Abstract
Yeast (Y) to germ-tube (GT) transition of Candida albicans is considered a putative virulence trait. On the other hand, interleukin-12 (IL-12) is a key promoter of T-helper type 1 protective immunity against this human opportunistic pathogen. We studied IL-12 production by human monocytes cocultured in vitro with Y or GT forms of C. albicans. Following stimulation by Y cells, monocytes produced appreciable levels of IL-12, which, upon addition of gamma interferon (IFN-γ), compared to those achievable by lipopolysaccharide (100 ng/ml) stimulation (140 ± 65 and 185 ± 80 pg/ml, respectively [mean ± standard deviation in four independent experiments]). In contrast, IL-12 production by GT cell-stimulated monocytes was much lower or absent (<5 pg/ml) and could not be brought to the level induced by Y cells by the addition of IFN-γ (30 ± 10 pg/ml in the four independent experiments above). Besides being observed as actual cytokine production, this lower response was also observed as specific IL-12 p40 mRNA transcript and was not associated with hyperproduction of the IL-12-competing cytokine IL-10. Phagocytosis and killing experiments in the presence of cytochalasin D showed that IL-12 production by Y cell-stimulated monocytes was phagocytosis dependent and that GT cells of C. albicans were not phagocytized by the human monocytes. Importantly, however, Y and GT cells were equally killed by the monocytes. Thus, the virulence trait attributed to the Y-GT transition of C. albicans might also be related to the lack of induction by GT cells of a protective anticandidal immunity through defective IL-12 production.
Several studies have provided convincing evidence that the induction of a T-helper type 1 (Th1) response during host-parasite relationship is critically regulated by interleukin-12 (IL-12), a heterodimeric cytokine produced by a variety of cells involved in antigen presentation, phagocytosis, and overall host defense against microbial invaders (2, 27, 29). There is also a rather wide consensus that Th1 response plays a critical role in the protection against Candida albicans, a major cause not only of severe opportunistic infections in immunocompromised patients but also of largely prevalent mucosal infections in otherwise healthy subjects (18). Overall, IL-12 has been considered a pivotal cytokine orchestrating anticandidal defense at both mucosal and systemic levels (7, 13–15, 22, 25, 26).
On the other hand, infections by C. albicans are facilitated by the expression of virulence traits enabling this fungus to somewhat evade or divert immune response (9). One of these factors is C. albicans's capacity of converting from a unicellular yeast (Y) to a filamentous habit of growth giving rise to true hyphal cells. Germ-tube (GT) formation is the critical step in this conversion (18). Importantly, GT formation promptly occurs in biological fluids so as to be prevalently found in infection, as opposed to the Y cells which are more commonly found in the saprophytic or commensal state (18). GT cells are phagocytized much less efficiently than Y cells by normal phagocytic effectors (31). They also express neoantigens on the cell surface, with loss of some dominant and protective immunodeterminants, which are expressed on Y surface (3, 4, 17, 20), a phenomenon which is readily appreciated in vivo (11).
Because of the critical role of IL-12 in the anticandidal response and the supposed immunoevasion properties of GT cells of C. albicans, as outlined above, we have investigated the production of this cytokine by human monocytes, a primary source of IL-12, upon stimulation by Y and GT forms of a virulent C. albicans strain in comparison with an avirulent, non-GT-forming strain of the fungus. We also examined whether IL-12 production was associated with phagocytosis and killing of the two forms of growth of the fungus by the activated human cells.
MATERIALS AND METHODS
Monocyte cultures and cytokine assays.
Human monocytes were obtained from leukocyte buffy coats, diluted in RPMI 1640 (Gibco-BRL, Grand Island, N.Y.) and separated by density gradient centrifugation on Lympholyte-H. Monocytes in the mononuclear cell population were let to adhere to plastic culture plates for 1 h at 37°C, under a 5% CO2 atmosphere in RPMI 1640 supplemented with 10% fetal calf serum, 2 mmol of l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin (Gibco-BRL) per ml (hereafter referred to as complete medium [CM]). After removal of nonadherent cells, monocytes were recovered by gentle scraping, resuspended at 106/ml in CM, and cultured in multiwell microplates with or without Candida cells, gamma interferon (IFN-γ) (R&D Systems, Minneapolis, Minn.), or cytochalasin D (Sigma Chemical Co., St. Louis, Mo.), as specified for each single experiment. Cultures were incubated 18 h at 37°C under 5% CO2. Cytokine production was evaluated by assaying culture supernatants by indirect enzyme-linked immunosorbent assay (ELISA) (Endogen Inc., Woburn, Mass.). In particular, for IL-12 measurements, two different ELISAs were employed, one selectively recognizing only the p70 bioactive heterodimer and one measuring both p70 and the free p40 monomer of the cytokine.
Fungal cells.
