Abstract
We compared cellular immune responses to rectal, subcutaneous, and intradermal administration of Mycobacterium bovis BCG for 5 to 20 weeks in mice, guinea pigs, and macaques. Strong lymphoproliferative responses were induced in spleen cells after in vitro stimulation with purified protein derivative in guinea pigs and macaques, whatever the route of immunization. Comparable high numbers of gamma interferon- and tumor necrosis factor alpha-producing cells were found in the spleen after rectal, subcutaneous, and intradermal immunization of mice and macaques. Similar levels of precursors of cytotoxic T lymphocytes specific for mycobacterial antigens were observed in mice for all immunization routes. In macaques, cytotoxic activity, determined only at the end of the experiment (20 weeks), was similar after rectal and intradermal immunization. Six months after immunization, rectal and subcutaneous routes induced in mice similar levels of protective immunity against challenge with a virulent Mycobacterium tuberculosis strain (H37Rv). Rectal immunization gave immune responses and protective capacity similar to those for parenteral immunization and seemed to be a promising new route of vaccination against tuberculosis; in our study, immunization via the rectal route never induced side effects associated with parenteral routes (axillary adenitis) and could also effectively reduce the risks of viral transmission associated with unsafe injections in the developing world.
Tuberculosis is still a major health problem. Its affects developing countries in particular (18) but industrialized countries as well, at least partly due to the rising prevalence of human immunodeficiency virus infection (13) but also due to social disintegration (2). The attenuated Mycobacterium bovis strain bacillus Calmette-Guérin (BCG) is widely administered as a vaccine to protect against tuberculosis. The protective efficacy of BCG vaccination is unclear, with clinical trials estimating it to be between 0 and 80%. Meta-analysis of the efficacy of BCG vaccine suggested that BCG vaccination reduces the risk of pulmonary tuberculosis by 50%, the number of deaths due to tuberculosis by 71%, and the number of meningitis cases by 85% (3, 4).
Development of a more effective vaccine is a possible answer to the global threat of tuberculosis. Recently, interest has increased in culture filtrate proteins obtained from Mycobacterium tuberculosis cultures (1, 11, 22) and plasmid DNA encoding mycobacterial antigens (12, 28) as candidate vaccines against tuberculosis. Both have been shown to offer some degree of protection if used as vaccines in animal models of experimental infection with M. tuberculosis. However, the level of protection and the longevity of their effect must be increased, as must their safety, especially if potent adjuvants are required to obtain a protective immune response.
BCG has proved to be safe: more than 3 billion doses of BCG have been administered, with a very low incidence of serious side effects. However, the intradermal route of administration currently used requires trained personnel, particularly for the vaccination of newborns, and raises questions about the reuse of syringes in developing countries, with the associated risk of AIDS and hepatitis transmission (25, 26). Mucosal administration of BCG is easy, but its ability to induce potent immune responses without side effects has to be checked carefully.
The oral route used by Calmette in 1921 for initial BCG vaccination gave protective immunity but was abandoned due to its deleterious effects such as suppurative cervical adenitis (16). We have recently shown that BCG translocation across the oropharyngeal area after oral ingestion in mice is not critical to the induction of a protective immunity against a virulent challenge with M. tuberculosis (14). Microencapsulation of the bacteria could be used to minimize the side effects by preventing inadvertent pharyngeal inoculation. However, microencapsulation is not easy, as the BCG must be maintained stable and alive in the preparation for prolonged periods. We therefore explored the rectal route for BCG vaccination. The rectal route would be easy to use under field conditions, with human newborns, using an inexpensive pediatric cannula, and lyophilized, stabilized BCG which could be rehydrated immediately before use.
In this study, we immunized various animal species (mice, guinea pigs, and macaques) with lyophilized BCG by rectal and parenteral routes. Cellular immune responses (according to the immunological tools available) were compared for the various species for 20 weeks. Protection against a virulent challenge with M. tuberculosis was assessed in mice 6 months after immunization, to test long-term protective immune responses.
MATERIALS AND METHODS
Animals.
Specific pathogen-free BALB/c mice (9 weeks) and outbred Hartley guinea pigs (300 to 350 g) were obtained from the breeding center of the Pasteur Institute of Teheran, Iran. Cynomolgus, Macaca irus female monkeys (1.7 to 2.4 kg; 11 to 25 months), originating from Tanzania, were obtained from the Razi Institute, Karaj, Iran. These animals had previously received a sample of a new batch of the polio vaccine produced at the Razi Institute. This previous immunization, a control for vaccine innocuity, was considered unlikely to affect BCG experiments.
