering. However, its sequence could only be found on an unanchored scaffold in the PN8X version and was totally missing in PN12X.v0 and PN12X.v2. It is now present on chromosome 7 of the PN40024.v4 assembly and also on its allelic region, chromosome 7_ALT sequence. Similarly, the APRT3 gene, located in the sex determination locus of grapevine, was present on chromosome 2 in the PN8X version and was truncated in PN12X.v0 and PN12X.v2. It is now fully retrieved on chromosome 2 of PN40024.v4 assembly and on its allelic region, chromosome 2_ALT sequence. These 2 examples, along with the BUSCO analysis, show that the PN40024.v4 assembly is more complete, especially in the residual heterozygous regions that are now more accurately exposed.
Fig. 6.
Plant core genes of the PN40024.v4 and PN12X.v2 genome assemblies and their annotations. The 2,326 plant core genes of the database eudicots_odb10 were determined in the PN40024.v4 genome assembly, in its annotation PN40024.v4.1, in the PN12X.v2 genome assembly and in the VCost.v3 gene annotation. “PN40024.v4.2” is the PN40024.v4 gene annotation after manual curation of the fragmented and missing plant core genes.
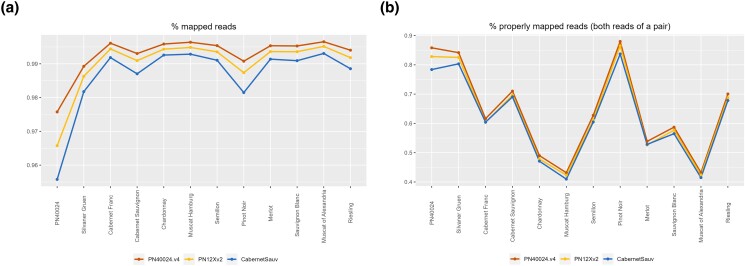
The alignment metrics of PN40024 genomic Illumina paired-end reads have always been better against PN40024.v4 compared to PN12X.v2, either for overall percentage of mapped reads (97.58% vs 96.58%) or for properly mapped pairs of reads (85.81% vs 82.82%) (Fig. 2). This confirms that the PN40024.v4 assembly is more complete and with a more accurate structure than PN12X.v2. Moreover, we compared alignments of 11 genomic Illumina paired-end read datasets from various cultivars against PN40024.v4 and PN12X.v2 assemblies, but also against “Cabernet Sauvignon” (Massonnet et al. 2020) haplotype 1, whose assembly metrics and technology were similar to PN40024.v4. Again, PN40024.v4 performs best for each dataset, even when “Cabernet Sauvignon” was aligned against its own assembly (Fig. 2). These results confirm that PN40024.v4 shows a quality suitable to become the new grapevine reference genome assembly, as it performs well with aligning genomic reads of various V. vinifera cultivars.
Fig. 2.
Percentage of mapped genomic reads (a) and percentage of properly paired genomic reads (b) between PN40024.v4, PN12X.v2 and cv. “Cabernet Sauvignon” (Massonnet et al. 2020) for 11 paired-end resequencing datasets of V. vinifera cultivars. The x-axis denotes the source (cultivar) of the genomic reads and the y-axis the percentage [%] of mapped reads. Note that the PN40024 dataset was obtained with Illumina Genome Analyzer IIx sequencing and all other samples with Illumina HiSeq 4000. The PN40024 dataset is therefore of lower quality than the others.
The error rate at nucleotide level was assessed by calling homozygous variations between PN40024 genomic Illumina paired-end reads aligned against the PN40024.v4 genome assembly. We identified 28.7 compared to 8.4 errors/Mb in the PN12X.v2 genome assembly. However, they are unevenly distributed along the chromosomes and they mostly co-localize with the newly assembled long read-based regions and the 7 heterozygous regions (Fig. 3). A higher density of errors was also detected in the heterozygous regions of the PN12X.v2 genome assembly (Supplementary File 1 and Supplementary Fig. 3). We detected 284.4 errors/Mb in PN40024.v4 heterozygous regions and 83.1 errors/Mb in PN12X.v2 heterozygous regions, which is, respectively, about 10 times denser than their average error rate. Thus, the overall increase of error rate in the PN40024.v4 assembly is mostly due to the use of SMRT long reads to improve the completeness of the reference genome assembly.
Using Merqury, the base level quality value (QV) of the PN40024.v4 genome assembly was estimated to be 36.02, which is slightly worse than QV of 37.43 of the PN12X.v2 genome assembly (Table 2). This result confirms that additional SMRT sequences are not as accurate as Sanger-based sequences and they slightly decrease overall accuracy of the assembly. Also, the error rate of the PN40024.v4 genome assembly was increased by 0.00006964% compared to PN12X.v2, but still represents an accuracy of 99.999749801%, a metric associated with high-quality genome assemblies.
Table 2.
