Abstract
Objectives
This study investigated whether the monitoring and control of clinical parameters are better among patients with newly compared with past recorded diabetes diagnosis.
Design
Retrospective cohort study.
Setting
MedicineInsight, a national general practice database in Australia.
Participants
101 875 ‘regular’ adults aged 18+ years with past recorded (2015–2016) and 9236 with newly recorded (2017) diabetes diagnosis.
Main outcome measures
Two different groups of outcomes were assessed in 2018. The first group of outcomes was the proportion of patients with clinical parameters (ie, glycated haemoglobin A1c (HbA1c), blood pressure (BP), total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate and albumin-to-creatinine ratio) monitored at least once in 2018. The second group of outcomes were those related to diabetes control in 2018 (HbA1c ≤7.0%, (BP) ≤140/90 mm Hg, total cholesterol <4.0 mmol/L and LDL-C <2.0 mmol/L). Adjusted ORs (ORadj) and adjusted probabilities (%) were obtained based on logistic regression models adjusted for practice variables and patients’ socio-demographic and clinical characteristics.
Results
The study included 111 111 patients (51.7% men; mean age 65.3±15.0 years) with recorded diabetes diagnosis (11.0% of all 1 007 714 adults in the database). HbA1c was monitored in 39.2% (95% CI 36.9% to 41.6%) of patients with newly recorded and 45.2% (95% CI 42.6% to 47.8%) with past recorded diabetes (ORadj 0.78, 95% CI 0.73 to 0.82). HbA1c control was achieved by 78.4% (95% CI 76.7% to 80.0%) and 54.4% (95% CI 53.4% to 55.4%) of monitored patients with newly or past recorded diabetes, respectively (ORadj 3.11, 95% CI 2.82 to 3.39). Less than 20% of patients with newly or past recorded diabetes had their HbA1c, BP and total cholesterol levels controlled (ORadj 1.08, 95% CI 0.97 to 1.21).
Conclusions
The monitoring of clinical parameters was lower among patients with newly than past recorded diabetes. However, diabetes control was similarly low in both groups, with only one in five monitored patients achieving control of all clinical parameters.
Keywords: diabetes & endocrinology, public health, primary health care, epidemiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This retrospective cohort used a large sample of patients attending primary healthcare services across all Australian states and territories.
A wide range of socio-demographic and clinical variables related to diabetes monitoring and control were included for adjustment.
Lifestyle variables were not included for adjustment, as they are not consistently recorded in the electronic medical records.
Patients may have had their diabetes parameters monitored somewhere else (eg, different practices or by specialists).
Introduction
Diabetes mellitus is a lifelong disease that requires regular monitoring and control to reduce the risk of diabetes-related complications.1–5 Microvascular and macrovascular complications of uncontrolled diabetes (eg, hypertension, dyslipidaemia, chronic kidney disease (CKD), cardiovascular disease (CVD)) increase the health burden worldwide.6 Blood glucose control is the most critical clinical goal of diabetes management, but other clinical variables also require regular monitoring.3 The Royal Australian College of General Practitioners (RACGP) guidelines recommend patients with diabetes should have their glycated haemoglobin A1c (HbA1c), blood pressure (BP) and lipid levels evaluated annually to improve management and control of these clinical parameters.7 Treatment options may vary depending on individual characteristics (eg, age, gender, presence of comorbidities)7 8 and the stage of diabetes progression (ie, recent or past diagnosis, presence of diabetes complications).9
Maintaining optimal levels of diabetes control with a combination of drug monotherapy and lifestyle changes is often possible for several years, after which a combination therapy may be necessary. The evaluation and modification of treatment plans in diabetes hinge on the information obtained from close monitoring of clinical parameters.10 However, gaps between real-world practice and guideline recommendations for diabetes management have been reported worldwide.4 11–13 For instance, a survey of 305 primary care physicians in the USA showed that only 38% of clinicians use guidelines in the management of diabetes.11
A systematic review of 123 Australian studies found that approximately 50% of patients with diabetes received ‘standard care’ (ie, assessment of HbA1c, BP, lipids, weight, eye health, foot health). Among those assessed, 40–60% met management targets for HbA1c, BP or lipid levels, but the study did not report the proportion that had all three parameters under control.13 Most studies included in that review used electronic health records (EHRs) to investigate diabetes control. However, these studies also tended to source data from specialised centres rather than primary healthcare settings, and used non-representative samples, hindering the generalisability of the results at a national level. Additionally, other potential determinants of diabetes management and control (eg, socio-demographic and clinical variables) were not widely investigated. Despite these limitations, figures in the review were consistent with measured data from the Australian Health Survey (AHS) (2011–2012), which reported that 54.7% of adults with known diabetes met HbA1c targets, 39% met recommended BP levels and 38% met total cholesterol targets.14
Despite concerns about the completeness and feasibility of using EHR-based primary care databases in research, studies conducted in countries such as the USA, Canada, the UK, France, Sweden, India and Australia have shown EHRs can provide accurate information on diabetes prevalence,15–17 management and control.13 18–20 EHR-based research can improve diabetes management without increasing overall treatment costs.21 22 Moreover, EHR databases minimise self-report bias by providing information on doctor-reported diagnoses, objective laboratory results and prescribed medications.15 16 23
Thus, this study used retrospective data from a national general practice database to investigate if (1) the monitoring of clinical parameters for diabetes management (HbA1c, BP, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, estimated glomerular filtration rate (eGFR), albumin-to-creatinine ratio (ACR)) is better among patients with newly than past recorded diabetes diagnosis, and (2) the proportion of those monitored who achieved diabetes control (ie, HbA1c, BP, total cholesterol, LDL-C) is higher in patients with newly recorded compared with those with past recorded diabetes diagnosis.
