Abstract
Introduction:
The spectrum of bladder health and the factors that promote bladder health and prevent lower urinary tract symptoms (LUTS) among women are not well understood. This manuscript describes the rationale, aims, study design, sampling strategy, and data collection for the RISE FOR HEALTH (RISE) study, a novel study of bladder health in women conducted by the Prevention of Lower Urinary Tract Symptom (PLUS) Research Consortium.
Methods and Results:
RISE is a population-based, multi-center, prospective longitudinal cohort study of community-dwelling, English- and Spanish-speaking adult women based in the US. Its goal is to inform the distribution of bladder health and the individual factors (biologic, behavioral, and psychosocial) and multi-level factors (interpersonal, institutional, community, and societal) that promote bladder health and/or prevent LUTS in women across the life course. Key study development activities included the: (1) development of a conceptual framework and philosophy to guide subsequent activities, (2) creation of a study design and sampling strategy, prioritizing diversity, equity, and inclusion, and (3) selection and development of data collection components. Community members and cross-cultural experts shaped and ensured the appropriateness of all study procedures and materials. RISE participants will be selected by simple random sampling of individuals identified by a marketing database who reside in the 50 counties surrounding nine PLUS clinical research centers. Participants will complete self-administered surveys at baseline (mailed paper or electronic) to capture bladder health and LUTS, knowledge about bladder health, and factors hypothesized to promote bladder health and prevent LUTS. A subset of participants will complete an in-person assessment to augment data with objective measures including urogenital microbiome specimens. Initial longitudinal follow-up is planned at one year.
Discussion:
Findings from RISE will begin to build the necessary evidence base to support much-needed, new bladder health promotion and LUTS prevention interventions in women.
Keywords: Bladder health, women’s health, lower urinary tract symptoms, epidemiology, longitudinal study, social ecological framework, health promotion
Introduction
The Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium is a transdisciplinary team of researchers established to build the necessary scientific evidence base to support future interventions to promote bladder health and prevent lower urinary tract symptoms (LUTS) in women.1
Rationale for Studying Bladder Health and LUTS
Bladder health, as defined by the PLUS Research Consortium, is “a complete state of physical, mental, and social well-being related to bladder function and not merely the absence of LUTS.” Healthy bladder function “permits daily activities, adapts to short-term physical or environmental stressors, and allows optimal well-being (e.g., travel, exercise, social, occupational, or other activities).”2 Little is known about the spectrum of bladder health in women or the individual factors (biologic, behavioral, and psychosocial) and multi-level factors (e.g., interpersonal, institutional, community, and societal) that promote bladder health across the life course.3
LUTS, a different but related concept to bladder health, has been studied more extensively. In prevalence studies, estimates as high as 73% have been observed for reporting at least one urinary symptom and as high as 18% for reporting moderate-to-severe urinary symptoms in women 30 years of age or older.4 Studies also show compromised physical function related to LUTS and negative impact on economic, social, emotional, and medical aspects of women’s lives.5 The prevalence, as well as the individual and societal burden of LUTS, are only anticipated to grow as the population continues to age.5 LUTS research has traditionally focused on estimating the burden of LUTS and treating the most highly affected subsets of women. However, little is known about how and why LUTS develop over time.
The dearth of studies on bladder health, and incomplete understanding of factors that promote bladder health and prevent LUTS in women, motivated the development of the RISE FOR HEALTH (RISE) cohort study. The goal of this study is to address gaps in our understanding of bladder health and LUTS by investigating: (1) individual and multi-level factors that promote bladder health and/or prevent LUTS, focusing primarily on modifiable factors; and (2) the distributions of bladder health (from very healthy to very unhealthy) and knowledge, attitudes, and beliefs (KAB) related to bladder health in women of all ages across the life course. Together, these data will improve our understanding of the full spectrum of bladder health among US women and begin to build the necessary evidence base to support much-needed, new educational and other bladder health promotion and LUTS prevention strategies. We present an overview of the RISE study design and methods.
Methods and Results
Foundational Work: Investigating Factors that Promote Bladder Health and Prevent LUTS
A foundational component for studying the etiology of bladder health and LUTS was the development of the conceptual framework shown in Appendix Figure 1.3,6 This framework considers factors across multiple levels of social ecology including biology, the environment (e.g., individual behaviors, interpersonal relationships, workplaces, and communities), and the life course (e.g., critical time periods) to ensure that PLUS investigators consider all levels of biology and social context in their research. Investigators used this framework to identify important themes for bladder health and LUTS research, as well as the most promising factors within each theme for bladder health promotion and LUTS prevention (Figure 1).7 These factors were identified by literature review and expert opinion and prioritized by expert consensus, focusing on factors with the greatest potential for future prevention interventions. The final set of RISE research questions includes factors from many research themes and levels of social ecology (Figure 1).
