Abstract
Background
As set out in the Climate Change Act (2008), the UK National Health Service (NHS) has made a commitment to halve greenhouse gas emissions by 2025 and reach net zero by 2050. Research forms a core part of NHS activity and reducing the carbon footprint of clinical trials is a core element of the National Institute for Health and Care Research Carbon Reduction Strategy (2019).
Key arguments
However, support from funding organisations on how to achieve these targets is lacking. This brief communication article reports the reduction in the carbon footprint of the NightLife study, an ongoing multicentre randomised controlled trial assessing the impact of in-centre nocturnal haemodialysis on quality of life.
Conclusion
By using remote conferencing software and innovative data collection methods, we demonstrated a total saving of 136 tonnes of carbon dioxide equivalent over three workstreams during the first 18 months of the study, following grant activation on 1 January 2020. In addition to the environmental impact, there were additional benefits seen to cost as well as increased participant diversity and inclusion. This work highlights ways in which trials could be made less carbon intensive, more environmentally sustainable and better value for money.
Keywords: Nephrology, Dialysis, End stage renal failure, Quality of Life
Introduction
The social distancing restrictions implemented during the COVID-19 pandemic had a significant impact on the delivery and conduct of health research in the UK. Trial management teams played a key role in rapidly adjusting the way clinical trials were designed and undertaken.1 Although reductions in the carbon footprint of research activities were not the driving force for the changes required during the pandemic, it was nevertheless a significant and positive outcome. It is important that, where possible, clinical trials use these approaches to ensure carbon dioxide equivalent (CO2e) savings and demonstrate an ongoing, responsible commitment to sustainability.
Overview of the NightLife study
The NightLife study is an ongoing randomised controlled trial (RCT) using mixed methods to assess the clinical and cost effectiveness of three times a week, extended hours, in-centre nocturnal haemodialysis in comparison to standard care (ISRCTN87042063;2 see study website3). The study includes three main workstreams: an RCT and internal pilot (workstream 1), an ongoing process evaluation (workstream 2) and a QuinteT Recruitment Intervention (workstream 3).4
Adjustments to the NightLife study delivery in response to the COVID-19 pandemic
Prior to the COVID-19 pandemic, it was planned to conduct all meetings and qualitative study elements in a face-to-face manner by ≥20 collaborators across the UK. This included in-person study launch and oversight committee meetings. Following UK Government instruction, staff worked from home wherever possible. All meetings, including trial management, oversight committee, patient experience and site feasibility were reconfigured and held online. Queries and outstanding actions were resolved via email correspondence. While study processes were conducted remotely, the patient population (adults receiving thrice weekly in-centre haemodialysis) enabled in-person recruitment for workstream 2, however, all qualitative data were collected remotely. Workstream 1 and workstream 3 (which were due to run in parallel) were paused for 9 months due to the impact of COVID-19 on research delivery.
Qualitative data collection through ethnographic methods (in-person observations and real-time field notes) and interviews with the research team, dialysis unit staff and individuals with kidney disease were paused and additional data collection techniques were considered to reduce face-to-face contact. This included virtual interviews using common conference software programmes and ‘photovoice’; a participatory research method that uses participant-led photography of the phenomena being researched (in this case, the lived experience of haemodialysis) and allows remote access to experiences and phenomena outside of the immediate field of study.5 6
Calculation of carbon footprint
The original grant application outlined the total number of planned face-to-face meetings and related costings for the duration of the study. This was used to map the study activities which were reconfigured to virtual methods. Using a web-based carbon footprint calculator,7 the CO2e saved by converting to virtual approaches, home working and alternative qualitative data collection techniques were estimated. The calculator took into account: travel modality (rail, car, bicycle, air travel), specific features such as vehicle and fuel type, number of people travelling and distance in miles. For air travel, airport codes and flight class were considered. Estimated CO2e savings were calculated over the first 18 months of the NightLife study.
What have we learnt?
Carbon reduction
To date, innovative changes to the management of the NightLife study have resulted in an estimated net CO2e saving of 136 tonnes. The saving of each workstream is outlined below, with real-life equivalent values detailed in table 1.7
Table 1.
