Abstract
Diffuse idiopathic skeletal hyperostosis (DISH) is a noninflammatory progressive disease resulting in ossification of the anterior longitudinal ligament of the spine and tendons. Herein, we describe a case of DISH in a patient on long-term hemodialysis. The patient was a 79-year-old man undergoing hemodialysis for chronic kidney disease due to diabetic nephropathy. He presented to the emergency department complaining of back pain after a slip and fall. Radiographs revealed bamboo spine-like findings, extending from the cervical to the lumbar spine. Computed tomography and magnetic resonance imaging revealed a compression fracture of thoracic vertebra 12, with abnormal ossification of the anterior longitudinal ligament. Inter-vertebral vertical osseous bridges were also observed at the cervical 7 and lumbar 2 vertebrae. There was no obvious spinal cord compression. Leukocytosis and C-reactive protein levels were not elevated and the human leukocyte type antigen HLA-B27 test was negative. Based on this finding, a diagnosis of DISH was made. In the absence of neurological findings, the patient was treated conservatively. Our findings show an overlap between the clinical features of DISH and those of hemodialysis patients, including older age, male sex, and diabetes.
Keywords: Diffuse idiopathic skeletal hyperostosis (DISH), Hemodialysis, Aging
Introduction
Diffuse idiopathic skeletal hyperostosis (DISH) is a clinical term proposed by Resnick et al. [1] which describes a noninflammatory, progressive, disease resulting in ossification of the anterior longitudinal ligament of the spine and tendons. In the spine, the progression of ossification causes the bridging of the intervertebral discs and ankylosis of the vertebral bodies, also known as ankylosing spondyloproliferative disease or Forestier’s disease [2]. Although most patients with ankylosing spondyloproliferative disease are asymptomatic, in some cases, severe ossification and associated vertebral body fractures can lead to complications, including arterial injury and nerve palsy [3]. Ossification of the cervical spine and the associated inflammation increases the risk of compression of the esophageal wall and trachea, causing dysphagia and dyspnea [3]. DISH is more common in males over the age of 50 years, with the incidence increasing with aging. While the cervical spine is the most common site of DISH (63% of cases), the thoracic spine, especially the mid and lower thoracic (Th) segments (Th7-10), and the lumbar spine can also be affected, in that order [1]. As DISH is generally asymptomatic, it has not received much attention in clinical research. However, in recent years, there is emerging clinical evidence that DISH may be associated with metabolic changes and cardiovascular disease [3]. Herein, we describe a case of DISH presenting with vertebral ankylosis and back pain in a patient on long-term hemodialysis.
Case presentation
A 79-year-old man was brought to the emergency department of Showa University Koto Toyosu Hospital due to back pain sustained during a slip and fall, resulting in bruising of his lower back. His medical history included type 2 diabetes, hyperlipidemia, hypertension, and cerebral infarction. At the age of 68 years, his renal function deteriorated, secondary to diabetic nephropathy, and hemodialysis was initiated. The patient was further treated with teneligliptin, pitavastatin, amlodipine, clopidogrel, lansoprazole, and triazolam.
On admission, the patient was conscious and complained of severe back pain. His blood pressure was 146/54 mmHg, with a heart rate of 74 beats/min. There was no evidence of anemia or jaundice. On chest examination, right respiratory sounds were slightly diminished but there were no abnormal heart sounds. Abdominal examination was also normal. There was no abnormal posture or paralysis of the extremities, although mild bilateral lower leg edema was noted. Tapping over the lower back produced pain but with no abnormal neurological findings noted. On the initial radiography examination, there was no evidence of fractures or other abnormalities. A diagnosis of lumbar contusion was made and the patient was treated conservatively. However, the patient’s back pain persisted and he had difficulty moving.
