Significance
The human language network is represented across frontal and temporal cortices. It is unclear whether subregions of this network differentially contribute to sentence comprehension. We monitored neural activity of patients implanted with intracranial electrodes as they read regular sentences and sentences deficient of meaning or structure. We uncovered two functionally distinct networks spanning frontotemporal cortex. Activity of the first network progressively increased across sentences, but not word lists, indexing the buildup of meaning across sentences. The second network showed reduced activity for words in sentences relative to word lists, suggesting that sentential context facilitates the processing of individual words. Our study exposes previously unknown organizational principles of the language network, parcellating core components of sentence comprehension across spatiotemporally distributed networks.
Keywords: human, language, reading, intracranial recording, electrophysiology
Abstract
Reading a sentence entails integrating the meanings of individual words to infer more complex, higher-order meaning. This highly rapid and complex human behavior is known to engage the inferior frontal gyrus (IFG) and middle temporal gyrus (MTG) in the language-dominant hemisphere, yet whether there are distinct contributions of these regions to sentence reading is still unclear. To probe these neural spatiotemporal dynamics, we used direct intracranial recordings to measure neural activity while reading sentences, meaning-deficient Jabberwocky sentences, and lists of words or pseudowords. We isolated two functionally and spatiotemporally distinct frontotemporal networks, each sensitive to distinct aspects of word and sentence composition. The first distributed network engages the IFG and MTG, with IFG activity preceding MTG. Activity in this network ramps up over the duration of a sentence and is reduced or absent during Jabberwocky and word lists, implying its role in the derivation of sentence-level meaning. The second network engages the superior temporal gyrus and the IFG, with temporal responses leading those in frontal lobe, and shows greater activation for each word in a list than those in sentences, suggesting that sentential context enables greater efficiency in the lexical and/or phonological processing of individual words. These adjacent, yet spatiotemporally dissociable neural mechanisms for word- and sentence-level processes shed light on the richly layered semantic networks that enable us to fluently read. These results imply distributed, dynamic computation across the frontotemporal language network rather than a clear dichotomy between the contributions of frontal and temporal structures.
Reading a sentence entails rapidly combining meaning from multiple lexical objects while taking into account their syntactic relationships. It is generally believed that this process is distributed between the lateral frontal and temporal cortices as suggested by functional imaging and lesion symptom analyses. Functional imaging studies, generally using a contrast of activation to sentences > pseudowords, primarily implicate two regions in deriving meaning from sentences—the inferior frontal gyrus (IFG) and the superior temporal sulcus/middle temporal gyrus (STS/MTG) (1–3).
These regions are thought to have distinct roles (4, 5) but are richly connected (6, 7), enabling them to function as part of the general-purpose distributed semantic network (8, 9). Activity of the IFG varies with sentence complexity (10), with subregions thought to be specifically engaged in syntax (11), and with lesions of IFG resulting in agrammatism (12). The MTG, on the contrary, is thought to be engaged in hierarchical sequence processing (13, 14) and is functionally segregated along the antero–posterior axis (3, 15–17), with the anterior MTG/temporal pole believed to integrate conceptual information over long timescales, while the posterior STS/MTG encodes morphosyntactic structure (18, 19). Indeed, MTG lesions are associated with paragrammatisms (12) and morphosyntactic deficits (19). Prior work (3, 11, 20–23) has revealed monotonically ramping activation in both IFG and STS/MTG with increasing sentence length while reading. This is believed to index a buildup of semantic and syntactic information and may be linked to sentence complexity and syntactic tree structure (18, 20, 23, 24).
The specific and distinct mechanisms by which these two major hubs of the language network enable the derivation of meaning remain debated (3, 25, 26). These are the open questions that we address here—do these two closely interconnected hubs display distinct contributions to sentence processing and how do they dynamically interact within the language network?
To derive a spatiotemporal map of the processes that enable structure building during reading, we used intracranial recordings in 36 individuals, with 2,675 electrodes in their left, language-dominant hemisphere, as they silently read sentences and lists made either of real words or pseudowords (Fig. 1). We developed a unique method for mixed-effects analysis on the cortical surface to isolate neural correlates of sentence-level processing while accounting for characteristics of individual words and for the uneven sampling intrinsic to cortical recordings in humans. We sought to isolate regional and network-based activation profiles that correlated specifically with either single word reading or the assimilation of words into meaningful sentences.
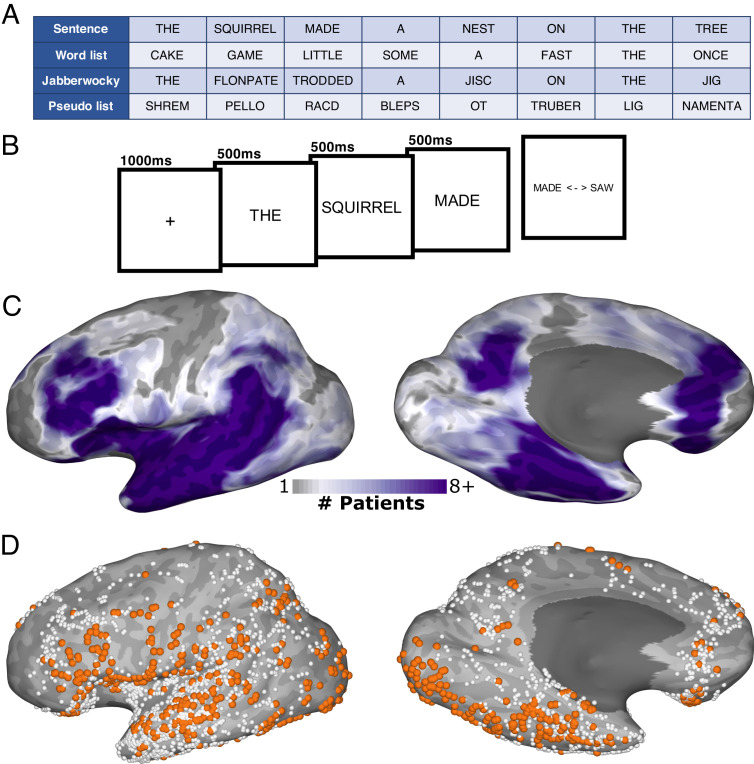
Fig. 1.
Experimental design and electrode coverage. (A) Example stimuli and (B) schematic representation of the sentence-reading task. (C) Representative coverage map (36 patients) and (D) individual electrode locations (2,675 electrodes) for the left hemisphere, highlighting electrodes responsive over baseline in orange [536 electrodes; ln(BF10) > 2.3, BGA > 5%].
Results
Thirty-six participants with intracranial electrodes placed for the localization of intractable epilepsy were visually presented with structured real-word or Jabberwocky sentences, or with unstructured word or pseudoword lists (Fig. 1A). Words were presented in rapid serial visual presentation format at a comfortable reading pace (500 ms/item), followed by a forced choice decision between a presented and nonpresented word (Fig. 1B). Thirty-four participants had stereotactic electroencephalography electrodes (sEEGs) and two had subdural grid electrodes (SDEs) (Fig. 1 C and D).
Behavioral Performance.
Participants identified the target word correctly in the two-alternative forced choice at a rate of (mean ± SD): sentences, 97 ± 3%; word lists, 93 ± 5%; Jabberwocky, 90 ± 6%; pseudoword lists, 86 ± 8%. Response accuracy was significantly affected by the presence of sentence structure [binomial generalized linear mixed model (GLMM), t(12,297) = 8.6, β = 0.64, P < 0.001, 95% CI = 0.49 to 0.78] and lexicality [t(12,297) = 13.3, β = 1.01, P < 0.001, 95% CI = 0.85 to 1.15]. Only correctly answered trials were used for further analysis. Response times (RTs) were: sentences, 1,672 ± 402 ms; word lists, 1,755 ± 402 ms; Jabberwocky, 1,909 ± 448 ms; pseudoword lists, 1,963 ± 456 ms. RT was significantly affected by the presence of sentence structure [GLMM, t(10,768) = −6.6, β = −72.1 ms, P < 0.001, 95% CI = −94 to −51 ms] and lexicality [t(10,768) = −18.1, β = −199.2 ms, P < 0.001, 95% CI = −221 to −178 ms].