To assess cellular immune responses, mice (n = 6) or guinea pigs (n = 4) were killed at various times after immunization, and spleen biopsies were performed on three different macaques at each time point, with a total of 15 macaques immunized by each route. To perform spleen biopsies, macaques were intramuscularly anesthetized (16 mg of alphaxalone-alphadolone per kg of body weight; Saffan-Mallinckrodt), and the spleen was removed under strict surgical conditions; at the end of the experiment all macaques were in good health.
Specific-pathogen-free BALB/c mice (6 to 7 weeks old) obtained from Iffa-Credo, Saint-Germain sur l'Arbresle, France, were used for the M. tuberculosis challenge.
Microorganisms.
The BCG Pasteur strain 1173P2 was grown as a dispersed culture in Beck-Proskauer medium supplemented with 6% glucose and 0.05% Triton-1331 (Sigma). The vaccine suspension was prepared as previously described (10), lyophilized in 2% sodium glutamate, and stored at −30°C. Just before animal immunization, BCG was rehydrated in Beck-Proskauer medium supplemented with 6% glycerol and 0.05% Triton-1331, and the vial was vigorously shaken to obtain dispersed bacilli without clumps.
The M. tuberculosis H37Rv strain used to challenge mice was grown on Sauton medium and stored at −80°C.
Immunizations.
For rectal immunization, mice, guinea pigs and macaques were deprived of food overnight; mice and guinea pigs were anesthetized with pentobarbital (Sigma), and macaques with ketamine HCl (Parke-Davis). They then received 2 × 109 (mice), 2 × 1010 (guinea pigs), and 6 × 1010 (macaques) viable units of BCG inserted into the rectum with a tip of an Eppendorf pipette (mice) or a pediatric cannula (guinea pigs and macaques).
For the subcutaneous immunization of mice, 100 μl of BCG (108 viable units) suspended in saline solution was injected into the base of the tail. For intradermal immunization, guinea pigs received 5 × 106 viable units in the flank, and macaques received 109 viable units of BCG in the inguinal region.
Lymphoproliferative assay.
At various times after immunization, splenocytes were collected from four guinea pigs or three macaque biopsy specimens and cultured in RPMI 1640 medium (Gibco BRL) containing 5% fetal calf serum (FCS), 1% l-glutamine (Gibco), 5 × 10−5 M β2-mercaptoethanol (Gibco), 100 U of penicillin, and 100 μg of streptomycin/ml (Roche-Hoffmann). Cells were cultured at a density of 106 cells per 0.2 ml/well, in 96-well flat-bottom culture plates (Nunc, Roskilde, Denmark) in the presence of 10 μg of purified protein derivative (PPD) per ml. The cells were incubated for 4 days at 37°C under 7% CO2; then, 0.5 μCi of [3H]thymidine (Amersham, Little Chalfont, United Kingdom) was added to each well, and the cells were incubated overnight. The cells were harvested and washed on fiberglass filters with a cell collector (Tomtec), and the incorporated radioactivity was measured in a liquid scintillation counter (Beckman). Results are given as geometric means of triplicate determinations, and stimulation indices (SI) calculated as (counts per minute with PPD stimulation)/(counts per minute without stimulation).
Cytokine ELISPOT assay.
