Abstract
Exposure to oocysts of the protozoan Cryptosporidium parvum causes intestinal epithelial cell dysfunction in vivo and in vitro, but effective means by which mucosal injury might be prevented remain unclear. We examined the ability of transforming growth factor β1 (TGF-β1)—a cytokine synthesized and released by cells in the intestine—to preserve the barrier function of human colonic epithelia when challenged with C. parvum oocysts and then studied the mechanisms involved. Epithelial barrier function was monitored electrophysiologically, receptors for TGF-β1 were localized by confocal microscopy, and TGF-β1-induced protein kinase C activation was detected intracellularly by translocation of its α isozyme. TGF-β1 alone enhanced intestinal epithelial barrier function, while exposure to C. parvum oocysts (≥105/monolayer) markedly reduced barrier function to ≤40% of that of the control. When epithelial monolayers were pretreated with TGF-β1 at 5.0 ng/ml, the barrier-disrupting effect of C. parvum oocysts was almost completely abrogated for 96 h. Further investigation showed that (i) the RI and RII receptors for TGF-β1 were present on 55 and 65% of human epithelial cell line cells, respectively, over a 1-log-unit range of receptor protein expression, as shown by flow cytometry and confirmed by confocal microscopy; (ii) only basolateral and not apical TGF-β1 exposure of the polarized epithelial monolayer resulted in a protective effect; and (iii) TGF-β1 had no direct effect on the organism in reducing its tissue-disruptive effects. In exploring mechanisms to account for the barrier-preserving effects of TGF-β1 on epithelium, we found that the protein kinase C pathway was activated, as shown by translocation of its 80-kDa α isozyme within 30 s of epithelial exposure to TGF-β1; the permeability of epithelial monolayers to passage of macromolecules was reduced by 42% with TGF-β1, even in the face of active protozoal infection; and epithelial cell necrosis monitored by lactate dehydrogenase release was decreased by 50% 70 h after oocyst exposure. Changes in epithelial function, initiated through an established set of surface receptors, likely accounts for the remarkable barrier-sparing effect of nanogram-per-milliliter concentrations of TGF-β1 when human colonic epithelium is exposed to an important human pathogen, C. parvum.
Cryptosporidium parvum is a parasitic protozoan that infects humans worldwide. Even after a prolonged period in a vegetative form, C. parvum can, upon ingestion, excyst and infect intestinal epithelium on contact. The lasting impact of cryptosporidial infections on nutritional status, childhood growth, and long-term physical fitness emphasize the functional importance of cryptosporidial infections in areas of endemicity (8, 15, 24). In immunodeficient or malnourished individuals, cryptosporidiosis may be ultimately fatal, while immunologically healthy persons generally expel C. parvum after a short illness (32, 36). The nature of the precise antiprotozoal element(s) that the normal individual is able to bring successfully to this interaction is not clear.
In vivo model systems have been used to study potential host protective mechanisms. These have shown that, after infection of intestinal epithelial cells, C. parvum remains extracytoplasmic but intracellular and undergoes development through asexual multiplication, gametogony, and oocyst formation (22). Recent investigation, particularly in mouse models with targeted disruption of important macromolecules, has shown the importance to resolution of C. parvum infection of elements of the cell-mediated immune response, including CD4+ αβ+ T lymphocytes secreting gamma interferon and interleukin-4, intestinal intraepithelial lymphocytes, and CD40+ splenocytes (4, 9, 10). However, immunocompetent small laboratory animals (such as the rat and mouse) are resistant to even high-dose exposure to C. parvum (13), and in vivo studies of individual components of host nonimmune (epithelial) resistance to this parasite remain difficult to carry out. To overcome these limitations, we studied a well-differentiated human intestinal epithelial cell monolayer that grows in a polarized fashion on a Nucleopore filter as a model of crypt epithelial cells in vivo. In this system, normal and altered physiological activity of the epithelium, particularly its barrier function, can be quantified through measurement of the apical-to-basal (transcellular) electrical potential difference which these cells are able to generate and sustain (2).
Transforming growth factor β1 (TGF-β1) stimulates the synthesis of extracellular matrix proteins (collagen and fibronectin) by up-regulating their gene expression (18) and alters the expression of integrins that act as receptors for these proteins, thereby enhancing the cell's ability to bind them (17). For the intestinal epithelial cell, these events have important implications for the function of its intercellular tight junctions, for its growth on matrix proteins composing the basement membrane, and, ultimately, for its barrier function. Furthermore, TGF-β1 has been shown to play a central role in restitution of rat intestinal epithelial cells after injury by promoting increased cell migration (11), and limited preliminary data suggest TGF-β1 may reduce barrier disruption caused by C. parvum (27). Nevertheless, investigation that directly examines the biology of barrier protection by TGF-β1; the location of, as well as signaling pathways activated by, the RI and RII receptors for TGF-β1 on the epithelium; and the mechanism of the TGF-β1 protective effect are not reported in the context of active epithelial cell infection, to our knowledge. We chose, therefore, to examine the biology of TGF-β1 in regulating the barrier function of the intestinal epithelium in the presence of a known intestinal pathogen, C. parvum.
The data presented show that TGF-β1 is a potent molecule for enhancing transmonolayer resistance for a prolonged period and in substantially retarding, by greater than 50%, the epithelial-barrier-disrupting effect of C. parvum infection. Our studies indicate that specific cell membrane receptors are present on human epithelial cell lines for interaction with TGF-β1, that the alpha isozyme of protein kinase C (PKC) is activated intracellularly by TGF-β1, and that the C. parvum-induced increase in epithelial permeability to macromolecules is prevented by TGF-β1 with little effect on oocyst viability.
MATERIALS AND METHODS
Characterization of epithelial receptors and barrier function. (i) Intestinal epithelial cell lines.
Passage 22 T84 cells were a generous gift of Kim Barrett (University of California at Los Angeles, Los Angeles). The cells were grown in 150-cm2 tissue culture flasks (Corning, Corning, N.Y.) with media consisting of Dulbecco's modified Eagle's medium and Ham's F-12 medium (1:1 ratio) (DMEM–F-12) supplemented with 15 mM sodium HEPES buffer, pH 7.5, 14 mM sodium bicarbonate, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 5% fetal calf serum (GIBCO, Grand Island, N.Y.). When the cells reached 90 to 95% confluence (10 to 14 days), a cell suspension was prepared by incubating the cells for 30 to 40 min at 37°C with 0.2% trypsin and 0.9 mM EDTA in a calcium- and magnesium-free phosphate-buffered saline (PBS). Trypan blue exclusion with a hemocytometer chamber indicated a viability greater than 95%. Mycoplasma testing with the mycotect kit (GIBCO) showed the cells to be mycoplasma free. Prior to being studied, the cells were maintained at 37°C in a 95% air–5% CO2 atmosphere with fresh medium (see above) added three times per week.
(ii) Specificity of study immunoglobulin.
