Abstract
Objectives
We investigated progression through the care cascade and associated factors for people with diabetes in sub-Saharan Africa to identify attrition stages that may be most appropriate for targeted intervention.
Design
Cross-sectional study.
Setting
Community-based study in four sub-Saharan African countries.
Participants
10 700 individuals, aged 40–60 years.
Primary and secondary outcome measures
The primary outcome measure was the diabetes cascade of care defined as the age-adjusted diabetes prevalence (self-report of diabetes, fasting plasma glucose (FPG) ≥7 mmol/L or random plasma glucose ≥11.1 mmol/L) and proportions of those who reported awareness of having diabetes, ever having received treatment for diabetes and those who achieved glycaemic control (FPG <7.2 mmol/L). Secondary outcome measures were factors associated with having diabetes and being aware of the diagnosis.
Results
Diabetes prevalence was 5.5% (95% CI 4.4% to 6.5%). Approximately half of those with diabetes were aware (54%; 95% CI 50% to 58%); 73% (95% CI 67% to 79%) of aware individuals reported ever having received treatment. However, only 38% (95% CI 30% to 46%) of those ever having received treatment were adequately controlled. Increasing age (OR 1.1; 95% CI 1.0 to 1.1), urban residence (OR 2.3; 95% CI 1.6 to 3.5), hypertension (OR 1.9; 95% CI 1.5 to 2.4), family history of diabetes (OR 3.9; 95% CI 3.0 to 5.1) and measures of central adiposity were associated with higher odds of having diabetes. Increasing age (OR 1.1; 95% CI 1.0 to 1.1), semi-rural residence (OR 2.5; 95% CI 1.1 to 5.7), secondary education (OR 2.4; 95% CI 1.2 to 4.9), hypertension (OR 1.6; 95% CI 1.0 to 2.4) and known HIV positivity (OR 2.3; 95% CI 1.2 to 4.4) were associated with greater likelihood of awareness of having diabetes.
Conclusions
There is attrition at each stage of the diabetes care cascade in sub-Saharan Africa. Public health strategies should target improving diagnosis in high-risk individuals and intensifying therapy in individuals treated for diabetes.
Keywords: General diabetes, PUBLIC HEALTH, International health services
Strengths and limitations of this study.
We present harmonised primary data on the diabetes care cascade from multiple countries in sub-Saharan Africa.
Our study included over 10 000 participants from eastern, western and southern Africa.
We did not perform glucose tolerance testing and therefore may not have identified individuals who met criteria for diabetes diagnosis only after a glucose challenge.
Glycaemic control was assessed using fasting plasma glucose which provides a point evaluation and may not be reflective of control over a longer period of time.
Introduction
Diabetes prevalence in adults in sub-Saharan Africa (SSA) is projected to increase from 23.6 million in 2021 to 54.9 million people in 2045.1 Inadequate control of blood sugar and other cardiovascular risk factors will impose an unsustainable burden of diabetes-related complications on already constrained regional healthcare systems. Existing data suggest that outcomes in individuals in SSA with diabetes are currently suboptimal with over 300 000 diabetes-related deaths before the age of 60 years in 2021,1 highlighting the need to improve clinical care. Optimisation of diabetes management is contingent on numerous factors including the diagnosis of diabetes, appropriate escalation of therapy and patient adherence to therapeutic interventions, but effective strategies to improve diabetes management in SSA are hampered by a lack of knowledge about the extent of the deficiencies in this care continuum.
The cascade of care model, frequently used to identify deficits in HIV care, may be applied to diabetes to identify opportunities for improved outcomes.2–4 The elements of the cascade, namely prevalence, awareness, treatment and control reflect aspects of the healthcare system, including effectiveness of prevention and detection strategies and the ability to implement and escalate therapy as necessary. On an individual level, diabetes awareness in particular is key to the adherence to lifestyle modification and medication that underpin glycaemic control. Evaluation of the diabetes care cascade allows policymakers to assess how well the healthcare system manages patients with diabetes and to identify areas for targeted interventions, particularly important in the resource-constrained lower-income and middle-income countries of SSA.
Despite the benefits of establishing the diabetes care cascade, there is a paucity of primary data on it in SSA. Studies have often been limited to diabetes prevalence and awareness and conducted in hospital-based populations, introducing selection bias, while multicountry studies that have reported on the entire cascade have meta-analysed data from heterogeneous studies with methodological differences in determining each cascade stage.2 3 We aimed to evaluate the diabetes cascade of care in four SSA countries, using harmonised data collected across six sites and performed exploratory analyses of the cascade stratified by sex and study site. We further investigated factors associated with the likelihood of having diabetes and being aware of a diagnosis of diabetes, the first two steps in the cascade.
