Abstract
We have previously demonstrated that a high proportion of RAG-2 SCID knockout mice, which lack T and B cells, develop orofacial abscesses and disseminated infections following pulpal infection, whereas immunocompetent control mice do not. In the present study, we sought to identify the components of the adaptive immune response which contribute to protection against disseminating anaerobic infections and sepsis. For this purpose, various genetically engineered immunodeficient mice were employed, including RAG-2 SCID, Igh-6 (B-cell deficient), Tcrb Tcrd (T-cell deficient) and Hc0 (C5 deficient). For abscess induction, the mandibular first molars were subjected to pulp exposure on day 0. Teeth were infected with a mixture of four anaerobic pathogens, including Prevotella intermedia, Streptococcus intermedius, Fusobacterium nucleatum, and Peptostreptococcus micros, and teeth were sealed to prevent communication with the oral cavity. The findings demonstrate that both RAG-2 SCID and B-cell-deficient mice, but not T-cell- or C5-deficient mice, have increased susceptibility to the development of disseminating anaerobic infections. Abscess-susceptible RAG-2 SCID and B-cell-deficient mice also showed a significant loss of body weight, splenomegaly, and absent antibacterial antibody production. Furthermore, dissemination was significantly reduced, from 74 to 25%, in susceptible RAG-2 mice by passively transferred antibody, predominantly immunoglobulin G2b (IgG2b) and IgM, against the infecting bacterial innoculum. Fractionated IgG-enriched preparations were more efficient in transferring protection than IgM preparations. We conclude that an antibody-mediated mechanism(s), most likely bacterial opsonization, is of importance in localizing anaerobic root canal infections and in preventing their systemic spread.
Bacterial infection of the dental pulp occurs as a consequence of caries, trauma, and operative dental procedures. These infections are mixed and anaerobic in nature, and are associated with high morbidity and mortality if bacterial dissemination occurs from the root canal into the tissues or the circulation. In this regard, pulpal infections may cause cellulitis, are the most common source of infecting microorganisms in Ludwig's angina (15), and have been implicated in cavernous sinus thrombosis, brain abscesses, mediastinitis, and osteomyelitis (7, 14). Oral bacteria are also a primary source of infections in transplant patients (13) and in immunodeficient and immunosuppressed individuals (22).
Pulpal infections induce local immune responses in the periapical region surrounding the root of the tooth. This response consists of a typical mixed inflammatory infiltrate and is composed of T cells, B cells, plasma cells, macrophages, and neutrophils (21, 23, 31, 32). However, it is unknown which of these responses protect the host against bacterial egress and dissemination. In previous studies, we developed a model of induced pulpal infection in RAG-2 severe combined immunodeficient (SCID) mice (34). RAG-2 SCID mice were found to develop orofacial abscesses and disseminating infections following pulpal infection, whereas immunocompetent control mice were resistant.
In the present study, we sought to determine whether T-cell or B-cell activities or both mediate resistance to infection dissemination in this model. Our results indicate that B-cell-deficient animals have increased susceptibility to infection dissemination, as do RAG2 SCID mice, and that dissemination is significantly reduced by passively transferred antibody against the infecting microorganisms.
MATERIALS AND METHODS
Animals.
Breeding pairs of RAG-2 mice produced by targeted gene disruption were kindly provided by Frederick Alt, Children's Hospital Medical Center, Boston, Mass. Igh-6 (B-cell-deficient), Tcrb Tcrd (T-cell-deficient), Hc0 (C5-deficient), and A/J and C57BL/6 immunocompetent mice were purchased from Jackson Laboratory, Bar Harbor, Maine. All immunodeficient strains are on a background of C57BL/6 except for RAG-2 (129/SvEvTac × C57BL/6). Animals were bred and/or maintained in laminar-flow isolators in the Forsyth Institute Animal Facility under pathogen-free conditions.
Pulp exposure.
RAG-2 (n = 11), Igh-6 (n = 12), Tcrb Tcrd (n = 24), Hc0 (n = 12), A/J (n = 8), and C57BL/6 (n = 12) mice, 8 to 12 weeks of age, were anesthetized by the intramuscular injection of ketamine (80 mg/kg of body weight) and xylazine (10 mg/kg) in sterile phosphate-buffered saline (PBS). The pulps of the mandibular first molars were exposed on day 0 using a portable variable-speed electric handpiece (Osada Electric, Los Angeles, Calif.) and a sterile size 1/4 round bur under a surgical microscope (model MC-M92; Seiler, St. Louis, Mo.). The pulp chambers were opened until the entrances of the canals could be visualized and probed with a no. 10 endodontic file.
Infection with pathogens.
