Abstract
Rationale & Objective
Heart failure and chronic kidney disease (CKD) frequently coexist reflective of the strong interplay between these organ systems. A better understanding of the prevalence of different types of heart failure (preserved and reduced ejection fraction) and their subsequent mortality risks among advanced CKD patients would provide important epidemiologic insights and may pave the way for more focused and proactive management strategies.
Study Design
Retrospective cohort study.
Setting & Population
Patients aged ≥18 years with incident CKD (estimated glomerular filtration rate ≤45 mL/min/1.73 m2) with and without heart failure in a large integrated health care system in Southern California.
Exposure
Heart failure, heart failure with preserved ejection fraction (HFpEF), heart failure with reduced ejection fraction (HFrEF).
Outcomes
All-cause and cardiovascular-related mortality within one year of CKD identification.
Analytical Approach
HRs were estimated using Cox proportional-hazards model for risk of all-cause mortality and Fine-Gray subdistribution hazard model for risk of cardiovascular-related mortality within 1 year.
Results
The study cohort included 76,688 patients with incident CKD between 2007 and 2017, of which 14,249 (18.6%) had prevalent heart failure. Among these patients, 8,436 (59.2%) had HFpEF and 3,328 (23.3%) had HFrEF. Compared with patients without heart failure, the HR for 1-year all-cause mortality was 1.70 (95% CI, 1.60-1.80) among patients with heart failure. The HRs were 1.59 (95% CI, 1.48-1.70) for patients with HFpEF and 2.43 (95% CI, 2.23-2.65) for patients with HFrEF. Compared with patients without heart failure, the 1-year cardiovascular-related mortality HR for patients with heart failure was 6.69 (95% CI, 5.93-7.54). Cardiovascular-related mortality HR was even higher among those with HFrEF (HR, 11.47; 95% CI, 9.90-13.28).
Limitations
Retrospective design with a short 1-year follow-up period. Additional variables including medication adherence, medication changes, and time-varying variables were not accounted for in this intention-to-treat analysis.
Conclusions
Among patients with incident CKD, heart failure was highly prevalent with HFpEF accounting for over 70% among patients with known ejection fraction. Although the presence of heart failure was associated with higher 1-year all-cause and cardiovascular-related mortality, patients with HFrEF were the most vulnerable.
Index Words: Cardiovascular mortality, chronic kidney disease, heart failure, mortality
Graphical abstract
Plain Language Summary.
Among a large diverse real-world population of patients with advanced chronic kidney disease (CKD) having estimated glomerular filtration rate <45 mL/min/1.73 m2, we found that 18.6% had prevalent heart failure where preserved ejection fraction accounted for over 70% among patients with known ejection fraction. Age and sex adjusted 1 year mortality rate was 11 times higher among CKD patients with heart failure than CKD patients without heart failure. All-cause mortality and cardiovascular-related mortality risk was highest among heart failure reduced ejection fraction followed by heart failure preserved ejection fraction. Given the advancements in therapies for heart failure, future studies are needed to assess the efficacy of drugs in real-world populations with CKD. We hope that such future work will provide more insights into the evolving care for heart failure and CKD, ultimately to help better understand and manage this vulnerable population.
Cardiovascular disease is the leading cause of death in patients with chronic kidney disease (CKD).1, 2, 3 An estimated 40%-50% of all deaths in patients with CKD stage 4 and 5 is attributable to cardiovascular-related mortality compared with only 26% in those with normal kidney function.2 Furthermore, the rate of hospitalizations among patients with CKD is 60% higher in the presence of cardiovascular disease compared with those without cardiovascular disease.4 One of the primary manifestations of cardiovascular disease is heart failure, which has a bidirectional interaction with CKD, termed “cardiorenal syndrome.” Up to 40% of CKD patients who initiate dialysis have heart failure, and among those without heart failure, the annual incidence is estimated at 20%.4,5 A better understanding of the epidemiology of patients with cardiorenal syndrome may improve ways to provide targeted management strategies to this vulnerable population.