Methods
Study setting and participants
The Genomic and Environmental Risk Factors for Cardiometabolic Disease in Africans (AWI-Gen) study and participating sites have been described in detail elsewhere.5 6 In brief, 10 700 individuals were recruited from six sites in SSA in a community-based, cross-sectional study conducted between August 2013 and August 2016. Individuals were eligible for inclusion if they were aged 40–60 years and resided permanently in the study sites. We excluded individuals who were pregnant and, given that one of the broader objectives of the AWI-Gen study was to investigate genomic determinants of cardiometabolic disease, we also excluded individuals who were closely related to an existing participant and who had recently immigrated into the study site. We selected individuals aged 40–60 years as this is a peak time for the development of cardiometabolic disease. Three of the study sites were in South Africa (Soweto, Agincourt and Dikgale), one was in Kenya (Nairobi), one in Ghana (Navrongo) and one in Burkina Faso (Nanoro). Participants were therefore included from southern, eastern and western Africa. The selected sites were also on a continuum of urbanisation: Nairobi and Soweto were urban sites, Agincourt and Dikgale were semi-rural and Nanoro and Navrongo were rural.
With the exception of Soweto, each study site is home to a health and socio-demographic surveillance system (HDSS) which enumerates all residents within the HDSS on a regular basis, ensuring a well-defined population sampling frame. In Nairobi, Agincourt, Navrongo and Nanoro, individuals were randomly sampled from the sampling frame, while in Dikgale, a convenience sampling strategy was employed. In Soweto, 700 women who were participants in the Study of Women Entering an Endocrine Transition study7 and caregivers of the Birth to Twenty+ cohort8 were recruited. Additional female and all male participants were randomly recruited, using a sampling frame which covered the Soweto region. Where necessary, there was oversampling to ensure equal numbers of women and men.
Patient and public involvement
Prior to the initiation of the AWI-Gen study, an extensive process of community engagement was conducted. This included meetings with civic and traditional leadership structures, household visits and group information sessions to discuss planned research activities. Study results were delivered annually to study participants, communities and community leaders.
Data collection and definitions
Data were collected by study staff trained on standardised protocols. Socio-demographic data and personal and family medical history were self-reported. Additionally, individuals were considered to have hypertension if the mean systolic blood pressure of the latter two of three readings at the study visit ≥140 mm Hg or the mean diastolic pressure ≥90 mm Hg (Omron M6, Omron, Kyoto, Japan).9 Individuals were classified as HIV positive if they reported a previous diagnosis of HIV or if they tested positive on the rapid HIV tests that were offered to participants in South Africa and Kenya (MD HIV 1/2 test (Medical Diagnostech, Cape Town, South Africa); One Step anti-HIV1+2 rapid screen test (InTec, Xiamen, China); Determine rapid test kit (Abbott Pharmaceuticals, Chicago, USA)). Rapid HIV tests were not offered in Ghana and Burkina Faso due to the low prevalence of HIV in those countries; individuals in these sites who did not know their HIV status were classified as HIV negative. Physical activity was assessed using the Global Physical Activity Questionnaire and occupational, leisure time and travel-related physical activity variables from this questionnaire were summed to give the total moderate–vigorous intensity physical activity (MVPA) in minutes per week. Individuals were classified as having no MVPA (0 min/week), insufficient MVPA (1–150 min/week) or sufficient MVPA (≥150 min/week).10
Standing height was measured with the participant barefoot or in light socks, using a Harpenden digital stadiometer (Holtain, Wales, UK). Weight was measured with the participant in light clothing, using a digital Physician Large Dial 200 kg capacity scale (Kendon Medical, South Africa) and body mass index was calculated as weight in kg divided by height in metres squared. Using a stretch-resistant measuring tape (SECA, Hamburg, Germany), hip circumference, as a measure of gluteofemoral fat, was measured around the most protruding part of the buttocks.
Visceral and subcutaneous adipose tissue, direct measures of central adiposity associated with insulin resistance, were measured using abdominal ultrasound (LOGIQ e ultrasound system (GE HealthCare, Connecticut, USA)). Study staff from all sites were centrally trained in Johannesburg, South Africa to perform the abdominal ultrasounds. Visceral adipose thickness was determined by the thickness of the fat pad between the anterior spine and peritoneal layer at end expiration, while subcutaneous adipose thickness was the thickness of the fat pad between the skin and the outer edge of the linea alba.
Venous blood was collected at study visits in potassium oxalate/sodium fluoride tubes and centrifuged immediately after collection, with the supernatant plasma stored at −80°C until analysis, according to a detailed sample processing protocol provided to all sites. Analyses for glucose were all performed at a central site, using colorimetric methods, on the Randox Plus clinical chemistry analyser (Randox, UK) with a range of 0.36–35 mmol/L and coefficient of variation <2.3%.
