Abstract
Renal invasion of T-cell lymphoma does not usually occur. The renal infiltration of peripheral T-cell lymphoma, not otherwise specified (PTCL–NOS), is rare. Therefore, the detailed pathology, clinical features, and effective therapy of this type of extranodal disease remain uncovered. Here, we report the rare case of acute kidney injury (AKI) caused by the renal infiltration of PTCL–NOS with no evidence of lymphadenopathy and extranodal lesions, except for the kidney. We mistakenly diagnosed our patient with drug-induced acute interstitial nephritis (AIN) at first, because his clinical features were similar to those of drug-induced AIN; however, we reached the correct diagnosis by detecting atypical T-cells in his urine. After the introduction of cyclophosphamide, doxorubicin, vincristine, and prednisone therapy his general condition improved rapidly. When suspecting drug-induced AIN as the cause of AKI, PTCL–NOS should also be recognized as one of the causes, and urine cytology may be useful to noninvasively distinguish between the two diseases.
Keywords: Peripheral T-cell lymphoma, Not otherwise specified, Acute interstitial nephritis, Acute kidney injury, Renal biopsy
Introduction
Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of T-cell lymphomas derived from mature T-cells away from the thymus or bone marrow. Based on the 2016 World Health Organization classification, PTCL includes six types: angioimmunoblastic T-cell lymphoma, enteropathy-associated T-cell lymphoma, extranodal NK/T-cell type lymphoma, subcutaneous panniculitis-like T-cell lymphoma, hepatosplenic T-cell lymphoma, PTCL, not otherwise specified (PTCL–NOS). PTCL that does not meet the criteria for the other specifically defined subtypes of PTCL is classified as PTCL–NOS. Although most PTCL–NOS patients present with generalized lymphadenopathy with or without extranodal disease, 13% of the patients have extranodal disease without evidence of nodal involvement [1]. While the skin and gastrointestinal tract are the most common extranodal sites [2, 3], renal involvement due to PTCL–NOS seems to be uncommon. In general, renal infiltration due to other types of lymphoma commonly occurs. In the case series of autopsies performed on lymphoma patients, 34% of patients showed signs of renal parenchymal invasion [4]. However, most cases of renal infiltration due to non-Hodgkin lymphoma are caused by B-cell lymphoma and ones due to T-cell lymphoma are relatively rare. Moreover, to our knowledge, there is only one previous paper that reports acute kidney injury (AKI) resulting from renal infiltration of PTCL–NOS [5]. Therefore, the detailed pathology, clinical features, and effective therapy of this type of extranodal disease remain uncovered.
Here, we report the renal invasion of PTCL–NOS that presented with clinical features similar to those of drug-induced acute interstitial nephritis (AIN). Although we had mistakenly diagnosed our patient with drug-induced AIN at first, urine cytology allowed us to reach a correct diagnosis.
Case report
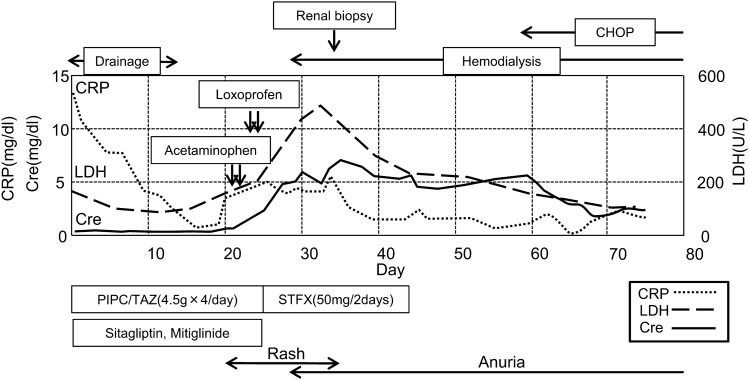
A 60-year-old Japanese male with a history of untreated type II diabetes mellitus was hospitalized with multiple abscesses in the lower lobe of his right lung, right chest wall, liver, and right retroperitoneum. He was put on antibiotics, piperacillin/tazobactam (PIPC/TAZ), and the abscess on his right chest wall was drained. With all these, he was recovering. However, on day 19, he suddenly developed a fever and itchy erythema of the trunk and extremities with pruritus. On day 25, renal dysfunction occurred progressively (Fig. 1), and he was referred to our department.
