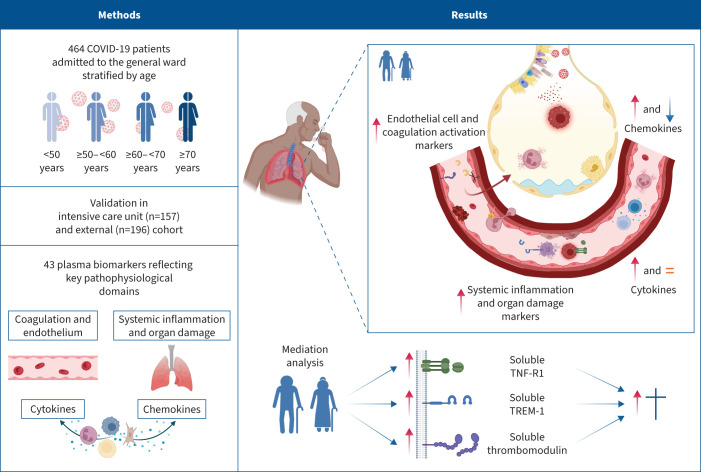
Graphical abstract
Overview of the study findings. TNF-R1: tumour necrosis factor receptor 1; TREM-1: triggering receptor expressed on myeloid cells 1. Figure partially created with BioRender.com.
Abstract
Background
Coronavirus disease 2019 (COVID-19)-induced mortality occurs predominantly in older patients. Several immunomodulating therapies seem less beneficial in these patients. The biological substrate behind these observations is unknown. The aim of this study was to obtain insight into the association between ageing, the host response and mortality in patients with COVID-19.
Methods
We determined 43 biomarkers reflective of alterations in four pathophysiological domains: endothelial cell and coagulation activation, inflammation and organ damage, and cytokine and chemokine release. We used mediation analysis to associate ageing-driven alterations in the host response with 30-day mortality. Biomarkers associated with both ageing and mortality were validated in an intensive care unit and external cohort.
Results
464 general ward patients with COVID-19 were stratified according to age decades. Increasing age was an independent risk factor for 30-day mortality. Ageing was associated with alterations in each of the host response domains, characterised by greater activation of the endothelium and coagulation system and stronger elevation of inflammation and organ damage markers, which was independent of an increase in age-related comorbidities. Soluble tumour necrosis factor receptor 1, soluble triggering receptor expressed on myeloid cells 1 and soluble thrombomodulin showed the strongest correlation with ageing and explained part of the ageing-driven increase in 30-day mortality (proportion mediated: 13.0%, 12.9% and 12.6%, respectively).
Conclusions
Ageing is associated with a strong and broad modification of the host response to COVID-19, and specific immune changes likely contribute to increased mortality in older patients. These results may provide insight into potential age-specific immunomodulatory targets in COVID-19.
Extract
From the start of the coronavirus disease 2019 (COVID-19) pandemic, epidemiological data indicate that COVID-19-related mortality sharply increases with old age [1–3]. In the Netherlands, individuals aged ≤50 years account for only ∼1% of COVID-19 mortality cases, while individuals aged ≥70 years account for ∼89% of these deaths [4]. The biological substrate behind this steep increase is currently unknown. Ageing-related comorbidities mediate only a fraction of the increased mortality [2, 5].
Tweetable abstract
In COVID-19, specific ageing-related alterations in the host response likely contribute to the increased mortality in older patients. Evidence is provided for potential age-specific immunomodulatory targets across four pathophysiological COVID-19 domains. https://bit.ly/3mIMt4l
Introduction
From the start of the coronavirus disease 2019 (COVID-19) pandemic, epidemiological data indicate that COVID-19-related mortality sharply increases with old age [1–3]. In the Netherlands, individuals aged ≤50 years account for only ∼1% of COVID-19 mortality cases, while individuals aged ≥70 years account for ∼89% of these deaths [4]. The biological substrate behind this steep increase is currently unknown. Ageing-related comorbidities mediate only a fraction of the increased mortality [2, 5].
COVID-19 is associated with increased concentrations of inflammation markers and cytokines, coagulation disturbances, and endotheliopathy [6, 7]. Multiple randomised controlled trials (RCTs) that sought to ameliorate these host response disturbances have reported improved clinical outcomes [8]. However, in the largest RCTs, the two current cornerstones of treatment for hospitalised COVID-19 patients, namely dexamethasone and tocilizumab, showed little if any beneficial effect in the oldest patients [9–11]. In general, ageing is associated with changes in innate and adaptive immunity; in this context, “inflammageing” refers to a state of sustained low-grade inflammation, while “immunosenescence” refers to a gradual decline in the ability to generate an effective immune response to new antigens [12]. Although the impact of ageing on the immune system has been implicated in the pathogenesis of COVID-19 in elderly patients [13, 14], studies analysing the influence of older age on host response disturbances in COVID-19 are scarce. Previous studies focused on plasma cytokine levels in a limited number of patients across various disease severities, showing an association of ageing with an increased inflammatory response [15–18]. The influence of ageing on endothelial and coagulation responses in COVID-19 has yet to be evaluated. Exploring age-driven differences in these key host response domains and their association with ageing-driven mortality in COVID-19 may lay a foundation for identifying new immunomodulating targets.
The primary objective of this study was to gain insight into the association between age and aberrations in key pathophysiological pathways implicated in COVID-19 in patients admitted to a general hospital ward (i.e. noncritically ill). To this end, we determined 43 biomarkers reflective of alterations in the endothelial and coagulation response, inflammation and organ damage, and cytokine and chemokine release. Our secondary objective was to associate the host response aberrations detected in older patients with the increased 30-day mortality rate in this age group.
Methods
Study design and population
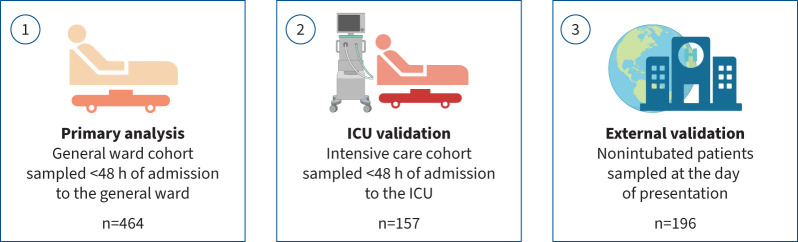
Data were derived from the ELDER-BIOME study (ClinicalTrials.gov: NCT02928367) and the Amsterdam University Medical Center (UMC) COVID-19 Biobank Study (AUMC 2020_065) (see supplementary methods for details). Both studies were approved by the Amsterdam UMC Ethics Committee. ELDER-BIOME is a prospective observational study in Amsterdam UMC (both locations: Academic Medical Center (AMC) and Vrije Universiteit Medical Center (VUMC), Amsterdam, The Netherlands) and Flevo Hospital (Almere, The Netherlands) [7]. The Amsterdam UMC COVID-19 Biobank Study is conducted in both AMC and VUMC, according to which leftover blood from clinical care is processed for plasma storage. Written informed consent was obtained from all patients or their legal representatives. Within the Amsterdam UMC Biobank Study, an additional option of deferred consent using an opt-out method was implemented for incapacitated patients. We included patients above 18 years of age with COVID-19-related symptoms and a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR or suspected COVID-19 with a CO-RADS computed tomography score ≥4 reflecting a high (4) or very high (5) suspicion for pulmonary involvement of COVID-19 [19]. Inclusion was done between February 2020 and September 2021. Patients were sampled within 48 h of general ward admission (or, in a separate cohort, intensive care unit (ICU) admission). Patients were divided according to four “waves” (for details, see supplementary methods and [20]). Biomarker data were validated in an external cohort (for details, see supplementary methods and [21]). Figure 1 depicts an overview of the COVID-19 cohorts. In addition, mortality data were also obtained from patients admitted to a general ward because of community-acquired pneumonia caused by pathogens other than SARS-CoV-2 (for details, see supplementary methods and [7]).
FIGURE 1.
Overview of the three COVID-19 cohorts in which plasma biomarkers were measured. The external validation cohort was derived from a publicly available dataset of nonintubated COVID-19 patients in whom plasma proteins were measured by the Olink proximity extension assay [21]. ICU: intensive care unit. Figure partially created with BioRender.com.