Diabetes was defined as a previous diagnosis of diabetes by a healthcare provider (which could include a doctor, nurse, community health worker or similar person), ever having received treatment for diabetes, or fasting plasma glucose (FPG) ≥7 mmol/L or random plasma glucose ≥11.1 mmol/L11 12 on the sample taken during the study visit. Samples were considered random if a participant had not fasted overnight or fasting status could not be confirmed. Participants were considered to be aware of a diagnosis of diabetes if they reported ever having been told by a healthcare provider that they had diabetes and were considered to have been treated for diabetes if they reported ever having received treatment for diabetes (dietary advice and/or glucose lowering agents) from a healthcare provider. Individuals were considered to have their diabetes controlled if fasting glucose was <7.2 mmol/L.11
Statistical analysis
Categorical participant characteristics of marital status, highest level of education, current smoking, known hypertension, known HIV positivity, family history of diabetes and physical activity category were described using frequencies and percentages, while medians and IQRs were used to describe continuous characteristics of age, body mass index, hip circumference, visceral fat and subcutaneous fat. The Mann-Whitney U, χ2 and Fisher’s exact tests were used to compare continuous and categorical variables, respectively, between groups defined by sex to investigate sex-related differences in potential determinants and groups defined by data missingness status to evaluate for bias between those who were included and those who were excluded from the analysis due to missing data.
Age-adjusted diabetes prevalence was determined using the United Nations African population distribution13 as the reference population structure. The proportion of those aware of having diabetes was calculated as a percentage of those with diabetes and similarly, the proportion of those ever receiving treatment for diabetes was calculated as a percentage of those aware of having diabetes. The proportion of those who had their diabetes controlled was calculated as a percentage of those who reported ever receiving treatment. The method for interval estimation described by Tiwari et al 14 was used to determine the 95% CIs. The Soweto site was excluded from the latter two stages of the cascade as the ‘ever receiving treatment’ variable was not collected.
Multivariable logistic regression was used to assess the relationship between the odds of having diabetes and socio-demographic and clinical characteristics including urbanicity. Independent variables for inclusion in the logistic regression were selected based on previous research.15 16 The Soweto site did not collect data on family history of diabetes and was therefore not included in this model, as family history of diabetes has been demonstrated in other settings to be strongly associated with higher odds of having the condition. Additional multivariable logistic regression models were also fit, using data from all sites, to investigate associations with awareness of a diagnosis of diabetes. In the model investigating associations with odds of having diabetes, we included visceral and subcutaneous fat as direct assessments of central obesity and hip circumference as a measure of gluteofemoral fat. In the model investigating associations with awareness, we used body mass index as the measure of obesity as we thought awareness was more likely to be associated with a global assessment of obesity rather than individual fat depots. We were underpowered to assess associations with diabetes treatment and control.
Sensitivity analyses were conducted in which associations with having diabetes and awareness of a diagnosis of diabetes were explored in analyses stratified by HIV prevalence, with the South African sites and Nairobi classified as high prevalence sites and Navrongo and Nanoro classified as low prevalence sites.
Missing data were handled using pairwise deletion. Analyses were conducted using Stata V.16 (StataCorp, USA).
Results
Sample characteristics
The characteristics of the 10 700 study participants are shown in online supplemental table S1. There were 5892 women (55%), with a median age of 50 years (IQR 45–55). There was some intersite variation in socio-demographic variables—while most participants in the urban and semi-rural sites had some formal education, between 70% and 80% of participants in the rural sites did not. Smoking prevalence ranged between 6% and 30% overall, with prevalence several fold higher in men than in women in all sites. There was a high prevalence of chronic disease with 3755 (37%) participants having hypertension and 1310 (12%) known as being HIV positive, although intersite variation was evident, with HIV prevalence being low, for example, in Nanoro and Navrongo. Family history of diabetes was highest in the urban and semi-rural areas. Anthropometric measures of obesity and subcutaneous fat were higher in women in urban and semi-urban areas, while there were no clear sex differences in Nanoro or Navrongo. Visceral fat was generally similar in both sexes. The majority of individuals (82%) were undertaking at least 150 min of moderate-to-vigorous physical activity weekly.
bmjopen-2022-069193supp001.pdf (171KB, pdf)
Missing outcome data
No participants had missing data on the diabetes status outcome, while 31 individuals had missing data on the awareness outcome and were slightly older (median age 54 vs 52 years; p=0.04), less likely to be employed (32 vs 64%; p<0.01) and had a different marital status distribution (p<0.01) than those who were not missing these data.
