Significance
We used a back-translational approach in mice to demonstrate the pronociceptive role of neutrophils in fibromyalgia. Adoptive transfer of neutrophils from mice with chronic widespread pain or from patients with fibromyalgia can confer mechanical pain to recipient naïve mice, sensitize evoked action potential firing of spinal cord neurons, and produce phenotypic changes in cell surface expression of neutrophil proteins that cause infiltration of neutrophils into dorsal root ganglia. These data provide the framework for an immunological basis of chronic widespread pain in fibromyalgia mediated by polymorphonuclear granulocytes.
Keywords: neutrophils, fibromyalgia, pain, dorsal root ganglia, neuroimmune
Abstract
Fibromyalgia is a debilitating widespread chronic pain syndrome that occurs in 2 to 4% of the population. The prevailing view that fibromyalgia results from central nervous system dysfunction has recently been challenged with data showing changes in peripheral nervous system activity. Using a mouse model of chronic widespread pain through hyperalgesic priming of muscle, we show that neutrophils invade sensory ganglia and confer mechanical hypersensitivity on recipient mice, while adoptive transfer of immunoglobulin, serum, lymphocytes, or monocytes has no effect on pain behavior. Neutrophil depletion abolishes the establishment of chronic widespread pain in mice. Neutrophils from patients with fibromyalgia also confer pain on mice. A link between neutrophil-derived mediators and peripheral nerve sensitization is already established. Our observations suggest approaches for targeting fibromyalgia pain via mechanisms that cause altered neutrophil activity and interactions with sensory neurons.
Although the etiology of chronic widespread pain in fibromyalgia syndrome is unknown, the concept of altered central processing of nociceptive information has dominated the literature and clinical treatment guidelines (1). Several studies point toward a peripheral drive fibromyalgia pain, e.g., peripheral administration of a localized lidocaine block of tender points reduces pain scores in patients with fibromyalgia (2). Moreover, abnormal activity-dependent slowing of unmyelinated nociceptors is observed in microneurography recordings (3), and pain-related evoked potentials reflect abnormalities in small fiber function (4).
Aberrant activity of immune cells and associated cytokine signaling has also been linked to fibromyalgia syndrome. Neutrophils are polymorphonuclear granulocytes that normally exist in circulation to function as primary mediators of rapid innate host defense, but are surprisingly found in higher number in the circulation of fibromyalgia patients, with a phenotype characterized by enhanced chemotactic and microbicidal properties (5–7). Systemic expression of proinflammatory cytokines, such as IL-6, IL-8, and tumour necrosis factor alpha (TNFα), is increased in patients with fibromyalgia, and these same cytokines are otherwise released by neutrophils and are known mediators of nociceptor sensitization (8–10).
Growing evidence demonstrates an intricate bidirectional interaction between immune cells and sensory neurons. Polarization of resident macrophages in dorsal root ganglia and mitochondrial transfer from infiltrating macrophages to somata can confer sensitization of peripheral sensory neurons (11, 12). Similarly, CD3+ T cells have also been described in the resolution pathway of inflammatory pain (13), and pharmacological blockade of T cell–derived leukocyte elastase significantly reduces the magnitude of behavioral hypersensitivity in a rodent model of neuropathic pain (14). Conversely, Nav1.8+ sensory neurons play a key role in psoriasis and CD8+ T cell responses to viral infection (15). Neutrophils are not normally resident cells within intact nervous tissue, but infiltration into peripheral nerves has been reported in rodent models of nerve injury (16, 17), diabetic neuropathy (18), and degenerative disease (19).
Here, we investigate a causal link between neutrophils and nociceptive activity underlying chronic widespread pain in fibromyalgia using a mouse model of hyperalgesic priming (20–22) and adoptive transfer of cells from mice and patients with chronic pain to recipient naïve mice. Integrating a combination of pain behavior, electrophysiological measures of neuronal excitability, and imaging of peripheral sensory ganglia, we demonstrate pronociceptive activity of neutrophils.
Results
Persistent Hypersensitivity in a Mouse Model of Chronic Widespread Pain.
A model of chronic widespread pain was induced in male mice using a hyperalgesic priming paradigm to trigger latent and persistent pain behavior with consecutive intramuscular injections of carrageenan, an acute inflammatory stimulus (Fig. 1A) (22). Control mice received only one intramuscular injection to induce acute inflammatory pain, whereas primed mice received two insults. Both primed and control mice developed mechanical hypersensitivity to von Frey stimulation of the hind paw after the first carrageenan administration that resolved by 4 d. In control mice, there was no observed ipsilateral or contralateral hypersensitivity for the remaining duration of the study (Fig. 1 B, i; 0.91 ± 0.1 g to 0.38 ± 0.1 g at 1 d in control mice; 1.2 ± 0.2 g to 0.37 ± 0.1 g at 1 d in primed mice), whereas primed mice developed persistent ipsilateral mechanical hypersensitivity to von Frey stimulation following the second intramuscular injection of carrageenan, and later development of contralateral hypersensitivity by ca. 2 wk after induction of the model (Fig. 1 B, ii). Control mice and primed mice developed weightbearing asymmetry after the first injection on D0 compared to respective baselines, but primed mice had sustained weightbearing asymmetry compared to control mice until D12, when contralateral mechanical hypersensitivity had developed in primed mice, resulting in equal weight bearing of both hindlimbs (Fig. 1C). In contrast to the development of mechanical hypersensitivity, heat pain thresholds of primed mice were comparable to those of control mice in the hot plate and Hargreaves’ assays for the duration of the hyperalgesic priming paradigm (Fig. 1 D and E). Moreover, priming did not affect motor function measured in the rotarod (SI Appendix, Fig. S1).
Fig. 1.
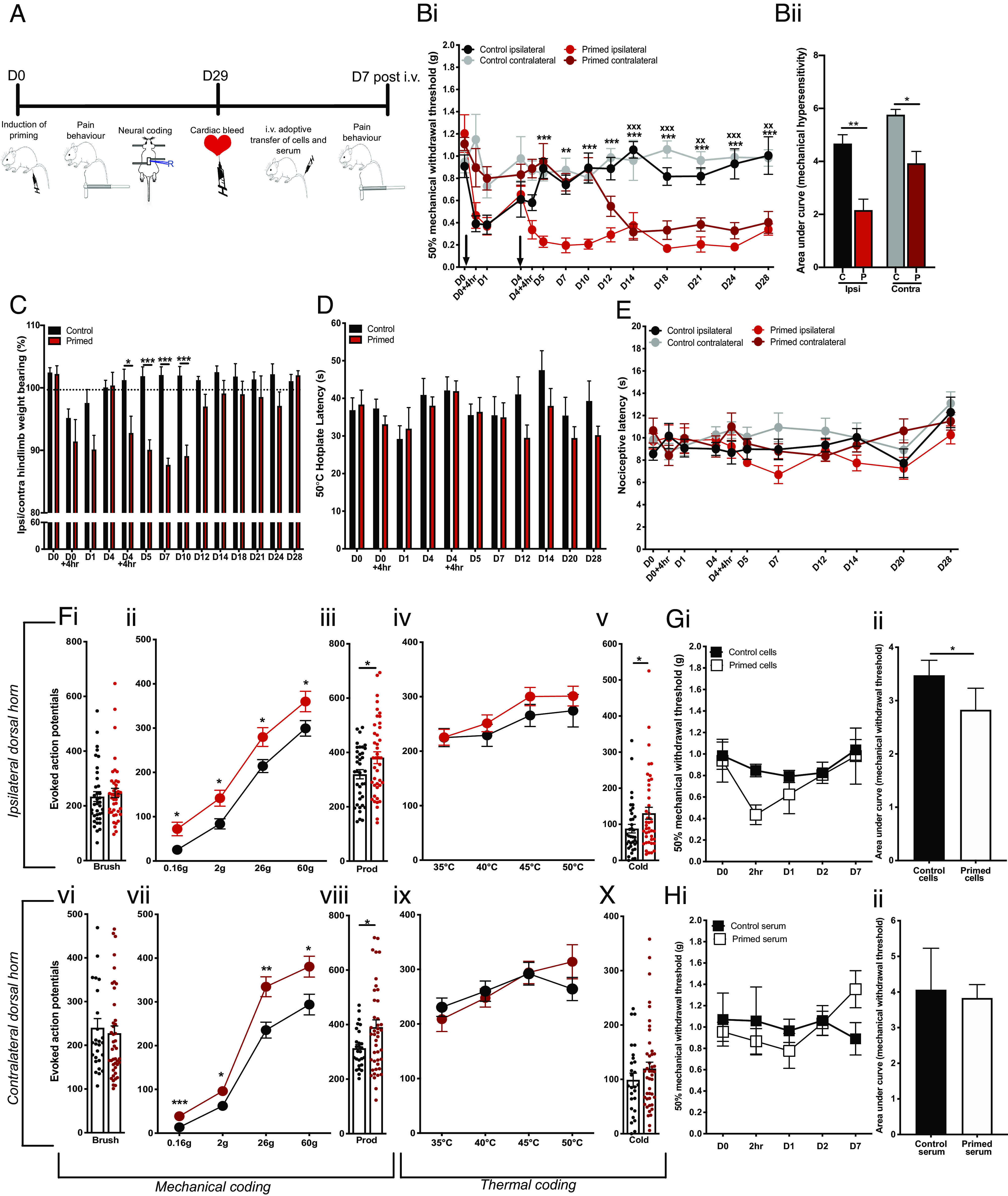
A hyperalgesic priming model for widespread pain in mice. (A) Schematic for study design: a hyperalgesic priming model of chronic widespread pain was induced in mice using intramuscular injections of carrageenan on D0 and D4 in primed mice (n = 7). Control mice (n = 7) received a single intramuscular injection on D0. After D29, cells and serum were obtained following cardiac bleed from primed and control mice and injected into recipient naïve mice whose pain behavior was monitored up to 7 d. (B, i) Mechanical hypersensitivity to von Frey stimulation of ipsilateral and contralateral limbs in primed mice (red) and control mice (**P < 0.01, ***P < 0.001 two-way RM ANOVA primed ipsilateral vs. control ipsilateral; xx P < 0.01, xxx P < 0.001 two way RM ANOVA primed contralateral vs. control contralateral). (B, ii) Area under the curve of mechanical pain thresholds illustrated in (B, i) of ipsilateral and contralateral hindlimbs in primed ‘P’ and control ‘C’ mice (*P < 0.05, **P < 0.01 unpaired t test). (C) Weightbearing asymmetry of hind limbs (*P < 0.05, ***P < 0.001 two-way RM ANOVA primed vs. control). (D) Latency to nociceptive behavior in the hot plate assay (two-way RM ANOVA primed vs. control). (E) Latency to nociceptive withdrawal in Hargreaves’ assay (two-way RM ANOVA primed ipsilateral vs. control ipsilateral, primed contralateral vs. control contralateral). (F) Evoked action potential firing of neurons in the (i–v) ipsilateral dorsal horn (control cells n = 37, n = 42 primed cells) and in the (vi–x) contralateral deep dorsal horn (control cells n = 27, n = 44 primed cells) of primed mice and control mice to (i and vi) innocuous brush stimulation, (ii and vii) von Frey stimuli, (iii and viii) noxious prod stimulation, (iv and ix) heat stimuli, and (v and x) noxious cold stimulation with ethyl chloride (*P < 0.05, **P < 0.01 unpaired t test). (G) Mechanical hypersensitivity following adoptive transfer of blood cells from primed mice (n = 4) and control mice (n = 4) with timecourse shown in (i) and area under the curve analysis of timecourse compared in (ii) (*P < 0.05 unpaired t test). (H) Mechanical hypersensitivity of naïve mice following adoptive transfer of blood serum from primed mice (n = 4) and control mice (n = 4) with timecourse shown in (i) and area under the curve analysis of timecourse compared in (ii) (unpaired t test).