Fig. 1.
Clinical course of the patient in this case. PIPC/TAZ piperacillin/tazobactam, STFX sitafloxacin. CHOP cyclophosphamide, doxorubicin, vincristine, and prednisone therapy
He had a moderate fever (37.7 °C). Except for the erythema, his physical examination was unremarkable. Laboratory data recorded at the time of consultation are presented in Table 1. Spot urine tests revealed high urine protein-to-creatinine ratio (UPCR) (4.3 g/gCr) and a high level of tubulointerstitial damage markers (β2 MG 113,170 μg/L) but no hematuria. Despite the high UPCR, the protein level in 24-h urine collection was only 680 mg/day, and the urine volume decreased to 200 mL/day. A blood test revealed a marked inflammatory reaction (CRP level of 9.42 mg/dL) and renal dysfunction (BUN 32 mg/dL, Cre 4.48 mg/dL). In a complete blood count, his eosinophil count increased to 648/μL. Computerized tomography (CT) scanning revealed bilaterally enlarged kidneys compared with the findings of CT examination on admission, while the abscesses were improving and there was no lymphadenopathy.
Table 1.
Laboratory data at the time of consultation
| Parameter | Level |
|---|---|
| Urinalysis | |
| Specific gravity | 1.015 |
| pH | 6 |
| Protein | 3 + |
| Occult blood | 1 + |
| Red blood cell, /HPF | 1–4 |
| White blood cell, /HPF | 5–9 |
| Tubular epithelial cell, /HPF | 5–9 |
| Granular cast | 3 + |
| Protein-to-creatinine ratio, g/gCr | 4.3 |
| β2-microglobulin, μg/L | 113,170 |
| N-acetyl-β-d-glucosaminidase, IU/L | 33.1 |
| Complete blood count | |
| White blood cell, μL | 6480 |
| Neutrophils, % | 80.5 |
| Lymphocyte, % | 3 |
| Eosinophils, % | 10 |
| Basophils, % | 2.5 |
| Monocyte, % | 4 |
| Hemoglobin, g/dL | 10.4 |
| Platelets, × 104μL | 19.1 |
| Serum biochemistry | |
| Total protein, g/dL | 6.7 |
| Albumin, g/dL | 2.5 |
| Urea nitrogen, mg/dL | 32 |
| Creatinine, mg/dL | 4.48 |
| Sodium, mmol/L | 136 |
| Potassium, mmol/L | 4.1 |
| Chloride, mmol/L | 101 |
| Calcium, mg/dL | 7.9 |
| Phosphate, mg/dL | 3.7 |
| Aspartate transaminase, U/L | 27 |
| Alanine aminotransferase, U/L | 23 |
| γ-Glutamyl transpeptidase, U/L | 46 |
| Lactate dehydrogenase, U/L | 198 |
| Hemoglobin A1c, % | 9.2 |
| C reactive protein, mg/dL | 9.42 |
| Immunoglobulin G, mg/dL | 1622 |
| Immunoglobulin A, mg/dL | 277 |
| Immunoglobulin M, mg/dL | 86.1 |
| Immunoglobulin E, IU/mL | 106.5 |
| Complement component 3, mg/dL | 118.8 |
| Complement component 4, mg/dL | 32.5 |
| Anti-nuclear antibody | Negative |
| Proteinase-3-anti-neutrophil cytoplasmic antibody, U/mL | < 1.0 |
| Myeloperoxidase–anti-neutrophil cytoplasmic antibody, U/mL | < 1.0 |
| Glomerular basement membrane, U/mL | < 2.0 |