Methods
Data source
We used retrospective data from an open cohort database (MedicineInsight) that includes de-identified EHRs from approximately 662 general practices (8.2% of all Australian practices) and over 2700 general practitioners (GPs) across Australia.23 Details of data extraction and database characteristics have been published elsewhere.23 Although practices in MedicineInsight were selected using a non-random process, all Australian states and territories, urban and rural settings and socioeconomic settings are represented in the database. Diagnostic algorithms used for identifying patients with chronic disease using MedicineInsight have been validated, showing sensitivity of 89% against the recording of diabetes in the original EHR.16
Study sample
This study was reported according to the REporting of studies Conducted using Observational Routinely-collected Data statement.20 Only data from practices with regular data provision (ie, no gap of more than 6 weeks in data provision in the previous 2 years) was included. The sample was adults (18+ years) who regularly attended the practice (ie, those with at least one consultation per year between 2015 and 2018) and who had a diagnosis of diabetes mellitus (either type 1 or type 2). Data from consultations between January 2015 and December 2017 were used to identify the level of exposure: patients with past (ie, diabetes diagnosis recorded in 2015 or 2016) or newly recorded diabetes (ie, first diagnosis recorded in 2017, but not during appointments in 2015 or 2016). The outcome was assessed using data from January to December 2018, considering all recordings of clinical parameters related to diabetes monitoring and control in that year.7
Three fields (‘diagnosis’, ‘reason for encounter’ and ‘reason for prescription’) were initially searched to identify patients with recorded diabetes diagnoses. The original search was based on the methods for data extraction used by MedicineInsight.16 23 It included standard clinical terminology, misspellings and abbreviations, and then expanded to include prescribed medications and laboratory results. Using as much information as possible from EHRs (ie, observations, medications, diagnostic information) can provide a more accurate picture for identifying diabetes.24 Besides, including laboratory results from EHRs are associated with higher rates of diabetes ascertainment.25 26
Patients were classified as having past recorded diabetes (ie, past diabetes) if between January 2015 and December 2016: (1) ‘diabetes’ was recorded in two different fields; or (2) antidiabetic medications were prescribed (Anatomical Therapeutic Chemical A10A or A10B27; metformin was considered only in the absence of polycystic ovary syndrome diagnosis); or (3) a diabetes diagnosis was recorded only once but there was at least one recorded laboratory result (fasting blood glucose, HbA1c or 2-hour oral glucose tolerance test) above the diabetes diagnosis threshold within the same time frame.7 Patients were classified as having newly recorded diabetes (ie, recent diabetes) if: (1) they did not meet the criteria for past recorded diabetes (ie, attended the practice in 2015 and 2016 but diabetes was not recorded) and (2) between January 2017 and December 2017 they presented any of the three criteria mentioned above for diabetes diagnosis (ie, ‘diabetes’ recorded in two fields, antidiabetic medications were prescribed OR ‘diabetes’ was recorded once only but there was at least one abnormal glycaemic result recorded in 2017).
Outcomes
The outcome was assessed considering data related to diabetes monitoring and control reported between January and December 2018. The first group of outcomes was the proportion of patients with diabetes who had their clinical parameters for diabetes management monitored at least once in 2018 (ie, HbA1c, BP, total cholesterol, LDL-C, HDL-C, triglycerides, eGFR or ACR).7 These clinical parameters were obtained from the fields ‘observations’ and ‘laboratory results’ using Logical Observation Identifiers Names and Codes.28
According to the RACGP guidelines, patients with diabetes should achieve recommended targets for all clinical parameters (ie, HbA1c, lipids (total cholesterol, HDL-C, LDL-C, non-HDL, triglycerides), BP and urine albumin excretion).7 However, three key parameters (HbA1c, BP and total cholesterol) can be used to define ‘well-controlled’ diabetes, since they indicate that patients can comprehensively manage their diabetes and reduce the risk of complications.12 13 Therefore, the second group of outcomes was the proportion of patients that achieved in 2018, among those checked, generally recommended targets (HbA1c ≤7.0%, BP ≤140/90 mm Hg and total cholesterol <4.0 mmol/L). Considering LDL-C is also commonly used to monitor cardiovascular risk,9 we performed additional analysis reporting the proportion of patients who achieved well-controlled LDL-C (<2.0 mmol/L). When multiple results were reported in 2018 for the same parameter and patient, the mean of these results was estimated and used for analysis.
‘Well-controlled’ diabetes was then explored using two different approaches. First, we analysed each clinical parameter as a different outcome: (1) controlled HbA1c, (2) controlled BP or (3) controlled total cholesterol or LDL-C. Second, based on whether each of these three parameters was controlled or not, we created an outcome variable with eight categories to explore the most frequent combination of parameters that were under control: (1) none controlled, (2) HbA1c only, (3) BP only, (4) total cholesterol only, (5) HbA1c and BP controlled, (6) HbA1c and total cholesterol controlled, (7) BP and total cholesterol controlled or (8) all controlled. The same combination was analysed considering LDL-C rather than total cholesterol and results were reported as supplementary material.
Covariates
Covariates included a group of socio-demographic and cardiovascular risk factors/history of CVD that may affect diabetes control.5 29 Practice data included practice remoteness (major cities, inner regional or outer regional/remote/very remote) and practice Index of Relative Socio-economic Advantage and Disadvantage (IRSAD quintiles). Remoteness and IRSAD were defined based on postcodes. Remoteness is determined according to the population size and average distance to services, while IRSAD is an area-level measure of socioeconomic status based on combined indicators (ie, household income, education and working status). Higher IRSAD scores indicate a more advantaged area.30 Patient variables included age (18–39, 40–64, 65+), gender (females, males), smoking status (smoker, ex-smoker or non-smoker), recorded history of hypertension, and recorded history of CVD (including heart failure, ischaemic heart disease or stroke), dyslipidaemia, CKD, liver disease and depressive symptoms during 2015–2017. Details on the data extraction methods for these variables have been published elsewhere.16 31
Statistical analysis
All analyses were performed in Stata V.16.1 (StataCorp, Texas, USA), considering the practices as clusters, using robust SEs and conditioned to the number of visits to the practice.
The distribution of socio-demographic and clinical characteristics among patients with past or newly recorded diabetes were presented as proportions with their corresponding 95% CI (categorical variables), or as means with their SD or median with their IQR (numerical variables).
Logistic regression models were used to assess differences in diabetes monitoring or diabetes control in 2018 (binary outcomes: each parameter controlled) between patients with past (ie, diagnosis recorded in 2015 or 2016, reference group) or newly recorded diabetes (ie, first diagnosis recorded in 2017). All results were adjusted for differences between these two groups in terms of practice characteristics (remoteness, IRSAD quintiles), patient socio-demographics (gender, age) or clinical characteristics (smoking status, history of hypertension, CVD, CKD, dyslipidaemia, liver disease and depressive symptoms). We reported adjusted ORs (ORadj) with their corresponding 95% CI, following recommendations of the American Statistical Association.32 Furthermore, results from the adjusted logistic regression models were also used to estimate adjusted predicted probabilities (ie, adjusted proportions) of the investigated outcomes using the command ‘margins’ in Stata.
