Abstract
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease worldwide and is a significant burden on healthcare systems. α-klotho (klotho) is a protein known for its anti-aging properties and has been shown to delay the onset of age-related diseases. Soluble klotho is produced by cleavage of the full-length transmembrane protein by a disintegrin and metalloproteases, and it exerts various physiological effects by circulating throughout the body. In type 2 diabetes and its complications DN, a significant decrease in klotho expression has been observed. This reduction in klotho levels may indicate the progression of DN and suggest that klotho may be involved in multiple pathological mechanisms that contribute to the onset and development of DN. This article examines the potential of soluble klotho as a therapeutic agent for DN, with a focus on its ability to impact multiple pathways. These pathways include anti-inflammatory and oxidative stress, anti-fibrotic, endothelial protection, prevention of vascular calcification, regulation of metabolism, maintenance of calcium and phosphate homeostasis, and regulation of cell fate through modulation of autophagy, apoptosis, and pyroptosis pathways. Diabetic retinopathy shares similar pathological mechanisms with DN, and targeting klotho may offer new insights into the prevention and treatment of both conditions. Finally, this review assesses the potential of various drugs used in clinical practice to modulate klotho levels through different mechanisms and their potential to improve DN by impacting klotho levels.
Keywords: klotho, diabetic nephropathy, type 2 diabetes mellitus, diabetic retinopathy, mechanism, drug, chronic kidney disease
1. Introduction
The incidence of Diabetic Nephropathy (DN), a microvascular complication caused by diabetes, has been on the rise globally and is currently the primary cause of end-stage renal disease (1). In most cases, the presence of persistent albuminuria in patients with type 2 diabetes mellitus (T2DM), along with concomitant retinopathy and the exclusion of other renal diseases, serves as the main diagnostic criteria for DN, as a renal biopsy can be traumatic for the patient. DN affects approximately 40% of patients with T2DM, and some studies indicate a percentage as high as 55% (2, 3). Patients with DN are at a significantly higher risk of experiencing cardiovascular events and all-cause mortality, requiring continuous renal replacement therapy when they progress to end-stage renal disease, which places a heavy burden on both society and families due to the scarcity of healthcare resources.
The pathogenesis of DN is multifactorial, involving various mechanisms such as activation of the renin-aldosterone system, glomerular hyperfiltration, altered renal hemodynamics, ischemia and hypoxia, oxidative stress, inflammation, and fibrosis (4). Several classes of drugs, including hypoglycemic, agents renin-angiotensin system (RAS) blockers, sodium-glucose cotransport protein-2 inhibitors (SGLT2i), and glucagon-like peptide-1 receptor (GLP-1R) agonists, have been shown to effectively manage diabetes and DN (5). However, the progression of DN can occur in patients who are transiently exposed to hyperglycemia, even when their glycemia is mostly controlled within the normal range by medication (6, 7). This indicates that the pathogenesis of DN is still not fully understood, and the available drugs cannot completely halt the persistence of the pathological state. Given the gravity of the impact of DN on patient prognosis and the healthcare system, it is imperative to pursue intensive research with a focus on developing drugs that can precisely intervene in the disease’s pathogenesis.
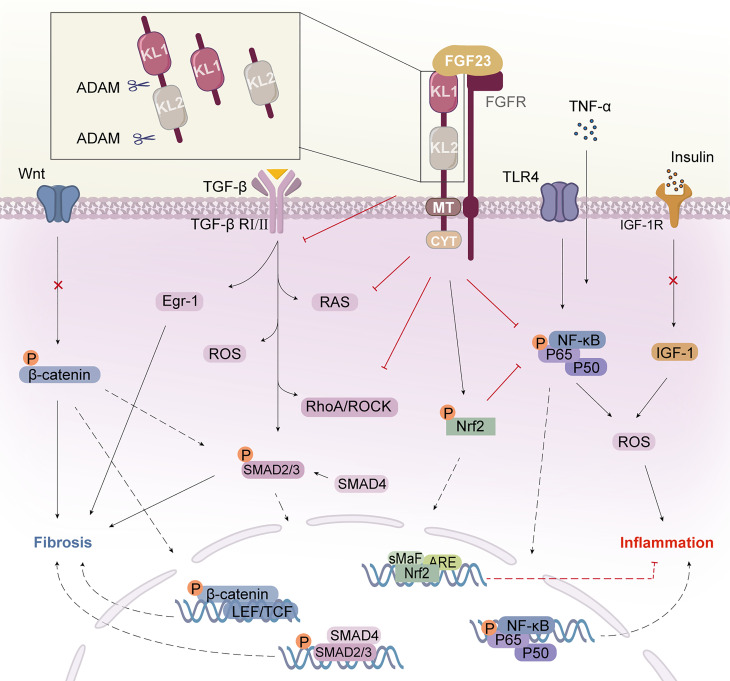
Klotho, first discovered in aging mice in 1997, has been linked to the control of illnesses associated with aging (8). Genetic defects in klotho have been found to cause vascular calcification, hyperphosphatemia, nephropathy, growth retardation, multi-organ atrophy, and fibrosis (8–10). Expression of klotho has been detected mainly in the kidney, brain, and pancreas, but also in human germ cells and sperm (11). The klotho family consists of three members: α-, β-, and γ-klotho (12). In this article, we will only focus on the molecular mechanisms of α-klotho in DN. Unless otherwise specified, the term “klotho” specifically refers to α-klotho. Three forms of klotho protein with different functions have been identified, including full-length transmembrane klotho (m-klotho), truncated soluble klotho (s-klotho), and secreted klotho, although no klotho receptor has been identified (13). M-klotho, which is the transmembrane form, binds to the fibroblast growth factor receptor (FGFR) and forms a ternary complex atomic structure with Fibroblast growth factor (FGF)23 and FGFR1c to confer stability (9, 14). It acts as a protein that primarily regulates renal phosphate excretion and aids in FGF23 signaling (15, 16). FGF23, secreted from bone, is known to reduce serum phosphate and 1,25-dihydroxy vitamin D levels through its action in the kidney (17). The extracellular structural domain of transmembrane klotho can be cleaved by a disintegrin and metalloproteinase (ADAM)-10 and ADAM-17, resulting in the formation of soluble klotho, which can be further cleaved to produce functionally independent Kl1 and Kl2 fragments and participate in the circulation (18) ( Figure 1 ). Although klotho is predominantly expressed in the renal distal convoluted tubules, both tubular and glomerular compartments are protected by this circulating hormone (19, 20). This is because secreted klotho circulates in the blood and acts on tissues or cells that do not express klotho (9, 13). Furthermore, Secretory klotho exhibits potential sialidase activity, which modifies cell surface glycans. This feature may be related to klotho’s ability to regulate multiple ion channels, including transforming growth factor-β1 (TGF-β1), insulin-like growth factor-1 (IGF-1), Wnt, and growth factors such as insulin (21). Circulating levels of soluble klotho decrease with age and are reduced in pathological conditions such as DN, acute kidney injury, and chronic wasting disease (22, 23).
Figure 1.
Klotho structure and its mechanism of action. Klotho consists of two extracellular structural domains (KL1 and KL2), a transmembrane segment (TM), and a short non-signaling cytoplasmic tail (CYT). Enzymes such as ADAM10 or ADAM17 can cleave Klotho to produce a soluble form called s-Klotho. FGF23 binds to the Klotho/FGFR1c receptor in the renal tubule to form the Klotho/FGFR/FGF23 signaling complex, which exerts multiple actions. Klotho binds to the TGF-β receptor, specifically the TβRII component, and inhibits TGF-β and its downstream pathways and molecular activation. Klotho inhibits the activation of the inflammatory NF-κB pathway and increases signaling in the Nrf2 pathway. Klotho block IGF-1 receptor signaling as well as the expression of target genes downstream of Wnt/β-catenin. Klotho inhibits inflammation and fibrosis in diabetic nephropathy through multiple pathways. KL, Klotho; FGF23, fibroblast growth factor 23; FGFR, FGF receptor; AMAD, a disintegrin and metalloproteases; TGF-β, transforming growth factor β; TβRII, TGF-β receptor type II; Egr-1, early growth response factor 1; RAS, agents renin-angiotensin system; ROS, reactive oxygen species; TLR4, toll-like receptor 4; Nrf2, nuclear factor-erythroid 2-related factor 2; NF-κB, nuclear factor κB; IGF-1, insulin-like growth factor 1; IGF-1R, IGF-1 receptor.
With further research, klotho levels may be linked to the development of various pathological mechanisms, such as metabolic disorders, oxidative stress, inflammation, and fibrosis. Despite extensive research on klotho, there is currently no comprehensive literature summarizing its specific mechanisms of action in DN. Therefore, in this paper, we provide the first analysis and description of the multiple ways in which klotho is involved in the development and progression of DN, based on existing studies. Importantly, targeting klotho may be a promising strategy for preventing and treating DR and DN, given their shared pathological mechanisms. In light of the high cost and long duration of drug development, we also compile a list of drugs commonly used in clinical practice, which may have potential in improving DN by modulating klotho levels.
2. Potential relationship between klotho and diabetes and DN
Klotho is involved in adipocyte maturation and systemic glucose metabolism and is closely related to the development of T2DM (24, 25). Klotho levels show a decreasing trend in both type 1 diabetes mellitus (T1DM) (26–28) and T2DM (29–31), and it is even depleted in the pancreas of diabetic patients (32). Surprisingly, it has been suggested that patients with poor glycemic control may have higher s-klotho levels, possibly because glycosuria increases the metabolic demands of the renal tubules, leading to increased expression and/or cleavage of klotho (33).
It should be noted that low levels of circulating klotho have a strong association with deteriorating renal function in patients with T2DM, with this trend being more pronounced in those receiving hemodialysis (33). Furthermore, studies have indicated that the expression of klotho in both plasma and urine is reduced in the early stages of DN (34–36) and that a further decrease may indicate the development of DN (37). In simpler terms, the levels of serum soluble klotho have a positive correlation with the estimated glomerular filtration rate (eGFR) and a negative correlation with urinary albumin, particularly in patients with diabetes (29, 30, 38–40). Meanwhile, one study mentioned that the ratio of klotho/Cr in random urine tends to decrease as eGFR decreases (41). Conversely, increasing klotho levels helped to delay the progression of diabetes and postpone the onset of diabetic complications (31, 32). All these results suggest that klotho plays a key role in the development of DN.
Nevertheless, it has been suggested that hyperglycemia does not directly impact klotho production in isolated human renal tubular epithelial cells (42). However, this conclusion may be premature as it fails to account for the effect of the entire renal system and its potential paracrine and endocrine regulators. There is evidence suggesting that the level of klotho is not related to kidney function, but is significantly associated with age (43). This may be attributed to the inconsistency of the primary disease and comorbidities in the study, which can contribute to a complex physiological and pathological process. In Addition, certain drugs, such as statins and angiotensin II receptor blockers, interfere with klotho expression, leading to inconsistent results (44–46). In summary, directing therapeutic efforts towards klotho presents a promising avenue for treating patients with DN and may also offer benefits for individuals with T2DM.
3. Inflammation and oxidative stress
Several inflammatory factors, such as monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), as well as oxidative stress factors, such as reactive oxygen species (ROS) and nitric oxide (NO), are involved in inflammation-related kidney injury in DN (47). The relationship between inflammation and oxidative stress is complex and interrelated, with inflammation leading to increased ROS production and ROS exacerbating inflammation, resulting in more severe oxidative stress and inflammatory response in DN patients (48). It has been observed that the levels of klotho decrease with increasing levels of inflammation and oxidative stress in DN patients (35). Studies have shown that inflammatory factors, including tumor necrosis factor-α (TNF-α), and the uremic toxin indolyl sulfate, can increase ROS production and downregulate klotho expression through a nuclear factor-kappaB(NF-κB)-dependent mechanism (49, 50). In addition, epigenetic involvement in klotho gene expression, such as m6A modification of the methyltransferase complex, has been found to reduce klotho expression and exacerbate renal injury and inflammation in DN (51).
Increased levels of klotho help to control levels of inflammation and oxidative stress. Klotho attenuates the inflammatory response induced by hyperglycemia by directly or indirectly regulating NF-κB, thereby reducing renal damage (35, 52, 53). It can be argued that klotho is an anti-inflammatory modulator that negatively regulates NF-κB, and thus reduces the transactivation of pro-inflammatory genes, primarily through a mechanism involving phosphorylation of Ser (536) in the transactivating structural domain of RelA (53). Klotho also inhibits the translocation of NF-κB to the nucleus and attenuates the activity of the NF-κB pathway, thereby reducing the production of downstream inflammatory factors (54).
Klotho has been demonstrated to possess an inhibitory effect on toll-like receptor 4 (TLR4), resulting in the alleviation of inflammation and fibrosis in mesangial cells cultured in high glucose (52). Activation of TLR4 is also known to contribute to the transcription of pro-inflammatory genes, production of inflammatory factors, and ROS, promoting the development of systemic inflammation through paracrine and systemic effects. Subsequent investigations have demonstrated that klotho and TLR4 counteract each other via a protein hydrolysis process related to deglycosylation, whereby klotho mitigates the downstream inflammatory response associated with TLR4 and reverses acute kidney inflammation (55).
In early DN, IGF-1 activity is increased and interacts with the RAS to co-regulate renal hemodynamics (56, 57). Specifically, in renal tubular epithelial cells, IGF-1 induces elevated levels of ROS, NADPH oxidase activity, and fibronectin expression, which trigger renal inflammation (58). Conversely, klotho inhibits intracellular insulin/IGF-1 signaling and enhances tissue resistance to oxidative stress by suppressing tyrosine phosphorylation of insulin and IGF1 receptors (19, 57, 59). In light of this, Takenaka et al. proposed that klotho protein supplementation may have the potential to alleviate hypertension and albuminuria (57).