Figure 1 shows the clinical course of this patient. We suspected drug-induced AIN at first; so, the drugs that had been used before the onset of his renal dysfunction, which included PIPC/TAZ, Sitagliptin, Mitiglinid, Acetaminophen, and Loxoprofen, were discontinued, and Sitafloxacin was started instead of PIPC/TAZ to continue the treatment of the abscesses. On day 27, since anuria had occurred, hemodialysis was started. On day 32, a percutaneous biopsy of his left kidney was performed to identify the cause of the renal dysfunction. Light microscopy revealed eight collapsed glomeruli that had no findings suggestive of glomerulonephritis (Fig. 2a); however, it revealed highly severe interstitial cellular infiltration by relatively large mononuclear cells and scattered eosinophils (Fig. 2b, c). As unique findings, the tubular basement membrane (TBM) was severely divided everywhere and these mononuclear cells existed between each divided membrane as if this structural change was caused by these cells’ infiltration into the TBMs (Fig. 2b). All immune-fluorescence tests, which included anti-IgG, IgA, IgM, C3, C4, and C1q, were negative. These results, except for the divided TBM, were compatible with drug-induced AIN. In addition, since the drug-induced lymphocyte stimulation test for PIPC/TAZ was positive (stimulation index 592%), we were convinced that the cause of the renal dysfunction was PIPC/TAZ-induced AIN. However, the result of urine cytology, which we had performed at the time of consultation to confirm the presence of eosinophils in urine, because a list of our potential diagnoses then had included drug-induced AIN, was reported after we made a diagnosis of drug-induced AIN. It revealed the presence of numerous atypical cells in the urine at the onset of AKI. We retrospectively added immunostaining to the kidney biopsy specimen, and it revealed that the infiltrating mononuclear cells were positive for CD3 and CD5, and negative for CD20, which meant these cells were T-cells (Fig. 3). In addition, some of them showed atypical mitosis (Fig. 3d). These findings strongly indicated that the infiltrating cells were T-cell lymphoma cells. Moreover, immunostaining in the cell block of atypical cells in the urine revealed that these cells were positive for CD2, CD3, and CD5 but weakly positive for CD7, which implied that the number of CD2 (+), CD3 (+), CD5 (+), and CD7 (−) cells was monoclonally increasing, supporting that these were T-cell lymphoma cells (Fig. 4). The concentration of soluble interleukin-2 receptor (sIL-2R) measured on day 46 increased to 8910 U/ML. The lactate dehydrogenase (LDH) concentration started increasing after the development of AKI, and peaked at 467 U/L on day 33 (Fig. 1). Based on these findings, we finally made a diagnosis of renal infiltration of PTCL–NOS.
Fig. 2.
Periodic acid–methenamine–silver staining (a, b) and hematoxylin and eosin staining (c) of the renal biopsy specimen. a glomerulus with no evidence of glomerulonephritis. b Tubulointerstitial infiltration of mononuclear cells and divided TBM (indicated by arrows). c Eosinophils infiltrating the tubulointerstitium
Fig. 3.