Assembly quality values of PN40024.v4 and 12X.v2. Assembly quality values measured by Merqury for PN40024.v4 and 12X.v2 genome assemblies. QV denotes base level quality value.
| 12X.v2 | PN40024.v4 | |
|---|---|---|
| QV | 37.4338 | 36.0171 |
| Error rate (%) | 0.000180559 | 0.000250199 |
| k-mer completeness (%) | 96.79 | 96.96 |
Nevertheless, the k-mer completeness was raised from 96.79% to 96.96% for the PN40024.v4 assembly. Based on k-mer profiles of PN40024 and its parents (see The origin of the PN40024 genotype section for details), Merqury computed the inheritance spectrum (Supplementary File 1 and Supplementary Fig. 4) showing a low portion of read-only missing k-mers that are unique for the child read set (paired-end short reads of PN40024). The few missing sequences are probably due to sequencing errors, k-mers of novel variations or contamination from microbiome in PN40024 short reads, indicating an almost fully complete PN40024.v4 genome sequence assembly. Also, as the spectrum shows a single 2-copy peak around 12× and that no 1-copy peak was observed at half the size, the k-mer analysis supports the assumptions of an almost homozygous grapevine genotype.
The origin of the PN40024 genotype
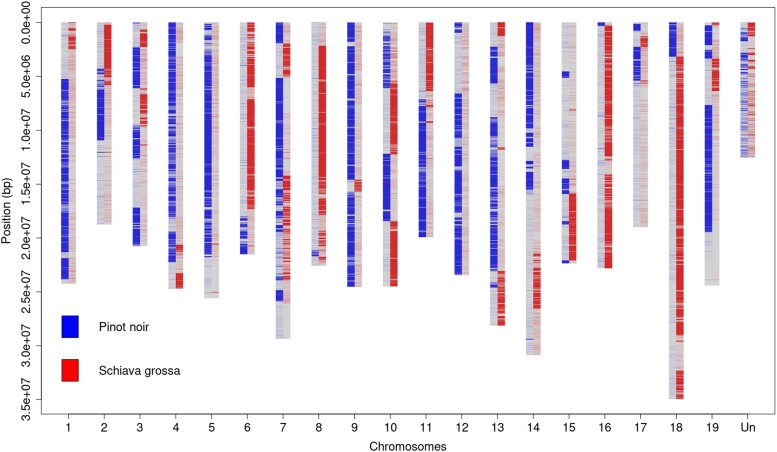
So far, the PN40024 genotype was supposed to be originally derived from cv. “Pinot noir” (Jaillon et al. 2007). However, we found 1,415,200 homozygous variants between “Pinot noir” and PN40024.v4 (versus 17,696 homozygous variants of PN40024 against its own assembly), meaning that “Pinot noir” haplotypes were completely missing at these locations. These homozygous “Pinot noir” variants were unevenly distributed along the chromosomes and formed blocks (Fig. 4). We identified that the haplotypes of unknown origins could be assigned to “Schiava grossa” (synonyms: “Trollinger” and “Frankenthal”) as already suspected by Jaillon et al. (2007). There were 953,735 homozygous variants found between cv. “Schiava grossa” and PN40024.v4 and the formed haplotype blocks were highly complementary to “Pinot noir” haplotype blocks (Fig. 4). As a negative control, the same analysis was performed with cv. “Araklinos” and 2,273,888 homozygous variants were identified, evenly distributed along the chromosomes (Supplementary File 1 and Supplementary Fig. 5).
Fig. 4.
Density of “Pinot noir” and “Schiava grossa” homozygous SNPs compared to the PN40024.v4 genome assembly. The x-axis shows the 19 main pseudochromosomes and the artificial chrUn (“Un”). The y-axis shows the base position in [bp]. Where density of “Pinot noir” SNPs is high, it means PN40024.v4 carries the “Schiava grossa” haplotype and vice versa. The regions where both “Pinot noir” and “Schiava grossa” SNP density is low correspond to regions where both genomes share a common haplotype.
Using Merqury, only a small portion of hap-mer specific k-mers (parental specific k-mers of the assembled F1) were found in the PN40024.v4 genome assembly (Supplementary File 1 and Supplementary Fig. 4). With the use of read data from both parents and child, Merqury was able to compute haplotype blocks by using the parental specific k-mers as anchors. A total of 1,454 haplotype blocks were computed for PN40024.v4 sequences with additional 289 haplotype blocks for alternative heterozygous sequence regions and 2,575 haplotype blocks for the 12X.v2 genome assembly (Table 3). The N50 was measured to 2.05 Mb (REF), 0.25 Mb (ALT), and 1.76 Mb (PN12X.v2). Compared to the PN12X.v2 genome assembly, PN40024.v4 presented less haplotype blocks, but comprised almost all bases showing a higher N50 value, that is its haplotype blocks are more contiguous.
Table 3.
Haplotype block statistics of PN40024.v4 and 12X.v2. Phasing accuracy estimation of Merqury for PN40024.v4 and 12X.v2 genome assembly. ALT denotes alternative heterozygous sequence parts of PN40024.v4.
| 12X.v2 | PN40024.v4 | ALT | |
|---|---|---|---|
| Number of blocks | 2,575 | 1,454 | 289 |
| Total bases in blocks (bp) | 474,845,411 | 468,703,133 | 19,519,697 |
| Block N50 size (kb) | 1,762 | 2,050 | 250 |
| Switch error rate (%) | 0.766002 | 0.959042 | 4.75944 |
A greater amount of paternal (“Schiava grossa”) than maternal (“Pinot Noir”) specific k-mers were identified. After identifying the origin of each haplotype block using segmentation, it is estimated that 41% of the genome harbors a “Schiava grossa”-specific haplotype and 27% a “Pinot noir”-specific haplotype. It is estimated that 32% of the genome shares a common haplotype between the 2 parents, that is that these regions could originate either from “Pinot noir” or “Schiava grossa” indicating that ∼57% could originate from “Schiava grossa” and ∼43% from “Pinot noir”.