Diabetes cascade of care
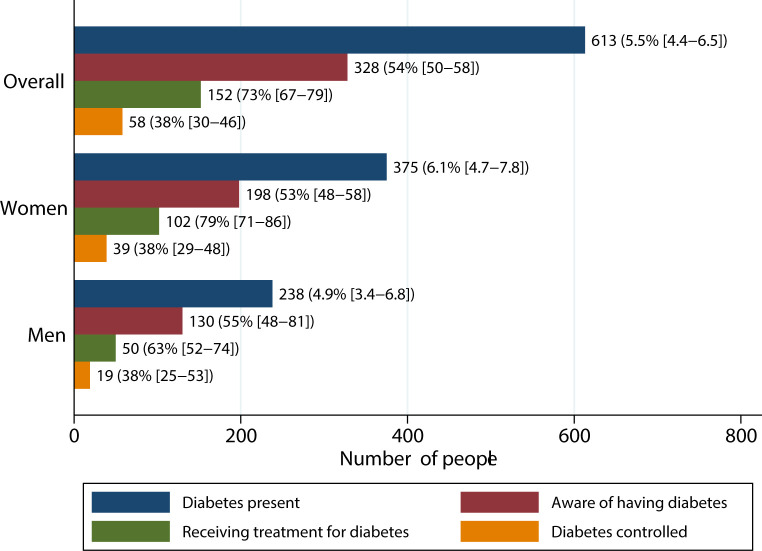
The diabetes cascade of care is shown in figure 1. The age-adjusted prevalence of diabetes in study participants was 5.5% (95% CI 4.4% to 6.5%) and was significantly higher in women (6.1% vs 4.9%; p<0.01). Prevalence varied by site, with highest prevalence in the urban site of Soweto (9.0%; 95% CI 7.8% to 10%) and the lowest in rural Navrongo (1.3%; 95% CI 0.7% to 1.9%) (online supplemental table S2). Diabetes prevalence was higher in women than men in Soweto and Nairobi (Soweto: 12% vs 6.3%, p<0.01; Nairobi: 9.1% vs 4.1%, p<0.01) while in Nanoro, the prevalence was higher in men (1.8% vs 4.7%, p<0.01).
Figure 1.
Diabetes cascade of care in six sub-Saharan African countries, overall and stratified by gender. Estimates given as counts and proportions with 95% CIs and proportions calculated as percentages of eligible individuals in previous stage. Estimates for ever receiving treatment and achieving glycaemic control (calculated as percentage of those who ever received treatment) exclude Soweto as the treatment variable was not collected at that site. Data on diabetes control were missing for a further 17 participants.
Overall, just over half of the 613 individuals with diabetes were aware of their condition (54%; 95% CI 50% to 58%), with the highest awareness in Navrongo (65%; 95% CI 43% to 84%) and the lowest in Nanoro (25%; 95% CI 16% to 37%), although CIs across the sites were wide and overlapping. Nearly 75% of individuals aware of having diabetes reported ever receiving treatment, but only 38% (95% CI 30% to 46%) were adequately controlled. More women reported ever being treated for diabetes (p=0.01), but there were no sex differences in participants achieving control (p=0.98).
In logistic regression models, increasing age (OR 1.1; 95% CI 1.0 to 1.1; p<0.01) and urban residence (OR 2.3; 95% CI 1.6 to 3.5; p<0.01) were associated with higher odds of having diabetes (table 1). Hypertension was also associated with having diabetes (OR 1.9; 95% CI 1.5 to 2.4; p<0.01), as was family history of diabetes (OR 3.9; 95% CI 3.0 to 5.1; p<0.01); conversely, known HIV positivity was associated with lower odds of diabetes (OR 0.6; 95% CI 0.4 to 0.9; p<0.01). Visceral and subcutaneous fat were also associated with higher odds, while there was a marginal negative association with hip circumference (table 1).
Table 1.
Factors associated with odds of having diabetes in five sub-Saharan African sites (Agincourt, Dikgale, Nairobi, Nanoro and Navrongo)*
| OR | 95% CI | P value | |
| Age | 1.1 | 1.0 to 1.1 | <0.01 |
| Sex | |||
| Women | Reference | ||
| Men | 1.1 | 0.8 to 1.5 | 0.65 |
| Location | |||
| Rural | Reference | ||
| Semi-rural | 1.5 | 1.0 to 2.3 | 0.08 |
| Urban | 2.3 | 1.6 to 3.5 | <0.01 |
| Marital status | |||
| Currently married or cohabitating | Reference | ||
| Never married or cohabitating | 1.4 | 0.9 to 2.0 | 0.15 |
| Previously married | 1.0 | 0.8 to 1.3 | 0.99 |
| Educational attainment | |||
| No formal education | Reference | ||
| Primary education | 1.2 | 0.9 to 1.7 | 0.29 |
| Secondary education | 1.0 | 0.7 to 1.5 | 0.84 |
| Tertiary education | 1.4 | 0.7 to 2.6 | 0.37 |
| Employment status | |||
| Unemployed | Reference | ||
| Employed | 1.1 | 0.8 to 1.5 | 0.48 |
| Smoking status | |||
| No history of smoking | Reference | ||
| Current smoker | 0.7 | 0.4 to 1.1 | 0.15 |
| History of hypertension | |||
| No | Reference | ||
| Yes | 1.9 | 1.5 to 2.4 | <0.01 |
| Known HIV positivity | |||
| No | Reference | ||
| Yes | 0.6 | 0.4 to 0.9 | 0.01 |
| Family history of diabetes | |||
| No | Reference | ||
| Yes | 3.9 | 3.0 to 5.1 | <0.01 |
| Physical activity categories | |||
| Absent | Reference | ||
| Insufficient | 0.9 | 0.5 to 1.5 | 0.61 |
| Sufficient | 0.7 | 0.5 to 1.0 | 0.08 |
| Hip circumference | 1.0 | 1.0 to 1.0 | 0.04 |
| Visceral fat | 1.2 | 1.1 to 1.2 | <0.01 |
| Subcutaneous fat | 1.3 | 1.1 to 1.4 | <0.01 |
*7425 participants were included in the analysis. Participants from the Soweto site were excluded as data on family history were not collected. Age was entered as a continuous variable.