Figure 1:
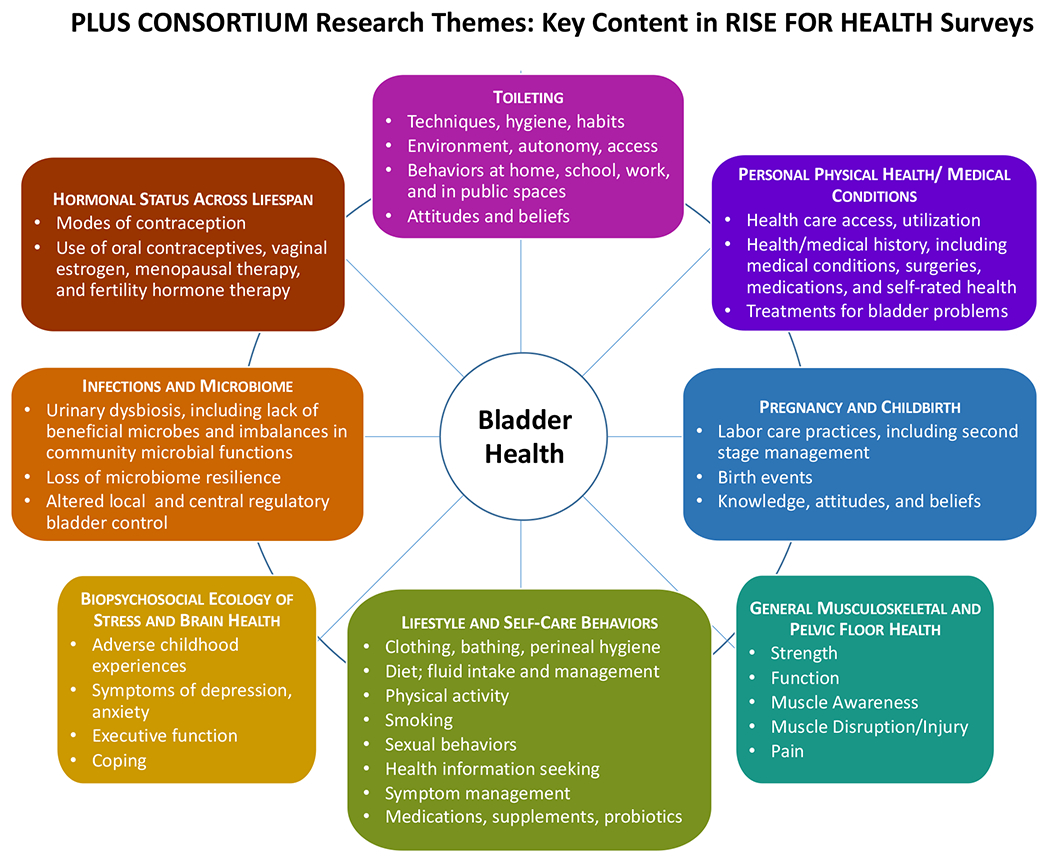
Eight research themes and associated individual factors (biologic, behavioral, and psychosocial) and multi-level factors (interpersonal, institutional, community, and societal) studied in RISE FOR HEALTH.
Foundational Work: Investigating the Spectrum of Bladder Health and Knowledge, Attitudes, and Beliefs in Women
As foundational work for measuring bladder health and bladder health-related knowledge, PLUS investigators conducted qualitative focus groups to begin to understand women’s perspectives on these novel concepts.8 Subsequently, studies were performed to develop, cognitively evaluate,9 and validate10, a new bladder health instrument, the Bladder Health Scales/Bladder Function Indices (BHS/BFI). Similar studies were also performed to develop a novel instrument to assess knowledge, attitudes and beliefs (KAB) surrounding bladder health. Finally, to inform the expected distribution of bladder health in the general female population, PLUS investigators used existing data from the Boston Area Community Health Survey to obtain preliminary estimates of the spectrum of bladder health in women. These analyses indicated a wide spectrum of bladder health among women, with only a small percentage classified as having optimal bladder health at baseline (~20%)11,12 and even fewer after five years of follow-up (~5%).13 Importantly, some women’s bladder health improved over time, demonstrating the potential for secondary prevention interventions.13
Guiding Philosophy for RISE
Throughout the development process, RISE was guided by a philosophy of enhancing diversity, equity, and inclusion (DEI), and encouraging participation by all women, including those traditionally under-represented in research. These include racial/ethnic minorities (e.g., Black or African-American, Hispanic/Latina/o/x/e [herein referred to as Latina/o], and Spanish-speaking individuals), individuals from lower educational and socioeconomic backgrounds, sexual orientation and gender identity minorities, and individuals from across the full adult age lifespan and much of the US. A DEI philosophy factored into all aspects of the study, including the study design and sampling scheme, recruitment and retention procedures and materials, and data collection procedures and materials. PLUS investigators implemented this philosophy by building a multi-tier infrastructure, including a community engagement group to incorporate diverse community member perspectives, and a cross-cultural review group to incorporate Hispanic/Latina/o perspectives at all levels of acculturation (Figure 2). The individual roles of these groups are described below.
Figure 2.
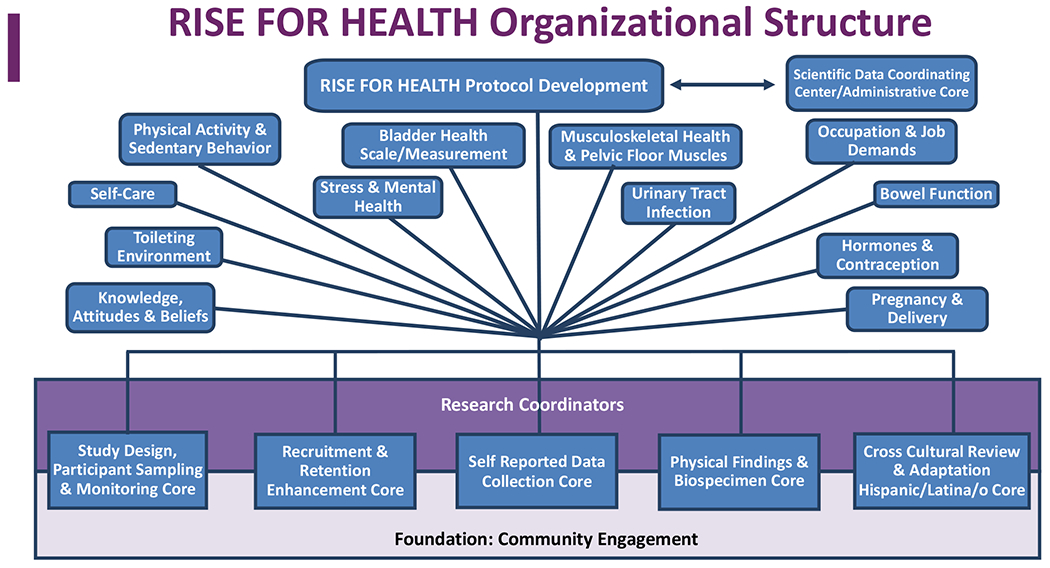
An organizational research infrastructure to effectively study bladder health was built upon traditional study design and sampling considerations to include a team science approach with community engagement and feedback, novel and innovative sampling strategies, cross cultural adaptation for special populations and Spanish language translation, and a focus on recruitment and retention strategies.