Tryptic soy broth with yeast agar plates of four common endodontic pathogens, Prevotella intermedia ATCC 25611, Streptococcus intermedius ATCC 27335, Fusobacterium nucleatum ATCC 25586, and Peptostreptococcus micros ATCC 33270, were grown under anaerobic conditions (80% N2, 10% H2, and 10% CO2), harvested, and cultured in mycoplasma liquid medium. The cells were centrifuged at 7,000 × g for 15 min and resuspended in prereduced anaerobically sterilized Ringer's solution under the influx of nitrogen. The final concentration of each organism was determined by spectrophotometry, and the four pathogens were mixed to yield a concentration of 1010 cells of each pathogen/ml in 0.01 g of methylcellulose/ml. The animals were infected by placing 1 to 2 μl of the inoculum mixture into the exposed pulp. Following infection, the teeth were sealed with composite resin (Zenith, Englewood, N.J.) to prevent superinfection of pulps with microorganisms from the oral cavity.
Abscess scoring and body weight measurements.
Grossly evident orofacial abscesses were scored as positive or negative following visual examination. Body weight was measured on day 0 and again on the day of sacrifice. Splenic weights were determined at sacrifice.
Abscess sampling.
All surviving mice were sacrificed on day 21, and abscess formation was confirmed by the aspiration of pus from lesions. Aspirated material was transferred to vials containing prereduced anaerobically sterilized Ringer's solution, serially diluted, plated on tryptic soy broth with yeast agar plates, and incubated under anaerobic conditions. After 1 week of growth, plates were counted for the number of CFU. The presence of pathogens in abscesses was assessed using checkerboard hybridization, as described by Socransky et al. (29).
Antibody transfer.
Antibodies directed against the four pathogens were produced by induction of immune ascitic fluids in C57BL/6 as described by Tung et al. (35), which involved repeated intraperitoneal injections of an emulsion of incomplete Freund's adjuvant with antigen in a ratio of 9:1. The ascitic fluids were collected from the abdominal cavity by cannulation with a sterile 19-gauge needle. The fluids were centrifuged at 4°C at 2,300 × g for 10 min to remove cells and fibrin clots and were frozen at −70°C.
For purification of immunoglobulin M (IgM)- and IgG-enriched fractions, ascitic fluids were pooled and Igs were precipitated with 45% ammonium sulfate, pH 7.0. IgM- and IgG-containing fractions were separated by fast-performance liquid chromatography using Superose 6 gel filtration chromatography (Amersham Pharmacia Biotech, Piscataway, N.J.), as previously described by Smith et al. (26). IgG aggregates contaminating the IgM-containing fractions were removed by passage over a protein A-Sepharose column (Pierce, Rockford, Ill.). The IgM- and IgG-enriched fractions were dialyzed against PBS, and their purity was confirmed by enzyme-linked immunosorbent assay (ELISA) (MAb typing kit; Calbiochem, San Diego, Calif.).
In the first experiment, one group of RAG-2 SCID mice (n = 12) received subcutaneous injections of mixtures of unfractionated antibodies (ascites) on days 1, 3, 7, 11, and 15 relative to pulp exposure; control RAG-2 SCID mice (n = 15) received injections of saline. In a second experiment, 0.5 to 1 mg of IgM (n = 10) and IgG (n = 11) were passively transferred to RAG-2 SCID mice on days −1, 3, 7, 11, and 15 after pulp exposure. Control animals (n = 10 each) received unfractionated ascites (positive) or saline (negative).
ELISA.
Serum samples from pulpally infected mice were obtained by orbital bleeding 21 days after pulpal exposure. Ninety-six-well plates were coated with formalin-killed microorganisms in PBS (optical density [OD] at 580 nm = 0.3) and incubated for 3 h at 37°C. After two days at 4°C, plates were washed three times with buffer II (0.9% NaCl, 0.05% Tween 20). For determination of specific antibody against pathogens, the plates were incubated with diluted serum (1/100) in buffer III (PBS with 0.05% Tween 20 and 0.02% NaN3) for 2 h at room temperature (RT) with shaking. The optimal serum dilution of 1/100 was determined after testing a range of dilutions of serum. The plates were washed three times with buffer II, and the bound Ig was detected by reaction with goat anti-mouse Ig to alkaline phosphatase (Biosource) and diluted 1/1,500 in buffer III overnight at RT. Conversion of substrate (p-nitrophenylphosphate [1 mg/ml]; Sigma) was determined at an OD at 405 nm using an enzyme-linked immunosorbent assay (ELISA) reader (BIO-TEK Instruments, Inc., Winooski, Vt.).
Identification of antibody subclasses IgG1, IgG2a, IgG2b, IgG3, IgM, and IgA was performed using a hybridoma subisotyping kit (Calbiochem). Following reaction of the primary antisera with bacterium-coated wells, typing sera were added and the plates were incubated for 1 h at RT. Peroxidase-conjugated goat anti-rabbit IgG (1/4,000) was then added to each well and incubated for 1 h at RT. The color was developed by a ready-to-use 3,3′,5,5′-tetramethylbenzidine substrate reagent supplied in the kit and quantified using an ELISA reader at 450 nm.
