Abstract
Antiphospholipid syndrome is a systemic autoimmune disorder characterized by vascular thrombosis and/or obstetric events in association with persistently elevated antiphospholipid antibodies. Antiphospholipid syndrome is typically considered a rare disease, but the true incidence is uncertain owing to the diverse antiphospholipid antibody-related clinical manifestations, inconsistent definitions of antiphospholipid antibody positivity, under-recognition of the disease, and limited population-based studies. Published estimates of the incidence of antiphospholipid syndrome range from approximately 2 to 80 per 100 000 person-years. A targeted literature review and applied methodology were performed to derive a best available estimate. Significant limitations of the published literature were observed, some of which have been previously reported. The incidence of antiphospholipid syndrome in the United States was estimated to be approximately 7.1 to 13.7 per 100 000 person-years in the general population. Although this estimate is likely more accurate than previously reported estimates, large, contemporary, population-based studies that reasonably adhere to the antiphospholipid syndrome classification criteria are needed to further refine estimates of the incidence of antiphospholipid syndrome.
Keywords: Antiphospholipid syndrome, antiphospholipid antibodies, incidence, epidemiology
Main Points
The true incidence of antiphospholipid syndrome (APS) is uncertain because of diverse APS-related clinical manifestations, inconsistent definitions of antibody positivity, under-recognition of the disease, and limited population-based studies.
Using a targeted literature review and applied methodology, this study estimated an incidence of 7.1 to 13.7 per 100 000 person-years.
Large, contemporary, population-based studies that reasonably adhere to the APS classification criteria are needed to further refine estimates of the incidence of APS.
Introduction
Antiphospholipid syndrome (APS) is a systemic autoimmune disorder characterized by vascular thrombosis and/or obstetric events in association with persistently elevated antiphospholipid antibodies (aPLs). According to international classification criteria for APS,1 APS requires: (i) vascular thrombosis (arterial, venous, or small vessel in any tissue or organ) or pregnancy morbidity (≥3 consecutive early pregnancy loses; pregnancy loss after 10 weeks gestation; or premature delivery prior to 34 weeks gestation due to preeclampsia or placental insufficiency) plus (ii) aPL, including positive lupus anticoagulant test, and/or anticardiolipin antibodies (aCLs), and/or anti-β2 glycoprotein-I antibodies (aβ2GPI) in medium to high titers for at least 12 weeks. It is uncommon that both thrombotic and obstetric events occur in the same person with APS2; therefore, these manifestations are often considered separately.
Antiphospholipid syndrome is typically considered a rare disease,3 but the true incidence is uncertain owing to the diverse aPL-related clinical manifestations, inconsistent definitions of aPL-positivity, under-recognition of the disease, and limited population-based studies. Because aPL can be transient, the APS classification criteria require that positive test results be present in “medium to high titer” and be repeated after 12 weeks to confirm APS1; however, very few published studies of the incidence of APS have used standardized cutoff values for aPL titers or performed repeated testing.4 Furthermore, older studies of APS did not assess for all 3 aPLs,4 because these studies preceded the inclusion of aβ2GPI in the APS classification criteria in 2006.1 Studies of the obstetric manifestations of APS did not consistently assess premature delivery prior to 34 weeks gestation due to preeclampsia or placental insufficiency, and studies of pregnancies that did not result in live births often used imprecise and inconsistent terminology (e.g., spontaneous abortion, miscarriage, fetal loss, and stillbirth) that made interpretation and comparison of results difficult.4 Lastly, APS commonly causes clinical manifestations that are currently not included in the classification criteria (e.g., thrombocytopenia and livedo5), but that nevertheless may be considered when making the clinical diagnosis of APS.6
To date, only 1 large population-based incidence study has rigorously and strictly applied the APS classification criteria with respect to qualifying obstetric events, repeat aPL testing, and minimum aPL titers.7 This study reported an incidence rate of 2.1 per 100 000 person-years but had several important limitations. Overall, the sample size was small; the incidence estimate was derived from 33 observed cases over the course of 16 years of observation in Olmsted County, Minn, USA. As acknowledged by the authors, this study, conducted between 2000 and 2015, was significantly limited by the lack of completeness of the clinical evaluation performed at the time of the clinical events. Patients who presented with clinical features consistent with APS but were not assessed for aβ2GPI or persistence of aPL could not satisfy the APS classification criteria even if they truly had APS. The number of incompletely evaluated and undiagnosed cases of APS in the study population is hence unknown. Therefore, this study most likely underestimated the incidence of APS and should be considered a minimum estimate.