The switch error rate was determined to 0.96% (REF), to 4.76% (ALT), and to 0.77% (PN12X.v2). Some of the switches are probably due to sequencing errors in the additional long read-based sequences. Moreover, as the error rate of ALT sequences was measured to ∼4.76%, portions of the alternative sequences are a mixture of the maternal and paternal haplotype, confirming that despite the improved separation of the 2 haplotypes in PN40024.v4, phasing is still not perfect.
By exploring the VIVC database (www.vivc.de), the “Helfensteiner” cultivar was found to originate from a cross between “Pinot noir precoce” (a clone of “Pinot noir”) and “Schiava grossa”. By performing the same variant calling analysis, 53,671 homozygous variants were found between cv. “Helfensteiner” and PN40024.v4, with 543 homozygous variants/Mb in the heterozygous regions and 93 homozygous variants/Mb in the homozygous regions (Fig. 5). As a negative control, “Araklinos” showed 3,967 homozygous variants/Mb in the heterozygous regions and 4,818 homozygous variants/Mb in the homozygous regions). Thus, the “Helfensteiner” homozygous variants are almost 6 times denser in error-prone regions of the PN40024.v4 assembly, which makes them probable “false positive” homozygous variants. Apart from heterozygous regions, no blocks of homozygous variants could be identified, meaning that one of the 2 “Helfensteiner” haplotypes is always present in the PN40024 genome. This confirms that the “Helfensteiner” variety is the true parent of the first selfing, from which the PN40024 genotype was created after 8 more selfings.
Fig. 5.
Density of “Helfensteiner” homozygous SNPs compared to the PN40024.v4 genome assembly. The x-axis shows the 19 main pseudochromosomes and the artificial chrUn (“Un”). The y-axis shows the base position in [bp]. The 7 regions squared are the heterozygous regions.
PN40024.v4.1 gene prediction, functional annotation, and manual curation
The PN40024.v4.1 gene annotation of REF haplotype comprises 35,922 gene models of which 35,197 are protein-coding and 725 encode for tRNAs (Table 4). In particular, 1,572 novel protein-coding genes were annotated in the newly assembled long read-based regions. For heterozygous regions, 1,855 and 1,809 protein-coding genes were predicted for REF and ALT haplotypes, respectively (Table 5). Most genes were predicted on the ∼11 Mb heterozygous region on chromosome 7 with 830 on the reference sequence and 792 on the alternative sequence followed by the ∼5 Mb region on chromosome 10 with 650 and 623 protein-coding genes.
Table 4.
Vcost.v3, PN40024.v4.1 REF haplotype and PN40024.v4.2 REF haplotype gene prediction overview.
| VCost.v3 | PN40024.v4.1 | PN40024.v4.2 | ||||
|---|---|---|---|---|---|---|
| Number | Mean length (bp) | Number | Mean length (bp) | Number | Mean length (bp) | |
| Protein-coding genes | 41,182 | 4,485 | 35,197 | 4,742 | 35,230 | 4,735 |
| ȃTranscripts | 47,363 | 1,383 | 41,160 | 1,433 | 41,173 | 1,440 |
| ȃExons | 239,165 | 273 | 208,581 | 282 | 208,719 | 283 |
| ȃCDS | 225,869 | 220 | 199,956 | 231 | 200,059 | 232 |
| ȃ5′ UTRs | 26,024 | 259 | 17,019 | 280 | 17,478 | 275 |
| ȃ3′ UTRs | 26,994 | 327 | 17,873 | 440 | 18,344 | 433 |
| tRNAs | 19 | 74 | 725 | 75 | 725 | 75 |
Table 5.
Gene numbers of heterozygous sequence regions. The abbreviation ALT denotes the alternative heterozygous sequence regions.
| Bases (bp) | Number of genes | |||
|---|---|---|---|---|
| PN40024.v4 | ALT | PN40024.v4.1 | ALT | |
| chr02 | 1,610,271 | 1,886,900 | 190 | 214 |
| chr03 | 288,001 | 287,774 | 14 | 13 |
| chr04 | 1,049,642 | 929,781 | 123 | 122 |
| chr07 | 11,422,405 | 10,851,409 | 830 | 792 |
| chr10 | 5,475,057 | 5,100,371 | 650 | 623 |
| chr11 | 733,078 | 630,772 | 43 | 41 |
| chr15 | 60,730 | 52,641 | 5 | 4 |
| Total | 20,639,184 | 19,739,648 | 1,855 | 1,809 |
To check for completeness of the gene models, the plant core genes of the database eudicots_odb10 were predicted with BUSCO (Fig. 6). Of the 2,326 searched plant core genes, 2,296 or 98.7% were classified as complete in the PN40024.v4.1 gene annotation. Only 16 were predicted as fragmented and only 14 were not found.
Compared to PN12X.v2 VCost.v3 gene annotation, PN40024.v4.1 counts less predictions (41,182 vs 35,197) but their size is longer on average (4,485 vs 4,742 bp) (Table 4). Also, the BUSCO analysis performed on VCost.v3 showed that 2,257 or 97.0% were classified as complete (Fig. 6). Thus, PN40024.v4.1 gene annotation represents PN40024 gene space in a more exhaustive and less fragmented manner compared to VCost.v3.
