Abstract
Previously we demonstrated that human neutrophils mediate potent and long-lasting fungistasis against Histoplasma capsulatum yeasts and that all of the fungistatic activity resides in the azurophil granules. In the present study, specific azurophil granule constituents with fungistatic activity were identified by incubation with H. capsulatum yeasts for 24 h and by quantifying the subsequent growth of yeasts via the incorporation of [3H]leucine. Human neutrophil defensins HNP-1, HNP-2, and HNP-3 inhibited the growth of H. capsulatum yeasts in a concentration-dependent manner with maximum inhibition at 8 μg/ml. At a concentration of 4 μg/ml, all possible paired combinations of defensins exhibited additive fungistatic activity against H. capsulatum yeasts. Cathepsin G and bactericidal-permeability-increasing protein (BPI) also mediated fungistasis against H. capsulatum in a concentration-dependent manner. The fungistatic activities of combinations of cathepsin G and BPI were additive, as were those of combinations of cathepsin G or BPI with HNP-1, HNP-2, and HNP-3. Lysozyme and elastase exhibited modest antifungal activity, and azurocidin and proteinase 3 exhibited no significant fungistasis against H. capsulatum yeasts. Thus, defensins, cathepsin G, and BPI are the major anti-H. capsulatum effector molecules in the azurophil granules of human neutrophils.
Histoplasma capsulatum is a dimorphic fungal pathogen of worldwide importance that causes a broad spectrum of disease activity. The course of H. capsulatum infection is mild in most immunocompetent individuals, but progressive disseminated infections occur in individuals immunocompromised by hematologic malignancies (6, 25, 30) or cytotoxic therapy (16, 32, 33) or in individuals infected with human immunodeficiency virus (15, 20, 31).
Infection with H. capsulatum is acquired by inhalation of microconidia into the terminal bronchioles and alveoli of the lung. Inhaled microconidia subsequently convert into yeasts that are responsible for the pathogenesis of histoplasmosis (17). H. capsulatum yeasts are phagocytized by alveolar macrophages (Mφ), within which they multiply (2, 7). Dividing yeasts destroy the alveolar Mφ, and subsequently the yeasts are ingested by other resident alveolar Mφ and by inflammatory phagocytes recruited to the locus of infection. Repetition of this cycle results in spread of infection to hilar lymph nodes and to other organs during the acute phase of primary histoplasmosis. Subsequently, the maturation of specific cell-mediated immunity against H. capsulatum activates Mφ to halt yeast proliferation with gradual resolution of the disease process in immunocompetent hosts (7, 22).
The role of polymorphonuclear neutrophils (PMNs) in the cell-mediated immune response against H. capsulatum is unclear (7). However, even the earliest studies in a murine model of histoplasmosis described PMNs as being the predominant inflammatory cell type in the lungs during the first 36 h after intranasal inoculation with H. capsulatum macroconidia. In these studies the PMNs were not observed to phagocytose the macroconidia, and the few yeasts that were present at 36 h were found only within Mφ (24). Baughman et al. (2) observed an intense PMN response in the lung at 1 week of infection after intranasal inoculation of C57BL/6 mice with yeasts of H. capsulatum strain G217B. By the second week, PMNs were largely supplanted by mononuclear cells characteristic of a granulomatous inflammatory response. Thus, these in vivo observations suggest that PMNs may play a role in host defense against H. capsulatum at early times postinfection.
Previously (23), we demonstrated that human PMNs mediate potent and long-lasting fungistatic activity against H. capsulatum yeasts and that all of the fungistatic activity is contained in the azurophil granules. The present study was designed to (i) identify which constituents of neutrophil azurophil granules mediate fungistasis against H. capsulatum yeasts and (ii) determine if there is additive or synergistic activity between various components. The data presented demonstrate that the defensins HNP-1, HNP-2, and HNP-3, as well as cathepsin G and bactericidal-permeability-increasing protein (BPI), mediate the majority of the fungistasis that is derived from PMN azurophil granules.