Both live and heat-inactivated Y and GT cells were used throughout this study. Fungal cells were obtained either from strain BP of C. albicans, which is virulent and competent for GT formation, or from another strain (CA2), which is a low-virulence mutant incapable of GT conversion (24, 25). Live Y cells of both strains of C. albicans were grown for 18 h in Winge broth at 28°C. Cells were harvested by centrifugation, extensively washed with phosphate-buffered saline (PBS), resuspended at the desired concentration in RPMI, and administered to freshly isolated monocyte cultures for IL-12 stimulation. During cocultivation with monocytes in CM at 37°C, more than 90% of the cells of the germinative strain BP developed, within 60 to 90 min, GT forms, i.e., hyphal filaments emerging from Y cells and 3 to 10 times as long as the Y cells of origin, growing by apical elongation. Under the same conditions, the agerminative strain CA2 grew by budding in a stable Y form.
For a direct comparison of Y and GT forms for IL-12 induction, Y cells were differentiated in GT-inducing medium as reported elsewhere (4). Briefly, washed Y cells as described above were resuspended at 2 × 106 cells/ml in Lee's medium and incubated at 28 or 37°C for 90 min. At 28°C, both Candida strains maintained the Y form in this medium. At 37°C strain BP, within 90 min of incubation, differentiated >90% GT cells, as defined above, while strain CA2 maintained its Y form. Y and GT cells were harvested by centrifugation, washed with H2O, and resuspended in PBS at the desired concentration. For heat inactivation, Y or GT cells prepared as above were resuspended in H2O and treated at 70°C for 40 min. After inactivation fungal cells were extensively washed and brought to the desired cell density in PBS.
Phagocytosis and killing assays.
To measure phagocytosis, we used a short-term 3H-glucose uptake inhibition assay based on the principle that free Candida cells efficiently take up the radiolabeled sugar while monocyte-ingested Candida cells do not. In these assays, monocytes (5 × 106/ml) were cocultured in microplates with live Y or GT cells (from the CA2 or BP strain, respectively) at various monocyte/fungal cell ratios, in the presence or in the absence of cytochalasin D (Sigma). Wells containing Candida Y or GT cells alone were also included in the experiment as controls. Each condition was assayed in triplicate. Cocultures were incubated 30 min at 37°C under 5% CO2, after which 0.5 μCi of 3H-glucose (specific activity, 50 Ci/mmol; Amersham-Pharmacia, Little Chalfont, United Kingdom) was added to the wells and the plates were incubated for an additional 1.5 h. Monocytes were lysed with Triton X-100 (0.2%; Sigma), and fungal cells were harvested from the plates and counted in a β-counter. Percent phagocytosis was calculated by comparing the radiolabel incorporation by fungal cells cocultured with monocytes with incorporation by control fungal cells in the absence of monocytes. Label incorporation by monocytes alone was always <100 cpm.
Alternatively, monocytes (5 × 106/ml) were cultured for 4 h at 37°C under 5% CO2 with heat-inactivated Y or GT cells from C. albicans strain BP at a monocyte/Candida ratio of 1:1. Samples of the cultures were smeared onto silanized microscope slides and fixed 30 min at room temperature with 4% formaldehyde in PBS. Slides were washed with absolute ethanol and stained by the periodic acid-Schiff technique for observation of fungal cell phagocytosis. Monocytes were counterstained with 1:10 diluted trypan blue. Slides were examined under a Leitz Diaplan microscope.
Killing of Y or GT cells by monocytes was evaluated by cocolturing monocytes (2 × 106/ml) with Y or GT cells at final monocyte-to-Candida ratios of 10:1 and 20:1, in the presence or absence of cytochalasin D (1 μg/ml). Triplicate samples of cocultures and control Candida cultures without monocytes were incubated 4 h at 37°C under 5% CO2. Monocytes were finally lysed by addition of Triton X-100 (0.2%), and residual live fungal cells were enumerated by CFU counts in duplicate Sabouraud agar plates. Percent killing was calculated by comparison of CFU counts from Candida-monocyte cocultures and CFU counts from control cultures of Candida cells alone.
RT-PCR analysis of cytokine gene expression.
The reverse transcriptase PCR (RT-PCR) analysis of cytokine gene expression was carried out as described elsewhere (30). Aliquots of cDNA yielding equivalent amounts of β-actin amplified band were used for the semiquantitative evaluation of cytokine mRNAs. PCR was performed in a 10-μl volume in a Perkin-Elmer 9600 thermal cycler running for 25 (for β-actin), 30 (for IL-10 and tumor necrosis factor alpha [TNF-α]), or 40 (for IL-12 p40) cycles of 1-min denaturation at 94°C; 40-s annealing at 62°C, and 1-min extension at 72°C. Cytokine-specific primer pairs were synthesized by Gibco-BRL according to published sequences (1, 12). The PCR products were visualized by electrophoresis and ethidium bromide staining, identified by their predicted molecular sizes, and quantitatively evaluated by densitometric scanning.