Multinomial logistic regression models were used to compare whether the most frequent combination of parameters under control differed between patients with past or newly recorded diabetes, using a similar approach for adjustment and then obtaining the ORadj and adjusted probabilities for each category of the outcome.
Patient and public involvement
No patient involved.
Results
Population characteristics
The database included 1 007 714 regular patients (at least one visit per year between 2015 and 2018) aged 18+ years attending 541 practices (figure 1 and table 1). Of these, 111 111 individuals (11.0%) had recorded diabetes diagnosis (51.7% men; mean age 65.3±15.0 years): 101 875 with past and 9236 with newly recorded diabetes. Table 1 shows that patients with past recorded diabetes were older (mean 65.9±14.6 vs 58.1±17.1 years), and had a higher proportion of men (52.4% vs 44.0%), and history of CKD (4.7% vs 2.9%) than those with newly recorded diabetes. However, diagnosis of hypertension (35.0% vs 36.8%), dyslipidaemia (17.6% vs 20.2%) or depressive symptoms (18.4% vs 20.9%) was less frequent in patients with past recorded diabetes. The distribution according to remoteness, IRSAD, smoking status, history of CVD or history of liver disease was similar in both groups.
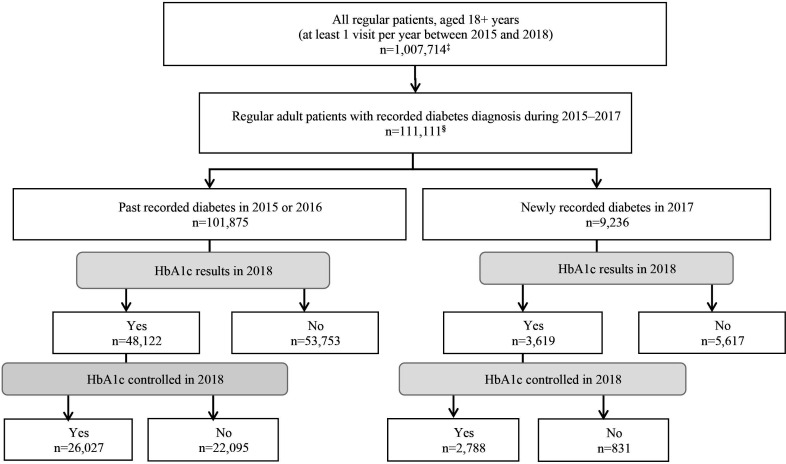
Figure 1.
Flowchart of the identification of ‘regular’ adult patients with recorded diabetes and HbA1c control†. †Results are shown as absolute numbers from the data set without adjusting or weighting. ‡At least one consultation per year between 2015 and 2018. §Patients were classified as recorded diabetes when (1) ‘diabetes’ was recorded on two different occasions (as a ‘diagnosis’, ‘reason for encounter’ or ‘reason for prescription’), or (2) antidiabetic medications were prescribed (Anatomical Therapeutic Chemical A10A or A10B; metformin was considered only in the absence of polycystic ovary syndrome diagnosis), or (3) diabetes diagnosis was recorded only once, but there was at least one laboratory result (fasting blood glucose, HbA1c or 2-hour oral glucose tolerance test) above the diabetes threshold. HbA1c, glycated haemoglobin A1c.
Table 1.
Socio-demographic and clinical profile of regular patients† aged 18+ years in the database
| Variables | All adults in MedicineInsight (N=1 007 714) | Patients with diabetes | |
| Past recorded diabetes (n=101 875) | Newly recorded diabetes (n=9236) | ||
| % (95% CI) | % (95% CI) | ||
| Practice characteristics | |||
| Geographical area of GP | |||
| Major cities | 63.8 (59.4 to 68.2) | 59.4 (54.6 to 64.2) | 62.3 (57.2 to 67.4) |
| Inner regional | 24.8 (20.6 to 28.9) | 27.4 (22.8 to 32.1) | 25.0 (20.4 to 29.7) |
| Outer regional/remote/very remote | 11.4 (8.5 to 14.4) | 13.2 (9.8 to 16.5) | 12.6 (9.1 to 16.0) |
| GP IRSAD | |||
| More disadvantaged | 33.8 (32.4 to 35.2) | 39.2 (34.0 to 44.4) | 38.3 (33.0 to 43.7) |
| Middle | 23.7 (22.4 to 25.1) | 25.0 (20.3 to 29.6) | 24.6 (19.8 to 29.4) |
| More advantaged | 43.8 (42.5 to 45.1) | 36.6 (32.1 to 41.0) | 38.1 (33.5 to 42.8) |
| Patient’s characteristics | |||
| Gender | |||
| Male | 40.4 (39.9 to 40.9) | 52.4 (51.9 to 53.0) | 44.0 (42.7 to 45.4) |
| Age, mean±SD | 54.0±19.1 | 65.9±14.6 | 58.1±17.1 |
| Age group (years) | |||
| 18–39 | 26.2 (25.1 to 27.2) | 5.8 (5.4 to 6.2) | 15.8 (14.6 to 17.1) |
| 40–64 | 40.9 (40.4 to 41.4) | 34.9 (34.0 to 35.7) | 43.0 (41.7 to 44.4) |
| 65+ | 33.0 (31.7 to 34.2) | 59.4 (58.2 to 60.5) | 41.2 (39.5 to 42.9) |
| Smoking status | |||
| Smoker | 12.0 (11.6 to 12.4) | 10.5 (10.1 to 10.8) | 10.8 (10.0 to 11.5) |
| History of hypertension | |||
| Yes | 19.0 (18.5 to 19.5) | 35.0 (33.9 to 36.2) | 36.8 (35.4 to 38.3) |
| History of CVD | |||
| Yes | 5.3 (5.2 to 5.4) | 13.2 (12.8 to 13.5) | 12.5 (11.7 to 13.3) |
| History of dyslipidaemia | |||
| Yes | 11.0 (10.5 to 11.4) | 17.6 (16.7 to 18.6) | 20.2 (19.0 to 21.3) |
| History of CKD | |||
| Yes | 1.3 (1.2 to 1.4) | 4.7 (4.3 to 5.1) | 2.9 (2.5 to 3.4) |
| History of liver disease | |||
| Yes | 0.2 (0.2 to 0.2) | 0.5 (0.5 to 0.6) | 0.6 (0.5 to 0.8) |
| History of depressive symptoms | |||
| Yes | 20.7 (20.1 to 21.4) | 18.4 (17.6 to 19.1) | 20.9 (19.9 to 22.0) |
| Consultations in 2018, median (IQR) | 4 (2–8) | 7 (4–13) | 6 (3–11) |
All results were adjusted for differences between these two groups in terms of practice characteristics (remoteness, IRSAD quintiles), patient socio-demographics (gender, age) or clinical characteristics (smoking status, history of hypertension, CVD, CKD, dyslipidaemia, liver disease and depressive symptoms).