MATERIALS AND METHODS
Yeasts.
H. capsulatum strain G217B was maintained as previously described (21). Yeasts were grown in HMM medium (36) at 37°C with orbital shaking at 150 rpm. After 2 days, log-phase yeasts were harvested by centrifugation, washed three times in Hank's balanced salt solution containing 20 mM HEPES and 0.25% bovine serum albumin, and resuspended to 50 ml in the same buffer. Large aggregates were removed by centrifugation at 200 × g for 5 min at 4°C. The top 5 ml was removed, and the single-cell suspension obtained was standardized to 5 × 104/ml in HMM medium diluted 1/25 in 10 mM phosphate buffer (pH 6.0 or 7.0 as designated below).
Azurophil granule components.
Lysozyme, elastase, and cathepsin G were purchased from Sigma Chemical Co., St. Louis, Mo. BPI was a gift from Incyte Pharmaceuticals, Palo Alto, Calif. Human neutrophil defensins HNP-1, HNP-2, and HNP-3 and azurocidin and proteinase 3 were purified as described previously (4, 10, 12, 27). All azurophil granule components were diluted in 10 mM citrate phosphate buffer (pH 7.0), except for cathepsin G and BPI, which were at pH 6.0. These pHs were chosen for optimum activity of the different granule components in the fungistasis assay as determined in preliminary experiments.
Quantitation of fungistatic activity against H. capsulatum yeasts.
The fungistatic activity of the various azurophil granule components was quantified by the incorporation of [3H]leucine into remaining viable yeasts (23). Viable H. capsulatum yeasts (5 × 103 in 0.1 ml) were added to the wells of a 96-well tissue culture plate and were incubated with 0.1 ml of various concentrations of individual components of neutrophil azurophil granules. Control wells contained H. capsulatum yeasts added to 0.1 ml of citrate phosphate buffer. After culture for 24 h at 37°C, the plates were centrifuged at 700 × g, the supernatant was carefully aspirated through a 27-gauge needle, and 50 μl of [3H]leucine (specific activity, 153 Ci/mmol; New England Nuclear, Boston, Mass.) in sterile water (1.0 μCi) and 5 μl of a 10× yeast nitrogen broth (Difco Laboratories, Detroit, Mich.) were added to each well. After further incubation for 24 h at 37°C, 50 μl of l-leucine (10 mg/ml) and 50 μl of sodium hypochlorite were added to each well. The contents of the wells were harvested onto glass fiber filters using an automated harvester (Skatron, Sterling, Va.). The filters were placed into scintillation vials, the scintillation cocktail was added, and the vials were counted on a Beckman LS 7000 liquid scintillation spectrometer (Beckman Instruments, Inc., Fullerton, Calif.).
As there was considerable variation in the counts per minute (cpm) obtained from yeasts multiplying in controls containing only medium, the data are presented as the means ± standard errors of the mean (SEMs) of percent inhibition, which is defined as 1 − (cpm in experimental wells/cpm in control wells) × 100. All experimental points were performed in triplicate, and all experiments were performed at least three times.
Statistics.
Statistical analysis of the data was performed using Sigma Stat (Jandel Scientific, San Rafael, Calif.). Results were considered significant at a P value of <0.05.
RESULTS
Human neutrophil defensins mediate fungistasis against H. capsulatum.
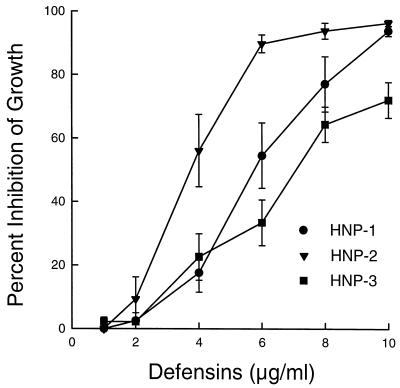
In initial studies, various concentrations of HNP-1, HNP-2, and HNP-3 were incubated with H. capsulatum yeasts for 24 h at 37°C. The data in Fig. 1 show that all three defensins mediated a concentration-dependent inhibition of the growth of H. capsulatum yeasts and that HNP-2 had the greatest inhibitory activity at all concentrations tested.