We performed multiunit extracellular recordings in the dorsal horn of live mice to determine how the induction of widespread pain in the hyperalgesic priming model affects the bilateral excitability of spinal cord neurons, which are otherwise responsible for mediating pain-related reflexes in the pain behavior assays we used. We quantified the number of action potentials fired by polymodal wide-dynamic-range neurons in the ipsilateral and contralateral dorsal horn in response to 10 s of peripheral stimulation of various modalities (Fig. 1F). Dorsal horn neurons in control and primed mice displayed graded intensity coding to innocuous and noxious mechanical punctate stimulation with von Frey hairs, although primed cells displayed a sensitized response and consistently fired more action potentials to all von Frey intensities (Fig. 1 F, ii and vii). Dorsal horn cells in primed mice were also sensitized to noxious prod stimulation of the hindpaw compared to cells in control mice (Fig. 1 F, vii–viii). Dorsal horn cells in primed mice did not show any significant difference in evoked activity to brush or heat stimuli (Fig. 1 F, i, iii, vi, and ix). These findings support our observation that primed mice did not develop behavioral hypersensitivity to noxious heat stimulation and further suggest that induction of the hyperalgesic priming model does not confer sensitization to innocuous dynamic mechanical stimulation. However, we did observe sensitization of primed cells triggered by application of a noxious cold stimulus, which was only statistically significant in ipsilateral cells (average spikes 88 ± 11 primed vs. 131 ± 16 control in the ipsilateral side; average spike 99 ± 12 primed vs. 120 ± 11 control cells in the contralateral side). Together, these data demonstrate that induction of the hyperalgesic priming model leads to the development of persistent pain behavior encoded by prolonged ipsilateral hypersensitivity at the spinal cord level and a spatiotemporal spread of mechanical hypersensitivity to the contralateral limb.
Circulating Neutrophils Are Essential for the Development of Hypersensitivity in a Mouse Model of Chronic Widespread Pain.
We further investigated whether a systemic circulating factor could underlie the observed contralateral hypersensitivity in primed mice. We first transferred isolated blood cells and serum from primed and control mice to recipient naïve mice. Transfer of blood cells, but not serum, from primed mice induced transient mechanical hypersensitivity lasting up to 2 h (Fig. 1 G and H). These observations suggest that circulating cells constitute the algogenic etiology of chronic widespread pain in this murine model. In order to determine the identity of these pain-producing cells, we flow sorted blood cells derived from primed mice displaying persistent widespread hypersensitivity and from control mice with normal pain thresholds (Fig. 2 A–C). Four different immune cell types—T cells, B cells, neutrophils, and monocytes—were administered i.v. to naïve recipient mice (Fig. 2 D and E). Mice that were administered neutrophils derived from primed mice displayed robust, transient mechanical hypersensitivity in the von Frey assay for up to 1 d compared to mice that were administered neutrophils derived from control mice (Fig. 2 D, iii). In order to validate the algogenic role of neutrophils in mediating chronic widespread pain, we pharmacologically depleted circulating neutrophils with systemic administration of 1A8 clone on D3, just prior to the second intramuscular injection in primed mice (Fig. 3). Neutrophil depletion lasted at least 24 h as confirmed by FACS of circulating CD45+ and Ly6G/Ly6C+ cells (Fig. 3E). Administration of the antineutrophil antibody did not affect acute pain behavior following an intramuscular injection of carrageenan (Fig. 3A). However, primed mice administered with this antibody failed to develop prolonged ipsilateral hypersensitivity and any contralateral hypersensitivity compared to control primed mice administered with PBS (phosphate-buffered saline) (Fig. 3B). Moreover, pretreatment with 1A8 clone before the priming stimulus (24 h before the first intramuscular carrageenan insult) delays the onset of persistent ipsilateral and contralateral hypersensitivity after the precipitation of latent hypersensitivity with the second carrageenan insult (SI Appendix, Fig. S2 A–C). In animals administered neutrophil sequestering antibody at a late (D28) timepoint in the hyperalgesic priming model, we observe a reduction in ipsilateral and contralateral mechanical hypersensitivity lasting at least one day as indicated by significant analgesia at D29 (SI Appendix, Fig. S2 D and E). Our observations suggest that neutrophil depletion can delay or attenuate the development of persistent and widespread pain, without affecting gross measures of inflammation and its resolution (Fig. 3 C and D).
Fig. 2.
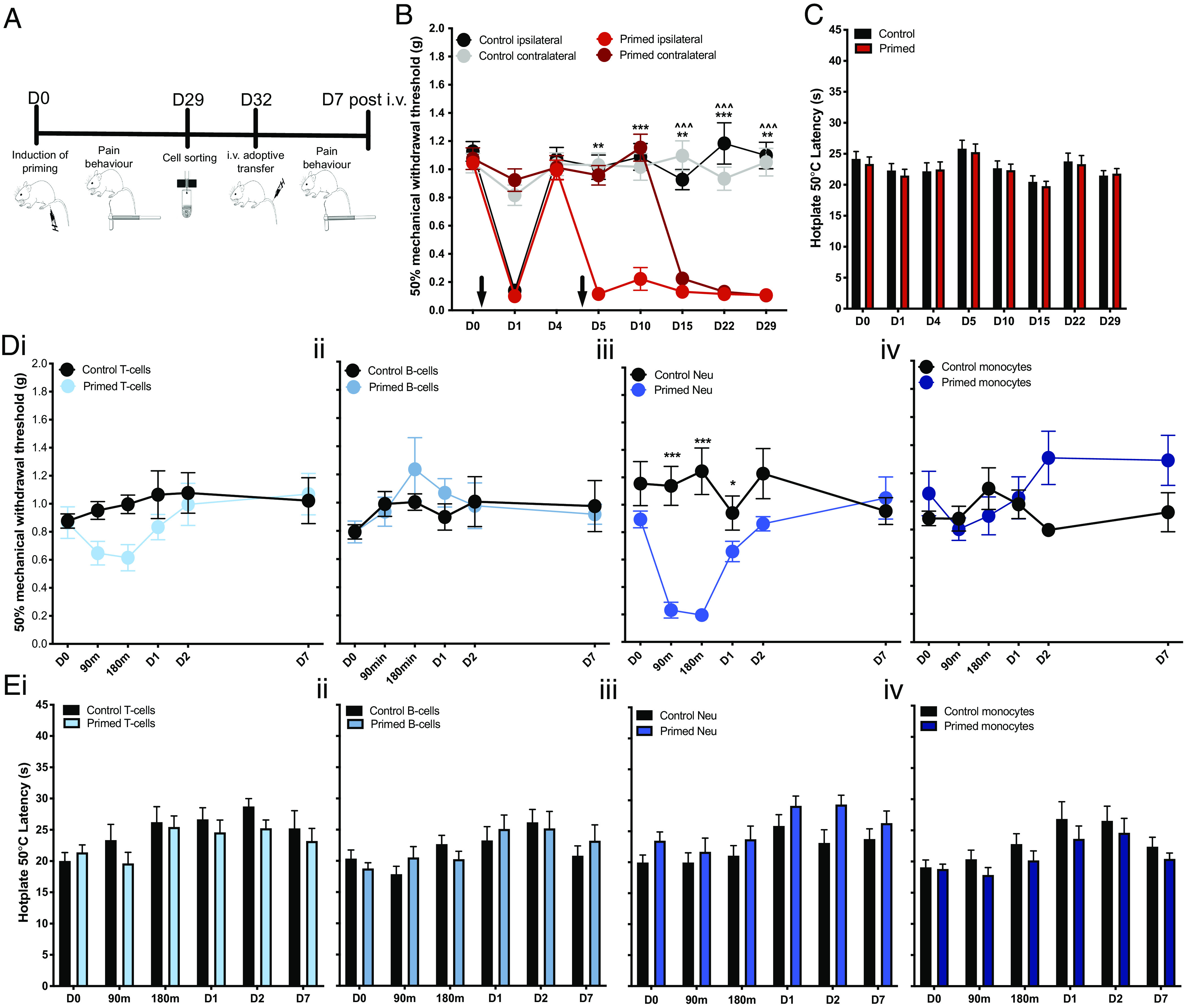
Adoptive transfer of neutrophils from primed mice confers widespread pain in naïve mice. (A) Schematic for study design: a hyperalgesic priming model of widespread pain was induced in mice (primed mice n = 24; control mice n = 24). On D29, blood cells were obtained through cardiac bleed from primed and control mice, flow sorted into B cells, T cells, neutrophils, and monocytes, and injected into naïve mice whose pain behavior was monitored up to 7 d. (B) Mechanical hypersensitivity to von Frey stimulation of ipsilateral and contralateral limbs of primed and control mice (**P < 0.01, ***P < 0.001 two-way RM ANOVA primed ipsilateral vs. control ipsilateral; ^^^P < 0.001 two-way RM ANOVA primed contralateral vs. control contralateral). (C) Latency to nociceptive behavior in the hot plate assay (two-way RM ANOVA primed vs. control). (D) Mechanical hypersensitivity to von Frey stimulation and (E) thermal hypersensitivity in the hot plate following adoptive transfer of (i) T cells, (ii) B cells, (iii) neutrophils, and (iv) monocytes from primed mice (n = 8) and control mice (n = 8) (*P < 0.05, ***P < 0.001 two-way RM ANOVA primed vs. control cells).