Spatiotemporal Representation of Sentence Structure.
We generated population-level maps to tease apart the factors driving cortical activation, via a unique grouped analysis of population electrocorticography (ECoG) data. This surface-based linear mixed-effects (sbLME) analysis builds on our prior univariate, population-level ECoG analytical tool, surface-based mixed-effects multilevel analysis (27). This model used the mean broadband gamma activity (BGA; 70 to 150 Hz) from 300 to 500 ms after the onset of each word (Fig. 2 and SI Appendix, Fig. S2) in normalized cortical surface space. sbLME enables the separation of contributions of multiple linguistic features to BGA while adjusting for unequal sampling and interindividual variations in activation. The features used for these analyses included sentence structure, word position, word frequency, and lexicality. The specific time window of 300 to 500ms after word onset was based on our prior work that shows this interval is critical for word reading (28, 29).
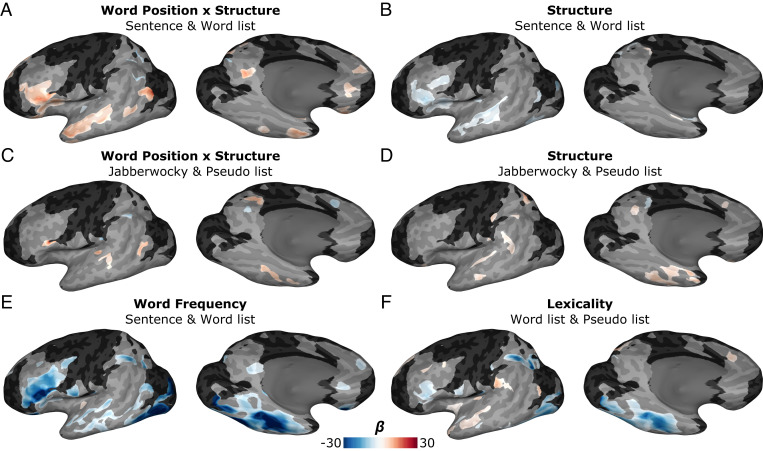
Fig. 2.
sbLME modeling of sentence reading. Spatial maps of cortical regions showing significant clusters (P < 0.01) of sensitivity to lexical and sentential factors for the 300 to 500 ms window. (A, B) Results from the combined sentence and word list model showing regressors for (A) the interaction between word position and sentence structure and (B) the main effect of the presence of sentence structure. (C, D) Results from the combined Jabberwocky sentence and pseudoword list model showing regressors for (C) the interaction between word position and sentence structure and (D) the main effect of the presence of sentence structure. (E) Word frequency and (F) lexicality regressors are also shown. Regions in black did not have sufficient coverage for reliable sbLME results (<3 patients). Full results of each sbLME model are shown in SI Appendix, Fig. S2.
First, we applied an sbLME model combining all words from the sentence and word list conditions. This revealed a positive interaction effect between word position and sentence structure, indexing a greater ramp-up of activity for sentences than word lists along the STS, the anterior IFG, and the medial surface of the frontal operculum (FO) (Fig. 2A). We also observed a main effect of sentence structure, independent of word position, with suppressed activity for sentences relative to word lists (Fig. 2B). In contrast, a comparable sbLME model for pseudowords, including all words from the Jabberwocky and pseudoword list conditions, revealed minimal effects of sentence structure, localized to a small portion of the posterior MTG (Fig. 2 C and D).
We also examined the main effects of frequency and lexicality. An sbLME model restricted to stimuli with real words (sentence and word list conditions) displayed a significant effect of word frequency across the ventral occipitotemporal cortex, IFG, and lateral temporal cortex (Fig. 2E). A model of words and pseudowords deficient of syntax (word list and pseudoword list conditions) revealed lexicality effects, with greater activation for pseudowords in the mid-fusiform cortex, inferior parietal sulcus, and anterior IFG, and greater activity for known words in the anterior lateral temporal lobe (Fig. 2F).
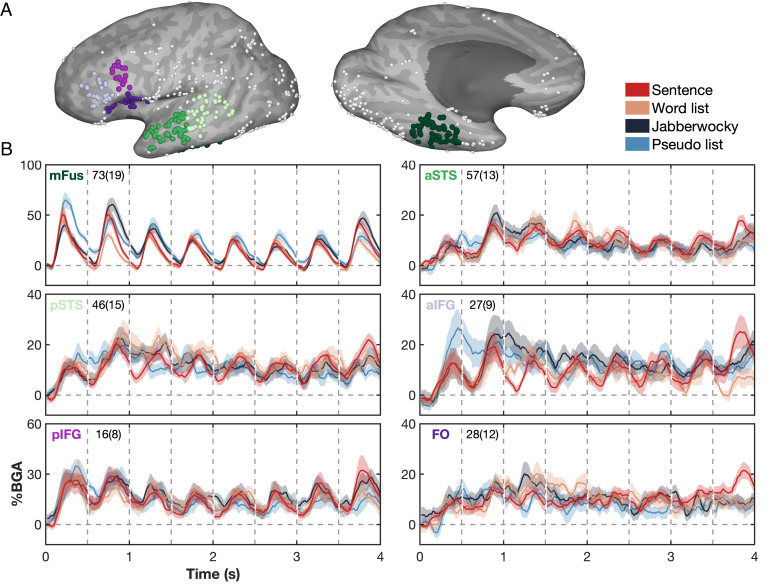
These sbLME analyses elaborated regions principally engaged in reading words and sentences, generally concordant with expectations from the literature (3, 28–30). To visualize the time course of their responses, we used Region of interests (ROIs) centered on six anatomical regions—mid-fusiform cortex (mFus), anterior and posterior superior temporal sulcus (aSTS and pSTS), anterior and posterior inferior frontal gyrus (aIFG and pIFG), and the medial FO—to isolate the spatiotemporal properties of the responses. All the six ROIs showed activity peaks locked to presentation of each word (Fig. 3B), across all the four conditions. In aIFG and mFus, there was greater activation for pseudowords than known words regardless of whether they occurred in sentences or in lists (Fig. 3B and SI Appendix, Fig. S3). By comparison, the pIFG showed little differentiation between pseudowords and known words. Note that pseudoword lists were the only condition that reliably contained complex and unknown words in word position 1, accounting for the prominent initial activation across ROIs.
Fig. 3.
Temporal dynamics of the six selected anatomical ROIs. (A) ROI definitions, using only responsive electrodes. (B) Between-patient average (mean ± SE) BGA responses of each ROI, to the four experimental conditions. Number of electrodes and patients per ROI is shown. mFus, midfusiform cortex; aSTS, anterior superior temporal sulcus; pSTS, posterior superior temporal sulcus; aIFG, anterior inferior frontal gyrus; pIFG, posterior inferior frontal gyrus; FO, frontal operculum.
Analysis of the right, nondominant hemisphere showed minimal activation time-locked to the presentation of each word across the lateral frontotemporal cortex, and no significant distinctions between conditions (SI Appendix, Fig. S4).