At the opposite end of the spectrum, a review of 120 studies of aPL positivity associated with thrombotic or obstetric events estimated an incidence of APS of approximately 80 per 100 000 person-years in the USA.4 This review highlighted the major limitations of the literature; fewer than 20% of the included studies required repeat aPL testing for confirmation, and there was no consistent use of cutoff values for medium or high titer aPL levels (i.e., low titer aPLs were often considered positive). Additionally, there was rarely consideration of other risk factors for thrombotic events (e.g., cardiovascular risk factors for stroke or myocardial infarction in older patients were not considered and all patients were tested for aPL indiscriminately). Because normal healthy persons can have transient or low-titer aPL, this study most likely overestimated the incidence of APS and should be considered a maximum estimate.
Thus, the true incidence of APS very likely lies between the minimum estimate of 2 per 100 000 person-years determined by strict adherence to the APS classification criteria7 and the maximum estimate of 80 per 100 000 person-years determined by any presence of aPL.4 This study aimed to perform a targeted review of the published literature to refine the estimated incidence of APS in the USA.
Methods
A targeted literature review of PubMed was conducted to identify the most relevant publications describing the incidence of APS. Searches were performed using keywords and Medical Subject Headings, title/abstract, and full-text designations. In addition, the reference sections of all identified articles were searched. The search was restricted to full-text articles published in English after 1980. The primary search was conducted in September 2019 and partially updated in January 2021 to assess for recently published articles. When considering which publications to include, priority was assigned to studies that were meta-analyses, systematic reviews, or review articles. When multiple original studies were identified, studies with larger sample sizes, more rigorous methods, more recent publication dates, and more frequently referenced by other publications were prioritized. Only the highest quality studies among the studies identified for each thrombotic or obstetric event were included. No human subjects were involved in this study, and ethics approval was not sought.
An indirect method was used to estimate the incidence of APS (Figure 1). The first step was to identify the published incidence in the general population of the USA for the most common thrombotic events [venous thromboembolism (VTE), myocardial infarction (MI), and stroke] and all 3 obstetric events included in the APS classification criteria. For the determination of incidence rates, the population of the USA was estimated to be 330 million (www.census.gov). The second step was to estimate the proportion of thrombotic events and obstetric events that may be attributable to APS based upon published studies, including studies of the prevalence of aPL near the time of thrombotic or obstetric events. Because aPL can be identified in asymptomatic individuals and because many identified studies did not properly apply APS criteria by restricting positive results to medium or high titers and by repeating testing 12 weeks later, the estimated proportion of patients with aPL but without APS was subtracted from the proportions reported with aPL in the included studies to determine the proportion of thrombotic or obstetric events truly attributable to APS. The proportion of aPL without APS was determined from general studies of healthy individuals without APS (e.g., healthy blood donors) and controlled studies assessing the specific outcomes (e.g., case–control studies reporting the proportion of persons without thrombotic or obstetric events found to have aPL). The third step was to multiply the incidence of thrombotic and obstetric events by the estimated proportion attributable to APS for each event to determine the event-specific APS incidence rate. The event-specific incidence rates were then summed to determine the incident rates for thrombotic APS, obstetric APS, and overall APS.
Figure 1.
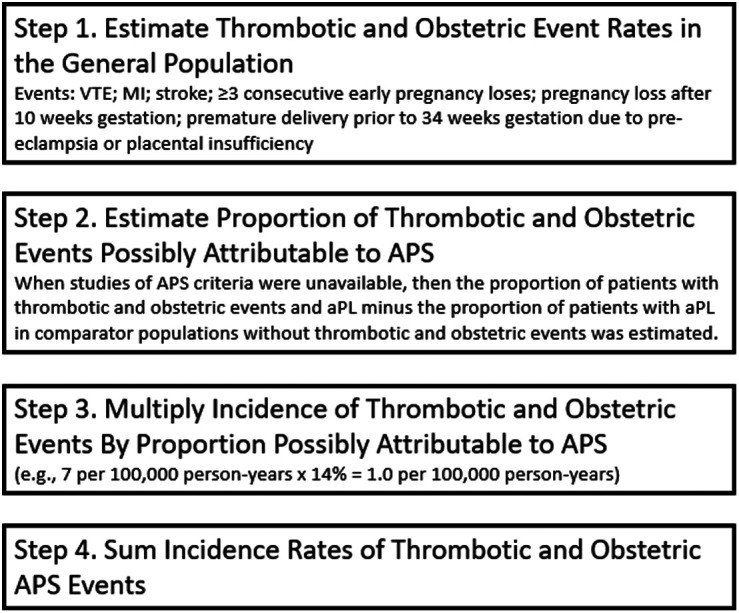
Indirect method to estimate the incidence of APS. aPL, antiphospholipid antibodies; APS, antiphospholipid syndrome; MI, myocardial infarction; VTE, venous thromboembolism.