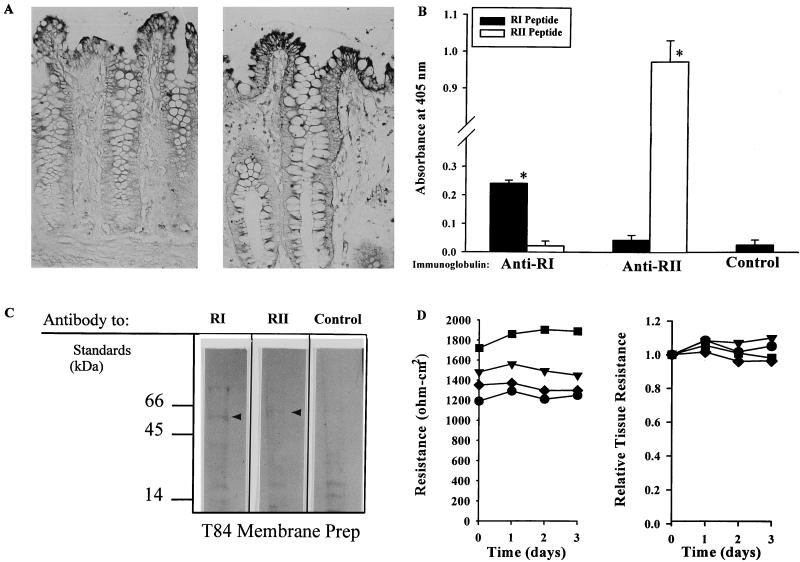
Evidence that the study antisera (kindly supplied by Santa Cruz Biotechnology, Santa Cruz, Calif.) were specific for the RI and RII receptors of TGF-β1 present on human colonic epithelial cells included (i) specific recognition, in immunohistochemical studies, of apical epithelial cells in human colonic mucosa (Fig. 1A), matching that previously reported (12); (ii) specific binding by each immunoglobulin of peptide constituents of one (but not both) human TGF-β1 receptors, as determined by enzyme-linked immunosorbent assay and reported previously (Fig. 1B) (peptides were plated at 0.4 μg/ml, and immunoglobulins were used at 0.2 μg/ml); and (iii) recognition of bands at 55 and 60 kDa of membrane glycoproteins solubilized from a human colon cell line (T84) and examined by Western blotting (Fig. 1C) as described previously (12).
FIG. 1.
Characterization of the specificities of immunoglobulins elicited by TGF-β1 receptors RI and RII and used in this study. (A) Selective in situ staining of human colonic epithelium by TGF-β1 RI (left)- and TGF-β1 RII (right)-elicited immunoglobulins. Five-micrometer-thick sections of normal human colonic mucosa were exposed to immunoglobulins (2 μg/ml), followed by alkaline phosphatase-conjugated anti-rabbit IgG (H and L) and substrate. Shown are surface epithelial cells expressing RI and RII receptor proteins; cells in the subepithelial areas, including those in the lamina propria, were receptor-negative. Magnification, ×250. (B) Specific immunoreactivity of immunoglobulins, elicited to human TGF-β1 RI and RII receptor proteins, as determined by enzyme-linked immunosorbent assay. Shown is the absorbance at 405 nm (mean ± 1 SD) as a measure of each immunoglobulin's ability to bind receptor protein 8 h after the addition of substrate. The plate-bound (“study”) antigens are defined in the key, and the identities of the immunoglobulins are indicated below the x axis. (C) Western blot of electrophoretically separated T84 plasma membrane proteins, using study immunoglobulins as indicated. Bands at 55 and 60 kDa (arrowheads) were bound by immunoglobulins elicited by human RI and RII, respectively. Molecular mass standards are shown; normal rabbit serum constituted the control. (D) Comparison of data on electrical potential differences of T84 epithelial monolayers over time, displayed as resistance (left), with the same data shown as relative tissue resistance (right). Each symbol designates one of four individual monolayers, monitored serially.
(iii) Confocal microscopic localization of receptors on T84 cells.
T84 cells were grown on nitrocellulose filters (3-μm pore size; 33.2 mm2) in transwells (Costar, Cambridge, Mass.) for 3 to 5 days and then incubated with immunoglobulin monospecific for TGF-β1 receptor RI or RII (characterized above and used at 2 μg/ml for 30 min at 20°C) followed by Cy3-conjugated, affinity-purified goat anti-rabbit immunoglobulin G (IgG) (7 μg/ml; 30 min; 20°C) (Jackson ImmunoResearch Laboratories, West Grove, Pa.). The preparations were examined on a Zeiss 410 scanning confocal microscope equipped with argon-krypton lasers and image enhancement software.
(iv) Membrane and cytosol fractions for PKC studies.
T84 cells (107/ml) were lysed in a Dounce homogenizer in a hypotonic buffer (20 mM Tris containing 0.25 M sucrose, 10 mM EGTA, and 22 mM EDTA) and then pelleted (15,000 rpm; 30 min) for separation of membrane and cytosolic fractions. The membrane and cytosol preparations were then added to boiling Laemmli sample buffer (20), mixed by repeated pipetting, and stored until used.
(v) Detection of PKC-α isozyme activation in T84 cells.
Membrane and cytosolic fractions of T84 cells were electrophoresed on a sodium dodecyl sulfate–8% polyacrylamide gel (5 mA; 16 h) and transblotted onto nitrocellulose as described previously (28). Protein-impregnated nitrocellulose strips were then incubated with immunoglobulin previously shown to be monospecific for PKC-α (21) followed by horseradish–peroxidase (HRP)-labeled immunoglobulin (NA 934; Amersham Life Sciences). Specific immunoglobulin binding was detected using an enhanced chemiluminescence system (Amersham Pharmacia Biotech).
(vi) Preparation of T84 monolayers on semipermeable supports.
Thirteen-millimeter-diameter polycarbonate filters with 5-μm pores (Nucleopore Corporation, Pleasonton, Calif.) were coated on one side with rat tail collagen prepared as described by Cereijido et al. (7). Once coated, the filters were attached to one end of a 5-mm-high polycarbonate ring standing on three 1-mm-long legs. When the filter was placed in a 12-well culture dish (Costar), 300 μl of DMEM–F-12 tissue culture medium was placed in the chamber above the filter, with 2,000 μl of medium in the well below the filter.
For initiation of monolayers, each filter mounted in a ring was seeded with a T84 cell suspension, prepared as described above, that delivered 7 × 105 cells per filter. The ring filters were then placed in petri dishes (six per dish) (the medium was changed every other day) and maintained at 37°C in a 95% air–5% CO2 atmosphere for a minimum of 7 days prior to study. Subsequently, filters with confluent monolayers were transferred to individual wells (one filter per well) of a 12-well sterile plate (Costar), and the monolayers were fed daily and assessed for electrical resistance as described below.
(vii) Flow cytometry of T84 cells.
Cells were harvested from flasks with 0.1% trypsin in PBS, permeabilized (PermWash; PharmMingen, San Diego, Calif.), and incubated (106 cells/tube) in 100 μl of medium containing primary unlabeled immunoglobulin to RI or RII (2 μg/ml; 40 min; 4°C). After the addition of Cy3-conjugated, affinity-purified goat anti-rabbit IgG (2 μg/ml; 30 min; 4°C), the cells were washed twice, and a final suspension was made in 400 μl of PBS with 0.5% paraformaldehyde. The cells were analyzed on a fluorescence-activated cell sorter (FACScan; Becton Dickinson, Mansfield, Mass.), and 6 × 103 to 8 × 103 gated living cells were acquired in list mode for each sample. Normal rabbit serum served as the primary unlabeled negative control.
(viii) Measurements of epithelial-barrier function.