To help the community in the transfer of information across versions (i.e. correspondences), we retained as many gene names from VCost.v3 in PN40024.v4.1 as possible. We adopted a strategy based on RBHs followed by some filtering steps which allowed us to transfer names for 66% (23,206) of PN40024.v4.1 gene models with the nomenclature VitviXXg0YYYY (XX being the chromosome number and YYYY a sequential number below 4,000). One third (11,991) of PN40024.v4.1 gene models could not be named with a VCost.v3 identifier and were named with the nomenclature VitviXXg04ZZZ (XX being the chromosome number and ZZZ a sequential number below 1,000). The detailed nomenclature for PN40024.v4.1 gene annotations is given in Supplementary File 2 and Supplementary Table 4.
The functional annotation of PN40024.v4.1 was performed using Blast2GO and resulted in at least one Gene Ontology term for 87% (30,689) of the genes and one Enzyme Code for 41% (14,512) of them. The main classes and ontologies are detailed in Supplementary File 1 and Supplementary Figs. 6 and 7.
A subset of the RNA-Seq data published by Palumbo et al. (2014) was used to compare the results of a differential gene expression analysis performed with PN12X.v2/VCost.v3 and PN40024.v4/PN40024.v4.1. In terms of mapping, the percentage of aligned reads was equivalent or slightly better when using PN40024.v4 genome assembly compared to PN12X.v2 (Supplementary File 2 and Supplementary Table 6). Additionally, the percentage of assigned reads, that is the percentage of reads aligned under an annotated gene, was 2.4% to 3% better with PN40024.v4/PN40024.v4.1 compared to PN12X.v2/VCost.v3, which confirms the improved quality of PN40024.v4.1 gene annotation. Moreover, after differential gene expression analysis, the use of PN40024.v4/PN40024.v4.1 allowed the identification of more differentially expressed genes than PN12X.v2/VCost.v3 (Supplementary File 1 and Supplementary Fig. 8). This result along with the exhaustive functional annotation of PN40024.v4.1 shows that this new version of the PN40024 reference genome and annotation is a very efficient resource to perform transcriptomics and functional enrichment analyses.
Despite marked improvement of the PN40024.v4.1 automated annotation with respect to the previous VCost.v3 annotation, some recently expanded gene families have not been comprehensively annotated, such as the stilbene synthase gene family. Therefore, 1,641 genes (1,579 edited and 62 deleted) were manually curated using a purpose-built Apollo server (http://138.102.159.70:8080/apollo) providing a wide range of transcriptomic and genomic data for PN40024.v4. In an effort to preserve previous VCost.v3 manual curation and functional annotation efforts, a particular focus was given to genes present in the reference catalogue (Navarro-Payá et al 2022). The PN40024.v4.1 automated annotation including the manually curated features was called PN40024.v4.2, which metrics are presented in Table 4. An automated annotation from PN40024.v4.1 that was manually curated was deleted and replaced by its curated version in PN40024.v4.2. Also, the same rules were applied for gene name transfer and nomenclature for PN40024.v4.1 and PN40024.v4.2. The BUSCO analysis performed on PN40024.v4.2 shows that the fragmented plant core genes were reduced to 6 and the missing genes to 8 (Fig. 6). Thus, PN40024.v4.2 gene models comprise 2,308 or 99.2% complete plant core genes.
In conclusion, the here provided PN40024.v4 assembly is the most suitable grapevine reference genome sequence assembly as it notably outperforms PN12X.v2. In terms of genomic and transcriptomic read mapping, the assembly also outperforms other high-quality V. vinifera genome assemblies, something that occurs even when reads from these recently sequenced cultivars are used. Having a fully resolved alternative haplotype sequence, more continuous sequences and resolving many up-to-now unknown bases, PN40024.v4 represents the near complete diploid genome of the PN40024 genotype. Despite many improvements and advances in PN40024.v4, the genome sequence is still not perfect in regard to haplotype switching and newly introduced errors by implementation of long genomic reads. Further improvements should focus on these regions. Nevertheless, the gene annotation of PN40024.v4 should be used as the most updated resource for transcriptomics and functional enrichment analyses, while the genes of heterozygous regions that are likely represented on both haplotypes will allow exploration of heterozygous genetic traits.
Supplementary Material
Acknowledgments
We thank INRAE department “Biologie et Amélioration des Plantes” for funding; experimental unit UEAV (INRAE, Colmar) for plant maintenance; Anne-Marie Digby (University of Verona) for English correction; Emilce Prado for plant DNA extractions; EPGV (INRAE, Evry) for library prep and DNA sequencing; CNRGV (INRAE, Toulouse) for long read DNA extractions; Gentyane platform (INRAE, Clermont-Ferrand) for SMRT sequencing; Dr. Timothée Flutre and Amandine Launay for their help in coordinating the FruitSelGen project and in acquiring GBS data; Mario Pezzotti, Anne-Françoise Adam-Blondon, Michele Morgante, Gabriele Di Gaspero and Gabriele Magris for helpful discussions throughout the project; Pablo Carbonell-Bejerano (Instituto de Ciencias de la Vid y el Vino—ICVV) for critically reviewing the manuscript. This work was supported by the BMBF-funded de.NBI Cloud within the German Network for Bioinformatics Infrastructure (de.NBI). This article is based upon work from COST Action CA17111 INTEGRAPE, supported by COST (European Cooperation in Science and Technology).
Contributor Information
Amandine Velt, SVQV, INRAE—University of Strasbourg, Colmar 68000, France.
Bianca Frommer, Genetics and Genomics of Plants, CeBiTec & Faculty of Biology, Bielefeld University, Bielefeld 33615, Germany.