Furthermore, overexpression of klotho has been shown to significantly induce the signaling of nuclear factor erythroid 2-related factor 2 (Nrf2), a core transcription factor that regulates antioxidant responses. By regulating Nrf2 and cytoprotective antioxidant enzymes, klotho inhibits high glucose-induced oxidative stress and podocyte apoptosis, thereby ameliorating DN (60, 61). Consequently, klotho may have the immunotherapeutic potential for the treatment of diabetes and its associated nephropathy by controlling levels of inflammation and oxidative stress.
4. Fibrosis
A decrease in klotho levels can have a significant impact on endocrine signaling in the body, and a reduced abundance of klotho in the kidneys may directly lead to renal fibrosis and impaired renal function (62–64). Specifically, the klotho protein inhibits the binding of TGF-β1 to cell surface receptors, thereby inhibiting the TGF-β1-induced epithelial-mesenchymal transition (EMT) response, which is a crucial mechanism underlying renal fibrosis (65). In other words, low levels of klotho can activate the TGF-β1 signaling pathway, which contributes to diabetes-related renal fibrosis (66, 67). Notably, when klotho is deficient, a large increase in circulating levels of FGF23 can also target cell types that lack klotho, and this becomes the pathological basis for promoting fibrosis. However, it has been demonstrated that recombinant klotho protein or exogenous klotho supplementation can inhibit high glucose-induced TGF-β signaling, downregulate the expression of pro-fibrotic genes in renal mesenchymal fibroblasts, and ultimately reverse fibrotic lesions (57, 68, 69).
In-depth studies have shown that klotho down-regulates early growth response factor 1 (Egr-1) downstream through the inhibition of TGF-β1 signaling. This mechanism prevents the proliferation of glomerular mesangial cells (MC) and the overproduction of extracellular matrix (ECM) under hyperglycemic conditions (70). Egr-1 is expressed in renal tubular fibroblasts, glomerular mesangial cells, and several other cell types, making it a critical factor in the EMT of DN (71, 72). In particular, klotho has been found to directly target the Egr1/TLR4/mammalian target of rapamycin (mTOR) axis, which reduces inflammation and fibrosis in the glomerulus, suggesting that it has multi-targeted effects (73). Importantly, klotho has significant upstream regulatory effects on TGF-β1. For example, klotho can downregulate TGF-β1 and connective tissue growth factor expression by inhibiting RhoA/ROCK activity, thereby reducing tubulointerstitial fibrosis and proteinuria in DN (74). The results reported by Deng et al. are consistent with these findings and suggest that klotho may inhibit ROCK1 mRNA transcription and protein activity by suppressing NF-κB activity (75).
The Wnt/β-catenin proteins frequently interconnect with other signaling pathways, including TGF-β1, that jointly contribute to a complex mechanism promoting interstitial fibrosis in the kidney. The endogenous antagonist klotho blocks Wnt-triggered β-catenin protein activation and nuclear translocation, inhibiting podocyte dedifferentiation and mesenchymal transformation and ultimately restoring podocyte integrity (76, 77). In another example, klotho-derived peptide 6 has been shown to possess similar properties to klotho by inhibiting the expression of downstream target genes of the Wnt/β-catenin pathway. This effect has been shown to improve DN and reduce lesions of glomerulosclerosis and interstitial fibrosis (78). It is worth noting that klotho can further suppress renin-angiotensin-aldosterone system (RAAS) activation by inhibiting the Wnt/β-catenin pathway, as the overexpression of β-catenin or Wnt ligands can activate all RAS genes (79). This mechanism, which involves the presence of klotho and its ability to inhibit the Wnt/β-catenin pathway, has the potential to significantly delay renal fibrosis. Despite these observations, given the complexity of fibrosis and the involvement of multiple signaling pathways, it is possible that klotho exerts its antifibrotic effects through other undiscovered mechanisms. Therefore, ongoing research on the mechanisms of klotho action may reveal new therapeutic targets for the prevention and treatment of DN fibrosis.
In summary, in addition to its ability to modulate TGF-β1 signaling, klotho also demonstrates the ability to inhibit other fibrosis-inducing signaling pathways such as Wnt/β-catenin, Egr-1, and ROCK. These findings suggest that secreted klotho may have the potential to exert endogenous antifibrotic effects by concurrently suppressing multiple growth factors signaling pathways (65). Despite the current findings, the intricate nature of fibrosis and the numerous signaling pathways involved suggest that klotho may exert its anti-fibrotic effects through undiscovered mechanisms. Therefore, further research is necessary to elucidate klotho’s mechanisms of action, potentially uncovering new therapeutic targets for preventing and treating fibrosis in individuals with DN.
5. Prevention of endothelial dysfunction
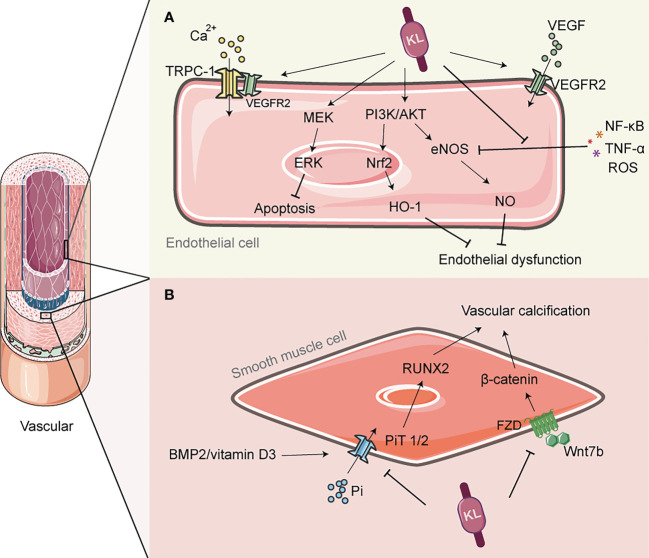
According to current understanding, glomerular endothelial cell (GEC) dysfunction is believed to be a key factor in the development of microangiopathy in DN, and it may also play a role in the failure of RAS inhibitors to effectively prevent the progression of DN (80). GEC dysfunction has several pathological manifestations, including endothelial cell apoptosis, impaired glomerular filtration barrier, and glycocalyx decomposition (81, 82), which may also contribute to the production of microalbuminuria (80). Previous research has shown that klotho gene expression is downregulated in several conditions of endothelial dysfunction associated with vascular disease, including DN (83). In addition, studies have shown that klotho protein or its metabolites promote endothelial NO production in small arteries through a humoral pathway, thereby maintaining normal endothelial function (83, 84) ( Figure 2A ). Further studies have shown that klotho plays an important role in the regulation of vascular function, vascular remodeling, and prevention of perivascular fibrosis through the upregulation of oxidative scavengers and activation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/nitric oxide synthase (eNOS) pathway, as well as stimulation of the MAPK/ERK kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway to attenuate endothelial apoptosis and senescence (85–87).
Figure 2.
The role of Klotho in vascular protection. (A) Klotho can activate PI3K/AKT/Nrf2/HO-1 to enhance endothelial antioxidant defense; It can also activate PI3K/Akt/eNOS pathway, and alleviate the inhibition of eNOS phosphorylation by inflammatory factors such as TNF-α, thereby promoting NO production and preventing endothelial cell dysfunction. Klotho is involved in the transmission of VEGF signaling and regulates Ca2+ influx, which helps to maintain endothelial integrity. (B) Klotho inhibits phosphate uptake by vascular smooth muscle cells (VSMCs), leading to improved vascular calcification. It also inhibits the PiT2/RUNX2 signaling pathway, which improves extracellular matrix calcification. Additionally, Klotho inhibits the Wnt7b/β-catenin signaling pathway, which prevents VSMC calcification. NO, nitric oxide; eNOS, endothelial nitric oxide synthase; PI3K, Phosphoinositide 3-kinase; AKT, protein kinase B; Nrf2, Nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; TRPC-1, transient-receptor potential canonical Ca(2+) channel 1; VEGF, vascular endothelial growth factor; PiT, Pi transporter; RUNX2, runt-associated transcription factor 2.
In addition to regulating NO release from the endothelium, klotho may also play a role in the expression of vascular endothelial growth factor (VEGF), which is produced by podocytes and regulates the structure and function of neighboring endothelial cells in a paracrine manner, as well as regulating angiogenesis. It has been shown that klotho transmits VEGF signaling to transient-receptor potential canonical Ca(2+) channel 1 (TRPC-1), which regulates Ca2+ influx and thus helps to maintain endothelial integrity (88). Conversely, mice deficient in klotho exhibit increased hyperpermeability due to enhanced apoptosis and reduced surface expression of vascular endothelial (VE)-cadherin, which is accompanied by hyperactivation of Ca2+-dependent calpain/caspase-3 (88). However, the role of VEGF in the kidney is complex, and excess VEGF may be damaging to the kidney (89). At least one recent study has reported that klotho can inhibit VEGF secretion from the basal membrane by inhibiting phosphorylation of VEGF receptor 2, suggesting that klotho may have a bidirectional role in regulating the renal VEGF system (90). Furthermore, oxidative stress and inflammatory responses induced by high glucose are thought to be among the determinants of endothelial dysfunction (91). The anti-inflammatory and antioxidant effects of klotho are fully described in subsection 4 of this paper, but it is worth adding that klotho can also enhance the antioxidant defense of endothelial cells by activating PI3K/AKT/Nrf2/heme oxygenase-1 (HO-1) (92). In addition, klotho inhibits the expression of vascular cell adhesion molecule-1 (VCAM-1) and suppresses the inflammatory response and vascular damage caused by the adhesion of leukocytes to endothelial cells; at the same time, klotho also deregulates the inhibition of eNOS phosphorylation by TNF-α, maintains NO production and regulates endothelial inflammation (93). Overall, klotho may indirectly reduce endothelial dysfunction induced by inflammatory factors and uremic toxins through its anti-inflammatory and antioxidant effects (94).
6. Regulation of calcium and phosphate homeostasis
Calcium and phosphate are vital in the composition of biological substances, signal transduction, and other life processes. Multiple molecules are responsible for regulating their balance in the body, including parathyroid hormone (PTH), vitamin D, and klotho. In patients with T2DM as well as DN, high blood phosphorus levels are not only associated with cardiovascular mortality but also independently with renal disease progression (95, 96). As an important pathway for calcium and phosphorus excretion, increased urinary phosphorus excretion by the kidneys seems to guarantee a lower risk of chronic kidney disease (CKD) progression in T2DM patients (97). The mechanism by which klotho regulates calcium and phosphate homeostasis involves the binding of skeletal-produced FGF23 to the klotho/FGFR1c receptor in the renal tubules, which leads to increased phosphate excretion in the urine (98). Conversely, defects in klotho or FGF23 contribute to hyperphosphatemia (99). However, one study has reported that klotho can transport renal phosphate independently of FGF23 (100). Although the exact mechanism is not fully understood, it is clear that klotho plays a role in regulating the balance of calcium and phosphate in the body.
Calcification is a small vessel disease characterized by extensive vascular calcification as the main pathological feature, and diabetes is one of the main risk factors predisposing patients with chronic kidney disease to calcification reactions (101, 102). Downregulation of the klotho gene in chronic renal failure is thought to be associated with the development of vascular calcification (VC) (103) Specifically, defects in klotho lead to phosphate retention, and elevated blood phosphate concentrations trigger calcium phosphate precipitation, a product that is adsorbed by the serum protein fetuin-A and induces ectopic calcification (104, 105). In the early stages of renal disease, a reduction in the co-receptor klotho can lead to an increased risk of vascular calcification and vascular senescence. This risk is further amplified by a compensatory increase in F23 (10, 106, 107). However, it is unclear whether this effect is due to circulating klotho or localized klotho in the vascular system. Rukov et al. demonstrated that neither normal nor calcified vascular systems in chronic renal failure express klotho (108). However, this finding contradicts the findings of Donate-Correa et al., who reported that klotho is expressed in calcified human arteries (109, 110). All studies conducted thus far have confirmed the beneficial effects of klotho on vascular calcification. Klotho can improve vascular calcification by inhibiting phosphate uptake by vascular smooth muscle cells (VSMC) and increasing urinary phosphorus excretion (103, 111). Further studies have revealed that this process may also involve the regulation of the Wnt7b/β-catenin signaling pathway by klotho, which indirectly inhibits hyper phosphate-induced VSMC calcification (112) ( Figure 2B ). It is worth noting that even after excluding the effect of hyperphosphatemia-induced calcification, calcification of the extracellular matrix can still occur in arteries due to the activation of bone morphogenetic protein (BMP2)/vitamin D3- (Pi transporters 2, PiT2)/runt-associated transcription factor 2 (RUNX2) signaling. However, klotho has been found to inhibit the activation of this signaling pathway and attenuate the calcification response (113). Considering these reasons, the replacement of klotho may have therapeutic potential for vascular calcification in DN, and this strategy should not be underestimated.