†People had at least one visit per year between 2015 and 2018.
CKD, chronic kidney disease; CVD, cardiovascular diseases, including heart failure, ischaemic heart disease and stroke; GP, general practitioner; IRSAD, Index of Relative Socio-economic Advantage and Disadvantage.
Diabetes monitoring
Table 2 reports the proportion and ORadj of individuals who had their clinical parameters monitored in 2018, according to whether they had past or newly recorded diabetes. The most frequently monitored parameter was BP (past diabetes, 84.3% (95% CI 83.3% to 85.3%); newly diagnosed diabetes, 81.4% (95% CI 80.0% to 82.8%)). The least monitored parameter was ACR (past diabetes, 17.4% (95% CI 16.8% to 18.0%); newly recorded diabetes, 13.5% (95% CI 12.6% to 14.3)). Although 45.2% (95% CI 42.6% to 47.8%) of those with past diabetes and 39.2% (95% CI 36.9% to 41.6%) with newly recorded diabetes had their HbA1c levels monitored in 2018 (table 2), an additional 15% points in each group (absolute difference) had their glycaemic parameters checked through fasting and/or random glucose levels (online supplemental table S1).
Table 2.
Clinical parameters monitored in 2018 according to whether patients had past (2015–2016) or newly recorded diabetes (2017)
| Clinical parameters monitored | Past recorded diabetes (n=101 875) | Newly recorded diabetes (n=9236) | Adjusted* OR (95% CI) |
| % (95% CI) | % (95% CI) | ||
| HbA1c | 45.2 (42.6 to 47.8) | 39.2 (36.9 to 41.6) | 0.78 (0.73 to 0.82) |
| Blood pressure¶ | 84.3 (83.3 to 85.3) | 81.4 (80.0 to 82.8) | 0.81 (0.75 to 0.87) |
| Total cholesterol | 42.3 (39.8 to 44.8) | 38.9 (36.4 to 41.4) | 0.86 (0.82 to 0.91) |
| HDL-C | 38.0 (35.7 to 40.2) | 34.5 (32.2 to 36.7) | 0.86 (0.81 to 0.91) |
| LDL-C | 35.8 (33.6 to 37.9) | 32.9 (30.5 to 34.8) | 0.87 (0.82 to 0.92) |
| Triglycerides | 41.3 (38.9 to 43.7) | 37.8 (35.4 to 40.1) | 0.86 (0.81 to 0.90) |
| Any type of kidney function# | 26.9 (26.3 to 27.5) | 25.5 (24.4 to 26.4) | 0.93 (0.88 to 0.98) |
| eGFR | 26.5 (25.9 to 27.1) | 25.1 (24.1 to 26.2) | 0.93 (0.88 to 0.98) |
| ACR | 17.4 (16.8 to 18.0) | 13.5 (12.6 to 14.3) | 0.74 (0.69 to 0.79) |
Results adjusted for differences between these two groups in terms of practice characteristics (remoteness, IRSAD quintiles), patient socio-demographics (gender, age) and CVD risk factors (smoking status, history of hypertension or CVD, CKD, dyslipidaemia, liver disease and depressive symptoms using logistic regression models).
*Past recorded diabetes was used as the reference category.
ACR, urine albumin-to-creatinine ratio; CKD, chronic kidney disease; CVD, cardiovascular diseases, including heart failure, ischaemic heart disease and stroke; eGFR, Estimated glomerular filtration rate; HbA1c, glycated haemoglobin A1C; HDL-C, high-density lipoprotein cholesterol; IRSAD, Index of Relative Socio-economic Advantage and Disadvantage; LDL-C, low-density lipoprotein cholesterol.
bmjopen-2022-069875supp001.pdf (105.2KB, pdf)
Table 2 also shows that ORadj of monitoring of any parameter (ie, HbA1c, BP, total cholesterol, HDL-C, LDL-C, triglycerides, eGFR or ACR) was lower among patients with newly than past recorded diabetes, especially HbA1c (ORadj 0.78, 95% CI 0.73 to 0.82) and ACR (ORadj 0.74, 95% CI 0.69 to 0.79). Online supplemental table S2 presents the ORadj of distribution of patients with all three clinical parameters non-tiered (HbA1c, BP and total cholesterol) according to socio-demographic and clinical characteristics among those with past or newly recorded diabetes.
Well-controlled diabetes
Table 3 shows the proportion of patients that achieved clinical goals for diabetes management in 2018 among those with available results for each of the three key parameters. Patients with newly recorded diabetes had a higher chance of having their HbA1c controlled than those with past diabetes (ORadj 3.11, 95% CI 2.82 to 3.39). Nevertheless, the odds of having diastolic BP (ORadj 0.72, 95% CI 0.63 to 0.82), total cholesterol (ORadj 0.63, 95% CI 0.57 to 0.69) and LDL-C (ORadj 0.58, 95% CI 0.53 to 0.63) controlled were lower among those with newly recorded diagnosis than their peers. Systolic BP control was not different across groups.
Table 3.