The electrical potential difference across the cell monolayer after passage of a defined current pulse was measured using a two-electrode model EVOM voltohmmeter (World Precision Instruments, New Haven, Conn.) and expressed in ohms per square centimeter. The development of an electrical resistance across each monolayer was determined serially in a sterile manner with this apparatus, and the value in ohms per square centimeter just prior to adding a potential modulator served as the control value for that monolayer. For the calculation of relative tissue resistance from the electrical potential difference (range, 1,200 to 3,200 ohm · cm2), the control value was set at 1.0. In contrast, the electrical potential difference across collagen-coated filters without a cellular monolayer was consistently less than 80 ohm · cm2. Preliminary experiments showed that data plotted as electrical potential difference and as relative tissue resistance gave parallel results when recorded from a series of epithelial monolayers (Fig. 1D); the latter was selected for use as a measure of epithelial barrier function in this study because it allowed normalization of values of individual monolayers, permitting calculation of group means and variances.
The concentration in medium of the intracellular enzyme lactate dehydrogenase (LDH) was used as a measure of epithelial cell injury and dysfunction and was determined in duplicate by the technique of Arnador et al. (5) as modified by the kit manufacturer (Sigma, St. Louis, Mo.). The LDH content of the supernatant from control and experimental monolayers was expressed as LDH activity percent, using a standard curve constructed with solutions with known LDH activities. Total cellular LDH was determined by exposing the monolayers to Triton X-100 detergent (1% [vol/vol]) for 20 min and quantitating the LDH released into the medium.
Epithelial monolayer permeability was also assessed using HRP (Sigma) as a probe. At the nadir of transepithelial resistance (2 to 4 days after the addition of oocysts), HRP (0.002 μg) was applied to the apical surface of the T84 monolayer, the plate was incubated at 37°C for 2 h, and aliquots of medium in the basolateral side of the monolayer were collected for analysis. HRP was measured by the technique originally described by Ortiz de Montellano et al. (26) as modified by Hecht et al. (16), with values determined from a standard curve constructed with known quantities of HRP.
Effects of C. parvum on epithelial function. (i) Source of oocysts.
C. parvum oocysts (strain AUCp-1) were obtained from the feces of orally infected newborn calves and were given to our laboratory by Ronald Fayer (Immunology Disease Resistance Laboratory, U.S. Department of Agriculture, Beltsville, Md.). From this 2.5% potassium dichromate-preserved preparation, oocysts were purified by a method modified from that of Ungar et al. (34) and were enumerated in a hemocytometer chamber before administration. Oocyst viability was estimated using an excystation procedure (greater than 80% in all cases) and by vital dye staining. The filtrates consisted of medium aspirated from C. parvum-infected monolayers (at ≥48 h) and passed through a 0.22-μm-pore-size filter (Miller-GV; Millipore, Bedford, Mass.).
(ii) Electron microscopy of C. parvum-infected monolayers.
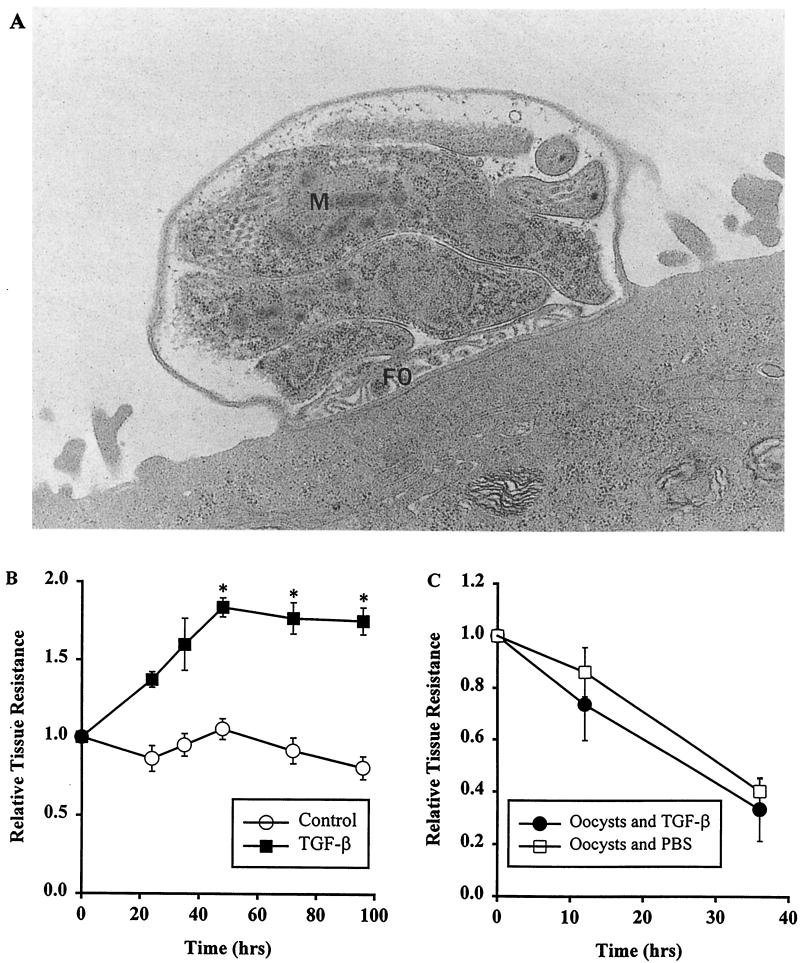
Monolayers on polycarbonate filters were fixed in situ by submerging them in 3% paraformaldehyde, 3% glutaraldehyde, and 0.1 M Sorenson's buffer, pH 7.4. Subsequently, the monolayers were rinsed in Sorenson's buffer, postfixed in osmium tetroxide, dehydrated through graded alcohols, and embedded in an Epon resin. Thick and thin sections were cut with a diamond knife on a microtome (Ultracut E; Reichert, Buffalo, N.Y.). The thick sections were stained with aqueous 1% toluidine blue; the thin sections were placed on nickel grids, stained with uranyl acetate, and examined under the electron microscope (model 100CK; Jeol, Peabody, Mass.). Figure 2A shows a schizont with multiple maturing merozoites and a feeder organelle, a specialized region where contact between parasite and host cell cytoplasm occurs.
FIG. 2.
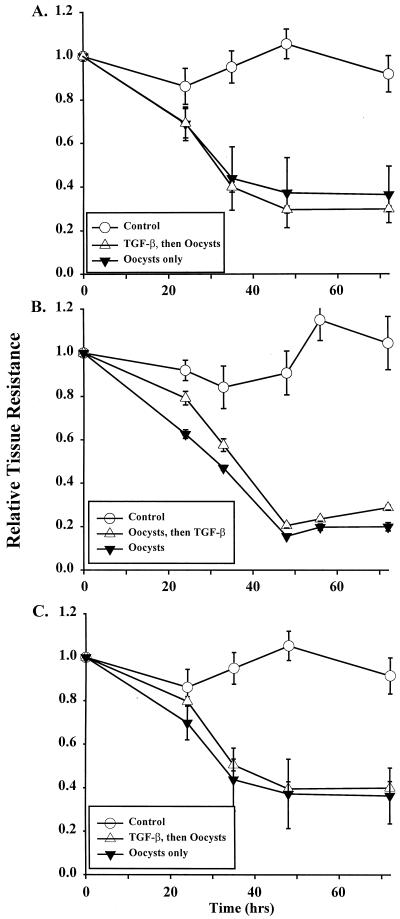
Findings after exposure of human epithelial monolayers to C. parvum oocysts or to TGF-β1. (A) Electron micrograph of experimental T84 epithelial monolayer 30 h after incubation of its microvillous (apical) surface with 106 C. parvum oocysts. Shown is a schizont with multiple maturing merozoites (M) and a feeder organelle (FO). Magnification, ×8,000. (B) Effect of TGF-β1 on the epithelial barrier function of human epithelial monolayers. Electrically stable, confluent sets of monolayers were exposed basolaterally to 2.5 ng of TGF-β1/ml or to medium for 72 h, and their transepithelial resistances were monitored daily. The results are shown as the relative tissue resistance (mean ± 1 SD) calculated as defined in Materials and Methods. Each data point represents a minimum of four monolayers. The asterisks indicate statistical significance (P < 0.05), comparing TGF-β1-treated monolayers with control monolayers. (C) Lack of a direct effect of TGF-β1 on C. parvum oocysts, monitored by oocysts' ability to disrupt epithelial function. Experimental monolayers were incubated with oocysts preexposed for 12 h to TGF-β1 (5 ng/ml); control monolayers were incubated with oocysts after preexposure to PBS for 12 h. There was no significant difference between oocysts treated and not treated with TGF-β1 (P ≥ 0.82).