To quantify antibody concentrations, standard curves were established for each antibody subclass. In brief, 96-well plates were precoated with a capture goat anti-mouse Ig antibody. Serial fivefold dilutions of purified IgG1, IgG2a, IgG2b, IgG3, IgM, and IgA (Calbiochem) were added to the precoated plates and incubated for 1 h at RT, and this was followed by the addition of subclass-specific typing antisera. Assays were conducted as above. Conversion from OD to antibody concentration was determined by reference to the linear portion of the standard curves generated for each antibody subclass.
Statistical analysis.
Evaluation of the effect of antibody treatment on abscess formation was made using chi-square analysis. Comparisons of body weights were carried out by t test.
RESULTS
Development of orofacial abscesses in immunodeficient mice.
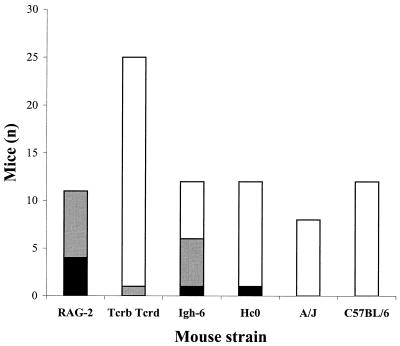
In order to determine whether immune mechanisms mediated by B or T cells serve to limit pulpal infections and prevent their dissemination, various immunodeficient mouse strains were screened. T-cell-deficient (Tcrb Tcrd), B-cell-deficient (Igh-6), and complement-deficient (Hc0) mice were subjected to pulpal exposure and a standard infection regimen. RAG-2 SCID mice served as positive controls, and immunocompetent wild-type A/J and C57BL/6 mice served as negative controls. All RAG-2 SCID mice developed evident orofacial abscesses, and four mice died (Fig. 1 and 2). A high frequency of abscesses also occurred in Igh-6 knockout mice (6 of 12), with one animal dying. In contrast, only 1 of 24 Tcrb Tcrd and 1 of 12 Hc0 knockout mice developed abscesses. Wild-type mice were uniformly abscess resistant, as previously reported (34). These data suggest that B-cell- but not T-cell-deficient animals have increased susceptibility to the development of disseminating infections in this model.
FIG. 1.
RAG-2 knockout mouse 21 days after pulp exposure and infection. Note orofacial abscess.
FIG. 2.
Abscess development in immunodeficient mice. Solid bar, dead mice with abscesses; grey bar, mice with abscesses only; open bar, mice without abscesses.
Eight abscesses from RAG-2 SCID mice were sampled microbiologically and analyzed using DNA checkerboard hybridization. The results revealed that various combinations of the four infecting pathogens were present in most of the abscesses sampled; however, P. intermedia and F. nucleatum were present in somewhat higher numbers than P. micros and S. intermedius (data not shown).
Effect of bacterial infection on body weight and splenomegaly.
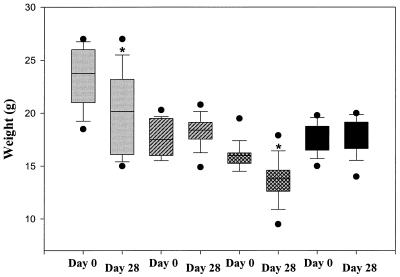
Loss of body weight is an indicator of disseminating infection and sepsis. The effect of bacterial infection on weight loss in immunodeficient mice was therefore assessed. As shown in Fig. 3, the RAG-2 knockout mice on day 28 experienced significant weight loss compared to their original weights on day 0, as did Igh-6 knockout mice (P < 0.05 [chi-square test]). In contrast, neither Tcrb Tcrd nor Hc0 knockout mice showed significant weight loss during the experimental period. In addition, we measured spleen weights of these infected mice on day 28. We also found significant splenomegaly in RAG-2 SCID and Igh-6 knockout mice, compared to Tcrb Tcrd and Hc0 knockout mice (Table 1), which further indicates the presence of sepsis in the former strains.
FIG. 3.
Effect of bacterial infection on weight loss in immunodeficient mice: comparison of body weight on days 0 and 28. Gray bars, RAG-2; hatched bars, Tcrb Tcrd; cross-hatched bars, Igh-6; and solid bars, Hc0. The bars encompass the 25th to 75th percentiles, the horizontal lines represent the medians, the brackets indicate standard errors of the means, and the closed circles represent outliers. ∗, P < 0.05 (day 28 versus day 0).
TABLE 1.
Spleen weights in immunodeficient mice
| Strain | Mean spleen wt (g) ± SD |
|---|---|
| RAG-2 | 0.12 ± 0.06a |
| Igh-6 | 0.14 ± 0.06a |
| Tcrb Tcrd | 0.06 ± 0.01 |
| Hc0 | 0.06 ± 0.02 |
P < 0.01 (t test).
Antibody response to pathogens.