Statistical analysis.
Data were evaluated by the nonparametric Mann-Whitney U test or by the parametric Student test, as indicated.
RESULTS
Differential stimulation of IL-12 production by Y and GT forms of C. albicans.
To examine the production of bioactive IL-12 p70 by human monocytes following stimulation with Y or GT forms of C. albicans, we performed four independent experiments with freshly isolated human monocytes, incubated with live or heat-inactivated Y or GT cells of the fungus, in the presence or absence of IFN-γ. Data from preliminary experiments (not shown) served to establish the optimal effector/target ratio and time of incubation for optimal cytokine production. Live fungal cells developing GT forms (>90% within 60 to 90 min) in monocyte-Candida cocultures were obtained from the virulent, GT-competent strain BP. Live, Y form-growing cells not developing GT were obtained from the low-virulence, nongerminative strain CA2. Since during cocultivation of monocytes with live fungal cells uncontrolled alterations of monocyte/Candida ratio and fungal growth-related toxicity could occur, we also used predifferentiated and heat-inactivated Y cells (from the BP and CA2 strains) and GT cells (from the BP strain) as IL-12 stimulants. This experimental approach also allowed us to detect any potential strain-dependent difference in the IL-12 response.
As shown in Table 1, monocytes alone were substantially unable to produce appreciable quantities of IL-12, even in the presence of IFN-γ. However, their incubation with heat-inactivated Y cells from both strains of C. albicans, but not with inactivated GT cells, greatly promoted heterodimer production, which, in three of the four experiments, reached values above 100 pg/ml in the presence of IFN-γ, which is comparable to the IL-12 production seen for some donors by such a potent stimulation as that achievable with 100 ng of lipopolysaccharide (LPS) per ml. However, if Y cells of the virulent, germinative strain were added in their viable state to the phagocyte cultures, in which they promptly (60 to 90 min) differentiate to GT cells, IL-12 production was much lower, even in the presence of IFN-γ. This event clearly paralleled the lower cytokine production following stimulation by the inactivated, predifferentiated GT cells (Table 1).
TABLE 1.
Efficacy of Y and GT Cells of C. albicans in triggering IL-12 p70 production by human monocyte cultures
| Stimulanta | IL-12 p70
production (pg/ml)b by donor:
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| None | <5 | <5 | <5 | <5 |
| IFN-γ only | 5 | <5 | 7 | 8 |
| Live Y cells, germinative strain | 5 | <5 | <5 | NDc |
| Live Y cells, agerminative strain | 12 | 8 | 15 | ND |
| Heat-inactivated Y cells, germinative strain | 13 | 36 | 40 | 51 |
| Heat-inactivated Y cells, agerminative strain | 10 | 45 | ND | ND |
| Heat-inactivated GT cells, germinative strain | 6 | 7 | 7 | <5 |
| Live Y cells, germinative strain, plus IFN-γ | 35 | 10 | 15 | ND |
| Live Y cells, agerminative strain, plus IFN-γ | 110 | 25 | 50 | ND |
| Heat-inactivated Y cells, germinative strain, plus IFN-γ | 125 | 130 | 75 | 230 |
| Heat-inactivated Y cells, agerminative strain, plus IFN-γ | 140 | 155 | ND | ND |
| Heat-inactivated GT cells, germinative strain, plus IFN-γ | 36 | 40 | 31 | 15 |
| LPS plus IFN-γ | 210 | 95 | ND | 250 |
Monocytes from four different donors were stimulated with live Y cells (monocyte/Candida ratio = 1:0.5) or with heat-inactivated Y or GT cells (monocyte/Candida ratio = 1:2) from the germinative (BP) or the agerminative (CA2) strain of C. albicans. LPS was used at 100 ng/ml. When indicated, IFN-γ (100 U/ml) was also added to the monocyte cultures.
All the differences of IL-12 production between monocytes stimulated with Y cells and GT or GT-forming cells were statistically significant (P < 0.005) as assessed by the Mann-Whitney U test (two tailed).
ND, not done.
Conversely, the quantity of IL-12 heterodimer produced upon monocyte stimulation by Y cells from the avirulent, non-GT-forming strain of C. albicans was detectable, even in the viable state, and was clearly superior to that produced upon stimulation by viable cells of the virulent, GT-forming strain, with or without IFN-γ as a costimulator (Table 1). Differences between the levels of IL-12 produced by stimulation with live Y cells of the two different Candida strains were highly significant (P < 0.01, Mann-Whitney U test), in parallel with the statistically significant difference between the levels of IL-12 stimulated by inactivated Y and GT cells of the germinative strain BP. On the contrary, no statistically significant difference in IL-12 production was found between inactivated Y cells of the germinative and the agerminative strains (Table 1).
