Abstract
Key Clinical Message
Cardiac ultrasound is recommended in investigating ischemic stroke events. There is increasing evidence that direct oral anticoagulants can be safely used instead of vitamin K antagonists in the setting of left ventricular thrombus.
Abstract
Cardioembolic stroke is responsible for an increasing number of ischemic strokes. Compared to other causes of stroke, cardioembolic strokes affect a larger brain area. Left ventricular (LV) thrombi account for up to 10% of cardioembolic strokes. It is essential to identify patients at high risk of LV thrombus formation, such as patients with a history of myocardial infarction, patients with reduced ejection fraction, or patients with cardiomyopathies. We present a patient with an ischemic stroke, and the cardiac ultrasound revealed a reduced ejection fraction and the presence of LV thrombus at the apex. The patient had no prior history of cardiovascular diseases. Even in a resource‐limited setting, cardiac ultrasound is recommended to investigate stroke or transient ischemic attack events, especially in patients with a prior history of myocardial infarction. Although patients with LV thrombus should be treated with oral anticoagulants for at least 3 months, the role of direct oral anticoagulants and the optimal period of anticoagulation in this setting needs further investigation.
Keywords: cardiac magnetic resonance, cardiomyopathies, echocardiogram, left ventricular thrombus, stroke
1. CASE REPORT
A 49‐year‐old male patient was admitted to the emergency department with confusion, right hemiplegia, right face drooping, and aphasia. The symptoms started at least 5 h before presentation. Neurological examination revealed 2/5 motor strength on the right upper and lower extremities and a positive Babinski sign on the right side. He was an active smoker with a history of ischemic stroke 10 years ago. He received only acetylsalicylic acid 100 mg orally. In his initial evaluation, brain computed tomography showed an acute ischemic infarct on the left frontal parietal region and an infarct on the M2 segment of the middle cerebral artery. The initial electrocardiogram showed sinus rhythm with q waves in the inferior leads, negative T waves in the inferior leads, and biphasic T waves in V3 and negative T waves in V4–V6 leads (Figure 1). Cardiac ultrasound was performed on the second day of hospitalization. It revealed a reduced left ventricular ejection fraction (LVEF) of 30%–35% with regional wall motion abnormalities (hypokinesia on the inferior, inferolateral wall, and the apex of the heart). Furthermore, a thrombus was noted at the apex of the left ventricle (LV) (thrombus diameter: 2.1 × 1.4 cm) (Figure 2). Blood tests for thrombophilia and autoimmune diseases were normal, while troponin levels were within normal range. The patient was treated with ramipril 2.5 mg once a day, spironolactone 25 mg once a day, metoprolol 25 mg twice a day and furosemide 20 mg once a day, acetylsalicylic acid 325 mg once a day and atorvastatin 40 mg once a day. After consulting with neurology regarding hemorrhagic transformation risk, enoxaparin 1 mg/kg twice a day was added to the treatment on the seventh day of hospitalization. The patient's motor strength progressively improved during the hospital stay. He was discharged on day 15 of hospitalization with complete restoration of muscle strength in the extremities and aphasia recovery. One month after the acute event, coronary angiography showed mildly atheromatous coronary arteries. Cardiac magnetic resonance was then performed and showed severe impairment of the LV systolic function (LVEF 30%) with regional wall motion abnormalities and infarct pattern scar with no viability of the apical and the mid inferior LV segments. Additionally, an LV thrombus was demonstrated at the apex (Figure 3). The patient deferred warfarin in favor of rivaroxaban, and a dose of 20 mg once a day was prescribed. During follow‐up, the patient remained asymptomatic, while the LV thrombus disappeared at 6 months of follow‐up.
FIGURE 1.
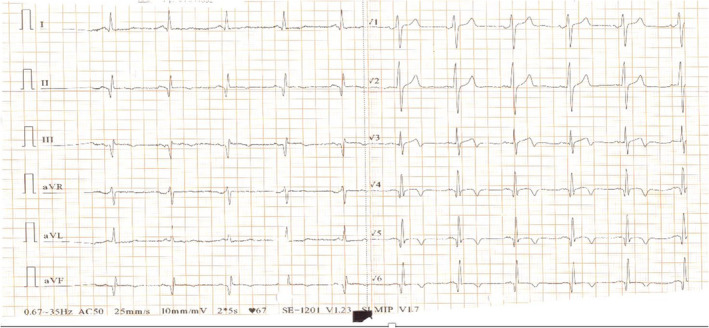
The initial electrocardiogram showed sinus rhythm with q waves in the inferior leads and negative T waves in the inferior leads, biphasic T waves in V3, and negative T waves in V4–V6 leads.
