Abstract
A left visual field (LVF) bias in perceptual judgments, response speed, and discrimination accuracy has been reported in humans. Cognitive factors, such as visual spatial attention, are known to modulate or even eliminate this bias. We investigated this problem by recording pupillometry together with functional magnetic resonance imaging (fMRI) in a cued visual spatial attention task. We observed that (i) the pupil was significantly more dilated following attend-right than attend-left cues, (ii) the task performance (e.g. reaction time [RT]) did not differ between attend-left and attend-right trials, and (iii) the difference in cue-related pupil dilation between attend-left and attend-right trials was inversely related to the corresponding difference in RT. Neuroscientically, correlating the difference in cue-related pupil dilation with the corresponding cue-related fMRI difference yielded activations primarily in the right hemisphere, including the right intraparietal sulcus and the right ventrolateral prefrontal cortex. These results suggest that (i) there is an asymmetry in visual spatial attention control, with the rightward attention control being more effortful than the leftward attention control, (ii) this asymmetry underlies the reduction or the elimination of the LVF bias, and (iii) the components of the attentional control networks in the right hemisphere are likely part of the neural substrate of the observed asymmetry in attentional control.
Keywords: left visual field bias, pseudoneglect, spatial attention, attentional cuing, pupillometry
Introduction
In humans, most models of visual spatial attention hold that the left and right hemispheres of the human brain differ in their contributions to the control of attention (Kinsbourne 1970; Heilman and Van Den Abell 1980; Mesulam 1981). Evidence in support of such models comes from studies in patients with unilateral brain damage, where right hemisphere lesions lead to more severe neglect of contralateral left visual field (LVF) stimuli than do left hemisphere lesions for right visual field stimuli (Mesulam 1981; Heilman et al. 1987; Beis et al. 2004; Becker and Karnath 2007; Duecker and Sack 2015), and studies in commissurotomy patients, where the 2 cerebral hemispheres are disconnected at the cortical level and can be investigated separately, revealing differences in left and right hemisphere attention performance (Mangun et al. 1994; Kingstone et al. 1995). In healthy individuals, functional brain imaging and neurophysiological recordings have also shown differences in brain activity between the 2 hemispheres in attention tasks (Jansen et al. 2004; Corbetta and Shulman 2011; Gallotto et al. 2020).
A classic method to assess asymmetry in attention is the line bisection task. Participants are asked to place a marker at the perceived center of a horizontal line drawn on a sheet of paper. Patients with right hemisphere lesions tend to place the bisection marker to the right of the horizontal line’s center, as though they were neglecting part of the left side of the line and thence misjudging the midpoint. Interestingly, however, when neurologically intact participants are asked to perform the line bisection task, Bowers and Heilman (1980) observed the opposite tendency: They tended to place the bisection marker to the left of the midpoint of the horizontal line, as though the healthy subjects were neglecting the right visual field portions of the line and hence misjudging the midpoint as being to the left of the line’s true center. This naturally occurring attentional asymmetry, where the left hemispace is favored over the right by healthy neurologically intact individuals, is referred to as the LVF bias, or pseudoneglect, and is thought to reflect the right hemisphere dominance in spatial attention (Heilman and Van Den Abell 1980; Reuter-Lorenz et al. 1990; Çiçek et al. 2009; Benwell et al. 2014).
The LVF bias has been revealed in many different experimental settings. In visual search, when instructed to identify and respond to target objects among distracting items in arrays that are spread across visual space, the participant often begins the exploration of the visual scene on the left side (Bartolomeo et al. 1994; Gigliotta et al. 2017). In the computer version of the line bisection task, when a prebisected line is shown, the participant often indicates the left segment to be longer when the bisection marker is actually at the midpoint (Rueckert and McFadden 2004; Dufour et al. 2007; Thomas and Elias 2011). In rapid serial visual search (RSVP) tasks, when asked to detect targets in a rapid sequence of stimuli presented at single locations in either the left or the right visual field, the participant often performs better for the left visual than the right visual field RSVP task (Holländer et al. 2005; Śmigasiewicz et al. 2010, 2014, 2016; Verleger et al. 2013).
Interestingly, the LVF bias is diminished or absent when the stimulus presentation is preceded by cues that direct attention to the visual hemifield of the upcoming visual stimulus (Gitelman et al. 1999; Giesbrecht et al. 2003, 2006; McCourt et al. 2005; Wilson et al. 2005; Corbetta et al. 2008). In a dual-stream RSVP task, Śmigasiewicz et al. (2016) compared the responses to left-visual field stimuli versus right-visual field stimuli with or without having instructional cueing preceding the stimuli. In the absence of cueing, there is evidence of a LVF bias, which is consistent with prior studies (Holländer et al. 2005; Verleger et al. 2009; Śmigasiewicz et al. 2010). With cueing, however, the difference in behavioral performance between attended right stimuli and attended left stimuli was reduced. The mechanism underlying the reduction of the LVF bias in performance with attention cuing remains to be understood. We posit that the reduction of the LVF bias results from an asymmetry in the allocation of spatial attention (i.e. in attentional control) triggered by attentional cuing. Specifically, we hypothesize that directing attention to the right visual field requires more effort than directing it to the left, and the increased attention effort toward the right visual field underlies the reduction or the elimination of the LVF bias in subsequent target stimulus processing through a mechanism akin to neural compensation (Kahneman 1973; Hockey 1997; Wang et al. 2016).
To test the hypothesis, measures beyond the traditional reaction time (RT) and response accuracy are needed because these behavioral measures reflect the cumulative effects of both attentional control (cue-related) and attentional selection (target-related) rather than attentional control alone. Pupillometry offers a potential solution. Past work has shown that pupil diameter is a reliable physiological index of effort (Kahneman and Beatty 1966; Beatty 1982; Ebitz and Moore 2018) and can be used to index the state of attention and other task parameters (Hoeks and Levelt 1993; Mathôt et al. 2013; Kang et al. 2014; Binda and Murray 2015; Liao et al. 2016; Irons et al. 2017; Wainstein et al. 2017). For example, Irons et al. (2017) showed that attentional cues (both auditory and visual) elicit pupil dilation, and this increase in pupil size reflects task difficulty; more difficult task conditions elicit larger pupil dilations. If directing and maintaining covert visual spatial attention to the right visual field requires more attentional effort, as we hypothesized, one would expect that the pupil be more dilated following attend-right cues than attend-left cues, and the larger the difference in pupil dilation, the smaller the LVF bias in subsequent target stimulus processing.