Table summarising the net CO2e saving for each workstream and equivalent kilometres driven in a car7
| Workstream | Net CO2e saving | Original method as per grant application | Adaptations implemented | Equivalent kilometres driven in a standard (non-electric) car |
| Workstream 1 | 12 tonnes | Face-to-face trial management, oversight committee, patient experience, site feasibility and study launch meetings | Virtual trial management, oversight committee, patient experience, site feasibility and study launch meetings. Queries and actions resolved via email | 37 015 |
| Workstream 2 | 20 tonnes | In-person observations; real-time field notes; face-to-face interviews; regular researcher travel to base hospitals and satellite haemodialysis units | ‘Photovoice’; virtual interviews; reduced researcher travel to base hospitals and satellite haemodialysis units | 61 692 |
| Workstream 3 | 0.32 tonnes | Face-to-face interviews; in-person attendance at, and observations of, trial management, investigator and site initiation meetings; face-to-face provision of feedback to ‘recruiters’ at participating units | Virtual interviews; remote attendance at, and observations of, trial management, investigator and site initiation meetings; virtual provision of feedback to ‘recruiters’ at participating units | 987 |
| Other benefits | 104 tonnes | In-person conference attendance; onsite working | Virtual conference attendance; home-working | 320 797 |
| Total | 136 tonnes | – | – | 419 503 |
CO2e, carbon dioxide equivalent.
Workstream 1
The net saving for workstream 1 was 12 tonnes of CO2e (emissions saved 12 tonnes; emissions used 0). Key savings were related to travel due to online reconfiguration of study meetings, UK-wide site visits.
Workstream 2
The net saving for workstream 2 was 20 tonnes of CO2e (total emissions saved 20 tonnes; emissions used 0.74 tonnes). Fifty per cent of participants opted for virtual interviews/‘photovoice’ in place of traditional ethnographic methods such as face-to-face semi-structured interviews. Subsequently, researcher travel to base hospitals and satellite haemodialysis units was also reduced by 50%. The purchase of a smartphone and two electronic tablets incurred 0.74 tonnes of CO2e.
Workstream 3
The net saving for workstream 3 was 0.32 tonnes of CO2e (total emissions saved 0.32; emissions used 0). Researcher travel was reduced by 100% as semi-structured interviews, attendance and observations of, trial management meetings, investigator meetings and site visits, and provision of feedback regarding recruitment to participating units were completed remotely.
Cost savings
All adaptations to the study organisation, management and design were made within the original study budget and resulted in significant cost savings. This included costs for travel, consumables and researcher time. In the first 18 months, the estimated total travel saving was £9659 across all workstreams, meaning 93% of the travel budget (£10 391) and 24% of the entire non-staff costs (£40 603) were saved. The underspend was repurposed for researcher training, participant benefit and further opportunities for scientific communication (conference attendance and publication open access dissemination costs) following funder approval.
Other benefits
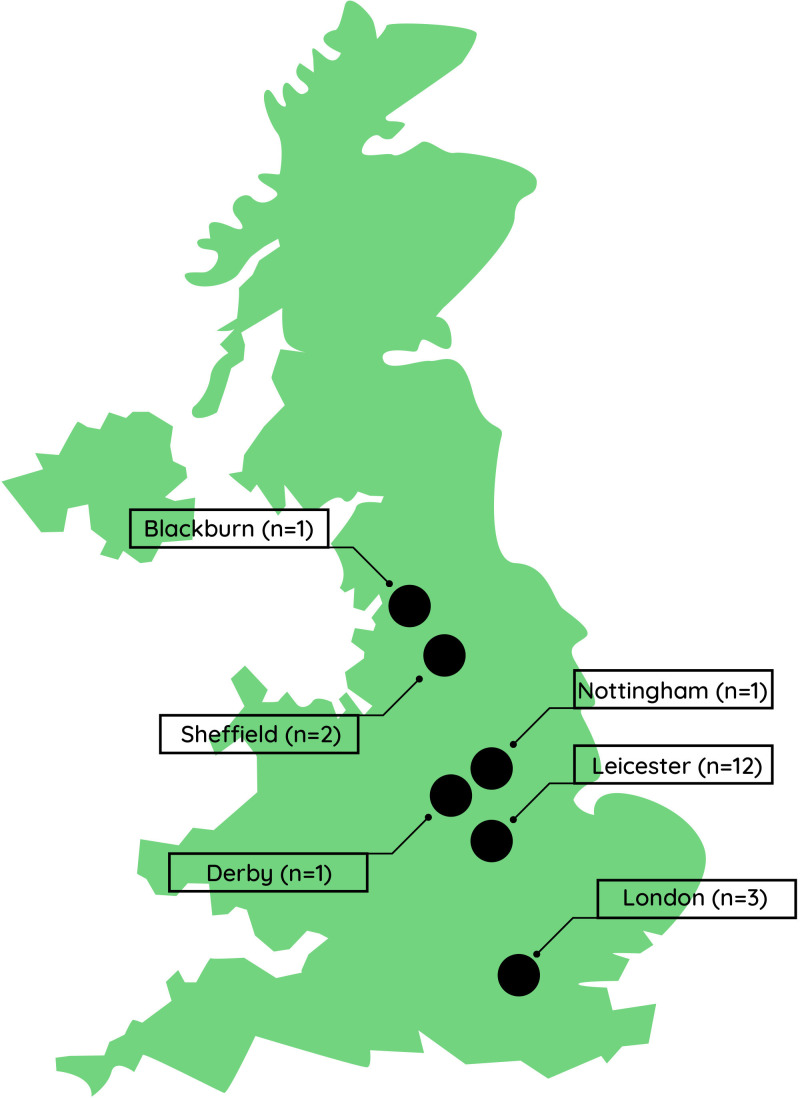
Additional CO2e savings were incurred through virtual attendance at national and international conferences and reduced travel due to home-working, saving 71 tonnes and 33 tonnes of CO2e, respectively across all workstreams. Virtual patient experience activities resulted in geographical and ethnic diversity of group members as individuals joined from various locations across the UK (see figure 1).
Figure 1.
Map of the UK showing geographical locations of patient experience group members.
Value of this experience