FIGURE 2.
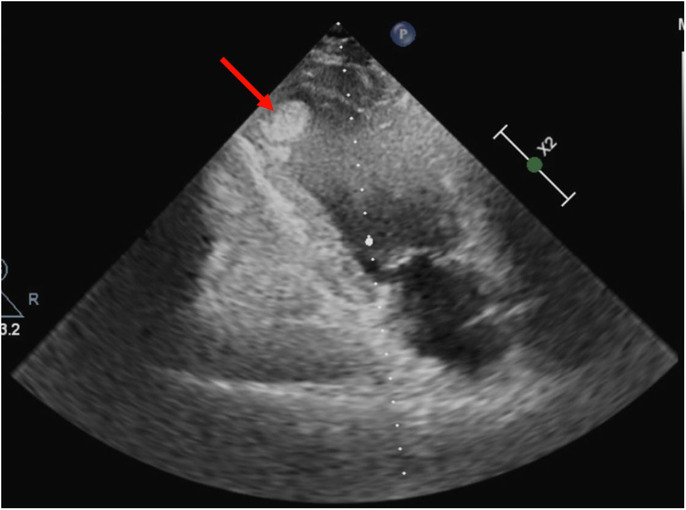
Cardiac ultrasound revealed a reduced left ventricular ejection fraction with regional wall motion abnormalities (hypokinesia on the inferior and inferolateral wall and the apex of the heart) and the presence of thrombus at the apex of the left ventricle (thrombus diameter: 2.1 × 1.4 cm).
FIGURE 3.
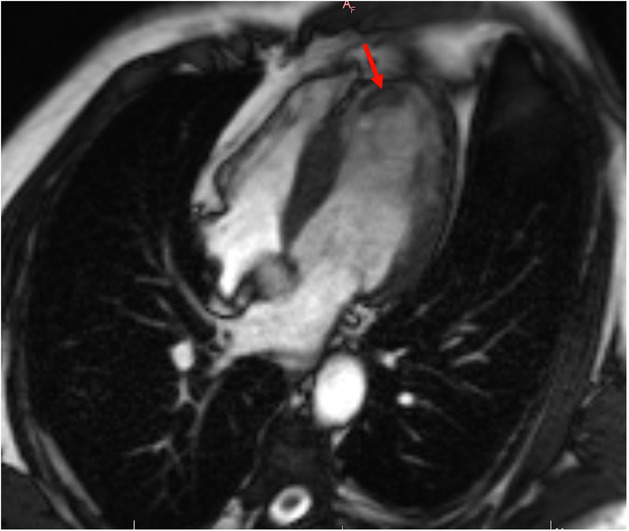
The cardiac MRI revealed the presence of late gadolinium enhancement (LGE) in the apical segments and the presence of thrombus in the apex of the left ventricle (red arrow).
2. DISCUSSION
Cardioembolic stroke is related to a worse prognosis because it usually affects a larger brain area. 1 The presence of LV thrombus accounts for up to 10% of cardioembolic strokes. It is often seen in patients with a history of myocardial infarction, especially patients with anterior STEMI, reduced LVEF, non‐ischemic cardiomyopathy, and a lower TIMI score. 2 The risk of thrombus formation is highest during the first 3 months after acute myocardial infarction. In addition, the risk for cerebral emboli persists in patients with chronic left ventricular dysfunction. 3 The pathophysiological mechanisms that can explain the thrombus formation in these patients include Intracavitary blood pooling, endocardial injury with inflammatory changes, collagen exposure, elevated fibrinogen and neutrophils, and hypercoagulability. 4
According to guidelines, cardiac ultrasound is included in the diagnostic workup of stroke or TIA, or peripheral embolism. 5 It is also recommended in patients with known or suspected cardiomyopathy to determine the extent of LV or RV dysfunction. Transthoracic and transesophageal echocardiograms are recommended when symptoms of a cardiac etiology are present. Cardioembolic sources of embolism are classified into cardiac pathologies that tend to form a thrombus, cardiac masses, and causes of paradoxical embolization, for example, patent foramen ovale. The main advantage of cardiac ultrasound is the feasibility of being performed on all patients with acute neurological events, even in low‐income countries. However, advanced cardiac imaging with cardiac MRI or contrast‐enhanced echocardiography with a microbubble contrast agent has shown to be superior to echocardiography without contrast for detecting left ventricular thrombus. 6 Transesophageal echocardiogram is an alternative, while CT is more accurate than transesophageal echocardiography for detecting thrombus in the LV. 7 Advanced imaging modalities can be implemented in patients with a high suspicion of LV thrombus and whose echocardiographic findings are inconclusive. It is recommended to perform a follow‐up echocardiogram at the end of the treatment to evaluate the resolution of the LV thrombus. 8
According to American Heart Association guidelines, anticoagulation with therapeutic warfarin is recommended in stroke patients with LV thrombus for at least 3 months until the thrombus has matured, to prevent a recurrent stroke event. 9 Post myocardial infarction patients with LV thrombus should also be treated with oral anticoagulant for 3 months. 10 In case the LV thrombus has been resolved and anticoagulation has been discontinued, a follow‐up cardiac echo exam 3 months later is advised. According to the AHA guidelines, in patients with LV thrombus in the setting of acute myocardial infarction, the addition of oral anticoagulation to dual antiplatelet therapy is recommended in the treatment of LV thrombus for a minimum period of 3 months. 9