Heart failure is a clinical entity that can be categorized as heart failure with preserved ejection fraction (HFpEF), defined as ejection fraction greater than 40%, or heart failure with reduced ejection fraction (HFrEF), defined as ejection fraction less and or equal to 40%.6 Multiple cohorts have suggested that HFpEF has been trending higher in the general community and now accounts for up to half of all cases of heart failure,7,8 with differences in prevalence based on age, sex, and comorbid conditions.4,9,10 A meta-analysis has suggested that the prevalence of HFpEF compared to HFrEF is similar in patients with advanced CKD, accounting for approximately 55%.11 The pathophysiologic mechanism of HFpEF is contributed by a proinflammatory state leading to stiffening of the myocardium with increased filling pressures, whereas in HFrEF, there is also decreased myocardial contractility.12 In patients with both heart failure and CKD, these mechanisms are further complicated by a complex, bidirectional neurohormonal interaction between the 2 organ systems often referred to as the cardiorenal syndrome.
Using a large diverse population within a real-world environment, we sought to determine the prevalence of heart failure (both HFpEF and HFrEF) in patients with incident advanced CKD. Furthermore, we compared 1-year all-cause and cardiovascular-related mortality within this population.
Methods
Study Design
A retrospective cohort study was conducted from January 1, 2007-December 31, 2017 within Kaiser Permanente Southern California (KPSC), which is a large integrated health care system with over 4.8 million members cared for at 14 hospitals and >200 outpatient offices. Information used in this study was collected from the KPSC electronic health records as part of routine clinical care, KPSC administrative files (Table S1). The study was approved by the KPSC institutional review board and exempted from informed consent (IRB # 10591).
Population
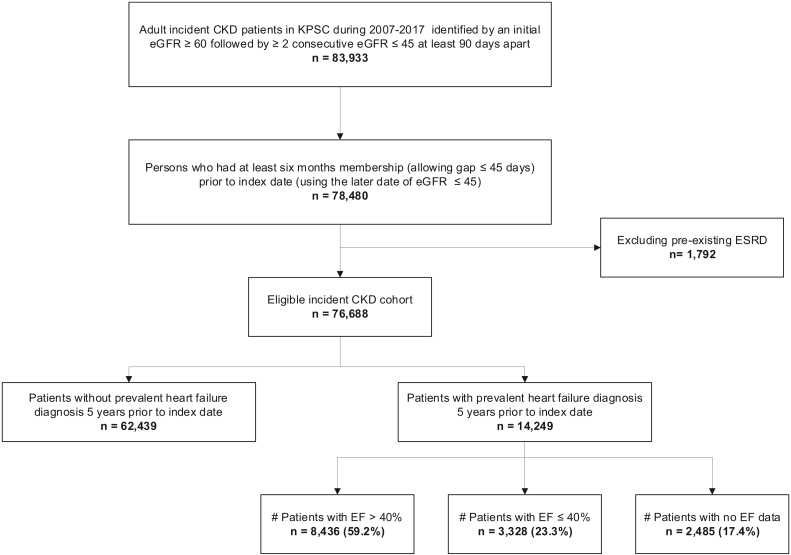
Patients (aged ≥18 years) were identified as having incident CKD if they had 2 consecutive estimate glomerular filtration rate (eGFR) measurements ≤ 45 (mL/min/1.73 m2) at least 90 days apart, with a prior eGFR ≥ 60. eGFR was estimated from serum creatinine using the 2009 CKD EPI (Chronic Kidney Disease Epidemiology Collaboration) equation.13 The second eGFR ≤ 45 (mL/min/1.73 m2) was defined as the index date. Patients requiring kidney replacement therapy with dialysis or kidney transplant were excluded. Prevalent heart failure was identified as having at least 1 inpatient or at least 3 separate outpatient visits using the International Classification of Diseases Ninth and Tenth Revisions diagnosis codes for heart failure in the preceding 5 years of incident CKD. Heart failure was further stratified using echocardiogram data (when available) as ejection fraction > 40% (HFpEF), ejection fraction ≤ 40% (HFrEF), or unknown if ejection fraction data was unavailable (Fig 1).
Figure 1.