(iii) Exposure of epithelial cell monolayers to oocysts and to TGF-β1.
T84 intestinal epithelial cells, as a polarized monolayer with a stable transmonolayer electrical potential difference of ≥1,200 ohm · cm2, were overlaid with C. parvum oocysts (numbers varied in individual experiments), applied directly without excystation to the apical (mucosal) surface. Each monolayer was then placed in a well in a 12-well plate (Costar) at 95% air–5% CO2 with a DMEM–F-12 medium with supplements (see above) in both the apical and basal chambers. TGF-β1 (Genzyme, Cambridge, Mass.) at a final concentration of 0.1 to 5.0 ng/ml was added to the basolateral or, in one study, apical chamber, usually for 48 h or as indicated for each study. Investigations were carried out in 95% air–5% CO2, with the transmonolayer electrical resistance measured at 37°C by the two-electrode EVOM voltohmmeter system described above. In preliminary studies, basolateral exposure of monolayers to TGF-β1 progressively raised their resistances over 40 h, after which resistance was stable (Fig. 2B). Controls included monolayers overlaid with (i) culture medium not containing oocysts, (ii) suspensions of oocysts applied apically in the absence of TGF-β1, and (iii) gamma interferon alone as the positive control, expected to consistently reduce transmonolayer resistance by ≥50%.
To determine if TGF-β1 had a primary effect on oocysts (rather than on epithelium), we studied whether a 12-h oocyst pretreatment with TGF-β1 (5 ng/ml) would reduce the oocysts' known capacity to alter T84 epithelial barrier function (Fig. 2C). No significant difference in relative tissue resistance between TGF-β1-treated and PBS-treated oocysts was seen at 10 or 35 h (P = 0.82), suggesting that any effect of TGF-β1 in enhancing barrier function was not due to an antiparasitic activity of the cytokine but rather to its ability to alter the physiology of the epithelial cell.
Statistical methods.
Data are shown as the mean ±1 standard deviation (SD) of quadruplicate values, representing epithelial monolayers at each time point and the oocyst dose. For comparison between experimental and control conditions, including the endpoints of relative tissue resistance, LDH activity, and HRP levels, the paired two-tailed Student's t test was used; a P value of <0.05 was designated statistically significant.
RESULTS
Effect of C. parvum on epithelial barrier function.
We first sought to confirm a prior report (3) that apical exposure of human epithelial monolayers to C. parvum is accompanied by a change in the physiological integrity of the monolayer. Monolayers incubated on their apical surfaces with 106 oocysts demonstrated a modest decline (35%) in relative tissue resistance in the first 18 to 24 h, followed by a marked decline by 48 h (Fig. 3). Compared with controls (monolayers exposed to medium without oocysts), the decrease in tissue resistance at 36, 48, 72, and 96 h after oocyst addition was statistically significant (P < 0.05). In prior work, monolayers incubated with 106 Cryptosporidium oocysts which had been heat inactivated (85°C; 30 min) showed no significant change from baseline (3). Filtrates of C. parvum, when placed on the apical surfaces of T84 epithelial cell monolayers, caused no significant reduction in barrier function (relative tissue resistance, >0.92 at 6, 12, 24, 36, and 48 h). Together with electron microscopic data showing life cycle forms of C. parvum budding from the microvillous surface of T84 cells 30 h after exposure of the cells to oocysts (Fig. 2A), these studies suggested that viable parasites, not their exotoxins or dead organisms, were necessary to disrupt epithelial barrier function.
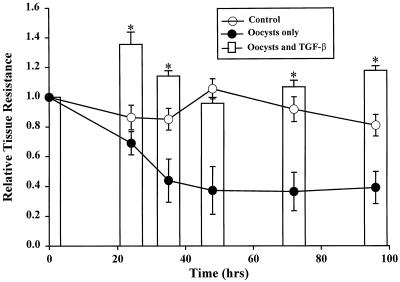
FIG. 3.
Effect of TGF-β1 on the abilities of epithelial monolayers to resist a C. parvum-induced reduction in barrier function. Individual experimental monolayers were exposed on their basolateral surfaces to TGF-β1 for 48 h and then challenged on their apical surfaces with 106 C. parvum oocysts (at 0 h). Controls consisted of monolayers incubated with medium only or with C. parvum oocysts only. Shown is the relative tissue resistance (± 1 SD), reflecting epithelial barrier function, over 96 h after oocyst addition. ∗, P < 0.05, comparing monolayers exposed to oocysts alone with monolayers pretreated with TGF-β1 before oocyst challenge.
TGF-β1 preserves barrier function from C. parvum-induced disruption.
Because preliminary studies suggested that basolateral exposure of human epithelial cell monolayers to TGF-β1 enhanced their level of barrier function (Fig. 2B), we sought to determine the effect of a 48-h TGF-β1 pretreatment on monolayer vulnerability to barrier change with C. parvum oocysts (Fig. 3). While the relative tissue resistance did decline by 58% at 36 h after the addition of oocysts alone, the resistance of monolayers pretreated with TGF-β1 and then exposed to oocysts (Fig. 3) did not decrease below 94% of its baseline value (1.0) and was not distinguishable statistically from that of monolayers incubated with medium only. For monolayers receiving a similar dose of oocysts applied alone, barrier function did not recover over the 96-h course of the study (Fig. 3). These results suggested that TGF-β1 can preserve the barrier function of human colonic epithelium in the presence of a potent pathogen, C. parvum.
Nonuniformity of TGF-β1 receptor expression on the epithelial cell membrane.
