Abstract
Introduction
Thoracic endovascular aortic repair (TEVAR) carries a 3%–6.1% stroke risk, including risk of ‘silent’ cerebral infarction (SCI). Stent-grafts are manufactured in room air and retain air. Instructions for use recommend saline flushing to ‘de-air’ the system prior to insertion, but substantial amounts of air are released when deploying them, potentially leading to downstream neuronal injury and SCI. Carbon dioxide (CO2) is more dense and more soluble in blood than air, without risk of bubble formation, so could be used in addition to saline to de-air stents. This pilot trial aims to assess the feasibility of a full-scale randomised controlled trial (RCT) investigating the neuroprotective benefit against SCI with the use of CO2-flushed aortic stent-grafts.
Methods and analysis
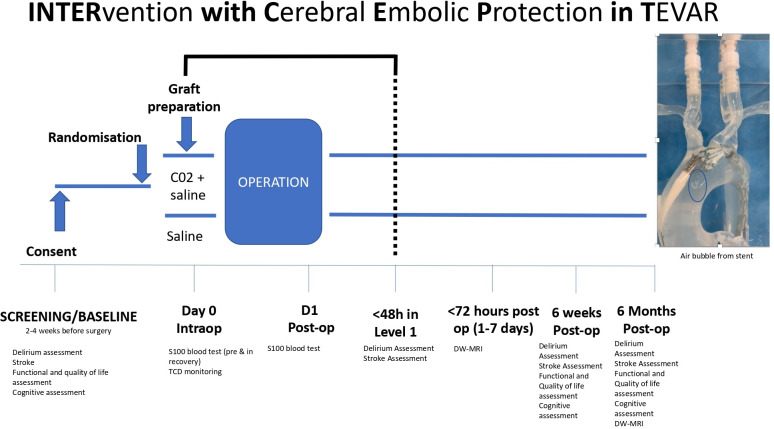
This is a multicentre pilot RCT, which is taking place in vascular centres in the UK, USA and New Zealand. Patients identified for TEVAR will be enrolled after informed written consent. 120 participants will be randomised (1:1) to TEVAR-CO2 or TEVAR-saline, stratified according to TEVAR landing zone. Participants will undergo preoperative neurocognitive tests and quality of life assessments, which will be repeated at 6 weeks, or first outpatient appointment, and 6 months. Inpatient neurological testing will be performed within 48 hours of return to level 1 care for clinical stroke or delirium. Diffusion-weighted MRI will be undertaken within 72 hours postoperatively (1–7 days) and at 6 months to look for evidence and persistence of SCI. Feasibility will be assessed via measures of recruitment and retention, informing the design of a full-scale trial.
Ethics and dissemination
The study coordination centre has obtained approval from the London Fulham Research Ethics Committee (19/LO/0836) and Southern Health and Disability Ethics Committee (NZ) and UK’s Health Regulator Authority (HRA). The study has received ethical approval for recruitment in the UK (Fulham REC, 19/LO/0836), New Zealand (21/STH/192) and the USA (IRB 019-264, Ref 378630). Consent for entering into the study will be taken using standardised consent forms by the local study team, led by a local PI. The results of the trial will be submitted for publication in an open access journal.
Trial registration number
Keywords: vascular surgery, stroke medicine, geriatric medicine, vascular medicine
Strength and limitations of this study
Multicentre pilot randomised controlled trial (RCT) will assess the feasibility and shape the design of a full-scale RCT, which will gather further information regarding the neurological risk associated with thoracic endovascular aortic repair (TEVAR) and the clinical significance of silent cerebral infarction, where a paucity of literature exists.
A cheap and readily available intervention is being studied.
Unprecedented levels of neurocognitive, neuroimaging and follow-up data will be collected to determine the clinical impact of cerebral infarction complicating TEVAR.
Blinding is incomplete, as the surgeons carrying out the procedure cannot be blinded to stent-graft flushing.
Introduction
There has been a significant increase in the number of thoracic endovascular aortic repairs (TEVARs) performed in the last decade. TEVAR is offered as preventative treatment to prevent rupture and death from aneurysmal aortic disease, aortic dissection and traumatic aortic injury. It has been adopted as the standard method for thoracic aortic repair as the avoidance of thoracotomy and aortic cross-clamping means morbidity is reduced and hospital stay is significantly decreased.1 Although TEVAR has successfully reduced periprocedural morbidity and mortality, stroke remains a significant risk. Several studies have identified risk factors contributing to neurological injury2 3 and further work is needed to investigate these risk factors to predict more accurately the patients at higher risk of neurological injury.