We recorded behavioral and pupillometry data from participants performing a cued visual spatial attention task. To avoid the confounding influences from the pupil’s reflexive responses to visual stimulation, we used auditory cues instead of visual cues, thereby ensuring that cue-related pupillary responses were entirely attributable to internally generated attentional processes and not related to changes in the visual environment. In addition, functional magnetic resonance imaging (fMRI) data were collected along with behavioral and pupillometry data, with the goal being to use the multimodal data to examine the neural basis of the hypothesized differential pupil responses to left versus right attention cuing.
Materials and methods
Overview
The experimental protocol was approved by the Institutional Review Board (IRB) of the University of Florida. Twenty (5 females; mean age = 24.65 ± 2.87) right-handed healthy individuals provided written informed consent and took part in the study. The participants reported no prior history of mental disorders and had normal or correct-to-normal vision. The data from this experiment have been used in previous publications to investigate different questions (Rajan et al. 2019, 2021; Meyyappan et al. 2021).
Procedure
Two sets of dots, 3.6° lateral to the upper left and upper right of the fixation cross, indicated the 2 locations where the stimuli would appear. See Fig. 1 for illustration. At the beginning of each trial, an auditory cue (500 ms) instructed the participants to covertly direct their attention to either a spatial location (“left” or “right”) or a color (“red” or “green”) while fixating the central plus sign. On 80% of such trials, following a random delay period ranging from 3,000 ms to 6,600 ms, 2 colored rectangles (red or green) were presented for a duration of 200 ms, with 1 in each of the 2 peripheral locations. For the remaining 20% of the trials, the cues were not followed by the stimuli (cue-only trials), and these cue-only trials were included to help with better modeling of and differentiation between cue-evoked versus target-evoked blood oxygen level–dependent (BOLD) activities. Following the presentation of the stimuli, the subject’s task was to report the orientation of the rectangle (target) appearing at the cued location or having the cued color and to ignore the other rectangle (distractor). For color trials, the 2 rectangles displayed were always of the opposite color; for spatial trials, the 2 rectangles were either of the same color or the opposite color. On 8% of the trials (invalid trials), only 1 rectangle was displayed, which was either not in the cued location for spatial trials or not having the cued color for color trials, and the participants were required to report the orientation of that rectangle. These invalidly cued trials were included to measure the behavioral benefits of attentional cuing (Posner 1980). An intertrial interval, which was varied randomly from 8,000 ms to 12,800 ms following the target onset, elapsed before the start of the next trial. In addition to spatial and color cues (80% of all the trials), there was a third type of cue (20% of all the trials) consisting of the word “none,” which informed the subject to prepare to respond to the orientation of the rectangle being placed on a gray patch (neutral attention). Trials were organized into blocks, with each block consisting of 25 trials and lasting approximately 7 minutes. Each participant completed 10–14 blocks over 2 days. For this study, given the stated purpose of investigating the top-down control of the LVF bias in visual spatial attention, only spatial trials were considered.
Fig. 1.
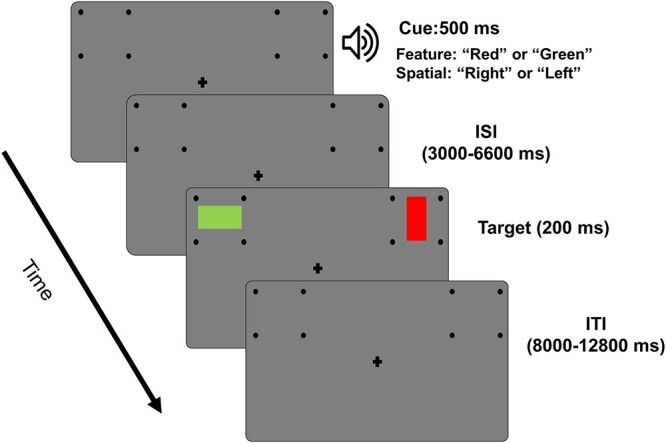
Experimental paradigm. Each trial starts with an auditory cue (500 ms) instructing the subject to covertly attend to a spatial location (“left” or “right”) or to a color (“red” or “green”). Following a variable cue-to-target delay (3,000–6,600 ms), 2 colored rectangles were displayed (200 ms), 1 in each of the 2 peripheral locations. Participants were asked to report the orientation of the rectangle (horizontal or vertical) displayed in the cued location or having the cued color. On some of the trials (8%), the cues were not valid, i.e. only 1 target appeared, which was either not at the cued location or not having the cued color, and participants were required to report the orientation of the rectangle. An intertrial interval, varied randomly from 8,000 to 12,800 ms following the target onset, elapsed before the start of the next trial.
The participants went through a training session prior to scanning in which they were introduced to the task and became comfortable performing it. Since the study required participants to maintain central fixation for long durations and pay covert attention to the periphery while fixating the central plus sign, they were screened based on their ability to maintain eye fixation throughout the training session. An SR Research EyeLink 1000 eye tracker system was used for that purpose. In addition, participants who attained behavioral accuracy above 70% at the end of the training session proceeded to fMRI-pupillometry recordings.
fMRI data acquisition and preprocessing
fMRI data were collected on a 3T Philips Achieva scanner with a 32-channel head coil (Philips Medical Systems, the Netherlands). The echo-planar imaging (EPI) sequence parameters were: time repetition, 1.98 s; echo time, 30 ms; flip angle, 80°; field of view, 224 mm; slice number, 36; voxel size, 3.5 mm × 3.5 mm × 3.5 mm; matrix size, 64 × 64. The slices were oriented parallel to the plane connecting the anterior and posterior commissures.
The fMRI BOLD data were preprocessed using the Statistical Parametric Mapping toolbox (SPM-12) as well as custom scripts written in MATLAB. Preprocessing steps included slice timing correction, realignment, spatial normalization, and smoothing. Slice timing correction was carried out using sinc interpolation to correct for differences in slice acquisition time within an EPI volume. The images were then spatially realigned to the first image of each session by a 6-parameter rigid-body spatial transformation to account for head movement during acquisition. Each participant’s images were then normalized and registered to the Montreal Neurological Institute space. All images were further resampled to a voxel size of 3 mm × 3 mm × 3 mm and were spatially smoothed using a Gaussian kernel with 7-mm full width at half maximum. Slow temporal drifts in the baseline were removed by applying a high-pass filter with a cutoff frequency set at 1/128 Hz.