Between 2007-2017, incident CKD patients identified if they had 2 consecutive eGFR measurements ≤45 (mL/min/1.73 m2) at least 90 days apart, with a prior eGFR ≥60. A total of 76,688 patients (52.0% non-Hispanic White, 23.4% Hispanic, 13.3% Black, and 8.8% Asian) were identified with incident CKD, of which 14,249 (18.6%) had a diagnosis of heart failure. Among CKD patients with heart failure, 8,436 (59.2%) were HFpEF, whereas 3,328 (23.3%) were HFrEF. Abbreviations: CKD, chronic kidney disease; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Outcomes and Covariates
The primary outcomes were all-cause and cardiovascular-related mortality within 1 year of CKD identification. Mortality was identified from the KPSC mortality database, which combines information from California State Death Master Files, California State Multiple Cause of Death Master Files, Social Security Administration Death Master Files, KPSC Hospital and Emergency Room records, KPSC Membership System, Perinatal Data Mart, and Outside Claims Processing System. The cause of mortality was primarily obtained by linkage to the California State Multiple Cause of Death Master Files using International Classification of Diseases, Tenth Revision codes.1
Members’ demographic information such as age, sex, and race/ethnicity were identified from KPSC membership files. Preselected comorbid conditions such as hypertension, diabetes, coronary artery disease, atrial fibrillation, and weighted Charlson comorbidity score were determined by International Classification of Diseases Ninth and Tenth Revisions codes using electronic health record utilization database.14
Statistical Analysis
Patients’ characteristics were compared among CKD patients without heart failure and CKD with heart failure further categorized as HFrEF, HFpEF, or unknown ejection fraction. Age- and sex-adjusted mortality rates were calculated by direct standardization using United States 2010 Census data. Hazard ratios (HRs) and their 95% confidence intervals (95% CIs) for 1-year all-cause mortality were estimated using a Cox proportional-hazards model adjusting for preselected confounders including age, sex, race/ethnicity, hypertension, diabetes, CKD stage, coronary artery disease, atrial fibrillation, and weighted Charlson comorbidity score at baseline. The subdistribution HRs of cardiovascular-related mortality within 1 year was estimated using the Fine-Gray subdistribution hazard model after adjustment for the same set of confounders, accounting for all other-cause mortality as a competing risk.
Results
A total of 76,688 patients (52.0% non-Hispanic White, 23.4% Hispanic, 13.3% Black, and 8.8% Asian) were identified with incident CKD, of which 14,249 (18.6%) had a diagnosis of heart failure (Table 1). Among CKD patients with heart failure, 8,436 (59.2%) were HFpEF, whereas 3,328 (23.3%) were HFrEF (Fig 1). Compared with patients without heart failure, those with heart failure had higher Charlson comorbidity scores, co-existence of diabetes, hypertension, atrial fibrillation, and other cardiovascular diseases. There were a higher proportion of males and Black patients among CKD patients with heart failure. Mean systolic blood pressure was lower among the heart failure population and lowest among HFrEF patients (Table 1).
Table 1.
Baseline Characteristics of Incident Chronic Kidney Disease Patients Based on Heart Failure Status (2007-2017)
| Heart Failure |
No HF | Total | |||
|---|---|---|---|---|---|
| No EF | EF > 40% | EF ≤ 40% | |||
| N | 2,485 | 8,436 | 3,328 | 62,439 | 76,688 |
| Age at index date, mean (SD) | 75.9 (11.04) | 75.6 (11.31) | 73.9 (11.22) | 71.7 (12.36) | 72.4 (12.24) |