Sophie Blanc, SVQV, INRAE—University of Strasbourg, Colmar 68000, France.
Daniela Holtgräwe, Genetics and Genomics of Plants, CeBiTec & Faculty of Biology, Bielefeld University, Bielefeld 33615, Germany.
Éric Duchêne, SVQV, INRAE—University of Strasbourg, Colmar 68000, France.
Vincent Dumas, SVQV, INRAE—University of Strasbourg, Colmar 68000, France.
Jérôme Grimplet, Unidad de Hortofruticultura, Centro de Investigación y Tecnología Agroalimentaria de Aragón (CITA), Zaragoza 50059, Spain.
Philippe Hugueney, SVQV, INRAE—University of Strasbourg, Colmar 68000, France.
Catherine Kim, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724, USA.
Marie Lahaye, SVQV, INRAE—University of Strasbourg, Colmar 68000, France.
José Tomás Matus, Institute for Integrative Systems Biology (I2SysBio), Universitat de València-CSIC, Paterna 46908, Valencia, Spain.
David Navarro-Payá, Institute for Integrative Systems Biology (I2SysBio), Universitat de València-CSIC, Paterna 46908, Valencia, Spain.
Luis Orduña, Institute for Integrative Systems Biology (I2SysBio), Universitat de València-CSIC, Paterna 46908, Valencia, Spain.
Marcela K Tello-Ruiz, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724, USA.
Nicola Vitulo, Dipartimento di Biotecnologie, Università degli Studi di Verona, Verona 37134, Italy.
Doreen Ware, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724, USA; USDA ARS NEA Robert W. Holley Center for Agriculture and Health, Agricultural Research Service, Ithaca, NY 14853, USA.
Camille Rustenholz, SVQV, INRAE—University of Strasbourg, Colmar 68000, France.
Data availability
Supplemental files are provided with the manuscript. Supplementary File 1 contains additional figures and Supplementary File 2 additional tables. Raw sequencing data and the PN40024.v4 genome assembly are available at ENA under BioProject PRJEB45423. Also, the PN40024.v4 genome assembly with structural and functional gene annotation is available on the INTEGRAPE website (https://integrape.eu/resources/genes-genomes/genome-accessions), on the Grape Genomics Encyclopedia portal (http://grapedia.org/) and under the DOI number doi:10.57745/F9N2FZ (https://entrepot.recherche.data.gouv.fr/dataset.xhtml?persistentId=doi:10.57745/F9N2FZ). A Sequence Server v2.0.0 interface (http://138.102.159.70:4567/) was set up to perform BLAST analyses. A JBrowse interface (http://138.102.159.70/jbrowse/) was set up to visualize PN40024.v4 assembly and PN40024.v4.1 and v4.2 annotations, but also some previous annotation versions that were transferred, some RNA-Seq alignments and miscellaneous tracks. An Apollo interface (http://138.102.159.70:8080/apollo; training and account mandatory) was set up to manually curate gene annotations according to the dedicated guidelines (https://integrape.eu/resources/data-management/). Code used to analyze GBS data can be found at https://forgemia.inra.fr/sophie.blanc/gbs and code used to generate the PN40024.v4.2 version can be found at https://gitlab.com/MSVteam/pn40024-visualization-tools/-/tree/master/update_gff3_script.
Supplemental material available at G3 online.
Funding
This work was supported by INRAE department “Biologie et Amélioration des Plantes”, by the BMBF-funded de.NBI Cloud within the German Network for Bioinformatics Infrastructure (de.NBI) and by COST (European Cooperation in Science and Technology).
Author contributions
A.V. performed genome assembly and functional annotation. B.F. performed gene prediction. B.F., N.V., and D.H. built the annotation pipeline. S.B. performed GBS analysis and built the genetic maps. É.D. built the genetic map pipeline. A.V., B.F., and C.R. performed the analysis for quality assessments. C.R. performed the analysis on the origin of PN40024. V.D. performed plant material management and sampling. J.G. and B.F. worked on the gene name transfer. A.V., M.L., and D.N.-P. built the online tools. A.V., B.F., D.H., J.G., C.K., J.T.M, D.N.-P., L.O., M.K.T.-R., D.W., and C.R. worked on gene manual curation and writing of the dedicated guidelines. É.D., P.H., and C.R. looked for funding. C.R. supervised the project. A.V., B.F., D.H., and C.R. drafted and formatted the manuscript. All the authors read and helped improve the manuscript.
Literature cited
- Akiva E, Brown S, Almonacid DE, Barber AE 2nd, Custer AF, et al. The structure–function linkage database. Nucleic Acids Res. 2014;42(D1):D521–D530. doi: 10.1093/nar/gkt1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JE, Salzberg SL. JIGSAW: integration of multiple sources of evidence for gene prediction. Bioinformatics. 2005;21(18):3596–3603. doi: 10.1093/bioinformatics/bti609. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Andrews, S., 2010FASTQC. A quality control tool for high throughput sequence data.