Closer examination of radiographs revealed bamboo spine-like findings extending from the cervical to the lumbar spine (Fig. 1). Computed tomography revealed a compression fracture of Th12 and abnormal ossification of the anterior longitudinal ligament, with vertical osseous bridges between vertebrae, from cervical vertebra 7 (C7) to lumbar vertebra 2 (L2; Fig. 2). Ossification of the anterior longitudinal ligament with osteophyte formation was also visible in the cervical spine. Laboratory examination revealed impairment in renal function but with no leukocytosis or elevated C-reactive protein levels (Table 1). The human leukocyte type antigen HLA-B27 was negative. Spinal magnetic resonance (MR) imaging revealed a compression fracture of Th12, with no obvious spinal cord compression from the cervical to the lumbar spine(Fig. 3). Based on the above findings, a diagnosis of DISH, and not ankylosing spondylitis, was made. In the absence of obvious neurological findings at the time, the decision was made to proceed with a conservative plan of management.
Fig. 1.
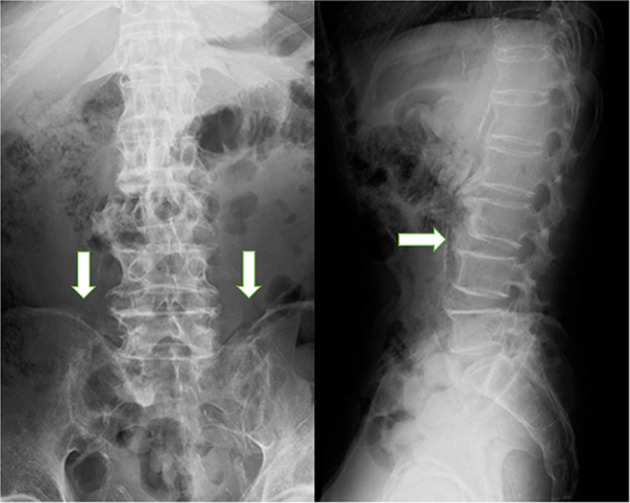
Lumbar spine radiographs. Ossification of the anterior longitudinal ligament is observable, extending from the lower thoracic spine to the lumbar spine, giving the appearance of a bamboo-like spine. The left sacroiliac joint is somewhat obscured but there is no ossification of the right sacroiliac joint
Fig. 2.

Computed tomography images of the cervical and thoracic spine. Arrows show ossification of the anterior longitudinal ligament in the cervical region. Marked ossification of the anterior longitudinal ligament is observable from the thoracic to the lumbar spine. Ossification of more than four vertebrae is seen in the frontal view
Table 1.
Blood test results on admission
| WBC | 4170/μL | CK | 54 U/L |
|---|---|---|---|
| RBC | 424 × 104/μL | CK-MB | 24 U/L |
| Hgb | 13.8 g/dL | Troponin I | 5.6 pg/mL |
| Hct | 41.2% | BNP | 123.4 pg/mL |
| Plt | 12.6 × 104/μL | Glucose | 84 mg/dL |
| TP | 6.5 g/dL | HbA1C (NGSP) | 5.1% |
| Alb | 3.3 g/dL | GA | 25.1% |
| AST | 20 IU/L | Insulin | 6 μU/mL |
| ALT | 12 IU/L | Insulin Ab | < 0.4% |
| T-Bil | 0.9 mg/dL | T-cho | 46 mg/dL |
| γ-GTP | 9 IU/L | TG | 20 mg/dL |
| ALP | 261 IU/L | HDL-c | 19 mg/dL |
| LDH | 247 IU/L | LDL-c | 23 mg/dL |
| Amylase | 21 IU/L | Fe | 26 μg/dL |
| BUN | 32.1 mg/dL | TIBC | 127 μg/dL |
| Cr | 4.48 mg/dL | TSAT | 20.4% |
| Na | 139 mEq/L | Ferritin | 132.8 ng/mL |
| K | 4.5 mEq/L | intact PTH | 90 pg/mL |
| CI | 103 mEq/L | 1–25(OH)2VD3 | < 4 pg/mL |
| cCa | 9.1 mg/dL | PTHrp-intact | < 1.1 pmol/L |
| IP | 3.0 mg/dL | OC | 153.1 ng/mL |