| Sex, n (%) | |||||
| Male | 1,242 (50.0%) | 3,964 (47.0%) | 2,166 (65.1%) | 27,688 (44.3%) | 35,060 (45.7%) |
| Female | 1,243 (50.0%) | 4,472 (53.0%) | 1,162 (34.9%) | 34,751 (55.7%) | 41,628 (54.3%) |
| Race/ethnicity, n (%) | |||||
| White | 1,524 (61.3%) | 4,751 (56.3%) | 1,687 (50.7%) | 31,879 (51.1%) | 39,841 (52.0%) |
| Asian | 139 (5.6%) | 576 (6.8%) | 235 (7.1%) | 5,832 (9.3%) | 6,782 (8.8%) |
| Black | 289 (11.6%) | 1,204 (14.3%) | 610 (18.3%) | 8,116 (13.0%) | 10,219 (13.3%) |
| Hispanic | 411 (16.5%) | 1,783 (21.1%) | 736 (22.1%) | 15,016 (24.0%) | 17,946 (23.4%) |
| Other/unknown | 122 (4.9%) | 122 (1.5%) | 60 (1.8%) | 1,596 (2.6%) | 1,900 (2.5%) |
| BMI, kg/m2 | |||||
| Mean (SD) | 30.1 (7.72) | 30.3 (7.68) | 28.1 (6.28) | 29.2 (6.66) | 29.3 (6.81) |
| < 18.5 | 26 (1.1%) | 147 (1.7%) | 57 (1.7%) | 900 (1.4%) | 1,130 (1.5%) |
| 18.5-24.9 | 463 (18.6%) | 1,723 (20.4%) | 942 (28.3%) | 13,111 (21.0%) | 16,239 (21.2%) |
| 25-29.9 | 618 (24.9%) | 2,208 (26.2%) | 982 (29.5%) | 18,061 (28.9%) | 21,869 (28.5%) |
| 30-34.9 | 382 (15.4%) | 1,518 (18.0%) | 528 (15.9%) | 11,373 (18.2%) | 13,801 (18.0%) |
| 35-39.9 | 210 (8.5%) | 891 (10.6%) | 236 (7.1%) | 5,289 (8.5%) | 6,626 (8.6%) |
| 40+ | 193 (7.8%) | 839 (10.0%) | 143 (4.3%) | 3,599 (5.8%) | 4,774 (6.2%) |
| Unknown | 593 (23.9%) | 1,110 (13.2%) | 440 (13.2%) | 10,106 (16.2%) | 12,249 (16.0%) |
| Average DBP within 1 y before index date | |||||
| Mean (SD) | 65.2 (9.44) | 64.8 (9.41) | 64.5 (9.76) | 68.6 (9.44) | 67.9 (9.56) |
| < 60 | 524 (21.1%) | 2,218 (26.3%) | 963 (28.9%) | 8,474 (13.6%) | 12,179 (15.9%) |
| 60 to < 80 | 1,306 (52.6%) | 4,779 (56.7%) | 1,764 (53.0%) | 38,829 (62.2%) | 46,678 (60.9%) |
| 80+ | 129 (5.2%) | 472 (5.6%) | 208 (6.3%) | 6,441 (10.3%) | 7,250 (9.5%) |
| Unknown | 526 (21.2%) | 967 (11.5%) | 393 (11.8%) | 8,695 (13.9%) | 10,581 (13.8%) |
| Average SBP within 1 y before index date | |||||
| Mean (SD) | 126.1 (15.85) | 1,26.9 (14.71) | 118.0 (15.66) | 129.5 (13.39) | 128.6 (13.96) |
| < 120 | 659 (26.5%) | 2,278 (27.0%) | 1,658 (49.8%) | 11,052 (17.7%) | 15,647 (20.4%) |
| 120 to < 140 | 959 (38.6%) | 3,926 (46.5%) | 1,019 (30.6%) | 32,593 (52.2%) | 38,497 (50.2%) |
| 140+ | 341 (13.7%) | 1,265 (15.0%) | 258 (7.8%) | 10,099 (16.2%) | 11,963 (15.6%) |
| Unknown | 526 (21.2%) | 967 (11.5%) | 393 (11.8%) | 8,695 (13.9%) | 10,581 (13.8%) |
| CKD stage/eGFR (mL/min/1.73 m2) | |||||
| <15 | 10 (0.4%) | 85 (1.0%) | 30 (0.9%) | 738 (1.2%) | 863 (1.1%) |
| 15-29 | 252 (10.1%) | 850 (10.1%) | 311 (9.3%) | 5,097 (8.2%) | 6,510 (8.5%) |
| 30-45 | 2,223 (89.5%) | 7,501 (88.9%) | 2,987 (89.75%) | 56,604 (90.7%) | 69,315 (90.4%) |
| Weighted Charlson comorbidity, median (IQR) | 5.0 (4.0, 7.0) | 6.0 (4.0, 7.0) | 6.0 (5.0, 7.0) | 4.0 (2.0, 5.0) | 4.0 (2.0, 5.0) |
| Elixhauser comorbidity, median (IQR) | 7.0 (6.0, 9.0) | 9.0 (7.0, 11.0) | 9.0 (7.0, 11.0) | 5.0 (4.0, 7.0) | 5.0 (4.0, 8.0) |
| Pre-existing comorbid conditions, n (%) | |||||
| Diabetes | 1,320 (53.1%) | 4,691 (55.6%) | 1,857 (55.8%) | 27,510 (44.1%) | 35,378 (46.1%) |
| Hypertension | 2,110 (84.9%) | 7,743 (91.8%) | 2,992 (89.9%) | 47,725 (76.4%) | 60,570 (79.0%) |
| Atrial fibrillation | 583 (23.5%) | 3,035 (36.0%) | 1,245 (37.4%) | 3,511 (5.6%) | 8,374 (10.9%) |
| Coronary artery disease | 1,252 (50.4%) | 3,875 (45.9%) | 2,066 (62.1%) | 10,022 (16.1%) | 17,215 (22.4%) |
| Malignancy | 281 (11.3%) | 1048 (12.4%) | 418 (12.6%) | 7524 (12.1%) | 9271 (12.1%) |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; EF, ejection fraction; eGFR, estimated glomerular filtration rate; body mass index; HF, heart failure; IQR, interquartile range; SBP, systolic blood pressure; SD, standard deviation.