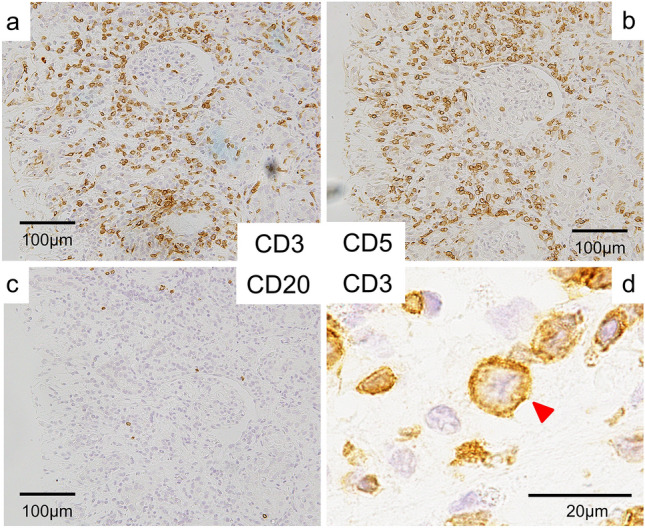
Immunostaining on the renal biopsy specimen. a, b A lot of CD3- and CD5-positive cells infiltrating the tubulointerstitium. c Immunostaining revealing CD20-negative infiltrating cells. d Atypical mitosis (indicated by an arrow) of a CD3-positive cell
Fig. 4.
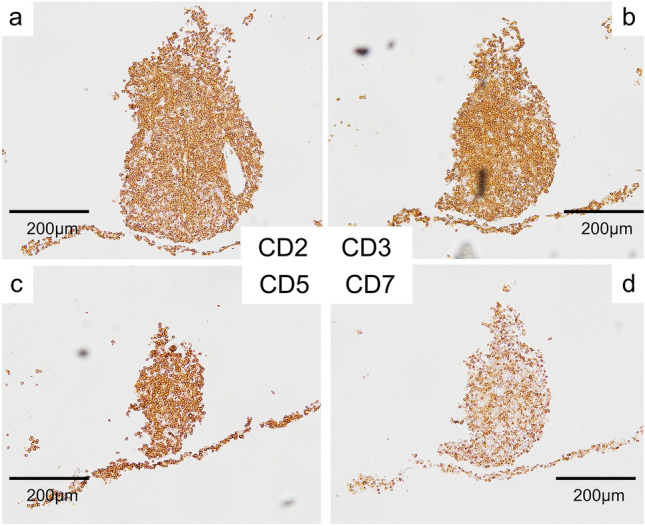
Immunostaining of the cell block made from atypical cells in the urine showing weak positivity for CD7 compared to the others, which implies the monoclonality of these atypical cells. a CD2. b CD3. c CD5. d CD7
Momentarily, cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) therapy was commenced and his general condition improved. After the start of CHOP therapy, the levels of sIL-2R and LDH were also improved to the normal range. Although the renal dysfunction did not improve and he required maintenance hemodialysis, he was discharged on day 78.
Discussion
We encountered a rare case of renal infiltration of PTCL–NOS. Typically, normal T-cells should be equally stained for typical T-cell-associated antigens, such as CD2, CD3, CD5, and CD7. However, immunostaining in the cell block prepared from urine sample in this case revealed that cells in the urine sample were positive for CD2, CD3, and CD5 but weakly positive for CD7. This implied that the number of T-cells expressing CD2, CD3, and CD5, but not CD7, was monoclonally increasing, and these cells were considered to be malignant. Because we first performed immunostaining of the renal biopsy tissue only for CD3, CD5, and CD20, we repeated immunostaining of the renal tissue not only for CD3, CD5, and CD20 but also for CD2 and CD7 to confirm whether the results of urine cells were also similar to those of the T-cells infiltrating the tubulointerstitium. Results showed that these T-cells were also positive for CD2, CD3, and CD5 but weakly positive for CD7 (Fig. 5), confirming that most T-cells in the tubulointerstitium were also T-cell lymphoma cells. Based on these results, we believe that renal infiltration of PTCL–NOS primarily caused AKI and mimicked clinical features similar to those of drug-induced AIN. Certainly, it may be difficult to completely exclude the possibilities of coexisting drug-induced AIN, considering the fact that immediately after stopping PIPC/TAZ, the skin rash and CRP level were spontaneously improving without the initiation of chemotherapy (Fig. 1). In fact, some previous case reports showed that several types of PTCL occurred with or mimicked allergic diseases [6–9]. It is also known that PTCL itself can cause eosinophilia [10]. Therefore, it may be difficult to clearly exclude allergic diseases from PTCL. However, based on immunostaining results, we believe that it was T-cell lymphoma that played a major role in this case, even if drug-induced AIN was slightly related to the condition.