Results
This targeted literature review identified 8 studies that provided best estimates of the incidence of the thrombotic and obstetric events of interest.8-15 Three studies were identified that assessed for APS in persons with VTE,16-18 6 studies assessed aPL in persons with thrombotic and obstetric events,4,12,19-22 and 3 studies assessed aPL without APS.21,23,24 One broadly applicable estimate from a study of healthy blood donors reported the presence of IgG aCL in approximately 6.5% of persons.23 This estimate was considered in the determination of the proportion of events attributable to APS in the thrombotic and obstetric outcomes.
Table 1 lists the findings of the targeted literature review and the determination of the estimates for the incidence of thrombotic and obstetric APS events.
Table 1.
Estimated Incidence of Potential Thrombotic APS and Obstetric APS Events in the USA
| Potential APS Event | Annual Event Incidence in the USA per 100 000 Person-Years | Percentage of Events Attributed to APS | Incidence of Event Attributable to APS in General Population per 100 000 person-years | References |
|---|---|---|---|---|
| Venous thromboembolism | 120 (total) | 6% | All events 2.9-7.2 |
(4, 8, 15-18) |
| 7 (first VTE, unprovoked, <50 years old) | 14% | |||
| 37 (first VTE, unprovoked, >50 years old) | 5% | |||
| 44 (first VTE, unprovoked, all ages) | 9% | |||
| Myocardial infarction | 15 (<45 years old) | 4.5%-8% | 0.7-1.2 | (1, 4, 9, 23, 24) |
| Stroke | 15 (<50 years old) | 11%-16% | 1.7-2.4 | (1, 9, 19, 20, 23, 24) |
| Early pregnancy loss | 13 | 8.5%-11.5% | 1.1-1.5 | (10-12, 23, 24) |
| Late pregnancy loss | 7 | 4%-8% | 0.3-0.6 | (13, 21, 23) |
| Pre-term delivery due to preeclampsia | 4 | 10%-20% | 0.4-0.8 | (14, 22) |
APS, antiphospholipid syndrome; VTE, venous thromboembolism.
Incidence of Thrombotic Antiphospholipid Syndrome Events
Venous Thromboembolism: Based upon a published review of 11 studies,8 the annual number of VTE (including deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE) in the USA is approximately 400 000 (approximately 120 per 100 000 person-years in the general population). The proportion of VTE caused by APS is age dependent and drops sharply with increasing age because of the development of other important risk factors for VTE,4 and this was considered in the estimate. According to a large prospective registry of patients with VTE,15 6% of all VTE episodes were first VTE episodes in patients less than 50 years old without typical risk factors for VTE (i.e., idiopathic or unprovoked VTE), and 31% of all VTE were first, unprovoked events in patients greater than 50 years old. The corresponding incidence rates for first, unprovoked VTE in patients less than and greater than 50 years old are 7 and 37 events per 100 000 person-years in the general population, respectively.
Studies that applied the APS criteria to populations of patients with VTE, rather than assessing only positive aPL results, were identified. A cross-sectional study of data from an oral anticoagulation dosage program reported that 9% of patients less than age 50 with a first, unprovoked VTE met APS criteria.16 A smaller, prospective study, also of patients less than age 50 with a first, unprovoked VTE, reported that 19% of patients met APS criteria.17 Taking the midpoint of these 2 reports, an estimated 14% of patients less than age 50 years with a first, unprovoked VTE had APS. Multiplying this estimate by the incidence of VTE in this patient population (7 per 100 000 person-years) results in an estimate of approximately 1.0 per 100 000 person-years in the general population.
Considering patients older than 50 years, a prospective study of all adult patients with first, unprovoked VTE reported that 9% of patients met APS criteria, irrespective of age (the mean age of patients in the study was 52 years).18 Because an estimated 14% of patients younger than 50 years old with a first, unprovoked VTE have APS (see above), an estimated approximately 5% of patients older than 50 years had APS, which would be expected to produce the resultant 9% reported for the entire study population of all adult patients.18 Multiplying this estimate (5%) by the incidence of VTE attributable to this patient population (37 per 100 000 person-years) results in an estimate of approximately 1.9 per 100 000 person-years in the general population.