For each ROI, we also analyzed activity over time using linear mixed-effects (LME) models, combining sentences and word lists, to disentangle sensitivities to word frequency and sentence structure (SI Appendix, Fig. S5). We observed significant, consistent, and prominent modulation of activity in the mFus and aIFG by word frequency. Across STS, aIFG, and FO, we observed significant effects of sentence structure: The activity was initially smaller for sentences relative to word lists, but starting at word 2, there was a progressive ramping up of activity, ending up with significantly greater activation for the final word of sentences relative to word lists.
Functional Clustering of Sentence Structure Responses.
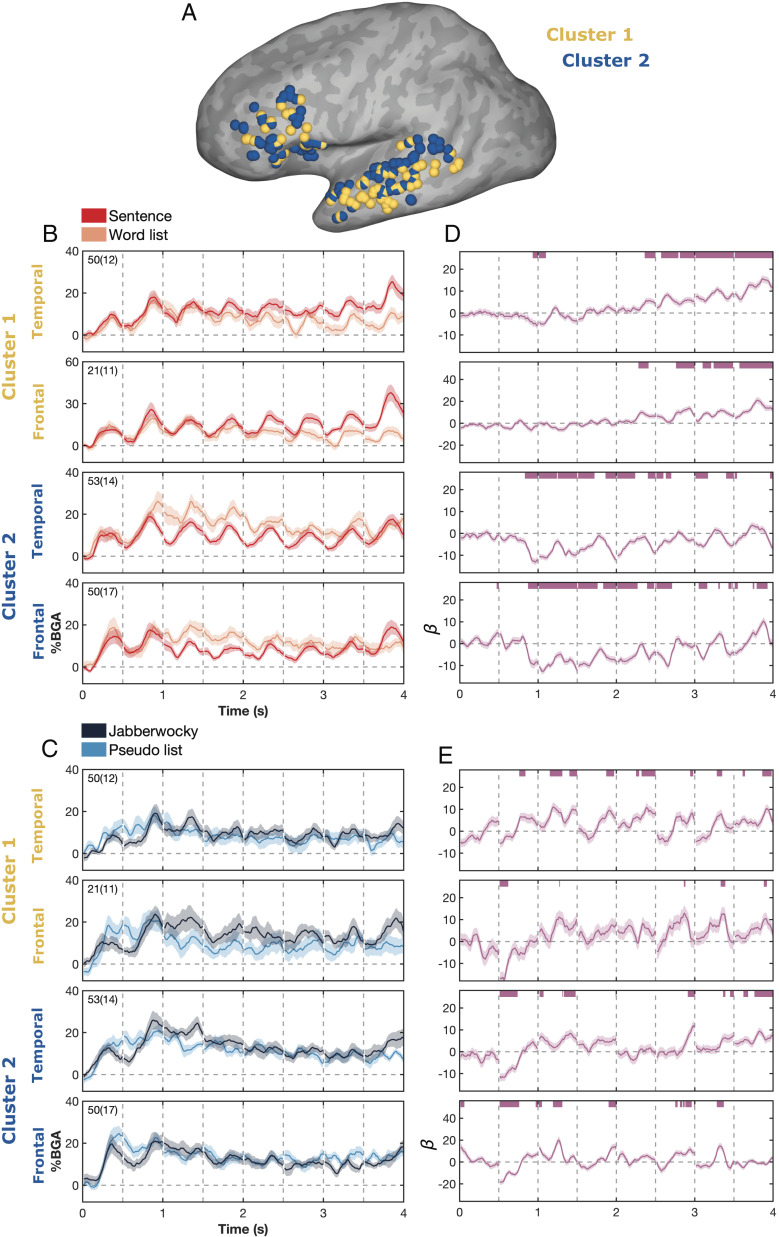
The ROI-based analysis provides insights into regional functions but amalgamates responses across many electrodes within prespecified anatomical bounds. However, the organization of integrative lexical semantics may not respect such prespecified boundaries and likely shows substantial interindividual variability in localization (1, 15). To overcome this, and perform a data-driven analysis, we clustered the 174 active lateral temporal and frontal electrodes based on their functional properties. For each electrode, we calculated the LME-derived time course of their structure sensitivity (sentence vs. word list). From this, we calculated the principal components of these time courses and grouped electrodes using k-means clustering on the principal component loadings, with k determined from the results of a silhouette test.
This revealed two functionally separable response types in electrodes located in the lateral frontal and temporal cortices (Fig. 4A and SI Appendix, Figs. S6 and S7). Both clusters showed word-induced activity, with a peak in activity after each item, but in cluster 1 (71 electrodes, 17 patients), there was a smooth, slow ramping increase in activity over time for sentences relative to word lists, especially after word 5 (Fig. 4D). Testing at a single electrode level, 23/71 electrodes (32%) in cluster 1 showed a significant ramping response (q < 0.05; SI Appendix, Fig. S8). Cluster 2 (103 electrodes, 21 patients), while also exhibiting some ramping, showed primarily a lower activity for sentences, starting at word 2, and greater sustained activity for word lists. In lateral temporal regions, this effect was stable across the duration of the sentence, with a structure-related suppression after each word presentation. Such structure effects were not observed when contrasting Jabberwocky sentences with pseudoword lists. There was a tendency for Jabberwocky to evoke greater activity than the corresponding list of pseudowords in both the frontal and temporal electrodes of cluster 1; however, this was only significant at restricted time points (Fig. 4E).
Fig. 4.
Functional clustering of responses to sentence structure vs. word lists. (A) Spatial map of electrode membership to the two derived functional clusters. (B and C) Mean BGA (± across-patient SE) for (B) sentences and word lists, and (C) Jabberwocky and pseudoword lists, and (D and E) LME beta values (β ± SE) for the structure regressor-contrasting (D) sentences and word lists, and (E) Jabberwocky and pseudoword lists, for electrodes in each cluster in lateral temporal or frontal ROIs. Word length and frequency effects were regressed out of the real-word LME, and word length and orthographic neighborhood were regressed out of the Jabberwocky LME. Responses to real-words and function words were removed from the LME for Jabberwocky sentences. Number of electrodes and patients per cluster is shown. Colored bars represent regions of significance from the LME analyses (q < 0.01).
In addition to their distinct functional response profiles, these clusters also showed partial anatomical dissociations. In the lateral temporal lobe, this manifested as a superior–inferior organization, with electrodes in the MTG mostly in cluster 1 and those in the STG mostly in cluster 2 (Fig. 4A) (Talairach coordinates: center of mass; Temporal 1, -53 -18 -4; Temporal 2, -55 -19 1). This segregation was less organized in the IFG, with the two clusters colocalizing to the same general center of mass coordinates (Frontal 1, -41 23 16; Frontal 2, -40 24 15). The clusters did not represent across-patient variations, but rather within-patient variability—eight patients with coverage of lateral temporal cortex and 10 patients with frontal cortex coverage had at least one electrode in each cluster within that region.
Running a comparable clustering analysis on the Jabberwocky vs. pseudoword list, contrast did not produce a clear functional separation, with 154/174 electrodes being assigned to a single cluster and no clear, interpretable response types observed in the principal components (SI Appendix, Fig. S9).
To attempt to further explain the factors modulating activity within individual sentences, we regressed the activity against the number of open nodes and number of closing nodes, as derived from a left-corner X-Bar parsing model (31) (SI Appendix, Fig. S10). When contrasting between words where a node closure occurred vs. words where there was no node closure, there initially appeared to be enhancement of activation in the IFG. However, node closures occur almost exclusively after low-frequency content words and when word frequency and length were regressed out, there was no significant effect of open nodes or node closures at any word position.