There is a reported 3%4–6.1%5 risk of stroke with TEVAR. Our own observational study has detected a 13% stroke rate in patients undergoing TEVAR.6 Furthermore, 68% of the patients developed covert brain injury as evidenced by new areas of brain infarction (BI) seen on diffusion-weighted MRI (DW-MRI) following TEVAR.6 Covert brain injury occurs in aortic surgical and cardiovascular catheter-based interventions6 7 and because these lesions do not manifest as clinical stroke with motor, sensory or speech deficits, they are termed ‘silent’ cerebral infarction (SCI). The American Heart and Stroke Association8 and the Neurological Academic Research Group (NeuroARC)9 now recognise the evolving definition of ‘stroke’ into a tissue-based diagnosis even in the absence of clinical symptoms. Incidentally identified SCI is a predictor of future development of clinically overt stroke,10 dementia11 and depression.12 There is also a direct clinical consequence of SCI with cognitive deficits demonstrated by neuropsychometric testing11 and in our own study, 88% of patients with SCI suffered with neurocognitive decline.6 Indeed, several studies have shown that radiologically detected cerebral infarcts tend to occur in those parts of the brain responsible for memory, mood and cognition. These procedurally related lesions are therefore not ‘silent’ but have clinically significant consequences.
Aetiological mechanisms of SCI in TEVAR remain uncharacterised, although several neuroimaging studies have detected evidence of SCI within a few days postprocedure, suggesting that periprocedural cerebral embolisation may be a cause.7 13 Further support for this hypothesis comes from continuous Transcranial Doppler (TCD) monitoring of the cerebral vessels for microembolic signals (MESs) during TEVAR whereby high-risk phases for cerebral embolisation have been shown to occur at specific time points during TEVAR.6 14 Stent-graft deployment is the phase most associated with embolisation, followed by wire manipulation in the aortic arch.6
Through the use of embolic differentiation software, we have deduced that >90% of MESs throughout TEVAR are gaseous in nature, with 81% of gaseous MESs apparent at stent-graft deployment. Once deployment is complete, TCD monitoring typically detects no further embolic activity. We also found a positive association between number of gaseous MESs and number of new DW-MRI BI.15 This suggests that cerebral air embolisation may be a significant cause of SCI in TEVAR and provides us with a basis on which to target preventative strategies.
Stent-grafts are manufactured in room air conditions and retain air. According to instructions for use (IFU), saline flushing is recommended to de-air the system. Emerging experimental studies have shown a substantial amount of air release from all commercially available grafts with bubbles ranging from 0.34 to 0.79 mL, despite saline flushing (see figure 1).16 17 This is a cause for concern given that cerebral arterioles are 40–250 µm in diameter.18 Large bubbles would be expected to cause downstream ischaemia and neuronal injury, while smaller bubbles may incite endothelial damage and activation of inflammatory and clotting cascades that may then cause secondary ischaemia.19 These small bubbles have been implicated in causing postoperative cognitive delirium (POCD).20
Figure 1.
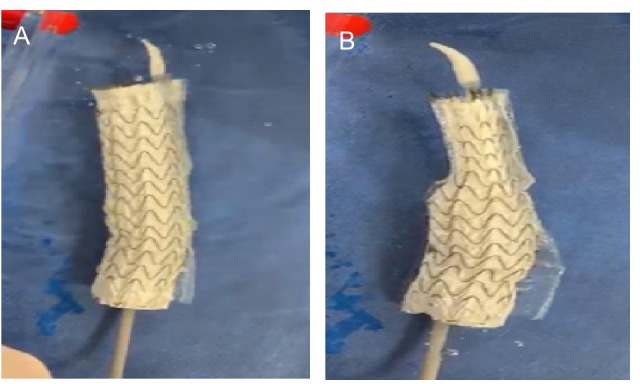
(A) Air bubble release during stent-graft deployment from the proximal end of the stent-graft as it opens in a benchtop experiment carried out by our group. (B) Air bubble release during stent-graft deployment from the distal end of the stent-graft as it opens in a benchtop experiment carried out by our group.
