Abstract
Background
The correlation between cognitive function and lipid profiles, including total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and triglycerides, is inconsistent.
Aims
This cross-sectional study investigated the association between serum lipid levels and the prevalence of cognitive impairment among community-dwelling older adults and explored this difference in association by gender and urban-rural residency.
Methods
Participants aged 65 and above in urban and rural areas were recruited between 2018 and 2020, selected from the Hubei Memory and Aging Cohort Study. Detailed neuropsychological evaluations, clinical examinations and laboratory tests were conducted in community health service centres. Multivariate logistic regression was used to analyse the correlation between serum lipid profiles and the prevalence of cognitive impairment.
Results
We identified 1 336 cognitively impaired adults (≥65 years)—1 066 with mild cognitive impairment and 270 with dementia—from 4 746 participants. Triglycerides level was correlated with cognitive impairment in the total sample (χ2=6.420, p=0.011). In gender-stratified multivariate analysis, high triglycerides in males reduced the risk of cognitive impairment (OR: 0.785, 95% CI: 0.623 to 0.989, p=0.040), and high LDL-C in females increased the risk of cognitive impairment (OR: 1.282, 95% CI: 1.040 to 1.581, p=0.020). In both gender-stratified and urban-rural stratified multivariate analyses, high triglycerides reduced the risk of cognitive impairment in older urban men (OR: 0.734, 95% CI: 0.551 to 0.977, p=0.034), and high LDL-C increased the risk of cognitive impairment in older rural women (OR: 1.830, 95% CI: 1.119 to 2.991, p=0.016).
Conclusions
There are gender and urban-rural differences in the correlation of serum lipids with cognitive impairment. High triglycerides levels may be a protective factor for cognitive function in older urban men, while high LDL-C levels may be a risk factor for cognitive function in older rural women.
Keywords: cross-sectional studies, cognitive dysfunction, risk factors
WHAT IS ALREADY KNOWN ON THIS TOPIC
The correlation between lipid profiles and cognitive function remains controversial. Most epidemiological studies were performed in high-income countries with generally well-educated participants. Less is known about the correlation in rural areas of less developed areas like Central China.
WHAT THIS STUDY ADDS
This study found that the correlation between blood lipids and cognitive impairment differed by gender and urban-rural regions.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Different strategies should be developed for men and women when it comes to preventing cognitive impairment. Rural-dwelling older adults should be considered a priority for the prevention of cognitive impairment.
Introduction
Cognitive impairment includes mild cognitive impairment (MCI) and dementia and is increasing rapidly with the ageing of the population. In 2018, China had 15.07 million patients with dementia, including 9.83 million with Alzheimer’s disease and 38.77 million patients with MCI.1 This has brought a huge health burden to Chinese society and has become one of the major health issues in the country.2 Existing studies suggest that dyslipidaemia is associated with cognitive impairment.3 4 The level of serum lipids gradually increases as individuals age and their lifestyles undergo changes; thus, with the increasing number of older persons, the prevalence of dyslipidaemia has increased significantly.5 A national survey in China showed that the prevalence of hypercholesterolaemia and hypertriglyceridaemia among adults was 6.9% and 13.8%, respectively.6 Therefore, it is crucial to understand the correlation between serum lipid levels and cognitive performance in the Chinese population, especially among elders.
Dyslipidaemia, as a modifiable risk factor, is one of the most important factors leading to atherosclerosis. It is also an independent risk factor for coronary heart disease and ischaemic stroke,7 which increase the risk of cognitive impairment in older adults.8 However, conflicting results have been found regarding the correlation of cognitive impairment with serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and triglycerides levels.9 10 A case–control study showed that cognitive performance test results changed with levels of serum TC and LDL-C concentrations in older adults.11 Another study showed that high LDL-C is a risk factor for cognitive impairment in older women.12 To the contrary, a meta-analysis did not find a significant relationship between TC, HDL-C or LDL and the risk of cognitive decline in older adults averaging 76 years of age.13 We speculate that the inconsistency is related to gender and urban-rural differences. However, only a few studies with relatively small samples have analysed the relationship between serum lipids and cognitive impairment while looking at differences in gender and urban-rural regional disparities. There are no reports on the correlation between cognitive function and lipid profiles in older individuals from rural areas in Central China; most reports are from relatively developed regions of the country. Therefore, further studies are necessary to clarify the correlation between cognitive impairment and lipid profiles by gender and urban-rural regional differences in Central China. Based on a cross-sectional investigation of the Hubei Memory and Aging Cohort Study (HMACS), this study explored the relationship between serum lipids and cognitive impairment in community-dwelling older adults in China and evaluated the gender and urban-rural differences.
Methods
Study participants
The HMACS was designed as a community-based cohort study with urban and rural settings, which was carried out between 2018 and 2020; it was designed to assess the prevalence and incidence of cognitive impairment, particularly Alzheimer’s disease, and the prevalence of related risk factors in older adults.14 In this study, cluster random sampling method was used to select four community health service centers in Wuhan and 48 villages in Dawu County. This study included participants who met the following eligibility criteria: (1) aged 65 and older; (2) with electronic medical records kept in community health centres or hospitals; (3) with completed cognitive evaluation data; and (4) with blood TC, LDL-C, HDL-C and triglyceride information. Potential participants were excluded if they (1) were not traceable; (2) showed mental retardation or dementia on their medical record; (3) had severe hearing and vision impairment and were not able to participate actively in the neuropsychological assessments. Written informed consent or witnessed oral consent was obtained from all participants after the nature of the procedures had been fully explained. Among 8 221 older residents, 2 109 were excluded due to illness or other reasons and 1 366 were unable to complete the survey. Of the enrollees, 4 746 eligible participants completed the full-length survey (figure 1).
Figure 1.