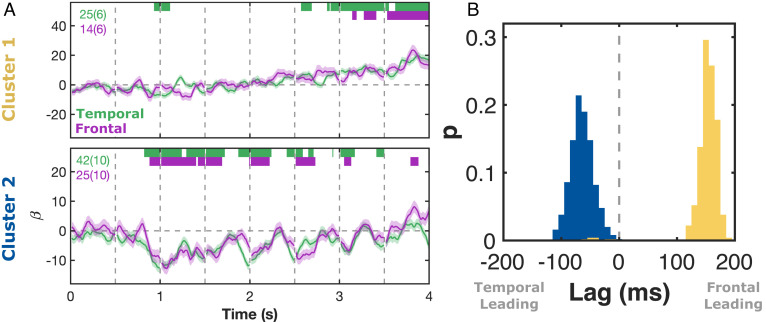
It stands to reason that there should be interactions within each of these two clusters during fluent reading. To investigate the interactions between frontal and temporal cortical areas, we considered only patients with electrodes in both anatomical regions within each functional cluster (Fig. 5A). We used the time course of the structure regressor within each electrode to probe interactions between the frontal and temporal electrodes by finding the time lag that maximized the crosscorrelation between regions (Fig. 5B). Within cluster 1 (62 electrode pairs), this analysis demonstrated that activity in the frontal cortex significantly preceded that in the temporal cortex (median lag = 150 ms, P = 0.016, 95% CI = 120 to 180 ms). However, within cluster 2 (101 electrode pairs), structure-related activity in the temporal electrodes preceded that in the frontal electrodes (median lag = −70 ms, P = 0.002, 95% CI = −100 to −20 ms).
Fig. 5.
Temporal interactions of structure-based functional clusters. (A) LME beta values (β ± SE) for the structure regressor-contrasting sentences and word lists, for patients with electrodes within each cluster in both frontal and temporal cortices. Word length and frequency effects were regressed out of the LME. Number of electrodes and patients per cluster is shown. Colored bars represent regions of significance from the LME analyses (q < 0.01). (B) Probability distribution of the time lag of maximum likelihood crosscorrelation of the structure responses between pairs of electrodes (500 iterations), within-patients, within-clusters, across regions.
Discussion
Our characterization of the cortical substrates underlying sentence structure and semantics reveals two functionally and spatiotemporally distinct frontotemporal networks sensitive to different aspects of sentence comprehension. In agreement with prior research (3, 21, 23), in network 1, we observed ramping increases in activation along the duration of the sentences, as compared to word lists. This ramping effect is seen only in the latter portions of the sentence, from word 5 onward. However, the principal component analysis suggests that this ramping unfolds starting with the second word, but the effect size is likely too small to achieve significance in the early stages of sentence construction. Crucially, the ramping activation was much weaker or absent in Jabberwocky sentences, and therefore likely represents predominantly the overall buildup of semantic information across a sentence, rather than the syntactic operations which could still be performed, to a large extent, during Jabberwocky. Our experimental design was based on the functional MRI (fMRI) results of Pallier et al. (3) who reported that, during Jabberwocky reading, a syntactic buildup still occurred, but was restricted to a relatively narrow subset of regions within the pSTS and IFG. Indeed, we did find small such signals in the posterior STS/MTG (Fig. 2C), a region which we have also shown is a good candidate for the elementary syntactic “merge” operation (3, 23, 32, 33). We cannot be certain that ramping-up during Jabberwocky would not be significant at this and other sites if our electrode sampling was higher. Nevertheless, our data thus far show that when sentences are delexicalized (as in Jabberwocky), sentence-related activation collapses in the vast majority of, if not all the language comprehension network, in broad agreement with previous positron emission tomography, fMRI, and intracranial findings (3, 21, 34). Beyond what is available to fMRI, we also observed that structure-related activity in the frontal regions of cluster 1 preceded that of the temporal cortex, potentially suggesting that this signature originates in the IFG before being inherited by the MTG. This timing is compatible with the hypothesis that IFG comprises one or several mechanisms for binding or unifying lexical representations into tree-like phrasal structures before sending them back to posterior temporal areas (35, 36).
Our second functional cluster in the STG and IFG demonstrates greater sustained activity following each lexical item in word lists as compared to sentences. We could not find prior published examples documenting a directly analogous functional response type in the context of sentence comprehension. The close spatial proximity and interindividual variability of the two clusters likely hamper population-level dissociation, as our sbLME analyses demonstrated population-level colocalization of these two functional profiles, meaning that such separation should be performed within individuals. The functional dissociation is also temporal in nature, with dissociation between sentences and word lists only strongly apparent midsentence and less so when integrating across the entire sentence, reducing its visibility to fMRI studies of reading. These dissociations occur after each presented word, rather than as an integration across the sentence as in cluster 1, suggesting an influence of sentential context on the representation of each individual word rather than an ongoing buildup of information during the construction of sentence structure. This would fit with linguistic models proposing that individual lexical representations are updated based on the syntactic role they play within a given sentence (37, 38). The reduced activity in this cluster may reflect the influence of predictive mechanisms that exist at multiple levels of the language-processing system (17, 39–44) and lead to a reduction in activity for words whose sentential context makes them partially predictable. Alternatively, it may also reflect a reduced effort of working memory specifically for known lexical items (45), as this region shows lexical selectivity, and behavioral data demonstrate easier recall of words from sentences than lists. The frontal portion of this cluster also shows a comparable localization to part of the fMRI-defined multiple demand network, a domain-general working memory system (46, 47), as well to areas whose fMRI dynamics during sentence comprehension suggest a role as a transient working-memory buffer (48). In contrast to cluster 1, this functional response appears to occur first in the temporal cortex before propagating to the IFG, compatible with a prefrontal buffering of lexical information first extracted in the temporal areas. The opposing streams of information between frontal and temporal language regions highlight the degree of interconnectivity between these hubs of the language network.
As the second cluster shows greater activation for word lists over sentences, it could be suggested that this region is not specialized for sentence processing. However, neural activation has been postulated to reflect a combination of both engagement and efficiency in an “inverted-U” response profile (49). For example, as we have previously shown, mFus does not engage for false letter stimuli (29) and shows strong activation for word-like pseudowords which are challenging to process, but low activation for high-frequency words, which are efficiently processed. This could suggest that, while presentation of multiple lexical objects in a sequence engages cluster 2, the network can more efficiently process sequences in the presence of sentential syntactic structure, resulting in reduced activation for sentences relative to word lists.
Many prominent language models ascribe distinct semantic and syntactic functions to frontal and temporal components of the language network (2, 4, 5, 18, 41, 50). Here, we show however that computational properties relating to semantic integration are distributed across frontotemporal cortices, with both regions sharing functional properties across a distributed network. These results suggest that neuroanatomical models of language need to evolve beyond simple localizationist models, to incorporate the dynamic interactions between hubs of multiple networks. While cortical sites within the two clusters exhibit local representation of information, they also belong to overlapping networks with synergy between frontal and temporal cortices. Here, we are presenting these functional types as distinct clusters; however, it is likely we are representing two poles of a multidimensional continua, with each electrode varying along a functional axis rather than representing dichotomous categories (SI Appendix, Fig. S6C). The primary distinction we observed between the frontal and temporal regions was in the sensitivity to the properties of single words, in the form of lexical frequency. Lateral frontal cortex was highly sensitive to both sentence structure and word frequency, whereas lateral temporal and medial frontal opercular cortices were insensitive to single word statistics but were sensitive to sentence structure. This could suggest that the anterior IFG may gate inputs to the language network while reading, by interfacing between visual word recognition networks and networks engaged in higher-order sentential processes.
The high temporal resolution of intracranial recordings clarifies the dynamics of sentence processing. Our results point to an early indexing of structure initiation at word 2 (the earliest possible time at which the difference between sentences and lists could arise), followed by a ramping effect in cluster 1, which increased continuously with each consecutive word (SI Appendix, Fig. S6) but became particularly significant from word 5 onward, and finally a wrap-up or consolidation effect following the final word. These effects appear to mark the major, elementary steps in sentence processing.