One-year all-cause mortality occurred in 17.8% of CKD patients with heart failure compared with 6.3% of CKD patients without heart failure (Table 2). The age, sex-adjusted all-cause mortality rate (events per 1,000 person-years) in CKD patients with heart failure was 125.1 (95% CI, 100.1-150.2), compared with 35.9 (95% CI, 32.4-39.4) in those without heart failure. The highest age and sex-adjusted all-cause mortality rate per 1,000 person-years within 1 year was found among patients with HFrEF at 137.0 (95% CI, 92.7-181.2), followed by the patients with HFpEF, whose adjusted rate was 125.2 (95% CI, 90.7-159.8).
Table 2.
One Year All-Cause and Cardiovascular-Related Mortality Incidence Rate
| One Year All-Cause and Cardiovascular-Related Mortality by Heart Failure and Chronic Kidney Disease Status |
|||||
|---|---|---|---|---|---|
| Outcome | Crude Incidence Ratea (95% CI) | Adjusted Incidence Ratea,b (95% CI) | |||
| All-cause death | No heart failure (N=62,439) | 3,928 (6.3%) | 66.7 (64.6, 68.8) | 35.9 (32.4, 39.4) | |
| Heart failure (N=14,249) | Total | 2,534 (17.8%) | 201.9 (194.0, 209.8) | 125.1 (100.1, 150.2) | |
| No EF (N=2,485) | 335 (13.5%) | 148.6 (132.7, 164.5) | 116.8 (49.9, 183.7) | ||
| EF > 40% (N=8,436) | 1,414 (16.8%) | 189.5 (179.6, 199.4) | 125.2 (90.7, 159.8) | ||
| EF ≤ 40% (N=3,328) | 774 (23.3%) | 278.3 (258.7, 298.0) | 137.0 (92.7, 181.2) | ||
| Cardiovascular-related death | No heart failure (N=62,439) | 572 (0.9%) | 9.7 (8.9, 10.5) | 4.4 (3.3, 5.5) | |
| Heart failure (N=14,249) | Total | 1,449 (10.2%) | 116.0 (110.0, 121.9) | 52.4 (40.5,64.3) | |
| No EF (N=2,485) | 160 (6.4%) | 71.0 (60.0, 82.0) | 54.5 (8.2, 100.8) | ||
| EF > 40% (N=8,436) | 754 (8.9%) | 101.1 (93.8, 108.3) | 47.1 (30.1, 64.1) | ||
| EF ≤ 40% (N=3,328) | 535 (16.1%) | 192.4 (176.1, 208.7) | 72.3 (57.3, 87.4) | ||
Abbreviations: CI, confidence interval; EF, ejection fraction.
Rates are per 1,000 person-years
Age, sex-adjusted using US 2010 Census Data
One-year cardiovascular-related mortality was 10.2% in CKD patients with heart failure compared with 0.9% in CKD patients without heart failure. The age and sex-adjusted incidence rate of cardiovascular-related mortality was 11 times higher among CKD patients with heart failure than CKD patients without heart failure, 52.4 (95% CI, 40.5-64.3) versus 4.4 (95% CI, 3.3-5.5) per 1,000 person-years, respectively. The incidence of cardiovascular-related mortality was highest among CKD patients with HFrEF.
