Abstract
Focal segmental glomerulosclerosis is a rare complication of acromegaly. A 74-year-old man was found to have acromegaly features such as enlargement of the forehead, nose, and hands. Laboratory tests showed a urine protein/creatinine ratio of 3.16 g/gCr and serum creatinine of 1.34 mg/dL. The levels of growth hormone and insulin-like growth factor I were markedly elevated, and the growth hormone level was not suppressed after 75 g oral glucose loading. Magnetic resonance imaging revealed a pituitary tumor with a diameter of 1.2 cm. Renal biopsy confirmed the diagnosis of focal segmental glomerulosclerosis. Transsphenoidal resection of the pituitary tumor led to remission of acromegaly and reduction in proteinuria highlighting the causal link between growth hormone overproduction and proteinuria. Treatment of acromegaly may be effective for acromegaly-associated focal segmental glomerulosclerosis.
Keywords: Acromegaly, Growth hormone, Insulin-like growth factor-1, Focal segmental glomerulosclerosis, Podocyte injury
Introduction
Focal segmental glomerulosclerosis (FSGS) is one of the main causes of chronic kidney disease worldwide, and is characterized by lesions of some glomeruli (i.e., focal) with partial involvement of the glomerular tuft (i.e., segmental). It may clinically present with marked proteinuria and steroid-resistant nephrotic syndrome. FSGS can be classified into primary and secondary forms. While primary FSGS has no known or apparent cause, secondary FSGS is associated with genetic mutations that affect podocyte function, drugs such as interferon, viral infections, unilateral renal dysplasia, or obesity [1–3].
Acromegaly is caused by overproduction of growth hormone (GH) usually from pituitary adenomas, presenting with enlargement of the hands and feet secondary to both bone expansion and soft tissue swelling [4, 5]. Moreover, acromegaly is associated with other serious complications such as cardiovascular diseases, diabetes, hypertension, and cancers, which contributes to increased mortality [4–6]. Although rare, acromegaly has been also reported to cause unique kidney injury [7]. Patients with active acromegaly present with renal hypertrophy and microalbuminuria [8]. To the best of our knowledge, there are very few reports describing secondary FSGS associated with acromegaly [9–13].
Here, we report the successful treatment of proteinuria in a patient with acromegaly-associated FSGS through transsphenoidal resection of a pituitary adenoma and present a literature review of similar reported cases.
Case presentation
A 74-year-old man had a 14-year history of hypertension and surgery for spinal stenosis 9 years ago. Proteinuria was positive for 5 years and serum creatinine was more than 1 mg/dL for 3 years. The patient had no family history of renal or endocrine diseases. He had been treated for hypertension and renal dysfunction by his previous physician for the past 6 months. For hypertension, 80 mg of valsartan, 5 mg of amlodipine, and 1 mg of indapamide were administered and his systolic blood pressure was controlled at 120–140 mmHg. His serum creatinine had remained 1.1–1.3 mg/dL but proteinuria increased from around 1 g/gCr to 4–6 g/gCre. A renal echo demonstrated that the right and left kidneys were sized 105×48 mm and 109×53 mm, with no apparent renal atrophy and hypertrophy. In addition to the increase of proteinuria, acromegaly was suspected due to the presence of distinct facial features and elevated GH levels leading to the referral to our hospital.
On admission, he had a blood pressure of 129/79 mmHg, a heart rate of 85/min, a height of 165.0 cm, and a weight of 63.6 kg. Physical examination revealed frontal bossing and enlargement of the tongue, hands, and feet. Urinalysis showed a urine protein/creatinine ratio of 3.16 g/gCr. Laboratory tests revealed an elevated blood urea nitrogen (27 mg/dL), an elevated serum creatinine (1.34 mg/dL) with low estimated glomerular filtration rate (43 mL/min/1.73 m2), and a normal serum albumin (4.3 g/dL). Laboratory tests also showed marked elevation of serum GH (28.2 ng/mL, normal range: 0–2.47 ng/mL) and insulin-like growth factor-1 (IGF-1, 733 ng/mL; age- and gender-specific normal range: 54–190 ng/mL) levels. The other laboratory findings are presented in Table 1. The GH levels remained elevated after 75 g oral glucose loading, suggestive of GH overproduction (Table 2). Pituitary magnetic resonance imaging revealed a 1.2 cm pituitary tumor classified as Knosp grade 1 (Fig. 1A). These findings were consistent with the diagnosis of acromegaly. No other endocrinopathy except impaired glucose tolerance and acromegaly was detected during hormonal evaluation.