Fig. 5.
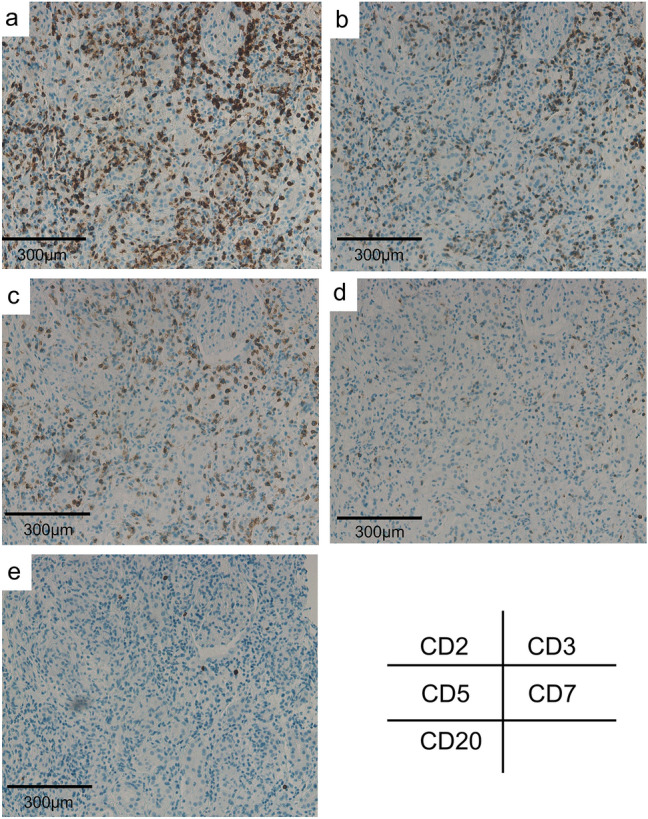
Immunostaining of the renal biopsy specimen revealing that infiltrating lymphocytes were weakly positive for CD7 (d), compared with CD2 (a), CD3 (b), and CD5 (c), which is compatible with the result of immunostaining of the cell block prepared from atypical cells in the urine. e Immunostaining revealing CD20-negative infiltrating cells
In this case, although the high UPCR and hypoalbuminemia, both of which fulfilled the criteria of nephrotic syndrome, were observed at the time of consultation, we believe that nephrotic syndrome could be excluded due to two reasons. First, the urine volume already declined to 200 mL/day at the time, and the protein excretion in the 24-h urine collection was 680 mg/day. Hence, we believe that the UPCR could incorrectly estimate the 24-h protein excretion. Second, as hypoalbuminemia had been persistent since the time of admission, we believe that the hypoalbuminemia was associated not with the nephrotic syndrome but with the inflammation caused by multiple abscesses.
One of the most important points to learn from this case report is that the renal involvement of PTCL–NOS can occur with clinical features, just like those of drug-induced AIN. To our knowledge, there has been only one previous case report of AKI due to PTCL–NOS; so, the clinical features of this condition are still unknown. The patient in the case report by Matsuda et al. presented with multiple extranodal lesions not only in the kidney but also in the skin, liver, and eyes [5]. However, the patient in our case had no evidence of lymphadenopathy and extranodal lesions, except for the kidney. The lack of organ damage except for AKI and the existence of systemic symptoms similar to those of drug-induced AIN were the causes of our initial misdiagnosis. Although renal invasion of PTCL–NOS is a rare clinical situation, this case reminds clinicians that when they encounter a case of suspected drug-induced AIN, they should add PTCL–NOS to the list of potential diagnoses.