| CRP | 0.45 mg/dL | ucOC | 37.93 ng/mL |
WBC white blood cell, RBC red blood cell, Hgb hemoglobin, Hct hematocrit, Plt platelet, TP total protein, Alb albumin, AST aspartate aminotransferase, ALT alanine aminotransferase, T-Bil total bilirubin, γ-GTP γ-glutamyltransferase, ALP alkaline phosphatase, LDH lactate dehydrogenase, BUN blood urea nitrogen, Cr creatinine, Na sodium, Cl chloride, K potassium, cCa corrected calcium, IP inorganic phosphorus, CRP C-reactive protein, CK creatine kinase, BNP brain natriuretic peptide, NGSP National Glycohemoglobin Standardization Program, GA glycoalbumin, Ab antibody, T-cho total cholesterol, TG triglyceride, HDL-c high-density lipoprotein cholesterol, LDL-c low-density lipoprotein cholesterol, Fe iron, TIBC total iron binding capacity, TSAT transferrin saturation, PTH parathyroid hormone, VD vitamin D, PTHrp parathyroid hormone-related peptide, OC osteocalcin, ucOC undercarboxylated osteocalcin
Fig. 3.
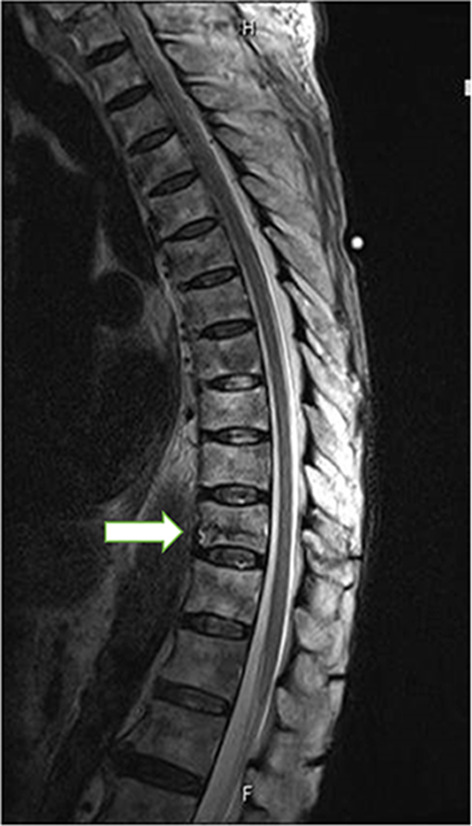
Magnetic resonance images. A compression fracture is observable at thoracic vertebra (Th) 11, with no evidence of associated spinal cord compression or spinal canal stenosis
Discussion
Herein, we describe a case of DISH in a patient on long-term hemodialysis. DISH is a progressive non-inflammatory disorder characterized by calcification and ossification of the enthesis and of the area of attachment of tendons, ligaments, and joint capsules, mainly of the spine. DISH, first described as senile ankylosing hyperostosis of the spine developing in elderly individuals, is a pathological condition distinct from ankylosing hyperostosis [1, 3]. Moreover, changes observed in DISH may also occur in peripheral joints other than the spine [1].
Race-specific differences in the prevalence of DISH have been reported. Among Caucasians, the prevalence rate of DISH ranges between 20 and 30% among men and 10–20% among women [4, 5]. By comparison, in the Japanese population, the overall prevalence rate of DISH is 10.8%, with a rate of 22.0% among males and 4.8% among females, reported in a study sample of 1690 patients with osteoarthritis (mean age, 65.3 years) who underwent whole spine radiography examination [6]. DISH occurs most commonly in the cervical spine and in the mid and lower thoracic spine, where ossification of the anterior longitudinal ligament and lateral ossification occur, with lateral ossification usually developing bilaterally. Although disc protrusion is generally greater on the right side of the thoracic spine, in patients with visceral retroversion, left-side protrusion is more prominent; therefore, the protrusion may be related to the location of the aorta [3].