Compared with CKD patients without heart failure, the HR of 1-year all-cause mortality was 1.70 (95% CI, 1.60-1.80) for CKD patients with heart failure in the multivariable model (Table 3). Further analysis by ejection fraction demonstrated that the 1-year all-cause mortality HR was 2.43 (95% CI, 2.23-2.65) for HFrEF and 1.59 (95% CI, 1.48-1.70) for HFpEF, compared with CKD patients without heart failure. The 1-year cardiovascular mortality HR was 6.69 (95% CI, 5.93-7.54) for CKD patients with heart failure compared with CKD patients without heart failure after considering other causes of mortality as a competing risk. Compared with CKD patients without heart failure, the 1-year cardiovascular mortality HR was 11.47 (95% CI, 9.90-13.28) and 6.10 (95% CI, 5.36-6.94) for CKD patients with HFrEF and HFpEF, respectively.
Table 3.
One Year All-Cause and Cardiovascular-Related Mortality Hazard Ratio
| 1 year All-Cause and Cardiovascular-Related Mortality by Heart Failure and Chronic Kidney Disease Status |
||||||
|---|---|---|---|---|---|---|
| Outcome | HRa (95% CI) | Adjusted HRb (95% CI) | ||||
| All-cause death rowhead | No heart failure (N=62,439) | 3,928 (6.3%) | Ref | Ref | ||
| Heart failure (N=14,249) | Total | 2,534 (17.8%) | 3.00 (2.86, 3.16) | 1.70 (1.60, 1.80) | ||
| No EF (N=2,485) | 335 (13.5%) | 2.22 (1.98, 2.48) | 1.39 (1.24, 1.56) | |||
| EF > 40% (N=8,436) | 1,414 (16.8%) | 2.82 (2.66, 3.00) | 1.59 (1.48, 1.70) | |||
| EF ≤ 40% (N=3,328) | 774 (23.3%) | 4.12 (3.81, 4.45) | 2.43 (2.23, 2.65) | |||
| Outcome | HRc (95% CI) | Adjusted HRd (95% CI) | |||
|---|---|---|---|---|---|
| Cardiovascular-related death | No heart failure (N=62,439) | 572 (0.9%) | Ref | Ref | |
| Heart failure (N=14,249) | Total | 1,449 (10.2%) | 11.66 (10.59, 12.85) | 6.69 (5.93, 7.54) | |
| No EF (N=2,485) | 160 (6.4%) | 7.21 (6.05, 8.59) | 4.46 (3.70, 5.38) | ||
| EF > 40% (N=8,436) | 754 (8.9%) | 10.17 (9.13, 11.34) | 6.10 (5.36, 6.94) | ||
| EF ≤ 40% (N=3,328) | 535 (16.1%) | 19.21 (17.07, 21.61) | 11.47 (9.90, 13.28) |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; EF, ejection fraction, HR, hazard ratio; Ref, reference.
Unadjusted Cox proportional-hazards model
Cox proportional-hazards model adjusted for age, sex, race/ethnicity, diabetes, hypertension, atrial fibrillation, coronary artery disease, CKD stage, and weighted Charlson comorbidity score.
Unadjusted Fine-Gray subdistribution hazard model
Fine-Gray subdistribution hazard model adjusted for age, sex, race/ethnicity, diabetes, hypertension, atrial fibrillation, coronary artery disease, CKD stage, and weighted Charlson comorbidity score.