Fig. 3.
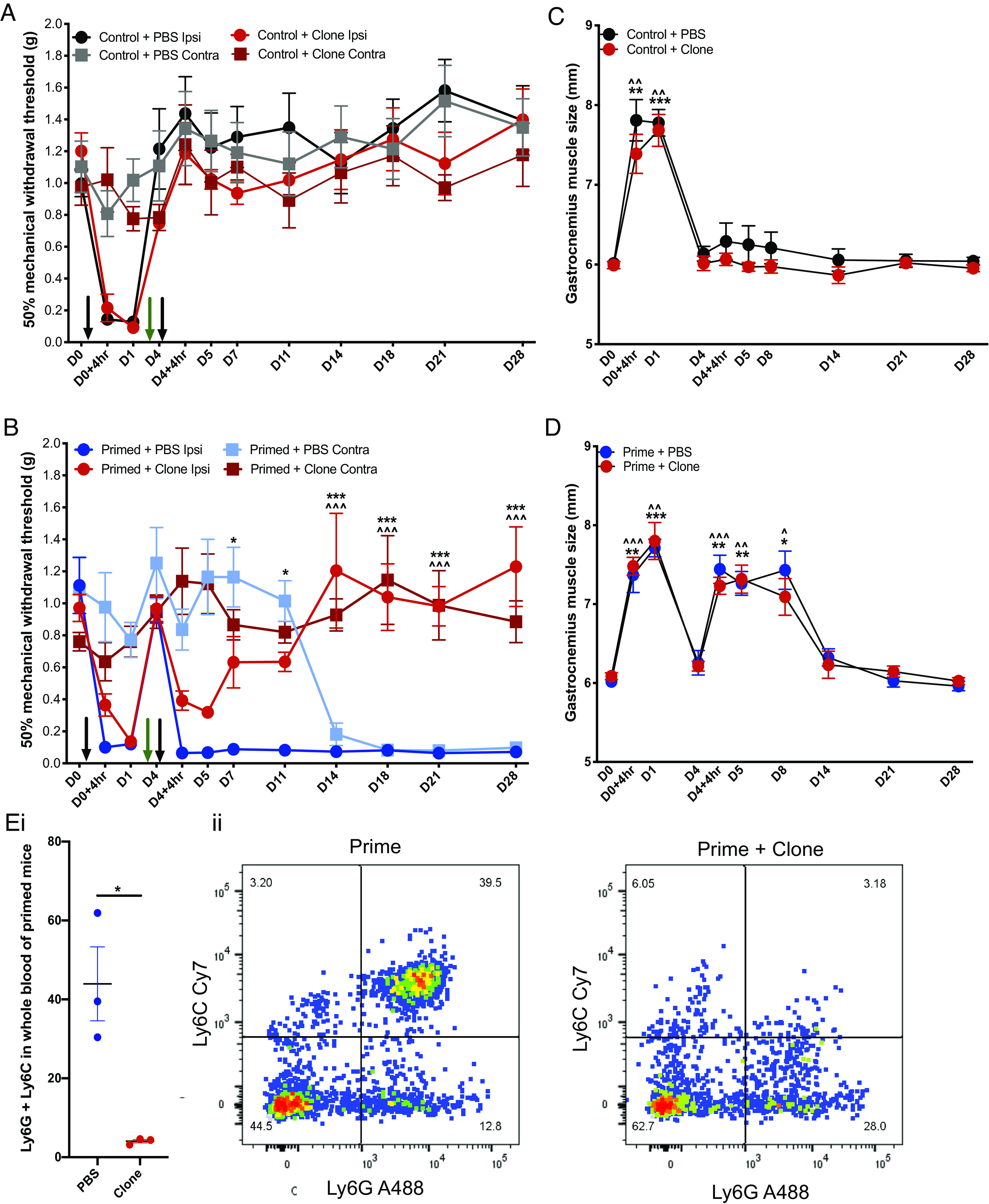
Neutrophil depletion prevents development of widespread pain. A hyperalgesic priming model of widespread pain was induced in mice with intramuscular injections of carrageenan on D0 and D4 (black arrows) and neutrophil sequestering antibody (50 µg i.v. 1A8 Clone) was administered on D3 (green arrow) (primed + clone n = 8; primed + PBS n = 8; control + clone n = 8; control + PBS n = 8). (A) Mechanical hypersensitivity to von Frey stimuli in control groups receiving one intramuscular injection of carrageenan on D0 and clone on D3. (B) Mechanical hypersensitivity to von Frey stimuli in primed groups receiving two intramuscular injections of carrageenan on D0 and D4 and clone on D3 (*P < 0.05, ***P < 0.001 two-way RM ANOVA primed + PBS ipsilateral vs. primed + clone ipsilateral; ^^^P < 0.001 two-way RM ANOVA primed + PBS contralateral vs. primed + clone contralateral). (C and D) Hindleg inflammation measured through gastrocnemius muscle size (P > 0.05 all timepoints two-way RM ANOVA; (* or ^P < 0.05, ** or ^^P < 0.01, *** or ^^^P < 0.001 one-way ANOVA Dunnett’s control+ PBS or primed + PBS vs. control + clone or primed + clone). (E, i) FACS confirmation of neutrophil depletion using 1A8 clone and (ii) forward and side scatter for Ly6G and Ly6C markers of neutrophils.
Neutrophils Derived from Mice with Chronic Widespread Pain Infiltrate Sensory Ganglia.
To test the hypothesis that circulating neutrophils in primed mice have the capacity to sensitize peripheral sensory neurons, we measured whether neutrophils were directly interacting with somata within dorsal root ganglia. We observed significant de novo infiltration neutrophils into L4 DRG of primed mice compared to control and naïve animals (Fig. 4A). Similarly, we used FACS to confirm greater number of Ly6G/Ly6C double-positive CD45+ cells in L3-L5 DRGs of primed mice compared to controls (Fig. 4B). We then characterized neutrophils from blood and from DRG in control and primed mice and compared the prevalence of subpopulations or “clusters” of Ly6G/Ly6C+ cells using an unsupervised t-distributed stochastic neighbor embedding (tSNE) analysis (SI Appendix, Fig. S3 and Fig. 4 C–F). Distinct neutrophil subpopulations from blood and DRG tSNE were defined and clustered by cell surface markers CXCR2, CD62L, CD11b, Ly6G, and Ly6C (SI Appendix, Fig. S3 i and ii). We applied tSNE analyses to neutrophil populations derived from control and primed mice to identify 11 distinct subpopulations (clusters) in the blood (Fig. 4C) and DRG (Fig. 4E) based on density of expression. The most up-regulated and down-regulated clusters of neutrophils derived from blood (Fig. 4D) and from DRG (Fig. 4F) illustrate differences in the expression of CXCR2 and CD62L, which was further confirmed by independent FACS analysis of Ly6G/Ly6C double-positive cells in blood (Fig. 4G) and DRG (Fig. 4H) of control and primed mice. Furthermore, tSNE was performed for all neutrophils (blood and DRG pooled together) to illustrate an enriched neutrophil subpopulation in DRG that is characterized by decreased levels of CXCR2, CD62L, CD11b, Ly6G, and Ly6C (SI Appendix, Fig. S3iii).
Fig. 4.