FIG. 1.
Concentration-dependent inhibition of the growth of H. capsulatum yeasts by HNP-1, -2, and -3. H. capsulatum yeasts (5 × 103 cells) were incubated with various concentrations of defensins for 24 h at 37°C. At the end of the incubation period, the plates were centrifuged, the supernatants were removed, and the yeasts were incubated for a further 24 h with 1 μCi of [3H]leucine in 50 μl of distilled water containing 10% yeast nitrogen broth. Numbers of cpm from control wells containing yeasts only were compared to those from wells containing yeasts cultured in the presence of defensins, and the percent inhibition of growth was calculated. The data are means ± SEMs (n = 4). The mean ± SEM cpm in control wells was 45,182 ± 8,697.
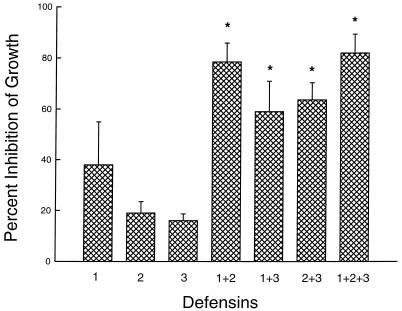
Next, defensins were incubated with H. capsulatum yeasts for 24 h either singly or in paired combinations at 4 μg/ml. All combinations of defensins demonstrated essentially an additive capacity to inhibit the replication of H. capsulatum yeasts (Fig. 2).
FIG. 2.
Inhibition of the growth of H. capsulatum yeasts by combinations of defensins. The procedure was as described in the legend to Fig. 1. Defensins were tested at 4 μg/ml each. The data are means ± SEMs (n = 4). ∗, P < 0.05 compared to HNP-2 and HNP-3, Student-Newman-Keuls method of multiple comparison.
Cathepsin G and BPI mediate fungistasis against H. capsulatum.
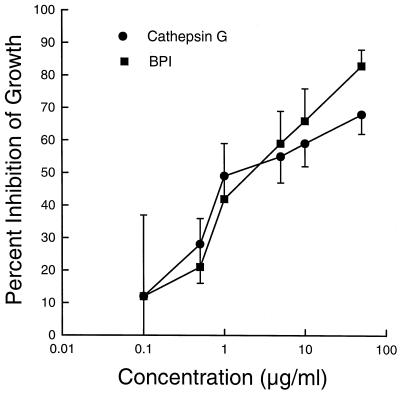
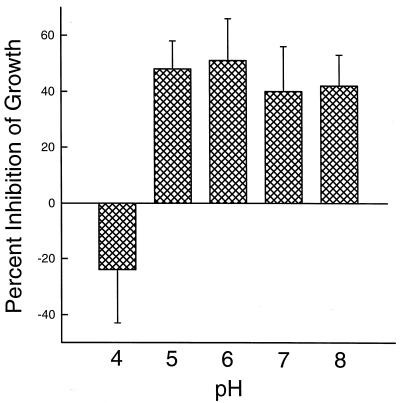
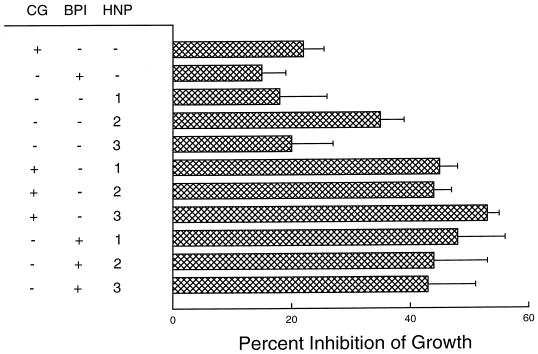
As in the defensin experiments, various concentrations of cathepsin G and BPI were incubated with H. capsulatum yeasts for 24 h. Both BPI and cathepsin G inhibited the growth of yeasts in a concentration-dependent manner over a 4-log range, and their inhibitory activity was equivalent at all concentrations tested (Fig. 3). When studied in combination, the inhibitory activities of cathepsin G and BPI were additive (Table 1). In addition, BPI mediated fungistatic activity against H. capsulatum over a wide range of pH (Fig. 4). Only at pH 4 was BPI inactive. Similar results were obtained with cathepsin G (data not shown). Finally, in another series of experiments, it was demonstrated that the inhibitory activities of combinations of cathepsin G or BPI with either HNP-1, HNP-2, or HNP-3 were additive (Fig. 5).