An adaptation of the enzyme-linked immunospot (ELISPOT) assay (27) was used to count gamma interferon (IFN-γ)- and tumor necrosis factor alpha (TNF-α)-specific spot-forming cells (SFC) in splenocytes. To detect cytokine-producing cells, 96-well nitrocellulose plates (Multiscreen, HA; Millipore, Molsheim, France) were coated with 4 μg of anti-mouse IFN-γ monoclonal antibody (MAb) (RA-6A2), anti-mouse-TNF-α MAb (MP6-XT22) (Pharmingen), anti-human IFN-γ MAb (25718.11), or anti-human TNF-α MAb (TA-31) (Sigma) per ml in bicarbonate buffer (100 μl/well). Nonspecific binding was blocked by adding RPMI 1640 medium containing 10% FCS to the wells and incubating the plates for 60 min at 37°C. Splenocytes pooled from six mice or from three macaque spleen biopsies were stimulated by incubation for 24 h with 10 μg of PPD per ml. They were then added to the wells at concentrations of 2.5 × 105 to 1 × 106 cells/100 μl/well. The cells were incubated for 20 h at 37°C under 7% CO2, in the presence of 10 μg of PPD per ml. The wells were washed once with 0.05% Tween 20 in H2O and five times with 0.05% Tween 20 in phosphate-buffered saline (PBS-Tween). One hundred microliters of biotinylated anti-mouse IFN-γ MAb (XMG1-2), polyclonal anti-mouse TNF-α antibody (4 μg/ml; Pharmingen), polyclonal anti-monkey IFN-γ, or anti-monkey TNF-α antibodies (4 μg/ml; Chemicon) was added to the corresponding wells and the plates were incubated overnight at 4°C. The plates were washed with PBS-Tween. Avidin-alkaline phosphatase conjugate (Sigma) diluted 1:2,000 was added, and the plates were incubated overnight at 4°C. The plates were washed with PBS-Tween, spots (corresponding to cytokine-secreting cells) were developed with 100 μl BCIP-NBT (5-bromo-4-chloro-3-indolyl phosphate–nitro blue tetrazolium) (20 mg/ml; Sigma) in water. The number of SFC was determined using a dissecting microscope.
Cytotoxicity assay.
At various times after the rectal or subcutaneous immunization of BALB/c mice, single-cell suspensions were prepared from six pooled spleens, in RPMI 1640 medium supplemented with 10% FCS, 1% l-glutamine, 5 × 10−5 M β2-mercaptoethanol, antibiotics, and 10 U of interleukin 2 (Genzyme, Cambridge, Mass.) per ml. Cells (4 × 106/ml) were plated in 24-well flat-bottom plates (Nunc) in the presence of PPD (10 μg/ml). Effector cells were incubated for 5 days at 37°C under 7% CO2 and then tested for cytotoxic activity against target cells. Mastocyma P815 cells (MHC-H2d), into which mycobacterial antigens contained in crude BCG culture filtrate (23) had been introduced by an osmotic shock, were used as target cells (17). In summary, the P815 target cells were harvested 24 h before the assay and suspended (2 × 106 cells) in 0.5 ml of hypertonic RPMI 1640 medium containing 0.5 M sucrose, 10 mM HEPES, 10% polyethylene glycol, and 100 μg of the crude BCG culture filtrate. After 10 min at 37°C under 7% CO2, 15 ml of hypotonic RPMI 1640 medium was added. The target cells were recovered by centrifugation, suspended in RPMI 1640 medium containing 10% FCS, and incubated at 37°C under 7% CO2 until the assay.
Effector cells obtained from six pooled BALB/c mouse spleens at various times after BCG immunization were incubated for 4 h with 51Cr-labeled P815 target cells at 37°C under 7% CO2, in appropriate effector/target cell ratios. The radioactive chromium released into the supernatant was counted, and the percentage of specific lysis was calculated as 100 × [(cpm released from target cells in the presence of effector cells − cpm released from target cells alone)/(cpm released from target cells lysed with 10% Triton X-100 − cpm released from target cells alone)], where cpm is counts per minute.
In order to characterize cytotoxic effector cells, the cells were incubated for 1 h at 37°C with either medium alone, GK1-5 anti-CD4, or H-35-17-2 anti-CD8 MAbs (20 μg/ml). Effector cells were then washed three times and placed in contact, in the presence of GK1-5 or H-35-17-2 (1 μg/ml), with P815 target cells prepared as previously described, and the cytotoxicity assay was performed. To determine the H2 restriction, EL4 cells (lymphoma cells of C57BL/6 mice [MHC-H2b]) were prepared as P815 cells and used as target cells in the cytotoxicity assay. These characterizations were performed only at 12 weeks after immunization.
Twenty weeks after rectal or intradermal immunization, spleen biopsies were performed on 3 macaques. Splenic single-cell suspensions were prepared in RPMI 1640 medium supplemented with 10% human serum (Transfusion and Plasmaphoresis Organization of Iran), 1% l-glutamine, 1% gentamicin (Sigma), 5 × 10−5 M β2-mercaptoethanol. Effector cells (4 × 106/ml) were plated in 24-well flat-bottom plates (Nunc) in the presence of PPD (10 μg/ml), incubated for 5 days at 37°C under 7% CO2, and tested for cytotoxic activity against target cells. Macrophages obtained from the same anesthetized macaques by bronchoalveolar lavage with PBS supplemented with 20 μg of gentamicin per ml were used as target cells. Macrophages (104/well) suspended in complete RPMI 1640 medium with 10% human serum and 1% gentamicin were incubated for 5 days at 37°C in 96-well flat-bottom plates (Nunc). Twenty-four hours before the assay, 105 viable units of BCG were added to each well. After phagocytosis had occurred, the plates were washed twice; 2 μCi of 51Cr in complete RPMI medium was added per well, and the plates were incubated overnight.