Table 1.
Laboratory findings of the present case
| Urinalysis | |
| Dipstick | |
| Protein | 3+ |
| Blood | – |
| Protein | 3.16 g/g Cr |
| Red blood cell | < 4 cells/high-power field |
| White blood cells | < 4 cells/high-power field |
| Cast | – |
| NAG | 30.6 IU/g Cr (0–5.6) |
| β2 microglobulin | 192 ug/L (< 150) |
| Blood counts | |
| White blood cell | 5.6 × 103 cells/μL |
| Red blood cell | 4.28 × 106 cells/μL |
| Hemoglobin | 13.1 g/dL |
| Hematocrit | 40.2% |
| Platelet | 197 × 103 platelets/μL |
| Biochemistry | |
| Blood urea nitrogen | 27 mg/dL (8–20) |
| Creatinine | 1.34 mg/dL (0.46–0.79) |
| eGFR | 43 mL/min/1.73 m2 |
| AST | 24 U/L (13–30) |
| ALT | 16 U/L (7–23) |
| Uric acid | 6.0 mg/dL (2.6–5.5) |
| Total protein | 7.4 g/dL (6.6–8.1) |
| Albumin | 4.3 g/dL (3.7–5.4) |
| Total cholesterol | 216 mg/dL (0–219) |
| Triglycerides | 189 mg/dL (0–149) |
| Glucose | 93 mg/dL (70–109) |
| HbA1c | 5.7% (4.6–6.2) |
| C-reactive protein | 0.03 mg/dL (0–0.14) |
| IgG | 1454 mg/dL (861–1747) |
| IgG4 | 24 mg/dL (11–121) |
| IgA | 252 mg/dL (93–393) |
| IgM | 82 mg/dL (50–269) |
| C3 | 80 mg/dL (73–138) |
| C4 | 22.8 mg/dL (11–31) |
| CH50 | 49.0 U/mL (31.6–57.6) |
| Anti-nuclear antibody | 160× (0–80) |
| Homogeneous | Positive |
| Speckled | Positive |
| Anti-dsDNA antibody | 2.2 IU/mL (0–12.0) |
| MPO-ANCA | < 1.0 U/mL |
| PR3- ANCA | < 1.0 U/mL |
| Endocrinology | |
| GH | 28.2 ng/mL (0–2.47) |
| IGF-1 | 733 ng/mL (54–190) |
| ACTH | 80.9 pg/mL (7.2–63.3) |
| Cortisol (baseline) | 23.0 μg/dL (7.07–19.6) |
| Cortisol at 1 mg DST | 1.6 μg/dL (< 1.8) |
| TSH | 1.05 μIU/mL (0.5–5.0) |
| FT3 | 2.49 pg/mL (2.3–4.0) |
| FT4 | 1.50 ng/dL (0.9–1.7) |
| PRL | 7.70 ng/mL (3.7–16.3) |
| LH | 3.10 mIU/mL (2.2–8.4) |
| FSH | 9.11 mIU/mL (1.8–12.0) |
| Total testosterone | 257 ng/dL (131–871) |
| whole PTH | 57.7 pg/mL (8.3–38.7) |
| Viral infection | |
| HBs antigen | Negative |
| HBs antibody | Negative |
| HCV antibody | Negative |
| HIV antibody | Negative |
Parentheses indicate reference values
NAG N-acetyl-β-D-glucosaminidase, eGFR estimated glomerular filtration rate, AST aspartate transaminase, ALT alanine transaminase, CH50 total complement activity, anti-dsDNA anti-double stranded deoxyribonucleic acid, MPO-ANCA myeloperoxidase-antineutrophil cytoplasmic antibody, PR3-ANCA proteinase 3- antineutrophil cytoplasmic antibody, GH growth hormone, IGF-1 insulin-like growth factor-1, ACTH adrenocorticotropic hormone, DST dexamethasone suppression test, TSH thyroid-stimulating hormone, FT3 free triiodothyronine, FT4 free thyroxine, LH luteinizing hormone, FSH follicle-stimulating hormone, PRL prolactin, PTH parathyroid hormone, HBs hepatitis B surface, HCV hepatitis C virus, HIV human immunodeficiency virus
Table 2.