Our initial choice of ROIs was based on prior fMRI studies where differential function within the lateral temporal cortex is typically segregated along the anterior-to-posterior axis (3, 15–17). The data-driven clustering analysis, however, demonstrated functional dissociations along the superior-to-inferior axis of the temporal lobe. We also replicate the commonly observed anterior-to-posterior dissociation within the IFG (5, 51). Strong effects of word frequency and lexicality were only observed in the anterior IFG but not posterior IFG, consistent with previous intracranial studies of reading (28). We observed negligible activity related to sentence structure in the right hemisphere, consistent with previous fMRI contrasts of narrative reading against scrambled word reading, and distinct from auditory narratives which show bilateral activation contrasts (52).
The medial FO, whose activity may be misattributed to the anterior insula by fMRI studies (53), is typically associated with its role in speech (28, 54, 55). However, disruption of this region can result in broader linguistic deficits (56, 57). The results of the current study suggest a role in linguistic structure processing, distinct from the activity of lateral IFG and potentially independent of the conceptual or semantic properties of individual lexical items.
This work also buttresses our prior claim that mFus is the earliest region to encode word frequency (28, 29). This implies that word frequency sensitivity in the IFG is dependent on inputs from mFus, rather than mFus’s frequency sensitivity being a result of top-down modulation from the IFG (58–61).
Functional imaging studies commonly use a contrast of sentences > pseudoword lists as a localizer of the “language network” (1). This network typically contains IFG, precentral sulcus, and lateral temporal cortex. We observed greater activity for sentences not only in the precentral sulcus and lateral temporal cortex but also in the medial parietal cortex (62, 63), most commonly associated with the default mode network (64). At population level in IFG, we consistently found greater activity for pseudoword lists over sentences, inconsistent with fMRI findings of sentence reading (1, 15, 25, 65, 66) but consistent with prior intracranial (28) and fMRI (49, 67) studies of single-word and pseudoword reading. fMRI and intracranial BGA typically show a high correspondence of effect localizations (68–70), making this divergence unexpected and meritorious of further study.
The stimuli for the present study were adapted from Fedorenko et al. (21), who reported exploratory intracranial data from four individuals, primarily constrained to electrodes that showed monotonic ramping activation of sentences > pseudoword lists, and did not observe a clear spatial organization across the cortex. Within those electrodes, they reported a consistent ordering of average activation (sentences > word lists > Jabberwocky > pseudoword lists) across all the tested cortical regions. With a much larger number of patients and electrodes, as well as improved stimuli (Materials and Methods), the present results suggest that their findings likely resulted from a mixture of two distinct response profiles—both of which we find tightly localized within classical left-hemisphere language areas. Our cluster 1 exhibits greatest activation for sentences, followed by Jabberwocky, then the lowest activation for the two list conditions, while cluster 2 shows greatest activity for word lists, followed by Jabberwocky and lowest activation for sentences and pseudoword lists.
Within our data, it appears that the role of semantics (e.g., compositional meaning, situation model construction, the establishment of thematic information) can be detected to a greater extent than more structural components of representation. The more restricted periods of significant difference between Jabberwocky and pseudoword lists (compared to the differences between sentences and word lists) also point to a more punctuated role for structure encoding, in contrast to the consistent and monotonic impact of semantic composition. At the same time, in whatever form this structure is processed, it does not appear to be clearly aligned with the parsing models we tested here (i.e., a left-corner X-bar parsing model). It is also possible that structural features may be more robustly encoded in distinct scales of neural organization (either at larger or smaller scales of system complexity), potentially across slower cortical dynamics (71). Presently, our results are more in accord with psycholinguistic models that place a greater computational burden on complex meaning construction rather than syntactic levels of representation (50), with syntax possibly being represented as stored knowledge and semantics being represented as a form of active inference with more readily detectable neural signals.
Beyond the overall linear ramping signals that were replicated here, Nelson et al. (23) suggested that neural activity also fluctuated in response to each successive word in a sentence, and that these fluctuations could be predicted by a bottom-up and left-corner X-bar parsing model, with BGA primarily relating to the number of open and closing nodes. Here, however, we did not replicate this finding, as the number of open nodes and node closures was not predictive of word-by-word variations in activation. This nonreplication could result from the high correlation, in the present stimuli, between those parameters and other variables that we controlled for. Indeed, the current sentences were much less variable in length and syntactic structure than those of Nelson et al. Consequently, the number of open nodes highly correlated with word position, and indeed the regions in the study by Nelson et al. which displayed a significant relationship with open nodes are highly comparable in localization to our ramping cluster 1. Furthermore, in the present stimuli, node closures were triggered almost exclusively by low-frequency content words. In the study by Nelson et al., multiple regression was used to control for word category (content vs. function words) but not for frequency, a feature to which the IFG is highly sensitive. Future work should therefore reassess which of the parameters of local phrase structure, word category, and frequency are the main drivers of brain activity in different nodes of the language network (72, 73). Meanwhile, the present results confirm that, at the scale of an entire eight-word sentence, ramping-up signals in the IFG and MTG track the integration of meaningful words into a coherent meaning, and thereby this gamma activity constitutes a plausible biomarker of semantic compositionality.
Materials and Methods
Participants.
Thirty-six patients (17 males, 21 to 50 y, three left-handed, IQ 96 ± 11, age of epilepsy onset 20 ± 11 y) participated in the experiments after giving written informed consent. All participants were semichronically implanted with intracranial electrodes for seizure localization of pharmacoresistant epilepsy. Participants were excluded if they had confirmed right-hemisphere language dominance, only had electrode coverage of right hemisphere, or had a significant additional neurological history (e.g., previous resections, MRI abnormalities such as malformations or hypoplasia). All experimental procedures were reviewed and approved by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center at Houston as Protocol Number HSC-MS-06-0385.
Electrode Implantation and Data Recording.
Data were acquired from either SDEs (two patients) or stereotactically placed depth electrodes (sEEGs; 34 patients). SDEs were subdural platinum–iridium electrodes embedded in a silicone elastomer sheet (PMT Corporation; top-hat design; 3 mm diameter cortical contact) and were surgically implanted via a craniotomy (74–76). sEEGs were implanted using a Robotic Surgical Assistant (ROSA; Medtech, Montpellier, France) (77, 78). Each sEEG probe (PMT Corporation) was 0.8 mm in diameter and had 8 to 16 electrode contacts. Each contact was a platinum–iridium cylinder, 2.0 mm in length, and separated from the adjacent contact by 1.5 to 2.43 mm. Each patient had 12 to 20 such probes implanted. Following surgical implantation, electrodes were localized by coregistration of preoperative anatomical 3T MRI and postoperative computerized tomography scans in AFNI (79). Electrode positions were projected onto a cortical surface model generated in FreeSurfer (80) and displayed on the cortical surface model for visualization (75). Intracranial data were collected during research experiments starting on the first day after electrode implantation for sEEGs and 2 d after implantation for SDEs. Data were digitized at 2 kHz using the NeuroPort recording system (Blackrock Microsystems), imported into Matlab, initially referenced to the white matter channel used as a reference for the clinical acquisition system, and visually inspected for line noise, artifacts, and epileptic activity. Electrodes with excessive line noise or localized to sites of seizure onset were excluded. Each electrode was rereferenced to the common average of the remaining channels. Trials contaminated by interictal epileptic spikes were discarded.
Stimuli and Experimental Design.