Carbon dioxide (CO2) is 1.5 times denser than air and can fill an enclosed space and displace air. It is 25 times more soluble in blood than air and does not lead to bubble formation.21 CO2 has been used extensively in cardiac surgery and shown to significantly reduce intracardiac air22 and POCD.23 CO2 can also significantly reduce the average amount of released air from a TEVAR stent in an experimental setting (0.79 vs 0.51 mL, p=0.005),17 and has been used clinically in a small series of patients with TEVAR where the authors describe a 3% clinical stroke rate. However, none of these patients underwent any formal cognitive or neuroimaging assessment and there was no control group, which has prompted the present study.24 25
We know that more proximal zones are associated with higher stroke rates. What remains unknown is whether CO2 flushing is enough to prevent neurological brain injury in these riskier zones, or whether solid embolisation from the manipulation of instruments close to atherosclerotic aortic valves and carotid vessels in more proximal zones is the main risk factor for neurological injury. This information will be used to aid refinement of the inclusion/exclusion criteria for the full-scale randomised controlled trial (RCT) and will be used to refine the sample size calculation for use in the trial.
We carried out a pilot study of 20 patients with TEVAR who underwent CO2 flushing and used TCD to detect cerebral embolisation rates and DW-MRI to assess for SCI. Intraoperatively, there were no MES detected at stent-graft deployment. The SCI rate was 25% and there was no clinical stroke in any of the patients (in comparison to 81% SCI and 13% stroke rate in patients with saline flushing).6 Although encouraging, we recognise the need for level 1 evidence in the form of a robust RCT to answer the question ‘is there a neuroprotective benefit against SCI and POCD with the use of CO2 flushed aortic stent-grafts.’
A review of registries on 28 January 2019 (www.clinicaltrials.gov and www.isrctn.com) found no similar studies in TEVAR.
Research influence
We have produced the largest case series to date regarding SCI in TEVAR and continue to highlight the magnitude of the problem by our ongoing study of neuroimaging, TCD, neurological and neurocognitive data on these patients. These data initially led us to believe that solid embolisation of particulate atherosclerotic matter dislodged from the thoracic aorta was responsible for SCI. Accordingly, we trialled the use of a cerebral embolic protection device designed to capture particulate matter ‘en-route’ to the brain in a cohort of 20 patients. This established feasibility and safety, and a 98% capture rate of embolic debris and a reduction in the number of lesions on DW-MRI. However, all patients still had lesions, with the majority concentrated in the posterior circulation territory.15
We suspect that both solid and gaseous emboli cause SCI. However, our TCD data continuously demonstrate an overwhelming occurrence of gaseous MES at stent-deployment in patients with TEVAR with and without filters, that amounts to a greater contribution of total MES than cumulative solid MES throughout TEVAR. Particulate embolism appeared to numerically correlate with the size of infarct, while gaseous emboli numerically correlated with the number of infarcts. These findings warrant our attention into investigating cerebral air embolism (CAE) as a cause of SCI and into CO2-flushed stent-grafts as a stand-alone intervention first, particularly as it is cheap, safe and easily implemented.
While the different ultrasonic reflective properties of solid and gaseous emboli provide the basis for discriminating between the two, we are aware of scepticism regarding the sensitivity and specificity of TCD embolic differentiation software during an embolic shower.26 We have sufficient recorded TCD data to demonstrate that the ‘shower’ of emboli seen at stent-graft deployment with resultant SCI on DW-MRI with saline flushing is reduced when stent-grafts are flushed with CO2, even when cerebral embolic protection devices are used to capture solid emboli. Reducing the contribution of gaseous embolic events will pave the way for future studies to tackle the residual problem of solid emboli, which will likely require the use of invasive devices, rather than a simple bench-top flushing procedure.
Objectives
This pilot trial aims to assess the feasibility of a full-scale RCT investigating the neuroprotective benefit against SCI with the use of CO2-flushed aortic stent-grafts. The results of this research will be used to gather further information regarding the neurological risks associated with TEVAR and the clinical significance of SCI, where a paucity of literature currently exists. It will also facilitate a more comprehensive and individualised consent process, allowing patients to make more informed decisions. We hope to inform the cardiovascular community about a potential prevention strategy against SCI. Stroke, dementia and neurocognitive decline are enormous burdens on healthcare resources, and any reduction in the incidence of these complications will have a positive effect on health economics, which is vital in the current financial climate.
Methods and analysis
Study design
Type of study: multicentre pilot RCT (see figure 2 for trial flowchart).
Figure 2.
Patient flowchart for the pilot trial.
Duration: estimated duration is 36 months for patient recruitment, from June 2021 to June 2024.
Participants: all elective patients undergoing TEVAR for aortic pathology.
Target total sample size: 120 (60 in each intervention arm).