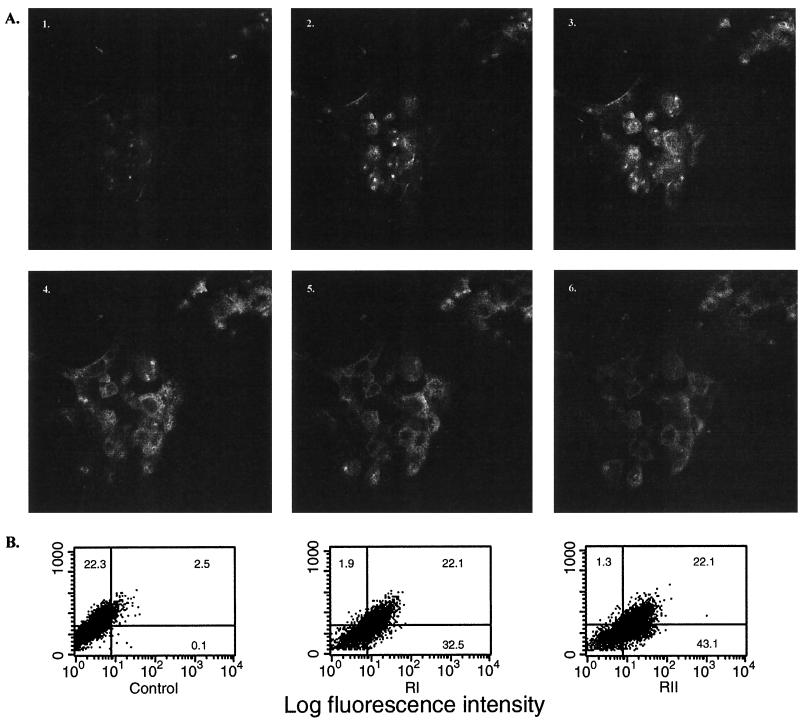
While TGF-β1 appeared to be protective of epithelial cell function in the studies described above, little is known about the expression on human colonic cell lines of either of its two specific receptors (designated RI and RII), necessary for a physiological effect of TGF-β1 on cells. Using receptor-specific immunoglobulin with confocal microscopy, sequential images showed that immunoreactive RII was expressed by T84 cells (Fig. 4A) in a pattern suggesting the receptor was present at the cell apex (image 2) but more widely evident on the basolateral cell surfaces (images 4 and 5). Similar results were obtained with the RI receptor (not shown). These findings were confirmed by flow cytometry, where T84 cells were found to be heterogeneous with respect to RI-RII receptor expression, with 55% of cells RI positive and 65% RII positive (Fig. 4B). These results mirrored findings in situ (Fig. 1A), where epithelial cells at the crypt apex were RI and RII positive while those in the basal regions were receptor negative. Our results suggested that two distinct receptors for TGF-β1 are present on a majority of human colonic epithelial cells and that they are available to mediate TGF-β1's barrier protective effects on epithelia.
FIG. 4.
TGF-β1 receptor RI and RII expression by human colonic (T84) epithelial cells, detected as immunoreactive protein. (A) Confocal microscopy of T84 cells growing on a Nucleopore filter and exposed to a TGF-β1 RII-specific immunoglobulin (2 μg/ml) (characterized in the text) followed by a Cy3-conjugated, affinity-purified anti-rabbit IgG (7 μg/ml). Shown are six images, taken 1 μm apart, from apex to base of individual colonic epithelial cells. White indicates the locations on the cells of RII protein; the clear spaces within the cells indicate the locations of nuclei. Magnification, ×900. (B) Flow cytometric analysis of a suspension of T84 cells incubated with normal serum (control), anti-RI (RI), or anti-RII (RII) followed by a Cy3-conjugated anti-immunoglobulin. Forward scatter (y axis) is graphed versus intensity of fluorescence, and the percentage of cells constituting each gated area is indicated. The majority of epithelial cells expressed both RI and RII.
Importance of duration of TGF-β1 treatment.
Since a 48-h preexposure of monolayers to TGF-β1 resulted in epithelial barrier protection in the experiments described above (Fig. 3), we next determined whether TGF-β1 incubation with monolayers for shorter periods would be similarly beneficial (Fig. 5A and B). Neither a 30-min preexposure to TGF-β1 nor incubation with it beginning 2 h after the addition of oocysts resulted in a reduction in C. parvum-induced epithelial barrier dysfunction. In both cases, by 48 h after the addition of oocysts, the relative tissue resistance was less than 40% of that of the control whether or not TGF-β1 was present basolaterally (P = 0.74 [comparing oocyst-challenged monolayers with or without TGF-β1 pretreatment]). In neither study did the transmonolayer resistance recover to baseline after oocysts were added, in contrast to findings with 48-h TGF-β1 preincubation (Fig. 3). These experiments suggested that time-dependent, TGF-β1-induced cellular events in the epithelial monolayer must occur prior to exposure to C. parvum oocysts in order for barrier function to be protected.
FIG. 5.
Modes of exposure of epithelial cell monolayers to TGF-β1 that do not prevent C. parvum-induced derangement of barrier function. (A) Experimental monolayers were incubated basolaterally with TGF-β1 for 30 min, followed by oocysts placed apically. Control monolayers were incubated with medium alone (control) or with C. parvum oocysts only. (B) Monolayers were continuously incubated on their basolateral surfaces with TGF-β1, beginning 2 h after oocysts were added to monolayers. Controls were the same as in panel A. (C) Epithelial cell monolayers were incubated on their apical surfaces with 5.0 ng of TGF-β1/ml for 48 h before exposure to C. parvum oocysts (106/monolayer) and subsequent daily monitoring of transepithelial resistance. Monolayer controls were incubated with medium alone (control) or with C. parvum oocysts. Time (x axis) is the interval after oocyst inoculation of monolayers. The values for relative tissue resistance are shown as the mean ± 1 SD, with n = 4 monolayers for each data point.
TGF-β1 exposure of the apical epithelial membrane is not protective.
Although immunohistochemical studies (Fig. 4A) of human intestinal epithelium demonstrated that TGF-β1 receptors were localized to one or more surfaces of the epithelial cell, one could propose that apically placed receptors for TGF-β1 on epithelia might be beneficial for the host, particularly for enhancing epithelial barrier function. This is because the microvillous apical surface is a common entry point for the organism and because trophozoites of C. parvum are found in a subapical but extracytoplasmic location in infected epithelial cells (22). Interestingly, a 48-h apical preexposure of epithelial cell monolayers did not result in preservation of barrier function upon incubation with C. parvum oocysts (Fig. 5C). Thus, at no time after the addition of oocysts was the relative tissue resistance of experimental monolayers significantly different from that of those exposed to C. parvum oocysts alone (P = 0.84). A 30-min apical preexposure to TGF-β1 yielded the same result (resistance less than 40% of that of the control at 48 h). In addition, these experiments suggested that coincubation (in the apical chamber) of oocysts and TGF-β1 did not result in a parasiticidal event sufficient to reduce the oocysts' ability to disrupt barrier function.
Change in monolayer permeability prevented by TGF-β1.
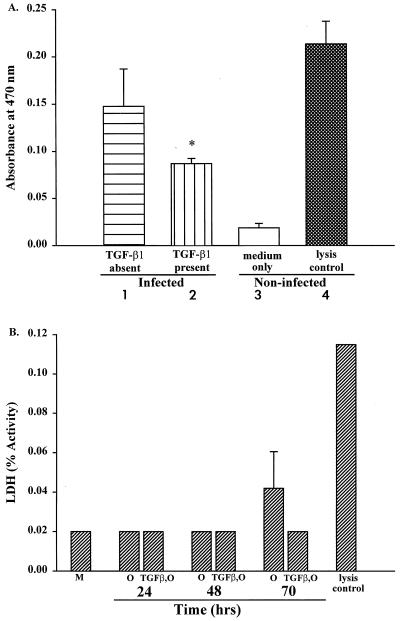
Exposure of human epithelial monolayers to C. parvum oocysts is reported to increase epithelial permeability to macromolecules, such as HRP, by 48 to 72 h (3). We wondered whether TGF-β1 might ameliorate this permeability change when monolayers are preexposed to TGF-β1. To determine this, we quantified the amount of HRP able to pass through the monolayer from its apical to its basolateral side on day 3 following the addition of oocysts, when the transmonolayer resistance is at its nadir, as shown in Fig. 3. Compared with monolayers incubated with oocysts alone (Fig. 6A, bar 1), those preexposed to TGF-β1 prior to the addition of oocysts showed a 42% reduction in the quantity of HRP able to pass through the monolayer into the basolateral chamber (Fig. 6A, bar 2). This difference was statistically significant (P < 0.05) [comparing oocyst-exposed monolayers with and without TGF-β1 pretreatment]). Controls consisted of monolayers exposed to medium only or to Triton X-100 (lysis control). These findings suggested that TGF-β1 is able to protect against a permeability change in epithelia when they are invaded by the protozoan C. parvum.