FIG. 3.
Concentration-dependent inhibition of the growth of H. capsulatum yeasts by cathepsin G and BPI. The procedure was as described in the legend to Fig. 1. The data are means ± SEMs of five experiments with cathepsin G and three experiments with BPI.
TABLE 1.
Additive effects of cathepsin G and BPI in inhibiting the growth of H. capsulatum yeastsa
| Concn (μg/ml) of:
|
% Inhibition of growthb | |
|---|---|---|
| Cathepsin G | BPI | |
| 0.1 | 13.4 ± 4.6 | |
| 0.5 | 14.6 ± 2.1 | |
| 1.0 | 23.3 ± 7.4 | |
| 0.1 | 0.5 | 39.0 ± 8.1 |
| 0.1 | 1.0 | 43.4 ± 5.8 |
H. capsulatum yeasts (5 × 103) were incubated with cathepsin G, BPI, or both for 24 h at 37°C. The yeasts then were pulsed with [3H]leucine for an additional 24 h to quantitate the growth of the remaining viable yeasts.
Mean ± SEM; n = 4.
FIG. 4.
The fungistatic activity of BPI occurs over a wide range of pH. The procedure was as described in the legend to Fig. 1 except that BPI was diluted in 10 mM citrate-phosphate buffer of various pHs. The media containing the yeasts also were at various pHs. The data are means ± SEMs (n = 4).
FIG. 5.
Additive effects of cathepsin G or BPI and defensins in inhibiting the growth of H. capsulatum yeasts. The procedure was as described in the legend to Fig. 1. Cathepsin G was used at 0.1 μg/ml, BPI at 1 μg/ml, and defensins at 4 μg/ml. The data are means ± SEMs (n = 3–4). There was no statistical significance between any of the groups by analysis of variance.
Lysozyme and elastase but not azurocidin and proteinase 3 have modest antifungal activity against H. capsulatum yeasts.
Incubation of various concentrations of lysozyme and elastase for 24 h with H. capsulatum yielded modest but not statistically significant inhibition of yeast replication (Table 2). In contrast, coculture of yeasts with azurocidin and proteinase 3 actually led to enhanced growth of H. capsulatum yeasts (Table 3). Various combinations of these azurophil components did not result in enhanced anti-Histoplasma activity, nor did they enhance the activity of defensins, cathepsin G, or BPI (data not shown).
TABLE 2.
Inhibition of the growth of H. capsulatum yeasts by lysozyme and elastasea
| Reagent | Concn (μg/ml) | % Inhibition of growthb |
|---|---|---|
| Lysozyme | 1 | 16 ± 4 |
| 10 | 20 ± 5 | |
| 100 | 36 ± 8 | |
| Elastase | 1 | 17 ± 6 |
| 10 | 22 ± 8 | |
| 100 | 25 ± 8 |
H. capsulatum yeasts (5 × 103) were incubated with various concentrations of lysozyme and elastase for 24 h at 37°C. The yeasts then were pulsed with [3H]leucine for an additional 24 h to quantitate the growth of the remaining yeasts.
Mean ± SEM (n = 10 for lysozyme and 6 for elastase). The data were not statistically significantly different from the controls (Student's t test).
TABLE 3.