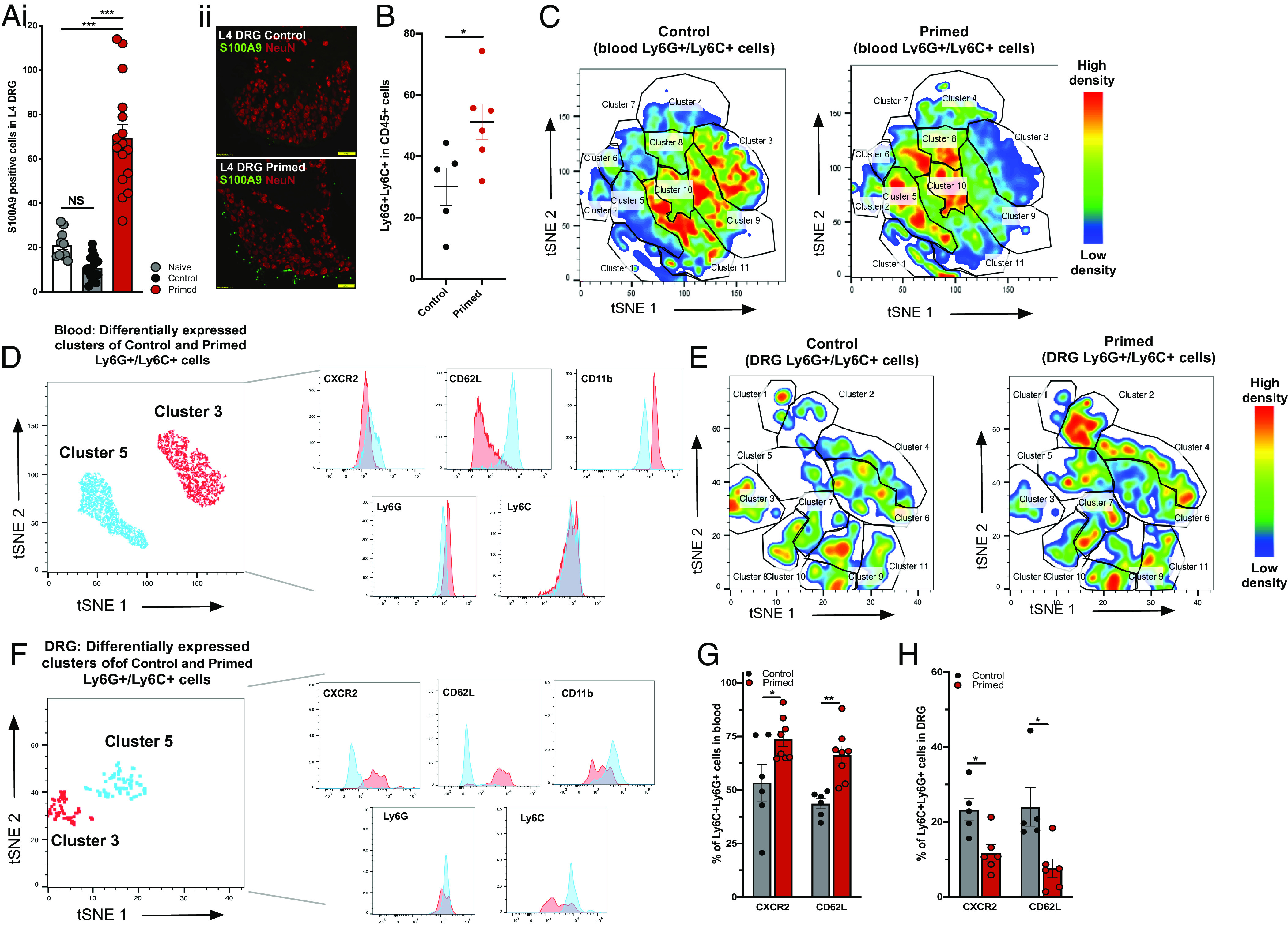
Chronic widespread pain in mice increases neutrophil trafficking into sensory ganglia. (A) Immunohistochemical staining for S100A9 in L4 DRG sections from control and primed mice (i) demonstrating increased numbers of neutrophils in DRG of primed mice (sections from n = 3 to 4 mice per group; ***P < 0.001 one-way ANOVA). Yellow line in representative L4 DRG images (ii) indicates 100 µm. (B) FACS sorted number of neutrophils (Ly6G/Ly6C double-positive cells) derived from DRG in control (n = 5) vs. primed (n = 6) mice (*P < 0.05 unpaired t test). (C) tSNE analysis of Ly6C/Ly6G double-positive neutrophils obtained from blood of control (n = 6) mice and primed (n = 8) mice. Neutrophil subpopulations labeled for CXCR2, CD62L, CD11b, Ly6G, and Ly6c are illustrated with colors in the expression level heatmap representing median intensity values for relative clustering based on cell density plots. (D) Differential expression of CXCR2, CD62L, CD11b, Ly6G, and Ly6C based on cluster 3 and cluster 5, with the most decreased (−55%) and increased (+101%) numbers of neutrophil subpopulations in primed cells, respectively. (E) tSNE analysis of Ly6C/Ly6G double-positive neutrophils obtained from DRG of control mice (n = 5) and primed (n = 6) mice, with CXCR2, CD62L, CD11b, Ly6G, and Ly6c markers. (F) Differential expression of CXCR2, CD62L, CD11b, Ly6G, and Ly6C based on clusters 3 and 5, with the most decreased (−60%) and increased (+178%) numbers of neutrophil subpopulations in primed cells, respectively (G and H) FACS quantification of CXCR2-positive and CD62L-positive neutrophils (double positive for Ly6G and Ly6C) in (G) blood vs. (H) DRG (*P < 0.05, **P < 0.01 unpaired t test).
Neutrophils Derived from Patients with Fibromyalgia Syndrome Induce Mechanical Hypersensitivity in Mice.
In order to further evaluate the pronociceptive activity of neutrophils, we used a back-translational model of adoptive transfer using neutrophils–derived from patients diagnosed with fibromyalgia syndrome–administered to naïve mice (Fig. 5A). All patients had a minimum of visula analogue score (VAS) 50 for scoring their pain on the day they were consented to provide blood (mean VAS 68.8 ± 4.2), or their overall pain during the week (mean VAS 78.8 ± 3.6). Healthy subjects were largely pain free (mean VAS for the day 0.8 ± 0.5 and mean VAS for the week 1.4 ± 0.9) (Fig. 5B). Neutrophils derived from patients with widespread pain conferred robust mechanical hypersensitivity in recipient naïve mice compared to neutrophils from healthy control subjects (Fig. 5C). In order to determine effects on neuronal excitability, we measured the effects of adoptive neutrophil transfer on neural coding of wide dynamic range deep dorsal horn neurons (Fig. 5E). Spinal cord cells of naïve mice demonstrated marked sensitization to noxious mechanical stimulation of receptive fields in the hindpaw with von Frey hairs, prod and pinch, within 1 h of administration of neutrophils (Fig. 5 E, iii and iv), but no marked change in the coding of innocuous dynamic stimulation with brush (Fig. 5 E, i). In addition, we observed sensitization of dorsal horn neurons to noxious cold stimulation, and surprisingly, also noxious heat stimulation with 50 °C despite the lack of effect on 50 °C hot plate–evoked behaviors (Fig. 5 D and E, ii).
Fig. 5.