Adding together the estimates for patients below and above age 50 years, the total incidence of APS manifested by VTE is approximately 2.9 per 100 000 person-years in the general population. If one instead disregards patient age and assumes that overall 9% of all patients with a first, unprovoked VTE have APS,18 then the estimate is 4.0 per 100 000 person-years (9% of 44 per 100 000 person-years). The true incidence of APS manifested by VTE is likely higher than this estimate because patients may have both a provoking risk factor for VTE and APS concurrently, as has been demonstrated.15 In fact, the proportion of patients with VTE attributable to APS may be as high as 6% across all patients based upon the median result from 5 studies that conducted confirmatory aPL testing of patients with DVT.4 If true, this would place the incidence of APS manifested as VTE at approximately 7.2 per 100 000 person-years in the general population (6% of 120 per 100 000 person-years).
Taken altogether, the incidence of APS manifested as VTE is most likely between approximately 2.9 and 7.2 per 100 000 person-years in the general population.
Myocardial Infarction: For the determination of the incidence of MI potentially attributable to APS, only events in young adults (less than 45 years old) were considered. Current recommendations advise against aPL testing in patients with MI unless the patient’s young age and lack of identifiable risk factors suggest rare etiology.1 Based upon epidemiologic data from the American Heart Association,9 the annual number of MI in the USA in persons less than 45 years old is approximately 50 000 (approximately 15 per 100 000 person-years in the general population).
The proportion of MI events associated with aPL was estimated to be approximately 11% based upon a review of 24 studies.4
A review of 11 controlled studies of the association between aPL and MI reported aPL detection in 3% of control patients,24 somewhat lower than the 6.5% reported among healthy blood donors.23 Considering the estimate for aPL above,4 approximately 4.5% (11% minus 6.5%) to 8% (11% minus 3%) of MI was attributable to APS among persons less than 45 years old. Although when restricted to 4 studies with confirmatory aPL testing, the aforementioned critical review reported a much higher median value of 18%,4 suggesting that the proportion of MI attributable to APS may be higher.
Multiplying the incidence of MI in persons less than 45 years old (15 per 100 000 person-years in the general population) by the proportion attributable to APS (4.5%-8%), the estimated incidence of APS manifesting as MI is approximately 0.7-1.2 per 100 000 person-years in the general population.
Stroke
For the determination of strokes potentially attributable to APS, only events in patients less than 50 years old were considered. Current recommendations state that it is unclear what proportion of ischemic stroke can be attributed to APS, especially in older patients with other risk factors present.1 The annual number of strokes in the USA in persons less than 50 years old is approximately 50 000 (approximately 15 per 100 000 person-years in the general population) based on epidemiologic data from the American Heart Association.9
Regarding the proportion of stroke events associated with aPL, a review of 15 studies assessing laboratory evidence of thrombophilia following ischemic stroke reported that 21% of patients less than 50 years old had aPL.19 A different review of 38 studies reported a median frequency of aPL of 17.2% among stroke patients less than 50 years old.20
A review of 14 controlled studies of the association between aPL and stroke reported aPL detection in 5% of control patients,24 which was very similar to the 6.5% reported among healthy blood donors.23 Considering the estimates for aPL above,19,20 approximately 11% (17.2% minus 6.5%) to 16% (21% minus 5%) of stroke is attributable to APS among patients less than 50 years old.
Multiplying the incidence of stroke in persons less than 50 years old (15 per 100 000 person-years in the general population) by the proportion attributable to APS (11%-16%), the estimated incidence of APS manifesting as stroke is approximately 1.7-2.4 per 100 000 person-years.
Incidence of Obstetric Antiphospholipid Syndrome Events
Recurrent Early Pregnancy Loss
An estimate of the number of women with recurrent early pregnancy loss in the USA as defined by the APS classification criteria1 was not identified. However, it has been estimated (presumably by expert opinion) that 1% of all women attempting pregnancy have recurrent early pregnancy loss.12 Approximately 3.4 million women have intentional pregnancies each year,10 and this represents approximately 80% of women attempting to become pregnant.11 Therefore, approximately 4.3 million women are attempting to become pregnant each year in the USA (80% of 4.3 million is 3.4 million). Applying the 1% estimate from above,12 the estimated number of women with recurrent early pregnancy loss is approximately 43 000 per year (approximately 13 per 100 000 person-years in the general population).
The proportion of recurrent early pregnancy loss associated with aPL was estimated to be approximately 15% based upon a summary of results from 2 studies.12
A review of 2 controlled studies of the association between aPL and early pregnancy loss reported aPL detection in 3.5% of control patients,24 somewhat lower than the 6.5% reported among healthy blood donors.23 Considering the estimate for aPL above,12 approximately 8.5% (15% minus 6.5%) to 11.5% (15% minus 3.5%) of early pregnancy loss is attributable to APS.