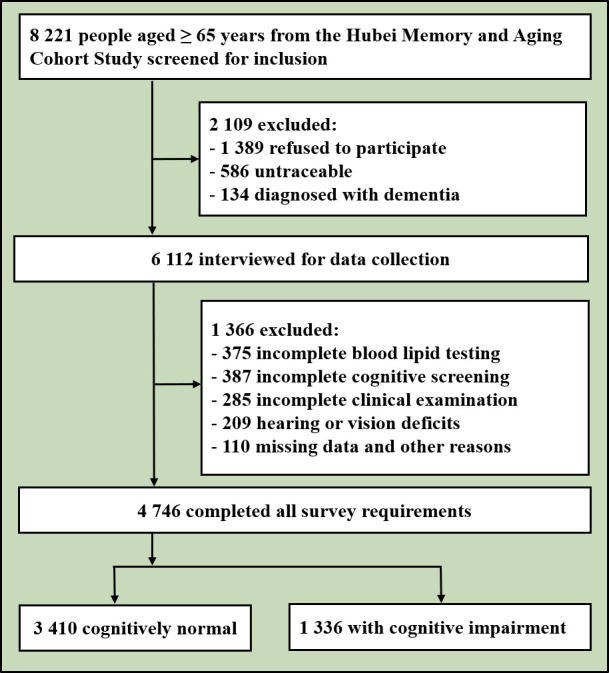
Flowchart of the study.
Neuropsychological assessment
The overall cognitive function and subdomains of the older adults were assessed with a structured neuropsychological scale. Tools used for assessment included the Mini-Mental State Examination (MMSE)15 and the Chinese version of the Montreal Cognitive Assessment-Basic (MOCA-BC)16 to assess global cognition, the Auditory Verbal Learning Test17 to assess memory, the Trail-Making Tests A (TMT-A) and B (TMT-B) to assess executive function, the forward and backward of the Digit Span Test to assess attention, the Boston Naming Test18 and the Animal Fluency Test to assess language, and the Clock-Drawing Test to assess visuospatial ability.19
The Activities of Daily Living Scale (ADL)20 was used to assess participants’ day-to-day life skills and activities, and the Geriatric Depression Scale 1521 was used to assess depression in the past week.
Diagnosis of cognitive impairment
Cognitive impairment here refers to MCI and dementia. After each participant was examined, a panel of two neuroscientists and two neuropsychologists with expertise in dementia diagnosed dementia and MCI. HMACS reached a diagnostic consensus for dementia using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria22: (1) previous normal cognition, (2) cognitive decline or abnormal mental behaviour, (3) decline affects work ability or daily life and (4) cannot be explained by delirium or other mental illness. For MCI, Petersen’s criteria were used23: (1) concern of a cognitive change by the participant, informant or clinician based on information obtained during the clinical interview; patients, insiders and/or clinicians report or find cognitive impairment; (2) objective impairment on any neuropsychological test within a cognitive domain (mainly based on MMSE and MOCA-BC scores); (3) essentially normal functional activities (as assessed by ADL); and (4) absence of dementia.
Serum lipid test
Participants were asked to abstain from alcohol and high-fat foods 24 hours before blood collection. After fasting for 10–12 hours, 3 mL of fasting venous blood was extracted in the early morning, and the levels of TC, triglycerides, LDL-C and HDL-C were detected by an automatic biochemical analyser. According to the 2016 Chinese Adult Dyslipidemia Prevention Guideline, normal blood lipids were defined as TC <5.2 mmol/L, triglycerides <1.7 mmol/L, LDL-C <3.4 mmol/L and HDL-C >1.0 mmol/L. For participants taking lipid-lowering drugs, they were defined as having normal lipid levels if their lipid levels were within the normal range.
Other covariants
Other covariants included demographic characteristics (age, sex, education level, marital status and residence), lifestyle (smoking, drinking, physical exercise and intellectual activity) and disease history (hypertension and diabetes), which were collected using a dedicated form. Physical and clinical examinations (height, weight, blood pressure, head circumference, waist circumference and hip circumference) were performed by nurses.
Sex was divided into male and female. Age was divided into four groups: 65–69, 70–74, 75–79 and 80 years old and above. The level of education included three categories: primary school or below, middle school, and university or above. Marital status was divided into two groups: married and unmarried. Residence was divided into rural and urban areas. Current smoking referred to smoking ≥1 cigarette/day within the last 6 months, consistent with the definition of the 2010 National Smoking Survey of Chinese Adults.24 Drinking was defined as consuming >14 drinks/week for men (seven drinks/week for women) for ≥1 year, and one drink was defined as 14 g of pure alcohol, which equates to a 150 mL glass of wine, a 350 mL can of beer or two shots of spirits.25 Physical activities were estimated by recalling the frequency and time spent on nine activities, such as walking, dancing, jogging or playing sports in a typical week, according to the guidelines for Chinese adults.26 Intellectual activities were defined as activities like reading, writing, playing chess/poker/mahjong or using an app for playing games ≥3 times/week for ≥30 min/activity.27 Hypertension was self-reported as a diagnosis and/or use of antihypertensive medication within 2 weeks prior to the study.28 Diabetes was self-reported as a diagnosis previously determined by a healthcare professional or current use of antidiabetic medications.29
Statistical analyses
SPSS V.26.0 was used for all statistical analyses (SPSS). Continuous variables were denoted as mean (standard deviation (SD)) or median (interquartile range (IQR)). Categorical variables were expressed as numbers (percentages). Differences between study groups were assessed using Student’s t-tests for continuous variables with normal distribution, rank tests for continuous variables with skewed distribution and χ2 tests for categorical variables.
Univariate logistic regression was used to analyse the relationship between lipid levels and the rate of cognitive impairment, and the above-mentioned various factors were included in the multivariate logistic regression analysis. Based on single-factor analysis, we used multiple logistic regression analysis in all study participants to adjust for confounding factors and to further explain the effects of serum lipids on cognitive impairment.
We also performed stratified analysis. The total population was divided into two gender subgroups and two residence subgroups according to gender and urban-rural regions. Then, these groups were further divided into four subgroups according to both genders and urban-rural regions. A stratified logistic regression model was used to evaluate the relationship between serum lipids and cognitive impairment among different genders and regions. All p values were bilateral, and results were considered to be statistically significant when p<0.05.