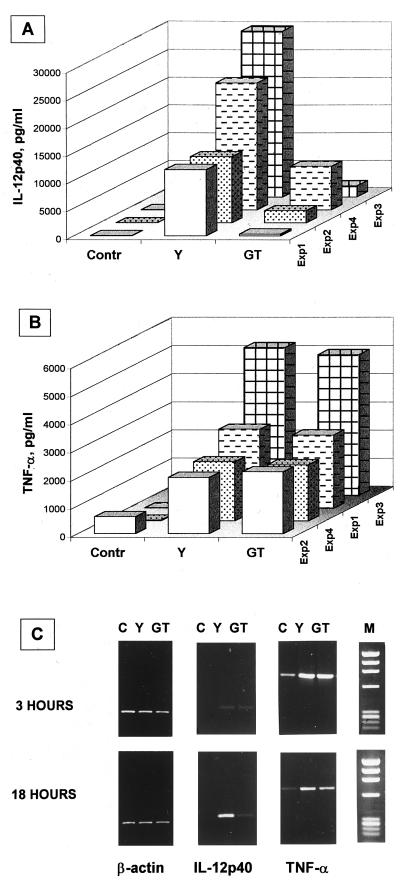
IL-12 production was also assessed as cytokine gene expression. As shown in Fig. 1, the inability of GT cells to induce IL-12 p70 clearly paralleled a similar inability to stimulate the expression of the IL-12 p40 gene transcript (Fig. 1C) and actual subunit production (Fig. 1A). As a control, Y and GT cells were equally able to trigger gene transcription and actual production of TNF-α (Fig. 1B and C), indicating that the lower amount of IL-12 produced under GT cell stimulation was specific and was not due to some adsorption of the protein to GT cells or its increased degradation.
FIG. 1.
Gene expression and actual production of IL-12 p40 subunit and TNF-α by human monocytes stimulated with Y or GT cells of C. albicans. (A and B) Production of IL-12 p40 subunit and TNF-α by Y- or GT-stimulated monocytes. Monocytes (106/ml) from four different donors were cultured in four different experiments (Exp1 to Exp4) in the presence of heat-inactivated Y or GT cells of the germinative strain BP of C. albicans, at a monocyte/Candida ratio of 1:2, or in medium only as a control (Contr). Production of IL-12 p40 (A) and TNF-α (B) was evaluated by ELISA after 18 h of culture. (C) Analysis of IL-12 p40 and TNF-α mRNA expression in Candida-stimulated monocytes. Monocytes (5 × 106) were either unstimulated (C) or stimulated with Y cells (Y) or GT cells (GT) of C. albicans, as described above. After 3 or 18 h, as indicated, IL-12 p40 and TNF-α gene expression was evaluated by semiquantitative RT-PCR analysis, as described in the text. Lane M, molecular weight markers. A statistically significant difference (P < 0.01 [Mann-Whitney U test]) was measured between Il-12 p40 production by monocytes stimulated with Y cells and those stimulated with GT cells. Differences of TNF-α production were not statistically significant.
Recognizing that IL-12 expression is regulated by other cytokines, among which IL-10 plays a prominent role (10), we also measured gene expression and actual production of IL-10 upon stimulation of monocytes with the two different forms of growth of the fungus. Both Y and GT cells induced a significant IL-10 gene transcription and actual cytokine production, and this was clearly downregulated in the presence of IFN-γ (Fig. 2). High-level IL-12 production, mediated by IFN-γ addition, was indeed coupled with significantly reduced IL-10 production, suggesting a complex interplay of these three cytokines in the monocyte response to Candida.
FIG. 2.
Comparison of IL-10 and IL-12 p70 production and IL-10 gene expression by human monocytes cocultured with Y or GT cells of C. albicans. (A) IL-10 and IL-12 p70 production upon stimulation with Y or GT cells of C. albicans. Monocytes were cultured for 18 h in medium only (Control) or in the presence of heat-inactivated Y or GT cells of C. albicans strain BP (monocyte/Candida ratio = 1:2). Where indicated, IFN-γ (100 U/ml) was added to the cultures as an IL-12 costimulant. IL-10 and IL-12 p70 production was evaluated by ELISA. (B) IL-10 gene expression in Candida-stimulated monocytes. Unstimulated control (C), Y cell-stimulated (Y), or GT cell-stimulated (GT) monocytes were analyzed, at the indicated times, for IL-10 mRNA expression by semiquantitative RT-PCR, as described in the text. Lane M, molecular weight markers. Data are from one representative experiment out of two performed with similar results.
Phagocytosis and killing of C. albicans by monocytes and relationship with IL-12 induction.
Since Y cells of C. albicans are easily phagocytized by human monocytes while GT cells are not (31), and knowing the importance of phagocytosis for non-T-cell-dependent, early IL-12 production in other host-parasite interactions (12), we also asked whether differences in phagocytosis might indeed account for differential cytokine production. In addition, we investigated whether there was any relationship between IL-12 production and the phagocytes' ability to kill the two forms of growth of Candida.