Assays
43 host response biomarkers were measured in EDTA anticoagulated plasma using Luminex (R&D, Minneapolis, MN, USA) and the Bio-Plex 200 System (Bio-Rad, Hercules, CA, USA) (for the selection rationale, see supplementary methods). Biomarkers were stratified into four pathophysiological domains (for details, see supplementary table S1). Reference values were obtained from 10 heathy and 19 age- and sex-matched noninfectious outpatient clinic controls (ClinicalTrials.gov: NCT02928367).
Statistics
Patients were stratified according to age decades: <50, ≥50– <60, ≥60– <70 and ≥70 years. This method was chosen to facilitate visualisation and clinical interpretation. Differences in 30-day survival were visualised by Kaplan–Meier curves. Biomarker data were log-transformed. Overall differences between age decades among biomarkers within one host response domain were visualised by a principal component analysis (PCA), as described previously [7]. Differences in the principal component (PC) scores between age groups were analysed by ANOVA. The correlation of PC scores with ageing on a continuous scale was determined by Spearman's correlation test.
Differences in individual biomarker levels between age decades were quantified using Hedges’ g effect size and visualised using heatmaps [22]. Additionally, in a regression analysis, age was modelled as a continuous variable. The association's strength was analysed using Spearman's correlation. In a separate analysis, the association of individual biomarkers with ageing was also explored upon admission to the ICU.
We investigated if age-driven alterations in biomarker concentrations were associated with the age-driven increase in 30-day mortality rates by mediation analysis [5, 23]. A biomarker or PC score needed to be significantly associated with both ageing and 30-day mortality to enter the mediation analysis (for assumptions and details, see supplementary methods) [24]. Biomarkers associated with both ageing and mortality were also externally validated in an independent cohort entailing 196 hospitalised nonintubated COVID-19 patients (for cohort description, see supplementary methods) (figure 1) [21]. Lastly, we performed a cluster analysis on patients aged ≥70 years to assess the uniformity of their host response, using Ward's method [25]. Furthermore, we investigated whether the mediating effects of biomarkers were influenced by the patient's host response phenotype.
The regression and mediation models all consisted of an unadjusted and an adjusted approach. Adjusted models evaluating the association of ageing with biomarker concentrations contained covariates associated with either ageing or COVID-19 care and included: demographics (inclusion hospital, sex and inclusion wave), age-related comorbidities (hypertension, diabetes, malignancies, immunosuppression, and chronic cardiac, neurological, respiratory and kidney disease), age- and biomarker-related chronic medication prior to admission (antiplatelet and anticoagulant drugs), and COVID-19-related immunomodulating treatments before sampling (corticosteroids, anti-interleukin (IL)-6 and imatinib). When evaluating the association of ageing with mortality, we used the same covariates plus covariates that may impact mortality in the adjusted model: immunomodulating treatments on admission but after sampling and the use of antibiotics and remdesivir (for details and assumption testing, see supplementary methods).
Details on missingness and handling of missingness are described in the supplementary methods, supplementary Excel file (sheet 1), and supplementary tables S2 and S3. p-values of all analyses were multiple testing corrected using the Benjamini–Hochberg (BH) procedure. A BH-adjusted p-value <0.05 was considered statistically significant.
Results
Patients, presentation and outcome
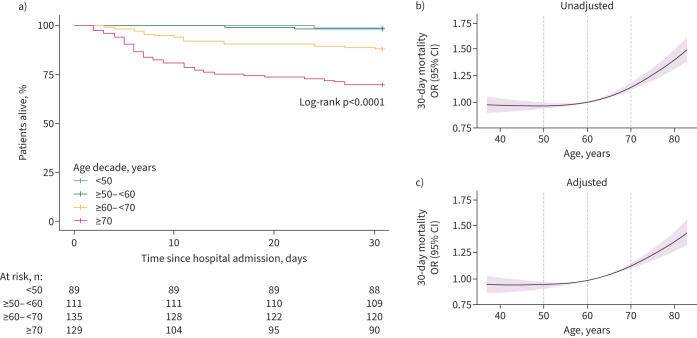
464 patients with COVID-19 admitted to a general hospital ward were included (table 1). Of these, 89 patients (19.2%) were aged <50 years at hospital admission, 111 (23.9%) ≥50– <60 years, 135 (29.1%) ≥60– <70 years and 129 (27.8%) ≥70 years. The distribution of sex did not differ between age decades. Most patients were enrolled during the second and third COVID-19 waves in the Netherlands, in which the SARS-CoV-2 alpha variant occurred and became dominant. The age distribution was similar between waves (supplementary table S4). The proportion of patients with comorbidities increased with age. While the duration of symptoms prior to admission did not differ between age groups, older patients presented with higher disease severity scores [26]. Routine laboratory values were comparable between age groups except for an age-dependent decrease in lymphocyte counts and an age-dependent increase in the neutrophil/lymphocyte ratio and creatinine. COVID-19-related treatments, primarily supplemental oxygen and dexamethasone, were comparable between age groups (table 2). Mortality rates increased with age in a nonlinear fashion (table 2 and figure 2). For example, an increase in age from 60 to 70 years was associated with an increased 30-day mortality (OR 1.15, 95% CI 1.12–1.18) (figure 2b), which was independent of demographics, age-related comorbidities and COVID-related treatments (adjusted OR 1.14, 95% CI 1.11–1.17) (figure 2c). In pneumonia patients with pathogens other than SARS-CoV-2 (supplementary table S5), a similar increase in age (from 60 to 70 years) was not associated with an increase in 30-day mortality odds (OR 1.01, 95% CI 0.996–1.025). Moreover, older patients with pneumonia caused by pathogens other than SARS-CoV-2 showed significantly lower mortality rates compared with those with SARS-CoV-2: ≥60– <70 years (p<0.01) and ≥70 years (p<0.001) (supplementary figure S1).
TABLE 1.
Baseline characteristics of COVID-19 patients on admission to the general ward
| Age decade | p-value | ||||
| <50 years (n=89) | ≥50– <60 years (n=111) | ≥60– <70 years (n=135) | ≥70 years (n=129) | ||
| Demographics | |||||
| Age, years | 44 (37–47) | 55 (53–57) | 64 (62–67) | 76 (72–82) | <0.001 |
| Male | 49 (55.1) | 68 (61.3) | 83 (61.5) | 78 (60.5) | 0.784 |
| BMI, kg·m−2 | 30.0 (27.4–33.2) | 27.8 (25.2–32.1) | 28.7 (25.6–32.8) | 27.5 (24.3–30.1) | 0.002 |
| Duration of symptoms#, days | 8 (7–10) | 9 (7–11) | 8 (6–11) | 8 (5–10) | 0.109 |
| Smoking status | 0.009 | ||||
| Current smoker | 4 (4.5) | 4 (3.6) | 8 (5.9) | 8 (6.2) | |
| Ex-smoker | 17 (19.1) | 27 (24.3) | 48 (35.6) | 53 (41.1) | |
| Never-smoker | 60 (67.4) | 70 (63.1) | 73 (54.1) | 55 (42.6) | |
| Unknown | 8 (9.0) | 10 (9.0) | 6 (4.4) | 13 (10.1) | |
| COVID-19 vaccination status | 0.262 | ||||
| Yes | 2 (2.2) | 2 (1.8) | 8 (5.9) | 7 (5.4) | |
| No | 86 (96.6) | 106 (95.5) | 120 (88.9) | 119 (92.2) | |
| Unknown | 1 (1.1) | 3 (2.7) | 7 (5.2) | 3 (2.3) | |