Preoperative and postoperative growth hormone suppression test results
| Time after 75 g glucose loading (minute) | Baseline | 30 | 60 | 90 | 120 |
|---|---|---|---|---|---|
| At diagnosis | |||||
| Growth hormone (ng/mL) | 34.2 | 32.1 | 33.2 | 31.5 | 28.7 |
| Glucose (mg/dL) | 91 | 161 | 192 | 206 | 154 |
| Three months after surgery | |||||
| Growth hormone (ng/mL) | 2.29 | 1.1 | 0.63 | 0.42 | 0.32 |
| Glucose (mg/dL) | 94 | 153 | 191 | 174 | 171 |
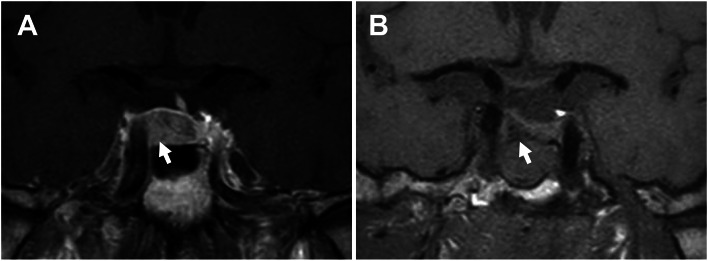
Fig. 1.
Pre- and postoperative findings of pituitary magnetic resonance imaging (MRI). A A preoperative T1-weighted image with contrast enhance. This image shows a 12 mm pituitary tumor as a less enhancing lesion (white arrow). The normal pituitary gland is shoved to the upper left of the sella turcica. B A postoperative T1-weighted image without contrast. After tumor resection (white arrow), the normal pituitary gland is decompressed
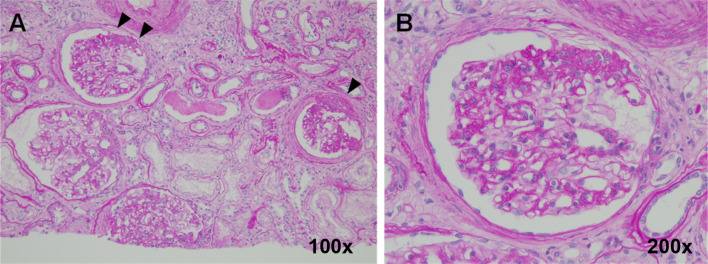
Among the 26 glomeruli retrieved via percutaneous renal biopsy, histopathologic findings include global sclerosis in 6 glomeruli, segmental sclerosis in 3 glomeruli, and adhesion in 2 glomeruli. Others exhibited glomerular hypertrophy (the maximum diameter; 250 μm) with mild mesangial cell proliferation. Moreover, tubular atrophy with interstitial fibrosis and inflammatory cell infiltration was observed in 50% of the renal cortex. Arteriolar wall thickening was also identified (Fig. 2). Immunostaining for immunoglobulins and complement proteins showed negative or non-specific deposition. Electron microscopy revealed the foot process effacement of podocytes and widening of the subendothelial spaces (Fig. 3). These findings were consistent with a diagnosis of FSGS, not otherwise specified.
Fig. 2.