Community Engagement
PLUS uses a community-engaged approach that includes community partners or members, representing a wide range of races and ethnicities, and socio-ecological experiences and identities. Each PLUS clinical research center (CRC) identified and cultivated relationships with community members who provided vital insights and recommendations on multiple aspects of RISE methodology, including study procedures (e.g., recruitment and retention strategies appealing to diverse participants, acceptability and feasibility of in-person assessment procedures), instrument development (e.g., prioritization and clarity of items), and study materials (e.g., study name, study logo [as shown in Figure 3], and invitation letters).
Figure 3:
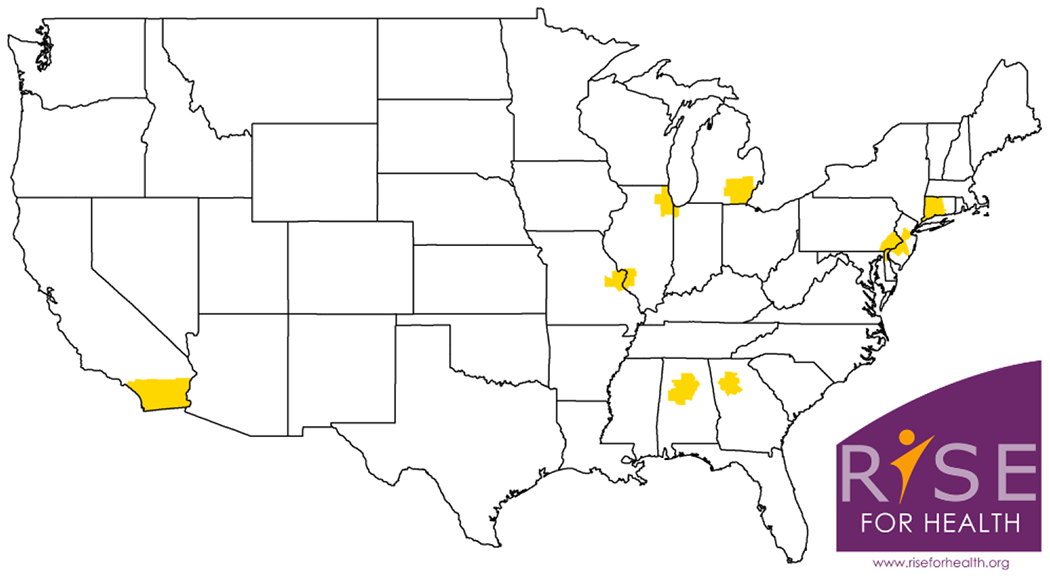
RISE FOR HEALTH study base: Areas comprising the study base for RISE FOR HEALTH are shaded in yellow. Eligible individuals are sampled from the civilian, non-institutionalized population residing in the 50 counties including or surrounding the nine PLUS CRCs (These include Emory University, Atlanta; Loyola University/University of Chicago, Chicago; Northwestern University, Chicago; University of Alabama at Birmingham; University of Michigan, Ann Arbor; University of Pennsylvania, Philadelphia; University of Southern California – San Diego; Washington University in St. Louis; and Yale University, New Haven).The Study logo was developed through community engagement.
Cross Cultural Review
Following Non-Hispanic whites, Hispanic/Latina/o populations are the largest racial/ethnic group in the US. To ensure cultural and linguistic equivalence for Hispanic/Latina/o study participants, all participant-facing materials (e.g., recruitment materials, data collection instruments) underwent a process of cross-cultural review and adaptation by PLUS team members, and translation into Spanish by professional translation services. The PLUS cross-cultural review group consisted of investigators and staff: (1) familiar with each instrument, (2) experienced in clinical care and public health, and/or (3) with Hispanic/Latina/o cultural backgrounds and Spanish language capacity. Because ~70% of US Hispanic/Latina/o populations speak English, reviewers evaluated all materials in both English and Spanish, taking into consideration degrees of acculturation from highly acculturated English speakers to less acculturated Spanish speakers. Professional translation services translated all participant-facing materials into Spanish. The BHS/BFI and other items without existing Spanish translations were adapted using published procedures, including two forward translations, reconciliation of the forward translations, back translation, and cognitive interviewing.14,15 All other participant-facing materials, such as recruitment flyers, were adapted using one forward translation.