As shown in Fig. 3A and 4, the human monocytes were much less able to phagocytize the GT cells than the Y cells of C. albicans. CFU enumeration showed, however, that the monocytes were equally capable of killing the two forms of growth (Fig. 3C). The addition of cytochalasin D to the monocyte cultures dose dependently inhibited phagocytosis of Y cells (from 70 to 0% in the representative experiment shown in Fig. 3B [also Fig. 4B]). This drug also inhibited the killing of Y cells but not that of the GT cells (Fig. 3C), which thus predominantly occurred by phagocytosis-independent, extracellular mechanisms (Fig. 3C). No difference was found in the extent of phagocytosis of heat-inactivated Y cells of the germinative and the agerminative strains (data not shown).
FIG. 3.
Phagocytosis and killing of Y ░⃞ and GT ▩ cells of C. albicans by human monocytes: effect of cytochalasin D. (A) Monocytes (5 × 106/ml) were cocultured with live Y or GT cells of C. albicans at the indicated monocyte/Candida (E:T) ratios. Phagocytosis was evaluated after 2 h by a 3H-glucose uptake inhibition assay, as described in Materials and Methods. ∗, P < 0.01; ∗∗, P < 0.05 (both determined by Student's t test, two tailed [comparing percent phagocytosis of Y and GT cells]). (B) Effect of cytochalasin D on the phagocytosis of Y cells by monocytes. Cytochalasin D (ctcl D), at the indicated doses, was added to monocyte cultures 30 min before Candida cells (E:T ratio = 1:1), and phagocytosis was measured as described in the legend to Fig. 3A. No ctclD, control culture (not treated with the drug). ∗, P < 0.01; ∗∗, P < 0.05 (both determined by Student's t test [comparing values measured in the absence of cytochalasin D]). (C) Killing of Y cells and GT cells by monocytes and effect of phagocytosis blockade with cytochalasin D. Monocytes (2 × 106/ml) were cultured for 4 h with Y cells or GT of C. albicans, at the indicated monocyte/Candida (E:T) ratios, in the presence or absence of cytochalasin D (1 μg/ml). Killing activity was evaluated by Candida CFU counts, as reported in Materials and Methods. ∗, P < 0.01 (Student's t test) as compared to killing values measured in the absence of cytochalasin D. Differences of killing activity against Y and GT cells were not significant. Values are means ± standard deviations (error bars) of three independent determinations.
FIG. 4.
Microscopic evaluation of phagocytosis of Y and GT cells of C. albicans by human monocytes. Monocytes (5 × 106/ml) were cultured for 4 h at 37°C under 5% CO2, with heat-inactivated Y or GT cells of C. albicans strain BP (5 × 106/ml). Samples of the cocultures were smeared onto silanized microscope slides, fixed, and periodic acid-Schiff stained. (A) Y cells ingested by monocytes; (B) Y cells uningested by monocytes in the presence of cytochalasin D (1 mg/ml); (C) GT cells uningested by monocytes. Magnification for all panels, ×400.
When cytochalasin D was used in experiments of IL-12 production upon stimulation by Y cells, at the concentration totally inhibiting Y cell phagocytosis, IL-12 heterodimer production was also practically abolished. This was seen to occur through inhibition of p40 monomer production (Table 2). Cytochalasin D did not cause any inhibition of IL-12 p40 or p70 production by LPS-stimulated monocytes, which is known to be independent of phagocytosis, attesting to the specificity of the action exerted by the phagocytosis inhibitor for the Y Candida cell-induced IL-12 production (Table 2).
TABLE 2.
Effect of cytochalasin D on IL-12 p70 and IL-12 p40 production by monocyte cultures stimulated with Candida cells or LPSa
| Stimulus | Production (pg/ml)
|
|||
|---|---|---|---|---|
| Donor
1
|
Donor 2
|
|||
| IL-12 p70 | IL-12 p40 | IL-12 p70 | IL-12 p40 | |
| None | 8 | 30 | 5 | 20 |
| Ctcl Db | 5 | 22 | <5 | 15 |
| Y cells | 230 | 12,000 | 290 | 13,700 |
| Y cells plus ctcl D | 14 | 300 | 25 | 220 |
| LPS | 250 | 13,500 | ND | 11,000 |
| LPS plus ctcl D | 200 | 10,000 | ND | 9,600 |
Monocytes from two different donors were stimulated with heat-inactivated Y cells of C. albicans strain BP at a Candida/monocyte ratio of 2:1, or with LPS (100 ng/ml) in the presence of IFN-γ (100 U/ml).
Ctcl D, cytochalasin D (1 μg/ml) was added to monocyte cultures 30 min before Y cells or LPS.