Histopathologic examination of the renal biopsy specimen. Periodic acid-Schiff staining of the renal biopsy specimen reveals focal segmental glomerulosclerosis (arrowheads). Original magnification: A 100 × and B 200 ×
Fig. 3.
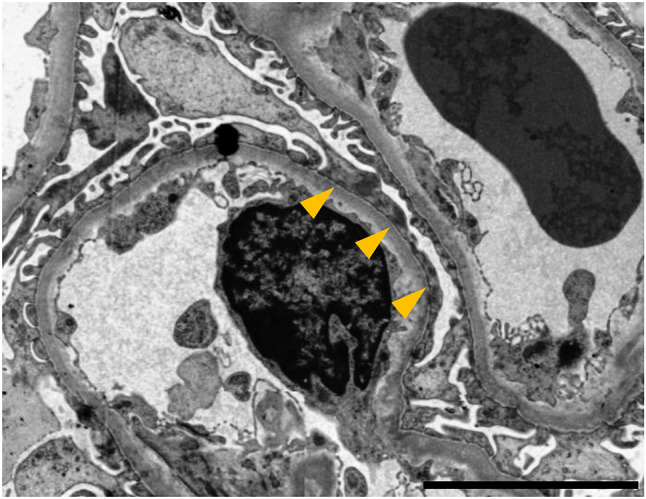
The findings of electron microscopy. The foot process effacement of podocytes (arrowheads) is observed in electron microscopy study. Scale bar indicates 5 µm
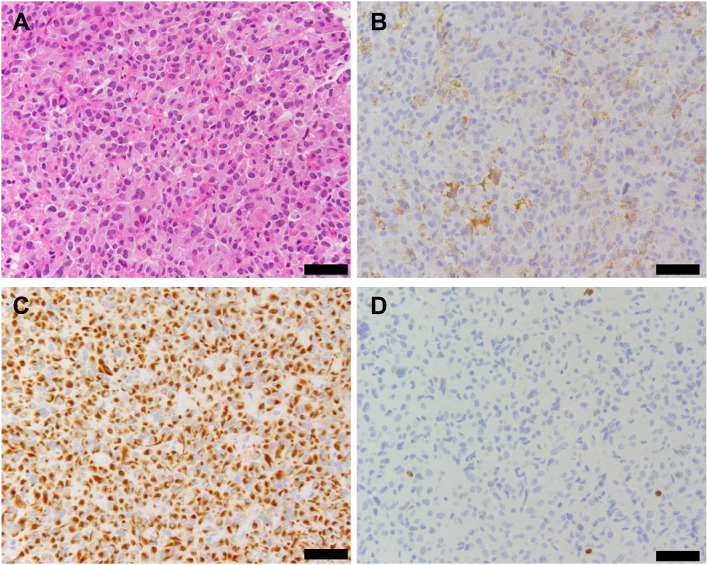
The pituitary tumor was completely removed by transsphenoidal surgery, confirmed by magnetic resonance imaging (Fig. 1B). Based on histopathological analysis, the tumor was diagnosed with a GH-producing adenoma (Fig. 4).
Fig. 4.
Histopathological findings of the resected pituitary tumor. Staining images of hematoxylin and eosin (A), growth hormone (B), CAM5.2 (C) and MIB-1 (D). The tumor cells harbor eosinophilic cytoplasm (A), 40% of which were positive for growth hormone (B). CAM5.2 staining showed a dotted perinuclear pattern in the tumor cells (C). MIB-1 labeling index was calculated as 0.6% (D). Scale bars indicate 50 µm
A 1-month follow-up showed improvement of the urinary protein/creatinine ratio (0.71 g/gCr) and GH level (6.41 ng/mL). A 3-month follow-up revealed further reduction of the urinary protein/creatinine ratio (0.3 g/gCr) under the same antihypertensive agents as before (blood pressure, 116/77 mmHg). The IGF-1 level was normalized (153 ng/mL). Finally, the GH level after 75 g oral glucose loading decreased to less than 0.4 ng/mL, indicating remission of acromegaly (Table 2). One year after surgery, proteinuria remained at about 0.7–1.0 g/gCr and serum creatinine was 1.53 mg/dL. Valsartan was maintained at 80 mg and no immunosuppressive therapy was used.