Azurocidin and proteinase 3 do not inhibit the growth of H. capsulatum yeastsa
| Reagent | Concn (μg/ml) | % Inhibition of growthb |
|---|---|---|
| Azurocidin | 1 | −38 ± 34 |
| 5 | −14 ± 30 | |
| 10 | −23 ± 35 | |
| Proteinase 3 | 0.1 | −47 ± 32 |
| 0.5 | −40 ± 30 | |
| 1.0 | −33 ± 36 |
H. capsulatum yeasts (5 × 103) were incubated with various concentrations of azurocidin and proteinase 3 for 24 h at 37°C. The yeasts then were pulsed with [3H]leucine for an additional 24 h to quantitate the growth of the remaining yeasts.
Mean ± SEM (n = 7). The minus sign indicates that there was increased growth compared to yeasts cultured in control medium.
DISCUSSION
In exploring a possible role for neutrophils in host defense against H. capsulatum, we initially demonstrated that human PMNs bind unopsonized yeasts via CD18 as do human monocytes and Mφ (3, 21). Unlike Mφ, PMNs phagocytose few unopsonized yeasts. However, phagocytosis is significantly enhanced by heat-labile and heat-stable serum opsonins. Most interesting is the fact that although the phagocytosis of opsonized H. capsulatum yeasts stimulates a potent respiratory burst, superoxide anion (O2−) is trapped intracellularly and not released into the extracellular milieu (26). Thus, we were surprised when subsequent studies revealed that the potent and long-lasting fungistatic activity that neutrophils mediate against H. capsulatum is not mediated by toxic oxygen metabolites but that all of the antifungal activity resided in the azurophil granules (23).
Human neutrophil azurophil granules contain two families of antimicrobial proteins, defensins and serprocidins, each with four members, and two antimicrobial proteins with unique primary structures, lysozyme and BPI (9, 11, 34). These azurophil granule proteins have been found to have a broad spectrum of antimicrobial activity against gram-negative and gram-positive bacteria, protozoans, enveloped viruses, and fungi, including Cryptococcus neoformans, Candida albicans, Aspergillus fumigatus, and Rhizopus oryzae.
HNPs are basic peptides that are 29 to 30 amino acids in length and contain three characteristic intramolecular disulfide bonds. HNP-1, HNP-2, and HNP-3, all of which mediated a concentration-dependent fungistasis against H. capsulatum yeasts, differ only in a single N-terminal amino acid. The peptides have a cyclic structure with spatial segregation of charged and hydrophobic residues. This amphiphilic structure may equip defensins for insertion into the phospholipid membrane of their target organism (27, 34, 35). Thus, the mechanism by which defensins might inhibit the replication of H. capsulatum yeasts is unclear, particularly because of its thick cell wall. However, as microorganisms may encounter defensin concentrations as high as 10 mg/ml (28), the observed fungistatic activity of defensins against H. capsulatum yeasts is well within the physiologic range.
The second major family of azurophil granule antimicrobial proteins, the serprocidins, consists of azurocidin, cathepsin G, elastase, and proteinase 3. Serprocidins are cationic glycoproteins of 25 to 29 kDa that exhibit considerable homology with serine proteases. Serprocidins are relatively abundant in PMNs (1 to 2 μg/106 PMNs) and also demonstrate a broad spectrum of antimicrobial activity against gram-positive and gram-negative bacteria and fungi (9). Of the four members, only cathepsin G was found to inhibit the growth of H. capsulatum yeasts, and cathepsin G activity was additive in its inhibitory activity when mixed with defensins HNP-1, -2, and -3. Cathepsin G is a neutral protease of molecular mass 29 to 31 kDa (14) and has been reported to kill Neisseria gonorrhoea (29) and Listeria monocytogenes (1) by a nonenzymatic mechanism and to kill microorganisms that cause dental caries by both enzymatic and nonenzymatic mechanisms (19). Cathepsin G also can synergize with azurocidin in killing the oral microorganism Capnocytophaga sputigena (18).