General linear model analysis of cue-evoked response
The general linear model (GLM) method, as implemented in the SPM toolbox, was used to analyze the BOLD responses to cues. Eight task-related events were included in the GLM analysis as regressors. Five of them were used to model the cue-related BOLD activity; only trials with correct responses were included. We used 2 additional regressors to account for the BOLD responses evoked by target stimuli; 1 for valid and 1 for invalidly cued targets; recall that the trials could be either validly cued or invalidly cued, and the subject was expected to respond to both. Finally, 1 regressor was used to model the trials with incorrect responses. The hemodynamic response function (HRF) used in the GLM analysis was the default HRF in the SPM toolbox where the delay was 6 s. At the group level, cue-evoked fMRI activations were obtained by applying a parametric 1-sample t-test and thresholding the results at P < 0.05 after correcting for multiple comparisons using the false discovery rate (FDR) method.
Estimating single-trial BOLD data
A beta-series regression method (Rissman et al. 2004) was used to estimate the BOLD response on each trial in every voxel. In this method, cues and targets in trials with correct responses were assigned individual regressors and 1 regressor was assigned for all the trials with incorrect responses. The regressors were modeled in the conventional GLM framework using custom MATLAB scripts developed within the SPM toolbox.
Pupillometry data acquisition and preprocessing
Pupil diameter data were collected simultaneously with fMRI data using an MR-compatible eye tracker at a sampling rate of 1,000 Hz (EyeLink 1000, SR Research). During preprocessing, eye blinks were detected, and the pupil diameter during eye blinks was determined by a linear interpolation algorithm (Bradley et al. 2008; Siegel et al. 2008). For cue-related analysis, the continuous pupil data were epoched from 200 ms before cue onset to 3,000 ms after cue onset (−200 ms to 3,000 ms). The pre-cue (−200 ms to 0 ms) period was used as a baseline to compute the percentage change of cue-related pupil dilation.
Cue-evoked pupillary response
To detect whether the pupil diameter time courses following attend-left cues and attend-right cues differed, we applied a nonparametric permutation method (Maris and Oostenveld 2007), which included the following steps. (i) For every subject, attend-left and attend-right labels from (all the runs) were randomly shuffled to yield surrogate datasets. (ii) The pupil diameter within the surrogate attend-left and attend-right conditions were averaged, and a mean surrogate-left and surrogate-right pupil diameter were computed. (iii) A paired t-test was performed between mean surrogate-right and surrogate-left pupil diameters. (iv) The resulting P-values as a function of time were analyzed, the contiguous time periods in which the difference was significantly different at P < 0.05 was determined, and the duration, denoted x, of each of these time periods was recorded. (v) Steps (i)–(iv) were repeated 105 times to yield an empirical distribution of x. (vi) For the actual data, the time periods (or clusters) in which the pupil diameter difference between attend-left and attend-right trials was significantly different at P < 0.05 were determined, and the duration of each such cluster was compared to the empirical distribution to determine its significance. (vii) Clusters whose empirical P-value as determined in Step (vi) which were <P = 0.0001 were considered to be statistically significant.
Pupil versus behavior
Recall that the experiment consisted of multiple blocks (~12 blocks per participant), also known as runs. The natural occurrence of behavioral fluctuations across these runs provided the opportunity to relate pupil dynamics and behavioral performance in an intrasubject manner. Specifically, the RT difference between attend-right and attend-left trials and the corresponding average cue-evoked pupil diameter differences were computed for each run. The runs were then sorted based on the RT difference and split (median) into low RT difference runs and high RT difference runs. The pupil differences in each of the 2 groups of runs was computed within each subject and averaged across subjects. A paired t-test was then used to compare the pupil dilation differences estimates between the low and high RT difference runs.
Pupil-BOLD coupling
The trial-by-trial fMRI beta-series data for the attend-right and attend-left trials were subjected to a 2-sampled t-test to yield a voxel-wise t-statistic for all the subjects. This voxel-wise estimate of fMRI activation difference was then correlated with the averaged pupil dilation difference between the attend-right and attend-left, across subjects, using the Pearson correlation technique. The P-values from the correlation analysis were subjected to cluster thresholding; clusters containing >200 continuous voxels that were significantly coupled with pupil size (P < 0.05) were then extracted to yield the brain maps. The cluster threshold of 200 was determined using Monte Carlo simulations (Slotnick et al. 2003; Slotnick 2017). The simulations were run 10,000 times, and acquisition parameters, such as the number of slices, voxel, and matrix dimensions (X and Y) as well as the kernel width for spatial smoothing, were used in the process.
Results
Behavioral analysis
RT for attend-left and attend-right trials were 998 ± 150 ms and 1,037 ± 169 ms, respectively, with RT for attend-right trials marginally longer than that for attend-left trials (P < 0.09); see Fig. 2A. The response accuracy (percentage of correct trials) was comparable between the 2 attention conditions (attend-left: 94.0 ± 1.2, attend-right: 93.9 ± 1.2; P < 0.95; see Fig. 2B). These results are consistent with previous reports that, in cued visual spatial attention paradigms, there is no obvious LVF bias in behavioral performance (Giesbrecht et al. 2003; Liu et al. 2016).
Fig. 2.
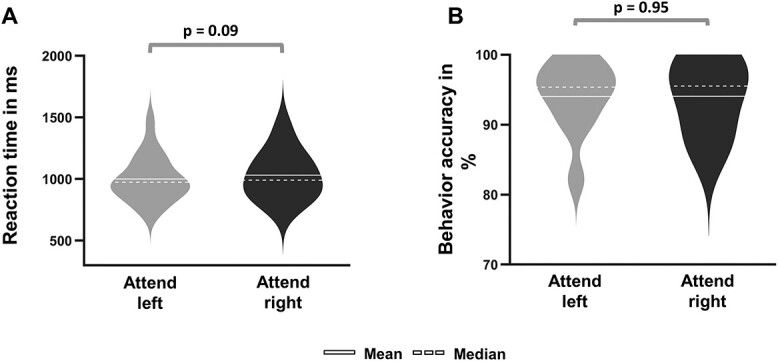
Comparison of behavioral results between spatial attention conditions (attend-right versus attend-left). A) RT for attend-right trials was marginally longer than attend-left trials (P = 0.09). B) Accuracy was not different between the 2 types of trials (P = 0.95).