Results
Characteristics of study participants
As shown in table 1, a total of 4 746 community adults aged ≥65 were included in this study, with a mean (SD) age of 71.6 (5.5), including 2 166 males (45.6%) and 2 580 females (54.4%). 80.7% of the participants were married; 1 323 (27.9%) were from remote rural areas, 3 423 (72.1%) were from urban areas; 927 (19.5%) had a college education or above; 3 972 (83.7%) and 2 980 (62.8%) regularly participated in physical exercise and intellectual activities; 3 134 (66.0%) of them had hypertension, 807 (17.0%) had diabetes, 708 (14.9%) had coronary heart disease and 882 (18.6%) had cerebrovascular disease; 1 767 (37.2%) had abnormal TC levels, 1 477 (31.1%) had abnormal triglyceride levels, 1 094 (23.1%) had abnormal LDL-C levels and 821 (17.3%) had abnormal HDL-C levels.
Table 1.
General characteristics of participants
| Characteristics | Total (%) | Rural (%) | Urban (%) | |||
| Normal cognition (n=3 410) |
Cognitive impairment (n=1 336) |
Normal cognition (n=849) |
Cognitive impairment (n=474) |
Normal cognition (n=2 561) |
Cognitive impairment (n=862) |
|
| Gender | p=0.179 | p=0.702 | p=0.176 | |||
| Male | 1 577 (46.2) | 589 (44.1) | 389 (45.8) | 212 (44.7) | 1 188 (46.4) | 377 (43.7) |
| Age (years) | p<0.001*** | p<0.001*** | p<0.001*** | |||
| 65–69 | 1 672 (49.0) | 409 (30.6) | 343 (40.4) | 133 (28.1) | 1 329 (51.9) | 276 (32.0) |
| 70–74 | 1 038 (30.4) | 413 (30.9) | 287 (33.8) | 166 (35.0) | 751 (29.3) | 247 (28.7) |
| 75–79 | 441 (12.9) | 255 (19.1) | 137 (16.1) | 99 (20.9) | 304 (11.9) | 156 (18.1) |
| ≥80 | 259 (7.6) | 259 (19.4) | 82 (9.7) | 76 (16.0) | 177 (6.9) | 183 (21.2) |
| Marital status | p<0.001*** | p=0.053 | p<0.001*** | |||
| Married | 2 851 (83.6) | 978 (73.2) | 559 (65.8) | 310 (65.4) | 2 252 (87.9) | 668 (77.5) |
| Education level | p<0.001*** | p=0.235 | p<0.001*** | |||
| Primary school or below | 941 (27.6) | 553 (41.4) | 687 (80.9) | 365 (77.0) | 254 (9.9) | 188 (21.8) |
| Middle school | 1 767 (51.8) | 558 (41.8) | 149 (17.6) | 101 (21.3) | 1 618 (63.2) | 457 (53.0) |
| University or above | 702 (20.6) | 225 (16.8) | 13 (1.5) | 8 (1.7) | 689 (26.9) | 217 (25.2) |
| Smoking history | p=0.053 | p=0.384 | p=0.003** | |||
| Yes | 990 (29.0) | 356 (26.6) | 258 (30.4) | 155 (32.7) | 732 (28.6) | 201 (23.3) |
| Drinking history | p=0.112 | p=0.908 | p=0.009** | |||
| Yes | 793 (23.3) | 282 (21.1) | 225 (26.5) | 127 (26.8) | 568 (22.2) | 155 (18.0) |
| Physical exercise | p<0.001*** | p=0.111 | p<0.001*** | |||
| Yes | 2 913 (85.4) | 1 059 (79.3) | 631 (74.3) | 333 (70.3) | 2 282 (89.1) | 726 (84.2) |
| Cognitive activity | p<0.001*** | p=0.345 | p<0.001*** | |||
| Yes | 2 263 (66.4) | 717 (53.7) | 334 (39.3) | 174 (36.7) | 1 929 (75.3) | 543 (63.0) |
| Eating animal fat | p<0.001*** | p=0.828 | p<0.001*** | |||
| Yes | 1 558 (45.7) | 498 (37.3) | 279 (32.9) | 153 (32.3) | 1 279 (49.9) | 345 (40.0) |
| Hypertension | p=0.002** | p=0.426 | p=0.006** | |||
| Yes | 2 207 (64.7) | 927 (69.4) | 584 (68.8) | 336 (70.9) | 1 623 (63.4) | 591 (68.6) |
| Diabetes | p=0.008** | p=0.339 | p<0.001*** | |||
| Yes | 549 (16.1) | 258 (19.3) | 101 (11.9) | 63 (13.3) | 448 (17.5) | 193 (22.4) |
| TC | p=0.719 | p=0.567 | p=0.324 | |||
| High TC | 1 265 (37.1) | 502 (37.6) | 309 (36.3) | 164 (34.5) | 956 (37.3) | 338 (39.2) |
| TG | p=0.011* | p=0.313 | p=0.075 | |||
| High TG | 1 098 (32.2) | 379 (28.4) | 227 (26.7) | 114 (24.1) | 871 (34.0) | 265 (30.7) |
| LDL-C | p=0.746 | p=0.540 | p=0.578 | |||
| High LDL-C | 798 (23.4) | 296 (22.2) | 104 (12.2) | 54 (11.4) | 694 (27.1) | 242 (28.1) |
| HDL-C | p=0.186 | p=0.564 | p=0.994 | |||
| Low HDL-C | 617 (18.1) | 204 (15.3) | 74 (8.7) | 39 (8.2) | 282 (11.0) | 95 (11.0) |
*p<0.05; **p<0.01; ***p<0.001.