Similar associations were evident in sensitivity analyses restricted to sites with high HIV prevalence (online supplemental table S3). However, only family history remained significantly associated with diabetes in low HIV prevalence settings, although previously unobserved associations with male sex and physical activity emerged (online supplemental table S4). These analyses were however limited by the low prevalence of diabetes in these settings which meant they were underpowered.
Increasing age (OR 1.1; 95% CI 1.0 to 1.1; p=0.02), semi-rural environment (OR 2.5; 95% CI 1.1 to 5.7; p=0.02) and secondary education (OR 2.4; 95% CI 1.2 to 4.9; p=0.02) were all associated with greater likelihood of awareness of diabetes, as were the chronic conditions of hypertension (OR 1.6; 95% CI 1.0 to 2.4; p=0.04) and known HIV positivity (OR 2.3; 95% CI 1.2 to 4.4; p=0.02) (table 2). In sensitivity analyses in high HIV prevalence sites, only hypertension and known HIV positivity remained associated with higher awareness of diabetes (online supplemental table S5). The sample size in low HIV prevalence sites was too small to perform meaningful analyses.
Table 2.
Factors associated with awareness of diabetes in six sub-Saharan African sites (Agincourt, Dikgale, Nairobi, Nanoro, Navrongo and Soweto)*
| OR | 95% CI | P value | |
| Age | 1.0 | 1.0 to 1.1 | 0.02 |
| Sex | |||
| Women | Reference | ||
| Men | 1.1 | 0.7 to 1.8 | 0.59 |
| Location | |||
| Rural | Reference | ||
| Semi-rural | 2.5 | 1.1 to 5.7 | 0.02 |
| Urban | 1.5 | 0.7 to 3.1 | 0.34 |
| Marital status | |||
| Currently married or cohabitating | Reference | ||
| Never married or cohabitating | 0.9 | 0.4 to 2.0 | 0.84 |
| Previously married | 1.0 | 0.6 to 1.7 | 0.86 |
| Educational attainment | |||
| No formal education | Reference | ||
| Primary education | 1.8 | 0.9 to 3.5 | 0.09 |
| Secondary education | 2.4 | 1.2 to 4.9 | 0.02 |
| Tertiary education | 2.1 | 0.7 to 6.1 | 0.17 |
| Employment status | |||
| Unemployed | Reference | ||
| Employed | 0.8 | 0.5 to 1.3 | 0.45 |
| History of hypertension | |||
| No | |||
| Yes | 1.6 | 1.0 to 2.4 | 0.04 |
| Known HIV positivity | |||
| No | Reference | ||
| Yes | 2.3 | 1.2 to 4.4 | 0.02 |
| Body mass index | 1.0 | 1.0 to 1.0 | 0.97 |
*Four hundred and seventy-two participants were included in the analysis. Age and body mass index were entered as continuous variables.
Discussion
In this multicountry study of the diabetes care cascade in SSA, we demonstrate attrition at each stage of the cascade with just over half of those with diabetes being aware of their condition and only approximately one-third of those who reported ever receiving treatment achieving optimal glycaemic control. We also report socio-demographic and clinical factors associated with increased odds of having diabetes including older age, urban residence and having hypertension and factors associated with awareness of having diabetes which included increasing age, semi-rural environment, secondary education and having hypertension or known HIV positivity.