| Comorbidities and (selected) chronic medication | |||||
| Charlson score¶ | 0.0 (0–1.0) | 1.0 (1.0–2.0) | 3.0 (2.0–4.0) | 4.0 (4.0–6.0) | <0.001 |
| Hypertension | 17 (19.1) | 32 (28.8) | 64 (47.4) | 68 (52.7) | <0.001 |
| Cardiac disease | 13 (14.6) | 16 (14.4) | 44 (32.6) | 51 (39.5) | <0.001 |
| Respiratory disease | 14 (15.7) | 20 (18.0) | 25 (18.5) | 33 (25.6) | 0.261 |
| Diabetes | 19 (21.3) | 13 (11.7) | 35 (25.9) | 41 (31.8) | 0.003 |
| Kidney disease | 5 (5.6) | 4 (3.6) | 8 (5.9) | 19 (14.7) | 0.005 |
| Neurological disease | 5 (5.6) | 3 (2.7) | 9 (6.7) | 21 (16.3) | 0.001 |
| Prior malignancy | 3 (3.4) | 1 (0.9) | 13 (9.6) | 14 (10.9) | 0.005 |
| Immunosuppression+ | 10 (11.2) | 4 (3.6) | 16 (11.9) | 5 (3.9) | 0.016 |
| Antiplatelet drugs | 1 (1.1) | 5 (4.5) | 28 (20.7) | 19 (14.7) | <0.001 |
| Anticoagulant drugs | 5 (5.6) | 4 (3.6) | 7 (5.2) | 24 (18.6) | <0.001 |
| Disease severity on admission | |||||
| 4C Mortality§ | 4 (3–5) | 3 (2–5) | 4 (3–6) | 6 (4–7) | <0.001 |
| qSOFA | 1 (0–1) | 1 (0–1) | 1 (0–1) | 1 (0–1) | 0.002 |
| MEWS | 3 (2–4) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.102 |
| CURBƒ | 0 (0–1) | 0 (0–1) | 0 (0–1) | 1 (0–1) | <0.001 |
| Treatment at day of admission | |||||
| Supplementary oxygen therapy | 80 (89.9) | 103 (92.8) | 121 (89.6) | 115 (89.1) | 0.784 |
| High-flow nasal cannula | 0 (0.0) | 0 (0.0) | 2 (1.5) | 2 (1.6) | 0.382 |
| Noninvasive ventilation | 1 (1.1) | 0 (0.0) | 2 (1.5) | 1 (0.8) | 0.647 |
| Routine laboratory markers | |||||
| Leukocyte count, ×109 L−1 | 6.1 (4.6–8.3) | 6.0 (4.8–8.6) | 6.4 (4.9–7.9) | 6.9 (5.2–8.6) | 0.306 |
| Neutrophil count, ×109 L−1 | 4.8 (3.3–5.9) | 4.4 (3.2–6.6) | 5.0 (3.4–6.3) | 5.2 (3.9–6.9) | 0.074 |
| Lymphocyte count, ×109 L−1 | 1.00 (0.70–1.20) | 0.97 (0.70–1.20) | 0.93 (0.70–1.20) | 0.75 (0.50–1.05) | 0.001 |
| Neutrophil/lymphocyte ratio | 4.8 (2.8–7.2) | 4.5 (2.9–8.6) | 4.8 (3.3–7.5) | 6.4 (4.1–10.5) | 0.002 |
| C-reactive protein, mg·L−1 | 98 (49–126) | 76 (43–136) | 83 (51–141) | 100 (57–147) | 0.369 |
| Platelet count, ×109 L−1 | 220 (184–255) | 225 (181–276) | 215 (149–281) | 207 (151–266) | 0.434 |
| Creatinine, µmol·L−1 | 74 (63–92) | 79 (65–90) | 82 (67–101) | 87 (70–113) | 0.001 |
Data are presented as median (interquartile range) or n (%), unless otherwise stated. BMI: body mass index; qSOFA: quick Sequential Organ Failure Assessment; MEWS: Modified Early Warning Score; CURB: confusion, urea >7 mmol·L−1, respiratory rate ≥30 breaths·min−1, blood pressure <90 mmHg (systolic) or ≤60 mmHg (diastolic). #: prior to admission; ¶: Charlson score was calculated without the age component; +: defined as a history of an organ transplant, immune deficiency or chronic use of immunosuppressants (see supplementary table S7 for details); §: 4C Mortality, a validated COVID-19 score [26], was calculated without the age and obesity component; ƒ: CURB score was calculated without the age component.
TABLE 2.
Treatments, disease course and outcome of COVID-19 patients after admission to the general ward
| Age decade | p-value | ||||
| <50 years (n=89) | ≥50– <60 years (n=111) | ≥60– <70 years (n=135) | ≥70 years (n=129) | ||
| Treatment | |||||
| Supplementary oxygen therapy | 84 (94.4) | 107 (96.4) | 131 (97.0) | 125 (96.9) | 0.737 |
| High-flow nasal cannula | 2 (2.2) | 8 (7.2) | 19 (14.1) | 17 (13.2) | 0.012 |
| Noninvasive ventilation | 1 (1.1) | 0 (0.0) | 3 (2.2) | 8 (6.2) | 0.015 |
| Invasive ventilation | 8 (9.0) | 10 (9.0) | 22 (16.3) | 9 (7.0) | 0.073 |
| Remdesivir | 12 (13.5) | 10 (9.0) | 14 (10.4) | 17 (13.2) | 0.670 |
| Chloroquine | 0 (0.0) | 2 (1.8) | 0 (0.0) | 1 (0.8) | 0.284 |
| Monoclonal antibodies against SARS-CoV-2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.8) | 0.457 |
| Antibiotics in the first 7 days of admission | 31 (34.8) | 40 (36.0) | 70 (51.9) | 65 (50.4) | 0.010 |
| Immunomodulating therapies | |||||
| Dexamethasone 6 mg | 72 (80.9) | 89 (80.2) | 102 (75.6) | 103 (79.8) | 0.730 |
| Of which before sampling | 62 (69.7) | 79 (71.2) | 96 (71.1) | 93 (72.1) | 0.985 |
| Other corticosteroids# | 4 (4.5) | 3 (2.7) | 6 (4.4) | 3 (2.3) | 0.713 |
| Of which before sampling | 3 (3.4) | 2 (1.8) | 5 (3.7) | 1 (0.8) | 0.392 |
| Interleukin-6 inhibitors | 10 (11.2) | 11 (9.9) | 13 (9.6) | 12 (9.3) | 0.971 |
| Of which before sampling | 4 (4.5) | 1 (0.9) | 3 (2.2) | 3 (2.3) | 0.426 |
| Anti-C5a antibody | 0 (0.0) | 0 (0.0) | 1 (0.7) | 1 (0.8) | 0.677 |
| Of which before sampling | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | >1.000 |
| Imatinib | 1 (1.1) | 5 (4.5) | 3 (2.2) | 11 (8.5) | 0.027 |
| Of which before sampling | 0 (0.0) | 1 (0.9) | 2 (1.5) | 4 (3.1) | 0.279 |
| Clinical course | |||||
| Thrombosis | 5 (5.6) | 12 (10.8) | 9 (6.7) | 18 (14.0) | 0.110 |
| Of which pulmonary embolism¶ | 5 (5.6) | 10 (9.0) | 9 (6.7) | 17 (13.2) | 0.175 |
| Of which deep venous thrombosis¶ | 0 (0.0) | 4 (3.6) | 0 (0.0) | 2 (1.6) | 0.054 |
| Length of hospital stay, days | 4 (2–7) | 4 (3–8) | 6 (3–11) | 7 (4–11) | <0.001 |
| ICU admission+ | 9 (10.1) | 15 (13.5) | 26 (19.3) | 13 (10.1) | 0.113 |
| ICU stay, days | 15 (8–29) | 11 (9–13) | 8 (5–14) | 8 (1–17) | 0.450 |
| Readmission§ | 4 (4.5) | 5 (4.5) | 6 (4.4) | 10 (7.8) | 0.581 |
| Mortalityƒ | |||||
| 30-day | 1 (1.1) | 2 (1.8) | 16 (11.9) | 39 (30.2) | <0.001 |
| 90-day | 2 (2.2) | 2 (1.8) | 16 (11.9) | 43 (33.3) | <0.001 |
Data are presented as median (interquartile range) or n (%), unless otherwise stated. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ICU: intensive care unit. #: prednisolone and hydrocortisone; ¶: numbers do not add up to 100% as some patients suffered from both pulmonary embolism and deep venous thrombosis; +: ICU admission after sampling; §: for any cause within 28 days of the initial admission; ƒ: for 98.2% of patients, worsening of COVID-19 was reported as a causal element for mortality by the treating physician.
FIGURE 2.
Mortality analysis of COVID-19 patients admitted to the general ward stratified by age decade. a) Kaplan–Meier plot of patients stratified by age decade. b) Risk of 30-day mortality with age modelled as a continuous variable. Given the nonlinear relationship between age and mortality, a restricted cubic spline function with three inner knots at default quantile locations was used. To calculate the odds ratio, the reference was set to 60 years of age. c) The same method as b), but now the 30-day mortality odds ratio is adjusted for demographics (inclusion hospital, sex and inclusion wave), age-related comorbidities (hypertension, diabetes, malignancies, immunosuppression, and chronic cardiac, neurological, respiratory and kidney disease), age-related chronic medication (antiplatelet and anticoagulant drugs), and COVID-19-related treatments both before and after sampling (corticosteroids including dexamethasone, anti-interleukin-6, imatinib, remdesivir and antibiotics).