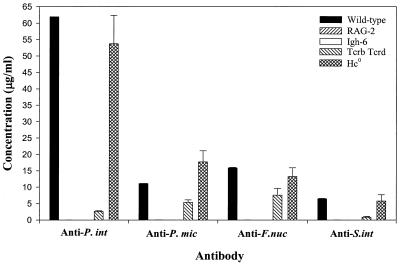
ELISAs were carried out to assess the production of specific antibodies against the four pathogens in infected immunodeficient mice. As seen in Fig. 4, in wild-type mice P. intermedia elicited the highest levels of antibody, followed by F. nucleatum, P. micros, and S. intermedius. The antibody levels thus paralleled the levels of each bacterium isolated from abscess samples, as described above. Hc0 knockout mice had responses that were similar to those seen in wild-type mice. Tcrb Tcrd knockout mice showed modest levels of antibody production, with the highest response to F. nucleatum. As expected, neither RAG-2 SCID nor Igh-6 knockout mice had detectable levels of circulating antibody, confirming their immunodeficient status.
FIG. 4.
Antibody production in immunodeficient mice. Levels of antibody produced against P. intermedia (P. int), P. micros (P. mic), F. nucleatum (F. nuc), and S. intermedius (S. int) were determined by ELISA. T-cell-deficient Tcrb Tcrd mice had modest levels of antibody against all pathogens.
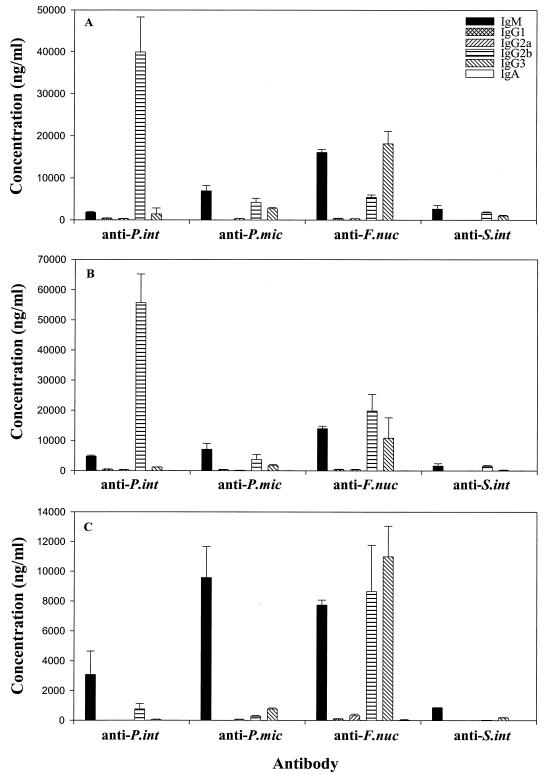
The subclasses of serum antibody produced by wild-type, Hc0, and Tcrb Tcrd knockout mice were determined 3 weeks after infection. As shown in Fig. 5A and B, infected wild-type and Hc0 knockout mice, respectively, produced predominantly IgG2b responses to P. intermedia, whereas responses to the other three bacteria included similar levels of IgM, IgG2b, and IgG3. In contrast, responses in Tcrb Tcrd knockout mice (Fig. 5C) against most of the pathogens were predominantly IgM, although one or more IgG subclasses were also produced against all bacteria. Indeed, the response to F. nucleatum was mainly IgG2b and IgG3, consistent with a T-independent type 1 (TI1) antibody response. Interestingly, some IgG was also produced against gram-positive pathogens (P. micros and S. intermedius), indicating that class switching also occurred against organisms that normally induce a TI2 response.
FIG. 5.
Subclasses of serum antibody produced by infected wild-type (A), Hc0 (B), and Tcrb Tcrd knockout (C) mice. Subclasses of antibody against P. intermedia (P. int), P. micros (P. mic), F. nucleatum (F. nuc), and S. intermedius (S. int) in serum were determined by ELISA.
Effect of passive antibody transfer on disseminating infections in RAG-2 SCID mice.
Having observed a high frequency of abscess formation and absent antibody production in mice deficient in B cells, we assessed the effect of passively transferred antibody on abscess formation in RAG-2 SCID mice. The transferred antibodies, derived from immune ascitic fluids, were dominated by IgG2b and IgM, although varying amounts of IgG1, IgG2a, and IgG3 were also present (Table 2). The levels of antibody against P. intermedia and P. micros were generally higher than those against F. nucleatum and S. intermedius. Following passive transfer, the levels of circulating antibody against the four pathogens in infected RAG-2 knockout mice were approximately fivefold higher than in wild-type mice immunized by pulpal infection (data not shown). As expected, no antibody was present in the control RAG-2 SCID animals not receiving passive antibody.
TABLE 2.