Clinical parameters controlled in 2018 according to whether patients had past (2015–2016) or newly recorded diabetes (2017) among those with available results for the three key parameters (HbA1c, blood pressure and total cholesterol/LDL-C)
| Clinical parameter controlled | Past recorded diabetes n=40 008 | Newly recorded diabetes n=2912 | Adjusted*†OR (95% CI) |
| % (95% CI) | % (95% CI) | ||
| HbA1c (≤7.0% or ≤53 mmol/mol) | 54.4 (53.4 to 55.4) | 78.4 (76.7 to 80.0) | 3.11 (2.82 to 3.39) |
| Systolic blood pressure (≤140 mm Hg) | 70.6 (69.5 to 71.6) | 71.4 (69.6 to 73.3) | 1.04 (0.96 to 1.14) |
| Diastolic blood pressure (≤90 mm Hg) | 94.6 (94.2 to 94.9) | 92.8 (91.9 to 93.6) | 0.72 (0.63 to 0.82) |
| Total cholesterol (<4.0 mmol/L) | 43.9 (43.0 to 44.9) | 33.8 (31.9 to 35.6) | 0.63 (0.57 to 0.69) |
| LDL-C (<2.0 mmol/L) | 47.1 (46.1 to 48.1) | 34.7 (32.7 to 36.6) | 0.58 (0.53 to 0.63) |
Results adjusted for differences between these two groups in terms of practice characteristics (remoteness, IRSAD quintiles), patient sociodemographics (gender, age) and CVD risk factors (smoking status, history of hypertension or CVD, CKD, dyslipidaemia, liver disease and depressive symptoms using logistic regression models).
*Past recorded diabetes was used as the reference category.
CKD, chronic kidney disease; CVD, cardiovascular diseases, including heart failure, ischaemic heart disease and stroke; HbA1c, glycated haemoglobin A1c; IRSAD, Index of Relative Socio-economic Advantage and Disadvantage; LDL-C, low-density lipoprotein cholesterol.
Table 4 shows the combination of the three key parameters that were more frequently controlled in 2018. The proportion of individuals that met the three recommended targets was clinically similar, whether they had past (17.4%, 95% CI 16.7% to 18.1%) or newly recorded diabetes (18.8%, 95% CI 17.2% to 20.3%). Patients with newly recorded diabetes were more likely to have their HbA1c controlled, either alone (ORadj 1.62, 95% CI 1.40 to 1.87) or in combination with BP controlled (ORadj 1.64, 95% CI 1.45 to 1.86) than their peers. In contrast, the odds of total cholesterol being controlled (either alone or with BP) was ~65% lower among those with newly recorded diabetes than their counterpart. Analyses using LDL-C rather than total cholesterol showed similar results to those presented above (online supplemental table S3).
Table 4.
Combination of clinical parameters controlled in 2018 according to whether patients had past (2015–2016) or newly recorded diabetes (2017) among those with available results for all three parameters (HbA1c, blood pressure and total cholesterol)
| Parameter(s) controlled | Past recorded diabetes (n=40 008) | Newly recorded diabetes (n=2912) | Adjusted* OR (95% CI) | ||
| n | % (95% CI) | n | % (95% CI) | ||
| None controlled | 3521 | 8.8 (8.3 to 9.3) | 149 | 5.1 (4.3 to 5.9) | 0.54 (0.45 to 0.66) |
| Only HbA1c | 3961 | 9.9 (9.4 to 10.4) | 492 | 16.9 (15.4 to 18.3) | 1.62 (1.40 to 1.87) |
| Only BP | 6761 | 16.9 (16.3 to 17.5) | 259 | 8.9 (7.9 to 9.9) | 0.49 (0.42 to 0.57) |
| Only total cholesterol | 2360 | 5.9 (5.5 to 6.2) | 61 | 2.1 (1.6 to 2.6) | 0.33 (0.25 to 0.43) |
| HbA1c and BP | 8202 | 20.5 (19.8 to 21.1) | 1031 | 35.4 (33.5 to 37.3) | 1.64 (1.45 to 1.86) |
| HbA1c and total cholesterol | 2641 | 6.6 (6.2 to 7.0) | 210 | 7.2 (6.1 to 8.4) | 1.02 (0.84 to 1.24) |
| BP and total cholesterol | 5601 | 14.0 (13.6 to 14.5) | 163 | 5.6 (4.7 to 6.5) | 0.37 (0.30 to 0.45) |
| All controlled | 6961 | 17.4 (16.7 to 18.1) | 547 | 18.8 (17.2 to 20.3) | 1.08 (0.97 to 1.21) |
†Past recorded diabetes was used as the reference category. Results adjusted for differences between these two groups in terms of practice characteristics (remoteness, IRSAD quintiles), patient socio-demographics (gender, age) and CVD risk factors (smoking status, history of hypertension or CVD, CKD, dyslipidaemia, liver disease and depressive symptoms using multinomial logistic regression models).
*Past recorded diabetes was used as the reference category.
BP, blood pressure; CKD, chronic kidney disease; CVD, cardiovascular diseases, including heart failure, ischaemic heart disease and stroke; HbA1c, glycated haemoglobin; IRSAD, Index of Relative Socio-economic Advantage and Disadvantage.
The association between socio-demographic and clinical variables with the monitoring of the three key parameters (HbA1c, BP and total cholesterol or LDL-C) are presented as supplementary materials (online supplemental tables S4 and S5).
Discussion
General findings
Based on a large retrospective cohort study of the national general practice database, three main findings can be highlighted. First, less than half of patients with diabetes had their HbA1c levels assessed over 12 months, and the monitoring of HbA1c or other clinical parameters was less frequent among patients with newly than past recorded diabetes. Second, although patients with newly recorded diabetes were less likely to be monitored, 8 out of 10 of these patients achieved HbA1c control. Third, in general, less than 20% of patients with diabetes who were monitored in 2018 had their HbA1c, BP and total cholesterol within targeted levels considered well-controlled.
Comparison with literature
Current Australian guidelines recommend annual monitoring of clinical parameters for all patients with diabetes.7 Nonetheless, we found that only 45.2% of those with past diabetes and 39.4% of those with newly recorded diabetes had their HbA1c levels monitored in 2018. Our results are consistent with the ‘Rule of Halves’ discussed in an Australian review, showing that half of patients with diabetes receive appropriate diabetes monitoring.13 On the other hand, another recent Australian retrospective study not included in that review and using EHRs from patients attending 50 practices in the inner eastern region of Melbourne (MAGNET database, period 2009–2014) found a higher proportion of monitoring. Findings showed that 66.5% of patients aged 65+ years with type 2 diabetes had their HbA1c checked within the last 2 years.33 However, it is important to note that the population in that study was older, probably triggering a more frequent monitoring.