7. Regulation of glucose metabolism
In diabetic rat kidneys, insulin-like growth factor 1 (IGF1) and insulin-like growth factor-1 receptor (IGF-1R) expression are significantly elevated, which can lead to lipid accumulation in rat renal mesangial cells and dysfunction (114, 115). However, overexpression of klotho in mice can bind to cell surface receptors and inhibit the intracellular transmission of insulin and IGF1 (19, 116). Specifically, klotho antagonizes IGF-1R and subsequently inhibits downstream PI3K/AKT/mTOR signaling, which contributes to increased peroxisome proliferator-activated receptor-α(PPAR-α)transcriptional activity of genes that regulate metabolism, improve insulin sensitivity, and maintain glucolipid homeostasis (117). While klotho overexpression has been shown to inhibit insulin signaling, it does not necessarily result in insulin resistance and can still effectively harness its anti-aging properties. On the contrary, a deficiency in klotho has been demonstrated to worsen insulin resistance and raise blood glucose levels (116, 117). This phenomenon may be attributed to the expression of klotho in pancreatic islets and insulinoma β-cells, which boosts insulin secretion by upregulating the plasma membrane level of transient receptor potential V2 (32, 118). Nevertheless, some studies have proposed an alternate view that klotho regulates metabolism and enhances whole-body energy expenditure by promoting glucose uptake and glycogen synthesis, and by enhancing insulin sensitivity through unknown mechanisms (116).
The discovery of klotho in the cerebral choroid plexus revealed that its functions are not limited to regulating glucose metabolism in the peripheral system. According to a study, central klotho does not have any impact on fasting insulin or glucose levels. Instead, its influence on insulin secretion may depend on glucose levels. Furthermore, the study suggests that this regulatory effect remains unaffected by any changes in body weight (119). However, recent studies have challenged previous findings and suggest that impaired central α-klotho function may play a role in abnormal glucose metabolism and development of obesity (120). It is worth noting that abnormalities in glucose metabolism can also impact klotho expression. For instance, s-klotho concentrations are significantly lower in children with T1DM compared to healthy children when controlling for related diseases such as aging (28). Despite controversy, this suggests that klotho may exert its anti-diabetic and anti-obesity effects through multiple pathways. In summary, the bioavailability of klotho may directly impact glucose metabolism, which in turn affects the development and progression of T2DM and DN. Further studies are required to assess potential adverse effects associated with the use of this soluble hormone, and to monitor whether the body becomes dependent on this exogenous hormone after prolonged use.
8. Regulation of cell death
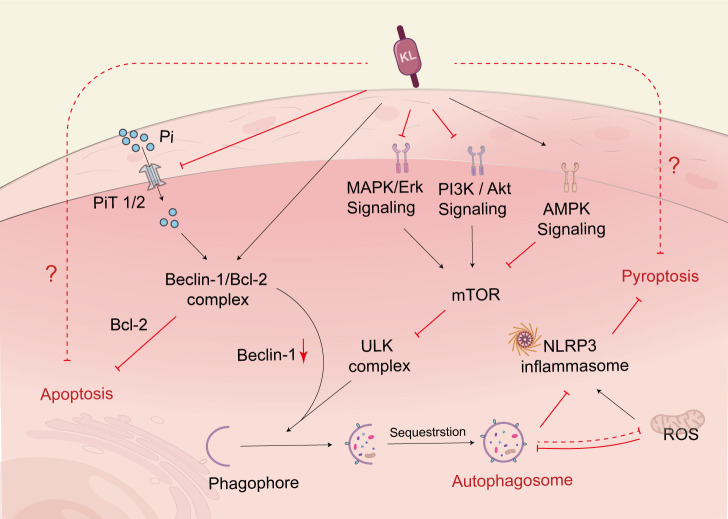
Autophagy is a lysosomal degradation pathway that aids cells in adapting to or counteracting stress responses, primarily by breaking down and recycling cytoplasmic components. Nutrient-sensing pathways, such as the IGF-1 signaling pathway, the mammalian target of rapamycin (mTOR) signaling pathway, and the adenylate-activated protein kinase (AMPK) pathway, regulate autophagy, with the target of mammalian rapamycin complex 1 (mTORC1) being the primary negative regulator of autophagy. A significant amount of evidence supports a regulatory role for autophagy in DN. For instance, cells exposed to high glucose levels for a prolonged period experience inhibited autophagy, which accelerates the development of DN (121). However, klotho can upregulate autophagy and mitigate renal cell injury and subsequent renal fibrosis (122). More specifically, klotho regulates the upregulation of autophagic flux by inhibiting the AKT/mTOR pathway or the IGF-1-mediated PI3K/Akt/mTOR pathway to improve diabetes and kidney injury (123, 124) ( Figure 3 ). In parallel, the AMPK and MAPK pathways are also targets of klotho’s intervention to inhibit hyperglycemic damage to renal tubules through improved autophagy (121, 125).In addition to the autophagic pathways mentioned above, klotho also regulates Beclin-1, a crucial autophagy regulator, and may modulate autophagy levels by affecting the relative levels of the Beclin-1/Bcl-2 protein complex (124, 126). Specifically, klotho increases the binding of Beclin-1 to Bcl-2 and decreases the interactions of Beclin-1 with other autophagy-related proteins, thereby inhibiting autophagic activity in DN (127). It is important to note that the autophagic process is not only regulated by klotho but also influenced by Pi, which promotes the binding of Beclin-1 to its negative regulator BCL-2, impairing autophagic flux (128). Therefore, klotho can indirectly regulate autophagic flux by regulating Pi excretion as well.
Figure 3.
Klotho regulates cell death. Klotho regulates autophagic flux through PI3K/AKT/mTOR, MAPK, and AMPK pathways. Klotho can increase the binding of Beclin-1 to Bcl-2, reduce Beclin-1’s interaction with other autophagy-related proteins, and thereby regulate autophagy. Additionally, Klotho indirectly upregulates autophagy by inhibiting the activity of NLRP3 inflammatory vesicles, which helps prevent renal tubular cell death. Klotho also indirectly regulates autophagy by controlling the excretion of Pi. PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; MAPK, mitogen-activated protein kinase; AMPK, adenosine monophosphate-activated protein kinase; NLRP3, nucleotide-binding oligomerization domain-like pyrin domain-containing protein 3; PiT, phosphate transporter.
In addition to its effects on the autophagic pathway, klotho is also known to rescue hyperglycemia-mediated apoptosis of podocytes, glomerular endothelial cells, and renal tubular epithelial cells (51, 60, 129). Interestingly, klotho can even inhibit apoptosis or necrosis caused by ischemia-reperfusion injury and nephrotoxicity (130, 131). It is widely recognized that apoptosis is primarily mediated by the mitochondrial pathway, the death receptor pathway, and endoplasmic reticulum stress. However, studies investigating the mechanisms by which klotho intervenes in apoptosis have only begun to scratch the surface of this complex process. As a molecular chaperone of klotho, HSP expression increases in response to various stresses and thus functions as a cytoprotective agent. Studies have shown that klotho reduces apoptosis via HSP-70 in experimental ischemic acute kidney injury, and the mechanism may involve HSP70 relying on the immunoprecipitated anti-apoptotic proteins Bcl-2 and Bcl-xl to provide protection to cells (132, 133). Recent research has also shown that HSP70 promotes renal cell survival by inhibiting nucleophosmin (NPM) phosphorylation and reducing its accumulation in the cytoplasm (134).
Pyroptosis is a type of programmed cell death that is characterized by cell lysis and is triggered by inflammatory vesicles. The canonical pathway for inducing pyroptosis is through activation of the nucleotide-binding oligomerization domain-like pyrin domain-containing protein 3 (NLRP3) inflammasome-mediated caspase-1. It is important to note that both pyroptosis and necroptosis pathways essentially result in inflammatory lytic cell death (135). Klotho was found to inhibit the Pyroptosis of renal tubular cells through the inhibition of NLRP3 inflammatory vesicles. The study also suggested that klotho might indirectly suppress the activity of NLRP3 inflammatory vesicles and the maturation of their downstream pathway molecules by upregulating autophagy (123).
In fact, autophagy, apoptosis, and pyroptosis are all part of programmed cell death. On the one hand, autophagy inhibits apoptosis as a pathway for cell survival, while on the other hand, autophagy itself or autophagy and apoptosis acting together can induce cell death. Apoptosis can be converted to pyroptosis via the cysteine-3-GSDME (caspase-3) signaling pathway (136), and autophagy in turn can inhibit pyroptosis by reducing mitochondrial ROS to suppress NLRP3 inflammatory vesicle activation (123). Therefore, these three mechanisms can act as both allies and adversaries, and the dominant mechanism is determined by the cellular environment in which the cell exists. Klotho is closely associated with cell fate and can exert its influence on the regression of DN by interfering with all three signaling pathways. However, the specific mechanisms through which it regulates cell death in the context of DN remain to be investigated in depth.
9. Emerging connection between klotho and diabetic retinopathy
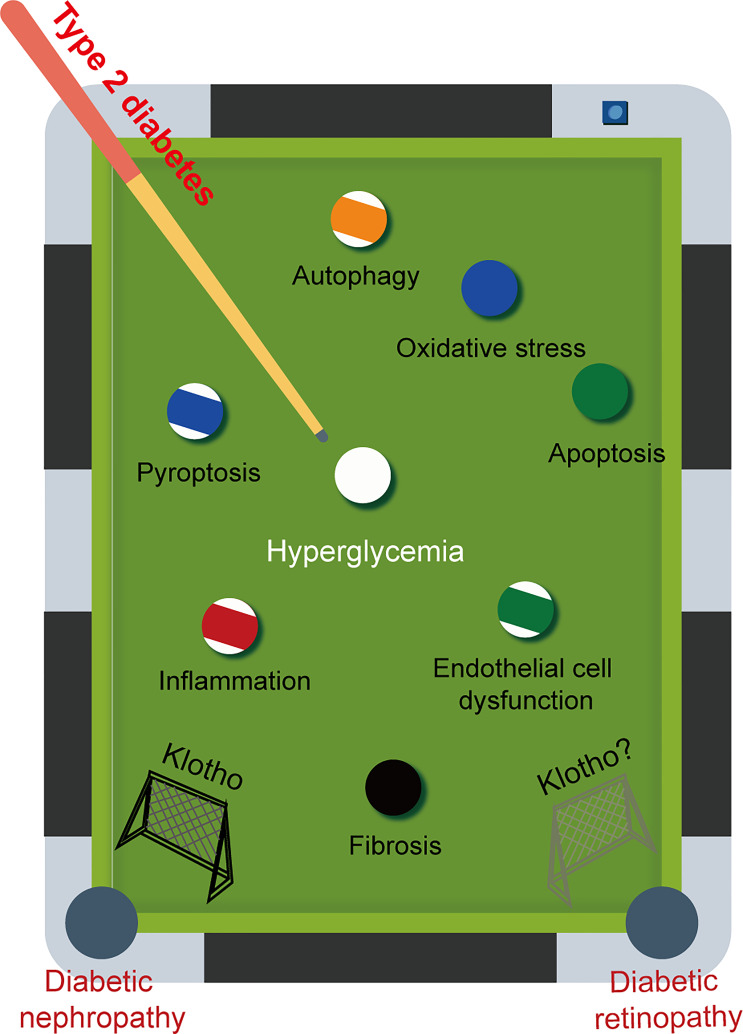
Klotho is found in every nuclear layer of the retina and is strongly linked to retinal function. For example, in klotho knockout mice, the amplitude of a- and b-waves is reduced, retinal signaling is weakened, and synaptic function is altered. Although there is no evidence of retinal degeneration, there is a definite tendency toward faster retinal aging (137). However, only a small number of therapeutic approaches targeting klotho have been explored for the treatment of DR. Late-stage diabetes can result in microvascular complications such as DR and DN. It is important to recognise that these complications share common pathogenic mechanisms and are often predictive of each other. Consistent with our expectation that decreased levels of klotho are also found in DR, and low levels of klotho are strongly associated with a high risk of progression to DR (138). Given the various advantageous effects of klotho on DN, it is speculated that klotho may also have a positive impact on DR ( Figure 4 ).
Figure 4.
The game between pathogenic factors and Klotho. Diabetic retinopathy (DR) and diabetic nephropathy (DN) are two major microvascular complications that commonly occur in late-stage diabetes. These complications share common pathogenic mechanisms. Given the multiple beneficial effects of Klotho on DN, it is reasonable to speculate that Klotho may also have a positive effect on DR.
Several studies revealed that decreased levels of α-klotho in the lens of diabetic rats lead to a reduction in Nrf2-mediated antioxidant defense, thereby contributing to increased NF-κB-mediated inflammatory responses. On the contrary, when klotho was supplemented, it resulted in elevated levels of antioxidants such as superoxide dismutase, glutathione peroxidase, glutathione, and other oxidants, thus effectively preventing oxidative stress and inflammation in the lens of diabetic rats (139, 140). The retinal pigment epithelium (RPE) is a polarized layer of cells containing pigment that is essential for normal visual function in the retina. The protein klotho plays a crucial role in maintaining the viability of the RPE by promoting mitochondrial biogenesis and scavenging ROS (141). In addition to its early effects on retinal function, klotho can also impact the late-stage morphology of the retina in DR. Specifically, klotho can indirectly inhibit secretion of VEGF from RPE cells by inhibiting insulin-like growth factor-3 (IGF-3) signaling and phosphorylation of VEGF receptor 4 (90). This function ultimately prevents neovascularization and helps to maintain the normal morphology of the choroidal layer, which is composed of blood vessels.
It is worth mentioning that klotho can exert a protective effect against DR by inhibiting retinal endothelial cell apoptosis. It achieves this by affecting the Bcl-2/Bax ratio and activating the PI3k/Akt signaling pathway (142). Indeed, autophagy, another form of programmed death, has an equally important role in the maintenance of retinal homeostasis. The relationship between apoptosis and autophagy is intricate. Since klotho can affect apoptosis, could it also exert a positive effect on DR by regulating autophagy? In addition, microvascular endothelial cells are considered to be the main target of hyperglycemic injury and diabetic endothelial dysfunction is a major cause of DR and DN development (82). However, there is still a lack of studies on the effects of klotho on microvascular endothelial cells in DR. In general, DR is the primary cause of blindness in adults with T2DM. But the specific mechanisms that contribute to this remain unknown. Given that klotho has demonstrated the ability to intervene in DN through various mechanisms and that there are similarities in the pathological mechanisms between DR and DN, targeting klotho as a therapeutic option may have the potential to intervene in DR.