Our prevalence estimate of 5.5% is similar to the 2019 International Diabetes Federation (IDF) estimate for SSA of 4.7% in adults aged 20–79 years.1 A subregional meta-analysis from western Africa revealed a lower prevalence (4.0% in urban adults and 2.6% in rural adults),17 in keeping with our study where prevalence in the western African sites was two to three times lower than in the Southern and eastern African sites. Factors in our study associated with higher odds of having diabetes, such as increasing age and urban residence, have been previously reported, with the western African meta-analysis reporting over a threefold increase in prevalence in people over 50 years17 and Werfalli et al reporting a prevalence of 20% in people living in urban areas versus 7.9% in those in rural areas.18 Our findings of associations with family history of diabetes, hypertension and adiposity support results from other country-level meta-analyses in Africa.19 20 We also noted lower odds of having diabetes in individuals with known HIV in keeping with other studies that have identified lower prevalence of cardiometabolic risk factors in individuals with HIV in SSA.21 22
While our estimate of the prevalence of diabetes unawareness of 47% was broadly similar to the 2019 IDF estimate of the prevalence of undiagnosed diabetes of 60% in SSA,1 it did contrast sharply with other studies. A meta-analysis of 23 studies from across Africa estimated a much lower pooled prevalence of undiagnosed diabetes of just under 4%.23 There was however significant heterogeneity in the included studies and the majority of the data originated from a single country, which may not be representative of other countries in the region. This itself differed considerably from data from 12 nationally representative surveys in SSA in which 73% of those with diabetes were unaware of their condition, with factors similar to our study, namely older age and higher level of educational attainment, associated with awareness.24 Our findings also suggest that those with chronic diseases such as HIV and hypertension may be more aware of having diabetes, which may be due to increased contact with the healthcare system.25
In a study reporting data from 15 SSA countries, approximately 40% of adults with diabetes received glucose-lowering medication, while approximately 25% received counselling on diet, exercise or weight loss.2 These proportions are lower than ours which may be due to the difference in denominators—we used a denominator of individuals aware of having diabetes rather than all those with diabetes. In another study reporting data from 12 SSA countries, just over 30% of those with diabetes were aware of their condition, with a similar percentage ever having received lifestyle advice or currently receiving diabetes medication and just over 20% achieving control.3 While this study also used a fixed denominator of the number of people with diabetes, the results support our finding that there is not a major fall-off between the stages of awareness and treatment and the most significant deficits are at the stages of awareness of having diabetes, that is, diagnosis and achieving glycaemic control. Of note, this study used a more liberal definition of glycaemic control than our study (FPG <10.1 mmol/L or glycosylated haemoglobin (HbA1c) <8% in the single study in which it was available) and may have identified a more drastic control deficit if a threshold for glycaemic control similar to ours had been used. A country-level meta-analysis of 22 studies from Ethiopia suggested a similar degree of glycaemic control as our study, with approximately one-third of those included achieving glycaemic targets, regardless of whether these were assessed using FPG or HbA1c.26
We describe, to our knowledge, the first study in SSA in which harmonised primary data on the diabetes care cascade have been collected from multiple countries. Previous multicountry research in SSA on this subject has relied on systematic reviews and meta-analyses and has therefore been limited by the methodological heterogeneity of the constituent studies, including the use of different biomarkers to define diabetes. In our work, data were collected in a standardised manner and in addition to self-report, we used venous blood samples, analysed at a single laboratory, to ascertain biochemical evidence of diabetes. Our study also included over 10 000 men and women from three subregions of SSA.
Our study does have limitations. We did not distinguish between type 1 and type 2 diabetes and the care cascade and associated factors may differ between these two conditions. While we used accepted and convenient diagnostic criteria for diabetes, we may have underestimated the prevalence of diabetes as we did not assess glucose tolerance and may therefore have excluded those who met the criteria for diabetes only after a glucose challenge, which may be particularly important in populations of African descent. Both oral glucose tolerance tests and HbA1c, appear to classify more African-ancestry individuals as having diabetes than FPG alone27 28 and use of either of these criteria may have increased diabetes prevalence in our study. Our research was conducted in HDSS sites and among a research cohort in Soweto, populations which may not be nationally representative. Indeed, individuals in these sites may have been told they had diabetes while taking part in previous studies, making the proportion of individuals with diabetes who know they have the condition higher than in the general population. We also used self-report rather than clinical records to determine ever receiving diabetes treatment. FPG was used to assess diabetes control and this provides an evaluation only at a single point in time and may be subject to more analytical variability than HbA1c, which has largely supplanted it in clinical use in well-resourced environments. Several large scale epidemiological studies have however used plasma glucose measures to assess glycaemic control.2 3 We collected data for this study between 2013 and 2016 and it is conceivable that some of the parameters in the cascade may have changed during or since that time.
Despite these limitations, our study provides valuable information on the burden of diabetes in SSA and the deficiencies which need to be addressed to improve outcomes. In areas where diabetes prevalence is low, primordial prevention strategies should be employed to reduce the likelihood of developing risk factors such as obesity, with particular focus on higher risk urban environments. Screening of at-risk populations needs to be enhanced and the low percentage of individuals attaining satisfactory glycaemic control suggests that more aggressive, treat to target strategies need to be promoted among healthcare workers, although we acknowledge this may be limited by drug availability in many parts of the continent.
Additional work is necessary to understand whether our findings are applicable to other SSA countries and subregions at different stages of the epidemiological transition and with variable access to healthcare. It is also essential to understand key determinants of ever receiving diabetes treatment and control, which we were underpowered to investigate, and care cascades for other important vascular risk factors in people with diabetes, such as elevated blood pressure and dyslipidaemia. Identification of the points in each of these care cascades at which significant attrition is occurring will assist public health officials in developing appropriate interventions to reduce diabetes-related morbidity and mortality.