FIG. 6.
Effect of TGF-β1 on permeability and cell necrosis of human epithelial monolayers exposed to oocysts of C. parvum. (A) Decrease in macromolecular flow across C. parvum-challenged epithelial monolayers after a 48-h basolateral preexposure to 5.0 ng of TGF-β1/ml (bar 2). Following a 70-h incubation with oocysts, the monolayers were pulsed with HRP apically, and medium in the basolateral chamber was analyzed spectrophotometrically for HRP 2 h later by established techniques (16, 26). Controls consisted of monolayers exposed to C. parvum oocysts only, in the absence of TGF-β1 pretreatment (bar 1), those exposed to medium only (bar 3), and those incubated with Triton X-100 (bar 4). The flow of HRP across the monolayer was significantly reduced by TGF-β1 pretreatment; ∗, P < 0.05, comparing infected monolayers with and without preincubation with TGF-β1. (B) Release of LDH from epithelial cell monolayers at three time points after exposure to C. parvum oocysts (106/monolayer). Experimental monolayers were preincubated with TGF-β1 for 48 h and then exposed to oocysts (TGF-β1,O) at time zero. The controls were incubated with medium alone (M), oocysts alone (O), or Triton X-100 (lysis control). Samples for LDH analysis were taken at the time points shown, and the data are depicted as the mean ± 1 SD. At 70 h, TGF-β1 pretreatment reduced LDH release by 50%.
C. parvum-induced monolayer cell necrosis mitigated by TGF-β1.
We have previously reported that human epithelial cell monolayers exposed to C. parvum oocysts undergo modest cell necrosis after 48 to 72 h, as shown by release of LDH from the cells (3). To determine whether pretreatment with TGF-β1 would reduce or prevent cell necrosis, monolayers were incubated basolaterally with TGF-β1-containing medium for 48 h and then exposed on their apical surfaces to 106 C. parvum oocysts (Fig. 6B). While an elevated LDH was found at 70 h in the medium of epithelium exposed to oocysts, monolayers preincubated with TGF-β1 and then exposed to oocysts showed a 50% reduction in LDH release at that time, to a level near that of control monolayers incubated with medium alone (Fig. 6B, bar M). Similarly, at 105 oocysts per monolayer, which is fully capable of disrupting monolayer barrier function (3), LDH release at 70 h also showed a substantial reduction, i.e., LDH (percent activity) of 0.045 decreasing to 0.031. Taken together, these studies suggested two mechanisms by which TGF-β1 can protect barrier function provided by intestinal epithelium: reduction of organism-induced permeability change and limiting the cell necrosis that takes place when epithelia are infected with C. parvum.
TGF-β1 activates PKC in human colonic T84 epithelial cells.
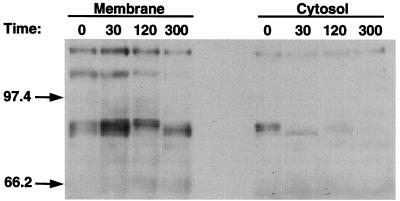
Although fresh mouse or rat epithelial cells, as well as the Caco-2 cell line, have been shown to activate PKC-α intracellularly (31, 33, 35), the identity of the induced PKC isozyme for the T84 cell line responding to TGF-β1 has not been defined. Therefore, we exposed T84 cells to TGF-β1 for 30, 120, and 300 s, lysed the cells, and analyzed cytosol and membrane fractions for translocation of the PKC-α isozyme. As seen in Fig. 7, the alpha isoform of PKC migrated from cytosol to membrane at 30 s, e.g., the amount of cytosol component detected at 86 kDa decreased at 30 s while, concomitantly, the amount of membrane component increased. These data suggest that the PKC-α isozyme is activated in T84 cells exposed to TGF-β1, and this could be important in the signaling pathway for TGF-β1 enhancement of epithelial barrier function.
FIG. 7.
TGF-β1 activates PKC-α isozyme in T84 epithelial cells. At the time intervals indicated (in seconds) after incubation of 106-cell aliquots of T84 cells with TGF-β1, cytosolic and membrane fractions were prepared and analyzed for immunoreactive PKC-α. Molecular mass markers (in kDa) are shown on the left. Translocation of the 85-kDa PKC-α isozyme was detected at 30 s.
DISCUSSION
The purpose of this study was to characterize the biology of TGF-β1 and its cell surface receptors in regulating the barrier function of intestinal epithelium exposed to a recognized tissue-invasive infectious agent, C. parvum. No medications are established for eradicating this protozoan from humans, and host cytokines which maintain or enhance intestinal barrier function in the presence of the organism have not been studied in detail. Published evidence documents the barrier-disruptive effect of C. parvum on human intestinal epithelial cell monolayers, and this has been proposed as a model of pathophysiological events in vivo (3, 14). Our experiments indicate that TGF-β1 strikingly protects barrier function in the face of a potent protozoan challenge (105 or 106 organisms/epithelial monolayer). Receptors necessary for a TGF-β1 effect were identified on the colonic epithelial cell by confocal microscopy and flow cytometry. Functionally, TGF-β1 initiated an intracellular signaling pathway involving PKC-α in these cells and was associated with limiting both protozoan-induced epithelial cell injury and increased permeability to macromolecules. This is the first report, we believe, in which the effect of an individual cytokine that enhances intestinal epithelial barrier function has been characterized in the setting of an agent that specifically infects the epithelium.
Prior studies of others (14), as well as our own (3), suggest that the C. parvum organism has a marked effect on intestinal epithelia, both structurally and functionally. Changes include the invasion of cell membranes (Fig. 1), release of intracellular enzymes (such as LDH), ready passage of macromolecules (such as HRP) across the previously intact epithelial cell monolayer, and loss of barrier function as quantitated electrophysiologically using the transmonolayer electrical potential difference. Our findings suggest a marked preservation of these parameters that reflect epithelial structure and function after incubation with TGF-β1 (Fig. 3 and 6), despite the presence of C. parvum. We suggest that these results are likely due to the effects of this cytokine on the epithelium itself, with alternative explanations for the protection of barrier function possible but less likely. First, one could propose that TGF-β1 directly retards the infectivity of C. parvum. However, prolonged incubation of TGF-β1 with oocysts did not alter their barrier-disrupting properties (Fig. 2C). Second, TGF-β1 could operate through other (nonepithelial) cells to protect barrier function. Our model, however, allows the component of epithelial cells in mucosa to be studied separately from other cell types, with changes in barrier function attributable to effects on epithelia alone.