Study Design and Sampling Scheme
RISE is a population-based prospective cohort study of community-dwelling English- and Spanish-speaking adult women based in the US. Eligible individuals will be adults (≥ 18 years of age) who were born or identify as female (i.e., cis-gendered women, trans-men, trans-women and non-binary individuals) from the civilian, non-institutionalized population residing in the 50 counties including or surrounding the nine PLUS CRCs (Figure 3). These include Emory University, Atlanta; Loyola University/University of Chicago, Chicago; Northwestern University, Chicago; University of Alabama at Birmingham; University of Michigan, Ann Arbor; University of Pennsylvania, Philadelphia; University of Southern California – San Diego; Washington University in St. Louis; and Yale University, New Haven. We chose to limit participants to areas near in proximity to the PLUS CRCs rather than more broadly across the country to: (1) capitalize on university recognition and branding, which has been shown to improve response and enhance longitudinal follow-up,16 and (2) facilitate in-person collection of clinical data and biologic specimens (described below). However, generalization to the broader US population should still be possible because the sociodemographic characteristics of women residing in the counties including or adjacent to the PLUS CRCs are similar to those of women in the broader US population, with the exception of rural geography (Table 1).
Table 1.
Comparison of Sociodemographic and Health Characteristics Between U.S. and RISE Base Populations
| United States | RISE Study Base | |
|---|---|---|
| Population, Census, April 1, 2020 | 331,449,288 | 36,755,096 |
| Persons under 18 years, percent | 22.30% | 22.20% |
| Persons 65 years and over, percent | 16.50% | 15.30% |
| Female persons, percent | 50.80% | 51.20% |
| White alone, percent | 76.30% | 69.10% |
| Black or African American alone, percent | 13.40% | 19.20% |
| American Indian and Alaska Native alone, percent | 1.30% | 0.60% |
| Asian alone, percent | 5.90% | 8.10% |
| Native Hawaiian and Other Pacific Islander alone, percent | 0.20% | 0.20% |
| Two or More Races, percent | 2.80% | 2.60% |
| Hispanic or Latino, percent | 18.50% | 18.00% |
| White alone, not Hispanic or Latino, percent | 60.10% | 53.50% |
| Foreign born persons, percent, 2015-2019 | 13.60% | 16.10% |
| Owner-occupied housing unit rate, 2015-2019 | 64.00% | 63.40% |
| Median value of owner-occupied housing units, 2015-2019 | $217,500 | $301,094 |
| Median gross rent, 2015-2019 | $1,062 | $1,224 |
| Persons per household, 2015-2019 | 2.62 | 2.68 |
| Living in same house 1 year ago, percent of persons age 1 year+, 2015-2019 | 85.80% | 86.70% |
| Language other than English spoken at home, percent of persons age 5 years+, 2015-2019 | 21.60% | 24.70% |
| Households with a computer, percent, 2015-2019 | 90.30% | 91.90% |
| Households with a broadband Internet subscription, percent, 2015-2019 | 82.70% | 85.40% |
| High school graduate or higher, percent of persons age 25 years+, 2015-2019 | 88.00% | 89.10% |
| Bachelor’s degree or higher, percent of persons age 25 years+, 2015-2019 | 32.10% | 38.20% |
| With a disability, under age 65 years, percent, 2015-2019 | 8.60% | 7.40% |
|
| ||
Potential study participants will be identified from the Acuity database, a commercial marketing database containing personally identifying information (e.g., name, address, telephone number), as well as demographic information (e.g., age, race, ethnicity, gender) on over 237,000,000 individual US residents. We selected this approach because large commercial databases have been used successfully for recruitment into population-based cohort studies for over a decade.17–21 They also permit: (1) personalized study invitations, which have been shown to increase response rates,22 and (2) adjustments in participant sampling based on demographic information to guide the characteristics of the final study population.
For the RISE study, we will select potential participants by simple random sampling from all individuals who meet study criteria and who are identified as female (as opposed to male) in the Acuity database. RISE study participation will involve completion of two mailed or electronic surveys at baseline, as well as one year later at follow-up. A subset of women will be recruited for a baseline in-person assessment and biologic specimen collection.
Recruitment Strategy
The RISE recruitment strategy was developed based on best practices from the literature,16,23,24 PLUS investigators’ experience, and community member input. Initial recruitment materials will consist of a mailed invitation packet followed by two mailed reminders to non-responders. Initial invitations will be delivered via the US Postal Service and will include: (1) an invitation letter and flyer with a brief study description in both English and Spanish, (2) a study website link and opportunity to enroll and complete baseline surveys electronically, (3) two $1 bills, and (4) a self-addressed and stamped return postcard with options to indicate lack of eligibility, lack of willingness to participate, paper survey preference, and Spanish language preference. Mailed reminders will include a letter and full baseline paper survey, as well as Spanish materials for all individuals identified as Hispanic or with Spanish as their primary language in the marketing database (Figure 4). Recruitment materials were designed carefully to make them engaging (e.g., by marketing techniques, such as use of color and visual appeal), and accessible and appealing to a diverse audience (e.g., by community member input). University branding was also used to enhance institutional recognition.
Figure 4:
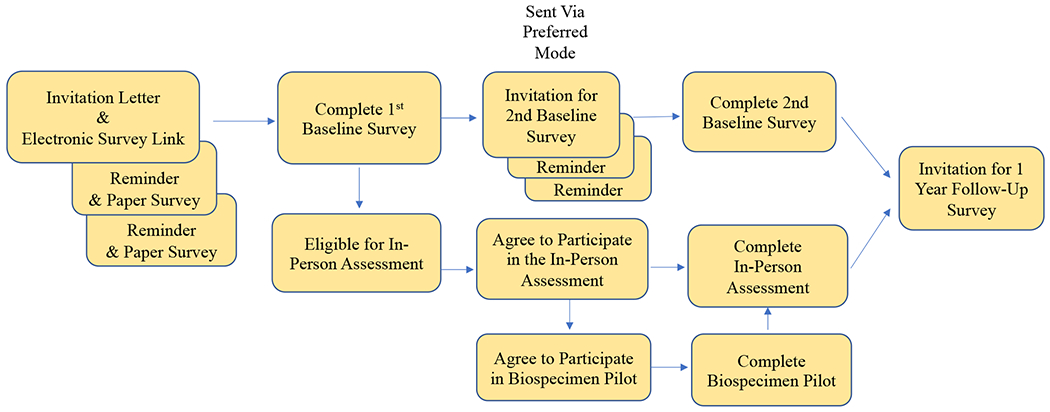
Overview of RISE FOR HEALTH study schematic: Invitation for enrollment, completion of 1st baseline survey, eligibility for in-person assessment and biospecimen pilot, completion of 2nd baseline survey, and follow-up. All participants will be asked to provide additional self-reported data one year from initial response.