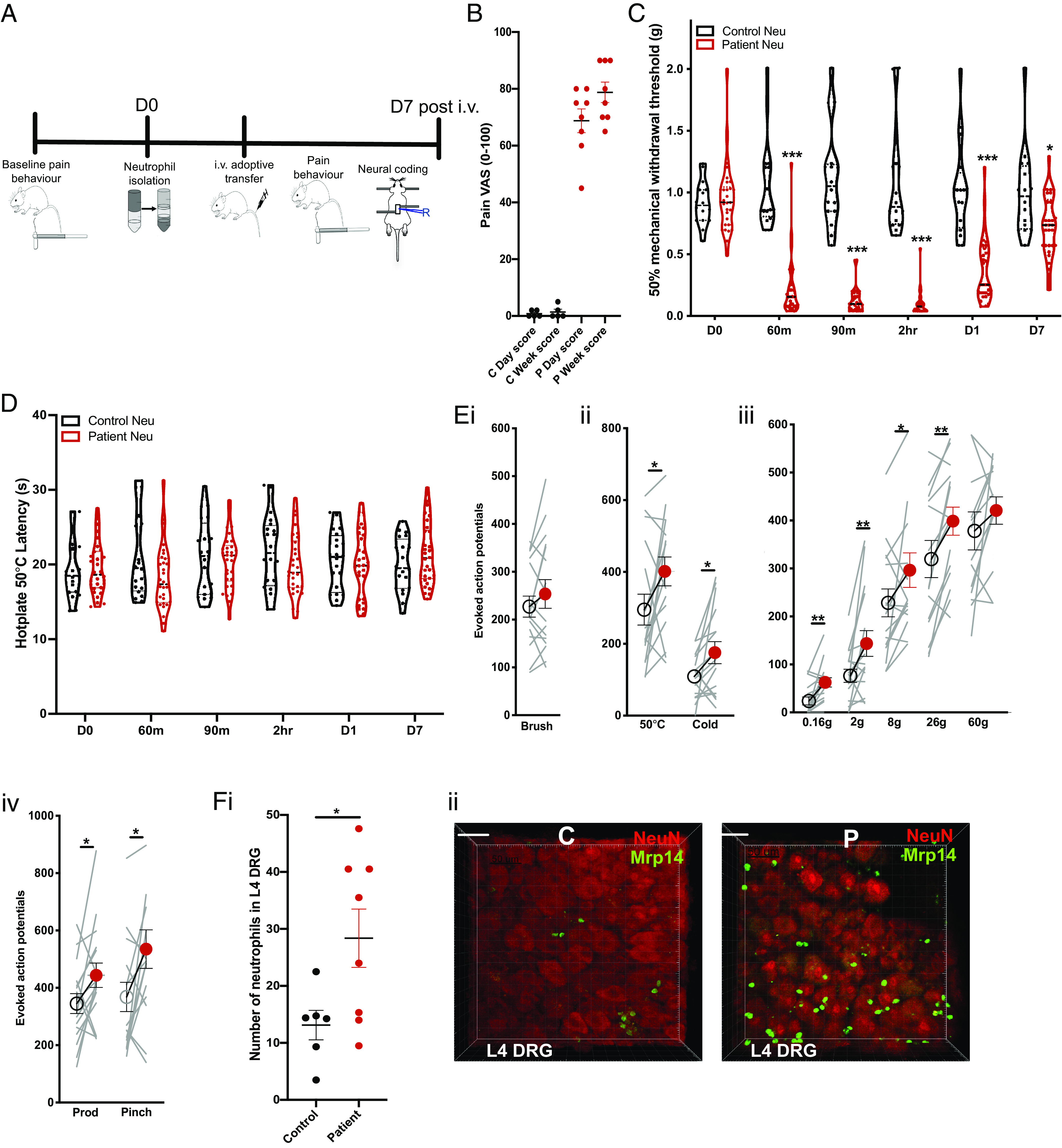
Adoptive transfer of neutrophils from primed mice confers widespread pain in naïve mice. (A) Neutrophils were isolated from patients with fibromyalgia (n = 9) and healthy control subjects (n = 5) and administered into naïve male and female mice (1 × 106 cells i.v. in PBS suspension). Cells from each subject were injected into four mice. (B) Pain visual analogue scores (VAS) of healthy control subjects and subjects with fibromyalgia. (C) Mechanical hypersensitivity to von Frey stimulation of mice following i.v. adoptive transfer of neutrophils (*P < 0.05, ***P < 0.001 two-way RM ANOVA control vs. patient cells). (D) Latency to nociceptive behavior in the hot plate assay (two-way RM ANOVA primed vs. control). (E) Evoked action potential firing of neurons in the deep dorsal horn of naïve mice (n = 16 cells) before (open circles) and 1 h after (red circles) administration of neutrophils from patients. Total number of action potentials fired over 10 s stimulation with (i) innocuous brush, (ii) noxious thermal stimuli, (iii) punctate von Frey stimuli, and (iv) noxious mechanical stimuli (*P < 0.05, **P < 0.01 paired t test). (F, i) Ex vivo imaging of neutrophil cell counts in L4 DRG following adoptive transfer of neutrophils from patient (n = 4 mice) or control blood (n = 3 mice). White line illustrates 50 µm. (*P < 0.05 unpaired t test). (ii) Representative of mean images for (F, i).
We used ex vivo imaging of L4 ganglia derived from naïve mice 24 h following i.v. administration of neutrophils from either a patient with fibromyalgia or pain-free control subject and observed greater numbers of neutrophils infiltrating ganglia following adoptive transfer of patient neutrophils (Fig. 5F). The neutrophils infiltrating sensory ganglia were both endogenous mouse neutrophils and exogenous human neutrophils, suggesting tissue-restricted innate mechanisms for the recruitment of endogenous and exogenous neutrophils into nervous tissue in chronic pain states (SI Appendix, Fig. S4).
Discussion
We used a mouse model of chronic widespread pain and a back-translational adoptive transfer model of neutrophils from patients with fibromyalgia syndrome to demonstrate that neutrophils are peripheral pathological drivers of chronic widespread pain. Our data reveal three main findings: 1) a hyperalgesic priming paradigm can be used to induce chronic widespread pain in mice, which reproduces the bilateral sensory dysfunction and mechanical hypersensitivity observed in patients with fibromyalgia; 2) systemic neutrophil sequestration in a mouse model of chronic widespread pain can prevent the development of persistent ipsilateral and contralateral hypersensitivity without affecting gross measures of inflammation induction and resolution following injury; 3) circulating neutrophils in mice and in patients with chronic widespread pain are pronociceptive.
We used a hyperalgesic priming paradigm to induce persistent and widespread pain in mice in line with other studies that have used priming of nociceptive pathways to precipitate latent, bilateral hypersensitivity and to model the transition from acute to chronic pain (20–23). Consecutive intramuscular injections of the acute inflammatory stimulus carrageenan induced prolonged ipsilateral, mechanical hypersensitivity in mice and a spatiotemporal spread of hypersensitivity to the contralateral limb lasting more than 4 wk (22) (Fig. 1B). Sensitization to von Frey stimulation of the hind paw indicates the development of secondary hypersensitivity, beyond the primary site of injury in the gastrocnemius muscle. Our observation of increased excitability of dorsal horn neurons, which have receptive fields in the hind paw, suggests a spinal mechanism underlying this secondary hyperalgesia (24, 25). Previous studies have also reported bilateral mechanical hypersensitivity with a single intramuscular injection of carrageenan (26) or acidic saline (27), as well as with consecutive injections of intramuscular carrageenan (28). Intramuscular injections of carrageenan also produced significant weightbearing asymmetry. Primed mice continued to display substantial weightbearing asymmetry following the second carrageenan administration until ca. 2 wk, corresponding to the development of contralateral mechanical hypersensitivity (Fig. 1 B and C). Contralateral dorsal horn neurons were also sensitized to mechanical stimuli indicating either top-down bilateral modulation of spinal excitability by supraspinal centers (29) or a systemic etiology for the development of bilateral hypersensitivity. We tested the hypothesis for the latter narrative and observed that transfer of blood cells, but not serum, from primed mice confers transient mechanical hypersensitivity to recipient naïve mice (Fig. 2). Although the hyperalgesic priming paradigm with repeated muscle insults mimics some aspect of sensory dysfunction observed in patients with fibromyalgia, such as widespread mechanical sensitivity of muscles, paws, and viscera (27, 28, 30, 31); a limitation of this paradigm is that it does not necessarily induce comorbid conditions commonly experienced by patients with fibromyalgia such as sleep dysfunction, stress, and anxiety (32).
Neutrophils are primary mediators of rapid innate host defense prior to the complex humoral and lymphocyte cellular processes of acquired immunity (33). The development of severe immune deficiency following iatrogenic neutropenia, e.g., from cancer-related chemotherapy, highlights the importance of neutrophils in the immune response to infection and injury (34). Some studies suggest an additional role for neutrophils in antinociceptive activity based on their atypical expression of opioid peptides (35, 36). In contrast, we observed that transfer of neutrophils from mice or patients with chronic widespread pain can induce transient hypersensitivity in otherwise healthy mice (Figs. 2 and 5). Moreover, antibody depletion of neutrophils can prevent the development of persistent mechanical hypersensitivity in mice that have been primed by a muscle insult (Fig. 3). When systemic neutrophils are depleted prior to induction of a hyperalgesic priming model of chronic widespread pain in mice, the onset of persistent widespread pain is delayed, and neutrophil depletion following the establishment of chronic widespread pain transiently attenuates ipsilateral and contralateral mechanical hypersensitivity (SI Appendix, Fig. S2). Together, these data suggest that circulating neutrophils can develop a pathological phenotype after a priming stimulus in the hyperalgesic priming model of chronic widespread pain and that neutrophils play a critical role in the transition from acute to chronic pain in fibromyalgia. The role of neutrophils in regulating acute inflammatory pain and resolution of inflammation is well established, but neutrophil-mediated modulation of nociceptive processing beyond the inflammatory resolution phase has not been explored (37). Neutrophils have been shown to play a role in the resolution of inflammatory pain (38), and neutrophil-derived products such as annexin A1 and resolvins are key proresolving mediators (39, 40). In contrast, several findings in animal studies also point toward a pronociceptive role of neutrophils ever since the first report of proalgesic effects of articular neutrophils in joints of dogs, promoted by administration of lipopolysaccharides (41). Subsequent studies by Levine et al. showed that intraplantar administration of LTB4 and the complement factor 5a in the rat paw produces mechanical hypersensitivity that is dependent on neutrophil migration (42, 43). In addition, thermal hypersensitivity induced by nerve growth factor is inhibited in neutrophil-depleted rats (44), and migrating neutrophils during carrageenan-induced inflammation contribute to mechanical hypersensitivity partly by release of PGE2 (45). In addition, neutrophil chemotaxis can be regulated by primary afferent-derived mediators, including neuropeptides GRP and VIP (46) as well as chemokines such as CXCL5 that can amplify nociceptive signaling through sensitization of nociceptors (47).