Another noteworthy point here is the diagnostic usefulness of urine cytology in the renal infiltration of PTCL–NOS. As mentioned above, the PTCL–NOS in this case presented with symptoms similar to those of drug-induced AIN. The diagnosis of drug-induced AIN is usually based on the patient’s clinical presentation or history, and renal biopsy is often omitted. This means there is the possibility of missing PTCL–NOS that clinically mimics drug-induced AIN. However, it must be unreasonable to perform a renal biopsy on all such patients to avoid missing such a rare disease. In this case, the detection of atypical cells in the urine led to the correct diagnosis. Actually, renal biopsy exhibited the infiltration of atypical cells into the tubular lumen (Fig. 2b). Therefore, although the sensitivity and specificity of the test are unknown, considering its non-invasiveness and simplicity, urine cytology may be one of the precise methods of detecting PTCL–NOS in clinically diagnosed drug-induced AIN.
The intensely divided TBMs were the unique kidney biopsy findings in this case that may not be detected in typical drug-induced AIN. Although it is not certain whether these TBM changes were PTCL–NOS-specific, according to previous studies, some diseases, including diabetic nephropathy, acute rejection after kidney transplantation, and some genetic disorders such as nephronophthisis and manifest morphological changes of TBMs such as double-layered TBM [11–15]. Among these diseases, only diabetes mellitus was confirmed in this case. Although thickened or double-layered TBMs are commonly found in diabetic nephropathy, these findings generally appear to be focal and not severe compared to the findings in our case. Moreover, we do not believe that advanced diabetic nephropathy developed in our patient, because the renal biopsy did not reveal any evidence of diabetic nephropathy in the glomeruli, such as Kimmelstiel–Wilson lesions and exudative lesions. However, considering the previous report that mentioned that TBM changes occur relatively early during clinically silent course of the disease [11], there exists a possibility that our patient already had focal double-layered TBM changes before the occurrence of PTCL–NOS. Under this assumption, if numerous T-cell lymphoma cells and other inflammatory cells infiltrated the spaces between focally duplicated TBMs and expanded the spaces from inside, these processes might cause the diffusely and intensely divided TBMs as those observed in this case. Nonetheless, this is a hypothetical assumption, and it is difficult to validate this hypothesis.
CHOP therapy seems to be the treatment of choice for PTCL. However, there are few established strategies, and the prognosis is poor due to the aggressive clinical course of the disease [16]. Although Matsuda et al. reported that the early introduction of steroid therapy led to the dramatic improvement of extranodal lesions and prevented AKI from progressing further [5], in our case, the presence of coexisting multiple abscesses was an obstacle to the early introduction of steroid therapy. Eventually, we could not stop the progression of AKI, and the CHOP therapy introduced after diagnosis also did not resolve the renal dysfunction. However, after the introduction of CHOP therapy our patient’s general condition improved rapidly, and he could be discharged.