Data Collection
Baseline
Baseline RISE data will be collected by self-administered paper or electronic surveys in either English or Spanish (Table 2). To minimize participant burden, baseline data collection was divided into two self-administered surveys designed to take no more than 30 minutes each to complete. These will be administered within eight weeks of each other.
Table 2.
Overview of Data Collection
| Baseline | 1 Year Follow-up Approximately 12 months after completion of first baseline survey |
|||
|---|---|---|---|---|
| 1st Baseline Survey | 2nd Baseline Survey | In-person Data Collection | 1st Follow-up Survey | 2nd Follow-Up Survey |
| Demographics/Social Determinants of Health/Health History | Toileting Environment & Behaviors | Height and Weight, Short Performance Battery | PLUS BHS/BFI | PLUS Knowledge, Attitudes, and Beliefs 2 |
| PLUS Bladder Health Scales/Bladder Function Indices (BHS/BFI) | Physical Activity and Sedentary Behavior | Musculoskeletal (MSK) Exam for Function and Pain | LUTS, Bladder Management/ Adaptive Behaviors | Self-Care Practices and Urinary Tract Infection History |
| LUTS and Bladder Management/Adaptive Behaviors | Occupation and Job Demands | Pelvic Organ Prolapse Assessment | Time-varying variables collected previously | Toileting Behaviors |
| PLUS Knowledge, Attitudes, and Beliefs (KAB) | Musculoskeletal Function and Pain | Pelvic Floor Muscle and Myofascial Pain Assessment | Stress and Brain Health | Hormones, Contraception and Sexual History |
| Additional Key Covariates (Body Mass Index, Smoking History) | Additional covariates (Fluid Intake, Menopause Symptoms) | Biospecimen Collection | Updated Health History and Demographics | Pregnancy and Delivery |
The first baseline survey was designed to capture information necessary to describe the distribution of bladder health and bladder health-related KAB and can be used for this purpose even if participants are subsequently lost to follow-up. It includes two outcome measures, the BHS/BFI and the LURN-SI-10 (Symptoms of Lower Urinary Tract Dysfunction Research Network Symptom Index – 10)25, a validated LUTS instrument; additional items to inform participants’ bladder health status (e.g., LUTS therapies and bladder disease diagnoses); a measure of bladder KAB; demographic characteristics, including social determinants of health; and known risk factors for LUTS and bladder disease (e.g., body mass index, tobacco use, mobility status, and medical history).
The second baseline survey was designed to capture an initial set of factors hypothesized to promote bladder health and/or prevent LUTS. These include measures of the toileting environment and toileting behaviors, physical activity and sedentary behavior, occupational demands, musculoskeletal health, bowel function, fluid intake, and sexual activity.
Whenever possible, we used existing validated instruments or items developed by PLUS (BHS/BFI, KAB, toileting environment, and bowel function) to measure relevant constructs. All other items were derived from previous national studies, such as the National Health and Nutrition Examination Survey,26 the literature, or expert opinion.
In-Person Assessment
Baseline data will also be collected in a subset of participants willing to participate in an in-person assessment that includes several clinical measures and tests, as well as collection of biological specimens. Detailed methods for the in-person assessment are described in a companion manuscript in this issue (Table 2).
Follow-up
Similar to baseline, two 30-minute surveys will be administered at the one-year follow-up. These will include the BHS/BFI and LURN SI-10 to assess changes in bladder health and LUTS over time, additional bladder-related KAB items, and an additional set of factors hypothesized to promote bladder health and/or prevent LUTS (stress and brain health, self-care practices, urinary tract infection history, hormone and contraceptive use, and childbirth-related factors).
Research Appreciation
Research appreciation will be demonstrated by a $15 gift card for each completed survey and a $100 gift card for the in-person assessment. These amounts were derived by input from PLUS investigators, research coordinators, and community members.
Participant Retention
RISE retention strategies were based on best practices from the literature and community member input, and will be refined as the study develops and as novel ideas arise. Initial retention strategies were designed to: (1) maintain interest and engagement in continuing RISE participation, (2) use RISE study branding to increase identification of RISE materials and communications, and (3) help participants remain engaged with RISE between study activities and over time. Specific retention strategies will include quarterly greeting cards, study updates, and thank you notes after each study activity. All participant communications will include the RISE logo and contact information for participants’ local CRC, indication of the approximate timing of the next RISE study activity and remuneration, and occasionally, small tokens of appreciation such as a branded magnet. Retention efforts will also highlight the RISE website, which offers video introductions of the research team at each CRC in both English and Spanish.
Sample Size Calculation and Replicates
RISE aims to recruit 4,750 individuals at baseline to retain 4,000 at one year (assuming ~85% retention). This sample size will give us sufficient power to detect relatively small differences in bladder health change over time (i.e., 0.148 standard deviations of change) between groups with prevalences of at least 10%. All factors hypothesized to promote bladder health and prevent LUTS (Figure 1) were selected to meet this criterion.