Discussion
Our study evaluated patients with incident advanced CKD (eGFR < 45 mL/min/1.73 m2) and found that 18.6% had prevalent heart failure. In addition, we found that over 70% of patients with CKD with heart failure and a known ejection fraction had preserved ejection fraction compared to the general population, in which about half of diagnosed heart failure patients described have reduced ejection fraction.15,16 Our findings were similar to the Chronic Renal Insufficiency Cohort, which demonstrated considerably higher incident HFpEF rates (8.6) compared with HFrEF (6.7 per 1000 person-years) among the CKD population.17
Our study using real-world data from a large integrated health system highlights the vulnerability of patients with both CKD and heart failure. Our study demonstrates that CKD patients with heart failure have a substantially higher risk of both short-term, all-cause and cardiovascular-related mortality compared with patients with CKD without heart failure. Among patients with CKD with HFrEF, there was a greater than 2-fold risk of 1-year all-cause mortality and up to 11-fold increased risk of cardiovascular-related mortality compared with patients with CKD without heart failure. The high rate of cardiovascular-related mortality likely reflects progressive heart failure or sudden cardiac death, which is not uncommon among the CKD population.18, 19, 20 Furthermore, our patients with CKD without heart failure may represent a much different subgroup given the commonality of heart failure among the CKD population. Among our study population of patients with CKD without heart failure, the cardiovascular mortality accounted for only 12.7% of all-cause mortality, which is lower than reported (up to 47%) among all patients with CKD, including those with kidney failure.1,3,4
The ideal management for CKD patients with heart failure is largely unknown. Current therapy recommendations for patients with heart failure are derived from evidence-based cardiovascular outcome trials, termed “guideline-directed medical therapy.” They were introduced in 2013 to optimize medical therapy for patients with heart failure.6 In addition, the optimal management of patients with HFpEF is uncertain. Overall, patients with CKD have been mostly excluded from trials that lead to guideline-directed medical therapy recommendations, with only a few trials demonstrating evidence of benefit for guideline-directed medical therapy medications in patients with HFrEF and CKD.21
There are potential limitations of our study that may confound the interpretation of our findings. First, this was a retrospective cohort study in a population with prevalent heart failure at CKD incidence. The heart failure may have caused the incident CKD in these patients (cardiorenal syndrome) and thus, that in and of itself may have accounted for the substantially higher risk of cardiovascular-related deaths given the short-term follow-up of our study. In addition, because we used the intent-to-treat method only using baseline information, the time-varying variables such as medication adherence and particularly guideline-directed medical therapy for the heart failure population during follow-up were not measured. However, because the follow-up was relatively short at 1 year, we expected the change to be small. We did not have comprehensive information on albuminuria and did not account for it in the outcomes analyses though albuminuria is a well described risk factor for cardiovascular and mortality outcomes. Our study population only included an insured population within Southern California and may not reflect the general CKD and heart failure populations in the community.
Despite these potential limitations, our study findings are derived from a large, diverse population of patients with CKD. The integrated health system and insured population minimizes many of the issues related to access to care and inconsistencies in health delivery. In addition, KPSC has standardized approaches to many chronic conditions such as hypertension and CKD, which can minimize heterogeneity of practice patterns among providers.22,23 We identified our incident CKD population using a comprehensive approach based on serial eGFR measurements over specified time periods. We used an electronic health record-based approach to identify heart failure but also had echocardiogram data on over 80% of the heart failure patients. Therefore, the findings from our diverse population of a real-world clinical practice environment may have greater translational value than more artificial environments (eg, clinical trials) in terms of reflecting what occurs in the community.
Among a large diverse real-world population of patients with incident CKD, we observed a high rate of prevalent heart failure. HFpEF was the predominant form of heart failure over HFrEF among CKD patients. One-year all-cause mortality and cardiovascular-related mortality risk was highest among HFrEF followed by HFpEF compared with CKD patients without heart failure. Given the advancements in therapies for heart failure such as neprilysin inhibitors, sodium/glucose cotransporter 2 inhibitors, and newer generation mineralocorticoid receptor antagonists, future studies are needed to assess the efficacy of these drugs in real-world populations. We hope that such future work will provide more insights into the evolving care for heart failure and CKD,24 ultimately to help better understand and manage this vulnerable population.
Article Information
Authors’ Full Names and Academic Degrees
Albert S. Yu, MD, Katherine J. Pak, MS, Hui Zhou, PhD, Sally F. Shaw, PhD, Jiaxiao Shi, PhD, Benjamin I. Broder, MD, and John J. Sim, MD.
Authors’ Contributions
Research idea and study design: JJS, ASY; data acquisition: HZ, KP, JS, JJS; analysis or interpretation of data: HZ, KP, ASY, JS, SFS, BB, JJS; study supervision: JJS; statistical analysis: HZ, KP, JS. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
This study was supported by the Kaiser Permanente Southern California (KPSC) internal research funds. The funder/supporter had no role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgments
The authors would like to thank the KPSC Renal Business Group for their assistance with identifying and providing comprehensive information for the kidney failure population. The authors would also like to acknowledge and thank the members of KPSC who are the source of our findings and the foundation for why we perform research.