Enrolment
Patients suitable for TEVAR as decided on by a vascular multidisciplinary meeting will be invited to participate and enrolled after informed written consent. Participants will be recruited by the research team at each site before surgery before their procedure (box 1).
Box 1. Intervention and control treatment.
TEVAR-S group
ALL stent-grafts used in a patient randomised to TEVAR-S are prepared according to their IFU including flushing of the device through the side flush port and with 60mls physiological saline solution.
TEVAR-CO2 group
ALL stent-grafts used in a patient randomised to TEVAR-CO2 are prepared according to their respective IFU. Flushing of the stent-graft will be performed first by flushing 100% CO2 at 2 L/min, 4 bar from a pressurised cylinder with 1.4 inch tubing connected to the side flush port for 1 minute followed by 60 mL of physiological saline.
Abbreviations: IFU, instructions for use; TEVAR, thoracic endovascular aortic repair.
Randomisation and interventions
Participants will be randomly assigned to TEVAR-CO2 or TEVAR-S group (box 1) providing they fulfil the entry criteria at screening (box 2). Participants will be randomised 1:1 via computerised randomisation tool via the INTERCEPT Redcap database with stratification by zone of TEVAR. The latter has been chosen because more proximal landing zones (PLZs) in the aortic arch for stent-graft placement are closer to the cerebral vessels and represent a greater risk factor for stroke (zone 0>1>2>3>4). Stratification by zones will ensure the groups are similar with respect to this potential confounding factor. Randomisation will occur on the day of surgery. The surgical team delivering the intervention in theatre will be unblinded but are not involved in assessing the outcomes of the study. Participants and outcome assessors will be blinded to group allocation. For sheathed devices, there is a side-port for flushing with saline and/or CO2. For unsheathed devices (eg, CTAG, Gore), bench top-models have shown that using a dry seal, can allow sufficient flushing of the stent with CO2 and saline.
Box 2. Inclusion and exclusion criteria.
Inclusion criteria
All patients suitable for TEVAR for any thoracic aortic pathology in zones 0–4.
Exclusion criteria
Stroke within the last 12 months
Pregnancy
<18 years
Unwilling or unable to provide informed consent
Contraindications to MRI, eg, Permanent Pacemaker (PPM), cerebral aneurysm clips, cochlear implant
Withdrawal criteria
Any patient has the right to withdraw from the study at any point; their treatment and management will not be altered in any way.
Abbreviation: TEVAR, thoracic endovascular aortic repair.
Primary objectives: evaluation of pilot RCT processes
Conduct an evaluation of the processes described in this pilot RCT, to inform the feasibility and design of a full-scale RCT. Evaluation outcome measures includes:
Recruitment (number eligible and willing to be randomised, identify challenges to randomisation).
Retention in follow-up assessments.
Study design for the full RCT (appropriateness of inclusion/exclusion criteria, study outcomes) and identification of important stratification variables.
Sample size refinement for a future full-scale RCT.
Secondary objectives: neurological outcomes
1. Primary neurological outcome: Incidence of DW-MRI SCI
MRI scans will be performed at each site where the patient is recruited from.
DW-MRI will be performed within 72 hours postoperatively (1-7 days will also be eligible for analysis) to look for new lesions using a 3-Tesla Discovery MR750w system (GE healthcare, UK) or equivalent system, and at 6 months routine outpatient appointment to look for residual disease. We have previously published the MRI protocol15 that we will use and these sequences may have to be modified where only a 1.5T scanner is available and discussions with the local MR department will be undertaken to ensure image accuracy. Chronic small vessel ischaemia will be classified using the Fazekas Scale.27 Preop MRI will not be carried out, with a Fazekas score carried out on their postop MRI to give an estimation of their chronic small vessel disease. This decision was made due to previous experience of loss of patients for follow-up scans, and the focus of the MRIs being on acute lesions, which will be easily identifiable using the MRI sequences chosen. MRIs will be compared for number, laterality and vascular territory (anterior or posterior circulation or border zone territory) of lesions. Maximum diameter and surface area of lesions will also be recorded and lesion surface area as measured on the slice of largest lesion diameter. Lesions are considered as separate if there is no continuity between them on the same slice and adjacent slices.