10. Re-evaluation of commonly used clinical drugs
Targeting klotho may offer therapeutic benefits for DN through various pathways. However, the development of new drugs can be a very expensive and time-consuming process for a variety of reasons, including failure, poor safety profile, and limited efficacy. We, therefore, look at existing or commonly used clinical agents to explore whether they can have a positive impact on DN via klotho.
10.1. Anti-diabetic drugs
According to reports, metformin, which is the most commonly used antidiabetic drug, can increase klotho levels (143, 144). In addition, entertain-based therapies such as glucagon-like peptide-1 receptor agonist (GLP-1RA) and dipeptidyl peptidase-4 (DPP-4) inhibitors have also been found to increase klotho levels (145–147). However, it remains unclear whether these hypoglycemic agents directly increase klotho levels, modulates the inhibition of klotho by controlling blood glucose levels or indirectly increase klotho production by protecting relevant cells. It is certain that restoring normal blood glucose through insulin supplementation alone can also reverse lower klotho expression levels (148). Therefore, strict glycemic control is an effective approach to prevent the suppression of the physiological function of klotho.
Thiazolidinediones (TZDs) are agonists of peroxisome proliferator-activated receptor γ (PPAR-γ) and have been reported to induce klotho gene expression in a time- and dose-dependent manner in various renal epithelial cells, which may contribute to improving the symptoms and pathology of DN (149–151). That being said, the use of TZDs is limited due to their side effects, such as cardiovascular complications, fluid retention, and obesity, which have severely limited their use in clinical settings (152). New PPAR γ agonists with genetic selectivity have emerged as the next generation of anti-diabetic drugs, and they have shown fewer adverse effects than TZDs, which is encouraging (153). Nevertheless, further studies are needed to determine whether the effect of the new PPAR γ agonists on klotho has changed. The effects of various oral hypoglycemic agents (glimepiride, metformin, and linagliptin) on serum klotho levels are being compared in a trial registered with the Clinical Trials Registry (NCT03585777).
10.2. Sodium-glucose co-transporter inhibitors
Sodium-glucose co-transporter 2 (SGLT-2) inhibitors are widely known for their cardiorenal protective effects. Studies have shown that SGLT-2 inhibitors can restore klotho levels and improve diabetes and its associated cardiorenal complications (154–156). Specifically, SGLT-2 inhibitors prevent the downregulation of klotho by reducing the effects of glucotoxicity and inflammation on renal cells (157), which may contribute to the renal protection provided by SGLT-2 inhibitors. Recent studies have suggested that SGLT-2 inhibitors may not have an effect on klotho or FGF-23 levels, and no changes in serum calcium or phosphate levels were observed. However, this single-center study had a small number of subjects, and further trials are needed to confirm these findings (158). The effects of Empagliflozine (NCT02918591) and Dapagliflozin (NCT05033054, NCT05359263) on soluble klotho are currently being investigated.
10.3. Renin-angiotensin system blockers
RAS inhibitors can provide cardiorenal benefits that are independent of blood pressure through the upregulation of klotho expression (45, 159, 160). In turn, klotho can block RAS activation triggered by angiotensinogen and renin, resulting in a significant renoprotective effect (161). Additionally, Angiotensin Converting Enzyme-2, a component of the unconventional RAS, is strongly associated with the restoration of endogenous klotho levels (162). However, although some studies have shown that dual blockade therapy for diabetic nephropathy RAAS is superior to monotherapy, the relationship between this benefit and klotho is unclear (163).
10.4. Statin drugs
According to a study, statins were found to have vasoprotective effects without significant impacts on blood pressure or lipid levels (44). This suggests that statins may have vasoprotective effects that are independent of lipid metabolism. Further research indicates that atorvastatin or pitavastatin completely prevented the reduction of klotho expression in endothelial cells (44, 164, 165), indicating that the positive effects of these drugs on the kidney and blood vessels may be partially due to their ability to regulate klotho (160, 166). However, it is still unclear what specific role the antioxidant effects of statins play in influencing changes in klotho levels (165, 167).
10.5. Vitamin D
Vitamin D supplementation can normalize klotho levels in children with mild to moderate CKD, but in children with advanced CKD, the kidney’s ability to synthesize klotho has been reduced. Therefore, vitamin D supplementation may primarily promote increased levels of FGF23 (168). Moreover, aside from its ability to enhance endothelial cell adhesion, vitamin D also helps maintain endothelial integrity and functional stability, partially by increasing local klotho levels (169, 170). Although the source of increased klotho concentrations is uncertain, vitamin D still holds promise as a potential therapeutic approach for managing calcium and phosphorus metabolism and endothelial dysfunction in CKD patients (107, 169). A trial of oral vitamin D (Cholecalciferol) affecting plasma klotho levels has been completed but the results have not been published (NCT01524874). It is noteworthy that non-selective vitamin D receptor activators (VDRAs), such as calcitriol, have been linked to elevated levels of blood calcium and phosphate. In contrast, selective VDRAs prevent the adverse effects of increased blood calcium and serum phosphate levels, making them a more viable option in the future (171).
10.6. Mammalian target of rapamycin inhibitor
As commonly used autophagy inducers, mTOR inhibitors, such as rapamycin, have proven effective in treating DN as well as immunosuppression after organ transplantation (172–174). Some studies have shown that mTOR inhibitors can increase klotho expression in transplant recipients, although the effect of the transplanted kidney on klotho expression levels cannot be ruled out (175). In addition to this, mTOR inhibitors have indeed been shown to be effective agents in enhancing klotho levels (176, 177). However, these drugs suppress normal immune function and tend to cause abnormal increases in blood glucose, so their use in clinical practice is still limited.
10.7. Pentoxifylline
Pentoxifylline (PTX) is primarily utilized for the management of ischaemic peripheral vascular disease and plays a crucial role in the treatment of diabetic-related vascular disease and degenerative vascular complications (178, 179). PTX has been found to induce a dose-dependent increase in klotho protein and mRNA expression, with a concomitant increase in klotho levels detected in serum and urine. Furthermore, urinary klotho levels were found to be higher in stage 3 CKD patients than in those with more advanced CKD, suggesting a direct correlation between changes in klotho levels and eGFR (180). Given the enormous burden of diabetes and its complications, PTX should not be disregarded as a potential and reasonably accessible therapeutic alternative for DN, even in the absence of definitive data on renal outcomes.
10.8. Phytotherapeutics
Natural compounds derived from traditional medicines, such as resveratrol, cordycepin, astaxanthin, ligustilide, and ginseng, have been found to regulate klotho expression. They achieve this effect either by directly regulating the klotho gene or indirectly by regulating downstream molecules. These compounds are believed to have potential anti-aging (181–184), anti-oxidative stress (185, 186), fibrosis-ameliorating (187), cardiovascular calcification-improving (188), and organ-protective effects (189), either individually or simultaneously, through this mechanism ( Table 1 ). Interestingly, research shows that Tetrahydroxystilbene glucoside, a major component of Polygonatum multiflorum, can prolong the lifespan of mice by modulating neuronal insulin signaling (190). In addition, baicalin and curcumin have been found to regulate endogenous klotho expression by modulating klotho promoter methylation, which can alleviate diabetes or cyclosporin A-induced kidney injury in mice (191, 192). Based on current evidence, it appears that both klotho and compounds targeting klotho have the potential to affect a variety of bodily systems, including the nervous, cardiovascular, and urinary systems. While traditional Chinese medical theory can guide the application of natural compounds in the human body, it remains unclear whether these compounds can effectively modulate klotho levels in humans. Therefore, further research is necessary to fully assess the potential of phytotherapeutics in targeting klotho.
Table 1.
Drugs already in clinical use targeting klotho.
| Drugs | Targets | References |
|---|---|---|
| Biguanide | ||
| Metformin | klotho protein↑ | (143, 144) |
| Dipeptidyl peptidase-4 (DPP-4) | ||
| Linagliptin | regulate FGF-23/klotho expression | (145) |
| Vildagliptin | klotho protein↑ | (146) |
| Sitagliptin | klotho protein↑ | (147) |
| Sodium-glucose co-transporter 2 (SGLT-2) inhibitors | ||
| Empagliflozine | increases or preserve klotho expression | (155, 156) |
| Canagliflozin | klotho protein↑, klotho mRNA↑ | (156) |
| Dapagliflozin | klotho protein↑, klotho mRNA↑ | (156) |
| Thiazolidinediones (TZDs) | ||
| Troglitazone | activates PPAR-γ, klotho protein↑, klotho mRNA↑ | (149) |
| Ciglitazone | activates PPAR-γ, klotho protein↑, klotho mRNA↑ | (149) |
| Pioglitazone | klotho protein↑, klotho mRNA↑ | (150) |
| Rosiglitazone | activates PPAR-γ, klotho protein↑, klotho mRNA↑ | (151) |
| Renin-angiotensin-aldosterone inhibitors | ||
| Losartan | klotho protein↑, klotho mRNA↑, preserve klotho expression | (45, 150) |
| Valsartan | klotho protein↑ | (159, 160) |
| Statins | ||
| Atorvastatin | preserve klotho expression | (44) |
| Pitavastatin | klotho protein↑, klotho mRNA↑, preserve klotho expression | (44, 164) |
| Simvastatin | klotho protein↑, klotho mRNA↑ | (166) |
| Fluvastatin | klotho mRNA↑ | (160) |
| Vitamin D Supplements | ||
| Vitamin D | klotho protein↑ | (168) |
| Calcitriol | klotho protein↑ | (170) |
| Paricalcitol | klotho protein↑ | (169) |
| mTOR inhibitor | ||
| Rapamycin | klotho protein↑, klotho mRNA↑ | (176, 177) |
| Nonspecific phosphodiesterase inhibitor | ||
| Pentoxifylline | klotho protein↑, preserve klotho expression | (180) |
| Phytotherapeutics | ||
| Cordycepin | klotho protein↑ | (181) |
| Resveratrol | klotho protein↑, klotho mRNA↑, mediated expression of klotho via SIRT1 | (182, 185, 188) |
| Astaxanthin | modulate klotho protein expression | (184) |
| Ligustilide | increases cleavage of full-length klotho by α-secretase | (183) |
| Ginseng | klotho protein↑, klotho mRNA↑ | (186, 187) |
| Tetrahydroxystilbene Glucoside | klotho protein↑ | (190) |
| Baicalin | regulates methylation of the klotho promoter | (191) |
| Curcumin | inhibits CpG hypermethylation of the klotho promoter | (192) |
| Dendrobium nobile Lindl. alkaloid | klotho protein↑ | (144) |
FGF-23, Fibroblast growth factor 23; peroxisome proliferator-activated receptor γ, PPAR-γ; Mammalian target of rapamycin, mTOR; silent information regulator factor 2-related enzyme 1, SIRT1.
11. Conclusion
Klotho plays a protective role in various cell types in DN, including renal tubular epithelial cells (72), renal glomerular and vascular endothelial cells (79, 86), mesenchymal fibroblasts (68, 69), and glomerular mesangial cells (70). Current evidence suggests that klotho can ameliorate DN through various mechanisms, including anti-fibrosis, anti-inflammatory and oxidative stress, vascular protection, metabolic modulation, anti-apoptosis, and regulation of autophagy. However, further research is needed to identify the primary targets of klotho’s action among these various pathways.
In recent years, sufficient attention has been paid to the study of the physiological role of klotho and related molecular mechanisms. Despite this, several questions surrounding its effects remain unresolved. For instance, a study found that serum glucose in the klotho overexpression group was significantly different from the control group 12 weeks after establishing a diabetic mouse model (193). However, it is still unclear whether klotho has the potential to impact serum glucose levels directly. In addition to blocking klotho downregulation or replacing deficient klotho, targeting microRNAs have been identified as an effective approach to modulating klotho levels (194, 195). Although, studies in this area are still in the preclinical stage. Furthermore, klotho has the potential to serve as a promising biomarker for the prediction of aging, endocrine or renal diseases. However, there is a need to refine its measurement techniques and diagnostic criteria.
Targeting klotho with approved drugs may be an effective approach to intervening in DN. Therefore, we have compiled several drugs in clinical use that modulate klotho levels through different mechanisms. According to current research, many drugs have been found to increase klotho concentrations, and klotho supplementation has not shown any signs of causing toxic side effects. While several clinical trials have been conducted to evaluate the efficacy of klotho in treating DN, further research is needed to determine the safety of long-term, high-dose administration of recombinant klotho or klotho supplements. Aside from assessing efficacy and safety, more research is necessary to examine the quantitative impact of these drugs in treating DN. Finally, although much of the fundamental research on klotho is based on animal models, further data from assays in healthy humans or patients with DN is required to refine our understanding.