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Older adults with cognitive impairment accounted for 35.8% of the rural-dwelling and 25.2% of the urban-dwelling participants, respectively. The cognitive impairment group was less educated, had more unmarried participants, was less likely to engage in physical exercise and intellectual activities and had a higher prevalence of high blood pressure, diabetes, coronary heart disease and cerebrovascular disease. There were significant differences in age, education, marital status, physical exercise habits, intellectual activity habits, eating animal fat, hypertension, diabetes and cerebrovascular disease between the normal cognition and the cognitive impairment groups (p<0.05) for the total and urban-dwelling participants, but not for rural-dwelling participants. For more details, please see the online supplemental file.
gpsych-2023-101009supp001.pdf (60.5KB, pdf)
Prevalence of cognitive impairment by dyslipidaemia rate in the gender and urban-rural residence groupings
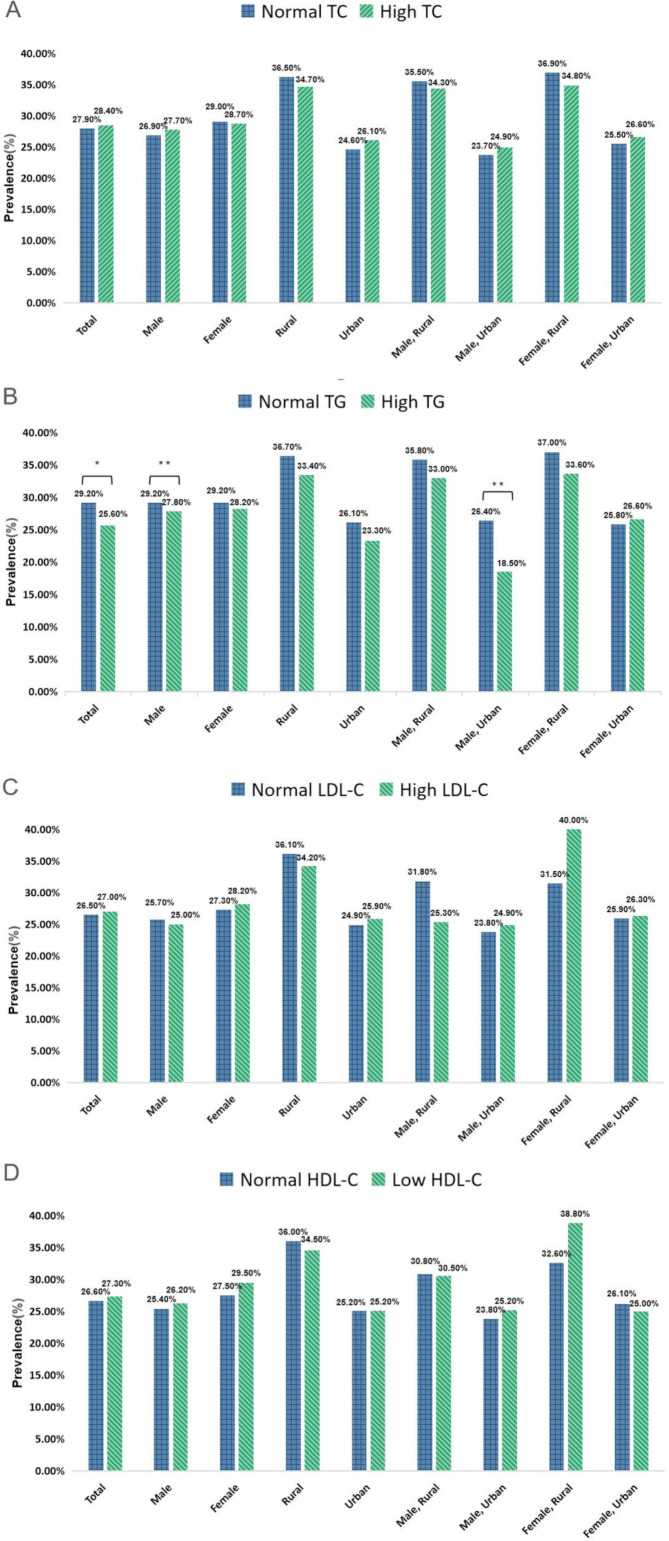
Figure 2 shows the prevalence of cognitive impairment by gender and urban-rural groups. There was no significant difference in the prevalence of cognitive impairment between the normal TC group and the high TC group in the total population. After gender and urban-rural stratification analyses, the prevalence of cognitive impairment between the normal TC group and the high TC group was also not significantly different (figure 2A). In the total population, the prevalence of cognitive impairment was lower in the high triglycerides group than in the normal triglycerides group (χ2=6.420, p=0.011), and the same result was observed in the male and urban male subgroups (χ2=11.837, p=0.001; χ2=11.190, p=0.001) (figure 2B). In the total population, there was no significant difference in the prevalence of cognitive impairment between the normal LDL-C group and the high LDL-C group. After being stratified by gender and urban-rural regions, the prevalence of cognitive impairment was not significantly different (figure 2C). This also held for the normal HDL-C group and low HDL-C group (figure 2D).
Figure 2.
Prevalence of cognitive impairment by dyslipidaemia rate in the gender and urban-rural residence groupings. (A) Total cholesterol (TC). (B) Triglyceride (TG). (C) Low-density lipoprotein cholesterol (LDL-C). (D) High-density lipoprotein cholesterol (HDL-C). *p<0.05; **p<0.01.
Relationships between serum lipids and cognitive impairment with multivariate analysis
In order to explore the relationship between serum lipids and cognitive impairment, we conducted multivariate stratified analysis (table 2). In model 1, no potential confounding variables were added. After stratified analysis, we found that high triglycerides levels were associated with a lower risk of cognitive impairment in the total population (OR: 0.836, 95% CI: 0.727 to 0.960, p=0.011), male subgroup (OR: 0.679, 95% CI: 0.544 to 0.847, p=0.001) and urban male subgroup (OR: 0.633, 95% CI: 0.483 to 0.823, p=0.001).
Table 2.