To date, adaptations to the management of the NightLife study have resulted in a net saving of 136 tonnes of CO2e. Key savings were related to travel due to reconfiguration of study meetings, UK-wide site visits. Extrapolating these data forward will lead to further increases in savings over the 5-year study period based on a hybrid approach now that restrictions have been lifted.
The benefits of the NightLife study adaptations go beyond the positive environmental impact. Interestingly, 50% of participants opted for virtual interviews and/or ‘photovoice’ in place of face-to-face semi-structured interviews, which revealed a holistic insight into the lived experience of haemodialysis. Photovoice allowed the researcher to approach the observational element differently, allowing participants to lead data collection and extend it into their home life; the experience of haemodialysis is a constant life disruption, not limited to the time spent in the clinical environment. This added richness in findings that may not have been achieved with traditional ethnographic methods alone.
All adaptations to the study organisation, management and design were made within the original study budget and resulted in significant cost savings which were repurposed following funder approval. Additional CO2e savings were incurred through virtual attendance at national and international conferences and reduced travel due to home-working.
Teleconferencing, video-conferencing and web-based training materials were found to be effective. The inaugural investigator meeting was held entirely remotely with more than 40 attendees from the research and nephrology community across the UK. The virtual nature of trial management and oversight committee meetings allowed more flexibility for meeting attendance, particularly for committee members based abroad. Indeed, the frequency of these meetings was increased to support the ongoing oversight of the study at no additional cost. However, an objective assessment of the impact of remote working and study activities is beyond the scope of this work. As we move away from the COVID-19 lockdown era, there is room and need for hybrid approaches to various clinical trial activities, with an acceptance of some CO2e emissions.
Debates are ongoing about how to incorporate a diverse range of patient voices in the design and delivery of research, highlighting a lack of diversity and inclusion.8 The use of alternative meeting techniques as part of the NightLife study resulted in both geographical and ethnic diversity of the patient experience group, enriching the feedback of the lived experience of kidney disease and haemodialysis.
The findings of our work are supported by a retrospective analysis of 12 pragmatic randomised control trials9; emissions are often generated in areas where steps could be taken to reduce them, such as travel and trial conduct. Resistance to such changes, however, is common. Trial-related travel is often comprised heavy emissions (particularly where multicentre studies are concerned). Traditionally this has included travel to site visits across the UK (by rail and road), as well as investigator meetings which often include international travel (by air), oversight committee meetings, training, onsite monitoring and closedown visits, as well as conference attendance throughout the study’s duration. These are travel related activities that most Clinical Trials Units (CTUs) cost for when considering the generation of a trial grant. This is generally done by aligning activities and associated costs with the risk of study. For example, clinical trials of investigational medicinal products are deemed higher risk, therefore onsite monitoring of participating sites and pharmacies and resulting travel is a necessity. However, where trials are not bound by such strict legislation, COVID-19 has presented an opportunity to change these practices in a way that reduces the trial’s carbon footprint, as reflected by our changes in the NightLife study.