The effector cells were incubated for 4 h with 51Cr-labeled autologous target cell macrophages at 37°C under 7% CO2 in appropriate effector/target cell ratios. The radioactive chromium released into the supernatant was counted, and the percentage of specific lysis was calculated as previously described.
Challenge with the virulent M. tuberculosis H37Rv strain.
Control and immunized mice that had received BCG via the rectal or subcutaneous route were challenged 6 months after immunization. Bacteria from the virulent M. tuberculosis strain H37Rv were obtained from stock vials containing 107 CFU per ml, stored at −80°C; 105 CFU was injected intravenously. Four weeks later, the mice were killed by carbon dioxide suffocation and their organs were collected. Appropriate dilutions were prepared from the dissociated organs and plated on Middlebrook 7H11 solid medium (Difco). The petri dishes were kept in sealed plastic bags at 37°C for 4 weeks, and then the colonies were counted.
Statistical analysis.
Statistical analysis, analysis of variance, and Student's t test were performed using the Instat package from GraphPad Software (San Diego, Calif.) A P of <0.05 was considered significant.
RESULTS
Specific proliferative responses in guinea pigs and macaques.
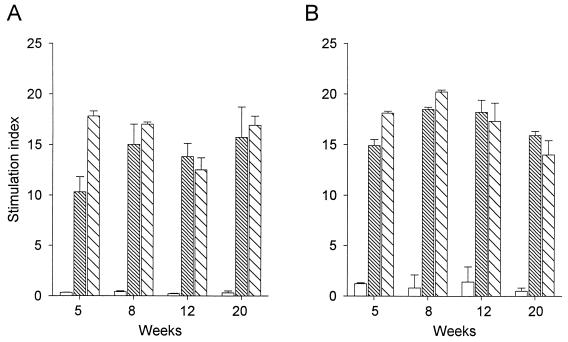
After the rectal or intradermal immunization of guinea pigs and macaques with BCG, the in vitro proliferative responses of splenocytes to PPD were analyzed. Specific proliferative responses were induced in guinea pigs and macaques 5 weeks after BCG immunization, and, at this time, the intradermal route induced particularly in guinea pigs the strongest responses (P < 0.01) (Fig. 1). At 8, 12, and 20 weeks after BCG immunization, proliferative responses remained strong (stimulation indexes > 10) with no significant differences among rectal or intradermal routes of immunization (P > 0.05).
FIG. 1.
Lymphoproliferative responses of guinea pig or macaque spleen cells stimulated with PPD, at various times after rectal and intradermal administration of BCG. Spleen cells collected from guinea pigs (A) or macaques (B) nonimmunized (empty bars) or immunized with BCG via rectal (fine hatches) or intradermal (coarse hatches) routes were stimulated by incubation for 4 days in vitro with PPD (10 μg/ml). The SI were calculated as (counts per minute with PPD)/(counts per minute without PPD). Results in guinea pigs (n = 4) and macaques (n = 3) (geometric means of triplicate determinations [error bars, standard deviations]) are representative of two different experiments.
Cytokine-producing spleen cells in mice and macaques.