Discussion
We encountered a case of massive proteinuria and renal dysfunction in a patient with acromegaly-associated FSGS confirmed via renal biopsy. Treatment with transsphenoidal surgery significantly improved proteinuria and lead to complete remission of acromegaly. These findings suggest a potential association between GH overproduction and glomerular injury in acromegaly.
GH receptor is expressed in glomerular cells such as podocyte and mesangial cells [14, 15]. Since FSGS is a condition associated with podocyte damage [16], the role of GH overproduction in podocyte injury has been demonstrated in a number of studies [7, 17]. One of which showed that GH-transgenic mice presented with glomerular hypertrophy and podocyte injury [18]. Furthermore, several studies have described that GH promotes mesenchymal-to-epithelial transition or apoptosis via Notch signaling or transforming growth factor-beta/Smad signaling pathway in podocytes [19, 20]. IGF-1 receptor is expressed in glomeruli, but overexpression of IGF-1 did not cause obvious glomerulosclerotic lesions [18, 21]. Based on the findings of these basic studies and the course of decreased proteinuria after acromegaly treatment, we speculate that GH overproduction directly causes podocyte injury and FSGS in our patient. There is no family history suggestive of hereditary FSGS, and the absence of a history of FSGS-causing drugs treatment or suspected viral infection such as HIV also supports the association of FSGS and acromegaly in this case. However, one limitation is that acromegaly was diagnosed just prior to referral, so the time of onset and duration of the disease are unknown. Thereby, we cannot accurately compare the onset of renal impairment.
The patient has a long history of hypertension, which can be associated with acromegaly [4, 5]. In addition, atherosclerotic lesions were also observed on the renal biopsy, suggesting the presence of hypertensive renal injury. It is possible that long-term hypertension may have affected renal function and the course of proteinuria in this case.
To the best of our knowledge, there are only six cases of FSGS with associated acromegaly, including the present case [9–13] (Table 3). Among these, all the patients were men with an average age of 58.2 years. The degree of proteinuria was variable with three cases of nephrotic-range proteinuria [9, 11]. In all the cases, treatment with surgery or pharmacologic therapy led to remission of acromegaly. In three cases with severe proteinuria, immunosuppressive therapy (e.g., corticosteroids) was administered [9, 11, 13]. Moreover, five cases received renin–angiotensin–aldosterone system inhibitors [9, 10, 12, 13]. Regarding the renal outcomes, reduction of proteinuria or partial remission of nephrotic syndrome was observed in five cases [9, 11–13].
Table 3.
Summary of FSGS cases in acromegaly patients
| Age/sex | Baseline renal function | Treatment | Renal function at final follow-up | References |
|---|---|---|---|---|
| 46/M | sCr 0.8 mg/dL | Corticosteroid | sCr 1.2 mg/dL | [9] |
| UP 8.2–15.0 g/day | Immunosuppressants | UP 1.0 g/day | ||
| Surgical treatment | ||||
| LDL adsorption | ||||
| Somatostatin analogue | ||||
| ACEi | ||||
| 53/M | sCr 132 μmol/L | Surgical treatment | UP 2 + | [10] |
| UP 3 + ; 1.4 g/day | ACEi | |||
| 63/M | sCr 130.4 μmol/L | Corticosteroid | sCr 80.0 μmol/L | [11] |
| Surgical treatment | UP 2.0 g/day | |||
| UP 9.1 g/day | ||||
| 64/M | cCr 0.6 mg/dL | Surgical treatment | UP 513 mg/day* | [12] |
| UP 967.3 mg/day* | ARB | |||
| 49/M | sCr 61 μmol/L | Corticosteroid | sCr 76 μmol/L | [13] |
| UP 6.29 g/day | ARB, TAC, Tripterygium | UP 2.85 g/day | ||
| Surgical treatment | ||||
| 74/M | sCr 1.34 mg/dL | Surgical treatment | sCr 1.53 mg/dL | Present case |
| UP 3.16 g/gCr | ARB | UP 0.7–1.0 g/gCr |
M male, sCr serum creatinine, UP urinary protein, ACEi angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, TAC tacrolimus
*Average of three examinations
Surgical excision of pituitary tumors is a standard therapy for acromegaly. In addition, pharmacologic therapy such as somatostatin analogs or GH receptor antagonists has also been widely used [4, 5]. Reduction of GH and/or IGF-1 is reported to improve cardiovascular comorbidities and mortality in patients with acromegaly [22, 23]. Previous study reported that immunosuppressive therapy improved the proteinuria of a patient with FSGS [13], however, in our case, transsphenoidal surgery was effective in treating acromegaly and reducing proteinuria, avoiding the potential side effects of immunosuppressants. There are some cases with FSGS in which reduction of proteinuria can be expected with surgical therapy alone, but further studies are required to identify the optimal management strategy for renal complications in patients with acromegaly.