Typically, most CTUs continue to use paper Investigator Site Files (ISFs). However, this approach to trial organisation and data management is being challenged and there is widespread recognition from the research community for significant improvements in environmental sustainability within clinical trials.10 There are many ways to reduce waste with increasing scope to switch from paper to electronic trial management systems (eg, ISFs) in order to (i) minimise paper usage and storage requirements; (ii) increase document accessibility; (iii) streamline management, monitoring and archiving of multicentre clinical trials and; (iv) reduce monetary costs. Adshead et al suggest that clinical trials with a lower carbon footprint should be prioritised by funders, and just as researchers have to justify to funders the budget for a trial, they should also have to justify the carbon footprint to their stakeholders and demonstrate that it as low as possible.10
Aid for future trial design and further work
To the authors’ knowledge, this is one of few articles to consider and evaluate the environmental improvements that can be made by remote working and virtual adaptations to study designs when establishing multicentre RCTs. This work has the potential to act as a guide for other clinical trials to reduce cost and their environmental impact. It also demonstrates how to enhance geographical diversity of research teams without excessive cost.
Take-home messages
The COVID-19 pandemic presented a need to adapt clinical trials to protect patients, carers, clinical teams and researchers, and accelerated a pre-existing drive to reduce the carbon footprint of research. Study processes needed to evolve rapidly to ensure they were robust and financially lean in the COVID-19 era. The legacy of such changes has been wide ranging but of note, the impact on CO2e saving experienced in the NightLife study is a benefit that should inspire and drive the reduction of the carbon impact of all clinical trials from now and into the future. We have highlighted opportunities for investigators and trial management teams to implement alternative approaches to designing and conducting clinical trials in order to make them less carbon intensive, more environmentally sustainable and better value for money.
Supplementary Material
Footnotes
Twitter: @NiamhQuann
Contributors: The paper was conceived by NQ and JOB. SB performed the carbon footprint calculations. NQ, JOB, KLH, VC, CR, KM, CC, LR and HE reviewed and approved the final draft submitted.
Funding: The NightLife study is funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme (funder reference: NIHR127440). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mitchell EJ, Ahmed K, Breeman S, et al. It is unprecedented: trial management during the COVID-19 pandemic and beyond. Trials 2020;21:784. 10.1186/s13063-020-04711-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ltd BC . ISRCTN registry - does NIGHT-time dialysis improve quality of LIFE? n.d. Available: https://www.isrctn.com/ISRCTN87042063
- 3.NightLife . The nightlife study. n.d. Available: https://nightlifestudy.co.uk/
- 4.Donovan JL, Rooshenas L, Jepson M, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the quintet recruitment intervention (QRI). Trials 2016;17:283. 10.1186/s13063-016-1391-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cluley V. Using photovoice to include people with profound and multiple learning disabilities in inclusive research. Br J Learn Disabil 2017;45:39–46. 10.1111/bld.12174 [DOI] [Google Scholar]
- 6.Cluley V, Pilnick A, Fyson R. Improving the inclusivity and credibility of visual research: interpretive engagement as a route to including the voices of people with learning disabilities in analysis. Visual Studies 2021;36:524–36. 10.1080/1472586X.2020.1807402 [DOI] [Google Scholar]
- 7.Carbon calculator - carbon footprint calculator for individuals and households. n.d. Available: https://www.carbonfootprint.com/calculator.aspx
- 8.Tierney S, Dawson S, Boylan A-M, et al. Broadening diversity through creative involvement to identify research priorities. Res Involv Engagem 2021;7:3. 10.1186/s40900-020-00244-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyle K, Dent L, Bailey S, et al. Carbon cost of pragmatic randomised controlled trials: retrospective analysis of sample of trials. BMJ 2009;339:b4187. 10.1136/bmj.b4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adshead F, Al-Shahi Salman R, Aumonier S, et al. A strategy to reduce the carbon footprint of clinical trials. Lancet 2021;398:281–2. 10.1016/S0140-6736(21)01384-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.