- Attwood TK, Coletta A, Muirhead G, Pavlopoulou A, Philippou PB, et al. The PRINTS database: a fine-grained protein sequence annotation and analysis resource-its status in 2012. Database. 2012;2012:bas019. doi: 10.1093/database/bas019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett DW, Garrison EK, Quinlan AR, Strömberg MP, Marth GT. BamTools: a C++ API and toolkit for analyzing and managing BAM files. Bioinforma Oxf Engl. 2011;27(12):1691–1692. doi: 10.1093/bioinformatics/btr174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brůna T, Hoff KJ, Lomsadze A, Stanke M, Borodovsky M. BRAKER2: automatic eukaryotic genome annotation with GeneMark-EP+ and AUGUSTUS supported by a protein database. NAR Genomics Bioinforma. 2021;3(1):lqaa108. doi: 10.1093/nargab/lqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brůna T, Lomsadze A, Borodovsky M. GeneMark-EP+: eukaryotic gene prediction with self-training in the space of genes and proteins. NAR Genomics Bioinforma. 2020;2(2):lqaa026. doi: 10.1093/nargab/lqaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12(1):59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10(1):421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MS, Holt C, Moore B, Yandell M. Genome annotation and curation using MAKER and MAKER-P. Curr Protoc Bioinforma. 2014;48(1):4.11.1–4.11.39. doi: 10.1002/0471250953.bi0411s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaguier A, Grimplet J, Di Gaspero G, Scalabrin S, Duchêne E, et al. A new version of the grapevine reference genome assembly (12X.v2) and of its annotation (VCost.v3). Genom Data. 2017;14(1):56–62. doi: 10.1016/j.gdata.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PP, Lin BY, Mak AJ, Lowe TM. tRNAscan-SE 2.0: improved detection and functional classification of transfer RNA genes. Nucleic Acids Res. 2021;49(16):9077–9096. doi: 10.1093/nar/gkab688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchêne É, Dumas V, Butterlin G, Jaegli N, Rustenholz C, et al. Genetic variations of acidity in grape berries are controlled by the interplay between organic acids and potassium. Theor Appl Genet. 2020;133(3):993–1008. doi: 10.1007/s00122-019-03524-9. [DOI] [PubMed] [Google Scholar]
- Dunn NA, Unni DR, Diesh C, Munoz-Torres M, Harris NL, et al. Apollo: democratizing genome annotation. PLoS Comput Biol. 2019;15(2):e1006790. doi: 10.1371/journal.pcbi.1006790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn KW, Lorenz DH. 1977. Phanologische Entwicklungsstadien der Rebe. Nachrichtenblatt Dtsch. Pflanzenschutzdienstes.
- Gao S, Bertrand D, Chia BKH, Nagarajan N. OPERA-LG: efficient and exact scaffolding of large, repeat-rich eukaryotic genomes with performance guarantees. Genome Biol. 2016;17(1):102. doi: 10.1186/s13059-016-0951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis HZ. Red: an intelligent, rapid, accurate tool for detecting repeats de-novo on the genomic scale. BMC Bioinformatics. 2015;16(1):227. doi: 10.1186/s12859-015-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girollet N, Rubio B, Lopez-Roques C, Valière S, Ollat N, et al. De novo phased assembly of the Vitis riparia grape genome. Sci Data. 2019;6(1):127. doi: 10.1038/s41597-019-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh O. A space-efficient and accurate method for mapping and aligning cDNA sequences onto genomic sequence. Nucleic Acids Res. 2008;36(8):2630–2638. doi: 10.1093/nar/gkn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36(10):3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough J, Karplus K, Hughey R, Chothia C. Assignment of homology to genome sequences using a library of hidden Markov models that represent all proteins of known structure. J Mol Biol. 2001;313(4):903–919. doi: 10.1006/jmbi.2001.5080. [DOI] [PubMed] [Google Scholar]
- Haas BJ, Salzberg SL, Zhu W, Pertea M, Allen JE, et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the program to assemble spliced alignments. Genome Biol. 2008;9(1):R7. doi: 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft DH, Selengut JD, Richter RA, Harkins D, Basu MK, et al. TIGRFAMs and genome properties in 2013. Nucleic Acids Res. 2013;41(D1):D387–D395. doi: 10.1093/nar/gks1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff KJ, Lange S, Lomsadze A, Borodovsky M, Stanke M. BRAKER1: unsupervised RNA-Seq-based genome annotation with GeneMark-ET and AUGUSTUS. Bioinforma Oxf Engl. 2016;32(5):767–769. doi: 10.1093/bioinformatics/btv661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff KJ, Lomsadze A, Borodovsky M, Stanke M. Whole-genome annotation with BRAKER. Methods Mol Biol. 2019;1962(1):65–95. doi: 10.1007/978-1-4939-9173-0_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C, Yandell M. MAKER2: an annotation pipeline and genome-database management tool for second-generation genome projects. BMC Bioinformatics. 2011;12(1):491. doi: 10.1186/1471-2105-12-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe KL, Chothia T, Durbin R. GAZE: a generic framework for the integration of gene-prediction data by dynamic programming. Genome Res. 2002;12(9):1418–1427. doi: 10.1101/gr.149502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Kang M, Xu A. HaploMerger2: rebuilding both haploid sub-assemblies from high-heterozygosity diploid genome assembly. Bioinformatics. 2017;33(16):2577–2579. doi: 10.1093/bioinformatics/btx220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H, Gotoh O. Benchmarking spliced alignment programs including Spaln2, an extended version of Spaln that incorporates additional species-specific features. Nucleic Acids Res. 2012;40(20):e161. doi: 10.1093/nar/gks708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillon O, Aury J-M, Noel B, Policriti A, Clepet C, et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007;449(7161):463–467. doi: 10.1038/nature06148. [DOI] [PubMed] [Google Scholar]