To recruit a sample size of 4,750 participants at baseline, we expect to send at least 30,000 invitations (response rate of no more than 16%). Invitations will be mailed in waves or “replicates” (replicates 1 and 2: 10% each of total planned invitations; replicates 3-6: 20% each) to allow us to stagger in-person assessments over one year and to monitor the demographic characteristics of RISE participants. If these are found to differ from the RISE study base population by age and race/ethnicity (particularly non-Hispanic Black and Hispanic/Latina/o), the sampling strategy will be altered to increase invitations to under-represented groups using demographic data from the Acuity database.
Discussion
RISE was conceptualized and designed to provide foundational evidence about bladder health and LUTS in women. Specifically, we aim to better understand the spectrum of bladder health and the burden of poor bladder health in the general female population, as well as to begin to elucidate factors that promote good bladder health and prevent the development of LUTS in women over time. Importantly, RISE will investigate not only traditional, individual biologic and behavioral factors, but also higher-level factors at multiple levels of social ecology, such as school and workplace toileting environments and norms. Together with foundational data on women’s KAB regarding bladder function and health, these findings will begin to build the necessary evidence base to support much-needed, new interventions for bladder health promotion and LUTS prevention in women across the life course (e.g., educational interventions, and programs and policies related to bathroom access in schools, workplaces, and public spaces).
To enhance the relevance of RISE data to as wide an array of women as possible, the RISE team adopted a DEI philosophy and incorporated numerous study development and design features to increase study participation among all women and especially those traditionally underrepresented in research. Diverse community members (with respect to age, race, ethnicity and socioeconomic status) and cross-cultural Hispanic/Latina/o experts reviewed all study procedures and materials to ensure their appropriateness and appeal to women of multiple races and ethnicities, sexual orientation and gender identities, and socioeconomic and educational backgrounds. Extensive efforts were also made to ensure that study participation was accessible to both Spanish- and English-speaking women. Finally, care was taken to ensure similar racial/ethnic diversity in the RISE study base (the 50 counties including and adjacent to the PLUS CRCs) compared with the overall US population and to select a sampling frame (Acuity database) that allows for adjustments to enhance the racial/ethnic diversity of the final sample. Together, we hope that these steps and our over-arching emphasis on broad representation, engagement, and accessibility will increase the relevance of our findings to a wide array of populations. Further, community engagement will not stop at study design and data collection, but will continue to inform interpretation and dissemination of results (e.g., lay summaries), helping to ensure that PLUS research findings are disseminated broadly rather than remaining housed in the scientific community.
Limitations
Despite our DEI focus, we recognize that our results may not be generalizable to populations outside of the US or to areas beyond the PLUS CRC regions, including rural areas. In addition, we will not specifically study certain groups of women, including those who are institutionalized, incarcerated, or homeless. Cis-men will also not be included in RISE.
Conclusion
Traditionally, LUTS research has focused on estimating the prevalence of various forms of LUTS and characterizing and treating the most affected subsets of women. Although this approach serves to optimize treatment outcomes, it is necessary to complement this work with an underlying understanding of the full spectrum of bladder health in women and how this spectrum changes and LUTS develop over time. With its population-based, longitudinal design, RISE will provide rich data to begin to broaden our understanding of the distribution of bladder health in women over the life course, and the individual and multi-level factors that promote bladder health and prevent the development of LUTS over time. This type of foundational data is critical to building the necessary evidence base to support future, innovative bladder health promotion and LUTS prevention interventions. Bladder health and LUTS prevention science will not only inform research and clinical care of women over their life course, but also improve public awareness of bladder health and LUTS, and empower women to make evidence-based choices about their wellness.
Acknowledgements:
We gratefully acknowledge the collegial research work of the Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium members, listed below. We also acknowledge the artistic talent of the late Michele (Shelli) Quackenboss and her contribution to the RISE FOR HEALTH participant facing materials.
Loyola University Chicago - Maywood, IL (U01DK106898)
Multi-Principal Investigators: Linda Brubaker, MD; Elizabeth R. Mueller, MD, MSME
Investigators: Marian Acevedo-Alvarez, MD; Colleen M. Fitzgerald, MD, MS; Cecilia T. Hardacker, MSN, RN, CNL; Jeni Hebert-Beirne, PhD, MPH; Missy Lavender, MBA.
Northwestern University - Chicago IL (U01DK126045)
Multi-Principal Investigators: James W. Griffith, PhD; Kimberly Sue Kenton, MD; Melissa Simon, MD, MPH; Investigator: Julia Geynisman-Tan, MD;
University of Alabama at Birmingham - Birmingham, AL (U01DK106858)
Principal Investigator: Alayne D. Markland, DO, MSc
Investigators: Tamera Coyne-Beasley, MD, MPH, FAAP, FSAHM; Kathryn L. Burgio, PhD; Cora E. Lewis, MD, MSPH; Gerald McGwin, Jr., MS, PhD; Camille P. Vaughan, MD, MS; Beverly Rosa Williams, PhD.
University of California San Diego - La Jolla, CA (U01DK106827)
Principal Investigator: Emily S. Lukacz, MD
Investigators: Sheila Gahagan, MD, MPH; D. Yvette LaCoursiere, MD, MPH; Jesse Nodora, DrPH.
University of Michigan - Ann Arbor, MI (U01DK106893)
Principal Investigator: Janis M. Miller, PhD, APRN, FAAN
Investigators: Lisa Kane Low, PhD, CNM, FACNM, FAAN.
University of Minnesota (Scientific and Data Coordinating Center) - Minneapolis MN (U24DK106786)
Multi-Principal Investigators: Bernard L. Harlow, PhD; Kyle D. Rudser, PhD
Investigators: Sonya S. Brady, PhD; Haitao Chu, MD, PhD; Cynthia S. Fok, MD, MPH; Peter Scal, PhD; Todd Rockwood, PhD.