There is insufficient data regarding prophylactic anticoagulation therapy in patients at high risk for LV thrombus formation. 8 According to the 2013 American College of Cardiology Foundation/AHA STEMI guidelines, a vitamin K antagonist (VKA) may be considered for patients with STEMI and anterior apical akinesis or dyskinesia (Class 2b, Level of Evidence: C). 11 Similarly, the American College of Chest Physicians guidelines recommend VKA therapy in addition to dual antiplatelet therapy for 3–6 months among patients with anterior myocardial infarction at high risk for LV thrombus (Grade 2C). 12 On the other hand, the 2017 European Society of Cardiology STEMI guidelines do not provide recommendations for prophylactic VKA therapy in patients post‐STEMI. 13
In patients with persistent LV thrombus despite treatment, an alternative oral anticoagulant or low molecular weight heparin can also be considered. 10 Recent meta‐analyses have shown that direct oral anticoagulants (DOACs) can be a reasonable treatment option in patients with LV thrombus. Specifically, DOACs are associated with lower bleeding and mortality rates than VKAs. 14 , 15 , 16 Additional advantages of DOACs include the lack of INR monitoring requirements, easy administration, and the absence of dietary restrictions. According to 2021 AHA guidelines, anticoagulation with DOACs is as effective as VKA in LV thrombus prevention. Although the thrombus resolution rates were similar between DOACs and VKA, resolution occurred significantly earlier with DOACs (within the first month). Furthermore, the combined endpoint of stroke of peripheral embolism was also lower with DOACs than with VKA. A previous meta‐analysis showed that LV thrombus detection after STEMI has an incidence of 20% in anterior STEMI with reduced LVEF. 17 Furthermore, patients with LV thrombus that received triple therapy had similar embolic but higher bleeding events than those with no LV thrombus treated with dual antiplatelet therapy. 17 On the other hand, another meta‐analysis showed a trend toward higher stroke or embolism with DOACs compared to VKAs. 18 Other studies showed no significant difference between major bleeding, systemic thromboembolic events, or failure of resolution of LV thrombus between patients taking VKA or DOCAs. 19 , 20 Further randomized controlled trials are needed to compare the use of VKA to DOACs in LV thrombus, although they seem to be a promising treatment.
3. CONCLUSIONS
Cardioembolic stroke is responsible for an increasing number of ischemic strokes. Cardiac ultrasound is recommended to investigate stroke or TIA events, especially in patients with a prior history of anterior myocardial infarction. Further studies are needed to elucidate the role of DOACs and the optimal duration of anticoagulation in the setting of LV thrombus.
AUTHOR CONTRIBUTIONS
Vasiliki Vanesa Stylianou: Writing – review and editing. Vasiliki Tsampasian: Writing – review and editing. Marios Pavlou: Writing – review and editing. Panagiota Georgiou: Writing – review and editing. Dimitrios Patestos: Writing – review and editing. Lorentzos Kapetis: Writing – review and editing. Vassilios S. Vassiliou: Writing – review and editing. Christos Eftychiou: Writing – review and editing. Michalis Tsielepis: Writing – review and editing. George Bazoukis: Supervision; writing – original draft; writing – review and editing.
FUNDING INFORMATION
Not received.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
None.