DISCUSSION
In this study we have shown that the GT forms of C. albicans are significantly less efficient than the Y forms in stimulating human monocytes to release IL-12, a key cytokine in the development of protective anti-Candida immunity. We measured the in vitro IL-12 production by human monocytes following stimulation with predifferentiated and heat-inactivated Y or GT cells of the fungus or with live cells from a virulent, GT-forming strain and compared the results to those for live cells of an avirulent, nongerminative strain. The results of these experiments demonstrated that the virulent germinative strain, in its viable state, was substantially unable to induce IL-12 production by the monocytes and that this inability could be only partially reversed by overstimulation with IFN-γ. The experiments also indicated that transition to the GT form was the cause of the lower efficiency of the virulent strain in stimulating IL-12 production, as heat-inactivated Y cells, but not the inactivated GT cells, of this virulent strain stimulated the monocytes to produce quantities of IL-12 comparable to those detected upon stimulation with the corresponding Y form of the avirulent, non-GT-forming strain.
Our observations appear to be of some importance for the supposed role of GT cells in C. albicans pathogenicity and immune response. IL-12 is a key promoter of protective immunity against this pathogen. Endogenous IL-12 production is a critical requirement for the development of a Th1, protective response in mice with candidiasis, and even its suboptimal production has been clearly linked to a nonhealing disease (22, 24, 25). On the other hand, GT formation is a key feature of the interaction of this fungus with its host. During this process, most of the host-impacting cell surface molecules of C. albicans are modified and consistent rearrangements of the fungal antigenic makeup, inclusive of antigens with protective value, are observed (3, 4, 16, 17). Mutation of genes involved in or regulating GT transition have been shown to obviously affect fungal virulence (8). Data presented in this paper suggest that the greater virulence attributed to the Y-GT transition of C. albicans might also be due, at least in part, to deficient induction, by GT cells, of protection-associated cytokine responses by the host. Importantly, GT cells proved to be suboptimal stimulators of IL-12 production even in the presence of IFN-γ, a Th1 cytokine that appears to be important not only in stimulating IL-12 production but also in maintaining responsiveness to this cytokine (6). We have recently observed that GT cells were also much less efficient than Y cells in stimulating chemokine (in particular MCP-1) production by human monocytes and neutrophils (27a). The two processes could evidently synergize in vivo, as the low IL-12 production by the monocytes encountering the GT cells could match the suboptimal influx of those natural immunoeffectors which are devoted, among other things, to an early production of IL-12 in the absence of activated T cells (23, 30).
The lower IL-12 response generated by GT cells might be due, in principle, to selective stimulation of IL-10, a major IL-12-regulating cytokine with anti-inflammatory and Th2 differentiation-promoting activity. This was not the case, since GT cells did not significantly differ from Y cells in their IL-10 stimulation ability. Since IL-10 has been recognized as a potent downregulator of the anti-Candida functions of human phagocytes (21), this observation is also in line with the preserved killing activity exerted by monocytes against the GT forms of C. albicans. Nevertheless, if the balance of IL-10 and IL-12 production is considered, it follows that while Y cell forms induce both cytokines, GT cells predominantly induce IL-10, a cytokine which has been associated with nonhealing disease in mice with candidosis (23). Although preliminary, these observations suggest that unbalanced IL-12 versus IL-10 induction might be an additional feature of GT forms of C. albicans, leading to increased pathogenicity and evasion from host protective immunity. They are also in keeping with the different IL-12 and IL-10 responses associated with spontaneously healing or nonhealing disseminated murine infections by avirulent, agerminative or virulent, germinative C. albicans strains, respectively (23). Additional in vivo studies are, however, necessary to assess the precise role of the different cytokine response induced by the two forms of growth of C. albicans in modifying fungal pathogenicity and the final outcome of the infection.
A clear relationship has emerged from our study between the lower efficiency of GT cells in IL-12 induction and the monocyte inability to ingest this form of growth of the fungus. This apparent association has been reinforced by the observation that Y cell-stimulated IL-12 production is totally suppressed by the phagocytosis inhibitor cytochalasin D. In this context, the process of phagocytosis seems to be a major signal for Candida-mediated IL-12 induction, as also suggested for other microorganisms by some authors (12).
Recently, Pitzurra et al. (19) have shown that an early, non-T-cell-dependent IL-12 production by human monocytes is stimulated by either secreted or cell wall-associated mannoproteins of C. neoformans, acting, at least in part, through an internalization process in the endocytic pathway. We have previously shown intense IL-12 production by human phagocytes from both human immunodeficiency virus-positive and -negative subjects stimulated with a mannoprotein fraction of C. albicans (5). Since the other main cell wall constituent of this fungus, i.e., glucan, does not stimulate IL-12 production (A. Torosantucci, unpublished observation), cell wall-expressed mannoproteins of the Y form of C. albicans are probably involved in the interaction between the fungal cell and the phagocyte, leading to the subsequent activation of endocytosis. Mannoproteins are cell wall components of C. albicans which are mostly secreted and rearranged during GT formation, with remarkable losses of Y cell-expressed molecules (3, 17). Further studies of specific fungal molecules mediating adhesion to the phagocyte, thus fostering fungal internalization, and how these molecules are differentially expressed on Y and GT forms of C. albicans, could greatly contribute to a further understanding of the mechanisms whereby GT and Y cells differ in IL-12 induction by monocytes.