Multiplying the incidence of recurrent pregnancy loss (13 per 100 000 person-years in the general population) by the proportion attributable to APS (8.5% to 11.5%), the estimated incidence of APS manifesting as recurrent pregnancy loss is approximately 1.1-1.5 per 100 000 person-years in the general population.
Late Pregnancy Loss
The number of women with late pregnancy loss is approximately 24 000 per year (approximately 7 per 100 000 person-years in the general population) based upon the US national fetal death data.13
The estimated proportion of late pregnancy loss associated with aPL was approximately 11% based upon a population-based case–control study of stillbirth in the USA.21
Because 5% of the control patients without late pregnancy loss in the population-based case–control study had aPL,21 an estimated approximately 6% (11% minus 5%) of late pregnancy loss is attributable to APS. To represent the uncertainty of this estimate derived from a single study, a range of 4%-8% was used to estimate the incidence.
Multiplying the incidence of late pregnancy loss (7 per 100 000 person-years in the general population) by the proportion attributable to APS (4%-8%), the estimated incidence of APS manifesting as late pregnancy loss is approximately 0.3-0.6 per 100 000 person-years.
Pre-term Delivery due to Preeclampsia
The number of pre-term deliveries due to preeclampsia is approximately 12 000 per year (approximately 4 per 100 000 person-years in the general population) based upon a review of 2 published studies.14
An estimate for the proportion of pre-term deliveries due to preeclampsia associated with aPL was not identified. Published studies did not use the APS classification criteria requirement for infant delivery prior to 34 weeks, but rather reported on preeclampsia in general, often irrespective of the timing of the onset of preeclampsia or the presence of pre-term delivery of the infant. The proportion of overall preeclampsia events associated with aPL was between 11% and 29% according to 4 case–control studies.22 An estimated approximately 10%-20% of pre-term deliveries due to preeclampsia are attributable to APS.
Multiplying the incidence of pre-term deliveries due to preeclampsia (4 per 100 000 person-years in the general population) by the proportion attributable to APS (10%-20%), the estimated incidence of APS manifesting as pre-term deliveries due to preeclampsia is approximately 0.4-0.8 per 100 000 person-years.
Incidence of Antiphospholipid Syndrome Overall
Table 2 lists the ranges of estimates of incidence of APS from this study and the minimum and maximum estimates from the 2 aforementioned studies.4,7
Table 2.
Estimated Incidence of APS Compared to Other Published Estimates in the USA
| Minimum Estimate7 | This Study | Maximum Estimate4 | |
|---|---|---|---|
| Incidence of thrombotic APS per 100 000 person-years | 1.8 | 5.3-10.8 | 65 |
| Incidence of obstetric APS per 100 000 person-years | 0.2 | 1.8-2.9 | 15 |
| Incidence of APS overall per 100 000 person-years | 2.1 | 7.1-13.7 | 80 |
APS, antiphospholipid syndrome.
Adding together the ranges of estimates for the most common manifestations of thrombotic APS, the estimated incidence of thrombotic APS is approximately 5.3-10.8 per 100 000 person-years in the general population. This is compared to the minimum estimate of 1.87 and the maximum estimate of 654 identified in the published literature. Adding together the ranges of estimates for the criteria manifestations of obstetric APS, the estimated incidence of obstetric APS is approximately 1.8-2.9 per 100 000 person-years in the general population. This is compared to the minimum estimate of 0.27 and the maximum estimate of 154 identified in the published literature. Combining the ranges of estimates for thrombotic and obstetric APS, the estimated incidence for APS overall is approximately 7.1-13.7 per 100 000 person-years in the general population. This is compared to the minimum estimate of 27 and the maximum estimate of 804 identified in the published literature.