Concentration of subclasses of transferred antibody against pathogens
| Subclass | Concn (μg/ml) of antibody against:
|
|||
|---|---|---|---|---|
| P. intermedia | P. micros | F. nucleatum | S. intermedius | |
| IgM | 315.1443 | 8,004.352 | 138.4867 | 9.9919 |
| IgG1 | 620.857 | 36.7445 | 0.3558 | 0.6021 |
| IgG2a | 570.1827 | 0.8915 | 0.3749 | 0.0827 |
| IgG2b | 7,509.297 | 7,360.838 | 48.4488 | 9.2755 |
| IgG3 | 568.7158 | 874.6132 | 38.7272 | 0.7298 |
| IgA | 290.5824 | 5.60 | 0.0002 | 0.0002 |
The effect of passive antibody transfer on abscess formation is summarized as follows. As shown, of the 15 RAG-2 control mice that did not receive antibody, 11 developed orofacial abscesses. In contrast, only 3 of 12 antibody-treated RAG-2 SCID mice developed abscesses (P < 0.05 [chi-square test]); two of these abscesses were very small. Furthermore, 5 of 15 RAG-2 control mice died with abscesses prior to day 21, compared to no deaths among the 12 antibody-treated mice.
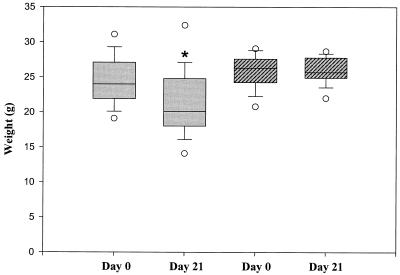
The effect of antibody treatment on weight loss in RAG-2 SCID mice was also assessed. As shown in Fig. 6, RAG-2 mice not receiving antibody showed significant weight loss on day 21 compared to their original weights on day 0 (P < 0.05 [chi-square test]). In contrast, RAG-2 mice receiving antibody showed no change in weight during the experimental period.
FIG. 6.
Effect of antibody treatment on body weight in RAG-2 knockout mice: comparison of weight loss in RAG-2 control mice without antibody treatment (shaded bars) and RAG-2 test mice with antibody treatment (hatched bars) on days 0 and 21 (surviving mice only). The bars encompass the 25th to 75th percentiles, the horizontal lines represent the medians, the brackets indicate standard errors of the means, and the open circles represent outliers. ∗, P < 0.05 (day 21 versus day 0).
Effect of transfer of fractionated IgM and IgG antibody on disseminating infections.
In order to identify the protective antibody isotype, immune ascites fluid was separated into IgM and IgG-enriched fractions. Following transfer of fractionated and unfractionated ascites fluid, RAG2 SCID mice were challenged by pulp exposure and infection with the four-pathogen mixture, and the effect on infection dissemination was determined. As summarized in Table 3, mice receiving PBS as a negative control developed a high frequency of abscesses (8 of 10), whereas those receiving unfractionated immune ascites fluid were largely protected (1 of 10) (P < 0.01 [chi-square test]). Of importance, animals infused with the IgG-enriched fraction (P < 0.01 versus PBS control) showed more protection than did animals infused with the IgM-enriched fraction (P > 0.05 [not significant]), suggesting that the IgG isotype is more effective in preventing dissemination in this model.
TABLE 3.
Efficacy of antibody isotypes in preventing infection dissemination
Results shown are number of mice with abscess/total number of mice.
P < 0.01 versus PBS control (chi-square test).
DISCUSSION
Bacteremia and sepsis of oral origin are common in immunocompromised patients, including neutropenic cancer patients (24, 37). In the present study, we evaluated various components of the adaptive immune response to determine their contribution to protection against disseminating oral anaerobic infections and sepsis originating from the root canal. Our findings demonstrate that both RAG-2 SCID and B-cell-deficient mice, but not T-cell- or complement C5-deficient mice, have increased susceptibility to the development of disseminating infections. Furthermore, dissemination was significantly reduced by passively transferred antibody against the infecting bacterial innoculum, with IgG-enriched fractions responsible for the bulk of the protection. These findings strongly support the conclusion that an antibody-mediated mechanism(s), possibly bacterial opsonization, is of importance in localizing infections to the root canal system and in preventing their systemic spread.
Tcrb Tcrd mice produced only modest levels of IgM and IgG in response to all pathogens yet were highly protected against infection dissemination. The response to the gram-negative pathogen F. nucleatum showed extensive class switching, typical of a TI1 antigen possessing inherent mitogenic activity, i.e., lipopolysaccharide (2, 27). The response to the gram-positive organisms P. micros and S. intermedius was dominated by IgM, typical of a TI2 response, although some class switching to IgG2b and IgG3 also occurred. The finding that IgG is more effective than IgM in transferring protection (Table 3) suggests that antibody class switching from IgM to IgG may be important in T-independent antibody-mediated protection in this model. TI2 antigens such as capsular polysaccharides (e.g., dextran) from gram-positive organisms, which activate B cells by receptor cross-linking, cannot induce class switching (27). However, NK cells and macrophages may stimulate TI2 antibody secretion and class switching through elaboration of granulocyte-macrophage colony-stimulating factor and gamma interferon (28). Further studies are needed to determine if this mechanism is involved in protection in this model.