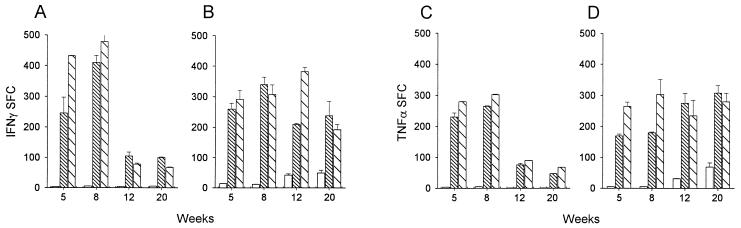
At 5, 8, 12, and 20 weeks after rectal or parenteral immunization, the in vitro cytokine production after PPD stimulation of mouse and macaque spleen cells was analyzed using the ELISPOT technique. High numbers of IFN-γ-producing cells were detected in the spleens of mice 5 and 8 weeks after BCG immunization (Fig. 2A). The number of IFN-γ-producing cells had decreased at 12 and 20 weeks after immunization by either rectal or subcutaneous route but remained at a similar, significant level compared to those in control mice (P < 0.05). At the beginning of the experiment, IFN-γ-producing cells were slightly less frequent in macaques (Fig. 2B) than in mice, particularly after parenteral immunization, but their numbers were sustained throughout the observation period (20 weeks). At the end of the experiment, no significant difference in IFN-γ production between the animals immunized via the rectal and parenteral routes was observed in mice and macaques (P > 0.05). The frequency of TNF-α-producing cells was slightly lower than that of IFN-γ-producing cells in the spleens of mice immunized 5 or 8 weeks previously (Fig. 2C). At 12 and 20 weeks the frequencies of TNF-α- and IFN-γ-producing cells in mice were similar, whatever the route of immunization. In macaques, the number of IFN-γ- and TNF-α-producing cells were in the same range (Fig. 2B and D), with no significant differences among the routes at the end of the experiment (P > 0.05).
FIG. 2.
IFN-γ- and TNF-α-producing cells in the spleens of mice and macaques at various times after rectal, intradermal, or subcutaneous administration of BCG. Spleen cells collected from mice (A and C) or macaques (B and D) nonimmunized (empty bars) or immunized with BCG via rectal (fine hatches) or parenteral (coarse hatches) routes were stimulated in vitro with PPD (10 μg/ml) in the wells of nitrocellulose plates that were coated with an anti-mouse IFN-γ, an anti-mouse TNF-α, an anti-human IFN-γ, or an anti-human TNF-α antibody. The SFC were developed as indicated in Materials and Methods. Results in mice (n = 6) and macaques (n = 3) are representative of two different experiments (error bars, standard deviations).
Specific cytotoxicity in mice and macaques.
The number of precursors of specific cytotoxic T lymphocytes present in the spleens of BALB/c mice (at 5, 8, 12, and 20 weeks) or macaques (at 20 weeks) was determined.
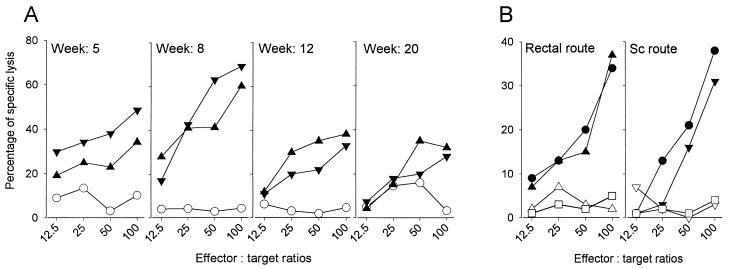
In BALB/c mice (H-2d), 5 weeks after rectal or subcutaneous immunization, cytotoxic T lymphocytes were detected in the spleen at frequencies of 34% (rectal) and 48% (subcutaneous). The frequency of cytotoxic precursor cells peaked at 8 weeks (60 and 69%, respectively). A level of cytotoxicity was maintained until the end of the experiment (33 and 28%, respectively) (Fig. 3A). We next determined, 12 weeks after immunization, if these cytotoxic responses were mediated by CD8 T cells. The mouse splenocyte cytotoxicity after rectal or subcutaneous immunization was abrogated by the addition of an anti-CD8 MAb; in contrast, the cytotoxic activity was not affected by the addition of an anti-CD4 MAb (Fig. 3B). The capacity of effector cells from BALB/c mice to lyse EL4 cells (major histocompatibility complex [MHC] class I H-2b) expressing mycobacterial antigens was also examined. Cytotoxic activity of effector cells from BALB/c mice (H-2d) could be demonstrated using P815 (H-2d) but not with EL4 (H-2b) target cells (Fig. 3B).
FIG. 3.
Precursors of cytotoxic T lymphocytes in the spleen after rectal or subcutaneous administration of BCG to BALB/c mice are mediated by CD8 T cells and are MHC class I restricted. (A) Spleens (n = 6) were collected at 5, 8, 12, or 20 weeks from nonimmunized (○) rectally (▴) or subcutaneously (▾) immunized mice. The cells were stimulated by incubating in vitro in the presence of PPD for 5 days. Their cytotoxic activity was tested against P815 cells presenting mycobacterial antigens after an osmotic shock in the presence of crude BCG culture filtrate. The results are representative of two different experiments. (B) Spleens (n = 6) were collected 12 weeks after immunization of BALB/c mice (H-2d) via the rectal or subcutaneous (Sc) routes. Cells were incubated in vitro in the presence of PPD (10 μg/ml) for 5 days and their cytotoxic activity was tested against P815 (H-2d) target cells in the presence of medium only (▴, ▾) or of anti-CD8 (▵, ▿) or anti-CD4 (●) MAbs. The cytotoxic activity of spleen cells was also tested against EL4 (H-2b) target cells (□).