Supplementary Material
Footnotes
Twitter: @shukrifmohamed, @EngelbertNonte1
Contributors: ANW—conceptualisation, writing—original draft, review and editing, funding acquisition. IM—formal analysis, writing—review and editing. GAg—data collection, investigation, writing—review and editing. GAs—data collection, investigation, writing—review and editing. PB—data collection, investigation, writing—review and editing, funding acquisition. SSRC—investigation, writing—review and editing. FXGO—data collection, investigation, writing—review and editing, funding acquisition. EM—investigation, writing—review and editing. LKM—investigation, writing—review and editing. SFM—investigation, writing—review and editing. EAN—data collection, investigation, writing—review and editing. SAN—conceptualisation, investigation, writing—review and editing. HS—data collection, investigation, writing—review and editing. MR—conceptualisation, writing—review and editing, project administration, funding acquisition. NJC—conceptualisation, writing—review and editing, funding acquisition. ANW, MR and NJC act as guarantors of the paper.
Funding: The AWI-Gen Collaborative Centre is funded by the National Human Genome Research Institute (NHGRI), Office of the Director (OD), the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), the National Institute of Environmental Health Sciences (NIEHS), the Office of AIDS Research (OAR) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) (grant number U54HG006938 and its supplements), as part of the H3Africa Consortium as well as by the Department of Science and Innovation, South Africa (grant number DST/CON 0056/2014). Funding for the Soweto site was also received from the South African Medical Research Council. ANW is supported by the Fogarty International Centre, National Institutes of Health (grant number K43TW010698).
Disclaimer: This paper describes the views of the authors and does not necessarily represent the official views of the National Institutes of Health (USA) or the South African Department of Science and Innovation who funded this research. The funders had no role in study design, data collection, analysis and interpretation, report writing or the decision to submit this article for publication.
Competing interests: ANW declares an honorarium received from Sanofi for serving as a panel member at an educational event on thyroid cancer. SAN declares participation in a data safety monitoring board of a Phase IV open-label trial to assess bone mineral density in a cohort of African women on Depo-Provera and tenofovir disoproxil fumarate switched to tenofovir alafenamide fumarate based antiretroviral therapy and Council membership in the International Society of Developmental Origins of Health and Disease.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. De-identified individual participant data from the AWI-Gen study are available from the European Genome-Phenome Archive (EGA) at study number EGA00001002482 (https://ega459archive.org/datasets/EGAD00001006425).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethical approval for the AWI-Gen study was provided by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand (M121029, M170880). Participants gave informed consent to participate in the study before taking part.
References
- 1. International Diabetes Federation . IDF Diabetes Atlas. Brussels, Belgium, 2021. [Google Scholar]
- 2. Flood D, Seiglie JA, Dunn M, et al. The state of diabetes treatment coverage in 55 low-income and middle-income countries: a cross-sectional study of nationally representative, individual-level data in 680 102 adults. Lancet Healthy Longev 2021;2:e340–51. 10.1016/s2666-7568(21)00089-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manne-Goehler J, Geldsetzer P, Agoudavi K, et al. Health system performance for people with diabetes in 28 low- and middle-income countries: a cross-sectional study of nationally representative surveys. PLoS Med 2019;16:e1002751. 10.1371/journal.pmed.1002751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kazemian P, Shebl FM, McCann N, et al. Evaluation of the cascade of diabetes care in the United States, 2005-2016. JAMA Intern Med 2019;179:1376–85. 10.1001/jamainternmed.2019.2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramsay M, Crowther N, Tambo E, et al. H3Africa AWI-gen collaborative centre: a resource to study the interplay between genomic and environmental risk factors for cardiometabolic diseases in four sub-Saharan African countries. Glob Health Epidemiol Genom 2016;1:e20. 10.1017/gheg.2016.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ali SA, Soo C, Agongo G, et al. Genomic and environmental risk factors for cardiometabolic diseases in Africa: methods used for phase 1 of the AWI-gen population cross-sectional study. Global Health Action 2018;11:1507133. 10.1080/16549716.2018.1507133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jaff NG, Norris SA, Snyman T, et al. Body composition in the study of women entering and in endocrine transition (sweet): a perspective of African women who have a high prevalence of obesity and HIV infection. Metabolism 2015;64:1031–41. 10.1016/j.metabol.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 8. Richter L, Norris S, Pettifor J, et al. Cohort profile: Mandela’s children: the 1990 birth to twenty study in South Africa. Int J Epidemiol 2007;36:504–11. 10.1093/ije/dym016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Unger T, Borghi C, Charchar F, et al. 2020 International Society of hypertension global hypertension practice guidelines. Hypertension 2020;75:1334–57. 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 10. World health organisation . Global physical activity questionnaire analysis guide. Available: https://www.who.int/ncds/surveillance/steps/GPAQ/en/ [Accessed 30 Oct 2020].