2. Secondary neurological outcome: detection of periprocedural cerebral solid and gaseous emboli
Continuous bilateral TCD insonation of the middle cerebral artery will be used to detect rates of intraoperative solid and gaseous cerebral MESs throughout all stages of TEVAR. For logistical reasons, this will likely be carried out at London centres only. Accepted criteria for emboli detection will be used.28 MESs will be differentiated between solid and gas through software using multifrequency TCD instrumentation which insonates simultaneously between 2.0MHz and 2.5MHz (EmboDop DWL, Compumedics Ltd, Germany). Manual offline analysis of the number of solid and gaseous emboli will be performed by trained assessors independent of each other. As it is impossible to characterise a solid or gas embolus manually during an 'embolic shower', the automated observations of the TCD equipment will be used.
3. Secondary neurological outcomes: neurological assessment, delirium, neurocognitive and quality of life testing
Preoperatively all patients will undergo:
Neurological assessment and outcome measurement with the National Institutes of Health Stroke (NIHSS)29 and disability assessment on modified Rankin scale (mRS).30–32
Baseline delirium test with the 4AT.33
Screening test for cognitive impairment with Montreal Cognitive Assessment (MOCA).34
-
Detailed neurocognitive assessment with a battery of validated tests categorised into visual memory, executive function, attention and decision-making. These have been devised after review of the literature, they are tests which we have used in our previous studies35 and have been pragmatically chosen in collaboration with a clinical psychologist.
Rey Auditory Verbal Learning.36
‘FAS’- Verbal fluency test (paper-based test).37
Grooved Pegboard Test (instrumentation based test to assess manual dexterity).38
Trail making test TMT39 (paper-based test to assess attention and switching).
Hospital Anxiety and Depression Scale40 to detect any psychological influence on the test results (paper-based).
National Adult Reading Test41 to test premorbid intelligence levels.
Quality of life assessment with Short Form Survey (SF-3642)and EQ5D5L.43
Within 48 hours of patients return to level 1 care (or prior to discharge if discharged from Intensive Therapy Unit (ITU):
NIHSS and mRs
4AT
MOCA
6-Week (or first outpatient appoitment) and 6 month follow-ups:
NIHSS and mRS
4AT.
MOCA and neurocognitive battery as above
SF-36 and EQ5D5L
4. Secondary neurological outcome: serial biomarker blood tests (eg, S100B)
A sample of the patient’s blood will be taken along with routine blood tests preoperatively, at the end of procedure and 24 hours later. We will study the upregulation of proinflammatory mediators in response to TEVAR between the two groups. Serial measurement of biomarkers will look at inflammatory pathway upregulation, modification of low-density lipoprotein (LDL) moieties inducing the modification of LDL into oxidised LDL and consumption of protection antibodies that work on maintaining homeostasis against danger-associated molecular patterns.44 S100B is regarded as a marker of brain damage. Reduced serum levels have been detected in patients who underwent CO2 field flooding in mitral valve operations with cardiopulmonary bypass where there is a risk of CAE.45 Further analysis will be done via a proteomic inflammatory panel analysis.46 We will also study the extent of neurological injury using S100B and markers of cell death: TNF receptor 1 (TNFR-1), TRAIL receptor 2 (TRAILR-2) and Fas.47 48
Levels of biomarkers will be correlated with DW-MRI SCI, neurological and neurocognitive assessments. For pragmatic reasons including transportation this test will only be conducted in participants recruited at London hospitals.
The samples will be centrifuged and stored at −80°C. Using Enzyme Linked Immunosorbent Assay, we will then analyse for S100B among a number of other biomarkers at the National Heart and Lung Institute by SC.
5. Secondary neurological outcome: risk factor assessment
Procedural risk factors such as conventional PLZs for the stent,45 coverage of arch vessel origins and intraoperative factors such as but not limited to, number of digital subtraction angiography runs and length of time of hypotension, stent type, length of procedure and post stent ballooning will be recorded for multivariate analysis to allow risk factor assessment.
Sample size
Observational data indicate that the incidence of SCI from TEVAR is 81%.6 Based on our CO2-pilot study that reduced SCI to 25%, a 50% reduction in SCI is possible. Taking a pragmatic and realistic approach to recruitment, we aim for an effect size of 40% reduction in incidence of SCI. Considering a 10% MRI dropout rate from our observational study, a total of 76 (38 per group) would be sufficient to detect an effect size. However, given that randomisation will be by zone of TEVAR, of which there are 5, and we expect a 20% MRI drop-out rate, we are aiming to recruit 120 cases (60 in each arm). This number has been chosen to ensure 10–12 patients in each of five arch landing zones in each of the two intervention groups, to allow us to quantify brain injury by zone between the two interventions in addition to establishing an overall measure of effect between the two interventions.