Association of ageing with aberrations in distinct host response domains
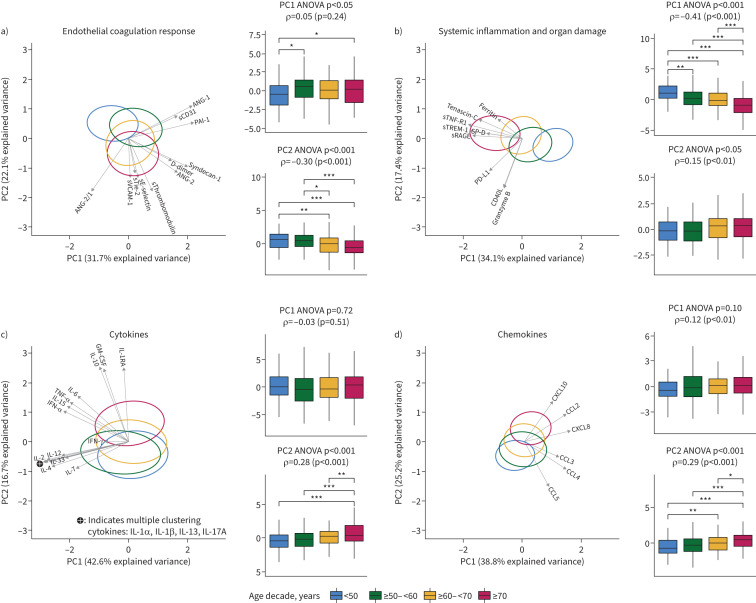
We determined 43 host response biomarkers in plasma obtained within 48 h after admission and stratified these in four pathophysiological domains. First, we performed PCA to visualise overall differences between age decades among biomarkers within each pathophysiological domain (figure 3). All domains showed significant differences between age groups. The strongest association with ageing was observed for PC1 of the systemic inflammation and organ damage domain (ρ= −0.41), which was mainly driven by increased plasma concentrations of soluble tumour necrosis factor receptor 1 (sTNF-R1), soluble triggering receptor expressed on myeloid cells 1 (sTREM-1) and soluble receptor for advanced glycation end-products (sRAGE) in older patients (figure 3b). The second strongest association was that of ageing with PC2 of the endothelial and coagulation domain (ρ=0.30), which was driven mainly by increased plasma angiopoietin (ANG)-2/1 ratios, soluble thrombomodulin (sThrombomodulin) and soluble E-selectin (sE-selectin) in older patients (figure 3a). The cytokine domain was characterised by two groups of cytokines: one in which the levels increased with ageing (figure 3c, PC2), primarily driven by granulocyte–macrophage colony-stimulating factor (GM-CSF) and the anti-inflammatory cytokines IL-1RA and IL-10, and one in which the levels were relatively similar between age groups (figure 3c; PC1). A mixed pattern was observed for the chemokine domain in which some chemokine levels increased with ageing (e.g. C-X-C motif chemokine ligand 10 (CXCL10)), while others decreased with ageing (e.g. C-C motif chemokine ligand 5 (CCL5)) (figure 3d; PC2). The complete contribution of each biomarker to a PC score is depicted in supplementary table S6. As a sensitivity analysis, we further stratified patients ≥70 years into patients ≥70– <80 years (n=86) and ≥80 years (n=43), which did not modify the age-dependent trends to a significant extent (supplementary figure S2).
FIGURE 3.
Principal component analysis (PCA) of host response domain differences between age groups: a) endothelial and coagulation response, b) systemic inflammation and organ damage, c) cytokines and d) chemokines. Principal components (PCs) 1 and 2 are plotted per domain. For each domain, the x- and y-axes are labelled with the percentage of the total variance within that domain that is explained by PC1 and PC2, respectively. The complete contribution of each biomarker to a PC score is depicted in supplementary table S6. The ellipse indicates the central 10% of each age group, colour coded as indicated in the key at the bottom of the figure. The arrows indicate the direction (arrow orientation) and strength (arrow length) of the correlation between each biomarker and the PCs. Next to each PCA plot are box plots with 1.5 interquartile range whiskers of PC1 and PC2. Herein, upper p-values were obtained by ANOVA between age groups: ρ-values with accompanying p-values were generated using a Spearman's correlation with ageing on a continuous scale. Note that a negative association of a PC with ageing may still reflect a positive association with biomarker concentration, as reflected by the direction of the arrows. Post-hoc testing was done with a Tukey test. *: p<0.05; **: p<0.01; ***: p<0.001. ANG: angiopoietin; sTie-2: soluble Tie-2; sE-selectin: soluble E-selectin; sThrombomodulin: soluble thrombomodulin; sVCAM-1: soluble vascular cellular adhesion molecule 1; PAI-1: plasminogen activator inhibitor 1; sCD31: soluble cluster of differentiation 31; sRAGE: soluble receptor for advanced glycation end-products; sTNF-R1: soluble tumour necrosis factor receptor 1; sTREM-1: soluble triggering receptor expressed on myeloid cells 1; SP-D: surfactant protein D; CD40L: CD40 ligand; PD-L1: programmed death ligand 1; CCL:C-C motif chemokine ligand; CXCL: C-X-C motif chemokine ligand; IL: interleukin; TNF: tumour necrosis factor; GM-CSF: granulocyte–macrophage colony-stimulating factor; IFN: interferon.
Association of ageing with individual host response biomarkers
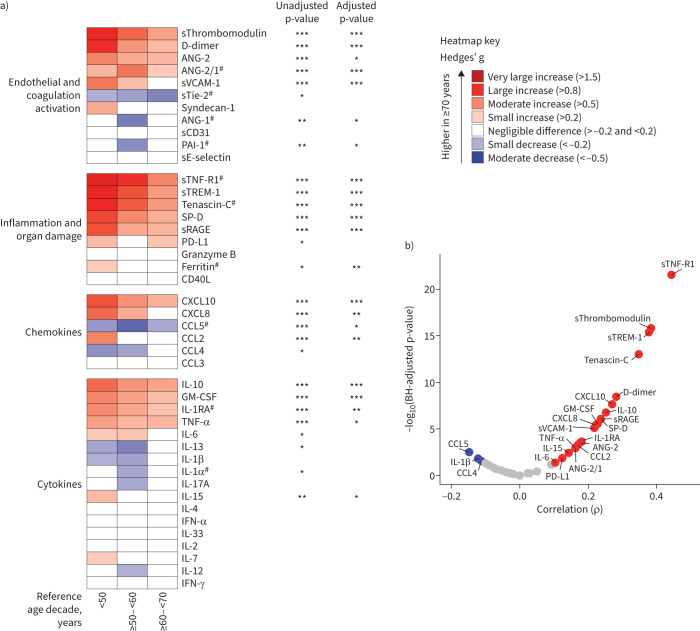
We next compared individual host response biomarkers across age decades. Of the 43 determined biomarkers, 36 were significantly different between patients and controls (supplementary Excel file, sheet 2). Figure 4a shows biomarker concentrations in patients ≥70 years compared with the other age groups, expressed as Hedges’ g, a commonly used effect size measure [22]. This analysis confirmed that most biomarkers reflective of endothelial and coagulation activation, as well as those indicating systemic inflammation and organ damage, were higher in patients ≥70 years, while chemokines and cytokines demonstrated a mixed pattern. A sensitivity analysis in which we further stratified patients ≥70 years into patients ≥70– <80 and ≥80 years yielded similar results (supplementary figure S2). For almost all biomarkers that had a significant association with ageing in the unadjusted model, significance was maintained after adjusting for demographics, comorbidities and COVID-19-related immunomodulating treatments before sampling (see Methods for details). On a continuous scale, sTNF-R1, sTREM-1, sThrombomodulin and tenascin-C demonstrated the strongest (positive) correlations with ageing (figure 4b). The strength and direction of the associations of each biomarker with ageing showed strong similarities across inclusion waves (supplementary figure S3).
FIGURE 4.