We used a back-translational paradigm to demonstrate that circulating neutrophils derived from patients with fibromyalgia can sensitize sensory neurons and elicit behavioral hypersensitivity in mice. Recent work indicates an immunological basis for fibromyalgia symptoms as passive IgG transfer from patients to mice can confer mechanical pain behavior (48), although we were unable to reproduce these findings (SI Appendix, Fig. S5). This may be due to differences in pain severity (measured through pain VAS) of the patient cohorts that blood samples were derived from and reflects the potential heterogeneity of disease pathogenesis among patients with fibromyalgia. In support of our findings, previous studies in humans demonstrate that recruitment of polymorphonuclear leukocytes by the potent chemoattractant LTB4 decreases pain thresholds, causing robust hyperalgesia in healthy subjects (49). Moreover, intraarticular steroids not only reduce neutrophil infiltration of knee joints in patients with osteoarthritis and inflammatory arthritis, but also reduce pain scores (50). The close correlation between reduction in pain VAS and neutrophil migration supports a causal link between neutrophil migration and nociception. Responses in the contralateral knee indicate a systemic effect of neutrophil migration on perceived nociception (50). We observed the development of mechanical hypersensitivity in both male and female mice following adoptive transfer of human neutrophils, and therefore the underlying mechanisms for sex differences in the prevalence of fibromyalgia remain to be determined (51). We did not observe any changes in thermally evoked pain behaviors in the 50 °C hot plate following administration of neutrophils from mice or patients with chronic widespread pain (Figs. 2E and 5D), but did observe marked sensitization of spinal neurons to 50 °C stimulation of peripheral receptive fields in the hindpaw. This discrepancy between integrated behavioral responses and the excitability of individual spinal cord neurons may relate to the recruitment of segmental inhibition of spinally mediated reflexes with suprathreshold stimulation following contact of all 4 hindpaws on the hot plate (52–54). Previous findings have observed that inhibition of neutrophil migration with selectin inhibitor fucoidin can reduce thermally evoked pain behaviors on the 51 °C hot plate following ovalbumin administration (55). Moreover, antibody depletion of circulating neutrophils significantly reduces endoneurium infiltration and attenuates the induction of thermal hyperalgesia induced by partial peripheral nerve injury (56).
Immune cell infiltration into nervous tissue beyond primary sites of injury has previously been observed in murine models of chronic pain (16–18, 57). Tcell and neutrophil infiltration in dorsal root ganglia in mice with diabetic peripheral neuropathy accompanies tonic pain (18). In the chronic constriction injury model of neuropathic pain, de novo neutrophil infiltration of ipsilateral dorsal root ganglia is observed with direct contact between polymorphonuclear granulocytes and neurons, despite no change in local expression of cytokine-induced neutrophil chemoattractant-1 (17). Moreover, nerve injury in mice leads to T cell and neutrophil infiltration of dorsal root ganglia and development of mechanical hypersensitivity that can be attenuated with inhibition of leukocyte-derived elastase (14, 58). We observed de novo infiltration of neutrophils into lumbar DRG in primed mice that could be prevented with antibody depletion of circulating neutrophils (Fig. 3). We also observed increased infiltration of endogenous mouse and exogenous human neutrophils into mouse sensory ganglia following adoptive transfer of neutrophils from a patient with fibromyalgia (Fig. 5 A, B, and F and SI Appendix, Fig. S4). The chemoattractant mechanisms underlying neutrophil infiltration into the sensory ganglia are likely related to our observation of an increased prevalence of subpopulations of circulating neutrophils with high expression of surface markers CD62L (L-selectin) and CXCR2 (receptor for IL-8 and other chemokines) in primed mice, whereas following infiltration into the DRG of primed mice, there is a greater prevalence of neutrophil subpopulations with lower CD62L and CXCR2 expression (Fig. 4 C–H), typical of an activated and migrated phenotype (59, 60). These observations are supported by previous work demonstrating increased L-selectin expression of neutrophils in patients with fibromyalgia syndrome, suggesting that a possible phenotypic population of neutrophils plays a role in chronic widespread pain (61). Other work in experimental autoimmune encephalomyelitis suggests that DRG infiltration of neutrophils can also be dependent on TLR-CXCL1 signaling (62). In line with these findings, adoptive transfer of myelin oligodendrocyte glycoprotein-stimulated neutrophils induces mechanical allodynia in recipient mice (63). Several studies also demonstrate that neutrophils release algesic mediators that activate and sensitize nociceptive afferents directly, or indirectly, by eliciting the release of algesic mediators from other resident cells, including the leukotriene 8R,15S-diHETE (64), superoxide radicals (65), and cyclooxygenase-2 activation that triggers release of prostaglandins (65, 66). Nerve growth factor-induced hyperalgesia is also dependent on circulating neutrophils (44, 67). Due to the short half-life of neutrophils (3 to 7 d), it is likely that these immune cells harbor a reactive capacity for phenotypic changes in chronic pain states. Other studies have shown an extension of neutrophil lifespans in pathological states (68, 69). These studies support our finding that neutrophils—when derived either from mice with persistent widespread pain lasting more than two weeks or from patients with enduring fibromyalgia pain over years—can undergo phenotypic changes that enable and sustain sensitization of peripheral sensory neurons.
Conclusion
We used a model of hyperalgesic priming to induce chronic widespread pain in mice that resulted in robust and persistent ipsilateral and contralateral behavioral hypersensitivity and sensitization of spinal cord neurons. Adoptive transfer of neutrophils from primed mice and from patients with fibromyalgia syndrome confers mechanical pain to recipient naïve mice, sensitizes evoked action potential firing of spinal cord neurons, and causes neutrophil infiltration into the dorsal root ganglia. These data demonstrate that neutrophils are fundamental for the development of chronic widespread pain through infiltration of peripheral sensory ganglia. Further studies characterizing the neutrophil phenotype in fibromyalgia syndrome may shed light on mediators of the cross talk between these polymorphonuclear granulocytes and sensory neurons. Our findings suggest that targeting neutrophils may be useful therapeutic targets for pain control in fibromyalgia.
Methods
Animals.
All animal assays performed in this study were approved by the United Kingdom Home Office Animals (Scientific Procedures) Act 1986. C57Bl/6J male and female mice aged 8 to 10 wk were kept on a 12-h light/dark cycle and maintained under standard conditions (21 ± 1 °C, food and water ad libitum).
Mouse Behavior.
All behavioral experiments were performed by an experimenter blind to randomized allocation of mice to experimental groups. Mechanical hypersensitivity was assessed using the up-down von Frey method as described previously (70). Thermal nociceptive thresholds were determined by measuring latencies to nociceptive behavior on a 50 °C hot plate (Ugo Basile). Hindlimb weightbearing was measured using the incapacitance tester (Bioseb).
Murine Model of Chronic Widespread Pain.
A model of hyperalgesic priming was used to induce chronic widespread pain in mice with consecutive injections of carrageenan into the right gastrocnemius muscle 4 d apart (i.m. 3% 30 μL, Sigma Aldrich). Control mice only received one injection on D0 (28).
In Vivo Electrophysiology.
Electrophysiological recordings were performed by an experimenter blind to experimental groups. Briefly, mice were anesthetized with isofluorane (4%; 0.5L/min N2O and 1.5 L/min O2) and secured in a stereotaxic frame. Anesthesia was reduced and maintained at 1.5% isoflurane for the remaining duration of the experiment. A laminectomy was performed to expose L3–L5 segments of the spinal cord, and extracellular recordings were made from wide dynamic range neurons in the deep dorsal horn (lamina III–V, 200 to 600 μm) using parylene-coated tungsten electrodes (A-M Systems, USA). Mechanical and thermal stimuli were applied to the peripheral receptive field of spinal neurons on the hindpaw glabrous skin and the evoked activity of neurons was visualized on an oscilloscope and discriminated on a spike amplitude and waveform basis using a CED 1401 interface coupled to Spike2 software (Cambridge Electronic Design, UK). Natural stimuli (dynamic brush, vF hairs 0.16 g to 60 g, noxious prod 100 g cm−2 mechanical stimulation; thermal water jet 35 to 50 °C) were applied in ascending order of intensity to receptive fields for 10 s and the total number of evoked spikes was recorded.
Neutrophil Depletion.
Neutrophil depletion was performed by i.v. injection of 50 µg anti-Ly6G antibody (clone 1A8 BioLegend #127601) or PBS on day 3 (24 h prior to the second injection in the primed group).
Patient Samples.
Ethical approval for the study was obtained from the UK National Research Ethics Service (West of Scotland Research Ethics Service subcommittee), REC 17/WS/0172, IRAS 60271. All subjects provided written informed consent, and samples were collected in compliance with NHS Health Research Authority approval. Blood samples were obtained from healthy, pain-free control subjects or from patients diagnosed with fibromyalgia according to ACR 2016 criteria (71) at Mile End Hospital, Barts NHS Trust. All subjects were asked to rate their overall pain score on a visual analog scale (VAS) from 0 to 100 (where 0 = no pain, 100 = maximum pain possible) for the day and over the past week. Healthy control subjects had pain scores of 5 or less. Blood samples were collected immediately after giving consent and record of pain VAS scores. Neutrophils were isolated by density gradient centrifugation at room temperature and red blood cells were removed using ACK lysing buffer. Naïve C57B/6 mice were injected with 1 × 106 neutrophils (i.v. 100 μL in PBS) from either patients or controls.
Adoptive Transfer of Whole Blood Components.