Among other clinical parameters, BP was the most frequently monitored regardless of having past (84.3%) or newly recorded diabetes (81.4%). In fact, having a newly recorded diagnosis of diabetes does not seem to affect BP monitoring in comparison with the general population, as a population-based study in South Australia found that 81.8% of individuals without diabetes, hypertension or CVD had their BP measured by a GP in the last 12 months.34
People with past recorded diabetes had a slightly higher proportion of kidney function monitoring than newly recorded diabetes. However, it is concerning that only one in four patients had these results reported in the last 12 months, even among those with past diabetes, considering that diabetes is one of the most important causes of CKD and annual kidney health checks (eGFR and urine ACR) are strongly recommended for patients living with diabetes.35
It is also concerning that a history of smoking or CVD did not affect the monitoring of the three main parameters (HbA1c, BP and total cholesterol) in any of the groups (past or newly recorded diabetes). These health conditions contribute to absolute CVD risk, diabetes-related comorbidities and, consequently, mortality.3 However, it is plausible that healthcare professionals have monitored these patients in other settings, such as smoking cessation programmes or CVD secondary prevention7 36 that would not be captured by our study.
Although patients with newly recorded diabetes were less likely to have their HbA1c monitored, 8 out of 10 of those monitored achieved HbA1c control. Patients with newly recorded diabetes were, on average, 8 years younger than those with past diabetes, which suggests their condition was at an earlier stage when complications are less frequent and diabetes control is more likely to be achieved with first-line medications.2 3 Additionally, medication adherence among patients with newly diagnosed diabetes can be as high as 65% then reduce over time, which, in turn, has been found to impact diabetes control.37 A previous study using the MedicineInsight database showed that greater regularity and continuity of care was associated with an increased likelihood of HbA1c monitoring, but it did not influence HbA1c control among patients with diabetes.38 Our results differ substantially from the findings of a longitudinal study carried out with newly diagnosed patients (within 6 months before screening) from 81 hospitals in China.39 The investigation found only 36.8% of HbA1c control (HbA1c <7%),39 but it is important to consider the different settings and patients characteristics in each study, as patients in hospital or specialised centres tend to need extra care or have a deteriorated health condition. Nonetheless, the possibility of information bias introduced by the less frequent HbA1c monitoring among those with newly recorded diabetes in our study cannot be discounted as an alternative explanation.
Despite the known effect of behavioural aspects40 such as denial or anxiety in the patient’s ability to monitor and manage their HbA1c when diabetes is diagnosed, according to our results, the management tends to weaken years after the diagnosis. The literature indicates that it happens due to the distress of living with diabetes and the high level of self-care needed to manage blood glucose, but also the lack of appropriate support or patient willpower over time.1 40–43 In our study, 54.4% of patients with past recorded diabetes achieved HbA1c control, very similar to results from the AHS (2011–2012), which reported 54.7% of control (HbA1c ≤7.0%) among adults with known diabetes.14 Results from the MAGNET database, 2009–2014, found that among patients monitored for HbA1c, 42.4% achieved control (ie, levels ≤7.0% in the most recent laboratory result).33
On the other hand, control of other clinical parameters in our study was better among patients with past than those with newly recorded diabetes. This could be related to the fact that patients with past diabetes were older (almost 60% were 65+ years compared with 41% among newly recorded diabetes), and older patients were at least twice more likely to achieve diabetes control than younger patients (online supplemental table S4). Results from the AHS (2011–2012)14 also found that the proportion of patients with well-controlled diabetes increased with age. The reason might be that older patients visit their GP more frequently, allowing more opportunities to have disease management monitored.
Our findings showed that among patients who had the three key parameters monitored (HbA1c, BP and total cholesterol or LDL-C), only one in five achieved targeted goals for the three parameters. A British EHR-based study indicated that despite optimal control of different CVD risk factors (HbA1c, systolic-BP, total cholesterol, triglycerides, smoking), patients with diabetes still had a 21% higher CVD risk than those without diabetes, reinforcing the need to monitor and control these parameters.44 Patients with a history of CVD were more likely to achieve well-controlled parameters, especially when they had newly recorded diabetes diagnosis. This finding might be related to the co-administration of antihypertensive and lipid-lowering therapy among patients with a history of CVD to reduce the risk of new CVD events.45 Moreover, the fear of own mortality increases the chances of compliance to medication in the short-term. Besides, this may be because patients with history of CVD were given more intensive treatments or combined use of antidiabetic medications.46 Discrepancies between patients with past or newly recorded diabetes diagnosis could result from prevalence-incidence bias, and prospective studies would be necessary to elucidate these findings.
Strengths and limitations
The study has significant strengths, such as the use of a large sample of patients attending primary healthcare services across all Australian states and territories. Furthermore, we explored socio-demographic and clinical variables related to diabetes monitoring and control that were not included in the most recent Australian studies on the same topic. Nonetheless, some other relevant covariates (eg, diet and exercise) were not explored, as they are not consistently recorded in EHRs, or are recorded in the progress notes which cannot be extracted because of confidentiality issues. This is a common limitation of EHR-based studies, as data from progress notes may affect completeness of information used for analysis. Additionally, patients may have had their diabetes parameters monitored somewhere else (eg, different practices or specialists). To minimise the effect of this, we used different fields to identify laboratory results that were not requested and automatically reported to the practice by the laboratories. Despite using widely accepted target levels for the clinical parameters investigated, they may be adjusted and tailored to individual characteristics, which may not be feasible to differentiate in large epidemiological studies. Finally, prevalence-incidence bias may have affected some of the investigated associations (eg, history of CVD and hypertension) among patients with past or newly recorded diabetes.
Conclusion
In Australia, monitoring and achieving clinical targets for diabetes management appears to be suboptimal. Consistent with previous research, we found half of the patients with diabetes had a record of their glycaemic levels being checked over 12 months. However, 80% of all those monitored did not achieve all targets of HbA1c, BP and total cholesterol recommended by the RACGP guidelines, regardless of the time of diabetes diagnosis. Multicomponent interventions for early detection and management of risk factors and complications, intensive glycaemic control and education on self-monitoring of blood glucose in persons with newly diagnosed diabetes, monitoring diabetes distress as part of routine care since the initial diagnosis, statin therapy for secondary CVD prevention and intensive hypertension control with ACE inhibitors or angiotensin receptor blockers to prevent end-stage renal disease are some of the cost-effective strategies highlighted in the literature that could be incorporated and emphasised in standard diabetes care programmes.40 42 43 47 48 Further studies are necessary to examine whether systematic implementation of these strategies in Australian primary healthcare settings, in addition to the continuous promotion of behaviour changes through clear and engaged communication within health professionals and patients, can optimise diabetes management in line with guidelines.