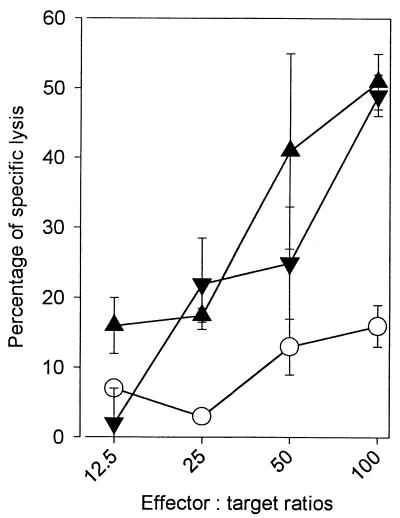
Cells isolated 20 weeks after immunization from macaque spleen biopsy samples showed high and identical levels of cytotoxicity against autologous macrophages which had phagocytized BCG (51 and 50% after rectal or intradermal immunization), whereas the cytotoxicity of the nonimmunized control group remained at a low level (15%) (Fig. 4).
FIG. 4.
Precursors of cytotoxic T lymphocytes in the spleen 20 weeks after rectal or intradermal administration of BCG to macaques. Cells were collected from spleen biopsies 20 weeks after immunization of macaques (n = 3) via the rectal (▴) or intradermal (▾) route. The cells were stimulated by incubating in vitro in the presence of PPD (10 μg/ml) for 5 days. Their cytotoxic activity was tested against syngenic macrophages obtained from bronchoalveolar lavages, which had taken up BCG by phagocytosis 24 h before the assay. The results are representative of two different experiments. Error bars, standard deviations.
Challenge with M. tuberculosis H37Rv in mice.
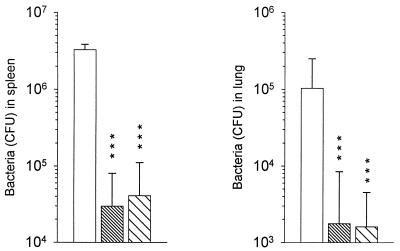
Six months after rectal or subcutaneous immunization, immunized and naive BALB/c mice were challenged intravenously with 105 CFU of M. tuberculosis (H37Rv). Four weeks after the challenge, there were significantly fewer H37Rv bacteria in the spleen and lungs of immunized mice than in nonimmunized control animals (Fig. 5). Similar levels of protection were observed for the two routes of immunization.
FIG. 5.
Assay of immune protection induced in mice 6 months after immunization by rectal or subcutaneous administration of BCG. Results are shown for BALB/c mice (n = 8-10) nonimmunized (empty bars) or immunized with BCG by the rectal (fine hatches) or by subcutaneous injection (coarse hatches) routes. Six months later, the mice were challenged intravenously with 105 viable units of the virulent M. tuberculosis strain H37Rv. The spleen and lungs were collected 4 weeks later and dissociated. Their bacterial loads were evaluated after appropriate dilutions and growth on Middlebrook 7H11 agar medium. Results are expressed as geometric mean + standard deviation (error bar) ∗∗∗, P < 0.001.
DISCUSSION
Rectal immunization with BCG, by inducing immune responses and protection against tuberculosis, may greatly facilitate BCG administration and reduce the cost of mass vaccination while avoiding the risks of AIDS and hepatitis transmission inherent in the reuse of syringes in developing countries (25, 26). Moreover, in our study immunization via the rectal route never induced any side effects in the animal species tested; on the other hand, immunization via the intradermal route, as related in human (16), induced in guinea pigs and particularly in macaques adenitis which spontaneously diminished in intensity at 12 weeks and became scarce at 20 weeks. BCG is the first vaccine given to newborns in developed countries, and suppurative axillary adenitis occurring after intradermal injection could compromise the other vaccinations of the Expanded Program on Immunization (EPI). In a recent study (14) we demonstrated that intragastrically delivered BCG induced protection against M. tuberculosis without inducing side effects due to the translocation of BCG into the oropharyngeal area after oral immunization. However, microencapsulation of BCG, which may prevent cervical adenitis by delivering the BCG to the stomach or duodenum, requires thorough investigation, and the delivery of encapsulated BCG to newborns by the oral route will also require the use of a feeding bottle. In contrast, the rectal route seems to be a cheap, easy, and safe route, by which the vaccine could be given without pain with a pediatric cannula or a suppository; this new route would be particularly suitable for newborns in developing countries. The accidental complications occurring after intradermal injection of high doses could be minimized after rectal vaccination because the majority of the BCG administered rectally is eliminated in the stool during the first 24 h after administration (data not shown).