Association of host response biomarkers with ageing. a) Heatmap depicting the magnitude of biomarker differences (Hedges’ g) between patients ≥70 years compared with other age groups. p-values were obtained from a linear (if linear) or cubic spline regression analysis (if nonlinear) in which age was modelled as a continuous variable. The adjusted model included demographics, age-related comorbidities, age and biomarker-related chronic medication, and COVID-19-related immunomodulating treatments before sampling (see Methods for details). Red indicates higher levels in patients ≥70 years and blue indicates lower levels in patients ≥70 years. #: biomarkers with a nonlinear relationship with ageing on a continuous scale. b) Volcano plot depicting the strength of the correlation between a biomarker and ageing. Red dots represent a significant positive correlation, blue dots represent a significant negative correlation and grey dots represent a nonsignificant correlation. Both the adjusted and unadjusted p-values are multiple testing corrected using the Benjamini–Hochberg (BH) procedure for testing 43 biomarkers. *: p<0.05; **: p<0.01; ***: p<0.001. ANG: angiopoietin; sTie-2: soluble Tie-2; sE-selectin: soluble E-selectin; sThrombomodulin: soluble thrombomodulin; sVCAM-1: soluble vascular cellular adhesion molecule 1; PAI-1: plasminogen activator inhibitor 1; sCD31: soluble cluster of differentiation 31; sRAGE: soluble receptor for advanced glycation end-products; sTNF-R1: soluble tumour necrosis factor receptor 1; sTREM-1: soluble triggering receptor expressed on myeloid cells 1; SP-D: surfactant protein D; CD40L: CD40 ligand; PD-L1: programmed death ligand 1; CCL: C-C motif chemokine ligand; CXCL: C-X-C motif chemokine ligand; IL: interleukin; TNF: tumour necrosis factor; GM-CSF: granulocyte–macrophage colony-stimulating factor; IFN: interferon.
In a separate analysis, we evaluated the age distribution of the same biomarkers in a cohort of critically ill COVID-19 patients sampled on ICU admission (n=157) (supplementary tables S8 and S9, and supplementary Excel file, sheet 3). Differences between patients ≥70 years and younger patients were largely reproduced in this cohort of critically ill patients, especially compared with the youngest age group (<50 years), although, due to the relatively low sample size, statistical significance was not always reached (supplementary figure S4a). Akin to the patients admitted to a general ward, sTNF-R1, sTREM-1, sThrombomodulin, tenascin-C and soluble vascular cellular adhesion molecule 1 (sVCAM-1) demonstrated a significant positive correlation with ageing (supplementary figure S4b).
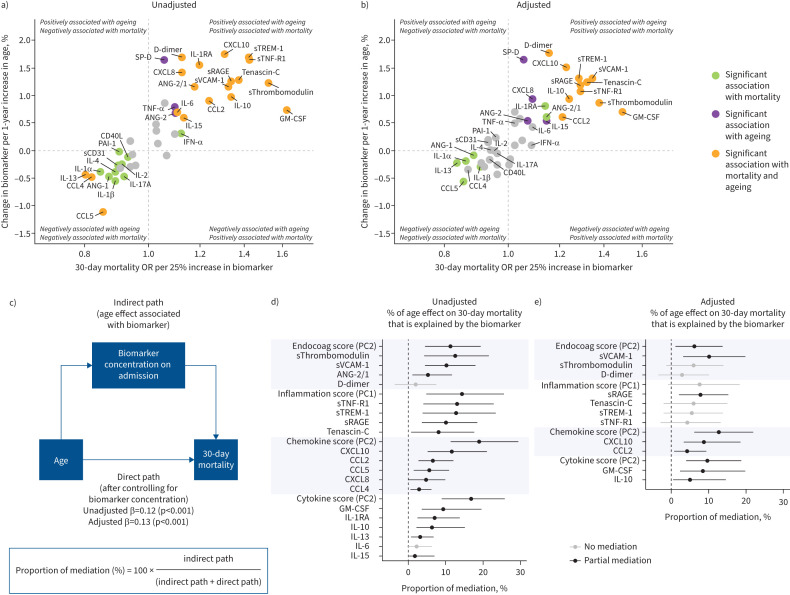
Association of age-driven host response alterations with mortality
We used mediation analysis to evaluate whether age-driven alterations in biomarker concentrations were associated with the age-driven increase in 30-day mortality rates [23]. First, we identified biomarkers associated with both ageing and 30-day mortality before and after adjustment for possible confounding and ageing-related factors (figure 5a and b). Then, we estimated which proportion of the age effect on mortality was associated with age-related alterations in PC scores of pathophysiological domains and individual host response biomarkers (figure 5c). In the unadjusted model (figure 5d), PC scores of the endothelial and coagulation (PC2), systemic inflammation and organ damage (PC1), and chemokine (PC2) and cytokine (PC2) domains explained a significant part of the age effect on mortality. With regard to individual biomarkers, ageing-related differences in sThrombomodulin, sVCAM-1, sTNF-R1, sTREM-1 and sRAGE were most associated with the age-driven increase in 30-day mortality. Notably, in an external cohort, sThrombomodulin, sVCAM-1, sTNF-R1 and tenascin-C showed the strongest association with ageing and 30-day mortality (supplementary figure S5); sTREM-1 was not measured [21]. In the adjusted model (figure 5e), only ageing-driven changes in sVCAM-1, sRAGE, CXCL10, CCL2, GM-CSF and IL-10 were associated with the ageing-driven increase in 30-day mortality. Collectively, these results demonstrate that a proportion of the association between age and mortality is explained by specific host response differences.
FIGURE 5.
Mediation analysis for ageing-associated mortality and host response biomarkers upon admission to the general ward. a) Quadrant plot. The x-axis depicts the increase in the 30-day mortality odds ratio per 25% increase of the biomarker derived from an unadjusted logistic regression with the log-transformed biomarker as the explanatory variable and 30-day mortality as the response variable. The y-axis shows the percentage change in the biomarker concentration per 1-year increase in age which was derived from an unadjusted linear regression analysis with the log-transformed biomarker as the response variable. Biomarkers in both the top-right and bottom-left corners are most likely associated with an age-dependent increase in 30-day mortality. The significance of the coefficient was multiple testing corrected using the Benjamini–Hochberg procedure for testing 43 biomarkers. b) Quadrant plot. The same method as in a), but both coefficients are now adjusted for demographics (inclusion hospital, sex and inclusion wave), age-related comorbidities (hypertension, diabetes, malignancies, immunosuppression, and chronic cardiac, neurological, respiratory and kidney disease), age- and biomarker-related chronic medication (antiplatelet and anticoagulant drugs), and COVID-19-related treatments both before and after sampling (corticosteroids including dexamethasone, anti-interleukin (IL)-6, imatinib, remdesivir and antibiotics). c) Diagram of mediation analysis. The adjusted model contained the same covariates as in b). d) Unadjusted and e) adjusted mediation analysis results. Only biomarkers and principal components (PCs) significantly associated with ageing and 30-day mortality were analysed. Confidence intervals were obtained from 1000 times bootstrapping. The higher the proportion of mediation, the stronger the association of the age-dependent differences in that biomarker and the age-dependent increase in 30-day mortality. The PCs and their contributing biomarkers are depicted in figure 3. The complete contribution of each biomarker to a PC score is depicted in supplementary table S6. Endocoag: endothelial and coagulation; ANG: angiopoietin; sTie-2: soluble Tie-2; sE-selectin: soluble E-selectin; sThrombomodulin: soluble thrombomodulin; sVCAM-1: soluble vascular cellular adhesion molecule 1; PAI-1: plasminogen activator inhibitor 1; sCD31: soluble cluster of differentiation 31; sRAGE: soluble receptor for advanced glycation end-products; sTNF-R1: soluble tumour necrosis factor receptor 1; sTREM-1: soluble triggering receptor expressed on myeloid cells 1; SP-D: surfactant protein D; CD40L: CD40 ligand; PD-L1: programmed death ligand 1; CCL: C-C motif chemokine ligand; CXCL: C-X-C motif chemokine ligand; IL: interleukin; TNF: tumour necrosis factor; GM-CSF: granulocyte–macrophage colony-stimulating factor; IFN: interferon.
Given that the host response in older patients may not be uniform, we performed a cluster analysis in patients ≥70 years. We identified three clusters (supplementary figure S6). Cluster 3 had the highest age and 30-day mortality, with high endothelial cell activation, inflammation and organ damage markers but low cytokine concentrations (supplementary tables S10 and S11, and supplementary Excel file, sheet 4). Cluster 1 had high coagulation markers and cytokine concentrations (supplementary figure S6). Cluster 2 had a mixed host response. The top three mediating biomarkers in the primary analysis (sThrombomodulin, sTNF-R1 and sTREM-1) were important for assigning patients to Cluster 3 (supplementary table S12). Furthermore, the majority of biomarkers and all PC scores that were significant mediators in the primary analysis remained predictors of mortality in patients ≥70 years, which was, with a few exceptions, independent of the assigned cluster (supplementary table S13).