Statistical analysis
Statistical analysis will be by intention to treat. Standard descriptive statistics will be used throughout (mean, range, SD and median, IQR), with comparative statistics for normally and non-normally distributed data with p<0.05 considered as significant. Cronbach’s alpha will be used to assess inter-rater reliability of MRI and TCD data. Subgroup analysis will be used to examine SCI and TCD MES rates with respect to PLZ, atheroma grade and stent-graft type.
The data monitoring committee will be made up of SC and LH. They will carry out interim analysis on an ad hoc basis, with no specific stopping guidelines. Any adverse events will be recorded in the trial management folder, and serious adverse events will be reviewed by the chief investigator (CI), with involvement of the local ethics committee if indicated. There will be no planned audits, but any audits will be undertaken by Imperial R&D if required.
Patient and public involvement
None.
Ethics and dissemination
The study coordination centre has obtained approval from the London Fulham Research Ethics Committee and Southern Health and Disability Ethics Committee (NZ) and UK’s Health Regulator Authority (HRA). The study will be conducted in accordance with Declaration of Helsinki. Any protocol modifications will be undertaken through the local ethics committee. Consent for entering into the study will be taken using standardised consent forms (see online supplemental materials) by the local study team, led by a local PI. For St Mary’s Hospital, St George’s Hospital and St Thomas’ Hospital, this includes consenting for blood sampling for biochemical marker analysis. Patients will be given an anonymised code on entering the trial, which will be stored on a secure hard drive to maintain confidentiality throughout.
bmjopen-2022-067605supp001.pdf (476.7KB, pdf)
The study has received ethical approval for recruitment in the UK (Fulham REC, 19/LO/0836), New Zealand (21/STH/192) and the USA (IRB 019264, Ref 378630). The trial is registered at ClinicalTrials.gov (NCT03886675).
The authors have no financial or competing interest to declare. The final trial dataset will be accessible by the trial coordinators (SC and LH), as well as the CI (RG). Post-trial provisions and compensation are covered by the policy with Gallagher insurance company. The results of the trial will be submitted for publication in an open access journal.
Protocol version
Based on protocol version 7 (6 February 2023).
Supplementary Material
Footnotes
Contributors: SC has been involved in the set-up, data collection and write up for this project. LH designed the trial, gained ethical approval and gained funding for the trial. AS developed the MRI protocol, and will be the blinded assessor of the MRIs for the trial. SG has developed the neurocognitive battery with LH, and helped in neurocognitive training for staff. RN, CB and MH were involved in the study design. DG was involved in study design and is PI for Baylor Scott & White (Texas). MS is the PI for St Thomas’ Hospital. BM has been involved in the study design, and data collection alongside SA. OL is the PI for CDHB (New Zealand). RG is the chief investigator for the study and led the study design, ethical approval and funding application.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Lee HC, Joo H-C, Lee SH, et al. Endovascular repair versus open repair for isolated descending thoracic aortic aneurysm. Yonsei Med J 2015;56:904–12. 10.3349/ymj.2015.56.4.904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feezor RJ, Martin TD, Hess PJ, et al. Risk factors for perioperative stroke during thoracic endovascular aortic repairs (TEVAR). J Endovasc Ther 2007;14:568–73. 10.1177/152660280701400420 [DOI] [PubMed] [Google Scholar]
- 3.Delafontaine J-L, Hu B, Tan T-W, et al. Outcome comparison of TEVAR with and without left subclavian artery revascularization from analysis of nationwide inpatient sample database. Ann Vasc Surg 2019;58:174–9. 10.1016/j.avsg.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 4.Chaikof EL, Mutrie C, Kasirajan K, et al. Endovascular repair for diverse pathologies of the thoracic aorta: an initial decade of experience. J Am Coll Surg 2009;208:802–16. 10.1016/j.jamcollsurg.2008.12.021 [DOI] [PubMed] [Google Scholar]