Antibodies may mediate host protection through a variety of mechanisms, including inhibition of microbial attachment, aggregation, opsonization, and activation of the complement system. Although IgM, IgG2b, and IgG3 can all fix complement (12), the finding that C5-deficient animals were not abscess susceptible suggests that lysis of the pathogenic microorganisms is not a primary protective mechanism in this model. Rather, we favor the interpretation that antibody-mediated aggregation and/or opsonization by IgG2b and possibly IgM may be responsible for the observed protection. The transferred antibodies against the four inoculated pathogens were opsonic in vitro for at least S. intermedius and P. intermedia. Opsonic activity also correlated with antibody levels (R. Teles, unpublished findings). The mechanism of IgM-mediated opsonization is not well understood but may require IgM complement activation to C3b, which functions as an opsonin (25). Additional studies are required to identify the IgG subclass(es) responsible for protection and to define the opsonic function of these antibodies in protection against infection dissemination.
The murine subcutaneous abscess and subcutaneous chamber models have been used extensively to examine the role of the host immune response in periapical and/or periodontal disease pathogenesis (1, 11, 20, 33, 34, 36). Results from these studies have been inconclusive. Reduced infection-stimulated bone destruction was reported in SCID (1) and in T-cell-deficient mice (33) compared to wild type, suggesting that T- and/or B-cell mechanisms may actually contribute to pathogenesis. In contrast, no difference in local bone destruction was reported in the periapical model in these strains in the absence of infection dissemination (34, 36). Similarly, Kesavalu et al. (20) found that SCID and B-cell- and T-cell-deficient mice challenged with Porphyromonas gingivalis had primary lesions of a size similar to that seen in the wild type in a subcutaneous abscess model.
Several studies have reported that active immunization with a variety of oral pathogens, such as P. gingivalis, F. nucleatum, and Wolinella recta, resulted in significant protection of mice from lesion progression, abscess formation, and death in these models (4, 5, 9, 19). Protection was correlated with IgG and IgM antibody production, but this was not confirmed in passive transfer experiments. A confounding factor in all studies was that cell-mediated immunity was also induced by the immunization protocols utilized (3, 9, 18, 19). These results also concur with the earlier demonstration by Dahlen et al. (8), who showed that immunized monkeys had a more distinct delimitation of the periapical resorptive process than nonimmunized monkeys.
In a previous study from this laboratory, the rate of abscess formation and dissemination in RAG2 SCID mice was approximately 33% (34), compared to 80 to 90% in the current investigation. This increased incidence is likely due to a modification in the infection protocol in which pathogens were sealed within the teeth. The lack of communication between the infected dental pulp and the oral cavity likely prevents drainage of the abscess through the tooth, thereby facilitating the systemization of infection. Given that most pulpal infections are closed to the oral cavity, this situation is likely to more closely mimic the clinical situation in humans. Moreover, contamination with other microorganisms from the oral environment is prevented, so that the immune response that occurs is directed only against the infecting inoculum.
It is noteworthy that only about 50% of B-cell-deficient mice exhibited abscess development, compared to 80 to 90% of RAG-2 SCID mice. In addition, despite the significant protection afforded by passively transferred antibody, this protection was incomplete. These data suggest the possibility that T-cell-mediated immune mechanisms that are distinct from helper activity for antibody formation may also exert some protective effect. In this regard, T-cell expression of interleukin 8 and granulocyte-macrophage colony-stimulating factor could stimulate polymorphonuclear lymphocyte emigration and activation (10) and increased antibacterial activity. Our previous findings demonstrated that polymorphonuclear leukocytes reduce infection-stimulated bone destruction and are protective in this model (17, 30).
A second possibility is that an innate response mediated by NK cells may actually contribute to disseminating infection and/or septic shock in RAG-2 SCID mice. The NK cell compartment is intact in RAG-2 SCID mice, and their numbers and function may even be increased (6). Much evidence suggests that NK cells play a critical role in septic shock through the elaboration of large amounts of gamma interferon and tumor necrosis factor alpha (16, 38). Whether T-cell-mediated immunity is protective and what the role of NK cells is in mediating increased susceptibility of RAG-2 SCID mice to disseminating infections need to be further defined.
ACKNOWLEDGMENTS
We thank R. L. Kent, Jr., for statistical consultation, W. King for help with immunoglobulin fractionation, S. Yoganathan for help with animal husbandry, and J. Buchanan for photography.
This work supported by grant DE-11664 from the National Institute of Dental and Craniofacial Research, NIH.