In conclusion, the renal infiltration of PTCL–NOS can clinically mimic drug-induced AIN, and urine cytology may be a useful tool in diagnosing this rare condition.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the participant included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weisenburger DD, Savage KJ, Harris NL, Gascoyne RD, Jaffe ES, MacLennan KA, et al. International peripheral T-cell lymphoma project peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood. 2011;117:3402–3408. doi: 10.1182/blood-2010-09-310342. [DOI] [PubMed] [Google Scholar]
- 2.Bekkenk MW, Vermeer MH, Jansen PM, van Marion AM, Canninga-van Dijk MR, Kluin PM, et al. Peripheral T-cell lymphomas unspecified presenting in the skin: analysis of prognostic factors in a group of 82 patients. Blood. 2003;102:2213–2219. doi: 10.1182/blood-2002-07-1960. [DOI] [PubMed] [Google Scholar]
- 3.Savage KJ, Ferreri AJ, Zinzani PL, Pileri SA. Peripheral T-cell lymphoma—not otherwise specified. Crit Rev Oncol Hematol. 2011;79:321–329. doi: 10.1016/j.critrevonc.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Richmond J, Sherman RS, Diamond HD, Craver LF. Renal lesions associated with malignant lymphomas. Am J Med. 1962;32:184–207. doi: 10.1016/0002-9343(62)90289-9. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda K, Fukami H, Saito A, Sato H, Aoki S, Takeuchi Y, et al. Rapidly progressive renal failure due to tubulointerstisial infiltration of peripheral T-cell lymphoma, not otherwise specified accompanied by uveitis: a case report. BMC Nephrol. 2018;19:312–318. doi: 10.1186/s12882-018-1125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramonell RP, Kuruvilla M, Sica G, Bag R, Eun-Hyung LF. A peripheral cause of asthma: peripheral T-cell lymphoma. Ann Allergy Asthma Immunol. 2019;122:420–422. doi: 10.1016/j.anai.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong LM, Fok KC, Tsui A, Qian C, Fisher L. Peripheral T-cell lymphoma mimicking 5-aminosalicylate hypersensitivity in ulcerative colitis. Intern Med J. 2013;43:1137–1140. doi: 10.1111/imj.12240. [DOI] [PubMed] [Google Scholar]
- 8.Schleinitz N, Veit V, Coso D, Aurran T, Berbis P, Harle JR. Drug-induced eosinophilia and systemic symptoms: hypersensitivity or peripheral T-cell lymphoma? Arch Dermatol. 2005;141:395–396. doi: 10.1001/archderm.141.3.395. [DOI] [PubMed] [Google Scholar]
- 9.Patel S, Patel R, Draikiwicz S, Capitle E. Peripheral T-cell lymphoma: a challenging mimicker of angioedema and urticaria. Ann Allergy Asthma Immunol. 2015;115:94–95. doi: 10.1016/j.anai.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Wang T, Feldman AL, Wada DA, Lu Y, Polk A, Briski R, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood. 2004;123:3007–3015. doi: 10.1182/blood-2013-12-544809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziyadeh FN. Renal tubular basement membrane and collagen type IV in diabetes mellitus. Kidney Int. 1993;43:114–120. doi: 10.1038/ki.1993.19. [DOI] [PubMed] [Google Scholar]
- 12.Mandache E, Gherghiceanu M, Serafinceanu C, Penescu M, Mircescu G. Myofibroblast involvement in tubular basement membrane remodeling in type II diabetic nephropathy. Rom J Morphol Embryol. 2011;52:75–79. [PubMed] [Google Scholar]
- 13.Bonsib SM, Abul-Ezz SR, Ahmad I, Young SM, Ellis EN, Schneider DL, Walker PD. Acute rejection-associated tubular basement membrane defects and chronic allograft nephropathy. Kidney Int. 2000;58:2206–2214. doi: 10.1111/j.1523-1755.2000.00395.x. [DOI] [PubMed] [Google Scholar]
- 14.Fujimaru T, Kawanishi K, Mori T, Mishima E, Sekine A, Chiga M, et al. Genetic background and clinicopathologic features of adult-onset nephronophthisis. Kidney Int Rep. 2021;6:1346–1354. doi: 10.1016/j.ekir.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwafuchi Y, Morioka T, Oyama Y, Nozu K, Iijima K, Narita I. A case of transforming growth factor-β-induced gene-related oculorenal syndrome: granular corneal dystrophy type II with a unique nephropathy. Case Rep Nephrol Dial. 2016;6:106–113. doi: 10.1159/000449129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abouyabis AN, Shenoy PJ, Sinha R, Flowers CR, Lechowicz MJ. A systematic review and meta-analysis of front-line anthracycline-based chemotherapy regimens for peripheral t-cell lymphoma. ISRN Hematol. 2011 doi: 10.5402/2011/623924. [DOI] [PMC free article] [PubMed] [Google Scholar]