Discussion
The incidence of APS is uncertain due to an inadequate number of studies rigorously analyzing aPL profiles that enable confirmation of persistency and fulfillment of current APS classification criteria. With these caveats in mind, a targeted review of the literature and applied methodology were performed to derive a best available estimate for the incidence of APS, which was found to be approximately 7.1-13.7 per 100 000 person-years in the general population of the USA. The range of estimated incidence lies near the geometric mean of the minimum estimate of 2 per 100 000 person-years7 and the maximum estimate of 80 per 100 000 person-years4 within the existing literature. Some expert authors previously estimated the incidence of APS to be approximately 5 cases per 100 000 person-years based upon personal interpretation of the published data and clinical experience, but no methodology or explanation was given for this estimate.25
Although published only in meeting abstract form to date, 2 additional studies on the incidence of APS have been reported. Using electronic medical records from a tertiary university hospital in Argentina, 1 study reported an overall incidence of 2.6 (95% CI 1.9-3.2) per 100 000 person-years.26 Another study from Brescia, Italy, reviewed medical records and estimated the incidence of primary APS among persons 18 to 50 years old to be 3.7 (95% CI 1.7-7.1) per 100 000 person-years.27 Details of the methodologies are limited by the meeting abstract format, but it appears likely that these studies had many of the same limitations as the study from the USA—most importantly, reliance on clinical evaluation and diagnosis of APS. In a Letter to the Editor, authors reported an incidence of 1.1 per 100 000 person-years according to data from the Rare Disease Registry of Piedmont and Aosta Valley, Italy.28 In addition to reliance on clinical evaluation and diagnosis of APS, this study may have been limited by incomplete reporting to the registry.29
Incomplete evaluation for APS is likely common in clinical practice. For example, aPLs are frequently not tested following VTE; in fact, the American College of Chest Physicians guidelines do not include APS among the criteria to determine the duration of anticoagulation following VTE.30 As noted, such practices result in lower estimates of the incidence of APS compared to the true incidence, as observed in the study from Olmstead County, USA,7 and presumably other studies.26-28
Although APS is known to occur in children,31 no estimates of the incidence of APS in children have been published,32 and this may influence estimates of the incidence of APS overall. For example, in the aforementioned study conducted in Olmsted County,7 the authors reported an incidence of 2.1 per 100 000 person-years among adults aged ≥18 years. Based on population figures published in the manuscript and assuming no cases of APS were identified in children, the incidence among all persons would be approximately 1.6 per 100 000 person-years. This is a considerable relative decrease in the estimated incidence rate, but compared to the wide range of incidence estimates reported in this current study, the overall impact of including versus excluding children is small. Because the age of patients was frequently not reported in the studies identified for this current study’s estimation, a general population incidence estimate that included persons of all ages was determined.
In addition to the limitations of the published literature, the clinical diagnosis of APS, and the current APS classification criteria,33 this current study’s methodology had limitations of its own. Importantly, it was assumed that the proportion of persons with aPL without true APS was similar between persons with and without thrombotic or obstetric events (i.e., the proportion of persons with asymptomatic or transient aPL in the general public was subtracted from the estimates of persons with thrombotic or obstetric events and aPL). This method assumed that no instances of MI or stroke that occurred in older patients were attributable to APS; this is unlikely to be true and may have led to an underestimate of the true incidence of APS. Transient ischemic attacks were not included in the assessment of stroke because they were not included in the best available estimates of the presence of aPL. Because transient ischemic attacks often precede strokes, this was an additional reason for not including transient ischemic attacks in the assessment of stroke (i.e., to avoid both transient ischemic attack and stroke being counted in the same person). Because thrombotic and obstetric events in APS may recur, some prevalent APS events (recurrent events) may have been categorized as incident events, and this would have overestimated the number of incident events. Although patients with obstetric APS may also have thrombotic manifestations of APS, this appears to occur in less than 10% of women2,34,35 and is unlikely to have substantially affected the accuracy of the incidence estimates. In general, some of the included studies were not population-based or had other methodological limitations; the potential effects on our incidence estimate are difficult to quantify. Finally, the targeted literature review was neither systematic nor exhaustive. The attempt to identify the best available estimates may have inadvertently omitted important published results. However, given the current challenges in assessing the incidence of APS, it appears unlikely that any omitted studies would have substantially improved the overall precision of the estimates.
In summary, the true incidence of APS remains unclear because of diverse aPL-related clinical manifestations, inconsistent definitions of aPL positivity, under-recognition of the disease, and limited population-based studies. Using a targeted literature review and applied methodology, this study estimated an incidence of APS of 7.1-13.7 per 100 000 person-years and likely represents a more accurate estimate than previously published estimates. Overall, an improved estimation was possible using this approach, but large, contemporary, population-based studies that reasonably adhere to the APS classification criteria are needed to further refine estimates of the incidence of APS.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept and Design – Y.I., T.B., M.M.; Literature Review, Data Collection, and Analyses – T.B.; Interpretation of Results – Y.I., T.B., M.M., D.E.; Writing – Y.I., T.B., D.E.; Critical Review – Y.I., T.B., M.M., D.E.