Cue-evoked pupillary response
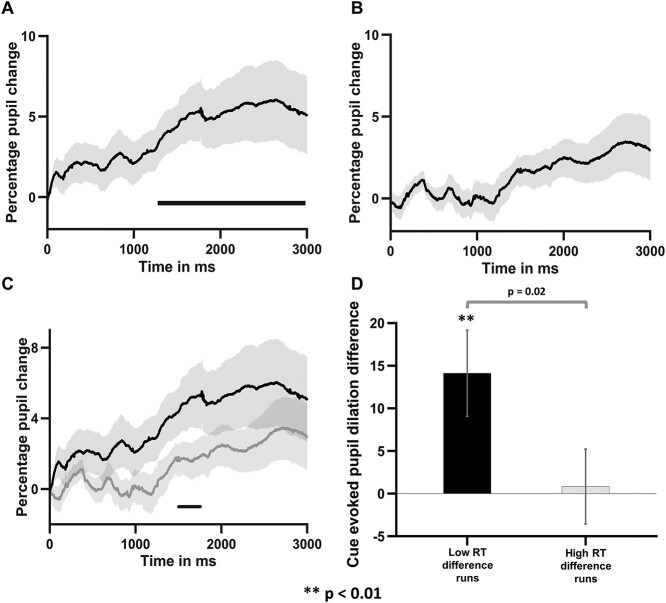
As shown in Fig. 3A, starting from 1,270 ms until the end of the time interval investigated (i.e. 3,000 ms pos-tcue), pupil dilation evoked by attend-right cues was significantly larger than that evoked by attend-left cues (attend-right > attend-left; cluster significance at P = 0.0001). At the population level, the averaged pupil dilation for attend-right was significantly larger than that for attend-left trials (attend-right > attend-left, P < 0.002); Figure 3B shows the individual subject data for pupil dilation averaged over the interval 1,270–3,000 ms for the attend-left and attend-right trials separately. These results support the hypothesis that the LVF is favored over the right visual field when deploying covert attention in advance of stimulus processing and more effort is required to attend to the right visual field.
Fig. 3.
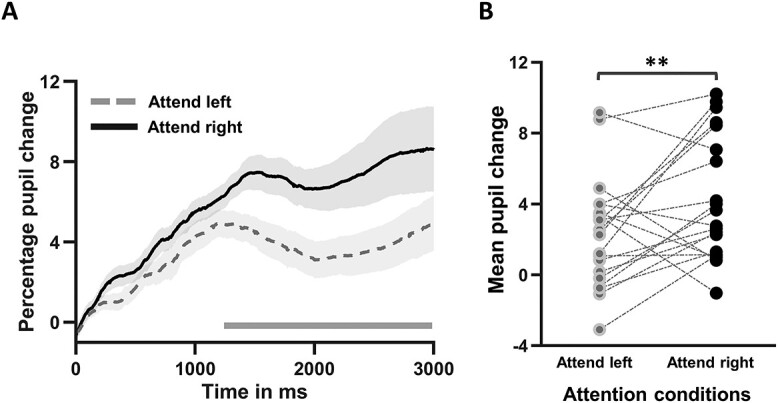
Cue-evoked pupillary response during spatial attention trials. A) Time course of pupil dilation measured by the percentage change relative to pre-cue baseline for attend-left and attend-right trials. The horizontal bar indicates the time window in which the pupil difference between attend-left and attend-right trials were significantly different (P < 0.0008; random permutation corrected). B) Subject-wise comparison of pupil dilation evoked by attend-left cue and attend-right cue averaged over the 1,270–3,000 ms interval. **P < 0.0002. The shaded regions around the pupil dilation timecourses denote SEM.
Cue-evoked pupillary response versus behavior
The increased attentional effort associated with initiating and maintaining anticipatory attention to the right visual field was hypothesized to underlie the reduction or elimination of the LVF bias in stimulus processing. To test this, we examined the relationship between pupil dilation and RT using an intrasubject analysis, which leverages the natural fluctuations of behavioral performance across different experimental runs. Specifically, for each run, we computed the RT difference between attend-right and attend-left trials. Based on this difference, the runs were divided into high RT difference runs and low RT difference runs. We then compared the corresponding pupil dilation differences between the 2 groups of runs.
As shown in Fig. 4A and B, for the low RT difference runs (Fig. 4A), the pupil dilation difference between the attend-right trials and attend-left trials was significantly >0 in the time period 1,300–3,000 ms pos-tcue onset (attend-right > attend-left), demonstrating that, when there was a greater attentional effort associated with attend-right trials (pupil difference being high), the LVF bias in stimulus processing was diminished (RT difference being low). By contrast, for the high RT difference runs (Fig. 4B), the pupil difference between the attend-right and attend-left trials were not significantly >0 for the entire duration of 0–3,000 ms, demonstrating that, when attentional effort during the cue-target interval was approximately equal between attend-left versus attend-right trials (pupil difference being low), there was a LVF bias in stimulus processing (RT difference being high). Figure 4C further shows that the pupil dilation for low RT difference runs were higher than high RT difference runs over the time period 1,550–1,800 ms post-cue (P < 0.002, random permutation corrected). In Fig. 4D, the averaged pupil dilation difference was shown to be significantly higher for low RT difference runs than for high RT difference runs (P < 0.02). These results support the hypothesis that the increased attentional effort associated with initiating and maintaining covert attention to the right visual field helps to reduce or eliminate the LVF bias in stimulus processing and equalize performance between left and right visual fields.
Fig. 4.
Relationship between cue-evoked pupil dilation and behavior. A) Time course of pupil dilation difference between attend-right and attend-left trials for the low RT difference runs. B) Time course of pupil dilation difference between attend-right and attend-left trials for the high RT difference runs. C) Comparison of cue-evoked pupil dilation difference between low and high RT difference groups. The horizontal bar indicates statistically significant time period (P < 0.002, random permutation corrected). D) Comparison of average cue-evoked pupil dilation difference for low and high RT difference runs. The shaded regions around the pupil dilation time courses denote SEM.
Cue-evoked BOLD activation
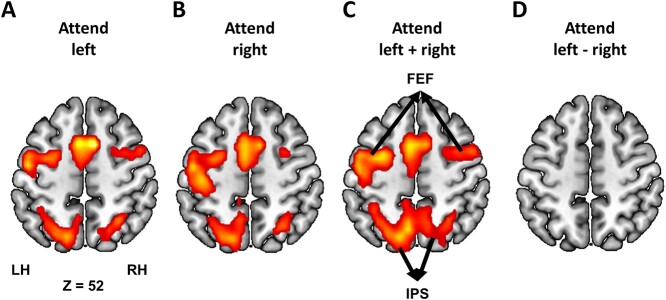
Neural responses to the cue were analyzed using the GLM method. Both attend-left and attend-right cues evoked strong bilateral activations in the dorsal attention network (DAN), consisting of FEF and SPL/intraparietal sulcus (IPS; P < 0.05, FDR-corrected; Fig. 5A–C); this is in line with the prior attention control literature (Corbetta et al. 2000; Hopfinger et al. 2000; Giesbrecht et al. 2003). Contrasting cue-left and cue-right resulted in no regions of activation (Fig. 5D), suggesting that the cue-related attention control activity is symmetric regardless of whether attention is directed (covertly) to the left or the right visual field.