- 11. American diabetes association . Standards of medical care in diabetes-2021. Diabetes Care 2021;44(Suppl 1):S73–84. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization . Diagnosis and management of type 2 diabetes (HEARTS-D). (WHO/UCN/NCD/20.1). Licence: CC BY-NC-SA 3.0 IGO. 2020. doi:Geneva [Google Scholar]
- 13. United Nations Department of Economic and Social Affairs . World Population Prospects. 2019. Available: https://population.un.org/wpp/DataQuery/ [Google Scholar]
- 14. Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res 2006;15:547–69. 10.1177/0962280206070621 [DOI] [PubMed] [Google Scholar]
- 15. Mbanya JCN, Motala AA, Sobngwi E, et al. Diabetes in sub-Saharan Africa. Lancet 2010;375:2254–66. 10.1016/S0140-6736(10)60550-8 [DOI] [PubMed] [Google Scholar]
- 16. Pinchevsky Y, Butkow N, Raal FJ, et al. Demographic and clinical factors associated with development of type 2 diabetes: a review of the literature. Int J Gen Med 2020;13:121–9. 10.2147/IJGM.S226010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abubakari AR, Lauder W, Jones MC, et al. Prevalence and time trends in diabetes and physical inactivity among adult West African populations: the epidemic has arrived. Public Health 2009;123:602–14. 10.1016/j.puhe.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 18. Werfalli M, Engel ME, Musekiwa A, et al. The prevalence of type 2 diabetes among older people in Africa: a systematic review. Lancet Diabetes Endocrinol 2016;4:72–84. 10.1016/S2213-8587(15)00363-0 [DOI] [PubMed] [Google Scholar]
- 19. Bigna JJ, Nansseu JR, Katte J-C, et al. Prevalence of prediabetes and diabetes mellitus among adults residing in Cameroon: a systematic review and meta-analysis. Diabetes Research and Clinical Practice 2018;137:109–18. 10.1016/j.diabres.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 20. Asamoah-Boaheng M, Sarfo-Kantanka O, Tuffour AB, et al. Prevalence and risk factors for diabetes mellitus among adults in Ghana: a systematic review and meta-analysis. Int Health 2019;11:83–92. 10.1093/inthealth/ihy067 [DOI] [PubMed] [Google Scholar]
- 21. Gaziano TA, Abrahams-Gessel S, Gomez-Olive FX, et al. Cardiometabolic risk in a population of older adults with multiple co-morbidities in rural South Africa: the HAALSI (health and aging in Africa: longitudinal studies of indepth communities) study. BMC Public Health 2017;17:206. 10.1186/s12889-017-4117-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nonterah EA, Boua PR, Klipstein-Grobusch K, et al. Classical cardiovascular risk factors and HIV are associated with carotid intima-media thickness in adults from sub-Saharan Africa: findings from h3africa AWI-gen study. J Am Heart Assoc 2019;8:e011506. 10.1161/JAHA.118.011506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dessie G, Mulugeta H, Amare D, et al. A systematic analysis on prevalence and sub-regional distribution of undiagnosed diabetes mellitus among adults in African countries. J Diabetes Metab Disord 2020;19:1931–41. 10.1007/s40200-020-00635-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manne-Goehler J, Atun R, Stokes A, et al. Diabetes diagnosis and care in sub-Saharan Africa: pooled analysis of individual data from 12 countries. Lancet Diabetes Endocrinol 2016;4:903–12. 10.1016/S2213-8587(16)30181-4 [DOI] [PubMed] [Google Scholar]
- 25. Manne-Goehler J, Montana L, Gómez-Olivé FX, et al. The art advantage: health care utilization for diabetes and hypertension in rural South Africa. J Acquir Immune Defic Syndr 2017;75:561–7. 10.1097/QAI.0000000000001445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gebreyohannes EA, Netere AK, Belachew SA. Glycemic control among diabetic patients in Ethiopia: a systematic review and meta-analysis. PLoS One 2019;14:e0221790. 10.1371/journal.pone.0221790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jagannathan R, DuBose CW, Mabundo LS, et al. The ogtt is highly reproducible in Africans for the diagnosis of diabetes: implications for treatment and protocol design. Diabetes Res Clin Pract 2020;170:108523. 10.1016/j.diabres.2020.108523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wade AN, Crowther NJ, Abrahams-Gessel S, et al. Concordance between fasting plasma glucose and HbA1c in the diagnosis of diabetes in black South African adults: a cross-sectional study. BMJ Open 2021;11:e046060. 10.1136/bmjopen-2020-046060 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069193supp001.pdf (171KB, pdf)
Data Availability Statement
Data are available upon reasonable request. De-identified individual participant data from the AWI-Gen study are available from the European Genome-Phenome Archive (EGA) at study number EGA00001002482 (https://ega459archive.org/datasets/EGAD00001006425).