- Jones P, Binns D, Chang H-Y, Fraser M, Li W, et al. InterProScan 5: genome-scale protein function classification. Bioinforma Oxf Engl. 2014;30(9):1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ. BLAT–the BLAST-like alignment tool. Genome Res. 2002;12(4):656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killick R, Eckley IA. Changepoint: an R package for changepoint analysis. J Stat Softw. 2014;58(3):1–19. doi: 10.18637/jss.v058.i03. [DOI] [Google Scholar]
- Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37(5):540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27(5):722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf I. Gene finding in novel genomes. BMC Bioinformatics. 2004;5(1):59. doi: 10.1186/1471-2105-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5(2):R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Bork P. 20 Years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018;46(D1):D493–D496. doi: 10.1093/nar/gkx922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34(18):3094–3100. doi: 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The sequence alignment/map format and SAMtools. Bioinforma Oxf Engl. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinforma Oxf Engl. 2014;30(7):923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- Lodhi MA, Reisch BI. Nuclear DNA content of Vitis species, cultivars, and other genera of the Vitaceae. Theor Appl Genet. 1995;90(1):11–16. doi: 10.1007/BF00220990. [DOI] [PubMed] [Google Scholar]
- Lomsadze A, Burns PD, Borodovsky M. Integration of mapped RNA-Seq reads into automatic training of eukaryotic gene finding algorithm. Nucleic Acids Res. 2014;42(15):e119. doi: 10.1093/nar/gku557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomsadze A, Ter-Hovhannisyan V, Chernoff YO, Borodovsky M. Gene identification in novel eukaryotic genomes by self-training algorithm. Nucleic Acids Res. 2005;33(20):6494–6506. doi: 10.1093/nar/gki937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, et al. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res. 2020;48(D1):D265–D268. doi: 10.1093/nar/gkz991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Liu B, Xie Y, Li Z, Huang W, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. GigaScience. 2012;1(1):18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Majoros WH, Pertea M, Salzberg SL. TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-finders. Bioinforma Oxf Engl. 2004;20(16):2878–2879. doi: 10.1093/bioinformatics/bth315. [DOI] [PubMed] [Google Scholar]
- Massonnet M, Cochetel N, Minio A, Vondras AM, Lin J, et al. The genetic basis of sex determination in grapes. Nat Commun. 2020;11(1):2902. doi: 10.1038/s41467-020-16700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdinoglu D, Butterlin G, Bevilacqua L, Chiquet V, Adam-Blondon A-F, et al. Development and characterization of a large set of microsatellite markers in grapevine (Vitis vinifera L.) suitable for multiplex PCR. Mol Breed. 2005;15(4):349–366. doi: 10.1007/s11032-004-7651-0. [DOI] [Google Scholar]
- Mi H, Ebert D, Muruganujan A, Mills C, Albou L-P, et al. PANTHER Version 16: a revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 2021;49(D1):D394–D403. doi: 10.1093/nar/gkaa1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar GA, et al. Pfam: the protein families database in 2021. Nucleic Acids Res. 2021;49(D1):D412–D419. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Payá D, Santiago A, Orduña L, Zhang C, Amato A, et al. The grape gene reference catalogue as a standard resource for gene selection and genetic improvement. Front Plant Sci. 2022;12:803977. doi: 10.3389/fpls.2021.803977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necci M, Piovesan D, Dosztányi Z, Tosatto SCE. MobiDB-lite: fast and highly specific consensus prediction of intrinsic disorder in proteins. Bioinforma Oxf Engl. 2017;33(9):1402–1404. doi: 10.1093/bioinformatics/btx015. [DOI] [PubMed] [Google Scholar]
- Palumbo MC, Zenoni S, Fasoli M, Massonnet M, Farina L, et al. Integrated network analysis identifies fight-club nodes as a class of hubs encompassing key putative switch genes that induce Major transcriptome reprogramming during grapevine development. Plant Cell. 2014;26(12):4617–4635. doi: 10.1105/tpc.114.133710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedruzzi I, Rivoire C, Auchincloss AH, Coudert E, Keller G, et al. HAMAP In 2013, new developments in the protein family classification and annotation system. Nucleic Acids Res. 2013;41(D1):D584–D589. doi: 10.1093/nar/gks1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinforma Oxf Engl. 2010;26(6):841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, Walenz BP, Koren S, Phillippy AM. Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21(1):245. doi: 10.1186/s13059-020-02134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet E, Gouzy J, Schiex T. EuGene: an automated integrative gene finder for eukaryotes and prokaryotes. Methods Mol Biol. 2019;1962(1):97–120. doi: 10.1007/978-1-4939-9173-0_6. [DOI] [PubMed] [Google Scholar]
- Salmela L, Rivals E. LoRDEC: accurate and efficient long read error correction. Bioinformatics. 2014;30(24):3506–3514. doi: 10.1093/bioinformatics/btu538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumate A, Salzberg SL. Liftoff: accurate mapping of gene annotations. Bioinformatics. 2021;37(12):1639–1643. doi: 10.1093/bioinformatics/btaa1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist CJA, de Castro E, Cerutti L, Cuche BA, Hulo N, et al. New and continuing developments at PROSITE. Nucleic Acids Res. 2013;41(D1):D344–D347. doi: 10.1093/nar/gks1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe I, Dawson N, Lewis TE, Das S, Lees JG, et al. CATH: expanding the horizons of structure-based functional annotations for genome sequences. Nucleic Acids Res. 2019;47(D1):D280–D284. doi: 10.1093/nar/gky1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- Slater GSC, Birney E. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics. 2005;6(1):31. doi: 10.1186/1471-2105-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AFA, Hubley R, Green P. 2013. RepeatMasker Open-4.0.