In our case, the patient presented with severe tubulointerstitial damage in the absence of a history of treatment with medications known to cause tubular injury (e.g., non-steroidal anti-inflammatory drugs and antimicrobials) [24]. Moreover, other systemic diseases (e.g., IgG4-related disease and sarcoidosis) that could have complicated tubular injury [24] were excluded. Although the cause of tubular injury in the present case is unknown, it might be possible that the GH overproduction contributed to the tubular injury since the expression of GH receptors was demonstrated in renal tubular cells [25]. Furthermore, aged GH-transgenic mice exhibited tubular changes in addition to glomerulosclerosis [18].
We have described a rare case of acromegaly-associated FSGS. This study highlights the utility of surgery in treating acromegaly to improve the clinical outcomes of FSGS.
Acknowledgements
We thank Kiyomi Kisu, Tohoku University, for her technical assistance.
Declarations
Conflict of interest
The author(s) declare that they have no conflict of interest.
Informed consent
Written informed consent was obtained from this patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Vriese AS, Wetzels JF, Glassock RJ, Sethi S, Fervenza FC. Therapeutic trials in adult FSGS: lessons learned and the road forward. Nat Rev Nephrol. 2021;17:619–630. doi: 10.1038/s41581-021-00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355:2558–2573. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- 5.Katznelson L, Laws ER, Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3933–3951. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
- 6.Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C, et al. A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metab. 2020;105:e937–e946. doi: 10.1210/clinem/dgz096. [DOI] [PubMed] [Google Scholar]
- 7.Haffner D, Grund A, Leifheit-Nestler M. Renal effects of growth hormone in health and in kidney disease. Pediatr Nephrol. 2021;36:2511–2530. doi: 10.1007/s00467-021-05097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auriemma RS, Galdiero M, De Martino MC, De Leo M, Grasso LF, Vitale P, et al. The kidney in acromegaly: renal structure and function in patients with acromegaly during active disease and 1 year after disease remission. Eur J Endocrinol. 2010;162:1035–1042. doi: 10.1530/EJE-10-0007. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida H, Akikusa B, Saeki N, Hasegawa S, Iesato K, Yamamoto S, et al. Effect of pituitary microsurgery on acromegaly complicated nephrotic syndrome with focal segmental glomerulosclerosis: report of a rare clinical case. Am J Kidney Dis. 1999;33:1158–1163. doi: 10.1016/S0272-6386(99)70156-3. [DOI] [PubMed] [Google Scholar]
- 10.Takai M, Izumino K, Oda Y, Terada Y, Inoue H, Takata M. Focal segmental glomerulosclerosis associated with acromegaly. Clin Nephrol. 2001;56:75–77. [PubMed] [Google Scholar]