The most unexpected results were obtained with BPI. BPI is a major component of the azurophil granules of PMNs and is found only in myeloid cells. BPI has a molecular mass of 50 to 60 kDa and contains a highly cationic, lysine-rich amino-terminal half and a very hydrophobic, much less charged, carboxy-terminal half (13). BPI has a strong affinity for lipopolysaccharide (LPS), and, therefore, its cytotoxic activity is directed almost exclusively to gram-negative bacteria. Indeed, the susceptibility of gram-negative bacteria appears to be determined primarily by the structure of the envelope LPS, specifically the length of the polysaccharide chains (8). However, despite the fact that H. capsulatum yeasts do not contain LPS, BPI inhibited the growth of yeasts in a concentration-dependent manner. Furthermore, the anti-Histoplasma activity of BPI was additive when BPI was mixed with cathepsin G or any of the three defensins. It is unclear what BPI might recognize on the surface of the yeasts to mediate its inhibitory effects.
While both our previous study (23) and the current study have demonstrated potent growth-inhibitory activity against H. capsulatum yeasts by whole PMNs and azurophil granule constituents, we have not actually quantified killing of the fungus. However, overall the data suggest that many of the yeasts are being killed. Thus, when neutrophils and yeasts are cocultured at a ratio of 50:1, no external yeasts are observed until after 6 days of culture, at which time most of the PMNs have disintegrated (23). In addition, in the current study, we observed that yeasts “shrunk” to small pinpoints during the 24 h of culture with defensins, cathepsin G, or BPI. Attempts to quantify the remaining viable yeasts by culture on HMM agar plates (36) were unsuccessful, whereas the CFU from control wells indicated that the yeasts had replicated over the 24-h incubation period. Since we can quantitatively plate 100 to 200 yeasts on these plates, these results suggest that perhaps greater than 95% of the yeasts may have been killed. Furthermore, when RAW 264.7 cells (a murine macrophage cell line) were transduced with cDNA encoding HNP-1, not only was the intracellular growth of H. capsulatum yeasts inhibited, but degraded ghost forms were frequently observed (5).
The potent fungistatic activity against H. capsulatum exhibited by human PMNs in vitro suggests that they may play an important role in host defense against H. capsulatum in vivo. Certainly PMNs may be capable of slowing the course of the infection, and, under certain circumstances, they may prevent dissemination of the yeasts from the lung. It also is possible that PMNs may damage the yeasts in such a manner as to render them vulnerable to inflammatory macrophages. Thus, inflammatory macrophages may phagocytose yeast-containing neutrophils and subsequently kill the partially damaged yeasts. Either or both of these postulated mechanisms of defense involving neutrophils during the early phase of the inflammatory response to H. capsulatum may explain, at least in part, the fact that a substantial percentage of pulmonary infections by this organism are subclinical and self-limiting.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI-32368, AI-37639, HL-55948, and AI-22931 from the National Institutes of Health.
We thank Marian Marra, Incyte Pharmaceuticals, for the generous gift of BPI.