Relationships between serum lipid and cognitive impairment by multivariate analysis
| Variable | Total | Rural | Urban | |||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Model 1 | ||||||
| Total | ||||||
| High TC | 1.024 (0.899 to 1.167) | 0.719 | 0.934 (0.738 to 1.182) | 0.567 | 1.083 (0.924 to 1.269) | 0.324 |
| High TG | 0.836 (0.727 to 0.960) | 0.011* | 0.875 (0.674 to 1.134) | 0.313 | 0.860 (0.728 to 1.015) | 0.075 |
| High LDL-C | 1.026 (0.879 to 1.197) | 0.746 | 1.119 (0.780 to 1.607) | 0.540 | 1.050 (0.884 to 1.247) | 0.578 |
| Low HDL-C | 1.038 (0.841 to 1.282) | 0.726 | 1.130 (0.746 to 1.710) | 0.564 | 1.001 (0.782 to 1.281) | 0.994 |
| Male | ||||||
| High TC | 1.039 (0.840 to 1.285) | 0.727 | 0.948 (0.654 to 1.375) | 0.779 | 1.064 (0.819 to 1.383) | 0.640 |
| High TG | 0.679 (0.544 to 0.847) | 0.001** | 0.884 (0.592 to 1.321) | 0.548 | 0.633 (0.483 to 0.823) | 0.001** |
| High LDL-C | 0.963 (0.747 to 1.242) | 0.771 | 0.729 (0.397 to 1.339) | 0.309 | 1.059 (0.799 to 1.404) | 0.690 |
| Low HDL-C | 1.045 (0.795 to 1.374) | 0.751 | 0.984 (0.542 to 1.786) | 0.958 | 1.081 (0.794 to 1.472) | 0.622 |
| Female | ||||||
| High TC | 0.987 (0.832 to 1.171) | 0.880 | 0.912 (0.670 to 1.243) | 0.561 | 1.057 (0.859 to 1.301) | 0.598 |
| High TG | 0.954 (0.796 to 1.142) | 0.606 | 0.863 (0.613 to 1.216) | 0.401 | 1.042 (0.840 to 1.292) | 0.709 |
| High LDL-C | 1.042 (0.856 to 1.269) | 0.678 | 1.445 (0.913 to 2.289) | 0.116 | 1.020 (0.818 to 1.271) | 0.861 |
| Low HDL-C | 1.028 (0.729 to 1.449) | 0.875 | 1.315 (0.736 to 2.352) | 0.335 | 0.940 (0.613 to 1.440) | 0.776 |
| Model 2 | ||||||
| Total | ||||||
| High TC | 1.096 (0.956 to 1.256) | 0.194 | 1.013 (0.795 to 1.291) | 0.914 | 1.136 (0.961 to 1.343) | 0.136 |
| High TG | 0.940 (0.813 to 1.088) | 0.409 | 0.929 (0.711 to 1.215) | 0.591 | 0.959 (0.804 to 1.143) | 0.641 |
| High LDL-C | 1.159 (0.986 to 1.362) | 0.073 | 1.225 (0.845 to 1.776) | 0.285 | 1.127 (0.940 to 1.351) | 0.198 |
| Low HDL-C | 0.966 (0.774 to 1.204) | 0.759 | 1.081 (0.706 to 1.657) | 0.719 | 0.939 (0.724 to 1.218) | 0.635 |
| Male | ||||||
| High TC | 1.073 (0.859 to 1.340) | 0.536 | 0.986 (0.670 to 1.452) | 0.945 | 1.036 (0.784 to 1.369) | 0.803 |
| High TG | 0.785 (0.623 to 0.989) | 0.040* | 0.944 (0.622 to 1.432) | 0.785 | 0.734 (0.551 to 0.977) | 0.034* |
| High LDL-C | 1.009 (0.774 to 1.315) | 0.948 | 0.721 (0.385 to 1.351) | 0.308 | 1.074 (0.797 to 1.448) | 0.638 |
| Low HDL-C | 1.009 (0.759 to 1.341) | 0.951 | 0.957 (0.518 to 1.767) | 0.887 | 1.022 (0.737 to 1.418) | 0.638 |
| Female | ||||||
| High TC | 1.008 (0.808 to 1.257) | 0.944 | 1.057 (0.766 to 1.460) | 0.735 | 1.225 (0.982 to 1.528) | 0.072 |
| High TG | 1.067 (0.881 to 1.292) | 0.506 | 0.901 (0.629 to 1.291) | 0.571 | 1.152 (0.915 to 1.450) | 0.229 |
| High LDL-C | 1.282 (1.040 to 1.581) | 0.020* | 1.830 (1.119 to 2.991) | 0.016* | 1.165 (0.922 to 1.473) | 0.201 |
| Low HDL-C | 0.830 (0.577 to 1.194) | 0.316 | 1.231 (0.660 to 2.298) | 0.513 | 0.797 (0.504 to 1.260) | 0.331 |
*p<0.05; **p<0.01.
Model 1: crude model. Model 2: adjusted model (adjusted variables: age, education level, marital status, eating animal fat, hypertension, diabetes, physical and cognitive activities).
CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; TC, total cholesterol; TG, triglyceride.
After controlling for age, education level, marital status, physical exercise, intellectual activity, eating animal fat, hypertension, diabetes and cerebrovascular disease in model 2, there was no significant correlation between high TC, high TG, high LDL-C, low HDL-C and cognitive impairment. However, gender-stratified multivariate analysis indicated that in the male subgroup, high TG was negatively associated with cognitive impairment (OR: 0.785, 95% CI: 0.623 to 0.989, p=0.040), while high LDL-C increased the risk of cognitive impairment in the female subgroup (OR: 1.282, 95% CI: 1.040 to 1.581, p=0.020). In the urban-rural stratified multivariate analysis, the results were similar to those in the total sample, and there seemed to be no correlation between the serum lipid indexes and cognitive impairment. Both gender-stratified and urban-rural stratified multivariate analyses showed that high TG reduced the risk of cognitive impairment in the urban male subgroup (OR: 0.734, 95% CI: 0.551 to 0.977, p=0.034). High LDL-C increased the risk of cognitive impairment in the rural female subgroup (OR: 1.830, 95% CI: 1.119 to 2.991, p=0.016). However, no significant correlation was found between the remaining serum lipid parameters and cognitive impairment.