Fig. 5.
Cue-evoked BOLD activation (P < 0.05, FDR). Bilateral DAN structures were activated by A) attend-left cues, B) attend-right cues, and C) attend left + right cues. D) No activation was found when attend-left cue was contrasted against attend-right cue.
Neural substrate of differential cue-evoked pupillary response
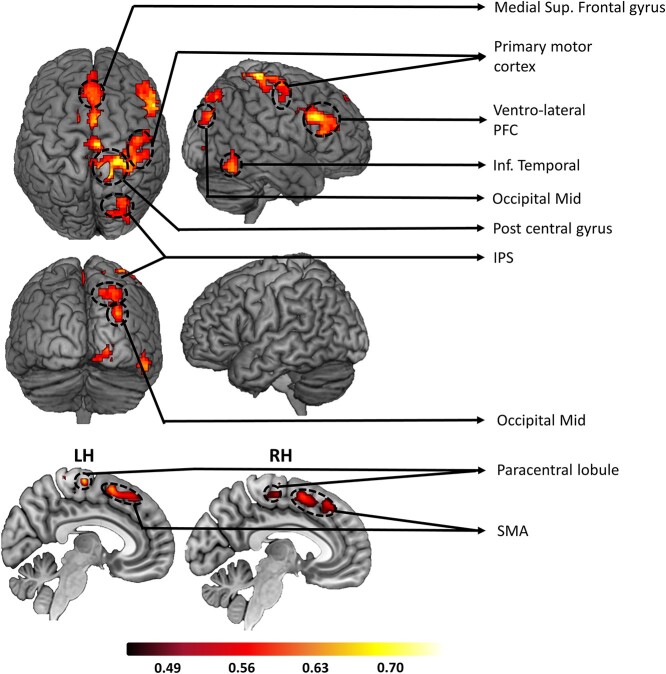
The LVF bias in stimulus processing is thought to result from the dominant role played by the right hemisphere in spatial attention (Bowers and Heilman 1980; Benwell et al. 2014). In line with this framework, we expected the differential pupil response to attention-directing cues to be mediated by the right hemisphere brain structures compared to left in the cue-to-target interval. To test the hypothesis, the pupil difference between the attend-left and attend-right in the 1,270–3,000-ms interval post-cue and the corresponding BOLD activity difference were computed at the individual subject level and then correlated across subjects for all voxels. Voxels showing significant positive correlation at P < 0.05 and being part of a cluster containing ≥200 contiguous such voxels are shown in Fig. 6. Here, the cluster threshold of 200 voxels was determined based on Monte-Carlo simulations (Slotnick et al. 2003; Slotnick 2017). As expected, more regions in the right hemisphere were positively correlated with pupil dilation difference, including the right IPS of the DAN and the right ventrolateral prefrontal cortex (VLPFC) of the ventral attention network (VAN). See Table 1 for a complete list of activated areas in the pupil-BOLD correlation analysis.
Fig. 6.
Pupil-BOLD coupling. Regions showing positive coupling between cue-evoked pupil dilation difference between attend-right and attend-left trials and the corresponding cue-evoked fMRI activation difference. These across-subject correlation maps were thresholded at P < 0.05 (clusters containing >200 voxels). Warmer colors reflect stronger correlation.
Table 1.
Regions where differential BOLD activities (attend right - attend left) are positively coupled with cue-evoked pupil dilation difference (attend right - attend left). P < 0.05 (cluster corrected; k = 200).
| Left hemisphere | Right Hemisphere |
|---|---|
| Medial superior frontal gyrus | Medial superior frontal gyrus |
| Supplementary motor cortex (SMA) | Supplementary motor cortex (SMA) |
| Paracentral lobule | Intra parietal sulcus (IPS; DAN) |
| Primary visual cortex | |
| Occipital Mid | |
| Inferior temporal cortex | |
| Ventro-lateral prefrontal cortex (VLPFC; VAN) | |
| Primary motor cortex | |
| Postcentral gyrus |
Discussion
We investigated whether the visual spatial attention control modulated the LVF bias in stimulus processing in healthy human volunteers. Pupillometry was combined with fMRI to provide measures of both attentional effort and the underlying brain networks involved in leftward versus rightward attentional orienting during anticipatory attention. Analogous to the LVF bias in stimulus processing, we posited that there is a LVF bias in attentional control, with attention to the right visual field being more effortful than directing it to the left, and the result of this increased attentional control effort for rightward attention would be to reduce the LVF bias (pseudoneglect) in subsequent target processing.
In support of our hypothesis, attend-right cues evoked a larger dilation of the pupil than attend-left cues, demonstrating that there is an inherent asymmetry in the effort required for the control of leftward versus rightward visual spatial attention. In addition, trials with larger pupil dilation differences in the cue-target interval were associated with smaller RT differences time-locked to the appearance of the target between attend-right and attend-left trials. Finally, combining fMRI and pupillometry data, the difference in attentional control effort in the cue-to-target interval between the 2 types of attention trials was found to be mediated mainly by structures in the right hemisphere, including the right IPS of the DAN and the right VLPFC of the VAN.
LVF bias in stimulus processing and attention control
The LVF bias in stimulus processing (pseudoneglect) has been demonstrated since the early 1980s, including in such paradigms as the line bisection task, visual search, and tasks involving the processing of lateralized stimuli. It is thought to be the consequence of the dominant role played by the right hemisphere in spatial attention. Interestingly, when the lateralized visual stimulus is preceded by an attention-directing cue, the LVF bias is often reduced or not observed (Reuter-Lorenz et al. 1990; Hopfinger et al. 2000; Giesbrecht et al. 2003; Śmigasiewicz et al. 2014; Liu et al. 2016). In our data, this general finding was replicated, where neither accuracy nor RTs were significantly different between attend-left and attend-right trials.