Acknowledgments: The authors thank Veronica Porkess of UCB Pharma for editorial support during the development of the manuscript.
Declaration of Interests: The authors have no conflicts of interest to declare.
Funding: This study was funded by UCB Pharma. Medical writing support was provided by Epi Excellence LLC (Garnet Valley, PA, USA) and funded by UCB Pharma, in accordance with Good Publication Practice (GPP3) guidelines (ismpp.org/gpp3).
References
- 1. Miyakis S, Lockshin MD, Atsumi T.et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295 306. ( 10.1111/j.1538-7836.2006.01753.x) [DOI] [PubMed] [Google Scholar]
- 2. Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R.et al. The European Registry on Obstetric Antiphospholipid Syndrome (EUROAPS): A survey of 1000 consecutive cases. Autoimmun Rev. 2019;18(4):406 414. ( 10.1016/j.autrev.2018.12.006) [DOI] [PubMed] [Google Scholar]
- 3. Tektonidou MG, Andreoli L, Limper M, Tincani A, Ward MM. Management of thrombotic and obstetric antiphospholipid syndrome: a systematic literature review informing the EULAR recommendations for the management of antiphospholipid syndrome in adults. RMD Open. 2019;5(1):e000924. ( 10.1136/rmdopen-2019-000924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andreoli L, Chighizola CB, Banzato A, Pons-Estel GJ, Ramire de Jesus G, Erkan D. Estimated frequency of antiphospholipid antibodies in patients with pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Arthritis Care Res (Hoboken). 2013;65(11):1869 1873. ( 10.1002/acr.22066) [DOI] [PubMed] [Google Scholar]
- 5. Cervera R, Piette JC, Font J.et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46(4):1019 1027. ( 10.1002/art.10187) [DOI] [PubMed] [Google Scholar]
- 6. Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018;378(21):2010 2021. ( 10.1056/NEJMra1705454) [DOI] [PubMed] [Google Scholar]
- 7. Duarte-García A, Pham MM, Crowson CS.et al. The epidemiology of antiphospholipid syndrome: a population-based study. Arthritis Rheumatol. 2019;71(9):1545 1552. ( 10.1002/art.40901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):3 14. ( 10.1007/s11239-015-1311-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benjamin EJ, Muntner P, Alonso A.et al. Heart disease and stroke Statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56 e528. ( 10.1161/CIR.0000000000000659) [DOI] [PubMed] [Google Scholar]
- 10. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843 852. ( 10.1056/NEJMsa1506575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol. 2004;103(1):51 56. ( 10.1097/01.AOG.0000100153.24061.45) [DOI] [PubMed] [Google Scholar]
- 12. Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010;376(9751):1498 1509. ( 10.1016/S0140-6736(10)60709-X) [DOI] [PubMed] [Google Scholar]
- 13. Hoyert DL, Gregory EC. Cause of fetal death: data from the fetal death report, 2014. Natl Vital Stat Rep. 2016;65(7):1 25. [PubMed] [Google Scholar]
- 14. Publications Committee, Society for Maternal-Fetal Medicine Simons foundation, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191 198. ( 10.1016/j.ajog.2011.07.017) [DOI] [PubMed] [Google Scholar]
- 15. Roldan V, Lecumberri R, Muñoz-Torrero JF.et al. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res. 2009;124(2):174 177. ( 10.1016/j.thromres.2008.11.003) [DOI] [PubMed] [Google Scholar]
- 16. Miranda S, Park J, Le Gal G.et al. Prevalence of confirmed antiphospholipid syndrome in 18-50 years unselected patients with first unprovoked venous thromboembolism. J Thromb Haemost. 2020;18(4):926 930. ( 10.1111/jth.14720) [DOI] [PubMed] [Google Scholar]
- 17. García-Fuster MJ, Forner MJ, Fernández C, Gil J, Vaya A, Maldonado L. Long-term prospective study of recurrent venous thromboembolism in patients younger than 50 years. Pathophysiol Haemost Thromb. 2005;34(1):6 12. ( 10.1159/000088541) [DOI] [PubMed] [Google Scholar]