Discussion
We report the association of ageing with 43 biomarkers reflective of host response disturbances in four key pathophysiological domains relevant for the pathogenesis of COVID-19 and relate these immune deviations to 30-day mortality. While our results confirm previously reported increased plasma concentrations of IL-6, IL-10, IL-15, TNF-α and CXCL8 in older COVID-19 patients [15–18], we additionally show that many cytokines are hardly affected by age. More importantly, we provide novel evidence that ageing is associated with greater activation of the endothelial cell and coagulation system and elevation of inflammation and organ damage markers in COVID-19. Mediation analysis indicated that specific host response alterations explained part of the age effect on mortality. Our study is the most comprehensive analysis of the association between ageing, the host response and mortality in COVID-19.
Older COVID-19 patients showed strongly increased plasma concentrations of several biomarkers in the endothelial cell and coagulation activation and inflammation and organ damage domains. Likely, alterations in these pathophysiological areas are at least in part intertwined. These (and all other) analyses were done in an unadjusted model and a model adjusted for variables associated with ageing. While these two types of analyses yielded largely similar results, we consider the unadjusted analyses a better reflection of the clinical setting of older patients, who (as an example) inherently have more comorbidities and comedication than young patients. Ageing results in endothelial senescence, characterised by vascular inflammation and endothelial dysfunction; age-associated increases in endothelial cell NF-κB activity have been implicated herein [27, 28]. Endotheliopathy is a key feature of COVID-19, and earlier studies reported elevated plasma levels of a variety of biomarkers indicating endothelial cell activation and dysfunction [29]. The current results suggest that COVID-19 is associated with more profound endotheliopathy in older patients. Indeed, patients ≥70 years had higher plasma levels of biomarkers reflective of endothelial cell activation (sThrombomodulin and sVCAM-1), a more disturbed glycocalyx function (syndecan) and a more disrupted barrier function (ANG-2). Loss of glycocalyx is an important feature of endothelial dysfunction in COVID-19 [29, 30]. The glycocalyx consists of proteoglycans and glycosaminoglycans that inhibit immune activation and provide an anticoagulant surface [30]. Hence, a more disturbed glycocalyx function in older COVID-19 patients may contribute to systemic hyperinflammation and coagulation activation. Higher ANG-2 and ANG-2/1 ratios in older COVID-19 patients point in the same direction: ANG-2 promotes endothelial inflammation and vascular leakage, high plasma ANG-2 has been associated with a worse outcomes in COVID-19 [31], and high ANG-2/1 ratios strongly correlated with mortality in critically ill patients with acute lung injury [32]. Of note, the association between older age and increased endotheliopathy seems specific for COVID-19, since we recently reported lower endothelial cell activation and dysfunction in old compared with young patients with sepsis [33].
Ageing was an independent risk factor for mortality in this study group of noncritically ill COVID-19 patients, confirming previous investigations that analysed populations with more diverse baseline disease severities [1, 2, 5]. More importantly, we showed that the age-dependent increase in mortality at day 30 was associated with certain age-associated host response changes. sTNF-R1 has been identified as a cornerstone biomarker for the unfavourable “hyperinflammation” subtype of acute respiratory distress syndrome [34]. sTNF-R1 levels may be a surrogate marker for the activation of cell-associated TNF-R1, which can trigger vascular leakage and neutrophilic inflammation in the lung [35–37]. Blockade of TNF-R1 in healthy individuals led to a decrease in neutrophil transmigration and endothelial injury upon inhalation of endotoxin [35], and TNF-R1-deficient mice were strongly protected against the formation of pulmonary oedema in an acute lung injury model [37]. Collectively, these data suggest that targeting the TNF-R1 pathway may improve the outcome especially in older COVID-19 patients. TREM-1 is a receptor on innate immune cells that amplifies the immune response triggered by Toll-like and NOD receptors [38]. Increased concentrations of sTREM-1 are reflective of TREM-1 pathway activity [39], and are associated with increased cytokine levels and disease severity in patients with COVID-19 [40]. In our study, ageing was associated with enhanced sTREM-1 levels, which was a main determinant in the ageing-driven increase in 30-day mortality. Of interest, in a recently completed study in COVID-19 patients requiring ventilator support, infusion of an inhibitor of the TREM-1 pathway improved clinical outcomes, including 28-day mortality (data from a press release by the sponsor [41]).
Plasma IL-6 was associated with both ageing and COVID-19 mortality. However, in the mediation analysis, the age-dependent increase in 30-day mortality was not associated with increased IL-6 concentrations, suggesting that IL-6 blockade may not benefit older COVID-19 patients. In agreement, the beneficial effect of tocilizumab in the overall population of COVID-19 patients enrolled in a large RCT was not observed in the oldest patients [11]. Similarly, dexamethasone, the other cornerstone immunomodulatory treatment in COVID-19, did not improve outcome in older patients [9, 10]. Elevated inflammation in older patients may result in steroid resistance [42], which could contribute to a lower response to a standard dose of dexamethasone. Collectively, these findings suggest that age-associated changes in the host response during COVID-19 influence the effect of immune modulation in this disease and that age should be taken into account for patient selection.
Our study has strengths and limitations. We provide comprehensive insight into aberrations in host response pathways considered important for the pathogenesis of COVID-19 in a large population. Our primary analysis only included noncritically ill patients, yet we provided preliminary evidence that ageing is associated with similar host response changes in COVID-19 patients admitted to the ICU. Our study was purely observational and no definitive conclusion can be drawn on causality. Nonetheless, by using mediation analysis, we provide novel insights into which host response pathways contribute to ageing-associated mortality. While admission plasma biomarkers are used to guide immunomodulating trials [43], they do not cover the full spectrum of the host response and we may have missed host response alterations that were not (yet) visible on a protein level. Our study was conducted before the broad availability of vaccines and the dominance of the omicron variant. Yet, in populations with a high vaccination coverage, a significant portion of hospitalised COVID-19 patients remain unvaccinated [44]. While the omicron variant still disproportionally affects older patients [45], robustness of the ageing-associated alterations remains to be validated.
This study documents that ageing is associated with substantial and broad alterations in the host response to COVID-19 across several pathophysiological domains. Increased concentrations of several biomarkers (in particular, sTNF-R1 and sTREM-1) were associated with the ageing-driven increase in 30-day mortality. These results may lay a foundation for new immunomodulatory targets in older patients.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00011-2023.Supplement (1.4MB, pdf)
Supplementary Excel file ERJ-00011-2023.Excel (75.4KB, xlsx)
Shareable PDF
Acknowledgements
We thank Barbara Smids-Dierdorp, Tamara Dekker and Anita Tuip (Amsterdam UMC, location University of Amsterdam, Amsterdam, The Netherlands) for their technical assistance in the performance of the Luminex assays.