The profile of RI-RII receptors that the T84 epithelial cell line expresses was elucidated in our studies. As determined by flow cytometry, some T84 cells are receptor negative, while the majority, express immunoreactive receptors with a range of receptor densities (Fig. 4B). This finding is consistent with the noncloned nature of this cell line and with in situ studies of human colon, where surface epithelium (top of the crypt) expressed RI and RII on each cell while epithelia in the basal portions of the crypt demonstrated neither (Fig. 1A). Further, images from confocal microscopy (Fig. 4A), comparing apical and basal portions of the plasma membrane, suggested that RI-RII receptor expression on receptor-positive cells was not uniform. Despite the heterogeneity in receptor expression among cells belonging to this well-established cell line, a remarkable change in barrier function occurs when monolayers of these cells are exposed to nanogram-per-milliliter concentrations of TGF-β1. Possible explanations for this discrepancy include the following: (i) small amounts of receptor on cells are able to bind ligand and change cell function, although they are not detectable by current techniques, and (ii) the functional change that we found with monolayers in response to TGF-β1 reflects the activity of receptor-positive cells and would be even greater in amount if all cells were to express this receptor.
The focus of the current work was on TGF-β1-driven barrier function, using multiple measures for its detection (electrophysiological methods, passage of macromolecules, and indicators of cell necrosis) and examining associated mechanisms (cell surface receptors and signaling pathways). These methodologies are not designed to distinguish between a possible TGF-β1-induced reduction of infection (e.g., fewer organisms at a given life cycle stage in the subapical epithelium over time) and a decrease in the effect of infection on epithelia (e.g., the same or increased number of organisms, with or without TGF-β1). However, the absence of a direct effect of TGF-β on the ability of the organism to disrupt barrier function (Fig. 2C) in this model system would suggest that the number of organisms was not reduced by TGF-β and that its impact was primarily on the capacity of the epithelium to withstand infection. While TGF-β1's effect on the epithelial response to C. parvum was not studied beyond 4 days, the rapidity of turnover of epithelial cells in vivo (e.g., every 96 h) suggests that more long-term studies may have limited physiological significance. In addition, clarification of the in vivo significance of our observations will likely await the development of methodologies to deliver TGF-β to colonic or small-bowel epithelial cells and/or its induction locally in tissue prior to exposure to a gastrointestinal pathogen.
Our report is one of the first to implicate a specific PKC isozyme in the regulation of human colonic epithelial barrier function. Thus, Tai and coworkers used a broadly reactive protein kinase inhibitor (H7, displaying similar inhibitory activities toward C, A, and G kinases) to show that alterations in protein kinase activity and in intracellular Ca2+ decreased the tight junction resistance of T84 cell monolayers (33). More recently, in a lung epithelial cell system, Ohtsuki and Massague showed the importance of a protein kinase for the cell effects of TGF-β1 but were unable to implicate PKC (25). In the present study, we showed that the α isozyme of PKC is activated in T84 cells within 30 s of their incubation with TGF-β1. This is consistent with other studies showing that PKC-α is prevalent in colonic mucosa and that it is involved in the control of epithelial proliferation and differentiation (1, 31, 35). It is of interest that these same cell characteristics (e.g., proliferation and differentiation) are also felt to be strongly influenced by TGF-β1 (6, 19, 23).
At the cellular level, there are several potential mechanisms for TGF-β1's beneficial effect on epithelial function. One is its known ability to enhance matrix protein synthesis by cells as diverse as fibroblasts, osteoblasts, and endothelial cells (18). This suggests that TGF-β1's enhancement of epithelial barrier function may rely on matrix-dependent processes, leading to strengthening of tight junctions between adjacent epithelial cells as well as a firmer anchorage of epithelial cells to the basement membrane in vivo or to the collagen-coated Nucleopore filter in vitro. Another potential mechanism for TGF-β1's favorable effect on epithelial function is its influence on nonepithelial cell types, allowing them to enhance the level of mucosal immune function in vivo that protects epithelia. This includes TGF-β1's ability to stimulate secretion by B lymphocytes, enhance in vivo T-lymphocyte effector function, increase the number of phenotypic antigen-specific memory T helper cells, and promote mononuclear, as well as neutrophil, chemotaxis (29, 30, 37). Third, TGF-β1 has been shown to be pivotal for wound healing in a rat epithelial cell system by increasing the rate of cell migration that repairs defects in the epithelial monolayer (11). Note that the strain of C. parvum (AUCp-1) used in our studies is infectious and causes diarrhea in humans and calves; nevertheless, we cannot exclude the possibility that strain differences exist that may affect epithelial barrier function or morphology in a manner that is similar but not identical to that which we report here. Furthermore, our system did not permit an evaluation of the effect of TGF-β1 on the glycocalyx layer adjacent to the epithelium in vivo or on other polarizable human cell lines that develop a stable transmonolayer resistance.
In summary, a prevalent cytokine was tested for its effect on C. parvum-induced changes in epithelial barrier function, as modeled in a well-established human epithelial cell monolayer system. TGF-β1 was shown to enhance transmonolayer electrical potential difference when present in nanogram quantities and to oppose the barrier-disruptive effects of a tissue-invasive protozoan, C. parvum, an important epithelium-invasive organism. Our data suggest that TGF-β1 limits both organism-induced epithelial cell necrosis and increases in permeability and that this effect may be mediated by PKC after ligation of TGF-β1 with its specific receptors on the surface of the epithelial cell. These studies suggest mechanisms by which TGF-β1 may play an important role in preserving epithelial barrier function in vivo in both physiological and pathophysiological circumstances.
ACKNOWLEDGMENTS
This work was supported in part by a U.S. Public Health Service grant (DK24289) from NIDDK, as well as by the Crohn's and Colitis Foundation of America.
We are indebted to William Ross in studies involving flow cytometry, Julianne Sando in investigation of PKC activation, and Ida Garrison for preparing the manuscript.