Discussion
Main findings
The correlation between lipid profiles and cognitive function remains controversial because of conflicting results from different studies. We speculated that this inconsistency is related to gender and urban-rural regions. Thus, we investigated the gender and urban-rural-dependent association of serum lipid levels with the prevalence of cognitive impairment among community-dwelling older adults. The results of this study show that the prevalence of cognitive impairment in rural area adults aged 65 years and above was significantly higher than in urban areas (35.8% vs 25.2%), which resulted in an overall higher prevalence than a previously published national survey.30 Our data could also suggest that the prevalence of cognitive impairment among older adults in China is increasing. Additionally, this study found that the correlation between blood lipids and cognitive impairment was different between different genders and urban-rural regions. High TG levels reduced the risk of cognitive impairment in older men, while high LDL-C levels increased the risk of cognitive impairment in older women in fully adjusted models. Further, in the rural female group, the correlation was positive between LDL-C and cognitive impairment, while in the urban male group, the correlation was negative between high TG and cognitive impairment. But no correlation was found between serum lipid levels and cognitive impairment in other groups. These results show that serum lipid levels in older adults are closely related to cognitive impairment with differences in gender and urban-rural residence.
A number of prospective studies found no significant associations between cognitive impairment and TC, LDL-C, HDL-C and triglycerides,31 32 but none of those studies considered gender or region stratifications. One study based on a rural population indicated that high LDL-C in older women is a risk factor for cognitive impairment.12 However, a study in Shanghai reported that high levels of LDL-C were inversely associated with dementia and were a potential protective factor against cognitive decline.33 Goh and Hart found high TG levels were positively associated with short-term memory in Asian men.34 One study showed that triglycerides levels were associated with a decreased risk of Alzheimer’s disease in women.35 Another study found that high TG levels were negatively correlated with cognitive function in female patients with major depressive disorders.36 Results of the previous research reported that the relationship between serum lipid levels and cognitive impairment remains unclear. Inconsistencies could result from heterogeneity in study design and sample characteristics, as well as the diagnostic criteria for cognitive impairment. Therefore, in order to clearly explore the relationship between blood lipids and cognitive impairment, stratification analysis according to gender and urban-rural areas was conducted in this study. To our knowledge, this is the first comprehensive subnational assessment of the association between serum lipid and cognitive impairment in Central China to include data on gender and urban-rural areas. We demonstrated that there are gender and urban-rural differences in the correlation of serum lipids with cognitive impairment.
One possible reason for these differences in lipids and cognitive impairment is that in rural areas, older adults have a lower education level and there is a higher rate of hypertension. In urban areas, older adults have a relatively high level of education, healthy lifestyles (less smoking and drinking and more physical activities) and better health conditions (lower ratio of hypertension and diabetes).37 However, significant urban-rural differences remained between the serum lipid parameters and cognitive impairment in the adjusted model 2. This suggests that rural-dwelling older adults should be considered a priority for the prevention of cognitive impairment.
Gender-specific associations between lipids and cognitive function may depend on genetic vulnerability to dyslipidaemia in men and hormonal status in postmenopausal women.38 Broader support can be taken from studies of sex differences in serum lipid patterns. For example, fat moves twice as fast through the bloodstream in women as it does in men.39 Furthermore, age-graded changes in serum lipids occur earlier in men than in women.39 The physiological underpinning of these gender differences remains speculative. Different levels of sexual dimorphism have been reported for different lipid traits based on cholesterol fractions.40 An animal study has suggested sex differences are linked to a difference in LDL receptors.41 Therefore, different strategies should be developed for men and women.
Currently, there are very few studies on the direct association between cognitive function and serum lipids. Some think serum lipids, especially triglyceride, may be a meaningful indicator of nutritional status.42 Triglyceride can increase the blood–brain barrier transport of insulin, which can improve cognitive function.43 Additionally, higher serum triglyceride indicates an abundance of circulating fatty acids that can protect cognitive function and decrease dementia risk.44 Low triglyceride concentrations were correlated with brain inflammation, frailty and low nutrition levels. Low triglyceride levels may, in turn, reflect a low nutritional-level diet, implying pathological changes, or they may be a marker for early cognitive impairment.45 High concentrations of TC and LDL-C have been proven to be independent risk factors for cerebrovascular diseases, which may, in turn, result in cognitive impairment through cerebral ischaemia.46 Meanwhile, high TC and LDL-C levels may also be connected with small-vessel diseases by holding a vital role in maintaining the balance of cholesterol levels in the brain. Thus, high TC and LDL-C could promote cognitive impairment through a cerebrovascular pathway.47 However, the reasons for these relationships remain to be clarified.
Limitations
This study has certain limitations: (1) This is a cross-sectional study, so it cannot establish a causal relationship between lipid level and cognitive impairment. Future cohort studies will be designed to further explore the causal relationship between blood lipid levels and cognitive impairment. (2) The results cannot be generalised to the whole population in China because the sample population was selected from one province. (3) Some factors, such as physical exercise, sleep and medication, may affect lipid levels. Therefore, field measurements may not be a good representation of the true levels of lipids. (4) There may be some bias in the results due to the exclusion of people with dementia from the initial study population selection. Therefore, the findings cannot be generalised to the whole population and readers should be aware of these limitations. We will explore the characteristics of these excluded groups further in future studies.
Implications
This study suggests that there are gender and urban-rural differences in the correlation of serum lipids with cognitive impairment. Specifically, high triglyceride levels may be a protective factor for cognitive function in older urban men, while high LDL-C levels may be a risk factor for cognitive function in older rural women. These results suggest that gender-specific and region-specific effects should be taken into account when assessing the relationship between lipids and cognitive impairment. In addition, multicentred population-based studies with larger sample sizes are needed to verify whether changes in serum lipids lead to changes in cognitive performance. Therefore, the findings of this study should be considered preliminary and further studies are needed to validate and confirm them.
Acknowledgments
We thank all the study participants and all the graduate students for their participation. We also thank the physicians, nurses and clinical supervisors for their contributions, and the field coordinators at the Qinglinjie, Gangduhuayuan and Liyuan community health centres and Dawu County Traditional Chinese Medicine Hospital.