REFERENCES
- 1.Alford C E, Amaral E, Campbell P A. Listericidal activity of human neutrophil cathepsin G. J Gen Microbiol. 1990;136:997–1000. doi: 10.1099/00221287-136-6-997. [DOI] [PubMed] [Google Scholar]
- 2.Baughman R P, Kim C K, Vinegar A, Hendricks D E, Schmidt D J, Bullock W E. The pathogenesis of experimental pulmonary histoplasmosis. Correlative studies of histopathology, bronchoalveolar lavage, and respiratory function. Am Rev Respir Dis. 1986;134:771–776. doi: 10.1164/arrd.1986.134.4.771. [DOI] [PubMed] [Google Scholar]
- 3.Bullock W E, Wright S D. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987;165:195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campanelli D, Detmers P A, Nathan C F, Gabay J E. Azurocidin and a homologous serine protease from neutrophils. Differential antimicrobial and proteolytic properties. J Clin Invest. 1990;85:904–915. doi: 10.1172/JCI114518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couto M A, Liu L, Lehrer R I, Ganz T. Inhibition of intracellular Histoplasma capsulatum replication by murine macrophages that produce human defensin. Infect Immun. 1994;62:2375–2378. doi: 10.1128/iai.62.6.2375-2378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies S F, Khan M, Sarosi G A. Disseminated histoplasmosis in immunologically suppressed patients. Occurrence in a nonendemic area. Am J Med. 1978;64:94–100. doi: 10.1016/0002-9343(78)90183-3. [DOI] [PubMed] [Google Scholar]
- 7.Deepe G S, Bullock W E. Histoplasmosis: a granulomatous inflammatory response. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation: basic principles and clinical correlates. New York, N.Y: Raven Press; 1992. pp. 943–958. [Google Scholar]
- 8.Elsbach P, Weiss J. Bactericidal/permeability increasing protein and host defense against gram-negative bacteria and endotoxin. Curr Opin Immunol. 1993;5:103–107. doi: 10.1016/0952-7915(93)90088-a. [DOI] [PubMed] [Google Scholar]
- 9.Gabay J E, Almeida R P. Antibiotic peptides and serine protease homologs in human polymorphonuclear leukocytes: defensins and azurocidin. Curr Opin Immunol. 1993;5:97–102. doi: 10.1016/0952-7915(93)90087-9. [DOI] [PubMed] [Google Scholar]
- 10.Gabay J E, Scott R W, Campanelli D, Griffith J, Wilde C, Marra M N, Seeger M, Nathan C F. Antibiotic proteins of human polymorphonuclear leukocytes. Proc Natl Acad Sci USA. 1989;86:5610–5614. doi: 10.1073/pnas.86.14.5610. . (Erratum, 86:10133.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz T, Selsted M E, Lehrer R I. Defensins. Eur J Haematol. 1990;44:1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 12.Ganz T, Selsted M E, Szklarek D, Harwig S S, Daher K, Bainton D F, Lehrer R I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray P W, Flaggs G, Leong S R, Gumina R J, Weiss J, Ooi C E, Elsbach P. Cloning of the cDNA of a human neutrophil bactericidal protein. Structural and functional correlations. J Biol Chem. 1989;264:9505–9509. [PubMed] [Google Scholar]
- 14.Heck L W, Rostand K S, Hunter F A, Bhown A. Isolation, characterization, and amino-terminal amino acid sequence analysis of human neutrophil cathepsin G from normal donors. Anal Biochem. 1986;158:217–227. doi: 10.1016/0003-2697(86)90612-3. [DOI] [PubMed] [Google Scholar]
- 15.Johnson P C, Hamill R J, Sarosi G A. Clinical review: progressive disseminated histoplasmosis in the AIDS patient. Semin Respir Infect. 1989;4:139–146. [PubMed] [Google Scholar]
- 16.Kauffman C A, Israel K S, Smith J W, White A C, Schwarz J, Brooks G F. Histoplasmosis in immunosuppressed patients. Am J Med. 1978;64:923–932. doi: 10.1016/0002-9343(78)90445-x. [DOI] [PubMed] [Google Scholar]
- 17.Medoff G, Sacco M, Maresca B, Schlessinger D, Painter A, Kobayashi G S, Carratu L. Irreversible block of the mycelial-to-yeast phase transition of Histoplasma capsulatum. Science. 1986;231:476–479. doi: 10.1126/science.3001938. [DOI] [PubMed] [Google Scholar]