Author contributions
This research was conducted in collaboration with all authors. AT, and YZ were responsible for manuscript writing. LW, YL, LL, and LZ contributed to the manuscript revision. BX, YH, and ML contributed to the final approval. All authors contributed to the article and approved the submitted version.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81973831, 82274482), Sichuan Science and Technology (2020YFS0367), and the Research Project of Hospital of Chengdu University of Traditional Chinese (H2021107).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Reutens AT. Epidemiology of diabetic kidney disease. Med Clin North Am (2013) 97(1):1–18. doi: 10.1016/j.mcna.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 2. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol (2017) 12(12):2032–45. doi: 10.2215/CJN.11491116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG, Investigators D. Prevalence and risk factors for microalbuminuria in a referred cohort of type ii diabetic patients: a global perspective. Kidney Int (2006) 69(11):2057–63. doi: 10.1038/sj.ki.5000377 [DOI] [PubMed] [Google Scholar]
- 4. Tuttle KR, Agarwal R, Alpers CE, Bakris GL, Brosius FC, Kolkhof P, et al. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int (2022) 102(2):248–60. doi: 10.1016/j.kint.2022.05.012 [DOI] [PubMed] [Google Scholar]
- 5. Yamazaki T, Mimura I, Tanaka T, Nangaku M. Treatment of diabetic kidney disease: current and future. Diabetes Metab J (2021) 45(1):11–26. doi: 10.4093/dmj.2020.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chalmers J, Cooper ME. Ukpds and the legacy effect. N Engl J Med (2008) 359(15):1618–20. doi: 10.1056/NEJMe0807625 [DOI] [PubMed] [Google Scholar]
- 7. Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia (2015) 58(3):443–55. doi: 10.1007/s00125-014-3462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature (1997) 390(6655):45–51. doi: 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 9. Kuro OM. The klotho proteins in health and disease. Nat Rev Nephrol (2019) 15(1):27–44. doi: 10.1038/s41581-018-0078-3 [DOI] [PubMed] [Google Scholar]
- 10. Olauson H, Larsson TE. Fgf23 and klotho in chronic kidney disease. Curr Opin Nephrol Hypertens (2013) 22(4):397–404. doi: 10.1097/MNH.0b013e32836213ee [DOI] [PubMed] [Google Scholar]
- 11. Bollehuus Hansen L, Kaludjerovic J, Nielsen JE, Rehfeld A, Poulsen NN, Ide N, et al. Influence of Fgf23 and klotho on Male reproduction: systemic vs direct effects. FASEB J (2020) 34(9):12436–49. doi: 10.1096/fj.202000061RR [DOI] [PubMed] [Google Scholar]
- 12. Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol (2013) 75:503–33. doi: 10.1146/annurev-physiol-030212-183727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu Y, Sun Z. Molecular basis of klotho: from gene to function in aging. Endocr Rev (2015) 36(2):174–93. doi: 10.1210/er.2013-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, et al. Alpha-klotho is a non-enzymatic molecular scaffold for Fgf23 hormone signalling. Nature (2018) 553(7689):461–6. doi: 10.1038/nature25451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ide N, Olauson H, Sato T, Densmore MJ, Wang H, Hanai JI, et al. In Vivo Evidence for a limited role of proximal tubular klotho in renal phosphate handling. Kidney Int (2016) 90(2):348–62. doi: 10.1016/j.kint.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 16. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical fgf receptor into a specific receptor for Fgf23. Nature (2006) 444(7120):770–4. doi: 10.1038/nature05315 [DOI] [PubMed] [Google Scholar]
- 17. Haussler MR, Whitfield GK, Kaneko I, Forster R, Saini R, Hsieh JC, et al. The role of vitamin d in the Fgf23, klotho, and phosphate bone-kidney endocrine axis. Rev Endocr Metab Disord (2012) 13(1):57–69. doi: 10.1007/s11154-011-9199-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen CD, Tung TY, Liang J, Zeldich E, Tucker Zhou TB, Turk BE, et al. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry (2014) 53(34):5579–87. doi: 10.1021/bi500409n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone klotho. Science (2005) 309(5742):1829–33. doi: 10.1126/science.1112766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T, et al. Amelioration of progressive renal injury by genetic manipulation of klotho gene. Proc Natl Acad Sci U.S.A. (2007) 104(7):2331–6. doi: 10.1073/pnas.0611079104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuro-o M. Klotho and aging. Biochim Biophys Acta (2009) 1790(10):1049–58. doi: 10.1016/j.bbagen.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JH, Hwang KH, Park KS, Kong ID, Cha SK. Biological role of anti-aging protein klotho. J Lifestyle Med (2015) 5(1):1–6. doi: 10.15280/jlm.2015.5.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kale A, Sankrityayan H, Anders HJ, Gaikwad AB. Epigenetic and non-epigenetic regulation of klotho in kidney disease. Life Sci (2021) 264:118644. doi: 10.1016/j.lfs.2020.118644 [DOI] [PubMed] [Google Scholar]
- 24. Donate-Correa J, Martin-Nunez E, Delgado NP, de Fuentes MM, Arduan AO, Mora-Fernandez C, et al. Implications of fibroblast growth Factor/Klotho system in glucose metabolism and diabetes. Cytokine Growth Factor Rev (2016) 28:71–7. doi: 10.1016/j.cytogfr.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 25. Razzaque MS. The role of klotho in energy metabolism. Nat Rev Endocrinol (2012) 8(10):579–87. doi: 10.1038/nrendo.2012.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keles N, Dogan B, Kalcik M, Caliskan M, Keles NN, Aksu F, et al. Is serum klotho protective against atherosclerosis in patients with type 1 diabetes mellitus? J Diabetes Complications (2016) 30(1):126–32. doi: 10.1016/j.jdiacomp.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 27. Tarhani F, Heidari G, Nezami A. Evaluation of alpha-klotho level in insulin dependent diabetes mellitus (Iddm) children. J Pediatr Endocrinol Metab (2020) 33(6):761–5. doi: 10.1515/jpem-2019-0591 [DOI] [PubMed] [Google Scholar]
- 28. Zubkiewicz-Kucharska A, Wikiera B, Noczynska A. Soluble klotho is decreased in children with type 1 diabetes and correlated with metabolic control. Front Endocrinol (Lausanne) (2021) 12:709564. doi: 10.3389/fendo.2021.709564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nie F, Wu D, Du H, Yang X, Yang M, Pang X, et al. Serum klotho protein levels and their correlations with the progression of type 2 diabetes mellitus. J Diabetes Complications (2017) 31(3):594–8. doi: 10.1016/j.jdiacomp.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 30. Fountoulakis N, Maltese G, Gnudi L, Karalliedde J. Reduced levels of anti-ageing hormone klotho predict renal function decline in type 2 diabetes. J Clin Endocrinol Metab (2018) 103(5):2026–32. doi: 10.1210/jc.2018-00004 [DOI] [PubMed] [Google Scholar]
- 31. Zhang L, Liu T. Clinical implication of alterations in serum klotho levels in patients with type 2 diabetes mellitus and its associated complications. J Diabetes Complications (2018) 32(10):922–30. doi: 10.1016/j.jdiacomp.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 32. Lin Y, Sun Z. In Vivo Pancreatic beta-Cell-Specific expression of antiaging gene klotho: a novel approach for preserving beta-cells in type 2 diabetes. Diabetes (2015) 64(4):1444–58. doi: 10.2337/db14-0632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ciardullo S, Perseghin G. Soluble alpha-klotho levels, glycemic control and renal function in us adults with type 2 diabetes. Acta Diabetol (2022) 59(6):803–9. doi: 10.1007/s00592-022-01865-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, Yoshimoto S, et al. Decreased renal alpha-klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int (2012) 81(6):539–47. doi: 10.1038/ki.2011.423 [DOI] [PubMed] [Google Scholar]
- 35. Typiak M, Piwkowska A. Antiinflammatory actions of klotho: implications for therapy of diabetic nephropathy. Int J Mol Sci (2021) 22(2):956. doi: 10.3390/ijms22020956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xin C, Sun X, Li Z, Gao T. Relationship of soluble klotho and early stage of diabetic nephropathy: a systematic review and meta-analysis. Front Endocrinol (Lausanne) (2022) 13:902765. doi: 10.3389/fendo.2022.902765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim SS, Song SH, Kim IJ, Lee EY, Lee SM, Chung CH, et al. Decreased plasma alpha-klotho predict progression of nephropathy with type 2 diabetic patients. J Diabetes Complications (2016) 30(5):887–92. doi: 10.1016/j.jdiacomp.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 38. Zhang Z, Zhou X, Deng L, Jin K, Xiong X, Su X, et al. The association between serum soluble klotho and chronic kidney disease among us adults ages 40 to 79 years: cross-sectional study. Front Public Health (2022) 10:995314. doi: 10.3389/fpubh.2022.995314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim HR, Nam BY, Kim DW, Kang MW, Han JH, Lee MJ, et al. Circulating alpha-klotho levels in ckd and relationship to progression. Am J Kidney Dis (2013) 61(6):899–909. doi: 10.1053/j.ajkd.2013.01.024 [DOI] [PubMed] [Google Scholar]
- 40. Silva AP, Mendes F, Pereira L, Fragoso A, Goncalves RB, Santos N, et al. Klotho levels: association with insulin resistance and albumin-to-Creatinine ratio in type 2 diabetic patients. Int Urol Nephrol (2017) 49(10):1809–14. doi: 10.1007/s11255-017-1646-3 [DOI] [PubMed] [Google Scholar]
- 41. Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, et al. Characteristics of urinary and serum soluble klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol (2012) 13:155. doi: 10.1186/1471-2369-13-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Ark J, Hammes HP, van Dijk MC, Lexis CP, van der Horst IC, Zeebregts CJ, et al. Circulating alpha-klotho levels are not disturbed in patients with type 2 diabetes with and without macrovascular disease in the absence of nephropathy. Cardiovasc Diabetol (2013) 12:116. doi: 10.1186/1475-2840-12-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seiler S, Wen M, Roth HJ, Fehrenz M, Flugge F, Herath E, et al. Plasma klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int (2013) 83(1):121–8. doi: 10.1038/ki.2012.288 [DOI] [PubMed] [Google Scholar]
- 44. Kuwahara N, Sasaki S, Kobara M, Nakata T, Tatsumi T, Irie H, et al. Hmg-coa reductase inhibition improves anti-aging klotho protein expression and arteriosclerosis in rats with chronic inhibition of nitric oxide synthesis. Int J Cardiol (2008) 123(2):84–90. doi: 10.1016/j.ijcard.2007.02.029 [DOI] [PubMed] [Google Scholar]
- 45. Yoon HE, Ghee JY, Piao S, Song JH, Han DH, Kim S, et al. Angiotensin ii blockade upregulates the expression of klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant (2011) 26(3):800–13. doi: 10.1093/ndt/gfq537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lim SC, Liu JJ, Subramaniam T, Sum CF. Elevated circulating alpha-klotho by angiotensin ii receptor blocker losartan is associated with reduction of albuminuria in type 2 diabetic patients. J Renin Angiotensin Aldosterone Syst (2014) 15(4):487–90. doi: 10.1177/1470320313475905 [DOI] [PubMed] [Google Scholar]
- 47. Rayego-Mateos S, Morgado-Pascual JL, Opazo-Rios L, Guerrero-Hue M, Garcia-Caballero C, Vazquez-Carballo C, et al. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int J Mol Sci (2020) 21(11):3798. doi: 10.3390/ijms21113798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Inci A, Olmaz R, Sari F, Coban M, Ellidag HY, Sarikaya M. Increased oxidative stress in diabetic nephropathy and its relationship with soluble klotho levels. Hippokratia (2016) 20(3):198–203. [PMC free article] [PubMed] [Google Scholar]
- 49. Moreno JA, Izquierdo MC, Sanchez-Nino MD, Suarez-Alvarez B, Lopez-Larrea C, Jakubowski A, et al. The inflammatory cytokines tweak and tnfalpha reduce renal klotho expression through nfkappab. J Am Soc Nephrol (2011) 22(7):1315–25. doi: 10.1681/ASN.2010101073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shimizu H, Bolati D, Adijiang A, Adelibieke Y, Muteliefu G, Enomoto A, et al. Indoxyl sulfate downregulates renal expression of klotho through production of ros and activation of nuclear factor-kb. Am J Nephrol (2011) 33(4):319–24. doi: 10.1159/000324885 [DOI] [PubMed] [Google Scholar]