All patients undertook a sentence-reading task (29). Stimuli were presented on a 2,880 × 1,800, 15.4” liquid crystal display screen positioned at eye level, 2 to 3′ from the patient. Participants were presented with eight-word sentences and word lists using rapid serial visual presentation format (Fig. 1 A and B). A 1,000 ms fixation cross was presented followed by each word presented one at a time, each for 500 ms. Words were presented in all capital letters, in Arial font, with a height of 150 pixels (~2.2° visual angle). To maintain the participants’ attention, after each sentence, they were presented with a two-alternative forced choice, deciding which of the two presented words was present in the preceding sentence, responding via a key press. Only trials with a correct response were used for analysis.
Stimuli were presented in blocks containing 40 real sentences, 20 Jabberwocky sentences, 20 word lists, and 20 pseudoword lists in a pseudorandom order. Each participant completed between two and four blocks. Word choice was based on stimuli modified from a previous study (21). Jabberwocky sentences were designed as syntactically correct structures that could still be parsed but were made virtually meaningless by replacing each content word with a pseudoword, thus aiming to dissociate syntactic parsing (intact) vs. semantic composition (impaired). Jabberwocky pseudowords were selected as pronounceable pseudowords, designed to fill the syntactic role of nouns, verbs, and adjectives by inclusion of relevant functional morphemes. Normal and Jabberwocky sentences were then shuffled to yield word and pseudoword lists, i.e., control stimuli that used the same materials but could not be syntactically parsed nor semantically composed. To come as close as possible to those goals, we slightly modified the original stimuli in ref. 21 two ways. First, the original stimuli contained many Jabberwocky sentences that could barely be parsed (e.g., “The bralm strurps streff and boins smac nupal,” or “By marning we hien swoated perence’s hutty scivings”). Those were minimally modified such that syntactic structure was clearer, and each pseudoword was unambiguously assigned a (pseudo)grammatical category and thematic role. Second, conversely, the original word lists often contained partial phrases (e.g., in “stood the tied candle into shed the quickly,” “stood the tied candle” could be parsed as a four-word verb phrase). As in ref. 3, we reshuffled the lists pseudorandomly, primarily by grouping together the open-class and closed-class stimuli, until phrase composition became virtually impossible even for two consecutive words or pseudowords. Four patients used stimulus set A and the remaining 32 patients used stimulus set B (SI Appendix, Fig. S1). The most notable difference being that the pseudoword list condition in stimulus set A contained the high-frequency function words from the Jabberwocky condition, whereas set B only contained pseudowords.
Signal Analysis.
Analyses were performed by first bandpass filtering raw data of each electrode into BGA (70 to 150 Hz) following removal of line noise (zero-phase second-order Butterworth bandstop filters). A frequency domain bandpass Hilbert transform (paired sigmoid flanks with half-width 1.5 Hz) was applied and the analytic amplitude was smoothed (Savitzky–Golay finite impulse response, third order, frame length of 201 ms). The resultant BGA time course was then downsampled to 100 Hz using a nonoverlapping sliding window average. BGA is presented here as percentage change from baseline level, defined as the period −500 to −100 ms before word 1 presentation.
Statistical Analysis.
For ROI-based analyses, electrodes were tested to determine whether they were responsive during sentence presentation. We decided on an analysis window of 300 to 500 ms post word onset as our prior work shows that this interval is critical for word reading (28, 29). For each electrode, we calculated the Bayes factor (BF) of the mean word-induced activity for this window, combining across all trials, word positions, and experimental conditions, compared to the mean activity in each of the two 200 ms presentence baseline windows (−500 to −300 ms, −300 to −100 ms). Electrodes were considered responsive if they showed strong evidence [ln(BF10) > 2.3, BGA > 5%] of a deviation from baseline during stimulus presentation. Of the 2,675 electrodes located in the left, language-dominant cortex, 536 electrodes (in 29 patients) were considered responsive (Fig. 1D). Of the 1,472 electrodes located in the right, nonlanguage-dominant cortex, 242 electrodes (in 23 patients) were considered responsive (SI Appendix, Fig. S3). ROIs were selected based on prior intracranial studies of reading (28, 29, 53) and fMRI studies of sentence processing (3, 16). ROI centers were defined on the cortical surface and all responsive electrodes within a set geodesic radius of this point were included (81). This method was selected as currently available cortical surface parcellations have been shown to be inadequate at predicting functional boundaries of task-evoked activity (82). Centers of mass for each of the left-hemispheric ROIs, in Talairach space, were as follows: mFus, -32 -31 -19; aSTS, -54 -8 -6; pSTS, -54 -32 4; aIFG, -41 33 15; pIFG, -43 19 27; FO, -38 16 10.
LME models were used to dissociate multiple factors modulating BGA over time. Hierarchical random effects were used, with the random effect of individual electrodes grouped by patient. For time-resolved LME analyses, we used LME models at each time point (10 ms resolution) and corrected for multiple comparisons using a Benjamini–Hochberg false detection rate threshold of q < 0.01.
sbLME Modeling.
Electrode activations for each trial were mapped onto the standardized population brain surface using each electrode’s presumed “recording zone,” an exponentially decaying geodesic radius (27, 83). This resulted in a surface-based activation map for each patient for each trial. LME models were then used at each vertex of the standardized surface, providing a beta and t-statistic estimate at each vertex. LME models are an extension on a multiple linear regression, incorporating fixed effects for fixed experimental variables and random effects for uncontrolled variables. Fixed effects used include word frequency, lexicality, word length, and word position and are stated in the relevant analyses. Models included a random effect of patient, allowing a random intercept for each patient to account for differences in mean response size between patients. Results were thresholded at a t-statistic greater than 2 and coverage of at least three patients. Cluster significance was computed at a corrected alpha-level of 0.01, using family-wise error rate corrections for multiple comparisons. The minimum criterion for family-wise error rates was determined by white-noise clustering analysis (Monte Carlo simulations, 1,000 iterations) of data with the same dimension and smoothness as that analyzed (27).
Functional Clustering.
To isolate clusters of electrodes with comparable sentence structure-related responses, we performed k-means clustering on the principal components of individual electrode structure responses. Multiple linear regressions were performed on each responsive electrode within the frontal and lateral temporal ROIs (n = 174). Effects of word length, word frequency, and sentence structure were regressed from the sentence and word list conditions combined, at each time point, giving a temporal progression of structure beta values across the sentence. Principal component analysis was performed on the time courses of the beta values of the structure regressor. The first six principal components were selected for k-means clustering analysis, explaining 47% of the variance. A k of 2 was decided based on the results of a silhouette test, testing values of k from 2 to 10.
Interregional Crosscorrelation.
To quantify frontotemporal interactions of sentence structure-related effects between frontal and temporal cortices, we used the time course of the structure regressors within patients who had coverage of both frontal and temporal cortices within a cluster. We calculated the crosscorrelation between the time-varying structure regressor beta coefficients across the frontal and temporal electrode pairs. Combining across all pairs within each cluster, we derived the time lag corresponding to the maximal value of the crosscorrelation likelihood function (84). We repeated this procedure 500 times, using a random sample, without replacement, of 50% each of sentences and wordlists from each electrode in each iteration, to create a probability distribution of time lags. This method should be invariant to the electrode-referencing scheme used.
Linguistic Analysis.
We quantified word frequency as the base-10 log of the SUBTLEXus frequency (85). This resulted in a frequency of 1 meaning 10 instances per million words and 4 meaning 10,000 instances per million words. Given the inherent structure of sentences, certain word positions were more likely to contain high-frequency function words or low-frequency content words and as such, some word positions in the sentences showed significant differences in median frequency between the sentences and word lists (SI Appendix, Fig. S1).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We express our gratitude to all the patients who participated in this study; the neurologists at the Texas Comprehensive Epilepsy Program who participated in the care of these patients; and the nurses and technicians in the Epilepsy Monitoring Unit at Memorial Hermann Hospital who helped make this research possible. This work was supported by the National Institute of Neurological Disorders and Stroke NS098981 and NS128921.