Whole blood was collected from control or primed mice through terminal cardiac bleed. For blood cell isolation, ACK lysing buffer was used to remove red blood cells from heparin-treated blood, the sample was centrifuged at 300 × g for 5 min at RT, and the supernatant was discarded. The pellet was resuspended in 5 mL DMEM solution (Gibco) and centrifuged at 300 × g for 5 min at 4 °C. The supernatant was discarded and was used to isolate either serum or blood cells for adoptive transfer into naïve recipient mice and injected into naïve mice (i.v. 100 μL). Neutrophil (Ly6C+Ly6G+), T cell (CD3+), B cell (CD19+), and monocyte (Ly6C+Ly6G−) populations of cells were isolated from either primed or control mouse blood using FACS cell sorting and injected into naïve recipient animals (i.v. 1 × 106 cells in 100 μL PBS). IgG from whole blood was purified using protein A Sepharose beads (Merck). Serum was diluted 1:2 in PBS, passed through a protein A column, the bound IgG was eluted using Ab Buffer Kit (Merck), and then the eluate was dialyzed overnight at 4 °C in PBS using a 10 kDa dialysis membrane (Thermo Fisher Scientific). The concentration of IgG present after dialysis was determined using a BCA assay and adjusted by dilution with PBS. The IgG solution was stored at 4 °C until i.p. administration (8 mg) into naïve mice.
Flow Cytometry.
Flow-cytometry analysis was performed on mouse blood cells. Blood samples from mice were collected in 1.5 mL Eppendorf tubes with 50 mM EDTA and kept on ice. Red blood cells were removed by incubating the blood samples with 2 mL ammonium chloride-potassium lysing buffer (Thermo Fisher Scientific #A1049201) for 3 to 5 min at RT. Samples were centrifuged at 300 × g for 5 min at room temperature and the supernatant was discarded. This step was repeated once. The cell pellet was then gently resuspended, followed by the addition of 1 mL ice-cold DMEM media and centrifugation at 300 × g for 5 min at 4 °C. The supernatant was discarded, and the pellet was resuspended in 500 μL Hanks’ Balanced Salt solution. The cells were stained using Zombie Aqua Live/Dead kit (BioLegend #423101) for 15 min, washed, and incubated for 10 min with human Fc TruStain FcX (BioLegend #101320). The cells were stained for surface antigens. Antibodies used are listed in Table 1. All samples were acquired using a BD Fortessa and analyzed with FlowJo software (FlowJo, LLC).
Table 1.
FACS mouse antibodies
| Antibody | Clone | Fluorochrome | Company | Catalog number | Batch | Dilution |
|---|---|---|---|---|---|---|
| CD45 | 30-F11 | BV785 | BioLegend | 103149 | B263597 | 1 in 800 |
| CD11b | M1/70 | APC | BioLegend | 101212 | B269209 | 1 in 200 |
| Ly6G | 1A8 | APC/cy7 | BioLegend | 127624 | B348489 | 1 in 1,600 |
| Ly6C | HK1.4 | A488 | BioLegend | 128022 | B304228 | 1 in 1,600 |
| CD62L | MEL 14 | BV605 | BioLegend | 104438 | B266851 | 1 in 800 |
| CXCR2 | SA045E1 | PerCP/Cy5.5 | BioLegend | 149606 | B273375 | 1 in 400 |
tSNE Analysis.
tSNE analyses were performed with FlowJoTM version 10 software (FlowJo LLC), with Ly6G Ly6C double-positive neutrophils. All Ly6G/Ly6C double-positive neutrophils were merged with the concatenate tool and barcoded to track individual cells and to distinguish the condition. Finally, tSNE analyses were performed with the CXCR2, CD62L, CD11b, Ly6G, and Ly6C markers. The following settings were used: learning configuration: auto (opt-SNE), K-NN algorithm: Exact (vantage point tree), and gradient algorithm: Barnes–Hut.
Immunohistochemistry.
Mice were anesthetized by overdose with pentobarbital and perfused transcardially with PBS followed by 4% paraformaldehyde in 1X PBS. DRGs were dissected and fixed in 4% PFA for 2 h, followed by 30% sucrose with 0.02% azide in 1X PBS. DRGs were placed in OCT and cut on a cryostat at 10 μm, followed by mounting on SuperFrost Plus glass slides for staining. Infiltration of neutrophils into the DRG neurons in naïve, control, and primed mice was evaluated using immunohistochemical staining of S100A9 costained with NeuN, a marker for neurons. The tissue sections were blocked with 10% normal goat serum (Vector Labs S-1000) in PBSTx (0.3% Triton-X in 1×PBS). Sections were costained with a monoclonal rat anti-S100A9 (Abcam; ab105472) and recombinant rabbit anti-NeuN antibody (Abcam; ab177487) and their respective secondary antibodies (Alexa Fluor 594 anti-rabbit IgG, ThermoFisher; A11037 and Alexa Fluor 488 goat anti-rat, Abcam; ab150157). Sections were mounted and coverslipped with Vectashield Hardset Antifade Mounting Medium with DAPI (Vectorlabs; H-1500) and imaged using an Olympus fluorescent microscope. The total number of S100A9+ cell neurons was quantified in three DRG sections per slide and pooled per mouse using ImageJ. Neutrophils within 250 μm of the tissue periphery were counted by a blinded experimenter using ImageJ.
Wholemount Ex Vivo Multiphoton Microscopy.
Mice were culled with isoflurane overdose followed by immediate harvesting of lumbar DRG, which were then incubated for 4 h in 4% PFA at 4 °C followed by incubation with perm block solution overnight (20% serum, 0.5% Triton X-100 and 10 × PBS). The following day, DRGs were incubated overnight at 4 °C with primary antibodies against MRP14 (1:200; Abcam #ab105472), CD31-647 (1:200; BioLegend #102515), and NeuN (1:200; Abcam #ab177487). Anti-MRP14 and anti-NeuN were conjugated with A488 and A555, respectively, carried out using labeling kits (Thermo Fisher Scientific) according to the manufacturer’s instructions. Samples were prepared for imaging by PBS immersion and covered with a coverslip. DRGs were observed with a Leica SP8 DIVE multiphoton confocal microscope (Leica Microsystems) equipped with a 25× 1.0 NA WI IR objective lens and a pulsed infrared laser. Most experiments were performed at 795 nm and 1,045 nm (MP laser) excitation with an intensity between 17.2% and 25%, respectively. Images were acquired with a 0.53-μm z step size with approximate z depth of 130 μm. Predefined settings for laser power and detector gain (speed 8,000, pixel size 346.32 nm2) were used for all experiments. Three-dimensional images were then analyzed offline using IMARIS software (Bitplane, Switzerland), and the number of neutrophils per field of view was quantified by creating isosurfaces on the neutrophil channels.
Statistical Analysis.
All behavior, electrophysiology, and imaging studies were conducted by experimenters blinded to the treatment. Statistical analyses were performed using Prism9 and R software. Behavior studies were analyzed using two-way ANOVA with repeated measures and Bonferroni posthoc tests to compare experimental groups. Unpaired t tests were used for electrophysiology data and FACS events comparing experimental groups. Immunohistochemistry analyses involved a minimum of 3 DRG sections from at least three animals per experimental group using a one-way ANOVA.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work was supported by Versus Arthritis UK (21734), Barts Charity (MGU0532), Wellcome Trust, Rosetrees Trust, and FOREUM (1016807).
Author contributions
S.C., S.Y., D.G., M.-B.V., J.N.W., and S.S. designed research; S.C., S.B., A.M.F., R.E., B.T., P.C., S.Y., and S.S. performed research; F.D., M.-B.V., and S.S. contributed new reagents/analytic tools; S.C., S.B., A.M.F., R.E., and S.S. analyzed data; and S.C., J.N.W., and S.S. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
John N. Wood, Email: j.wood@ucl.ac.uk.