What may underlie the cue-related reduction/elimination of the LVF bias in stimulus processing? Prior explanations emphasized the informativeness of cues in reducing the spatial uncertainty of target locations (Śmigasiewicz et al. 2016). We proposed that (i) there is an inherent asymmetry in attentional control, with leftward attentional control being favored over the rightward attentional control, and (ii) this asymmetry in attentional control underlies the reduction or elimination of the LVF bias in subsequent stimulus processing through a compensatory neural mechanism by altering the cognitive effort (Kahneman 1973; Hockey 1997).
We tested the idea using pupillometry (Kahneman and Beatty 1966; Peavler 1974; Kang et al. 2014; Querino et al. 2015). The pupil diameter was found to increase following both types of cues, but importantly, attend-right cues evoked significantly larger pupil dilation compared to attend-left cues, thereby establishing the hypothesized asymmetry in attention control. The difference in pupil dilation, in light of the extensive literature linking pupil size to cognitive effort (Hess and Polt 1964; Kahneman and Beatty 1966; Brocher and Graf 2017; Brocher et al. 2018; van der Wel and van Steenbergen 2018), is compatible with the interpretation that attending the right visual field is more effortful than attending the left visual field; in other words, leftward attention control is favored over rightward attention control. In addition, the inverse relationship between pupil dilation difference and RT difference, meaning that a larger pupil dilation difference is associated with a smaller RT difference, suggests that increased attentional effort associated with rightward attention helps to compensate for the inherent deficit in the right visual field processing and equalize performance.
Neural substrate of asymmetric pupil dilation
Visualspatial attention is controlled by 2 major brain networks: the DAN, comprising bilateral FEF and IPS, is involved in top-down attention control, whereas the right-lateralized VAN, comprising right TPJ and right VLPFC, is involved in attentional reorienting (Corbetta and Shulman 2002). In particular, the DAN, when activated by attention-directing cues, maintains the attentional set and issues control signals to bias sensory processing. In our data, the DAN is symmetrically activated by both attend-left and attend-right cues, which is consistent with the majority of the prior literature (Corbetta et al. 2000; Hopfinger et al. 2000; Giesbrecht et al. 2003; Wilson et al. 2005; Shulman et al. 2010). By contrast, a set of primarily right-lateralized brain regions, including the right IPS of the DAN and the right VLPFC of the VAN, was revealed when the pupil dilation difference between attend-right and attend-left was correlated with the corresponding difference in fMRI activation. This result is similar to those found in the pseudoneglect literature where both VAN and the right DAN have been implicated in mediating the LVF bias in stimulus processing (Fink et al. 2001; Foxe et al. 2003; Çiçek et al. 2009; Benwell et al. 2014). In particular, contrasting the line bisection condition against a control condition, Çiçek et al. (2009) (see also, Fink et al. 2001) found right-lateralization activations in areas such as the right IPS and the right VLPFC. We thus suggest that the observed cue-related asymmetric pupil dilation shares common neural substrate with pseudoneglect in stimulus processing.
In addition to DAN and VAN regions, other brain regions, including the lingual gyrus and occipital cortex, were also found in the pupil-BOLD correlation map, which is consistent with reports in other pupil-BOLD coupling studies (Murphy et al. 2014; Yellin et al. 2015). For example, Murphy et al. (2014) coupled the spontaneous fluctuations in pupil diameter with fMRI activity in resting state, and the task-induced pupil dilation with the corresponding BOLD activity in an oddball task, and found occipital and lingual regions in both correlation maps. These regions might form a generic core network involved in modulating pupil size along with attentional control regions such as DAN and VAN. The nature of the interaction among these regions, however, remains unclear and should be a topic of future investigations.
Limitations and other considerations
First, in this study, we were not able to demonstrate the presence of a LVF bias in the absence of spatial cuing. In order to do so, one could examine the color trials where no spatial cuing occurred. To do this, we conducted a new behavioral control experiment using the same design as the one adopted here along with pupillometry. As shown in Supplementary Material, for color trials, RT was found to be significantly shorter for attended targets appearing in the left than the right visual field (Supplementary Fig. S1A), demonstrating a LVF bias in the absence of spatial cuing. For spatial trials, RT was equalized between attend-left and attend-right trials (Supplementary Fig. S1B), and the pupil was significantly more dilated following attend-right cues than attend-left cues (Supplementary Fig. S1C), thus replicating the main findings of the present study. Second, pupillary size can be affected by saccadic activity (Mathot et al. 2015). While we instructed participants to fixate and used auditory cues to minimize eye movements (Laubrock et al. 2010), it is still possible that systematic differences in eye movements exist between attend-right and attend-left trials, which in turn may impact the cue-related pupil analysis. We examined this issue using multivariate pattern analysis. As shown in Supplementary Material, eye positions (x and y coordinates) during the cue-to-target interval were subjected to MVPA decoding analysis (attend-left vs. attend-right) (Supplementary Fig. S2), and the decoding accuracy was found to be at chance level, suggesting that no systematic differences in eye movements existed between attend-left and attend-right conditions. Third, experimental design and stimulus types can affect the existence and the magnitude of the LVF bias (Asanowicz et al. 2013; Bergerbest et al. 2017). For example, as reported in Bergerbest et al. (2017), when letters are used as stimuli, one may even observe a right visual field bias, which may reflect possibly the left hemisphere lateralization for language-related processing. Thus, the LVF bias can be modulated or reversed by other factors, such as asymmetries related to linguistic processing; in the present study, we avoided this confounding factor by using nonlinguistic stimuli. Finally, prior research has shown that perceptual asymmetries may exist in the 2 hemispheres (Asanowicz et al. 2017), raising the question of the role of such asymmetries in the current findings on pupil size. While, in the present work, we cannot know whether perceptual asymmetries give rise to attentional asymmetries, perhaps via compensatory mechanisms, we do know that in our cuing design, the pupil effects in the post-cue/pre-target period are correlated with attentional control. Whether or not these are triggered by standing perceptual asymmetries remains a question for further research.
Conclusion
In the current study, a LVF bias in anticipatory visual spatial attention was revealed, which manifested as increased dilation of the pupil following cues to covertly attend the right than the left visual hemifield. Due to an innate LVF bias in spatial attention, covertly orienting attention to the right is more demanding, leading to increased attentional effort that is reflected in the increased pupil size. The increased allocation of attentional effort compensates for the LVF bias in stimulus processing, which results in similar behavioral performance in subsequent target discrimination. Right hemisphere brain structures, including the right IPS of the DAN and the right VLPFC of the VAN, appear to be the neutral substrate mediating the asymmetric attentional effort.
Supplementary Material
Contributor Information
Sreenivasan Meyyappan, J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, Gainesville, FL 32611, USA; Center for Mind and Brain, University of California, Davis, CA 95618, USA.