Supplementary Material
Acknowledgments
We are grateful to the general practices that participate in MedicineInsight, and the patients who allow the use of their de-identified information. We also thank all colleagues at the Discipline of General Practice, especially Dr Jessica Edwards and Dr Mumtaz Begum.
Footnotes
Twitter: @Mingyue Zheng
Contributors: MZ and DG-C contributed to the conception and design of the study. MZ performed the statistical analysis and prepared the manuscript. CB and DG-C assisted with data extraction, analysis and manuscript writing. NS and PH contributed to the design and structure of the manuscript. All authors critically revised the manuscript and provided intellectual support to enhance the manuscript. All authors approved the final version for publication. DG-C is responsible for the overall content and is the guarantor of the study.
Funding: MZ received a PhD Scholarship from the University of Adelaide for this study. Research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data used in this study was obtained from a third party (MedicineInsight) for this specific project and cannot be released. Information about MedicineInsight data and how they can be accessed is available on the website (https://www.nps.org.au/medicine-insight). The data extraction algorithms used in this study are available from the corresponding author upon request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The independent MedicineInsight Data Governance Committee approved the study (protocol 2016-007). The Human Research Ethics Committee of the University of Adelaide exempted the study from an ethical review as it used de-identified data.
References
- 1.Lambrinou E, Hansen TB, Beulens JW. Lifestyle factors, self-management and patient empowerment in diabetes care. Eur J Prev Cardiol 2019;26:55–63. 10.1177/2047487319885455 [DOI] [PubMed] [Google Scholar]
- 2.Holman RR, Paul SK, Bethel MA, et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–89. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 3.Aschner P. New idf clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res Clin Pract 2017;132:169–70. 10.1016/j.diabres.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 4.Li M-Z, Ji L-N, Meng Z-L, et al. Management status of type 2 diabetes mellitus in tertiary hospitals in Beijing: gap between guideline and reality. Chin Med J (Engl) 2012;125:4185–9. [PubMed] [Google Scholar]
- 5.Nazarzadeh M, Bidel Z, Canoy D, et al. Blood pressure lowering and risk of new-onset type 2 diabetes: an individual participant data meta-analysis. Lancet 2021;398:1803–10. 10.1016/S0140-6736(21)01920-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin X, Xu Y, Pan X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep 2020;10. 10.1038/s41598-020-71908-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Royal Australian College of General Practitioners . General practice management of type 2 diabetes: 2016–18. East Melbourne, Vic, 2016: https://www.racgp.org.au/FSDEDEV/media/documents/Clinical%20Resources/Guidelines/Diabetes/General-practice-management-of-type-2-diabetes_1.pdf. [Google Scholar]
- 8.Krämer HU, Raum E, Rüter G, et al. Gender disparities in diabetes and coronary heart disease medication among patients with type 2 diabetes: results from the diana study. Cardiovasc Diabetol 2012;11:88. 10.1186/1475-2840-11-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertoluci MC, Rocha VZ. Erratum to: cardiovascular risk assessment in patients with diabetes. Diabetol Metab Syndr 2017;9:70. 10.1186/s13098-017-0270-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee A, Draznin B, Aroda VR. 9. pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care 2022;45:S125–43. 10.2337/dc22-S009 [DOI] [PubMed] [Google Scholar]
- 11.Mehta S, Mocarski M, Wisniewski T, et al. Primary care physicians’ utilization of type 2 diabetes screening guidelines and referrals to behavioral interventions: a survey-linked retrospective study. BMJ Open Diabetes Res Care 2017;5:e000406. 10.1136/bmjdrc-2017-000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guthrie B, Emslie-Smith A, Morris AD. Which people with type 2 diabetes achieve good control of intermediate outcomes? population database study in a UK region. Diabet Med 2009;26:1269–76. 10.1111/j.1464-5491.2009.02837.x [DOI] [PubMed] [Google Scholar]
- 13.Sainsbury E, Shi Y, Flack J, et al. The diagnosis and management of diabetes in Australia: does the rule of halves’ apply? Diabetes Res Clin Pract 2020;170:S0168-8227(20)30781-6. 10.1016/j.diabres.2020.108524 [DOI] [PubMed] [Google Scholar]
- 14.Australian Bureau of Statistics . Australian Health Survey: Biomedical Results for Chronic Diseases. 2013. Available: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/australian-health-survey-biomedical-results-chronic-diseases/latest-release
- 15.Henderson J, Barnett S, Ghosh A, et al. Validation of electronic medical data: identifying diabetes prevalence in general practice. HIM J 2019;48:3–11. 10.1177/1833358318798123 [DOI] [PubMed] [Google Scholar]
- 16.Havard A, Manski-Nankervis JA, Thistlethwaite J, et al. Validity of algorithms for identifying five chronic conditions in medicineinsight, an Australian National general practice database. BMC Health Serv Res 2021;21:551. 10.1186/s12913-021-06593-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng M, Bernardo CDO, Stocks N, et al. Diabetes mellitus diagnosis and screening in australian general practice: A national study. J Diabetes Res 2022;2022:1566408. 10.1155/2022/1566408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varroud-Vial M. Improving diabetes management with electronic medical records. Diabetes Metab 2011;37 Suppl 4:S48–52. 10.1016/S1262-3636(11)70965-X [DOI] [PubMed] [Google Scholar]
- 19.Marson A, Raffoul N, Osman R, et al. Management of patients with type 2 diabetes and cardiovascular disease in primary care. Aust J Gen Pract 2021;50:238–45. 10.31128/AJGP-02-20-5222 [DOI] [PubMed] [Google Scholar]
- 20.Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med 2015;12:e1001885. 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulleyblank R, Mellace G, Olsen KR. Evaluation of an electronic health record system with a disease management program and health care treatment costs for danish patients with type 2 diabetes. JAMA Netw Open 2020;3:e206603. 10.1001/jamanetworkopen.2020.6603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah S, Yeheskel A, Hossain A, et al. The impact of guideline integration into electronic medical records on outcomes for patients with diabetes: a systematic review. Am J Med 2021;134:952–62. 10.1016/j.amjmed.2021.03.004 [DOI] [PubMed] [Google Scholar]