Biography
Yafu Yu is a master's student in the Brain Science and Advanced Technology Institute at Wuhan University of Science and Technology (WUST) in China. He graduated from WUST with a bachelor’s degree in Preventive Medicine in 2020. His main research interest includes longitudinal studies on cognitively impaired/demented older adults.

Footnotes
Contributors: Conception and design of the study: YY, PY, YZ, GC, DL. Acquisition of data: YY, PY, PL, MY, HX, XC. Analysis and/or interpretation of data: YY, PY, LX. Drafting of the manuscript: YY, PY. Revising the manuscript critically for important intellectual content: YZ, GC. The guarantor of the study: YZ. Approval of the version of the manuscript to be published: YY, PY, GC, DL, LX, MY, HX, XC, PL, YZ. The guarantor of the study:YZ.
Funding: Financial support for the present study was received from Ministry of Science and Technology of China (No 2020YFC2006000).
Disclaimer: The financial contributors had no role in the design, analysis or writing of this article.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Medical Ethics Committee of Wuhan University of Science and Technology (protocol code: 201845; approved on 22 October 2018). Participants gave informed consent to participate in the study before taking part.
References
- 1. Jia L, Quan M, Fu Y, et al. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol 2020;19:81–92. 10.1016/S1474-4422(19)30290-X [DOI] [PubMed] [Google Scholar]
- 2. Ren R, Qi J, Lin S, et al. The China Alzheimer report 2022. Gen Psychiatr 2022;35:e100751. 10.1136/gpsych-2022-100751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reynolds CA, Gatz M, Prince JA, et al. Serum lipid levels and cognitive change in late life. J Am Geriatr Soc 2010;58:501–9. 10.1111/j.1532-5415.2010.02739.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee J, Lee S, Min J, et al. Association between serum lipid parameters and cognitive performance in older adults. JCM 2021;10:5405. 10.3390/jcm10225405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qi L, Ding X, Tang W, et al. Prevalence and risk factors associated with dyslipidemia in Chongqing, China. Int J Environ Res Public Health 2015;12:13455–65. 10.3390/ijerph121013455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan L, Yang Z, Wu Y, et al. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis 2016;248:2–9. 10.1016/j.atherosclerosis.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 7. Hurtubise J, McLellan K, Durr K, et al. The different facets of dyslipidemia and hypertension in atherosclerosis. Curr Atheroscler Rep 2016;18:82. 10.1007/s11883-016-0632-z [DOI] [PubMed] [Google Scholar]
- 8. Liang X, Huang Y, Han X. Associations between coronary heart disease and risk of cognitive impairment: a meta-analysis. Brain Behav 2021;11:e02108. 10.1002/brb3.2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J, Jiao M, Wen J, et al. Association of body mass index and blood lipid profile with cognitive function in Chinese elderly population based on data from the China Health and Nutrition Survey, 2009-2015. Psychogeriatrics 2020;20:663–72. 10.1111/psyg.12559 [DOI] [PubMed] [Google Scholar]
- 10. Han KT, Kim SJ. Are serum cholesterol levels associated with cognitive impairment and depression in elderly individuals without dementia?: A retrospective cohort study in South Korea. Int J Geriatr Psychiatry 2021;36:163–73. 10.1002/gps.5410 [DOI] [PubMed] [Google Scholar]
- 11. McFarlane O, Kozakiewicz M, Kędziora-Kornatowska K, et al. Blood lipids and cognitive performance of aging polish adults: a case-control study based on the polsenior project. Front Aging Neurosci 2020;12:590546. 10.3389/fnagi.2020.590546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao B, Shang S, Li P, et al. The gender- and age- dependent relationships between serum lipids and cognitive impairment: a cross-sectional study in a rural area of Xi’an, China. Lipids Health Dis 2019;18:4. 10.1186/s12944-018-0956-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peters R, Xu Y, Antikainen R, et al. Evaluation of high cholesterol and risk of dementia and cognitive decline in older adults using individual patient meta-analysis. Dement Geriatr Cogn Disord 2021;50:318–25. 10.1159/000519452 [DOI] [PubMed] [Google Scholar]
- 14. Li L, Cheng G-R, Liu D, et al. The Hubei Memory and Aging Cohort Study: study design, baseline characteristics, and prevalence of cognitive impairments. JAD 2022;85:561–71. 10.3233/JAD-215129 [DOI] [PubMed] [Google Scholar]
- 15. Trivedi D. Cochrane review summary: Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Prim Health Care Res Dev 2017;18:527–8. 10.1017/S1463423617000202 [DOI] [PubMed] [Google Scholar]
- 16. Cao L, Hai S, Lin X, et al. Comparison of the Saint Louis University Mental Status examination, the Mini-Mental State Examination, and the Montreal Cognitive Assessment in detection of cognitive impairment in Chinese elderly from the geriatric department. J Am Med Dir Assoc 2012;13:626–9. 10.1016/j.jamda.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 17. Stricker NH, Lundt ES, Albertson SM, et al. Diagnostic and prognostic accuracy of the cogstate brief battery and auditory verbal learning test in preclinical Alzheimer’s disease and incident mild cognitive impairment: implications for defining subtle objective cognitive impairment. J Alzheimers Dis 2020;76:261–74. 10.3233/JAD-200087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knesevich JW, LaBarge E, Edwards D. Predictive value of the boston naming test in mild senile dementia of the Alzheimer type. Psychiatry Res 1986;19:155–61. 10.1016/0165-1781(86)90008-9 [DOI] [PubMed] [Google Scholar]
- 19. Miebach L, Wolfsgruber S, Polcher A, et al. Which features of subjective cognitive decline are related to amyloid pathology? Findings from the DELCODE study. Alzheimers Res Ther 2019;11:66. 10.1186/s13195-019-0515-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pavlik VN, Chan W, Darby E. Cohort effects in progression rate on cognitive and functional measures in an Alzheimer’s disease clinical cohort. J Alzheimers Dis 2019;71:659–69. 10.3233/JAD-190661 [DOI] [PubMed] [Google Scholar]