University of Pennsylvania – Philadelphia, PA (U01DK106892)
Principal Investigator: Multi-Principal Investigators: Diane K. Newman, DNP FAAN; Ariana L. Smith, MD
Investigators: Amanda Berry, MSN, CRNP; Heather Klusaritz, PhD, MSW; Ann E. Stapleton, MD; Jean F. Wyman, PhD.
Washington University in St. Louis - Saint Louis, MO (U01DK106853)
Principal Investigator: Siobhan Sutcliffe, PhD, ScM, MHS
Investigators: Aimee S. James, PhD, MPH;Jerry L. Lowder, MD, MSc; Melanie R. Meister, MD, MSCI.
Yale University - New Haven, CT (U01DK106908)
Principal Investigator: Leslie M. Rickey, MD, MPH
Investigators: Marie A. Brault, PhD (Dec. 2020-); Deepa R. Camenga, MD, MHS; Shayna D. Cunningham, PhD.
Steering Committee Chair: Linda Brubaker, MD. UCSD, San Diego. (January 2021-)
NIH Program Office: National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urologic, and Hematologic Diseases, Bethesda, MD.
NIH Project Scientist: Julia Barthold, M.D.
Funding
for this work was provided through the following NIH awards:
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health (NIH) by cooperative agreements [U24 DK106786, U01 DK106853, U01 DK126045, U01 DK106858, U01 DK106898, U01 DK106893, U01 DK106827, U01 DK106908, U01 DK106892]. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors affirm that all required disclosures are included here:
Ariana L Smith: None
Kyle Rudser: None
Bernard L Harlow - None
Jerry McGwin – None
Julia Barthold - None
Sonya S. Brady: None
Linda Brubaker: Editorial stipends from JAMA, Female Pelvic Medicine and Reconstructive Surgery and Up to Date.
Shayna Cunningham: None
Jamie Griffith - Remuneration for speaking engagements and research consulting. Research funding from Pfizer, the National Eczema Association, and Northwestern University.
Kim Kenton: Research support Axonics, Expert witness Butler Snow/Ethicon
Heather Klusaritz- None
Cora E. Lewis- None
Emily S. Lukacz –Axonics: Consultant; Boston Scientific: Research funding; Cogentix/Uroplasty: Research funding; Pathnostics: Consulting/Scientific Advisory Board; Urovant: Scientific Advisory Board; UpToDate: Royalties
Julia Maki- None
Alayne Markland - None
Elizabeth R. Mueller: UpToDate: Royalties
Diane K. Newman- Research funding: Society of Urology Nurses & Associates; Patient Survey Research: Holliste; Consulting/Scientific Advisory Board; Convatec, Coloplast, COSM, EBT Medical, Urovant Sciences; Editorial: Digital Science Press
Jesse Nodora: None
Leslie M. Rickey: UpToDate: Royalties; UroCure LLC: Quality and Safety Oversight Committee; Renovia Inc: Advisory Board
Todd Rockwood - None
Melissa Simon- None
Jean F. Wyman: None
Siobhan Sutcliffe: None
Appendix Figure 1:
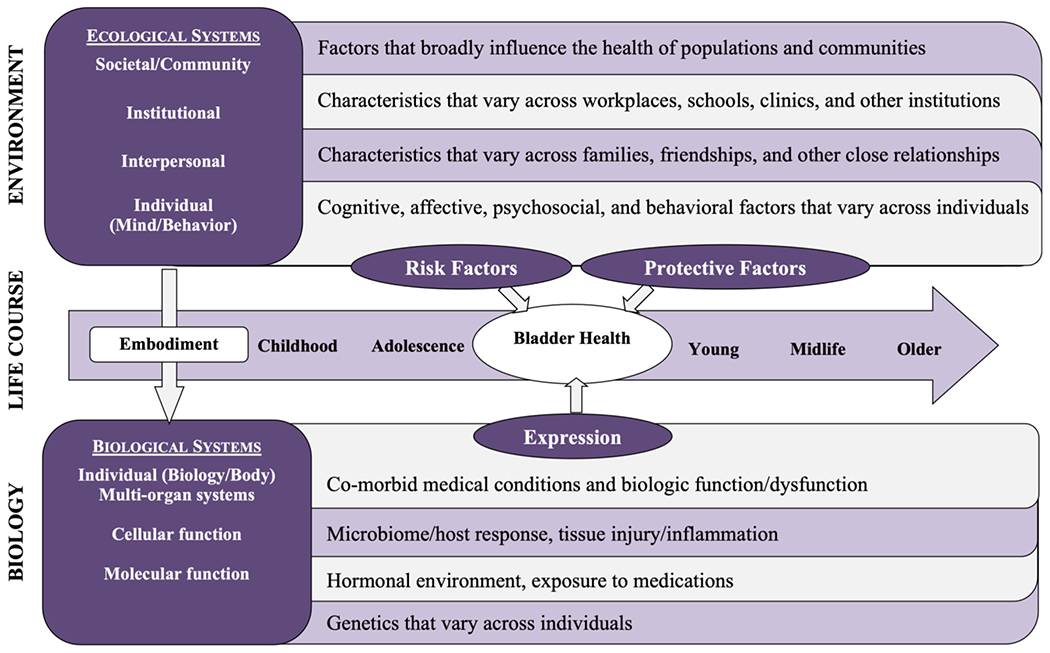
Prevention of Lower Urinary tract Symptoms (PLUS) Research Consortium Conceptual Framework. To facilitate research on bladder health promotion and LUTS prevention, the PLUS Consortium developed a framework for conceptualizing and organizing a broad array of candidate risk and protective factors for bladder health and LUTS. This conceptual framework, which was adapted from the Glass and McAtee Society-Behavior-Biology Nexus, considers multiple levels of social ecology (individual, divided into biology and behavior; interpersonal; institutional; and community/societal) as well as the full life course.