- 23.Busingye D, Gianacas C, Pollack A, et al. Data resource profile: medicineinsight, an australian national primary health care database. Int J Epidemiol 2019;48:1741–1741h. 10.1093/ije/dyz147 [DOI] [PubMed] [Google Scholar]
- 24.Anderson AE, Kerr WT, Thames A, et al. Electronic health record phenotyping improves detection and screening of type 2 diabetes in the general United States population: a cross-sectional, unselected, retrospective study. J Biomed Inform 2016;60:162–8. 10.1016/j.jbi.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flory JH, Roy J, Gagne JJ, et al. Missing laboratory results data in electronic health databases: implications for monitoring diabetes risk. J Comp Eff Res 2017;6:25–32. 10.2217/cer-2016-0033 [DOI] [PubMed] [Google Scholar]
- 26.Nishioka Y, Takeshita S, Kubo S, et al. Appropriate definition of diabetes using an administrative database: a cross-sectional cohort validation study. J Diabetes Investig 2022;13:249–55. 10.1111/jdi.13641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Pharmaceutical Benefits Scheme, Australian Department of Health and Aged Care . A10-drugs used in diabetes. 2023. Available: https://www.pbs.gov.au/browse/body-system
- 28.Stram M, Gigliotti T, Hartman D, et al. Logical observation identifiers names and codes for laboratorians potential solutions and challenges for interoperability. Arch Pathol Lab Med 2020;144:229–39. 10.5858/arpa.2018-0477-RA [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Mittal S, Aggarwal R, et al. Diabetes and cardiovascular disease: inter-relation of risk factors and treatment. Futur J Pharm Sci 2020;6. 10.1186/s43094-020-00151-w [DOI] [Google Scholar]
- 30.Australian Bureau of Statistics . Census of population and housing: socio-economic iIndexes for aAreas (SEIFA), Australia. 2016. Available: https://www.abs.gov.au/ausstats/abs@.nsf/mf/2033.0.55.001
- 31.Roseleur J, Gonzalez-Chica DA, Bernardo CO, et al. Blood pressure control in Australian general practice: analysis using general practice records of 1.2 million patients from the medicineinsight database. J Hypertens 2021;39:1134–42. 10.1097/HJH.0000000000002785 [DOI] [PubMed] [Google Scholar]
- 32.Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. Am Stat 2016;70:129–31. 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- 33.Xia T, Turner L, Enticott J, et al. Glycaemic control of type 2 diabetes in older patients visiting general practitioners: an examination of electronic medical records to identify risk factors for poor control. Diabetes Res Clin Pract 2019;153:125–32. 10.1016/j.diabres.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Chica DA, Bowden J, Miller C, et al. Patient-Reported GP health assessments rather than individual cardiovascular risk burden are associated with the engagement in lifestyle changes: population-based survey in South Australia. BMC Fam Pract 2019;20:173. 10.1186/s12875-019-1066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kidney Health Australia . Chronic kidney disease (CKD) management in primary carein, 4th edn. 2020. Available: https://kidney.org.au/uploads/resources/CKD-Management-in-Primary-Care_handbook_2020.1.pdf
- 36.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/apha/ASPC/NLA/PCNA guideline on the management of blood cholesterol: Executive summary: a report of the American College of cardiology/american heart association Task force on clinical practice guidelines. Circulation 2019;139:e1082–143. 10.1161/CIR.0000000000000624 [DOI] [PubMed] [Google Scholar]
- 37.Lin LK, Sun Y, Heng BH, et al. Medication adherence and glycemic control among newly diagnosed diabetes patients. BMJ Open Diab Res Care 2017;5:e000429. 10.1136/bmjdrc-2017-000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youens D, Robinson S, Doust J, et al. Associations between regular gp contact, diabetes monitoring and glucose control: an observational study using general practice data. BMJ Open 2021;11:e051796. 10.1136/bmjopen-2021-051796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv F, Cai X, Hu D, et al. Characteristics of newly diagnosed type 2 diabetes in chinese older adults: A national prospective cohort study. J Diabetes Res 2019;2019:5631620. 10.1155/2019/5631620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalra S, Jena BN, Yeravdekar R. Emotional and psychological needs of people with diabetes. Indian J Endocrinol Metab 2018;22:696–704. 10.4103/ijem.IJEM_579_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord 2013;12:14. 10.1186/2251-6581-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skinner TC, Joensen L, Parkin T. Twenty-five years of diabetes distress research. Diabet Med 2020;37:393–400. 10.1111/dme.14157 [DOI] [PubMed] [Google Scholar]
- 43.Czupryniak L, Barkai L, Bolgarska S, et al. Self-Monitoring of blood glucose in diabetes: from evidence to clinical reality in central and eastern Europe -- recommendations from the International central-eastern European expert group. Diabetes Technol Ther 2014;16:460–75. 10.1089/dia.2013.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright AK, Suarez-Ortegon MF, Read SH, et al. Risk factor control and cardiovascular event risk in people with type 2 diabetes in primary and secondary prevention settings. Circulation 2020;142:1925–36. 10.1161/CIRCULATIONAHA.120.046783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker IDI Heart and Diabetes Institute . National Evidence-Based Guideline on Secondary Prevention of Cardiovascular Disease in Type 2 Diabetes (Part of the Guidelines on Management of Type 2 Diabetes). Melbourne, Australia. 2015. Available: https://extranet.who.int/ncdccs/Data/AUS_D1_National%20Evidence-based%20Guideline%20on%20Secondary%20Prevention%20of%20Cardiovascu....pdf
- 46.Bashier A, Bin Hussain A, Abdelgadir E, et al. Consensus recommendations for management of patients with type 2 diabetes mellitus and cardiovascular diseases. Diabetol Metab Syndr 2019;11:80. 10.1186/s13098-019-0476-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li R, Zhang P, Barker LE, et al. Cost-Effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care 2010;33:1872–94. 10.2337/dc10-0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegel KR, Ali MK, Zhou XL, et al. Cost-Effectiveness of interventions to manage diabetes: has the evidence changed since 2008? Diabetes Care 2020;43:1557–92. 10.2337/dci20-0017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069875supp001.pdf (105.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data used in this study was obtained from a third party (MedicineInsight) for this specific project and cannot be released. Information about MedicineInsight data and how they can be accessed is available on the website (https://www.nps.org.au/medicine-insight). The data extraction algorithms used in this study are available from the corresponding author upon request.