- 11.Zheng J, Cui Z, Lv JC, Duan HZ, Wang SX, Zhang JQ, et al. Delayed diagnosis of acromegaly in a patient with focal segmental glomerulosclerosis: a rare case report and literature review. BMC Nephrol. 2019;20:435. doi: 10.1186/s12882-019-1626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamasaki A, Bito D, Eto E, Matsumoto K, Nakamura M, Miyazaki J, et al. Focal segmental glomerulosclerosis in which urinary protein improved after surgical treatment for acromegaly: a case report. J Med Case Rep. 2019;13:298. doi: 10.1186/s13256-019-2228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R, Wu Y, An D, Ma P, Guo Y, Tang L. Case report: glucocorticoids combined with immunosuppressant in the treatment of acromegaly complicated with focal segmental glomerulosclerosis. Front Med (Lausanne) 2020;7:563020. doi: 10.3389/fmed.2020.563020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy GR, Pushpanathan MJ, Ransom RF, Holzman LB, Brosius FC, Diakonova M, et al. Identification of the glomerular podocyte as a target for growth hormone action. Endocrinology. 2007;148:2045–2055. doi: 10.1210/en.2006-1285. [DOI] [PubMed] [Google Scholar]
- 15.Meinhardt U, Eblé A, Besson A, Strasburger CJ, Sraer JD, Mullis PE. Regulation of growth-hormone-receptor gene expression by growth hormone and pegvisomant in human mesangial cells. Kidney Int. 2003;64:421–430. doi: 10.1046/j.1523-1755.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- 16.Kopp JB, Anders HJ, Susztak K, Podestà MA, Remuzzi G, Hildebrandt F, et al. Podocytopathies. Nat Rev Dis Primers. 2020;6:68. doi: 10.1038/s41572-020-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhi D, Nishad R, Menon RK, Pasupulati AK. Novel actions of growth hormone in podocytes: implications for diabetic nephropathy. Front Med (Lausanne) 2017;4:102. doi: 10.3389/fmed.2017.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doi T, Striker LJ, Gibson CC, Agodoa LY, Brinster RL, Striker GE. Glomerular lesions in mice transgenic for growth hormone and insulinlike growth factor-I. I. Relationship between increased glomerular size and mesangial sclerosis. Am J Pathol. 1990;137:541–552. [PMC free article] [PubMed] [Google Scholar]
- 19.Nishad R, Mukhi D, Tahaseen SV, Mungamuri SK, Pasupulati AK. Growth hormone induces notch1 signaling in podocytes and contributes to proteinuria in diabetic nephropathy. J Biol Chem. 2019;294:16109–16122. doi: 10.1074/jbc.RA119.008966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishad R, Mukhi D, Singh AK, Motrapu M, Chintala K, Tammineni P, et al. Growth hormone induces mitotic catastrophe of glomerular podocytes and contributes to proteinuria. Cell Death Dis. 2021;12:342. doi: 10.1038/s41419-021-03643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin E, Bondy C. Insulin-like growth factor system gene expression in the human kidney. J Clin Endocrinol Metab. 1992;75:962–968. doi: 10.1210/jcem.75.3.1381376. [DOI] [PubMed] [Google Scholar]
- 22.Ramos-Leví AM, Marazuela M. Bringing cardiovascular comorbidities in acromegaly to an update. How should we diagnose and manage them? Front Endocrinol (Lausanne) 2019;10:120. doi: 10.3389/fendo.2019.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol. 2008;159:89–95. doi: 10.1530/EJE-08-0267. [DOI] [PubMed] [Google Scholar]
- 24.Joyce E, Glasner P, Ranganathan S, Swiatecka-Urban A. Tubulointerstitial nephritis: diagnosis, treatment, and monitoring. Pediatr Nephrol. 2017;32:577–587. doi: 10.1007/s00467-016-3394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathews LS, Enberg B, Norstedt G. Regulation of rat growth hormone receptor gene expression. J Biol Chem. 1989;264:9905–9910. doi: 10.1016/S0021-9258(18)81745-8. [DOI] [PubMed] [Google Scholar]