Footnotes
Amsterdam UMC COVID-19 Biobank Study Group: Michiel van Agtmael (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Anne Geke Algera (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Brent Appelman (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Floor van Baarle (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Martijn Beudel (Department of Neurology, Amsterdam UMC, Amsterdam Neuroscience, Amsterdam, The Netherlands), Harm Jan Bogaard (Department of Pulmonology, Amsterdam UMC, Amsterdam, The Netherlands), Marije Bomers (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Peter Bonta (Department of Pulmonology, Amsterdam UMC, Amsterdam, The Netherlands), Lieuwe Bos (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Michela Botta (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Justin de Brabander (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Godelieve de Bree (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Sanne de Bruin (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Marianna Bugiani (Department of Pulmonology, Amsterdam UMC, Amsterdam, The Netherlands), Esther Bulle (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), David T.P. Buis (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Osoul Chouchane (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Alex Cloherty (Experimental Immunology, Amsterdam UMC, Amsterdam, The Netherlands), Mirjam Dijkstra (Department of Clinical Chemistry, Amsterdam UMC, Amsterdam, The Netherlands), Dave A. Dongelmans (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Romein W.G. Dujardin (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Paul Elbers (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Lucas Fleuren (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Suzanne Geerlings (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Theo Geijtenbeek (Experimental Immunology, Amsterdam UMC, Amsterdam, The Netherlands), Armand Girbes (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Bram Goorhuis (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Martin P. Grobusch (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Laura Hagens (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Jorg Hamann (Amsterdam UMC Biobank Core Facility, Amsterdam UMC, Amsterdam, The Netherlands), Vanessa Harris (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Robert Hemke (Department of Radiology, Amsterdam UMC, Amsterdam, The Netherlands), Sabine M. Hermans (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Leo Heunks (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Markus Hollmann (Department of Anesthesiology, Amsterdam UMC, Amsterdam, The Netherlands), Janneke Horn (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Joppe W. Hovius (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Hanna K. de Jong (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Menno D. de Jong (Department of Medical Microbiology, Amsterdam UMC, Amsterdam, The Netherlands), Rutger Koning (Department of Neurology, Amsterdam UMC, Amsterdam Neuroscience, Amsterdam, The Netherlands), Bregje Lemkes (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Endry H.T. Lim (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Niels van Mourik (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Jeaninne Nellen (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Esther J. Nossent (Department of Pulmonology, Amsterdam UMC, Amsterdam, The Netherlands), Sabine Olie (Department of Neurology, Amsterdam UMC, Amsterdam Neuroscience, Amsterdam, The Netherlands), Frederique Paulus (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Edgar Peters (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Dan A.I. Pina-Fuentes (Department of Neurology, Amsterdam UMC, Amsterdam Neuroscience, Amsterdam, The Netherlands), Tom van der Poll (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Bennedikt Preckel (Department of Anesthesiology, Amsterdam UMC, Amsterdam, The Netherlands), Jan M. Prins (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Jorinde Raasveld (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Tom Reijnders (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Maurits C.F.J. de Rotte (Department of Clinical Chemistry, Amsterdam UMC, Amsterdam, The Netherlands), Michiel Schinkel (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Marcus J. Schultz (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Femke A.P. Schrauwen (Department of Clinical Chemistry, Amsterdam UMC, Amsterdam, The Netherlands), Alex Schuurman (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Jaap Schuurmans (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Kim Sigaloff (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Marleen A. Slim (Department of Intensive Care and Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Patrick Smeele (Department of Pulmonology, Amsterdam UMC, Amsterdam, The Netherlands), Marry Smit (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Cornelis S. Stijnis (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Willemke Stilma (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Charlotte Teunissen (Neurochemical Laboratory, Amsterdam UMC, Amsterdam, The Netherlands), Patrick Thoral (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Anissa M. Tsonas (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Pieter R. Tuinman (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Marc van der Valk (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Denise P. Veelo (Department of Anesthesiology, Amsterdam UMC, Amsterdam, The Netherlands), Carolien Volleman (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Heder de Vries (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands), Lonneke A. Vught (Department of Intensive Care and Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Michèle van Vugt (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Dorien Wouters (Department of Clinical Chemistry, Amsterdam UMC, Amsterdam, The Netherlands), A.H. (Koos) Zwinderman (Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Amsterdam UMC, Amsterdam, The Netherlands), Matthijs C. Brouwer (Department of Neurology, Amsterdam UMC, Amsterdam Neuroscience, Amsterdam, The Netherlands), W. Joost Wiersinga (Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands), Alexander P.J. Vlaar (Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands) and Diederik van de Beek (Department of Neurology, Amsterdam UMC, Amsterdam Neuroscience, Amsterdam, The Netherlands).
This article has an editorial commentary: https://doi.org/10.1183/13993003.00796-2023
Data sharing: Data can be shared upon reasonable request after approval of a proposal with a signed data access agreement and always in collaboration with the study group.
Author contributions: E.H.A. Michels, B. Appelman, H. Peters-Sengers and T. van der Poll contributed to the concept and design of the study. E.H.A. Michels, B. Appelman, J. de Brabander, R.B.E. van Amstel, C.C.A. van Linge, O. Chouchane, A.R. Schuurman, T.D.Y. Reijnders, T.A.L. Sulzer, A.M. Klarenbeek, R.A. Douma and the Amsterdam UMC COVID-19 Biobank Study Group were responsible for the collection of the data and samples and the sample preparation. E.H.A. Michels performed the data analysis. H. Peters-Sengers verified the analytical methods. E.H.A. Michels drafted the first manuscript in consultation with B. Appelman, H. Peters-Sengers and T. van der Poll. T. van der Poll supervised the project and provided the funding. All authors provided intellectual input and both revised and approved the final version of the manuscript.
Conflicts of interest: The authors declare no potential conflicts of interest.
Support statement: E.H.A. Michels, J. de Brabander and C.C.A. van Linge received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement number 847786 (FAIR). H. Peters-Sengers was supported by the Dutch Kidney Foundation (Kolff grant number 19OK009). O. Chouchane was supported by the Landsteiner Foundation (LSBR 1901). T.D.Y. Reijnders was supported by the research programme “NACTAR”, project “MDR-phage” (grant number 16447), which is financed by the Dutch Research Council (NWO). W.J. Wiersinga was supported by the Talud Foundation (Stichting Talud) for the Amsterdam UMC Corona Research Fund, the Netherlands Organisation for Health Research and Development (ZonMw; TURN-COVID grant number 10430142110001). L.D.J. Bos reports grants from the Dutch Lung Foundation, grants from the Dutch Lung Foundation and Health Holland (Public-Private Partnership grant), grants from the Dutch Lung Foundation (Dirkje Postma Award), grants from the IMI COVID19 initiative, and grants from Amsterdam UMC fellowship. The funders of this study had no role in study design, data collection, data analysis, data interpretation or writing of the report. Funding information for this article has been deposited with the Crossref Funder Registry.
Contributor Information
Amsterdam UMC COVID-19 Biobank Study Group:
Michiel van Agtmael, Anne Geke Algera, Brent Appelman, Floor van Baarle, Martijn Beudel, Harm Jan Bogaard, Marije Bomers, Peter Bonta, Lieuwe Bos, Michela Botta, Justin de Brabander, Godelieve de Bree, Sanne de Bruin, Marianna Bugiani, Esther Bulle, David T.P. Buis, Osoul Chouchane, Alex Cloherty, Mirjam Dijkstra, Dave A. Dongelmans, Romein W.G. Dujardin, Paul Elbers, Lucas Fleuren, Suzanne Geerlings, Theo Geijtenbeek, Armand Girbes, Bram Goorhuis, Martin P. Grobusch, Laura Hagens, Jorg Hamann, Vanessa Harris, Robert Hemke, Sabine M. Hermans, Leo Heunks, Markus Hollmann, Janneke Horn, Joppe W. Hovius, Hanna K. de Jong, Menno D. de Jong, Rutger Koning, Bregje Lemkes, Endry H.T. Lim, Niels van Mourik, Jeaninne Nellen, Esther J. Nossent, Sabine Olie, Frederique Paulus, Edgar Peters, Dan A.I. Pina-Fuentes, Tom van der Poll, Bennedikt Preckel, Jan M. Prins, Jorinde Raasveld, Tom Reijnders, Maurits C.F.J. de Rotte, Michiel Schinkel, Marcus J. Schultz, Femke A.P. Schrauwen, Alex Schuurman, Jaap Schuurmans, Kim Sigaloff, Marleen A. Slim, Patrick Smeele, Marry Smit, Cornelis S. Stijnis, Willemke Stilma, Charlotte Teunissen, Patrick Thoral, Anissa M. Tsonas, Pieter R. Tuinman, Marc van der Valk, Denise P. Veelo, Carolien Volleman, Heder de Vries, Lonneke A. Vught, Michèle van Vugt, Dorien Wouters, A.H. (Koos) Zwinderman, Matthijs C. Brouwer, W. Joost Wiersinga, Alexander P.J. Vlaar, and Diederik van de Beek
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324: 782–793. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 2.Romero Starke K, Reissig D, Petereit-Haack G, et al. The isolated effect of age on the risk of COVID-19 severe outcomes: a systematic review with meta-analysis. BMJ Glob Health 2021; 6: e006434. doi: 10.1136/bmjgh-2021-006434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 2021; 590: 140–145. doi: 10.1038/s41586-020-2918-0 [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Public Health and the Environment (RIVM) . COVID-19 deaths in the Netherlands by age. 2022. https://coronadashboard.government.nl/landelijk/sterfte Date last accessed: 22 April 2023.