Abhijit Rajan, J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, Gainesville, FL 32611, USA.
George R Mangun, Center for Mind and Brain, University of California, Davis, CA 95618, USA; Departments of Psychology and Neurology, University of California, Davis, CA 95616, USA.
Mingzhou Ding, J. Crayton Pruitt Family Department of Biomedical Engineering, University of Florida, Gainesville, FL 32611, USA.
Data availability
All data will be publicly available on the NIMH Data Archive.
Funding
This work was supported by National Institute of Mental Health (grant MH117991) to George R. Mangun and Mingzhou Ding.
Conflict of interest statement: The authors declare that there is no conflict of interest.
References
- Asanowicz D, Smigasiewicz K, Verleger R. Differences between visual hemifields in identifying rapidly presented target stimuli: letters and digits, faces, and shapes. Front Psychol. 2013:4:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanowicz D, Verleger R, Kruse L, Beier K, Smigasiewicz K. A right hemisphere advantage at early cortical stages of processing alphanumeric stimuli. Evidence from electrophysiology. Brain Cogn. 2017:113:40–55. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, D'Erme P, Gainotti G. The relationship between visuospatial and representational neglect. Neurology. 1994:44:1710–1714. [DOI] [PubMed] [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychol Bull. 1982:91:276–292. [PubMed] [Google Scholar]
- Becker E, Karnath H-O. Incidence of visual extinction after left versus right hemisphere stroke. Stroke. 2007:38:3172–3174. [DOI] [PubMed] [Google Scholar]
- Beis JM, Keller C, Morin N, Bartolomeo P, Bernati T, Chokron S, Leclercq M, Louis-Dreyfus A, Marchal F, Martin Y, et al. Right spatial neglect after left hemisphere stroke: qualitative and quantitative study. Neurology. 2004:63:1600–1605. [DOI] [PubMed] [Google Scholar]
- Benwell CSY, Harvey M, Thut G. On the neural origin of pseudoneglect: EEG-correlates of shifts in line bisection performance with manipulation of line length. NeuroImage. 2014:86:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergerbest D, Shilkrot O, Joseph M, Salti M. Right visual-field advantage in the attentional blink: asymmetry in attentional gating across time and space. Atten Percept Psychophys. 2017:79:1979–1992. [DOI] [PubMed] [Google Scholar]
- Binda P, Murray SO. Spatial attention increases the pupillary response to light changes. J Vis. 2015:15:1. [DOI] [PubMed] [Google Scholar]
- Bowers D, Heilman KM. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980:18:491–498. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008:45:602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocher A, Graf T. Decision-related factors in pupil old/new effects: attention, response execution, and false memory. Neuropsychologia. 2017:102:124–134. [DOI] [PubMed] [Google Scholar]
- Brocher A, Harbecke R, Graf T, Memmert D, Hüttermann S. Using task effort and pupil size to track covert shifts of visual attention independently of a pupillary light reflex. Behav Res Methods. 2018:50:2551–2567. [DOI] [PubMed] [Google Scholar]
- Çiçek M, Deouell LY, Knight RT. Brain activity during landmark and line bisection tasks. Front Hum Neurosci. 2009:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002:3:201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011:34:569–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000:3:292–297. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008:58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duecker F, Sack AT. The hybrid model of attentional control: new insights into hemispheric asymmetries inferred from TMS research. Neuropsychologia. 2015:74:21–29. [DOI] [PubMed] [Google Scholar]
- Dufour A, Touzalin P, Candas V. Time-on-task effect in pseudoneglect. Exp Brain Res. 2007:176:532–537. [DOI] [PubMed] [Google Scholar]
- Ebitz RB, Moore T. Both a gauge and a filter: cognitive modulations of pupil size. Front Neurol. 2018:9:1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Zilles K. The neural basis of vertical and horizontal line bisection judgments: an fMRI study of normal volunteers. NeuroImage. 2001:14:S59–S67. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, McCourt ME, Javitt DC. Right hemisphere control of visuospatial attention: line-bisection judgments evaluated with high-density electrical mapping and source analysis. NeuroImage. 2003:19:710–726. [DOI] [PubMed] [Google Scholar]
- Gallotto S, Duecker F, Oever ST, Schuhmann T, Graaf TA, Sack AT. Relating alpha power modulations to competing visuospatial attention theories. NeuroImage. 2020:207:116429. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. NeuroImage. 2003:19:496–512. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Weissman DH, Woldorff MG, Mangun GR. Pre-target activity in visual cortex predicts behavioral performance on spatial and feature attention tasks. Brain Res. 2006:1080:63–72. [DOI] [PubMed] [Google Scholar]
- Gigliotta O, Seidel Malkinson T, Miglino O, Bartolomeo P. Pseudoneglect in visual search: behavioral evidence and connectional constraints in simulated neural circuitry. eNeuro. 2017:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim Y-H, Meyer JR, Mesulam MM. A large-scale distributed network for covert spatial attention further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999:122:1093–1106. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Van Den Abell T. Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect). Neurology. 1980:30:327–330. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Valenstein E, Watson RT. Hemispace and hemispatial neglect. In: Jeannerod M, editors. Advances in psychology. North Holland: Advances in Psychology; 1987. pp. 115–150 [Google Scholar]
- Hess EH, Polt JM. Pupil Size in relation to mental activity during simple problem-solving. Science. 1964:143:1190–1192. [DOI] [PubMed] [Google Scholar]
- Hockey GR. Compensatory control in the regulation of human performance under stress and high workload; a cognitive-energetical framework. Biol Psychol. 1997:45:73–93. [DOI] [PubMed] [Google Scholar]
- Hoeks B, Levelt WJM. Pupillary dilation as a measure of attention: a quantitative system analysis. Behav Res Methods Instrum Comput. 1993:25:16–26. [Google Scholar]
- Holländer A, Corballis MC, Hamm JP. Visual-field asymmetry in dual-stream RSVP. Neuropsychologia. 2005:43:35–40. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000:3:284–291. [DOI] [PubMed] [Google Scholar]
- Irons JL, Jeon M, Leber AB. Pre-stimulus pupil dilation and the preparatory control of attention. PLoS One. 2017:12:e0188787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Floel A, Deppe M, Randenborgh J, Drager B, Kanowski M, Knecht S. Determining the hemispheric dominance of spatial attention: a comparison between fTCD and fMRI. Hum Brain Mapp. 2004:23:168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Attention and effort: Citeseer. New Jersey: Prentice-Hall; 1973.