REFERENCES
- 1.Abraham C, Scaglione-Sewell B, Skarosi S F, Qin W, Bissonnette M, Brasitus T A. Protein kinase C α modulates growth and differentiation in Caco-2 cells. Gastroenterology. 1998;114:503–509. doi: 10.1016/s0016-5085(98)70533-5. [DOI] [PubMed] [Google Scholar]
- 2.Adams R B, Planchon S M, Roche J K. Interferon-γ modulation of epithelial barrier function: time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–2362. [PubMed] [Google Scholar]
- 3.Adams R B, Zu S, Fang G, Guerrant R L, Roche J K. Cryptosporidium parvum infection of intestinal epithelium: morphologic and functional studies in an in vitro model system. J Infect Dis. 1994;169:170–177. doi: 10.1093/infdis/169.1.170. [DOI] [PubMed] [Google Scholar]
- 4.Aguire S A, Perryman L E, Davis W C, McGuire T C. IL-4 protects adult C57BL/6 mice from prolonged Cryptosporidium parvum infection: analysis of CD4+ αβ+ IFN-γ+ and CD4+ αβ+ IL-4+ lymphocytes in gut-associated lymphoid tissue during resolution of infection. J Immunol. 1998;161:1891–1900. [PubMed] [Google Scholar]
- 5.Arnador E, Dorfman L E, Wacher W E C. Serum lactate dehydrogenase: an analytical assessment of current assays. Clin Chem. 1963;9:391–399. [PubMed] [Google Scholar]
- 6.Barnard J A, Beauchamp R D, Coffey R J, Moses H L. Regulation of intestinal epithelial cell growth by transforming growth factor type β. Proc Natl Acad Sci USA. 1989;86:1578–1582. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cereijido M, Robbins E S, Dolan W J, Rotunno C A, Sabatini D D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978;77:853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Checkley W, Gilman R H, Epstine L D, Suarez M, Diaz J F, Cabrera L, Black R E, Sterling C R. Asymptomatic and symptomatic cryptosporidiosis: their acute effect on weight gain in Peruvian children. Am J Epidemiol. 1997;145:156–163. doi: 10.1093/oxfordjournals.aje.a009086. [DOI] [PubMed] [Google Scholar]
- 9.Cosyns M, Tsirkin S, Jones M, Flarell R, Hikutani H, Hayward A R. Requirement for CD40-CD40 ligand interaction for elimination of Cryptosporidium parvum for mice. Infect Immun. 1998;66:603–607. doi: 10.1128/iai.66.2.603-607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culshaw R J, Bancroft G J, McDonald V. Gut intraepithelial lymphocytes induce immunity against Cryptosporidium infection through a mechanism involving gamma interferon production. Infect Immun. 1997;65:3074–3079. doi: 10.1128/iai.65.8.3074-3079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dignass A U, Podolsky D K. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor-β. Gastroenterology. 1993;105:1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- 12.Eskinazi R, Resibois A, Svoboda M, Peny M O, Adler M, Robberecht P, Van Lacthem J L. Expression of transforming growth factor β receptors in normal human colon and sporadic adenocarcinoma. Gastroenterology. 1998;114:1211–1220. doi: 10.1016/s0016-5085(98)70427-5. [DOI] [PubMed] [Google Scholar]
- 13.Gardner A L, Roche J K, Weikel C S, Guerrant R L. Intestinal cryptosporidiosis: pathophysiologic alterations and specific cellular and humoral immune responses in rnu/+ and rnu/rnu (athymic) rats. Am J Trop Med Hyg. 1991;44:49–62. doi: 10.4269/ajtmh.1991.44.49. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths J K, Moore R, Dooley S, Keusch G, Tzipori S. Cryptosporidium infection of CaCo-2 cell monolayers induces an apical monolayer defect, selectively increases transmonolayer permeability, and causes epithelial cell death. Infect Immun. 1994;62:4506–4514. doi: 10.1128/iai.62.10.4506-4514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrant D I, Moore S R, Lima A A M, Patrick P D, Shorling J B, Guerrant R L. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four–seven years later in a poor urban community in Northwest Brazil. Am J Trop Med Hyg. 1997;61:707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 16.Hecht G, Koutsouris A, Pathoulakis C, LaMont J T, Madara J L. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992;102:416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- 17.Heine J, Ignotz R A, Hemler M E, Crouse C, Massague J. Regulation of cell adhesion receptors by transforming growth factor-β. Concomitant regulation of integrins that share a common β1 subunit. J Biol Chem. 1989;264:380–388. [PubMed] [Google Scholar]
- 18.Ignotz R A, Massague J. Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- 19.Kurokowa M, Lynch K, Podolsky D K. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor β inhibits proliferation and stimulates differentiation. Biochem Biophys Res Commun. 1987;142:775–782. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lu Z, Liu D, Hornia A, Devonish W, Pagano M, Foster D A. Activation of protein kinase C triggers its ubiquitization and degradation. Mol Cell Biol. 1998;18:839–845. doi: 10.1128/mcb.18.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcial M A, Madara J L. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology. 1986;90:583–594. doi: 10.1016/0016-5085(86)91112-1. [DOI] [PubMed] [Google Scholar]
- 23.Moses H L. TGF-β regulation of epithelial cell proliferation. Mol Reprod Dev. 1992;32:179–184. doi: 10.1002/mrd.1080320215. [DOI] [PubMed] [Google Scholar]
- 24.Newman R D, Sears C L, Moore S R, Nataro J P, Wuhib J, Agnew D A, Guerrant R L, Lima A A A. A longitudinal study of Cryptosporidium infection in northeast Brazilian children. J Infect Dis. 1999;100:167–175. doi: 10.1086/314820. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsuki M, Massague J. Evidence for the involvement of protein kinase activity in transforming growth factor-β signal transduction. Mol Cell Biol. 1992;12:261–265. doi: 10.1128/mcb.12.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oritz de Montellano P R, David S K, Ator M A, Tew D. Mechanism-based inactivation of horseradish peroxidase by sodium azide. Formation of meso-azidoprotoporphyrin IX. Biochemistry. 1988;27:5470–5476. doi: 10.1021/bi00415a013. [DOI] [PubMed] [Google Scholar]
- 27.Planchon S M, Martins C A P, Guerrant R L, Roche J K. Regulation of intestinal epithelial barrier function by TGF-β1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol. 1994;153:5730–5739. [PubMed] [Google Scholar]
- 28.Resnick M S, Luo X, Vinton G, Sando J J. Selective up-regulation of protein kinase Cz in phorbal ester-sensitive versus resistant EL4 mouse thymoma cells. Cancer Res. 1997;57:2200–2215. [PubMed] [Google Scholar]
- 29.Rizzino A. Transforming growth factor-β: multiple effects on cell differentiation and extracellular matrices. Dev Biol. 1988;130:411–422. doi: 10.1016/0012-1606(88)90337-5. [DOI] [PubMed] [Google Scholar]
- 30.Roberts A B, Sporn M B. Transforming growth factor-β. In: Aggarwal B B, Gubberman J U, editors. Human cytokines: handbook for basic and clinical research. Boston, Mass: Blackwell Scientific Publications; 1992. p. 399. [Google Scholar]
- 31.Scaglione-Sewell B, Abraham C, Bissonnette M, Skarosi S F, Hart J, Davidson N O, Wali R K, Davis B H, Sitrin M, Brasitus T A. Decreased PKC-α expression increases cellular proliferation, enhances the transformation phenotype and decreases differentiation in CaCo-2 cells. Cancer Res. 1998;58:1074–1081. [PubMed] [Google Scholar]
- 32.Stiehm E, Clin T W, Hass A, Peerless A G. Infectious complications of the primary immunodeficiencies. Clin Immunol Immunopathol. 1986;40:69–86. doi: 10.1016/0090-1229(86)90070-x. [DOI] [PubMed] [Google Scholar]
- 33.Tai Y-H, Flick J, Levine S A, Madam J L, Sharp G W E, Donowitz R. Regulation of tight junction resistance in T84 monolayers by elevation in intracellular Ca2+: a protein kinase C effect. J Membr Biol. 1996;149:71–79. doi: 10.1007/s002329900008. [DOI] [PubMed] [Google Scholar]
- 34.Ungar B L, Soave R, Fayer R, Nash T E. Enzyme immunoassay detection of immunoglobulin M and G antibodies to Cryptosporidium in immunocompetent and immunocompromised persons. J Infect Dis. 1986;153:570–578. doi: 10.1093/infdis/153.3.570. [DOI] [PubMed] [Google Scholar]
- 35.Verstovsek G, Byrd A, Frey M R, Petrelli N J, Black J D. Colonocyte differentiation is associated with increased expression and altered distribution of protein kinase C isozymes. Gastroenterology. 1998;115:75–85. doi: 10.1016/s0016-5085(98)70367-1. [DOI] [PubMed] [Google Scholar]
- 36.Weikel C S, Roche J K. Diarrheal diseases in the immunocompromised patient. In: Guerrant R, editor. Clinical tropical medicine and communicable diseases. W. B. London, United Kingdom: Saunders; 1988. pp. 401–415. [Google Scholar]
- 37.Weinberg A D, Whitham R, Swain S L, Morrison W J, Wyrick G, Hoy C, Vandenbark A A, Offner H. Transforming growth factor-β enhances the in vivo effector function and memory phenotype of antigen-specific T helper cells in experimental autoimmune encephalomyelitis. J Immunol. 1992;148:2109–2117. [PubMed] [Google Scholar]