Author contributions
O.W., C.D., S.D., and N.T. designed research; O.W., C.D., P.S.R., Z.J.R. and N.T. performed research; O.W., C.D., and E.M. contributed new reagents/analytic tools; O.W. analyzed data; and O.W., E.M., S.D., and N.T. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. C.H. is a guest editor invited by the Editorial Board.
Data, Materials, and Software Availability
Deidentified data supporting this paper are available at https://osf.io/f4p32/. Anonymized (Electrophysiological Recordings) data have been deposited in Open Science Foundation (86).
Supporting Information
References
- 1.Fedorenko E., Hsieh P. J., Nieto-Castañón A., Whitfield-Gabrieli S., Kanwisher N., New method for fMRI investigations of language: Defining ROIs functionally in individual subjects. J. Neurophysiol. 104, 1177–1194 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagoort P., The neurobiology of language beyond single-word processing. Science 366, 55–58 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Pallier C., Devauchelle A.-D., Dehaene S., Cortical representation of the constituent structure of sentences. Proc. Natl. Acad. Sci. U.S.A. 108, 2522–2527 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodgson V. J., Ralph M. A. L., Jackson R. L., Multiple dimensions underlying the functional organisation of the language network. NeuroImage 241, 118444 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friederici A. D., Language in Our Brain (The MIT Press, 2017), 10.7551/mitpress/11173.001.0001. [DOI] [Google Scholar]
- 6.Saur D., et al. , Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. NeuroImage 49, 3187–3197 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Glasser M. F., Rilling J. K., DTI tractography of the human brain’s language pathways. Cerebral Cortex 18, 2471–2482 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Binder J. R., Desai R. H., Graves W. W., Conant L. L., Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex 19, 2767–2796 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L., Lambon Ralph M. A., Rogers T. T., A unified model of human semantic knowledge and its disorders. Nat. Hum. Behav. 1, 0039 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santi A., Friederici A. D., Makuuchi M., Grodzinsky Y., An fMRI study dissociating distance measures computed by Broca’s area in movement processing: Clause boundary vs. identity. Front. Psychol. 6, 654 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giglio L., Ostarek M., Weber K., Hagoort P., Commonalities and asymmetries in the neurobiological infrastructure for language production and comprehension. Cereb. Cortex 32, 1405–1418 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matchin W., et al. , Agrammatism and paragrammatism: A cortical double dissociation revealed by lesion-symptom mapping. Neurobiol. Language 1, 208–225 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martins M. J. D., et al. , Recursive hierarchical embedding in vision is impaired by posterior middle temporal gyrus lesions. Brain 142, 3217–3229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng J., et al. , The cortical maps of hierarchical linguistic structures during speech perception. Cerebral Cortex 29, 3232–3240 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Branco P., Seixas D., Castro S. L., Mapping language with resting-state functional magnetic resonance imaging: A study on the functional profile of the language network. Hum. Brain Mapp. 41, 545–560 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pattamadilok C., Dehaene S., Pallier C., A role for left inferior frontal and posterior superior temporal cortex in extracting a syntactic tree from a sentence. Cortex 75, 44–55 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Matchin W., Hammerly C., Lau E., The role of the IFG and pSTS in syntactic prediction: Evidence from a parametric study of hierarchical structure in fMRI. Cortex 88, 106–123 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Matchin W., Hickok G., The cortical organization of syntax. Cerebral Cortex 30, 1481–1498 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee D. K., et al. , Neural encoding and production of functional morphemes in the posterior temporal lobe. Nat. Commun. 9, 1877 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding N., Melloni L., Zhang H., Tian X., Poeppel D., Cortical tracking of hierarchical linguistic structures in connected speech. Nat. Neurosci. 19, 158–164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorenko E., et al. , Neural correlate of the construction of sentence meaning. Proc. Natl. Acad. Sci. U.S.A. 113, E6256–E6262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaccarella E., Meyer L., Makuuchi M., Friederici A. D., Building by syntax: The neural basis of minimal linguistic structures. Cerebral Cortex 27, 411–421 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Nelson M. J., et al. , Neurophysiological dynamics of phrase-structure building during sentence processing. Proc. Natl. Acad. Sci. U.S.A. 114, E3669–E3678 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopopolo A., van den Bosch A., Petersson K.-M., Willems R. M., Distinguishing syntactic operations in the brain: Dependency and phrase-structure parsing. Neurobiol. Language 2, 152–175 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedorenko E., Blank I. A., Siegelman M., Mineroff Z., Lack of selectivity for syntax relative to word meanings throughout the language network. Cognition 203, 104348 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blank I., Balewski Z., Mahowald K., Fedorenko E., Syntactic processing is distributed across the language system. NeuroImage 127, 307–323 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadipasaoglu C. M., et al. , Surface-based mixed effects multilevel analysis of grouped human electrocorticography. NeuroImage 101, 215–224 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Woolnough O., et al. , A spatiotemporal map of reading aloud. J. Neurosci. 42, 5438–5450 (2022), 10.1523/JNEUROSCI.2324-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woolnough O., et al. , Spatiotemporal dynamics of orthographic and lexical processing in the ventral visual pathway. Nat. Hum. Behav. 5, 389–398 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huizeling E., Arana S., Hagoort P., Schoffelen J. M., Lexical frequency and sentence context influence the brain’s response to single words. Neurobiol. Language 3, 149–179 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stabler E., "Derivational minimalism" in Logical Aspects of Computational Linguistics, Retoré C., Ed. (Springer, Berlin, 1997), pp. 68–95. [Google Scholar]
- 32.Murphy E., et al. , Minimal phrase composition revealed by intracranial recordings. J. Neurosci. 42, 3216–3227 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy E., The Oscillatory Nature of Language (Cambridge University Press, 2020), 10.1017/9781108864466. [DOI] [Google Scholar]
- 34.Mazoyer B. M., et al. , The cortical representation of speech. J. Cogn. Neurosci. 5, 467–479 (1993). [DOI] [PubMed] [Google Scholar]
- 35.Dehaene S., Al Roumi F., Lakretz Y., Planton S., Sablé-Meyer M., Symbols and mental programs: A hypothesis about human singularity. Trends Cogn. Sci. 26, 751–766 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Hagoort P., MUC (Memory, Unification, Control) and beyond. Front. Psychol. 4, 416 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gwilliams L., How the brain composes morphemes into meaning. Philos. Trans. R. Soc. B Biol. Sci. 375, 20190311 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Embick D., The motivation for roots in distributed morphology. Annu. Rev. Linguist. 7, 69–88 (2021). [Google Scholar]
- 39.Piai V., et al. , Direct brain recordings reveal hippocampal rhythm underpinnings of language processing. Proc. Natl. Acad. Sci. U.S.A. 113, 11366–11371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heilbron M., Richter D., Ekman M., Hagoort P., de Lange F. P., Word contexts enhance the neural representation of individual letters in early visual cortex. Nat. Commun. 11, 321 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matchin W., Brodbeck C., Hammerly C., Lau E., The temporal dynamics of structure and content in sentence comprehension: Evidence from fMRI-constrained MEG. Hum. Brain Mapp. 40, 663–678 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shain C., Blank I. A., van Schijndel M., Schuler W., Fedorenko E., fMRI reveals language-specific predictive coding during naturalistic sentence comprehension. Neuropsychologia 138, 107307 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willems R. M., Frank S. L., Nijhof A. D., Hagoort P., Van Den Bosch A., Prediction during natural language comprehension. Cerebral Cortex 26, 2506–2516 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Caucheteux C., Gramfort A., King J.-R., Long-range and hierarchical language predictions in brains and algorithms (2021), 10.48550/ARXIV.2111.14232 (January 3, 2023). [DOI]