- Kahneman D, Beatty J. Pupil diameter and load on memory. Science. 1966:154:1583–1585. [DOI] [PubMed] [Google Scholar]
- Kang OE, Huffer KE, Wheatley TP. Pupil dilation dynamics track attention to high-level information. PLoS One. 2014:9:e102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingstone A, Enns JT, Mangun GR, Gazzaniga MS. Guided visual search is a left-hemisphere process in split-brain patients. Psychol Sci. 1995:6:118–121. [Google Scholar]
- Kinsbourne M. The cerebral basis of lateral asymmetries in attention. Acta Psychol. 1970:33:193–201. [DOI] [PubMed] [Google Scholar]
- Laubrock J, Kliegl R, Rolfs M, Engbert R. When do microsaccades follow spatial attention? Atten Percept Psychophys. 2010:72:683–694. [DOI] [PubMed] [Google Scholar]
- Liao H-I, Yoneya M, Kidani S, Kashino M, Furukawa S. Human pupillary dilation response to deviant auditory stimuli: effects of stimulus properties and voluntary attention. Front Neurosci. 2016:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bengson J, Huang H, Mangun GR, Ding M. Top-down modulation of neural activity in anticipatory visual attention: control mechanisms revealed by simultaneous EEG-fMRI. Cereb Cortex. 2016:26:517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun GR, Luck SJ, Plager R, Loftus W, Hillyard SA, Handy T, Clark VP, Gazzaniga MS. Monitoring the visual world: hemispheric asymmetries and subcortical processes in attention. J Cogn Neurosci. 1994:6:267–275. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007:164:177–190. [DOI] [PubMed] [Google Scholar]
- Mathôt S, Linden L, Grainger J, Vitu F. The pupillary light response reveals the focus of covert visual attention. PLoS One. 2013:8(10):e78168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathot S, Melmi JB, Castet E. Intrasaccadic perception triggers pupillary constriction. PeerJ. 2015:3:e1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Reuter-Lorenz PA. Unilateral visual cueing and asymmetric line geometry share a common attentional origin in the modulation of pseudoneglect. Cortex. 2005:41:499–511. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981:10:309–325. [DOI] [PubMed] [Google Scholar]
- Meyyappan S, Rajan A, Mangun GR, Ding M. Role of inferior frontal junction (IFJ) in the control of feature versus spatial attention. J Neurosci. 2021:41:8065–8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, O'Connell RG, O'Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum Brain Mapp. 2014:35:4140–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavler WS. Pupil size, information overload, and performance differences. Psychophysiology. 1974:11:559–566. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980:32:3–25. [DOI] [PubMed] [Google Scholar]
- Querino E, Dos Santos L, Ginani G, Nicolau E, Miranda D, Romano-Silva M, Malloy-Diniz L. Cognitive effort and pupil dilation in controlled and automatic processes. Transl Neurosci. 2015:6:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Siegel SN, Liu Y, Bengson J, Mangun GR, Ding M. Theta oscillations index frontal decision-making and mediate reciprocal frontal-parietal interactions in willed attention. Cereb Cortex. 2019:29:2832–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Meyyappan S, Liu Y, Samuel IBH, Nandi B, Mangun GR, Ding M. The microstructure of attentional control in the dorsal attention network. J Cogn Neurosci. 2021:33:965–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Kinsbourne M, Moscovitch M. Hemispheric control of spatial attention. Brain Cogn. 1990:12:240–266. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004:23:752–763. [DOI] [PubMed] [Google Scholar]
- Rueckert L, McFadden HG. Context effects in pseudo-neglect measured with a free vision Landmark task. Laterality. 2004:9:421–432. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Pope DLW, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 2010:30:3640–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron. 2008:60:709–719. [DOI] [PubMed] [Google Scholar]
- Slotnick SD. Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cogn Neurosci. 2017:8:150–155. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J Jr. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 2003:17:75–82. [DOI] [PubMed] [Google Scholar]
- Śmigasiewicz K, Shalgi S, Hsieh S, Möller F, Jaffe S, Chang C-C, Verleger R. Left visual-field advantage in the dual-stream RSVP task and reading-direction: A study in three nations. Neuropsychologia. 2010:48:2852–2860. [DOI] [PubMed] [Google Scholar]
- Śmigasiewicz K, Asanowicz D, Westphal N, Verleger R. Bias for the left visual field in rapid serial visual presentation: effects of additional salient cues suggest a critical role of attention. J Cogn Neurosci. 2014:27:266–279. [DOI] [PubMed] [Google Scholar]
- Śmigasiewicz K, Hasan GS, Verleger R. Rebalancing spatial attention: endogenous orienting may partially overcome the left visual field bias in rapid serial visual presentation. J Cogn Neurosci. 2016:29:1–13. [DOI] [PubMed] [Google Scholar]
- Thomas NA, Elias LJ. Upper and lower visual field differences in perceptual asymmetries. Brain Res. 2011:1387:108–115. [DOI] [PubMed] [Google Scholar]
- Wel P, Steenbergen H. Pupil dilation as an index of effort in cognitive control tasks: a review. Psychon Bull Rev. 2018:25:2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleger R, Sprenger A, Gebauer S, Fritzmannova M, Friedrich M, Kraft S, Jaskowski P. On why left events are the right ones: neural mechanisms underlying the left-hemifield advantage in rapid serial visual presentation. J Cogn Neurosci. 2009:21:474–488. [DOI] [PubMed] [Google Scholar]
- Verleger R, Dittmer M, Smigasiewicz K. Cooperation or competition of the two hemispheres in processing characters presented at vertical midline. PLoS One. 2013:8:e57421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainstein G, Rojas-Libano D, Crossley NA, Carrasco X, Aboitiz F, Ossandon T. Pupil size tracks attentional performance in attention-deficit/hyperactivity disorder. Sci Rep. 2017:7:8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Trongnetrpunya A, Samuel IB, Ding M, Kluger BM. Compensatory neural activity in response to cognitive fatigue. J Neurosci. 2016:36:3919–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KD, Woldorff MG, Mangun GR. Control networks and hemispheric asymmetries in parietal cortex during attentional orienting in different spatial reference frames. NeuroImage. 2005:25:668–683. [DOI] [PubMed] [Google Scholar]
- Yellin D, Berkovich-Ohana A, Malach R. Coupling between pupil fluctuations and resting-state fMRI uncovers a slow build-up of antagonistic responses in the human cortex. NeuroImage. 2015:106:414–427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be publicly available on the NIMH Data Archive.