Known risk factors for DISH include advanced age, male sex, obesity, diabetes, and hyperuricemia [6, 7]. Although the etiology of DISH has not yet been elucidated, genetic and metabolic factors (namely obesity and abnormal lipid metabolism) have been implicated [3]. There is a hypothesis that the process of angiogenesis is associated with atherosclerosis and osteogenesis [8], with significantly greater calcification of the thoracic aorta having been reported in patients with DISH [9]. In this case, on carotid artery ultrasound imaging, some plaques were observed, with an ankle Brachial Pressure Index of 0.85 on the right and 1.13 on the left, and a brachial-ankle pulse wave velocity of 2841 cm/s on the right and 3645 cm/s on the left. These results are suggestive of arterial stiffness, which cannot be completely ruled out in relation to the pathophysiology of DISH.
Although the majority of patients with DISH are asymptomatic, a decrease in spinal range of motion of about 50% has been reported [10], with back stiffness developing in more than half of patients due to decreased spinal mobility [11]. Bone proliferation extending to the cervical spine can cause dysphagia, hoarseness, and aspiration pneumonia. Bony bridges between vertebrae yield a continuous spine that limits the distribution of force. Therefore, external forces, even relatively minor trauma, are likely to cause spinal fractures; as such, the incidence of vertebral fractures is high among patients with DISH [12]. Moreover, pain threshold may be lower among patients with DISH [13].
The diagnosis of DISH requires differentiation from spondylolisthesis and AS [1]. In the case we present herein, as the patient is elderly, differentiating spondylolisthesis from AS may be particularly important. The following characteristics were observed and informed our diagnosis of DISH: evidence of spinal ossification from the lower cervical to the thoracic spine; continuous ossification of four or more vertebrae; and preservation of disc height, with only minor disc degeneration. The indications for surgical treatment were considered; however, in the absence of obvious neurological symptoms or signs or evidence of spinal cord compression on imaging studies, conservative treatment was preferred.
The overlapping clinical features between DISH and hemodialysis are noteworthy, including a higher incidence among elderly men, diabetes, and obesity as risk factors. Although most patients with DISH are asymptomatic, considering the high risk for spinal fractures with minor external forces and for associated neurological complications [12, 14], proactive diagnosis and management of DISH may be effective in avoiding fractures and their associated complications. It is undeniable that hypertension and renal failure in this case may be affected by vascular calcification through a process similar to that of osteogenesis in DISH [8]. Recently, treatment to delay vascular calcification in hemodialysis patients has been investigated [15] and the benefits of these treatments may be even greater for hemodialysis patients with DISH complications, although more cases will be needed for confirmation.