REFERENCES
- 1.Baker J P, Evans R T, Roopenian D C. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol. 1994;39:1035–1040. doi: 10.1016/0003-9969(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 2.Betts M, Beining P, Brunswick M, Inman J, Angus R D, Hoffman T, Golding B. Lipopolysaccharide from Brucella abortus behaves as a T-cell-independent type 1 carrier in murine antigen-specific antibody responses. Infect Immun. 1993;61:1722–1729. doi: 10.1128/iai.61.5.1722-1729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen P B, Davern L B, Aguirre A. Experimental Porphyromonas gingivalis infection in nonimmune athymic BALB/c mice. Infect Immun. 1991;59:4706–4709. doi: 10.1128/iai.59.12.4706-4709.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P B, Davern L B, Schifferle R, Zambon J J. Protective immunization against experimental Bacteroides (Porphyromonas) gingivalis infection. Infect Immun. 1990;58:3394–3400. doi: 10.1128/iai.58.10.3394-3400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen P B, Neiders M E, Millar S J, Reynolds H S, Zambon J J. Effect of immunization of experimental Bacteroides gingivalis infection in a murine model. Infect Immun. 1987;55:2534–2537. doi: 10.1128/iai.55.10.2534-2537.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christianson S W, Greiner D L, Schweitzer I B, Gott B, Meamer G L, Schweitzer P A, Hesselton R M, Shultz L D. Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scid mice and in C57BL/6J-scid bg mice. Cell Immunol. 1996;171:186–199. doi: 10.1006/cimm.1996.0193. [DOI] [PubMed] [Google Scholar]
- 7.Cogan M I C. Necrotizing mediastinitis secondary to a descending cervical cellulitis. Oral Surg. 1977;44:7–10. doi: 10.1016/0030-4220(73)90207-7. [DOI] [PubMed] [Google Scholar]
- 8.Dahlen G, Fabricius L, Heyden G, Holm S E, Moller A J. Apical periodontitis induced by selected bacterial strains in root canals of immunized and nonimmunized monkeys. Scand J Dent Res. 1982;90:207–216. doi: 10.1111/j.1600-0722.1982.tb00729.x. [DOI] [PubMed] [Google Scholar]
- 9.Ebersole J L, Feuille F, Kesavalu L, Holt S C. Host modulation of tissue destruction caused by periodontopathogens: effects on a mixed microbial infection composed of Porphyromonas gingivalis and Fusobacterium nucleatum. Microb Pathog. 1997;23:23–32. doi: 10.1006/mpat.1996.0129. [DOI] [PubMed] [Google Scholar]
- 10.Elbim C, Bailly S, Chollet-Martin S, Hakim J, Gougerot-Pocidalo M A. Differential priming effects of proinflammatory cytokines on human neutrophil oxidative burst response to bacterial N-formyl peptides. Infect Immun. 1994;62:2195–2201. doi: 10.1128/iai.62.6.2195-2201.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genco C A, Van Dyke T, Amar S. Animal models for Porphyromonas gingivalis-mediated periodontal disease. Trends Microbiol. 1998;6:444–449. doi: 10.1016/s0966-842x(98)01363-8. [DOI] [PubMed] [Google Scholar]
- 12.Goding J W. Monoclonal antibodies: principles and practice. 3rd ed. London, United Kingdom: Harcourt Brace and Company; 1996. pp. 79–89. [Google Scholar]
- 13.Heimdahl A, Mattsson T, Dahilof G, Lonnquist B, Ringden O. The oral cavity as a port of entry for early infections in patients treated with bone marrow transplantation. Oral Surg Oral Med Oral Path. 1989;68:711–716. doi: 10.1016/0030-4220(89)90160-6. [DOI] [PubMed] [Google Scholar]
- 14.Hollin S A, Hayashi H, Gross S W. Intracranial abscesses of odontogenic origin. Oral Surg Oral Med Oral Pathol. 1967;23:277–293. doi: 10.1016/0030-4220(67)90138-7. [DOI] [PubMed] [Google Scholar]
- 15.Hought R T, Fitzgerald B E, Latta J E, Zallen R D. Ludwig's angina: report of two cases and review of the literature from 1945 to January 1979. J Oral Surg. 1980;38:849–855. [PubMed] [Google Scholar]
- 16.Hunter C A, Ellis-Neyer L, Gabriel K E, Kennedy M K, Grabstein K H, Linsley P S, Remington J S. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii. J Immunol. 1997;158:2285–2293. [PubMed] [Google Scholar]
- 17.Kawashima N, Niederman R, Hynes R O, Ullmann-Cullere M, Stashenko P. Infection-stimulated infraosseus inflammation and bone destruction is increased in P-/E-selectin knockout mice. Immunolol. 1999;97:117–123. doi: 10.1046/j.1365-2567.1999.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesavalu L, Ebersole J L, Machen R L, Holt S C. Porphyromonas gingivalis virulence in mice: induction of immunity to bacterial components. Infect Immun. 1992;60:1455–1464. doi: 10.1128/iai.60.4.1455-1464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesavalu L, Holt S C, Crawley R R, Borinski R, Ebersole J L. Virulence of Wolinella recta in a murine abscess model. Infect Immun. 1991;59:2806–2817. doi: 10.1128/iai.59.8.2806-2817.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kesavalu L, Holt S C, Ebersole J L. Porphyromonas gingivalis virulence in a murine lesion model: effects of immune alterations. Microb Pathog. 1997;23:317–326. doi: 10.1006/mpat.1997.0161. [DOI] [PubMed] [Google Scholar]