- 5.Ehlert BA, Durham CA, Parker FM, et al. Impact of operative indication and surgical complexity on outcomes after thoracic endovascular aortic repair at national surgical quality improvement program centers. J Vasc Surg 2011;54:1629–36. 10.1016/j.jvs.2011.05.116 [DOI] [PubMed] [Google Scholar]
- 6.Perera AH, Rudarakanchana N, Monzon L, et al. Cerebral embolization, silent cerebral infarction and neurocognitive decline after thoracic endovascular aortic repair. Br J Surg 2018;105:366–78. 10.1002/bjs.10718 [DOI] [PubMed] [Google Scholar]
- 7.Fanning JP, Wesley AJ, Walters DL, et al. Neurological injury in intermediate-risk transcatheter aortic valve implantation. J Am Heart Assoc 2016;5:11.:e004203. 10.1161/JAHA.116.004203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2013;44:2064–89. 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lansky AJ, Messé SR, Brickman AM, et al. Proposed standardized neurological endpoints for cardiovascular clinical trials: an academic research consortium initiative. J Am Coll Cardiol 2017;69:679–91. 10.1016/j.jacc.2016.11.045 [DOI] [PubMed] [Google Scholar]
- 10.Gutsche JT, Cheung AT, McGarvey ML, et al. Risk factors for perioperative stroke after thoracic endovascular aortic repair. Ann Thorac Surg 2007;84:1195–200; 10.1016/j.athoracsur.2007.04.128 [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S, Okada K, Koide H, et al. Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke 1997;28:1932–9. 10.1161/01.str.28.10.1932 [DOI] [PubMed] [Google Scholar]
- 12.Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–22. 10.1056/NEJMoa022066 [DOI] [PubMed] [Google Scholar]
- 13.Kahlert P, Eggebrecht H, Jánosi RA, et al. Silent cerebral ischemia after thoracic endovascular aortic repair: a neuroimaging study. The Annals of Thoracic Surgery 2014;98:53–8. 10.1016/j.athoracsur.2014.03.037 [DOI] [PubMed] [Google Scholar]
- 14.Bismuth J, Garami Z, Anaya-Ayala JE, et al. Transcranial Doppler findings during thoracic endovascular aortic repair. Journal of Vascular Surgery 2011;54:364–9. 10.1016/j.jvs.2010.12.063 [DOI] [PubMed] [Google Scholar]
- 15.Grover G, Perera AH, Hamady M, et al. Cerebral embolic protection in thoracic endovascular aortic repair. J Vasc Surg 2018;68:1656–66. 10.1016/j.jvs.2017.11.098 [DOI] [PubMed] [Google Scholar]
- 16.Inci K, Koutouzi G, Chernoray V, et al. Air bubbles are released by thoracic endograft deployment: an in vitro experimental study. SAGE Open Med 2016;4:2050312116682130. 10.1177/2050312116682130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohlffs F, Tsilimparis N, Saleptsis V, et al. Air embolism during TEVAR: carbon dioxide flushing decreases the amount of gas released from thoracic stent-grafts during deployment. J Endovasc Ther 2017;24:84–8. 10.1177/1526602816675621 [DOI] [PubMed] [Google Scholar]
- 18.Pappano AJ, Wier WG. Cardiovascular physiology. 2019. [Google Scholar]
- 19.Mitchell S, Gorman D. The pathophysiology of cerebral arterial gas embolism. J Extra Corpor Technol 2002;34:18–23. [PubMed] [Google Scholar]
- 20.Borger MA, Peniston CM, Weisel RD, et al. Neuropsychologic impairment after coronary bypass surgery: effect of gaseous microemboli during perfusionist interventions. The Journal of Thoracic and Cardiovascular Surgery 2001;121:743–9. 10.1067/mtc.2001.112526 [DOI] [PubMed] [Google Scholar]
- 21.Martens S, Neumann K, Sodemann C, et al. Carbon dioxide field flooding reduces neurologic impairment after open heart surgery. The Annals of Thoracic Surgery 2008;85:543–7. 10.1016/j.athoracsur.2007.08.047 [DOI] [PubMed] [Google Scholar]
- 22.Svenarud P, Persson M, van der Linden J. Effect of CO2 insufflation on the number and behavior of air microemboli in open-heart surgery: a randomized clinical trial. Circulation 2004;109:1127–32. 10.1161/01.CIR.0000118501.44474.83 [DOI] [PubMed] [Google Scholar]
- 23.Chaudhuri K, Storey E, Lee GA, et al. Carbon dioxide insufflation in open-chamber cardiac surgery: a double-blind, randomized clinical trial of neurocognitive effects. J Thorac Cardiovasc Surg 2012;144:646–653. 10.1016/j.jtcvs.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 24.Kölbel T, Rohlffs F, Wipper S, et al. Carbon dioxide flushing technique to prevent cerebral arterial air embolism and stroke during TEVAR. J Endovasc Ther 2016;23:393–5. 10.1177/1526602816633705 [DOI] [PubMed] [Google Scholar]