Stylianou VV, Tsampasian V, Pavlou M, et al. Left ventricular thrombus in a patient with recurrent ischemic stroke events—The role of echocardiography. Clin Case Rep. 2023;11:e7300. doi: 10.1002/ccr3.7300
DATA AVAILABILITY STATEMENT
None.
REFERENCES
- 1. Kamel H, Healey JS. Cardioembolic stroke. Circ Res. 2017;120:514‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shacham Y, Leshem‐Rubinow E, Ben Assa E, et al. Frequency and correlates of early left ventricular thrombus formation following anterior wall acute myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2013;111:667‐670. [DOI] [PubMed] [Google Scholar]
- 3. Delewi R, Nijveldt R, Hirsch A, et al. Left ventricular thrombus formation after acute myocardial infarction as assessed by cardiovascular magnetic resonance imaging. Eur J Radiol. 2012;81:3900‐3904. [DOI] [PubMed] [Google Scholar]
- 4. Kushner A, West WP, Khan Suheb MZ, Pillarisetty LS. Virchow Triad. StatPearls ; 2022. [PubMed] [Google Scholar]
- 5. Pepi M, Evangelista A, Nihoyannopoulos P, et al. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr. 2010;11:461‐476. [DOI] [PubMed] [Google Scholar]
- 6. Abdelmoneim SS, Pellikka PA, Mulvagh SL. Contrast echocardiography for assessment of left ventricular thrombi. J Ultrasound Med. 2014;33:1337‐1344. [DOI] [PubMed] [Google Scholar]
- 7. Aimo A, Kollia E, Ntritsos G, et al. Echocardiography versus computed tomography and cardiac magnetic resonance for the detection of left heart thrombosis: a systematic review and meta‐analysis. Clin Res Cardiol. 2021;110:1697‐1703. [DOI] [PubMed] [Google Scholar]
- 8. Camaj A, Fuster V, Giustino G, et al. Left ventricular thrombus following acute myocardial infarction: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2022;79:1010‐1022. [DOI] [PubMed] [Google Scholar]
- 9. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364‐e467. [DOI] [PubMed] [Google Scholar]
- 10. Levine GN, McEvoy JW, Fang JC, et al. Management of patients at risk for and with left ventricular thrombus: a scientific statement from the American Heart Association. Circulation. 2022;146:e205‐e223. [DOI] [PubMed] [Google Scholar]
- 11. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78‐e140. [DOI] [PubMed] [Google Scholar]
- 12. Vandvik PO, Lincoff AM, Gore JM, et al. Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e637S‐e668S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119‐177. [DOI] [PubMed] [Google Scholar]
- 14. Mir T, Sattar Y, Attique HB, et al. Meta‐analysis of direct oral anticoagulants compared with vitamin K antagonist for left ventricle thrombus. Cardiovasc Revasc Med. 2022;35:141‐146. [DOI] [PubMed] [Google Scholar]
- 15. Kido K, Ghaffar YA, Lee JC, et al. Meta‐analysis comparing direct oral anticoagulants versus vitamin K antagonists in patients with left ventricular thrombus. PLoS One. 2021;16:e0252549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trongtorsak A, Thangjui S, Kewcharoen J, et al. Direct oral anticoagulants vs. vitamin K antagonists for left ventricular thrombus: a systematic review and meta‐analysis. Acta Cardiol. 2021;76:933‐942. [DOI] [PubMed] [Google Scholar]
- 17. Bulluck H, Chan MHH, Paradies V, et al. Incidence and predictors of left ventricular thrombus by cardiovascular magnetic resonance in acute ST‐segment elevation myocardial infarction treated by primary percutaneous coronary intervention: a meta‐analysis. J Cardiovasc Magn Reson. 2018;20:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdelaziz HK, Megaly M, Debski M, et al. Meta‐analysis comparing direct oral anticoagulants to vitamin K antagonists for the management of left ventricular thrombus. Expert Rev Cardiovasc Ther. 2021;19:427‐432. [DOI] [PubMed] [Google Scholar]
- 19. Shah S, Shah K, Turagam MK, et al. Direct oral anticoagulants to treat left ventricular thrombus‐a systematic review and meta‐analysis: ELECTRAM investigators. J Cardiovasc Electrophysiol. 2021;32:1764‐1771. [DOI] [PubMed] [Google Scholar]
- 20. Ali Z, Isom N, Dalia T, et al. Direct oral anticoagulant use in left ventricular thrombus. Thromb J. 2020;18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.