- 18.Miyasaki K T, Bodeau A L. Human neutrophil azurocidin synergizes with leukocyte elastase and cathepsin G in the killing of Capnocytophaga sputigena. Infect Immun. 1992;60:4973–4975. doi: 10.1128/iai.60.11.4973-4975.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyasaki K T, Bodeau A L. In vitro killing of Actinobacillus actinomycetemcomitans and Capnocytophaga spp. by human neutrophil cathepsin G and elastase. Infect Immun. 1991;59:3015–3020. doi: 10.1128/iai.59.9.3015-3020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neubauer M A, Bodensteiner D C. Disseminated histoplasmosis in patients with AIDS. South Med J. 1992;85:1166–1170. doi: 10.1097/00007611-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Newman S L, Bucher C, Rhodes J, Bullock W E. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages. Cellular cytoskeleton requirement for attachment and ingestion. J Clin Invest. 1990;85:223–230. doi: 10.1172/JCI114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman S L, Bullock W E. Interaction of Histoplasma capsulatum yeasts and conidia with human and animal macrophages. In: Zwilling B S, Eisenstein T K, editors. Macrophage-pathogen interactions. New York, N.Y: Marcel Dekker; 1994. pp. 517–532. [PubMed] [Google Scholar]
- 23.Newman S L, Gootee L, Gabay J E. Human neutrophil-mediated fungistasis against Histoplasma capsulatum. Localization of fungistatic activity to the azurophil granules. J Clin Invest. 1993;92:624–631. doi: 10.1172/JCI116630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Procknow J J, Page M I, Loosli C G. Early pathogenesis of experimental histoplasmosis. Arch Pathol. 1960;69:413–426. [PubMed] [Google Scholar]
- 25.Reddy P, Gorelick D F, Brasher C A, Larsh H. Progressive disseminated histoplasmosis as seen in adults. Am J Med. 1970;48:629–636. doi: 10.1016/0002-9343(70)90014-8. [DOI] [PubMed] [Google Scholar]
- 26.Schnur R A, Newman S L. The respiratory burst response to Histoplasma capsulatum by human neutrophils. Evidence for intracellular trapping of superoxide anion. J Immunol. 1990;144:4765–4772. [PubMed] [Google Scholar]
- 27.Selsted M E, Harwig S S, Ganz T, Schilling J W, Lehrer R I. Primary structures of three human neutrophil defensins. J Clin Invest. 1985;76:1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selsted M E, Ouellette A J. Defensins in granules of phagocytic and non-phagocytic cells. Trends Cell Biol. 1995;5:114–119. doi: 10.1016/s0962-8924(00)88961-8. [DOI] [PubMed] [Google Scholar]
- 29.Shafer W M, Onunka V C, Martin L E. Antigonococcal activity of human neutrophil cathepsin G. Infect Immun. 1986;54:184–188. doi: 10.1128/iai.54.1.184-188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith J W, Utz J P. Progressive disseminated histoplasmosis. A prospective study of 26 patients. Ann Intern Med. 1972;76:557–565. doi: 10.7326/0003-4819-76-4-557. [DOI] [PubMed] [Google Scholar]
- 31.Wheat J. Histoplasmosis and coccidioidomycosis in individuals with AIDS. A clinical review. Infect Dis Clin N Am. 1994;8:467–482. [PubMed] [Google Scholar]
- 32.Wheat L J, Slama T G, Norton J A, Kohler R B, Eitzen H E, French M L, Sathapatayavongs B. Risk factors for disseminated or fatal histoplasmosis. Analysis of a large urban outbreak. Ann Intern Med. 1982;96:159–163. doi: 10.7326/0003-4819-96-2-159. [DOI] [PubMed] [Google Scholar]
- 33.Wheat L J, Smith E J, Sathapatayavongs B, Batteiger B, Filo R S, Leapman S B, French M V. Histoplasmosis in renal allograft recipients. Two large urban outbreaks. Arch Intern Med. 1983;143:703–707. [PubMed] [Google Scholar]
- 34.White S H, Wimley W C, Selsted M E. Structure, function, and membrane integration of defensins. Curr Opin Struct Biol. 1995;5:521–527. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 35.Wimley W C, Selsted M E, White S H. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci. 1994;3:1362–1373. doi: 10.1002/pro.5560030902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worsham P L, Goldman W E. Quantitative plating of Histoplasma capsulatum without addition of conditioned medium or siderophores. J Med Vet Mycol. 1988;26:137–143. [PubMed] [Google Scholar]