- 5.Henkens MTHM, Raafs AG, Verdonschot JAJ, et al. Age is the main determinant of COVID-19 related in-hospital mortality with minimal impact of pre-existing comorbidities, a retrospective cohort study. BMC Geriatr 2022; 22: 184. doi: 10.1186/s12877-021-02673-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osuchowski MF, Winkler MS, Skirecki T, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med 2021; 9: 622–642. doi: 10.1016/S2213-2600(21)00218-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuurman AR, Reijnders TDY, van Engelen TSR, et al. The host response in different aetiologies of community-acquired pneumonia. EBioMedicine 2022; 81: 104082 doi: 10.1016/j.ebiom.2022.104082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, et al. A guide to immunotherapy for COVID-19. Nat Med 2022; 28: 39–50. doi: 10.1038/s41591-021-01643-9 [DOI] [PubMed] [Google Scholar]
- 9.The RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with covid-19. N Engl J Med 2021; 384: 693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA 2020; 324: 1307–1316. doi: 10.1001/jama.2020.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abani O, Abbas A, Abbas F, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397: 1637–1645. doi: 10.1016/S0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoro A, Bientinesi E, Monti D. Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Res Rev 2021; 71: 101422. doi: 10.1016/j.arr.2021.101422 [DOI] [PubMed] [Google Scholar]
- 13.Bartleson JM, Radenkovic D, Covarrubias AJ, et al. SARS-CoV-2, COVID-19 and the aging immune system. Nat Aging 2021; 1: 769–782. doi: 10.1038/s43587-021-00114-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunha LL, Perazzio SF, Azzi J, et al. Remodeling of the immune response with aging: immunosenescence and its potential impact on COVID-19 immune response. Front Immunol 2020; 11: 1748. doi: 10.3389/fimmu.2020.01748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou Y, Zhou Y, Jehi L, et al. Aging-related cell type-specific pathophysiologic immune responses that exacerbate disease severity in aged COVID-19 patients. Aging Cell 2022; 21: e13544. doi: 10.1111/acel.13544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y, Cheon S, Jeong H, et al. Differential association of viral dynamics with disease severity depending on patients’ age group in COVID-19. Front Microbiol 2021; 12: 712260. doi: 10.3389/fmicb.2021.712260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu C, Li J, Xing X, et al. The effect of age on the clinical and immune characteristics of critically ill patients with COVID-19: a preliminary report. PLoS One 2021; 16: e0248675. doi: 10.1371/journal.pone.0248675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin L, Li X, Shi J, et al. Gendered effects on inflammation reaction and outcome of COVID-19 patients in Wuhan. J Med Virol 2020; 92: 2684–2692. doi: 10.1002/jmv.26137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prokop M, Van Everdingen W, Vellinga T, et al. CO-RADS – a categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology 2020; 296: E97–E104. doi: 10.1148/radiol.2020201473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute for Public Health and the Environment (RIVM) . SARS-CoV2 variants in the Netherlands. 2022. https://coronadashboard.government.nl/landelijk/varianten Date last accessed: 22 April 2023.
- 21.Filbin MR, Mehta A, Schneider AM, et al. Longitudinal proteomic analysis of severe COVID-19 reveals survival-associated signatures, tissue-specific cell death, and cell-cell interactions. Cell Rep Med 2021; 2: 100287. doi: 10.1016/j.xcrm.2021.100287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Educat Stat 1981; 6: 107–128. doi: 10.3102/10769986006002107 [DOI] [Google Scholar]
- 23.Tingley D, Yamamoto T, Hirose K, et al. mediation: R package for causal mediation analysis. J Stat Softw 2014; 59: 1–38. doi: 10.18637/jss.v059.i0526917999 [DOI] [Google Scholar]
- 24.Corraini P, Olsen M, Pedersen L, et al. Effect modification, interaction and mediation: an overview of theoretical insights for clinical investigators. Clin Epidemiol 2017; 9: 331–338. doi: 10.2147/CLEP.S129728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murtagh F, Legendre P. Ward's hierarchical agglomerative clustering method: which algorithms implement Ward's criterion? J Classification 2014; 31: 274–295. doi: 10.1007/s00357-014-9161-z [DOI] [Google Scholar]
- 26.Knight SR, Ho A, Pius R, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C Mortality Score. BMJ 2020; 370: m3339. doi: 10.1136/bmj.m3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ting KK, Coleman P, Zhao Y, et al. The aging endothelium. Vasc Biol 2021; 3: R35–R47. doi: 10.1530/VB-20-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res 2018; 123: 825–848. doi: 10.1161/CIRCRESAHA.118.312563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flaumenhaft R, Enjyoji K, Schmaier AA. Vasculopathy in COVID-19. Blood 2022; 140: 222–235. doi: 10.1182/blood.2021012250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zha D, Fu M, Qian Y. Vascular endothelial glycocalyx damage and potential targeted therapy in COVID-19. Cells 2022; 11: 1972. doi: 10.3390/cells11121972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villa E, Critelli R, Lasagni S, et al. Dynamic angiopoietin-2 assessment predicts survival and chronic course in hospitalized patients with COVID-19. Blood Adv 2021; 5: 662–673. doi: 10.1182/bloodadvances.2020003736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong T, McClintock DE, Kallet RH, et al. Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit Care Med 2010; 38: 1845–1851. doi: 10.1097/CCM.0b013e3181eaa5bf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michels EHA, Butler JM, Reijnders TDY, et al. Association between age and the host response in critically ill patients with sepsis. Crit Care 2022; 26: 385. doi: 10.1186/s13054-022-04266-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Famous KR, Delucchi K, Ware LB, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 2017; 195: 331–338. doi: 10.1164/rccm.201603-0645OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proudfoot A, Bayliffe A, O'Kane CM, et al. Novel anti-tumour necrosis factor receptor-1 (TNFR1) domain antibody prevents pulmonary inflammation in experimental acute lung injury. Thorax 2018; 73: 723–730. doi: 10.1136/thoraxjnl-2017-210305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel BV, Wilson MR, O'Dea KP, et al. TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J Immunol 2013; 190: 4274–4282. doi: 10.4049/jimmunol.1202437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson MR, Goddard ME, O'Dea KP, et al. Differential roles of p55 and p75 tumor necrosis factor receptors on stretch-induced pulmonary edema in mice. Am J Physiol Lung Cell Mol Physiol 2007; 293: L60–L68. doi: 10.1152/ajplung.00284.2006 [DOI] [PubMed] [Google Scholar]
- 38.Bouchon A, Facchetti F, Weigand MA, et al. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 2001; 410: 1103–1107. doi: 10.1038/35074114 [DOI] [PubMed] [Google Scholar]
- 39.Jolly L, Carrasco K, Salcedo-Magguilli M, et al. sTREM-1 is a specific biomarker of TREM-1 pathway activation. Cell Mol Immunol 2021; 18: 2054–2056. doi: 10.1038/s41423-021-00733-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Silva-Neto PV, de Carvalho JCS, Pimentel VE, et al. sTREM-1 predicts disease severity and mortality in COVID-19 patients: involvement of peripheral blood leukocytes and MMP-8 activity. Viruses 2021; 13: 2521. doi: 10.3390/v13122521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inotrem . Inotrem announces that its ESSENTIAL phase II study for the treatment of critically ill COVID-19 patients meets its primary and key secondary endpoints. 2022. www.inotrem.com/2022/10/25/inotrem-announces-essential-phase-ii-study-treatment-critically-ill-covid-19-patients-meets-primary-key-secondary-endpoints Date last accessed: 21 April 2023.
- 42.Meduri GU, Annane D, Confalonieri M, et al. Pharmacological principles guiding prolonged glucocorticoid treatment in ARDS. Intensive Care Med 2020; 46: 2284–2296. doi: 10.1007/s00134-020-06289-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med 2021; 27: 1752–1760. doi: 10.1038/s41591-021-01499-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Havers FP, Pham H, Taylor CA, et al. COVID-19-associated hospitalizations among vaccinated and unvaccinated adults 18 years or older in 13 US States, January 2021 to April 2022. JAMA Intern Med 2022; 182: 1071–1081. doi: 10.1001/jamainternmed.2022.4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Havers FP, Patel K, Whitaker M, et al. Laboratory-confirmed COVID-19-associated hospitalizations among adults during SARS-CoV-2 omicron BA.2 variant predominance – COVID-19-Associated Hospitalization Surveillance Network, 14 States, June 20, 2021–May 31, 2022. MMWR Morb Mortal Wkly Rep 2022; 71: 1085–1091. doi: 10.15585/mmwr.mm7134a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-00011-2023.Supplement (1.4MB, pdf)
Supplementary Excel file ERJ-00011-2023.Excel (75.4KB, xlsx)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00011-2023.Shareable (978.1KB, pdf)