The presence of organized lymphoid tissue with M cells and germinal centers in the rectum of BALB/c mice (20) suggests that such tissue has the capacity to take up microorganisms. We were unable to recover BCG by plating homogenized rectal lymphoid tissues equivalent to Peyer's patches (one or two per mice) at various times after rectal immunization. In mesenteric lymph nodes, BCG (approximately 500 CFU per node) has been recovered 24 h after rectal administration but not thereafter (data not shown), suggesting that rectal and colonic follicles may be the entry points for the systemic dissemination of BCG and initiation of immune responses. Histologic studies are under way to determine the precise route of translocation of BCG in the rectal region.
In the three animal species studied, rectal and parenteral immunization induced long-term sustained systemic Th1 responses. Twenty weeks after immunization, lymphoproliferative responses and cytokine production (IFN-γ and TNF-α) remained at the same high level, particularly in macaques. The mycobacterial antigens recognized by CD4 and CD8 T cells, and the release of macrophage-activating cytokines such as IFN-γ are fundamental elements of the immune response to mycobacteria (5). In humans, susceptibility to tuberculosis is greater in individuals with a genetic defect in the gene encoding the IFN-γ receptor (19). In experimental mouse models, it has been shown that IFN-γ knockout (6, 8) greatly increases susceptibility to virulent mycobacterial challenge. TNF-α is another essential component of the protective immune response (24). TNF-α acts synergically with IFN-γ to stimulate macrophages. The CD8 lymphocytes have also been shown to play an important role in the control of BCG infection in vivo (21). Mice in which the β2-microglobulin gene has been knocked out have been shown to be more susceptible to M. tuberculosis (9). We and others have previously reported, in the mouse model (8, 14, 29), the development of CD8 T-cell-cytotoxic activity after immunization with BCG. CD8 T cells recognize mycobacterial antigens in the context of class I MHC molecules. In this study, we also found in mice a cytotoxic response mediated by CD8 MHC class I-restricted T cells after rectal or parenteral immunization. However, different results, including some from our laboratory (unpublished results), have shown that CD4 T cells may have cytotoxic activity in the context of class II MHC molecules (5). In our macaque model, the antigens are presented by alveolar macrophages after overnight BCG phagocytosis under conditions similar to those used previously for the detection of CD4 T-cell cytotoxicity in mice (15). The cells supporting cytotoxicity in macaque will be typed in the next series of experiments.
Rectal or parenteral routes of immunization provided similar levels of acquired immune protection. These results demonstrate for the first time to our knowledge, that BCG administered by the rectal route can induce potent protective immune responses against tuberculosis. The rectal route is very easy to use in the field, and immunization via this route did not induce side effects in any of the animal species tested during this study. It should be investigated in more detail, particularly in macaques, before undertaking clinical trials.