- 51. Li M, Deng L, Xu G. Mettl14 promotes glomerular endothelial cell injury and diabetic nephropathy Via M6a modification of alpha-klotho. Mol Med (2021) 27(1):106. doi: 10.1186/s10020-021-00365-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu C, Lv C, Chen F, Ma X, Shao Y, Wang Q. The function of mir-199a-5p/Klotho regulating Tlr4/Nf-kappab P65/Ngal pathways in rat mesangial cells cultured with high glucose and the mechanism. Mol Cell Endocrinol (2015) 417:84–93. doi: 10.1016/j.mce.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 53. Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, et al. Klotho depletion contributes to increased inflammation in kidney of the Db/Db mouse model of diabetes Via rela (Serine)536 phosphorylation. Diabetes (2011) 60(7):1907–16. doi: 10.2337/db10-1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buendia P, Ramirez R, Aljama P, Carracedo J. Klotho prevents translocation of nfkappab. Vitam Horm (2016) 101:119–50. doi: 10.1016/bs.vh.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 55. Bi F, Chen F, Li Y, Wei A, Cao W. Klotho preservation by rhein promotes toll-like receptor 4 proteolysis and attenuates lipopolysaccharide-induced acute kidney injury. J Mol Med (Berl) (2018) 96(9):915–27. doi: 10.1007/s00109-018-1644-7 [DOI] [PubMed] [Google Scholar]
- 56. Bach LA, Hale LJ. Insulin-like growth factors and kidney disease. Am J Kidney Dis (2015) 65(2):327–36. doi: 10.1053/j.ajkd.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 57. Takenaka T, Kobori H, Miyazaki T, Suzuki H, Nishiyama A, Ishii N, et al. Klotho protein supplementation reduces blood pressure and renal hypertrophy in Db/Db mice, a model of type 2 diabetes. Acta Physiol (Oxf) (2019) 225(2):e13190. doi: 10.1111/apha.13190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. New DD, Block K, Bhandhari B, Gorin Y, Abboud HE. Igf-I increases the expression of fibronectin by Nox4-dependent akt phosphorylation in renal tubular epithelial cells. Am J Physiol Cell Physiol (2012) 302(1):C122–30. doi: 10.1152/ajpcell.00141.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Olejnik A, Franczak A, Krzywonos-Zawadzka A, Kaluzna-Oleksy M, Bil-Lula I. The biological role of klotho protein in the development of cardiovascular diseases. BioMed Res Int (2018) 2018:5171945. doi: 10.1155/2018/5171945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xing L, Guo H, Meng S, Zhu B, Fang J, Huang J, et al. Klotho ameliorates diabetic nephropathy by activating Nrf2 signaling pathway in podocytes. Biochem Biophys Res Commun (2021) 534:450–6. doi: 10.1016/j.bbrc.2020.11.061 [DOI] [PubMed] [Google Scholar]
- 61. Prud’homme GJ, Kurt M, Wang Q. Pathobiology of the klotho antiaging protein and therapeutic considerations. Front Aging (2022) 3:931331. doi: 10.3389/fragi.2022.931331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Ostman Wernerson A, et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol (2014) 25(10):2169–75. doi: 10.1681/ASN.2013111209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Younis NN, Mohamed HE, Shaheen MA, Abdelghafour AM, Hammad SK. Potential therapeutic efficacy of pachymic acid in chronic kidney disease induced in rats: role of Wnt/Beta-Catenin/Renin-Angiotensin axis. J Pharm Pharmacol (2022) 74(1):112–23. doi: 10.1093/jpp/rgab129 [DOI] [PubMed] [Google Scholar]
- 64. Munoz-Castaneda JR, Rodelo-Haad C, Pendon-Ruiz de Mier MV, Martin-Malo A, Santamaria R, Rodriguez M. Klotho/Fgf23 and wnt signaling as important players in the comorbidities associated with chronic kidney disease. Toxins (Basel) (2020) 12(3):185. doi: 10.3390/toxins12030185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, et al. Klotho inhibits transforming growth factor-Beta1 (Tgf-Beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem (2011) 286(10):8655–65. doi: 10.1074/jbc.M110.174037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gu LY, Yun S, Tang HT, Xu ZX. Huangkui capsule in combination with metformin ameliorates diabetic nephropathy Via the Klotho/Tgf-Beta1/P38mapk signaling pathway. J Ethnopharmacol (2021) 281:113548. doi: 10.1016/j.jep.2020.113548 [DOI] [PubMed] [Google Scholar]
- 67. Oishi H, Doi S, Nakashima A, Ike T, Maeoka Y, Sasaki K, et al. Klotho overexpression protects against renal aging along with suppression of transforming growth factor-Beta1 signaling pathways. Am J Physiol Renal Physiol (2021) 321(6):F799–811. doi: 10.1152/ajprenal.00609.2020 [DOI] [PubMed] [Google Scholar]
- 68. Huang JS, Chuang CT, Liu MH, Lin SH, Guh JY, Chuang LY. Klotho attenuates high glucose-induced fibronectin and cell hypertrophy Via the Erk1/2-P38 kinase signaling pathway in renal interstitial fibroblasts. Mol Cell Endocrinol (2014) 390(1-2):45–53. doi: 10.1016/j.mce.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 69. Sugiura H, Yoshida T, Shiohira S, Kohei J, Mitobe M, Kurosu H, et al. Reduced klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol (2012) 302(10):F1252–64. doi: 10.1152/ajprenal.00294.2011 [DOI] [PubMed] [Google Scholar]
- 70. Li Y, Hu F, Xue M, Jia YJ, Zheng ZJ, Wang L, et al. Klotho down-regulates egr-1 by inhibiting tgf-Beta1/Smad3 signaling in high glucose treated human mesangial cells. Biochem Biophys Res Commun (2017) 487(2):216–22. doi: 10.1016/j.bbrc.2017.04.036 [DOI] [PubMed] [Google Scholar]
- 71. Li Y, Xue M, Hu F, Jia Y, Zheng Z, Yang Y, et al. Klotho prevents epithelial-mesenchymal transition through egr-1 downregulation in diabetic kidney disease. BMJ Open Diabetes Res Care (2021) 9(1):e002038. doi: 10.1136/bmjdrc-2020-002038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yang Z, Zhan YW, Huang YY, Huang W, Zhan F, Lin SD. Regulation of epithelial mesenchymal transition by the renin-angiotensin system: a role for klotho in renal tubular epithelial cells. J Biol Regul Homeost Agents (2020) 34(1):57–67. doi: 10.23812/19-410-A-27 [DOI] [PubMed] [Google Scholar]
- 73. Wu C, Ma X, Zhou Y, Liu Y, Shao Y, Wang Q. Klotho restraining Egr1/Tlr4/Mtor axis to reducing the expression of fibrosis and inflammatory cytokines in high glucose cultured rat mesangial cells. Exp Clin Endocrinol Diabetes (2019) 127(9):630–40. doi: 10.1055/s-0044-101601 [DOI] [PubMed] [Google Scholar]
- 74. Komers R, Oyama TT, Beard DR, Tikellis C, Xu B, Lotspeich DF, et al. Rho kinase inhibition protects kidneys from diabetic nephropathy without reducing blood pressure. Kidney Int (2011) 79(4):432–42. doi: 10.1038/ki.2010.428 [DOI] [PubMed] [Google Scholar]
- 75. Deng M, Luo Y, Li Y, Yang Q, Deng X, Wu P, et al. Klotho gene delivery ameliorates renal hypertrophy and fibrosis in streptozotocin-induced diabetic rats by suppressing the rho-associated coiled-coil kinase signaling pathway. Mol Med Rep (2015) 12(1):45–54. doi: 10.3892/mmr.2015.3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou L, Li Y, He W, Zhou D, Tan RJ, Nie J, et al. Mutual antagonism of wilms’ tumor 1 and beta-catenin dictates podocyte health and disease. J Am Soc Nephrol (2015) 26(3):677–91. doi: 10.1681/ASN.2013101067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Loss of klotho contributes to kidney injury by derepression of Wnt/Beta-catenin signaling. J Am Soc Nephrol (2013) 24(5):771–85. doi: 10.1681/ASN.2012080865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen X, Tan H, Xu J, Tian Y, Yuan Q, Zuo Y, et al. Klotho-derived peptide 6 ameliorates diabetic kidney disease by targeting Wnt/Beta-catenin signaling. Kidney Int (2022) 102(3):506–20. doi: 10.1016/j.kint.2022.04.028 [DOI] [PubMed] [Google Scholar]
- 79. Wang Q, Ren D, Li Y, Xu G. Klotho attenuates diabetic nephropathy in Db/Db mice and ameliorates high glucose-induced injury of human renal glomerular endothelial cells. Cell Cycle (2019) 18(6-7):696–707. doi: 10.1080/15384101.2019.1580495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nakagawa T, Tanabe K, Croker BP, Johnson RJ, Grant MB, Kosugi T, et al. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat Rev Nephrol (2011) 7(1):36–44. doi: 10.1038/nrneph.2010.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sward P, Rippe B. Acute and sustained actions of hyperglycaemia on endothelial and glomerular barrier permeability. Acta Physiol (Oxf) (2012) 204(3):294–307. doi: 10.1111/j.1748-1716.2011.02343.x [DOI] [PubMed] [Google Scholar]
- 82. Yang J, Liu Z. Mechanistic pathogenesis of endothelial dysfunction in diabetic nephropathy and retinopathy. Front Endocrinol (Lausanne) (2022) 13:816400. doi: 10.3389/fendo.2022.816400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, Nakamura T, et al. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci (2000) 57(5):738–46. doi: 10.1007/s000180050038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun (1998) 248(2):324–9. doi: 10.1006/bbrc.1998.8943 [DOI] [PubMed] [Google Scholar]
- 85. Yao Y, Wang Y, Zhang Y, Liu C. Klotho ameliorates oxidized low density lipoprotein (Ox-Ldl)-Induced oxidative stress Via regulating lox-1 and Pi3k/Akt/Enos pathways. Lipids Health Dis (2017) 16(1):77. doi: 10.1186/s12944-017-0447-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Maekawa Y, Ohishi M, Ikushima M, Yamamoto K, Yasuda O, Oguro R, et al. Klotho protein diminishes endothelial apoptosis and senescence Via a mitogen-activated kinase pathway. Geriatr Gerontol Int (2011) 11(4):510–6. doi: 10.1111/j.1447-0594.2011.00699.x [DOI] [PubMed] [Google Scholar]
- 87. Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, et al. In Vivo Klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun (2000) 276(2):767–72. doi: 10.1006/bbrc.2000.3470 [DOI] [PubMed] [Google Scholar]
- 88. Kusaba T, Okigaki M, Matui A, Murakami M, Ishikawa K, Kimura T, et al. Klotho is associated with vegf receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci U.S.A. (2010) 107(45):19308–13. doi: 10.1073/pnas.1008544107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Majumder S, Advani A. Vegf and the diabetic kidney: more than too much of a good thing. J Diabetes Complications (2017) 31(1):273–9. doi: 10.1016/j.jdiacomp.2016.10.020 [DOI] [PubMed] [Google Scholar]
- 90. Kokkinaki M, Abu-Asab M, Gunawardena N, Ahern G, Javidnia M, Young J, et al. Klotho regulates retinal pigment epithelial functions and protects against oxidative stress. J Neurosci (2013) 33(41):16346–59. doi: 10.1523/JNEUROSCI.0402-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes (2017) 9(5):434–49. doi: 10.1111/1753-0407.12521 [DOI] [PubMed] [Google Scholar]
- 92. Cui W, Leng B, Wang G. Klotho protein inhibits H(2)O(2)-induced oxidative injury in endothelial cells Via regulation of Pi3k/Akt/Nrf2/Ho-1 pathways. Can J Physiol Pharmacol (2019) 97(5):370–6. doi: 10.1139/cjpp-2018-0277 [DOI] [PubMed] [Google Scholar]
- 93. Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, Kida I, et al. Klotho suppresses tnf-Alpha-Induced expression of adhesion molecules in the endothelium and attenuates nf-kappab activation. Endocrine (2009) 35(3):341–6. doi: 10.1007/s12020-009-9181-3 [DOI] [PubMed] [Google Scholar]
- 94. Yang K, Nie L, Huang Y, Zhang J, Xiao T, Guan X, et al. Amelioration of uremic toxin indoxyl sulfate-induced endothelial cell dysfunction by klotho protein. Toxicol Lett (2012) 215(2):77–83. doi: 10.1016/j.toxlet.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 95. Chonchol M, Dale R, Schrier RW, Estacio R. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med (2009) 122(4):380–6. doi: 10.1016/j.amjmed.2008.09.039 [DOI] [PubMed] [Google Scholar]
- 96. Yoon CY, Park JT, Jhee JH, Noh J, Kee YK, Seo C, et al. High dietary phosphorus density is a risk factor for incident chronic kidney disease development in diabetic subjects: a community-based prospective cohort study. Am J Clin Nutr (2017) 106(1):311–21. doi: 10.3945/ajcn.116.151654 [DOI] [PubMed] [Google Scholar]
- 97. Duan S, Sun L, Zhu H, Nie G, Zhang C, Huang Z, et al. Association of urinary calcium and phosphorus excretion with renal disease progression in type 2 diabetes. Diabetes Res Clin Pract (2021) 178:108981. doi: 10.1016/j.diabres.2021.108981 [DOI] [PubMed] [Google Scholar]
- 98. Rausch S, Foller M. The regulation of Fgf23 under physiological and pathophysiological conditions. Pflugers Arch (2022) 474(3):281–92. doi: 10.1007/s00424-022-02668-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kuro-o M. A potential link between phosphate and aging–lessons from klotho-deficient mice. Mech Ageing Dev (2010) 131(4):270–5. doi: 10.1016/j.mad.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J (2010) 24(9):3438–50. doi: 10.1096/fj.10-154765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wilmer WA, Magro CM. Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment. Semin Dial (2002) 15(3):172–86. doi: 10.1046/j.1525-139x.2002.00052.x [DOI] [PubMed] [Google Scholar]
- 102. Gilbey SG, Walters H, Edmonds ME, Archer AG, Watkins PJ, Parsons V, et al. Vascular calcification, autonomic neuropathy, and peripheral blood flow in patients with diabetic nephropathy. Diabetes Med (1989) 6(1):37–42. doi: 10.1111/j.1464-5491.1989.tb01136.x [DOI] [PubMed] [Google Scholar]