- Song L, Sabunciyan S, Yang G, Florea L. A multi-sample approach increases the accuracy of transcript assembly. Nat Commun. 2019;10(1):5000. doi: 10.1038/s41467-019-12990-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M, Diekhans M, Baertsch R, Haussler D. Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics. 2008;24(5):637–644. doi: 10.1093/bioinformatics/btn013. [DOI] [PubMed] [Google Scholar]
- Stanke M, Schöffmann O, Morgenstern B, Waack S. Gene prediction in eukaryotes with a generalized hidden Markov model that uses hints from external sources. BMC Bioinformatics. 2006;7(1):62. doi: 10.1186/1471-2105-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Zhang X, Miao C, Zhang J, Ming R, et al. ALLMAPS: robust scaffold ordering based on multiple maps. Genome Biol. 2015;16(1):3. doi: 10.1186/s13059-014-0573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Butler D. R package ASMap: efficient genetic linkage map construction and diagnosis. J Stat Softw. 2017;79(6):1–29. doi: 10.18637/jss.v079.i06.30220889 [DOI] [Google Scholar]
- Torkamaneh D, Laroche J, Bastien M, Abed A, Belzile F. Fast-GBS: a new pipeline for the efficient and highly accurate calling of SNPs from genotyping-by-sequencing data. BMC Bioinformatics. 2017;18(1):5. doi: 10.1186/s12859-016-1431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varet H, Brillet-Guéguen L, Coppée J-Y, Dillies M-A. SARTools: a DESeq2- and EdgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS One. 2016;11(6):e0157022. doi: 10.1371/journal.pone.0157022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasimuddin MD, Misra S, Li H, Aluru S. Efficient architecture-aware acceleration of BWA-MEM for Multicore systems. In: 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS). 2019. p. 314–324.
- Velasco R, Zharkikh A, Troggio M, Cartwright DA, Cestaro A, et al. A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE. 2007;2(12):e1326. doi: 10.1371/journal.pone.0001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitulo N, Forcato C, Carpinelli EC, Telatin A, Campagna D, et al. A deep survey of alternative splicing in grape reveals changes in the splicing machinery related to tissue, stress condition and genotype. BMC Plant Biol. 2014;14(1):99. doi: 10.1186/1471-2229-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9(11):e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Kong L. Pblat: a multithread blat algorithm speeding up aligning sequences to genomes. BMC Bioinformatics. 2019;20(1):28. doi: 10.1186/s12859-019-2597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse RM, Seppey M, Simão FA, Manni M, Ioannidis P, et al. BUSCO applications from quality assessments to gene prediction and phylogenomics. Mol Biol Evol. 2018;35(3):543–548. doi: 10.1093/molbev/msx319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D, Pethica R, Zhou Y, Talbot C, Vogel C, et al. SUPERFAMILY–sophisticated comparative genomics, data mining, visualization and phylogeny. Nucleic Acids Res. 2009;37(suppl_1):D380–D386. doi: 10.1093/nar/gkn762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Nikolskaya A, Huang H, Yeh L-SL, Natale DA, et al. PIRSF: family classification system at the protein information resource. Nucleic Acids Res. 2004;32(90001):D112–D114. doi: 10.1093/nar/gkh097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TD, Watanabe CK. GMAP: a genomic mapping and alignment program for mRNA and EST sequences. Bioinformatics. 2005;21(9):1859–1875. doi: 10.1093/bioinformatics/bti310. [DOI] [PubMed] [Google Scholar]
- Xu G-C, Xu T-J, Zhu R, Zhang Y, Li S-Q, et al. LR_Gapcloser: a tiling path-based gap closer that uses long reads to complete genome assembly. GigaScience. 2019;8(1):giy157. doi: 10.1093/gigascience/giy157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplemental files are provided with the manuscript. Supplementary File 1 contains additional figures and Supplementary File 2 additional tables. Raw sequencing data and the PN40024.v4 genome assembly are available at ENA under BioProject PRJEB45423. Also, the PN40024.v4 genome assembly with structural and functional gene annotation is available on the INTEGRAPE website (https://integrape.eu/resources/genes-genomes/genome-accessions), on the Grape Genomics Encyclopedia portal (http://grapedia.org/) and under the DOI number doi:10.57745/F9N2FZ (https://entrepot.recherche.data.gouv.fr/dataset.xhtml?persistentId=doi:10.57745/F9N2FZ). A Sequence Server v2.0.0 interface (http://138.102.159.70:4567/) was set up to perform BLAST analyses. A JBrowse interface (http://138.102.159.70/jbrowse/) was set up to visualize PN40024.v4 assembly and PN40024.v4.1 and v4.2 annotations, but also some previous annotation versions that were transferred, some RNA-Seq alignments and miscellaneous tracks. An Apollo interface (http://138.102.159.70:8080/apollo; training and account mandatory) was set up to manually curate gene annotations according to the dedicated guidelines (https://integrape.eu/resources/data-management/). Code used to analyze GBS data can be found at https://forgemia.inra.fr/sophie.blanc/gbs and code used to generate the PN40024.v4.2 version can be found at https://gitlab.com/MSVteam/pn40024-visualization-tools/-/tree/master/update_gff3_script.
Supplemental material available at G3 online.