Finally, of interest is the observation that the lower production of IL-12 by the GT-stimulated monocytes did not affect their candidacidal capacity. Extracellular killing mechanisms have been shown to be possessed by most professional phagocytes, and GT cells were demonstrated to be highly sensitive to these nonphagocytic fungicidal mechanisms, particularly in the presence of opsonins and cytokine activators (31). It is also known that IL-12 does not, per se, enhance the candidacidal activity of both mononuclear and polymorphonucleate phagocytes (6). In the present investigation, low-level IL-12 stimulator GT cells appeared to be killed by human monocytes by nonphagocytic, extracellular mechanisms to exactly the same extent, in vitro, as the high-level IL-12 stimulator Y cells. Thus, the effect of GT cells on IL-12 production is uncoupled to monocyte candidacidal activity, suggesting that the process of GT formation and filamentation in vivo may critically impair the development of a protective response against the fungus rather than the immediate outcome of the local Candida-host encounter.
ACKNOWLEDGMENTS
Thanks are due to Antonio Cassone for helpful discussion during the course of this work and for critical reading of the manuscript. We also express our gratitude to A. Botzios for help in the preparation of the manuscript.
This work was partly supported by the National AIDS Program, contract 50C/B.
REFERENCES
- 1.Ausiello C M, Urbani F, Gessani S, Spagnoli G C, Gomez M J, Cassone A. Cytokine gene expression in human peripheral blood mononuclear cells stimulated by mannoprotein constituents from Candida albicans. Infect Immun. 1993;61:4105–4111. doi: 10.1128/iai.61.10.4105-4111.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biron C, Gazzinelli R T. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr Opin Immunol. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 3.Brawner D L, Cutler J E. Variability in expression of cell surface antigens of Candida albicansduring morphogenesis. Infect Immun. 1987;51:337–343. doi: 10.1128/iai.51.1.337-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromuro C, Torosantucci A, Gomez M J, Urbani F, Cassone A. Differential release of an immunodominant 65Kda mannoprotein antigen from yeast and mycelial forms of Candida albicans. J Med Vet Mycol. 1994;32:447–459. doi: 10.1080/02681219480000601. [DOI] [PubMed] [Google Scholar]
- 5.Cassone A, Chiani P, Quinti I, Torosantucci A. Possible participation of polymorphonuclear cells stimulated by microbial immunomodulators in the dysregulated cytokine patterns of AIDS patients. J Leukoc Biol. 1997;62:60–66. doi: 10.1002/jlb.62.1.60. [DOI] [PubMed] [Google Scholar]
- 6.Cenci E, Mencacci A, Del Sero G, d'Ostiani C F, Mosci P, Bacci A, Montagnoli C, Kopf M, Romani L. IFN-γ is required for IL-12 responsiveness in mice with Candida albicansinfection. J Immunol. 1997;161:3543–3550. [PubMed] [Google Scholar]
- 7.Cenci E, Mencacci A, Spaccapelo R, Tonnetti L, Mosci P, Enssle K H, Puccetti P, Romani L, Bistoni F. T helper type 1 (Th-1)- and Th-2 like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J Infect Dis. 1995;171:1279–1288. doi: 10.1093/infdis/171.5.1279. [DOI] [PubMed] [Google Scholar]
- 8.Corner B E, Magee P T. Candidapathogenesis: unravelling the threads of infection. Curr Biol. 1997;7:R691–694. doi: 10.1016/s0960-9822(06)00357-5. [DOI] [PubMed] [Google Scholar]
- 9.Cutler J E. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 10.D'Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin-10 inhibits human lymphocytes IFN-gamma production by suppressing natural killer stimulatory factor/interleukin-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1058. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bernardis F, Molinari A, Boccanera M, Stringaro A, Robert R, Senet J M, Arancia G, Cassone A. Modulation of cell surface-associated mannoprotein antigen expression in experimental Candidavaginitis. Infect Immun. 1993;62:509–519. doi: 10.1128/iai.62.2.509-519.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulton S A, Johnsen J M, Wolf S F, Sieburth D S, Boom W H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996;64:2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison T S, Levitz S M. Role of IL-12 in peripheral blood mononuclear cell responses to fungi in persons with and without HIV infection. J Immunol. 1996;156:4492–4497. [PubMed] [Google Scholar]
- 14.Lavigne L M, Schopf L R, Chung C L, Maylor R, Sypek J P. The role of recombinant murine IL-12 and IFN-γ in the pathogenesis of a murine systemic Candida albicansinfection. J Immunol. 1998;160:284–292. [PubMed] [Google Scholar]