- 18. Kearon C, Parpia S, Spencer FA.et al. Antiphospholipid antibodies and recurrent thrombosis after a first unprovoked venous thromboembolism. Blood. 2018;131(19):2151 2160. ( 10.1182/blood-2017-09-805689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bushnell CD, Goldstein LB. Diagnostic testing for coagulopathies in patients with ischemic stroke. Stroke. 2000;31(12):3067 3078. ( 10.1161/01.str.31.12.3067) [DOI] [PubMed] [Google Scholar]
- 20. Sciascia S, Sanna G, Khamashta MA.et al. The estimated frequency of antiphospholipid antibodies in young adults with cerebrovascular events: a systematic review. Ann Rheum Dis. 2015;74(11):2028 2033. ( 10.1136/annrheumdis-2014-205663) [DOI] [PubMed] [Google Scholar]
- 21. Silver RM, Parker CB, Reddy UM.et al. Antiphospholipid antibodies in stillbirth. Obstet Gynecol. 2013;122(3):641 657. ( 10.1097/AOG.0b013e3182a1060e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clark EA, Silver RM, Branch DW. Do antiphospholipid antibodies cause preeclampsia and HELLP syndrome? Curr Rheumatol Rep. 2007;9(3):219 225. ( 10.1007/s11926-007-0035-9) [DOI] [PubMed] [Google Scholar]
- 23. Vila P, Hernández MC, López-Fernández MF, Batlle J. Prevalence, follow-up and clinical significance of the anticardiolipin antibodies in normal subjects. Thromb Haemost. 1994;72(2):209 213. ( 10.1055/s-0038-1648840) [DOI] [PubMed] [Google Scholar]
- 24. Chighizola CB, Andreoli L, de Jesus GR.et al. The association between antiphospholipid antibodies and pregnancy morbidity, stroke, myocardial infarction, and deep vein thrombosis: a critical review of the literature. Lupus. 2015;24(9):980 984. ( 10.1177/0961203315572714) [DOI] [PubMed] [Google Scholar]
- 25. Gómez-Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. J Autoimmun. 2014;48-49:20 25. ( 10.1016/j.jaut.2014.01.006) [DOI] [PubMed] [Google Scholar]
- 26. Luissi A, Scolnik M, Soriano E. Incidence and prevalence of antiphospholipid syndrome in a health management organization (HMO): a 15-year study [abstract]. Arthritis Rheumatol. 2018;70(suppl 10) [Google Scholar]
- 27. Nalli C, Pascariello G, Zentilin A.et al. Primary antiphospholipid syndrome with vascular manifestations is a rare disease: a population-based, multi-source study assessing the prevalence and incidence in adults [abstract]. Arthritis Rheumatol. 2018;70(suppl 10). [Google Scholar]
- 28. Radin M, Sciascia S, Bazzan M.et al. Antiphospholipid syndrome Is Still a Rare Disease-Estimated Prevalence in the Piedmont and Aosta Valley Regions of Northwest Italy: comment on the Article by Duarte-Garcia et al. Arthritis Rheumatol. 2020;72(10):1774 1776. ( 10.1002/art.41401) [DOI] [PubMed] [Google Scholar]
- 29. Duarte-García A, Crowson CS, Warrington KJ, Matteson EL. Reply. Arthritis Rheumatol. 2020;72(10):1776. [DOI] [PubMed] [Google Scholar]
- 30. Kearon C, Akl EA, Ornelas J.et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315 352. ( 10.1016/j.chest.2015.11.026) [DOI] [PubMed] [Google Scholar]
- 31. Avcin T, Cimaz R, Silverman ED.et al. Pediatric antiphospholipid syndrome: clinical and immunologic features of 121 patients in an international registry. Pediatrics. 2008;122(5):e1100 e1107. ( 10.1542/peds.2008-1209) [DOI] [PubMed] [Google Scholar]
- 32. Aguiar CL, Soybilgic A, Avcin T, Myones BL. Pediatric antiphospholipid syndrome. Curr Rheumatol Rep. 2015;17(4):27. ( 10.1007/s11926-015-0504-5) [DOI] [PubMed] [Google Scholar]
- 33. Barbhaiya M, Zuily S, Ahmadzadeh Y.et al. Development of new international antiphospholipid syndrome classification criteria Phase I/II report: generation and reduction of candidate criteria. Arthritis Care Res (Hoboken). 2021;73(10):1490 1501. ( 10.1002/acr.24520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Drozdinsky G, Hadar E, Shmueli A, Gabbay-Benziv R, Shiber S. Obstetric antiphospholipid syndrome and long term arterial thrombosis risk. J Thromb Thrombolysis. 2017;44(3):371 375. ( 10.1007/s11239-017-1526-9) [DOI] [PubMed] [Google Scholar]
- 35. Ruffatti A, Calligaro A, Hoxha A.et al. Laboratory and clinical features of pregnant women with antiphospholipid syndrome and neonatal outcome. Arthritis Care Res (Hoboken). 2010;62(3):302 307. ( 10.1002/acr.20098) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a