- 103. Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol (2011) 22(1):124–36. doi: 10.1681/ASN.2009121311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kuro OM. Klotho and calciprotein particles as therapeutic targets against accelerated ageing. Clin Sci (Lond) (2021) 135(15):1915–27. doi: 10.1042/CS20201453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kuro OM. Phosphate as a pathogen of arteriosclerosis and aging. J Atheroscler Thromb (2021) 28(3):203–13. doi: 10.5551/jat.RV17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cianciolo G, Galassi A, Capelli I, Schillaci R, La Manna G, Cozzolino M. Klotho-Fgf23, cardiovascular disease, and vascular calcification: black or white? Curr Vasc Pharmacol (2018) 16(2):143–56. doi: 10.2174/1570161115666170310092202 [DOI] [PubMed] [Google Scholar]
- 107. Prie D, Friedlander G. Reciprocal control of 1,25-dihydroxyvitamin d and Fgf23 formation involving the Fgf23/Klotho system. Clin J Am Soc Nephrol (2010) 5(9):1717–22. doi: 10.2215/CJN.02680310 [DOI] [PubMed] [Google Scholar]
- 108. Rukov JL, Gravesen E, Mace ML, Hofman-Bang J, Vinther J, Andersen CB, et al. Effect of chronic uremia on the transcriptional profile of the calcified aorta analyzed by rna sequencing. Am J Physiol Renal Physiol (2016) 310(6):F477–91. doi: 10.1152/ajprenal.00472.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Donate-Correa J, Mora-Fernandez C, Martinez-Sanz R, Muros-de-Fuentes M, Perez H, Meneses-Perez B, et al. Expression of Fgf23/Klotho system in human vascular tissue. Int J Cardiol (2013) 165(1):179–83. doi: 10.1016/j.ijcard.2011.08.850 [DOI] [PubMed] [Google Scholar]
- 110. Chang JR, Guo J, Wang Y, Hou YL, Lu WW, Zhang JS, et al. Intermedin1-53 attenuates vascular calcification in rats with chronic kidney disease by upregulation of alpha-klotho. Kidney Int (2016) 89(3):586–600. doi: 10.1016/j.kint.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 111. Vervloet MG, Adema AY, Larsson TE, Massy ZA. The role of klotho on vascular calcification and endothelial function in chronic kidney disease. Semin Nephrol (2014) 34(6):578–85. doi: 10.1016/j.semnephrol.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 112. Chen YX, Huang C, Duan ZB, Xu CY, Chen Y. Klotho/Fgf23 axis mediates high phosphate-induced vascular calcification in vascular smooth muscle cells Via Wnt7b/Beta-catenin pathway. Kaohsiung J Med Sci (2019) 35(7):393–400. doi: 10.1002/kjm2.12072 [DOI] [PubMed] [Google Scholar]
- 113. Lin Y, Sun Z. Klotho deficiency-induced arterial calcification involves osteoblastic transition of vsmcs and activation of bmp signaling. J Cell Physiol (2022) 237(1):720–9. doi: 10.1002/jcp.30541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kong YL, Shen Y, Ni J, Shao DC, Miao NJ, Xu JL, et al. Insulin deficiency induces rat renal mesangial cell dysfunction Via activation of igf-1/Igf-1r pathway. Acta Pharmacol Sin (2016) 37(2):217–27. doi: 10.1038/aps.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Berfield AK, Andress DL, Abrass CK. Igf-1-Induced lipid accumulation impairs mesangial cell migration and contractile function. Kidney Int (2002) 62(4):1229–37. doi: 10.1111/j.1523-1755.2002.kid578.x [DOI] [PubMed] [Google Scholar]
- 116. Landry T, Shookster D, Huang H. Circulating alpha-klotho regulates metabolism Via distinct central and peripheral mechanisms. Metabolism (2021) 121:154819. doi: 10.1016/j.metabol.2021.154819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gu H, Jiang W, You N, Huang X, Li Y, Peng X, et al. Soluble klotho improves hepatic glucose and lipid homeostasis in type 2 diabetes. Mol Ther Methods Clin Dev (2020) 18:811–23. doi: 10.1016/j.omtm.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lin Y, Sun Z. Antiaging gene klotho enhances glucose-induced insulin secretion by up-regulating plasma membrane levels of Trpv2 in Min6 beta-cells. Endocrinology (2012) 153(7):3029–39. doi: 10.1210/en.2012-1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Landry T, Laing BT, Li P, Bunner W, Rao Z, Prete A, et al. Central alpha-klotho suppresses Npy/Agrp neuron activity and regulates metabolism in mice. Diabetes (2020) 69(7):1368–81. doi: 10.2337/db19-0941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Landry T, Li P, Shookster D, Jiang Z, Li H, Laing BT, et al. Centrally circulating alpha-klotho inversely correlates with human obesity and modulates arcuate cell populations in mice. Mol Metab (2021) 44:101136. doi: 10.1016/j.molmet.2020.101136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee J, Tsogbadrakh B, Yang S, Ryu H, Kang E, Kang M, et al. Klotho ameliorates diabetic nephropathy Via Lkb1-Ampk-Pgc1alpha-Mediated renal mitochondrial protection. Biochem Biophys Res Commun (2021) 534:1040–6. doi: 10.1016/j.bbrc.2020.10.040 [DOI] [PubMed] [Google Scholar]
- 122. Shi M, Flores B, Gillings N, Bian A, Cho HJ, Yan S, et al. Alphaklotho mitigates progression of aki to ckd through activation of autophagy. J Am Soc Nephrol (2016) 27(8):2331–45. doi: 10.1681/ASN.2015060613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhu X, Li S, Lin Q, Shao X, Wu J, Zhang W, et al. Alphaklotho protein has therapeutic activity in contrast-induced acute kidney injury by limiting Nlrp3 inflammasome-mediated pyroptosis and promoting autophagy. Pharmacol Res (2021) 167:105531. doi: 10.1016/j.phrs.2021.105531 [DOI] [PubMed] [Google Scholar]
- 124. Zhou H, Pu S, Zhou H, Guo Y. Klotho as potential autophagy regulator and therapeutic target. Front Pharmacol (2021) 12:755366. doi: 10.3389/fphar.2021.755366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Xue M, Yang F, Le Y, Yang Y, Wang B, Jia Y, et al. Klotho protects against diabetic kidney disease Via ampk- and erk-mediated autophagy. Acta Diabetol (2021) 58(10):1413–23. doi: 10.1007/s00592-021-01736-4 [DOI] [PubMed] [Google Scholar]
- 126. Li P, Shi M, Maique J, Shaffer J, Yan S, Moe OW, et al. Beclin 1/Bcl-2 complex-dependent autophagy activity modulates renal susceptibility to ischemia-reperfusion injury and mediates renoprotection by klotho. Am J Physiol Renal Physiol (2020) 318(3):F772–F92. doi: 10.1152/ajprenal.00504.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Naguib M, Rashed LA. Serum level of the autophagy biomarker beclin-1 in patients with diabetic kidney disease. Diabetes Res Clin Pract (2018) 143:56–61. doi: 10.1016/j.diabres.2018.06.022 [DOI] [PubMed] [Google Scholar]
- 128. Shi M, Maique J, Shaffer J, Davidson T, Sebti S, Fernandez AF, et al. The tripartite interaction of phosphate, autophagy, and alphaklotho in health maintenance. FASEB J (2020) 34(2):3129–50. doi: 10.1096/fj.201902127R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Xing L, Fang J, Zhu B, Wang L, Chen J, Wang Y, et al. Astragaloside iv protects against podocyte apoptosis by inhibiting oxidative stress Via activating ppargamma-Klotho-Foxo1 axis in diabetic nephropathy. Life Sci (2021) 269:119068. doi: 10.1016/j.lfs.2021.119068 [DOI] [PubMed] [Google Scholar]
- 130. Qian Y, Guo X, Che L, Guan X, Wu B, Lu R, et al. Klotho reduces necroptosis by targeting oxidative stress involved in renal ischemic-reperfusion injury. Cell Physiol Biochem (2018) 45(6):2268–82. doi: 10.1159/000488172 [DOI] [PubMed] [Google Scholar]
- 131. Panesso MC, Shi M, Cho HJ, Paek J, Ye J, Moe OW, et al. Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int (2014) 85(4):855–70. doi: 10.1038/ki.2013.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sugiura H, Yoshida T, Mitobe M, Yoshida S, Shiohira S, Nitta K, et al. Klotho reduces apoptosis in experimental ischaemic acute kidney injury Via hsp-70. Nephrol Dial Transplant (2010) 25(1):60–8. doi: 10.1093/ndt/gfp451 [DOI] [PubMed] [Google Scholar]
- 133. Seidberg NA, Clark RS, Zhang X, Lai Y, Chen M, Graham SH, et al. Alterations in inducible 72-kda heat shock protein and the chaperone cofactor bag-1 in human brain after head injury. J Neurochem (2003) 84(3):514–21. doi: 10.1046/j.1471-4159.2003.01547.x [DOI] [PubMed] [Google Scholar]
- 134. Wang Z, Havasi A, Beeler AA, Borkan SC. Mechanisms of nucleophosmin (Npm)-mediated regulated cell death elucidated by Hsp70 during renal ischemia. Apoptosis (2022) 27(1-2):22–33. doi: 10.1007/s10495-021-01696-8 [DOI] [PubMed] [Google Scholar]
- 135. Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ (2019) 26(1):99–114. doi: 10.1038/s41418-018-0212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hu L, Chen M, Chen X, Zhao C, Fang Z, Wang H, et al. Chemotherapy-induced pyroptosis is mediated by Bak/Bax-Caspase-3-Gsdme pathway and inhibited by 2-bromopalmitate. Cell Death Dis (2020) 11(4):281. doi: 10.1038/s41419-020-2476-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Reish NJ, Maltare A, McKeown AS, Laszczyk AM, Kraft TW, Gross AK, et al. The age-regulating protein klotho is vital to sustain retinal function. Invest Ophthalmol Vis Sci (2013) 54(10):6675–85. doi: 10.1167/iovs.13-12550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Corcillo A, Fountoulakis N, Sohal A, Farrow F, Ayis S, Karalliedde J. Low levels of circulating anti-ageing hormone klotho predict the onset and progression of diabetic retinopathy. Diabetes Vasc Dis Res (2020) 17(6):1479164120970901. doi: 10.1177/1479164120970901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ma Z, Li J, Jiang H, Chu Y. Expression of alpha-klotho is downregulated and associated with oxidative stress in the lens in streptozotocin-induced diabetic rats. Curr Eye Res (2021) 46(4):482–9. doi: 10.1080/02713683.2020.1805768 [DOI] [PubMed] [Google Scholar]
- 140. Ma Z, Liu J, Li J, Jiang H, Kong J. Klotho ameliorates the onset and progression of cataract Via suppressing oxidative stress and inflammation in the lens in streptozotocin-induced diabetic rats. Int Immunopharmacol (2020) 85:106582. doi: 10.1016/j.intimp.2020.106582 [DOI] [PubMed] [Google Scholar]
- 141. Zhou S, Hum J, Taskintuna K, Olaya S, Steinman J, Ma J, et al. The anti-aging hormone klotho promotes retinal pigment epithelium cell viability and metabolism by activating the Ampk/Pgc-1alpha pathway. Antioxid (Basel) (2023) 12(2):385. doi: 10.3390/antiox12020385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ji B, Wei H, Ding Y, Liang H, Yao L, Wang H, et al. Protective potential of klotho protein on diabetic retinopathy: evidence from clinical and in vitro studies. J Diabetes Investig (2020) 11(1):162–9. doi: 10.1111/jdi.13100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Xue J, Wang L, Sun Z, Xing C. Basic research in diabetic nephropathy health care: a study of the renoprotective mechanism of metformin. J Med Syst (2019) 43(8):266. doi: 10.1007/s10916-019-1412-4 [DOI] [PubMed] [Google Scholar]
- 144. Lv LL, Liu B, Liu J, Li LS, Jin F, Xu YY, et al. Dendrobium nobile lindl. alkaloids ameliorate cognitive dysfunction in senescence accelerated Samp8 mice by decreasing amyloid-beta aggregation and enhancing autophagy activity. J Alzheimers Dis (2020) 76(2):657–69. doi: 10.3233/JAD-200308 [DOI] [PubMed] [Google Scholar]
- 145. Manrique C, Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, et al. Dipeptidyl peptidase-4 inhibition with linagliptin prevents Western diet-induced vascular abnormalities in female mice. Cardiovasc Diabetol (2016) 15:94. doi: 10.1186/s12933-016-0414-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yossef RR, Al-Yamany MF, Saad MA, El-Sahar AE. Neuroprotective effects of vildagliptin on drug induced alzheimer’s disease in rats with metabolic syndrome: role of hippocampal klotho and akt signaling pathways. Eur J Pharmacol (2020) 889:173612. doi: 10.1016/j.ejphar.2020.173612 [DOI] [PubMed] [Google Scholar]
- 147. Liu W, Lau HK, Son DO, Jin T, Yang Y, Zhang Z, et al. Combined use of gaba and sitagliptin promotes human beta-cell proliferation and reduces apoptosis. J Endocrinol (2021) 248(2):133–43. doi: 10.1530/JOE-20-0315 [DOI] [PubMed] [Google Scholar]
- 148. Cheng MF, Chen LJ, Cheng JT. Decrease of klotho in the kidney of streptozotocin-induced diabetic rats. J BioMed Biotechnol (2010) 2010:513853. doi: 10.1155/2010/513853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, et al. Klotho is a target gene of ppar-gamma. Kidney Int (2008) 74(6):732–9. doi: 10.1038/ki.2008.244 [DOI] [PubMed] [Google Scholar]