- 21.Kontiainen S, Ranta H, Lautenschlager I. Cells infiltrating human periapical inflammatory lesions. J Oral Pathol. 1986;15:544–546. doi: 10.1111/j.1600-0714.1986.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 22.Laine P O, Lindqvist J C, Pyrhonen S O, Strand-Pettinen I M, Teerenhovi L M, Meurman J H. Oral infection as a reason for febrile episodes in lymphoma patients receiving cytostatic drugs. Eur J Cancer Ser B. 1992;26:103–107. doi: 10.1016/0964-1955(92)90036-z. [DOI] [PubMed] [Google Scholar]
- 23.Nilsen R, Johannessen A C, Skaug N, Matre R. In situ characterization of mononuclear cells in human dental periapical inflammatory lesions using monoclonal antibodies. Oral Surg Oral Med Oral Pathol. 1984;58:160–165. doi: 10.1016/0030-4220(84)90131-2. [DOI] [PubMed] [Google Scholar]
- 24.Parenti D M, Snydman D R. Capnocytophaga species: infections in nonimmunocompromised and immunocompromised hosts. J Infect Dis. 1985;151:140–147. doi: 10.1093/infdis/151.1.140. [DOI] [PubMed] [Google Scholar]
- 25.Schifferli J A, Ng Y C, Peters D K. The role of complement and its receptor in the elimination of immune complexes. N Engl J Med. 1986;315:488–495. doi: 10.1056/NEJM198608213150805. [DOI] [PubMed] [Google Scholar]
- 26.Smith D J, Taubman M A, King W F. Immunological features of minor salivary gland saliva. J Clin Immunol. 1987;7:449–455. doi: 10.1007/BF00915054. [DOI] [PubMed] [Google Scholar]
- 27.Snapper C M, Mond J J. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J Immunol. 1996;157:2229–2233. [PubMed] [Google Scholar]
- 28.Snapper C M, Moorman M A, Rosas F R, Kehry M R, Maliszewski C R, Mond J J. IL-3 and granulocyte-macrophage colony-stimulating factor strongly induce Ig secretion by sort-purified murine B cell activated through the membrane Ig, but not the CD40, signaling pathway. J Immunol. 1995;154:5842–5850. [PubMed] [Google Scholar]
- 29.Socransky S S, Smith C, Martin L, Paster B J, Dewhirst F E, Levin A E. “Checkerboard” DNA-DNA hybridization. BioTechniques. 1994;17:788–792. [PubMed] [Google Scholar]
- 30.Stashenko P, Wang C Y, Riley E, Wu Y, Ostroff G, Niederman R. Reduction of infection-stimulated periapical bone resorption by the biological response modifier PGG glucan. J Dent Res. 1995;74:323–330. doi: 10.1177/00220345950740010701. [DOI] [PubMed] [Google Scholar]
- 31.Stashenko P, Yu S M. T helper and T suppressor cell reversal during the development of induced rat periapical lesions. J Dent Res. 1989;68:830–834. doi: 10.1177/00220345890680051601. [DOI] [PubMed] [Google Scholar]
- 32.Stern M H, Dreizen S, Mackler B F, Selbst A G, Levy B M. Quantitative analysis of cellular composition of human periapical granuloma. J Endodontol. 1981;7:117–122. doi: 10.1016/S0099-2399(81)80125-2. [DOI] [PubMed] [Google Scholar]
- 33.Tani-Ishii N, Kuchiba K, Osada T, Watanabe Y, Umemoto T. Effect of T-cell deficiency on the formation of periapical lesions in mice: histological comparison between periapical lesion formation in BALB/c and BALB/c nu/nu mice. J Endodontol. 1995;21:195–199. doi: 10.1016/s0099-2399(06)80565-0. [DOI] [PubMed] [Google Scholar]
- 34.Teles R, Wang C Y, Stashenko P. Increased susceptibility of RAG-2 SCID mice to dissemination of endodontic infections. Infect Immun. 1997;65:3781–3787. doi: 10.1128/iai.65.9.3781-3787.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tung A S, Ju S T, Sato S, Nisonoff A. Production of large amounts of antibodies in individual mice. J Immunol. 1976;116:676–681. [PubMed] [Google Scholar]
- 36.Wallstrom J B, Torabinejad M, Kettering J, McMillan P. Role of T cells in the pathogenesis of periapical lesions. A preliminary report. Oral Surg Oral Med Oral Pathol. 1993;76:213–218. doi: 10.1016/0030-4220(93)90207-k. [DOI] [PubMed] [Google Scholar]
- 37.Wisplinghoff H, Reinert R R, Cornely O, Seifert H. Molecular relationships and antimicrobial susceptibilities of viridans group streptococci isolated from blood of neutropenic patients. J Clin Microbiol. 1999;37:1876–1880. doi: 10.1128/jcm.37.6.1876-1880.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wysocka M, Kubin M, Vieira L Q, Qzmen L, Garotta G, Scott P, Trinchieri G. IL-12 is required for IFNγ production and lethality in LPS-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]