- 21. Shin C, Park MH, Lee S-H, et al. Usefulness of the 15-item geriatric depression scale (GDS-15) for classifying minor and major depressive disorders among community-dwelling elders. J Affect Disord 2019;259:370–5. 10.1016/j.jad.2019.08.053 [DOI] [PubMed] [Google Scholar]
- 22. American Psychiatric Association . Diagnostic and statistical manual of mental disorders 4th edition, text revision (DSM-IV-TR). American Psychiatric Association, 2000. [Google Scholar]
- 23. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–94. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 24. Liu S, Zhang M, Yang L, et al. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Community Health 2017;71:154–61. 10.1136/jech-2016-207805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang G, Luo C, Cui Y, et al. Clustering of multiple health risk behaviors and its association with diabetes in a Southern Chinese adult population: a cross-sectional study. PeerJ 2020;8:e9025. 10.7717/peerj.9025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Composing and Editorial Board of Physical Activity Guidelines for Chinese . Physical activity guidelines for Chinese (2021). Zhonghua Yu Fang Yi Xue Za Zhi 2022;56:7–8. 10.11847/zgggws1137503 [DOI] [PubMed] [Google Scholar]
- 27. Li X, Ma C, Zhang J, et al. Prevalence of and potential risk factors for mild cognitive impairment in community-dwelling residents of Beijing. J Am Geriatr Soc 2013;61:2111–9. 10.1111/jgs.12552 [DOI] [PubMed] [Google Scholar]
- 28. Liu LS, Writing Group of 2010 Chinese Guidelines for the Management of Hypertension . 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2011;39:579–615. [PubMed] [Google Scholar]
- 29. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care 2021;44:S15–33. 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- 30. Jia J, Zhou A, Wei C, et al. The prevalence of mild cognitive impairment and its etiological subtypes in elderly Chinese. Alzheimers Dement 2014;10:439–47. 10.1016/j.jalz.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 31. Reitz C, Luchsinger J, Tang M-X, et al. Impact of plasma lipids and time on memory performance in healthy elderly without dementia. Neurology 2005;64:1378–83. 10.1212/01.WNL.0000158274.31318.3C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reitz C, Tang M-X, Manly J, et al. Plasma lipid levels in the elderly are not associated with the risk of mild cognitive impairment. Dement Geriatr Cogn Disord 2008;25:232–7. 10.1159/000115847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou F, Deng W, Ding D, et al. High low-density lipoprotein cholesterol inversely relates to dementia in community-dwelling older adults: the Shanghai Aging Study. Front Neurol 2018;9:952. 10.3389/fneur.2018.00952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goh VHH, Hart WG. The association of metabolic syndrome and aging with cognition in Asian men. The Aging Male 2014;17:216–22. 10.3109/13685538.2014.968772 [DOI] [PubMed] [Google Scholar]
- 35. Ancelin M-L, Ripoche E, Dupuy A-M, et al. Gender-specific associations between lipids and cognitive decline in the elderly. European Neuropsychopharmacology 2014;24:1056–66. 10.1016/j.euroneuro.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 36. Guan LY, Hou WL, Zhu ZH, et al. Associations among gonadal hormone, triglycerides and cognitive decline in female patients with major depressive disorders. J Psychiatr Res 2021;143:580–6. 10.1016/j.jpsychires.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 37. Liu D, Li L, An L, et al. Urban-Rural disparities in mild cognitive impairment and its functional subtypes among community-dwelling older residents in central China. Gen Psychiatr 2021;34:e100564. 10.1136/gpsych-2021-100564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Casiglia E, Tikhonoff V, Caffi S, et al. Menopause does not affect blood pressure and risk profile, and menopausal women do not become similar to men. J Hypertens 2008;26:1983–92. 10.1097/HJH.0b013e32830bfdd9 [DOI] [PubMed] [Google Scholar]
- 39. Knopp RH, Paramsothy P, Retzlaff BM, et al. Sex differences in lipoprotein metabolism and dietary response: basis in hormonal differences and implications for cardiovascular disease. Curr Cardiol Rep 2006;8:452–9. 10.1007/s11886-006-0104-0 [DOI] [PubMed] [Google Scholar]
- 40. Weiss LA, Pan L, Abney M, et al. The sex-specific genetic architecture of quantitative traits in humans. Nat Genet 2006;38:218–22. 10.1038/ng1726 [DOI] [PubMed] [Google Scholar]
- 41. Segatto M, Trapani L, Marino M, et al. Age- and sex-related differences in extra-hepatic low-density lipoprotein receptor. J Cell Physiol 2011;226:2610–6. 10.1002/jcp.22607 [DOI] [PubMed] [Google Scholar]
- 42. Weir CJ, Sattar N, Walters MR, et al. Low triglyceride, not low cholesterol concentration, independently predicts poor outcome following acute stroke. Cerebrovasc Dis 2003;16:76–82. 10.1159/000070119 [DOI] [PubMed] [Google Scholar]
- 43. Urayama A, Banks WA. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology 2008;149:3592–7. 10.1210/en.2008-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang CY, Ke DS, Chen JY. Essential fatty acids and human brain. Acta Neurol Taiwan 2009;18:231–41. [PubMed] [Google Scholar]
- 45. Hu P, Seeman TE, Harris TB, et al. Does inflammation or undernutrition explain the low cholesterol-mortality association in high-functioning older persons? Macarthur studies of successful aging. J Am Geriatr Soc 2003;51:80–4. 10.1034/j.1601-5215.2002.51014.x [DOI] [PubMed] [Google Scholar]
- 46. Xu Q, Lin Y, Geng J, et al. The prevalence and risk factors for cognitive impairment following ischemic stroke. Zhonghua Nei Ke Za Zhi 2008;47:981–4. [PubMed] [Google Scholar]
- 47. Ma C, Yin Z, Zhu P, et al. Blood cholesterol in late-life and cognitive decline: a longitudinal study of the Chinese elderly. Mol Neurodegener 2017;12:24. 10.1186/s13024-017-0167-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gpsych-2023-101009supp001.pdf (60.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.