- 45.Kucewicz M. T., et al. , Evidence for verbal memory enhancement with electrical brain stimulation in the lateral temporal cortex. Brain 141, 971–978 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Diachek E., Blank I., Siegelman M., Affourtit J., Fedorenko E., The domain-general multiple demand (MD) network does not support core aspects of language comprehension: A large-scale fMRI investigation. J. Neurosci. 40, 4536–4550 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blank I., Kanwisher N., Fedorenko E., A functional dissociation between language and multiple-demand systems revealed in patterns of BOLD signal fluctuations. J. Neurophysiol. 112, 1105–1118 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vagharchakian L., Dehaene-Lambertz G., Pallier C., Dehaene S., A temporal bottleneck in the language comprehension network. J. Neurosci. 32, 9089–9102 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor J. S. H., Rastle K., Davis M. H., Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol. Bull. 139, 766–791 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Pylkkänen L., The neural basis of combinatory syntax and semantics. Science 366, 62–66 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Price C. J., The anatomy of language: A review of 100 fMRI studies published in 2009. Ann. N.Y. Acad. Sci. 1191, 62–88 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Wilson S. M., Bautista A., McCarron A., Convergence of spoken and written language processing in the superior temporal sulcus. NeuroImage 171, 62–74 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolnough O., Forseth K. J., Rollo P. S., Tandon N., Uncovering the functional anatomy of the human insula during speech. eLife 8, e53086 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh A., Duerden E. G., Pang E. W., The role of the insula in speech and language processing. Brain Language 135, 96–103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fedorenko E., Fillmore P., Smith K., Bonilha L., Fridriksson J., The superior precentral gyrus of the insula does not appear to be functionally specialized for articulation. J. Neurophysiol. 113, 2376–2382 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mălîia M.-D., et al. , Functional mapping and effective connectivity of the human operculum. Cortex 109, 303–321 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Rolston J. D., et al. , Frontal operculum gliomas: Language outcome following resection. J. Neurosurg. 122, 725–734 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woodhead Z. V. J., et al. , Reading front to back: MEG evidence for early feedback effects during word recognition. Cerebral Cortex 24, 817–825 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Price C. J., Devlin J. T., The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn. Sci. 15, 246–253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heim S., Wehnelt A., Grande M., Huber W., Amunts K., Effects of lexicality and word frequency on brain activation in dyslexic readers. Brain Language 125, 194–202 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Liu Y., et al. , Early top-down modulation in visual word form processing: Evidence from an intracranial SEEG study. J. Neurosci. 41, 6102–6115 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lerner Y., Honey C. J., Silbert L. J., Hasson U., Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J. Neurosci. 31, 2906–2915 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy E., Forseth K. J., Donos C., Rollo P. S., Tandon N., The spatiotemporal dynamics of semantic integration in the human brain. bioRxiv (2022), 10.1101/2022.09.02.506386 (September 29, 2022). [DOI] [PMC free article] [PubMed]
- 64.Buckner R. L., DiNicola L. M., The brain’s default network: Updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 20, 593–608 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Blank I., Fedorenko E., Domain-general brain regions do not track linguistic input as closely as language-selective regions. J. Neurosci. 37, 9999–10011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schoffelen J. M., et al. , A 204-subject multimodal neuroimaging dataset to study language processing. Sci. Data 6, 17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fiebach C. J., Friederici A. D., Müller K., Von Cramon D. Y., fMRI evidence for dual routes to the mental lexicon in visual word recognition. J. Cogn. Neurosci. 14, 11–23 (2002). [DOI] [PubMed] [Google Scholar]
- 68.Esposito F., et al. , Cortex-based inter-subject analysis of iEEG and fMRI data sets: Application to sustained task-related BOLD and gamma responses. NeuroImage 66, 457–468 (2013). [DOI] [PubMed] [Google Scholar]
- 69.Conner C. R., Ellmore T. M., Pieters T. A., Disano M. A., Tandon N., Variability of the relationship between electrophysiology and BOLD-fMRI across cortical regions in humans. J. Neurosci. 31, 12855–12865 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacques C., et al. , Corresponding ECoG and fMRI category-selective signals in human ventral temporal cortex. Neuropsychologia 83, 14–28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu Y., Jin P., Pan X., Ding N., Delta-band neural activity primarily tracks sentences instead of semantic properties of words. NeuroImage 251, 118979 (2022). [DOI] [PubMed] [Google Scholar]
- 72.Frank S. L., Yang J., Lexical representation explains cortical entrainment during speech comprehension. PLoS One 13, e0197304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin P., Lu Y., Ding N., Low-frequency neural activity reflects rule-based chunking during speech listening. eLife 9, e55613 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tandon N., "Mapping of human language" in Clinical Brain Mapping, Yoshor D., Mizrahi E., Eds. (McGraw Hill Education, 2012), pp. 203–218. [Google Scholar]
- 75.Pieters T. A., Conner C. R., Tandon N., Recursive grid partitioning on a cortical surface model: An optimized technique for the localization of implanted subdural electrodes. J. Neurosurg. 118, 1086–1097 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Tong B. A., Esquenazi Y., Johnson J., Zhu P., Tandon N., The brain is not flat: Conformal electrode arrays diminish complications of subdural electrode implantation, a series of 117 cases. World Neurosurg. 144, e734–e742 (2020). [DOI] [PubMed] [Google Scholar]
- 77.Tandon N., et al. , Analysis of morbidity and outcomes associated with use of subdural grids vs stereoelectroencephalography in patients with intractable epilepsy. JAMA Neurol. 76, 672–681 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rollo P. S., Rollo M. J., Zhu P., Woolnough O., Tandon N., Oblique trajectory angles in robotic stereo-electroencephalography. J. Neurosurg. 135, 245–254 (2020). [DOI] [PubMed] [Google Scholar]
- 79.Cox R. W., AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996). [DOI] [PubMed] [Google Scholar]
- 80.Dale A. M., Fischl B., Sereno M. I., Cortical surface-based analysis: I. Segmentation and Surface Reconstruction. NeuroImage 9, 179–194 (1999). [DOI] [PubMed] [Google Scholar]
- 81.Kadipasaoglu C. M., et al. , Development of grouped icEEG for the study of cognitive processing. Front. Psychol. 6, 1008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhi D., King M., Hernandez-Castillo C. R., Diedrichsen J., Evaluating brain parcellations using the distance-controlled boundary coefficient. Hum. Brain Mapp. 43, 3706–3720 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCarty M. J., Woolnough O., Mosher J. C., Seymour J., Tandon N., The listening zone of human electrocorticographic field potential recordings. eNeuro 9, 1–13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zucker S., Cross-correlation and maximum-likelihood analysis: a new approach to combining cross-correlation functions. Mon. Not. R. Astron. Soc. 342, 1291–1298 (2003). [Google Scholar]
- 85.Brysbaert M., New B., Moving beyond Kučera and Francis: A critical evaluation of current word frequency norms and the introduction of a new and improved word frequency measure for American English. Behav. Res. Methods 41, 977–990 (2009). [DOI] [PubMed] [Google Scholar]
- 86.Woolnough O., Spatiotemporally distributed frontotemporal networks for sentence reading. Open Science Framework. osf.io/f4p32. Deposited 9 December 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Deidentified data supporting this paper are available at https://osf.io/f4p32/. Anonymized (Electrophysiological Recordings) data have been deposited in Open Science Foundation (86).