Shafaq Sikandar, Email: s.sikandar@qmul.ac.uk.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Mezhov V., Guymer E., Littlejohn G., Central sensitivity and fibromyalgia. Intern. Med. J. 51, 1990–1998 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Affaitati G., et al. , Effects of treatment of peripheral pain generators in fibromyalgia patients. Eur. J. Pain 15, 61–69 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Serra J., et al. , Hyperexcitable C nociceptors in fibromyalgia. Ann. Neurol. 75, 196–208 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Üçeyler N., et al. , Small fibre pathology in patients with fibromyalgia syndrome. Brain 136, 1857–1867 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Bote M. E., Garcia J. J., Hinchado M. D., Ortega E., Fibromyalgia: Anti-inflammatory and stress responses after acute moderate exercise. PLoS One 8, e74524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bote M. E., García J. J., Hinchado M. D., Ortega E., Inflammatory/stress feedback dysregulation in women with fibromyalgia. Neuroimmunomodulation 19, 343–351 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Aktürk S., Büyükavcı R., Evaluation of blood neutrophil-lymphocyte ratio and platelet distribution width as inflammatory markers in patients with fibromyalgia. Clin. Rheumatol. 36, 1885–1889 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Garcia J. J., Cidoncha A., Bote M. E., Hinchado M. D., Ortega E., Altered profile of chemokines in fibromyalgia patients. Ann. Clin. Biochem. 51, 576–581 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Bazzichi L., et al. , Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin. Exp. Rheumatol. 25, 225–230 (2007). [PubMed] [Google Scholar]
- 10.Wallace D. J., et al. , Cytokines play an aetiopathogenetic role in fibromyalgia: A hypothesis and pilot study. Rheumatology (Oxford). 40, 743–749 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Kiguchi N., Kobayashi D., Saika F., Matsuzaki S., Kishioka S., Pharmacological regulation of neuropathic pain driven by inflammatory macrophages. Int. J. Mol. Sci. 18, 2296 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Vlist M., et al. , Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron. 110, 613–626 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Laumet G., Edralin J. D., Dantzer R., Heijnen C. J., Kavelaars A., CD3(+) T cells are critical for the resolution of comorbid inflammatory pain and depression-like behavior. Neurobiol. Pain. 7, 100043 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vicuna L., et al. , The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat. Med. 21, 518–523 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riol-Blanco L., et al. , Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510, 157–161 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim C. F., Moalem-Taylor G., Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res. 1405, 95–108 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Morin N., et al. , Neutrophils invade lumbar dorsal root ganglia after chronic constriction injury of the sciatic nerve. J. Neuroimmunol. 184, 164–171 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Agarwal N., et al. , Evoked hypoalgesia is accompanied by tonic pain and immune cell infiltration in the dorsal root ganglia at late stages of diabetic neuropathy in mice. Mol. Pain 14, 1744806918817975 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khaw Y. M., Cunningham C., Tierney A., Sivaguru M., Inoue M., Neutrophil-selective deletion of Cxcr2 protects against CNS neurodegeneration in a mouse model of multiple sclerosis. J. Neuroinflammation 17, 49 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph E. K., Parada C. A., Levine J. D., Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain 105, 143–150 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Moy J. K., et al. , Temporal and sex differences in the role of BDNF/TrkB signaling in hyperalgesic priming in mice and rats. Neurobiol. Pain 5, 100024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandhi R., Ryals J. M., Wright D. E., Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J. Neurosci. 24, 9405–9413 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgos-Vega C. C., Quigley L. D., Avona A., Price T., Dussor G., Dural stimulation in rats causes brain-derived neurotrophic factor-dependent priming to subthreshold stimuli including a migraine trigger. Pain 157, 2722–2730 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy J. D., Wolff H. G., Goodell H., Pain Sensations and Reactions (Williams & Wilkins, 1952). [Google Scholar]
- 25.Simone D. A., et al. , Neurogenic hyperalgesia: Central neural correlates in responses of spinothalamic tract neurons. J. Neurophysiol. 66, 228–246 (1991). [DOI] [PubMed] [Google Scholar]
- 26.Radhakrishnan R., Moore S. A., Sluka K. A., Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain 104, 567–577 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sluka K. A., Kalra A., Moore S. A., Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 24, 37–46 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Vadakkan K. I., et al. , Genetic reduction of chronic muscle pain in mice lacking calcium/calmodulin-stimulated adenylyl cyclases. Mol. Pain 2, 7 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tillu D. V., Gebhart G. F., Sluka K. A., Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain 136, 331–339 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokoyama T., Maeda Y., Audette K. M., Sluka K. A., Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J. Pain 8, 422–429 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Miranda A., Peles S., Rudolph C., Shaker R., Sengupta J. N., Altered visceral sensation in response to somatic pain in the rat. Gastroenterology 126, 1082–1089 (2004). [DOI] [PubMed] [Google Scholar]
- 32.DeSantana J. M., da Cruz K. M., Sluka K. A., Animal models of fibromyalgia. Arthritis Res. Ther. 15, 222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhl B., et al. , Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood 128, 2327–2337 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford J., Dale D. C., Lyman G. H., Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer 100, 228–237 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Rittner H. L., et al. , Antinociception by neutrophil-derived opioid peptides in noninflamed tissue–role of hypertonicity and the perineurium. Brain Behav. Immun. 23, 548–557 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Rittner H. L., et al. , Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils. PLoS Pathog. 5, e1000362 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones H. R., Robb C. T., Perretti M., Rossi A. G., The role of neutrophils in inflammation resolution. Semin. Immunol. 28, 137–145 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Parisien M., et al. , Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci. Transl. Med. 14, eabj9954 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalli J., Chiang N., Serhan C. N., Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 21, 1071–1075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perretti M., et al. , Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat. Med. 2, 1259–1262 (1996). [DOI] [PubMed] [Google Scholar]
- 41.Weissmann G., Samuelsson B., Paoletti R., “Cell migration and hyperalgesia: A paradoxical effect of endotoxin” in Advances in Inflammation Research, International Congress of Inflamation 1978: Bologna, Italy, Eds. (Raven Press, 1979). [Google Scholar]
- 42.Levine J. D., Lau W., Kwiat G., Goetzl E. J., Leukotriene B4 produces hyperalgesia that is dependent on polymorphonuclear leukocytes. Science 225, 743–745 (1984). [DOI] [PubMed] [Google Scholar]
- 43.Levine J. D., Gooding J., Donatoni P., Borden L., Goetzl E. J., The role of the polymorphonuclear leukocyte in hyperalgesia. J. Neurosci. 5, 3025–3029 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett G., Al-Rashed S., Hoult S. J. R., Brain D. S., Nerve growth factor induced hyperalgesia in the rat hind paw is dependent on circulating neutrophils. Pain 77, 315–322 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Cunha T. M., et al. , Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J. Leukoc. Biol. 83, 824–832 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Czepielewski R. S., et al. , Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils. Proc. Natl. Acad. Sci. U.S.A. 109, 547–552 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawes J. M., et al. , CXCL5 mediates UVB irradiation-induced pain. Sci. Transl. Med. 3, 90ra60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goebel A., et al. , Passive transfer of fibromyalgia symptoms from patients to mice. J. Clin. Invest. 131, e144201 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bisgaard H., Kristensen J. K., Leukotriene B4 produces hyperalgesia in humans. Prostaglandins 30, 791–797 (1985). [DOI] [PubMed] [Google Scholar]
- 50.Jones A. K., et al. , In vivo leukocyte migration in arthritis. Arthritis Rheum. 34, 270–275 (1991). [DOI] [PubMed] [Google Scholar]
- 51.Wolfe F., Walitt B., Perrot S., Rasker J. J., Häuser W., Fibromyalgia diagnosis and biased assessment: Sex, prevalence and bias. PLoS One 13, e0203755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quevedo A. S., Mørch C. D., Andersen O. K., Coghill R. C., Lateral inhibition during nociceptive processing. Pain 158, 1046–1052 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takazawa T., MacDermott A. B., Synaptic pathways and inhibitory gates in the spinal cord dorsal horn. Ann. N. Y. Acad. Sci. 1198, 153–158 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.François A., et al. , A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and Enkephalins. Neuron 93, 822–839.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavich T. R., et al. , Neutrophil infiltration is implicated in the sustained thermal hyperalgesic response evoked by allergen provocation in actively sensitized rats. Pain 125, 180–187 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Perkins N. M., Tracey D. J., Hyperalgesia due to nerve injury: Role of neutrophils. Neuroscience 101, 745–757 (2000). [DOI] [PubMed] [Google Scholar]
- 57.Shaw S. K., et al. , Activated polymorphonuclear cells promote injury and excitability of dorsal root ganglia neurons. Exp. Neurol. 210, 286–294 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bali K. K., Kuner R., Therapeutic potential for leukocyte elastase in chronic pain states harboring a neuropathic component. Pain 158, 2243–2258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strausbaugh H. J., et al. , Painful stimulation suppresses joint inflammation by inducing shedding of L-selectin from neutrophils. Nat. Med. 5, 1057–1061 (1999). [DOI] [PubMed] [Google Scholar]
- 60.Silvestre-Roig C., Hidalgo A., Soehnlein O., Neutrophil heterogeneity: Implications for homeostasis and pathogenesis. Blood 127, 2173–2181 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Macedo J. A., et al. , Adhesion molecules and cytokine expression in fibromyalgia patients: Increased L-selectin on monocytes and neutrophils. J. Neuroimmunol. 188, 159–166 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Zhang J., Harada Y., Hayashi Y., A TLR-CXCL1 pathway in DRG neurons induces neutrophil accumulation in the DRG and mechanical allodynia in EAE mice. Sci. Rep. 9, 12003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harada Y., et al. , Cathepsin E in neutrophils contributes to the generation of neuropathic pain in experimental autoimmune encephalomyelitis. Pain 160, 2050–2062 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White D. M., Basbaum A. I., Goetzl E. J., Levine J. D., The 15-lipoxygenase product, 8R,15S-diHETE, stereospecifically sensitizes C-fiber mechanoheat nociceptors in hairy skin of rat. J. Neurophysiol. 63, 966–970 (1990). [DOI] [PubMed] [Google Scholar]
- 65.Harkema J. R., Hotchkiss J. A., Harmsen A. G., Henderson R. F., In vivo effects of transient neutrophil influx on nasal respiratory epithelial mucosubstances. Quantitative histochemistry. Am. J. Pathol. 130, 605–615 (1988). [PMC free article] [PubMed] [Google Scholar]
- 66.Fasano M. B., Wells J. D., McCall C. E., Human neutrophils express the prostaglandin G/H synthase 2 gene when stimulated with bacterial lipopolysaccharide. Clin. Immunol. Immunopathol. 87, 304–308 (1998). [DOI] [PubMed] [Google Scholar]
- 67.Schuligoi R., Effect of colchicine on nerve growth factor-induced leukocyte accumulation and thermal hyperalgesia in the rat. Naunyn Schmiedebergs Arch. Pharmacol. 358, 264–269 (1998). [DOI] [PubMed] [Google Scholar]
- 68.Pillay J., et al. , In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 116, 625–627 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Tak T., Tesselaar K., Pillay J., Borghans J. A., Koenderman L., What’s your age again? Determination of human neutrophil half-lives revisited. J. Leukoc Biol. 94, 595–601 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Sikandar S., et al. , Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain 141, 1028–1039 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salaffi F., et al. , Diagnosis of fibromyalgia: Comparison of the 2011/2016 ACR and AAPT criteria and validation of the modified fibromyalgia assessment status. Rheumatology (Oxford). 59, 3042–3049 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.