Footnotes
Ethics approval Statement: This manuscript is the original work of the authors and has not been submitted for publication elsewhere.
Patient (Participant) Consent Statement: None needed for methods paper
The RISE for Health study is registered at clinicaltrials.gov: NCT05365971
Data Availability Statement:
no data
References
- 1.Harlow BL, Bavendam TG, Palmer MH, et al. The Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium: A Transdisciplinary Approach Toward Promoting Bladder Health and Preventing Lower Urinary Tract Symptoms in Women Across the Life Course. J Womens Health (Larchmt). 2018;27(3):283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lukacz ES, Bavendam TG, Berry A, et al. A Novel Research Definition of Bladder Health in Women and Girls: Implications for Research and Public Health Promotion. J Womens Health (Larchmt). 2018;27(8):974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady SS, Bavendam TG, Berry A, et al. The Prevention of Lower Urinary Tract Symptoms (PLUS) in girls and women: Developing a conceptual framework for a prevention research agenda. Neurourol Urodyn. 2018;37(8):2951–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupelian V, Wei JT, O’Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166(21):2381–2387. [DOI] [PubMed] [Google Scholar]
- 5.Brady SS, Bavendam TG, Bradway CK, et al. Noncancerous Genitourinary Conditions as a Public Health Priority: Conceptualizing the Hidden Burden. Urology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady SS, Berry A, Camenga DR, et al. Applying concepts of life course theory and life course epidemiology to the study of bladder health and lower urinary tract symptoms among girls and women. Neurourol Urodyn. 2020;39(4):1185–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis J, Brady SS, Sutcliffe S, et al. Converging on bladder health through design thinking: from an ecology of influence to a focused set of research questions. Int J of Envir Res Pub Health. 2020;17(12):4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low LK, Williams BR, Camenga DR, et al. Prevention of Lower Urinary Tract Symptoms Research Consortium Focus Group Study of Habits, Attitudes, Realities, and Experiences of Bladder Health. J Adv Nurs. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rickey LM, Constantine ML, Lukacz ES, et al. Measuring Bladder Health: Development and Cognitive Evaluation of Items for a Novel Bladder Health Instrument. J Urol. 2021;205(5):1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukacz ES, Constantine ML, Kane Low L, et al. Rationale and design of the validation of bladder health instrument for evaluation in women (VIEW) protocol. BMC Womens Health. 2021;21(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutcliffe S, Bavendam T, Cain C, et al. The Spectrum of Bladder Health: The Relationship Between Lower Urinary Tract Symptoms and Interference with Activities. J Womens Health (Larchmt). 2019;28(6):827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutcliffe S, Cain C, Bavendam T, et al. Revisiting the Spectrum of Bladder Health: Relationships Between Lower Urinary Tract Symptoms and Multiple Measures of Well-Being. J Womens Health (Larchmt). 2020;29(8):1077–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutcliffe S, Cain C, Bavendam T, et al. Changes in Bladder Health over Time: A Longitudinal Analysis of Adult Women in the Boston Area Community Health Survey. J Urol. 2022;207(5):1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eremenco SL, Cella D, Arnold BJ. A comprehensive method for the translation and cross-cultural validation of health status questionnaires. Eval Health Prof. 2005;28(2):212–232. [DOI] [PubMed] [Google Scholar]
- 15.Wild D, Grove A, Martin M, et al. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8(2):94–104. [DOI] [PubMed] [Google Scholar]
- 16.Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009(3):MR000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. [DOI] [PubMed] [Google Scholar]
- 18.Connally NP, Yousey-Hindes K, Meek J. Selection of neighborhood controls for a population-based Lyme disease case-control study by using a commercial marketing database. Am J Epidemiol. 2013;178(2):276–279. [DOI] [PubMed] [Google Scholar]
- 19.Buck Louis GM, Schisterman EF, Sweeney AM, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development--the LIFE Study. Paediatr Perinat Epidemiol. 2011;25(5):413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanaya AM, Kandula N, Herrington D, et al. Mediators of Atherosclerosis in South Asians Living in America (MASALA) study: objectives, methods, and cohort description. Clin Cardiol. 2013;36(12):713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malek AM, Barchowsky A, Bowser R, et al. Exposure to hazardous air pollutants and the risk of amyotrophic lateral sclerosis. Environ Pollut. 2015;197:181–186. [DOI] [PubMed] [Google Scholar]
- 22.Scott P, Edwards P. Personally addressed hand-signed letters increase questionnaire response: a meta-analysis of randomised controlled trials. BMC Health Serv Res. 2006;6:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCluskey S, Topping AE. Increasing response rates to lifestyle surveys: a pragmatic evidence review. Perspect Public Health. 2011;131(2):89–94. [DOI] [PubMed] [Google Scholar]
- 24.Dillman DA, Smyth JD, Christian LM. Internet, Phone, Mail, and Mixed-Mode Surveys: The Tailored Design Method. Hoboken, NJ: John Wiley & Sons, Inc; 2014. [Google Scholar]
- 25.Cella D, Smith AR, Griffith JW, et al. A New Brief Clinical Assessment of Lower Urinary Tract Symptoms for Women and Men: LURN SI-10. J Urol. 2020;203(1):164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/questionnaires.aspx?Cycle=2021-2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
no data