Peer Review
Received August 22, 2022. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form January 16, 2023.
Footnotes
Complete author and article information provided before references.
Table S1: Diagnosis and Procedure Codes Associated With Interested Comorbid Conditions.
Supplementary Material
Table S1.
References
- 1.Bhandari S.K., Zhou H., Shaw S.F., et al. Causes of death in end-stage kidney disease: comparison between the United States renal data system and a large integrated health care system. Am J Nephrol. 2022;53(1):32–40. doi: 10.1159/000520466. [DOI] [PubMed] [Google Scholar]
- 2.Thompson S., James M., Wiebe N., et al. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26(10):2504–2511. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.US Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2022. USRDS 2022 Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 5.House A.A., Wanner C., Sarnak M.J., et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95(6):1304–1317. doi: 10.1016/j.kint.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(17):1757–1780. doi: 10.1016/j.jacc.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Tsao C.W., Lyass A., Enserro D., et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6(8):678–685. doi: 10.1016/j.jchf.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canepa M., Olivotto I. Evolving epidemiology of hypertrophic cardiomyopathy: shifting the focus from instant to lifetime risk awareness. Circ Heart Fail. 2022;15(9) doi: 10.1161/CIRCHEARTFAILURE.122.009873. [DOI] [PubMed] [Google Scholar]
- 9.Pfeffer M.A., Shah A.M., Borlaug B.A. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124(11):1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunlay S.M., Roger V.L., Redfield M.M. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 11.McAlister F.A., Ezekowitz J., Tarantini L., et al. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circ Heart Fail. 2012;5(3):309–314. doi: 10.1161/CIRCHEARTFAILURE.111.966242. [DOI] [PubMed] [Google Scholar]
- 12.Tromp J., Westenbrink B.D., Ouwerkerk W., et al. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2018;72(10):1081–1090. doi: 10.1016/j.jacc.2018.06.050. [DOI] [PubMed] [Google Scholar]
- 13.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Lam C.S.P., Gamble G.D., Ling L.H., et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur Heart J. 2018;39(20):1770–1780. doi: 10.1093/eurheartj/ehy005. [DOI] [PubMed] [Google Scholar]
- 16.Owan T.E., Hodge D.O., Herges R.M., Jacobsen S.J., Roger V.L., Redfield M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 17.Bansal N., Zelnick L., Go A., et al. Cardiac biomarkers and risk of incident heart failure in chronic kidney disease: the CRIC (Chronic Renal Insufficiency Cohort) Study. J Am Heart Assoc. 2019;8(21) doi: 10.1161/JAHA.119.012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Löfman I., Szummer K., Hagerman I., Dahlström U., Lund L.H., Jernberg T. Prevalence and prognostic impact of kidney disease on heart failure patients. Open Heart. 2016;3(1) doi: 10.1136/openhrt-2015-000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankowski J., Floege J., Fliser D., Böhm M., Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cubbon R.M., Gale C.P., Kearney L.C., et al. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ Heart Fail. 2011;4(4):396–403. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- 21.Edner M., Benson L., Dahlström U., Lund L.H. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insufficiency: a prospective propensity score-matched cohort study. Eur Heart J. 2015;36(34):2318–2326. doi: 10.1093/eurheartj/ehv268. [DOI] [PubMed] [Google Scholar]
- 22.Sim J.J., Handler J., Jacobsen S.J., Kanter M.H. Systemic implementation strategies to improve hypertension: the Kaiser Permanente Southern California experience. Can J Cardiol. 2014;30(5):544–552. doi: 10.1016/j.cjca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Sim J.J., Batech M., Danforth K.N., Rutkowski M.P., Jacobsen S.J., Kanter M.H. End-Stage renal disease outcomes among the Kaiser Permanente Southern California Creatinine Safety Program (Creatinine SureNet): opportunities to reflect and improve. Perm J. 2017;21(1):16–143. doi: 10.7812/TPP/16-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beldhuis I.E., Lam C.S.P., Testani J.M., et al. Evidence-based medical therapy in patients with heart failure with reduced ejection fraction and chronic kidney disease. Circulation. 2022;145(9):693–712. doi: 10.1161/CIRCULATIONAHA.121.052792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.