REFERENCES
- 1.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coker R. Lessons from New York's tuberculosis epidemic. BMJ. 1998;317:616. doi: 10.1136/bmj.317.7159.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colditz G A, Brewer T F, Berkey C S, Wilson M E, Burdick E, Fineberg H V, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 4.Comstock G W. Field trials of tuberculosis vaccines: how could we have done them better? Contr Clin Trials. 1994;15:247–276. doi: 10.1016/0197-2456(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 5.Conradt P, Hess J, Kaufmann S H E. Cytolytic T-cell responses to human dendritic cells and macrophages infected with Mycobacterium bovis BCG and recombinant BCG secreting listeriolysin. Microbes Infect. 1999;1:753–764. doi: 10.1016/s1286-4579(99)80077-x. [DOI] [PubMed] [Google Scholar]
- 6.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russel D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denis O, Iozes E, Huygen K. Induction of cytotoxic T-cell responses against culture filtrate antigens in Mycobacterium bovis bacillus Calmette-Guérin-infected mice. Infect Immun. 1997;65:676–684. doi: 10.1128/iai.65.2.676-684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gheorghiu M, Lagrange P H, Fillastre C. The stability and immunogenicity of a dispersed grown freeze-dried Pasteur BCG vaccine. J Biol Stand. 1988;16:15–26. doi: 10.1016/0092-1157(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz M A, Lee B E, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, Dewitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 13.Jereb J A, Kelly G D, Dooley S W, Jr, Cauthen G M, Snider D E., Jr Tuberculosis morbidity in the United States: final data, 1990. Morb Mortal Wkly Rep. 1991;40(SS-3):23–27. [PubMed] [Google Scholar]
- 14.Lagranderie M, Chavarot P, Balazuc A M, Marchal G. Immunogenicity and protective capacity of Mycobacterium bovis BCG after oral or intragastric administration in mice. Vaccine. 2000;18:1186–1195. doi: 10.1016/s0264-410x(99)00386-2. [DOI] [PubMed] [Google Scholar]
- 15.Lagranderie M R R, Balazuc A M, Dériaud E, Leclerc C D, Gheorghiu M. Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect Immun. 1996;64:1–9. doi: 10.1128/iai.64.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotte A, Wasz-Hockert O, Poisson N, Dimitrescu N, Vernon M, Couvet E. BCG complications: estimates of the risks among vaccinated subjects and statistical analysis of their main characteristics. Adv Tuberc Res. 1984;21:107–193. [PubMed] [Google Scholar]
- 17.Moore M W, Carbone F R, Bevan M J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 18.Murray C J L, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990;65:6–24. [PubMed] [Google Scholar]
- 19.Newport M J, Huxley C M, Huston S, Hawrylowicz C M, Oostra B A, Williamson R, Levin M. Mutation in the interferon-γ receptor and mycobacterial susceptibility in man. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 20.Owen R L, Piazza A J, Ermak T H. Ultrastructural and cytoarchitectural features of lymphoreticular organs in the colon and rectum of adult BALB/c mice. Am J Anat. 1991;190:10–18. doi: 10.1002/aja.1001900103. [DOI] [PubMed] [Google Scholar]
- 21.Pedrazzini T, Hug K, Louis J A. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette-Guérin in mice: assessment by elimination of T cell subsets in vivo. J Immunol. 1987;139:2032–2037. [PubMed] [Google Scholar]
- 22.Roberts A D, Sonnenberg M G, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 23.Romain F, Laqueyrerie A, Militzer P, Pescher P, Chavarot P, Lagranderie M, Auregan G, Gheorghiu M, Marchal G. Identification of a Mycobacterium bovis BCG 45/47-kilodalton antigen complex, an immunodominant target for antibody response after immunization with living bacteria. Infect Immun. 1993;61:742–750. doi: 10.1128/iai.61.2.742-750.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudolf L, Tacchini-Cottier F, Guler R, Vesin D, Jemelin S, Olleros M L, Marchal G, Browning J L, Vassalli P, Garcia I. A role for lymphotoxin I receptor in host defense against Mycobacterium bovis BCG infection. Eur J Immunol. 1999;29:4002–4010. doi: 10.1002/(SICI)1521-4141(199912)29:12<4002::AID-IMMU4002>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 25.Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull W H O. 1999;77:789–800. [PMC free article] [PubMed] [Google Scholar]
- 26.Singh J, Bhatia R, Gandhi J C, Kaswekar A P, Khare S, Patel S B, Oza V B, Jain D C, Sokhey J. Outbreak of viral hepatitis B in a rural community in India linked to inadequately sterilized needles and syringes. Bull W H O. 1998;76:93–98. [PMC free article] [PubMed] [Google Scholar]
- 27.Taguchi T, McGhee J R, Coffman R L, Beagley K W, Eldridge J H, Takatsu K, Kiyono H. Detection of individual mouse splenic T cells producing IFNγ and IL5 using the enzyme linked immunospot (ELISPOT) assay. J Immunol Methods. 1990;128:65–73. doi: 10.1016/0022-1759(90)90464-7. [DOI] [PubMed] [Google Scholar]
- 28.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregory D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 29.Zügel U, Kaufmann S H E. Activation of CD8 T cells with specificity for mycobacterial heatshock protein 60 in Mycobacterium bovis bacillus Calmette-Guérin-vaccinated mice. Infect Immun. 1997;65:3947–3950. doi: 10.1128/iai.65.9.3947-3950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]