In conclusion, we report on a case of DISH in an elderly male patient on long-term hemodialysis. We describe the clinical presentation, findings, and diagnostic process, as well as justify the conservative treatment approach used. To our knowledge, there are few case reports of DISH in hemodialysis patients, and thus, this case is valuable. Cases will need to be accumulated to develop a body of evidence to guide the diagnosis and management.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures were performed in accordance with the ethical standards of our institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient for publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Resnick D, Shaul SR, Robins JM. Diffuse idiopathic skeletal hyperostosis (DISH): Forestier’s disease with extraspinal manifestations. Radiology. 1975;115:513–524. doi: 10.1148/15.3.513. [DOI] [PubMed] [Google Scholar]
- 2.Forestier J, Rotes-Querol J. Senile ankylosing hyperostosis of the spine. Ann Rheum Dis. 1950;9:321–330. doi: 10.1136/ard.9.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mader R, Verlaan JJ, Buskila D. Diffuse idiopathic skeletal hyperostosis: clinical features and pathogenic mechanisms. Nat Rev Rheumatol. 2013;9:741–750. doi: 10.1038/nrrheum.2013.165. [DOI] [PubMed] [Google Scholar]
- 4.Weinfeld RM, Olson PN, Maki DD, Griffiths HJ. The prevalence of diffuse idiopathic skeletal hyperostosis (DISH) in two large American Midwest metropolitan hospital populations. Skeletal Radiol. 1997;26:222–225. doi: 10.1007/s002560050225. [DOI] [PubMed] [Google Scholar]
- 5.Westerveld LA, van Ufford HM, Verlaan JJ, Oner FC. The prevalence of diffuse idiopathic skeletal hyperostosis in an outpatient population in The Netherlands. J Rheumatol. 2008;35:1635–1638. [PubMed] [Google Scholar]
- 6.Kagotani R, Yoshida M, Muraki S, et al. Prevalence of diffuse idiopathic skeletal hyperostosis (DISH) of the whole spine and its association with lumbar spondylosis and knee osteoarthritis: the ROAD study. J Bone Miner Metab. 2015;33:221–229. doi: 10.1007/s00774-014-0583-9. [DOI] [PubMed] [Google Scholar]
- 7.Kiss C, Szilagyi M, Paksy A, Poor G. Risk factors for diffuse idiopathic skeletal hyperostosis: a case-control study. Rheumatology (Oxford) 2002;41:27–30. doi: 10.1093/rheumatology/41.1.27. [DOI] [PubMed] [Google Scholar]
- 8.Pappone N, Ambrosino P, Di Minno MND, Iervolino S. Is diffuse idiopathic skeletal hyperostosis a disease or a syndrome? Rheumatology. 2016;56:1635–1636. doi: 10.1093/rheumatology/kew451. [DOI] [PubMed] [Google Scholar]
- 9.Harlianto NI, Westerink J, Hol ME, Wittenberg R, Foppen W, van der Veen PH, et al. Patients with diffuse idiopathic skeletal hyperostosis have an increased burden of thoracic aortic calcifications. Rheumatol Adv Pract. 2022;6:rkac060. doi: 10.1093/rap/rkac060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mata S, Fortin PR, Fitzcharles MA, et al. A controlled study of diffuse idiopathic skeletal hyperostosis. Clinical features and functional status. Medicine (Baltimore) 1997;76:104–17. doi: 10.1097/00005792-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Resnick D, Shapiro RF, Wiesner KB, Niwayama G, Utsinger PD, Shaul SR. Diffuse idiopathic skeletal hyperostosis (DISH) [ankylosing hyperostosis of Forestier and Rotes-Querol] Semin Arthritis Rheum. 1978;7:153–187. doi: 10.1016/0049-0172(78)90036-7. [DOI] [PubMed] [Google Scholar]
- 12.Caron T, Bransford R, Nguyen Q, Agel J, Chapman J, Bellabarba C. Spine fractures in patients with ankylosing spinal disorders. Spine (Phila Pa 1976) 2010;35:E45864. doi: 10.1097/BRS.0b013e3181cc764f. [DOI] [PubMed] [Google Scholar]
- 13.Mader R, Novofastovski I, Rosner E, Adawi M, Herer P, Buskila D. Nonarticular tenderness and functional status in patients with diffuse idiopathic skeletal hyperostosis. J Rheumatol. 2010;37:1911–1916. doi: 10.3899/jrheum.091008. [DOI] [PubMed] [Google Scholar]
- 14.Westerveld LA, Verlaan JJ, Oner FC. Spinal fractures in patients with ankylosing spinal disorders: a systematic review of the literature on treatment, neurological status and complications. Eur Spine J. 2009;18:145–156. doi: 10.1007/s00586-008-0764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raggi P, Bellasi A, Bushinsky D, Bover J, Rodriguez M, Ketteler M, et al. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: Results of a randomized phase 2b study. Circulation. 2020;141:728–739. doi: 10.1161/CIRCULATIONAHA.119.044195. [DOI] [PubMed] [Google Scholar]