- 25.Lyons O, Schmidli J. Preventing stroke due to intervention in the aortic arch. European Journal of Vascular and Endovascular Surgery 2021;61:246–7. 10.1016/j.ejvs.2020.11.031 [DOI] [PubMed] [Google Scholar]
- 26.Markus HS, Punter M. Can transcranial doppler discriminate between solid and gaseous microemboli? assessment of a dual-frequency transducer system. Stroke 2005;36:1731–4. 10.1161/01.STR.0000173399.20127.b3 [DOI] [PubMed] [Google Scholar]
- 27.Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in alzheimer’s dementia and normal aging. AJR Am J Roentgenol 1987;149:351–6. 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 28.Basic identification criteria of doppler microembolic signals . Consensus committee of the ninth international cerebral hemodynamic symposium. Stroke 1995;26:1123. 10.1161/01.STR.26.6.1123 [DOI] [PubMed] [Google Scholar]
- 29.National Institute of Neurological, D. and Stroke . NIH stroke scale. National institute of neurological disorders and stroke, dept. of health and human services, USA, 2011. [Google Scholar]
- 30.RANKIN J. Cerebral vascular accidents in patients over the age of 60. II. prognosis. Scott Med J 1957;2:200–15. 10.1177/003693305700200504 [DOI] [PubMed] [Google Scholar]
- 31.Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke 1988;19:1497–500. 10.1161/01.str.19.12.1497 [DOI] [PubMed] [Google Scholar]
- 32.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. 10.1161/01.str.19.5.604 [DOI] [PubMed] [Google Scholar]
- 33.Saller T, MacLullich AMJ, Perneczky R. The 4AT-an instrument for delirium detection for older patients in the post-anaesthesia care unit. Anaesthesia 2020;75:410. 10.1111/anae.14937 [DOI] [PubMed] [Google Scholar]
- 34.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 35.Perera AH, Riga CV, Monzon L, et al. Robotic arch catheter placement reduces cerebral embolization during thoracic endovascular aortic repair (TEVAR). European Journal of Vascular and Endovascular Surgery 2017;53:362–9. 10.1016/j.ejvs.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 36.Bean J. Rey auditory verbal learning test, rey AVLT, in encyclopedia of clinical neuropsychology. New York, NY: Springer, 2011: 2174–5. [Google Scholar]
- 37.Patterson J. F-A-S test, in encyclopedia of clinical neuropsychology. 2011:1024–6.
- 38.Merker B, Podell K. Grooved pegboard test, in encyclopedia of clinical neuropsychology. New York, NY, 2011: 1176–8. 10.1007/978-0-387-79948-3 [DOI] [Google Scholar]
- 39.Llinàs-Reglà J, Vilalta-Franch J, López-Pousa S, et al. The TRAIL making test. Assessment 2017;24:183–96. 10.1177/1073191115602552 [DOI] [PubMed] [Google Scholar]
- 40.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 41.National adult reading test, in encyclopedia of clinical neuropsychology. New York, NY: Springer, 2011: 1705. 10.1007/978-0-387-79948-3 [DOI] [Google Scholar]
- 42.Ware JE, Brook RH, Davies-Avery A. Conceptualization and measurement of health for adults in the health insurance study: model of health and methodology. 1980. [Google Scholar]
- 43.EuroQol Group . EuroQol -- a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 44.Khan TZ, Hartley A, Haskard D, et al. Oxidised ldl and anti-oxidised ldl antibodies are reduced by lipoprotein apheresis in a randomised controlled trial on patients with refractory angina and elevated lipoprotein(a). Antioxidants (Basel) 2021;10:132. 10.3390/antiox10010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishimaru S. Endografting of the aortic arch. J Endovasc Ther 2004;11 Suppl 2:II62–71. 10.1177/15266028040110S614 [DOI] [PubMed] [Google Scholar]
- 46.Accelerated proteomics together. 28/07/20. n.d. Available: https://www.olink.com/products/inflammation)
- 47.Hartley A, Haskard D, Khamis R. Markers of apoptosis predict cardiovascular outcomes and point to “response to injury” as a common pathway leading to diabetes and cardiovascular events. EBioMedicine 2018;28:19–20. 10.1016/j.ebiom.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorla R, Erbel R, Eagle KA, et al. Systemic inflammatory response syndromes in the era of interventional cardiology. Vascul Pharmacol 2018:S1537-1891(18)30020-X. 10.1016/j.vph.2018.04.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067605supp001.pdf (476.7KB, pdf)