- 150. Maquigussa E, Paterno JC, de Oliveira Pokorny GH, da Silva Perez M, Varela VA, da Silva Novaes A, et al. Klotho and ppar gamma activation mediate the renoprotective effect of losartan in the 5/6 nephrectomy model. Front Physiol (2018) 9:1033. doi: 10.3389/fphys.2018.01033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Liu L, Liu Y, Zhang Y, Bi X, Nie L, Liu C, et al. High phosphate-induced downregulation of ppargamma contributes to ckd-associated vascular calcification. J Mol Cell Cardiol (2018) 114:264–75. doi: 10.1016/j.yjmcc.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 152. Sharma V, Patial V. Peroxisome proliferator-activated receptor gamma and its natural agonists in the treatment of kidney diseases. Front Pharmacol (2022) 13:991059. doi: 10.3389/fphar.2022.991059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Jia Z, Sun Y, Yang G, Zhang A, Huang S, Heiney KM, et al. New insights into the ppar gamma agonists for the treatment of diabetic nephropathy. PPAR Res (2014) 2014:818530. doi: 10.1155/2014/818530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kale A, Sankrityayan H, Anders HJ, Bhanudas Gaikwad A. Klotho: a possible mechanism of action of Sglt2 inhibitors preventing episodes of acute kidney injury and cardiorenal complications of diabetes. Drug Discovery Today (2021) 26(8):1963–71. doi: 10.1016/j.drudis.2021.04.007 [DOI] [PubMed] [Google Scholar]
- 155. Abbas NAT, El Salem A, Awad MM. Empagliflozin, Sglt(2) inhibitor, attenuates renal fibrosis in rats exposed to unilateral ureteric obstruction: potential role of klotho expression. Naunyn Schmiedebergs Arch Pharmacol (2018) 391(12):1347–60. doi: 10.1007/s00210-018-1544-y [DOI] [PubMed] [Google Scholar]
- 156. Mora-Fernandez C, Sanchez-Nino MD, Donate-Correa J, Martin-Nunez E, Perez-Delgado N, Valino-Rivas L, et al. Sodium-glucose Co-Transporter-2 inhibitors increase klotho in patients with diabetic kidney disease: a clinical and experimental study. BioMed Pharmacother (2022) 154:113677. doi: 10.1016/j.biopha.2022.113677 [DOI] [PubMed] [Google Scholar]
- 157. Rangaswami J, Bhalla V, de Boer IH, Staruschenko A, Sharp JA, Singh RR, et al. Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: a scientific statement from the American heart association. Circulation (2020) 142(17):e265–e86. doi: 10.1161/CIR.0000000000000920 [DOI] [PubMed] [Google Scholar]
- 158. Karalliedde J, Fountoulakis N, Stathi D, Corcillo A, Flaquer M, Panagiotou A, et al. Does dapagliflozin influence arterial stiffness and levels of circulating anti-aging hormone soluble klotho in people with type 2 diabetes and kidney disease? results of a randomized parallel group clinical trial. Front Cardiovasc Med (2022) 9:992327. doi: 10.3389/fcvm.2022.992327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L. Effect of renin-angiotensin system blockade on soluble klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin J Am Soc Nephrol (2013) 8(11):1899–905. doi: 10.2215/CJN.02700313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Janic M, Lunder M, Novakovic S, Skerl P, Sabovic M. Expression of longevity genes induced by a low-dose fluvastatin and valsartan combination with the potential to Prevent/Treat “Aging-related disorders”. Int J Mol Sci (2019) 20(8):1844. doi: 10.3390/ijms20081844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou FF, et al. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am J Pathol (2015) 185(12):3211–23. doi: 10.1016/j.ajpath.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kale A, Shelke V, Sankrityayan H, Dagar N, Gaikwad AB. Klotho restoration Via Ace2 activation: a potential therapeutic strategy against acute kidney injury-diabetes comorbidity. Biochim Biophys Acta Mol Basis Dis (2022) 1868(12):166532. doi: 10.1016/j.bbadis.2022.166532 [DOI] [PubMed] [Google Scholar]
- 163. Feng Y, Huang R, Kavanagh J, Li L, Zeng X, Li Y, et al. Efficacy and safety of dual blockade of the renin-Angiotensin-Aldosterone system in diabetic kidney disease: a meta-analysis. Am J Cardiovasc Drugs (2019) 19(3):259–86. doi: 10.1007/s40256-018-00321-5 [DOI] [PubMed] [Google Scholar]
- 164. Xia W, Zhang A, Jia Z, Gu J, Chen H. Klotho contributes to pravastatin effect on suppressing il-6 production in endothelial cells. Mediators Inflammation (2016) 2016:2193210. doi: 10.1155/2016/2193210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yoon HE, Lim SW, Piao SG, Song JH, Kim J, Yang CW. Statin upregulates the expression of klotho, an anti-aging gene, in experimental cyclosporine nephropathy. Nephron Exp Nephrol (2012) 120(4):e123–33. doi: 10.1159/000342117 [DOI] [PubMed] [Google Scholar]
- 166. Adeli S, Zahmatkesh M, Tavoosidana G, Karimian M, Hassanzadeh G. Simvastatin enhances the hippocampal klotho in a rat model of streptozotocin-induced cognitive decline. Prog Neuropsychopharmacol Biol Psychiatry (2017) 72:87–94. doi: 10.1016/j.pnpbp.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 167. Haendeler J, Hoffmann J, Zeiher AM, Dimmeler S. Antioxidant effects of statins Via s-nitrosylation and activation of thioredoxin in endothelial cells: a novel vasculoprotective function of statins. Circulation (2004) 110(7):856–61. doi: 10.1161/01.CIR.0000138743.09012.93 [DOI] [PubMed] [Google Scholar]
- 168. Lerch C, Shroff R, Wan M, Rees L, Aitkenhead H, Kaplan Bulut I, et al. Effects of nutritional vitamin d supplementation on markers of bone and mineral metabolism in children with chronic kidney disease. Nephrol Dial Transplant (2018) 33(12):2208–17. doi: 10.1093/ndt/gfy012 [DOI] [PubMed] [Google Scholar]
- 169. Vila Cuenca M, Ferrantelli E, Meinster E, Pouw SM, Kovacevic I, de Menezes RX, et al. Vitamin d attenuates endothelial dysfunction in uremic rats and maintains human endothelial stability. J Am Heart Assoc (2018) 7(17):e008776. doi: 10.1161/JAHA.118.008776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Takenaka T, Inoue T, Ohno Y, Miyazaki T, Nishiyama A, Ishii N, et al. Calcitriol supplementation improves endothelium-dependent vasodilation in rat hypertensive renal injury. Kidney Blood Press Res (2014) 39(1):17–27. doi: 10.1159/000355773 [DOI] [PubMed] [Google Scholar]
- 171. Cozzolino M, Stucchi A, Rizzo MA, Soldati L, Cusi D, Ciceri P, et al. Vitamin d receptor activation and prevention of arterial ageing. Nutr Metab Cardiovasc Dis (2012) 22(7):547–52. doi: 10.1016/j.numecd.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 172. Velagapudi C, Bhandari BS, Abboud-Werner S, Simone S, Abboud HE, Habib SL. The Tuberin/Mtor pathway promotes apoptosis of tubular epithelial cells in diabetes. J Am Soc Nephrol (2011) 22(2):262–73. doi: 10.1681/ASN.2010040352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Yu R, Bo H, Villani V, Spencer PJ, Fu P. The inhibitory effect of rapamycin on toll like receptor 4 and interleukin 17 in the early stage of rat diabetic nephropathy. Kidney Blood Press Res (2016) 41(1):55–69. doi: 10.1159/000368547 [DOI] [PubMed] [Google Scholar]
- 174. Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, et al. Mtorc1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest (2011) 121(6):2181–96. doi: 10.1172/JCI44771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Mizusaki K, Hasuike Y, Kimura T, Nagasawa Y, Kuragano T, Yamada Y, et al. Inhibition of the mammalian target of rapamycin may augment the increase in soluble klotho levels in renal transplantation recipients. Blood Purif (2019) 47 Suppl 2:12–8. doi: 10.1159/000496630 [DOI] [PubMed] [Google Scholar]
- 176. Zhao Y, Zhao MM, Cai Y, Zheng MF, Sun WL, Zhang SY, et al. Mammalian target of rapamycin signaling inhibition ameliorates vascular calcification Via klotho upregulation. Kidney Int (2015) 88(4):711–21. doi: 10.1038/ki.2015.160 [DOI] [PubMed] [Google Scholar]
- 177. Tataranni T, Biondi G, Cariello M, Mangino M, Colucci G, Rutigliano M, et al. Rapamycin-induced hypophosphatemia and insulin resistance are associated with Mtorc2 activation and klotho expression. Am J Transplant (2011) 11(8):1656–64. doi: 10.1111/j.1600-6143.2011.03590.x [DOI] [PubMed] [Google Scholar]
- 178. Lopes de Jesus CC, Atallah AN, Valente O, Moca Trevisani VF. Pentoxifylline for diabetic retinopathy. Cochrane Database Syst Rev (2008) 2008(2):CD006693. doi: 10.1002/14651858.CD006693.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Chahin J, Mendez ML, Gallego E, et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the predian trial. J Am Soc Nephrol (2015) 26(1):220–9. doi: 10.1681/ASN.2014010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Navarro-Gonzalez JF, Sanchez-Nino MD, Donate-Correa J, Martin-Nunez E, Ferri C, Perez-Delgado N, et al. Effects of pentoxifylline on soluble klotho concentrations and renal tubular cell expression in diabetic kidney disease. Diabetes Care (2018) 41(8):1817–20. doi: 10.2337/dc18-0078 [DOI] [PubMed] [Google Scholar]
- 181. Feng Y, Huang Q. Protective effects of cordycepin against d-Galactose-Induced aging in rats: a view from the heart. Geriatr Gerontol Int (2022) 22(5):433–40. doi: 10.1111/ggi.14376 [DOI] [PubMed] [Google Scholar]
- 182. Chu SH, Yang D, Wang YP, Yang R, Qu L, Zeng HJ. Effect of resveratrol on the repair of kidney and brain injuries and its regulation on klotho gene in d-Galactose-Induced aging mice. Bioorg Med Chem Lett (2021) 40:127913. doi: 10.1016/j.bmcl.2021.127913 [DOI] [PubMed] [Google Scholar]
- 183. Kuang X, Zhou HJ, Thorne AH, Chen XN, Li LJ, Du JR. Neuroprotective effect of ligustilide through induction of alpha-secretase processing of both app and klotho in a mouse model of alzheimer’s disease. Front Aging Neurosci (2017) 9:353. doi: 10.3389/fnagi.2017.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Kamel SS, Baky NAA, Karkeet RM, Osman AM, Sayed-Ahmed MM, Fouad MA. Astaxanthin extenuates the inhibition of aldehyde dehydrogenase and klotho protein expression in cyclophosphamide-induced acute cardiomyopathic rat model. Clin Exp Pharmacol Physiol (2022) 49(2):291–301. doi: 10.1111/1440-1681.13598 [DOI] [PubMed] [Google Scholar]
- 185. Chen CC, Chang ZY, Tsai FJ, Chen SY. Resveratrol pretreatment ameliorates concanavalin a-induced advanced renal glomerulosclerosis in aged mice through upregulation of sirtuin 1-mediated klotho expression. Int J Mol Sci (2020) 21(18):6766. doi: 10.3390/ijms21186766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Lim SW, Shin YJ, Luo K, Quan Y, Cui S, Ko EJ, et al. Ginseng increases klotho expression by Foxo3-mediated manganese superoxide dismutase in a mouse model of tacrolimus-induced renal injury. Aging (Albany NY) (2019) 11(15):5548–69. doi: 10.18632/aging.102137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Li SS, He AL, Deng ZY, Liu QF. Ginsenoside-Rg1 protects against renal fibrosis by regulating the Klotho/Tgf-Beta1/Smad signaling pathway in rats with obstructive nephropathy. Biol Pharm Bull (2018) 41(4):585–91. doi: 10.1248/bpb.b17-00934 [DOI] [PubMed] [Google Scholar]
- 188. Zhang P, Li Y, Du Y, Li G, Wang L, Zhou F. Resveratrol ameliorated vascular calcification by regulating sirt-1 and Nrf2. Transplant Proc (2016) 48(10):3378–86. doi: 10.1016/j.transproceed.2016.10.023 [DOI] [PubMed] [Google Scholar]
- 189. Long FY, Shi MQ, Zhou HJ, Liu DL, Sang N, Du JR. Klotho upregulation contributes to the neuroprotection of ligustilide against cerebral ischemic injury in mice. Eur J Pharmacol (2018) 820:198–205. doi: 10.1016/j.ejphar.2017.12.019 [DOI] [PubMed] [Google Scholar]
- 190. Zhou X, Yang Q, Xie Y, Sun J, Hu J, Qiu P, et al. Tetrahydroxystilbene glucoside extends mouse life span Via upregulating neural klotho and downregulating neural insulin or insulin-like growth factor 1. Neurobiol Aging (2015) 36(3):1462–70. doi: 10.1016/j.neurobiolaging.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 191. Zhang XT, Wang G, Ye LF, Pu Y, Li RT, Liang J, et al. Baicalin reversal of DNA hypermethylation-associated klotho suppression ameliorates renal injury in type 1 diabetic mouse model. Cell Cycle (2020) 19(23):3329–47. doi: 10.1080/15384101.2020.1843815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Hu Y, Mou L, Yang F, Tu H, Lin W. Curcumin attenuates cyclosporine a-induced renal fibrosis by inhibiting hypermethylation of the klotho promoter. Mol Med Rep (2016) 14(4):3229–36. doi: 10.3892/mmr.2016.5601 [DOI] [PubMed] [Google Scholar]
- 193. Jiang W, Xiao T, Han W, Xiong J, He T, Liu Y, et al. Klotho inhibits Pkcalpha/P66shc-mediated podocyte injury in diabetic nephropathy. Mol Cell Endocrinol (2019) 494:110490. doi: 10.1016/j.mce.2019.110490 [DOI] [PubMed] [Google Scholar]
- 194. Liu Y, Bi X, Xiong J, Han W, Xiao T, Xu X, et al. Microrna-34a promotes renal fibrosis by downregulation of klotho in tubular epithelial cells. Mol Ther (2019) 27(5):1051–65. doi: 10.1016/j.ymthe.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Kang WL, Xu GS. Atrasentan increased the expression of klotho by mediating mir-199b-5p and prevented renal tubular injury in diabetic nephropathy. Sci Rep (2016) 6:19979. doi: 10.1038/srep19979 [DOI] [PMC free article] [PubMed] [Google Scholar]