- 15.Leigh J E, Steele C, Wormley F L, Jr, Luo W, Clark R A, Gallaher W, Fidel P L., Jr Th1/Th2 cytokine expression in saliva of HIV-positive and HIV-negative individuals: a pilot study in HIV-positive individuals with oropharyngeal candidiasis. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:373–380. doi: 10.1097/00042560-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Mencacci A, Torosantucci A, Spaccapelo R, Romani L, Bistoni F, Cassone A. A mannoprotein constituent of Candida albicansthat elicits different levels of delayed-type hypersensitivity, cytokine production and anticandidal protection in mice. Infect Immun. 1994;62:5353–5360. doi: 10.1128/iai.62.12.5353-5360.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molinari A, Gomez M J, Crateri P, Torosantucci A, Cassone A, Arancia G. Differential cell surface expression of mannoprotein epitopes in yeast and mycelial forms of Candida albicans. Eur J Cell Biol. 1993;60:146–153. [PubMed] [Google Scholar]
- 18.Odds F C. Candida and candidosis. London, United Kingdom: Baillere-Tindall; 1988. [Google Scholar]
- 19.Pitzurra L, Cherniak R, Giammarioli M, Perito S, Bistoni F, Vecchiarelli A. Early induction of interleukin-12 by human monocytes exposed to Cryptococcus neoformansmannoproteins. Infect Immun. 2000;68:558–563. doi: 10.1128/iai.68.2.558-563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulain D, Hopwood V, Vernes A. Antigenic variability of Candida albicans. Crit Rev Microbiol. 1985;12:233–270. doi: 10.3109/10408418509104430. [DOI] [PubMed] [Google Scholar]
- 21.Roilides E, Anastasiou-Katsiardani A, Dimitriau-Georgiadou A, Kadiltsoglou I, Tsaparidou S, Panteliadis C, Walsh T J. Suppressive effects of interleukin-10 on human mononuclear phagocyte function against Candida albicans and Staphylococcus aureus. J Infect Dis. 1998;178:1734–1742. doi: 10.1086/314479. [DOI] [PubMed] [Google Scholar]
- 22.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Puccetti P, Wolf S F, Bistoni F. IL-12 is both required and prognostic in vivo for T helper type 1 differentiation in murine candidiasis. J Immunol. 1994;154:5167–5175. [PubMed] [Google Scholar]
- 23.Romani L, Puccetti P, Bistoni F. Interleukin-12 in infectious diseases. Clin Microbiol Rev. 1997;10:611–636. doi: 10.1128/cmr.10.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romani L, Mencacci A, Cenci E, Mosci P, Vitellozzi G, Grohnann U, Puccetti P, Bistoni F. Course of primary candidiasis in T-cell depleted mice infected with attenuated variant cells. J Infect Dis. 1992;166:1384–1392. doi: 10.1093/infdis/166.6.1384. [DOI] [PubMed] [Google Scholar]
- 25.Romani L, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Puccetti P, Bistoni F. CD4+ subset expression in murine candidiasis: Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993;150:925–931. [PubMed] [Google Scholar]
- 26.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Wolf S F, Puccetti P, Bistoni F. Interleukin-12 but not interferon-gamma production correlates with induction of T-helper type 1 phenotype in murine candidiasis. Eur J Immunol. 1994;24:909–915. doi: 10.1002/eji.1830240419. [DOI] [PubMed] [Google Scholar]
- 27.Sartori A, Ma X, Gri G, Showe L, Benjamin D, Trinchieri G. Interleukin-12: an immunoregulatory cytokine produced by B cells and antigen presenting cells. Methods. 1997;11:116–127. doi: 10.1006/meth.1996.0395. [DOI] [PubMed] [Google Scholar]
- 27a.Torosantucci, A., P. Chiani, and A. Cassone. Differential chemokine response of human monocytes to yeast and hyphal forms of Candida albicans and its relation to the β-1,6 glucan content of the fungal cell wall. J. Leukoc. Biol., in press. [PubMed]
- 28.Torosantucci A, Chiani P, Quinti I, Ausiello C M, Mezzaroma I, Cassone A. Responsiveness of human polymorphonuclear cells (PMNL) to stimulation with a mannoprotein fraction (MP-F2) of Candida albicans: enhanced production of IL-6 and tumor necrosis factor-alpha by MP-F2-stimulated PMNL from HIV-infected subjects. Clin Exp Immunol. 1997;107:451–457. doi: 10.1046/j.1365-2249.1997.2851176.x. [DOI] [PubMed] [Google Scholar]
- 29.Trinchieri G. Interleukin-12 and its role in the generation of Th-1 cells. Immunol Today. 1993;14:335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 30.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridges innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 31.Vazques-Torres A, Balish E. Macrophages in resistance to candidiasis. Microbiol Mol Biol Rev. 1997;61:170–192. doi: 10.1128/mmbr.61.2.170-192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]