Abstract
Background:
This study aimed to investigate the efficacy and safety of vonoprazan in the eradication of Helicobacter pylori (H. pylori).
Methods:
A total of 120 cases of H. pylori-infected outpatients were selected and randomly divided into the traditional quadruple therapy, vonoprazan triple therapy, and vonoprazan quadruple therapy groups. The traditional quadruple therapy group patients were orally treated with esomeprazole (20 mg) 30 minutes before breakfast and supper, amoxicillin (1000 mg orally) 30 minutes after breakfast and supper, furazolidone (100 mg orally) 30 minutes after breakfast and supper, and bismuth potassium citrate (0.6 g orally) 30 minutes before breakfast and supper. The vonoprazan triple therapy group patients were treated with vonoprazan (20 mg orally) 30 minutes following breakfast and supper, amoxicillin (1000 mg orally) 30 minutes following breakfast and supper, and bismuth potassium citrate (0.6 g orally) 30 minutes before breakfast and supper. The vonoprazan quadruple therapy group patients were treated with vonoprazan (20 mg orally) 30 minutes following breakfast and supper, amoxicillin (1000 mg orally) 30 minutes after breakfast and supper, furazolidone (100 mg orally) 30 minutes after breakfast and supper, and bismuth potassium citrate (0.6 g orally) 30 minutes before breakfast and supper. The 3 groups were treated for 14 days, and adverse reactions, such as vomiting and abdominal distension, were recorded during the treatment period. The 14C urea breath test was used to detect whether H. pylori was successfully eradicated in the patients.
Results:
The eradication rates of the vonoprazan triple therapy, vonoprazan quadruple therapy, and the traditional quadruple therapy groups were 80%, 95%, and 97.5%, respectively. The eradication rate was higher in the vonoprazan triple therapy and in the vonoprazan quadruple therapy groups compared with that noted in the control group. The adverse reactions were mild in these groups, and the main adverse reactions were nausea, abdominal distension, diarrhea, and constipation. The adverse reaction rate was 25%, 7.5%, and 15%, respectively. This rate was lower in the vonoprazan triple therapy and vonoprazan quadruple therapy groups than that noted in the control group.
Conclusion:
Both vonoprazan triple therapy and vonoprazan quadruple therapy regimens could increase the eradication rate of H. pylori. Vonoprazan triple therapy exhibited reduced side effects and could be applied in the eradication of H. pylori in the clinic.
Keywords: Adverse effects, eradication rate, Helicobacter pylori, quadruple therapy, vonoprazan
Main Points
A total of 120 cases of H. pylori-infected outpatients were selected and randomly divided into the traditional quadruple therapy, vonoprazan triple therapy, and vonoprazan quadruple therapy groups.
Both vonoprazan triple therapy and vonoprazan quadruple therapy regimens could increase the eradication rate of H. pylori.
Vonoprazan triple therapy exhibited reduced side effects and could be applied in the eradication of H. pylori in the clinic.
Introduction
Helicobacter pylori (H. pylori) is a spiral micro-aerophilic gram-negative bacterium. It is not only the common cause of chronic gastritis but also a pathogenic factor of peptic ulcer, atrophic gastritis, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma.1-4 It can be easily transmitted through fecal–oral and oral–oral routes or by contact with saliva from infected individuals and by contaminated food. Therefore, the infection rate in the population is very high.5-6 In China, the infection rate is as high as 56%. Kyoto Global Consensus recommends eradication treatment for these patients6 and drug treatment is the only method used to eradicate H. pylori infection.
Drug resistance and CYP2C19 gene polymorphisms noted in host organisms reduce the eradication rate of H. pylori. Specifically, the rate is lower than 80% following conventional triple treatment with proton pump inhibitor (PPI), amoxicillin, and furazolidone.7-13 The eradication rate can only be increased by 5% by prolonging the treatment to 10 days. The recommended use of antibiotics results in antibiotic resistance of H. pylori. Specifically, the resistance rate range of H. pylori for metronidazole is 60%-70%, for clarithromycin 20%-38%, and for levofloxacin 30%-38%.14 The treatment with high resistance rate drugs can significantly reduce the eradication rate.15,16 Quadruple treatment with bismuth is recommended as the first-line treatment.17 However, as the resistance of H. pylori to these drugs increases, the eradication rate caused by quadruple treatment methods is decreased.17-21 In certain patients, treatment is halted due to adverse effects. Therefore, it is imperative to identify a solution that can increase the eradication rate of H. pylori and reduce the adverse effects of the treatment.
Vonoprazan is a new proton pump inhibitor, which inhibits the binding of K+ ions and stops the exchange of H+-K+. It exerts sustained inhibition of acid secretion.22-25 Vonoprazan can inhibit both the activated and resilient proton pump, which can achieve stronger and longer-lasting suppression of gastric acid secretion than PPIs.23,26 This compound was approved for market sale in Japan in 2015 and it was recommended as a first-line treatment for H. pylori eradication by the Japanese H. pylori infection treatment guidelines in 2016. However, certain effects of the combination of vonoprazan plus bismuth triple therapy and vonoprazan plus bismuth quadruple therapy have not been previously investigated in the treatment of H. pylori eradication. Therefore, we discussed the effects of vonoprazan plus bismuth triple therapy and vonoprazan plus bismuth quadruple therapy in the eradication of H. pylori infection and the associated adverse effects.
Materials and Methods
The present study was approved by Ganzhou People’s Hospital Ethics Committee with approval number TY-ZKY2011-011-01.
A total of 120 H. pylori-positive patients who agreed to H. pylori eradication treatment were selected in our outpatient department between July and November 2021. The inclusion criteria used were as follows: (1) age between 18 and 60, with no restrictions on the gender; (2) confirmation of H. pylori infection by the 14C urea breath test; (3) patients who did not receive PPI, bismuth, vonoprazan, and antibiotics for the last month; and (4) patients who agreed to participate in the research and signed the informed consent form. The following exclusion criteria were used: (1) patients who had significant hepatic or renal disease; (2) patients who had taken PPI, bismuth, vonoprazan, and antibiotics in the last month; (3) patients who were allergic to penicillin, PPI, bismuth or vonoprazan; and (4) patients who did not accept to participate in the follow-up periods as required.
Treatment
A total of 120 H. pylori-infected patients were randomly divided into the traditional quadruple therapy, the vonoprazan triple therapy, and the vonoprazan quadruple therapy groups. Each group contained 40 patients.
The traditional quadruple therapy group patients were provided with esomeprazole (AstraZenaca Pharmaceutical Corporation London UK) administered at 20 mg orally 30 minutes before breakfast and supper, amoxicillin (Federal drug Corporation Santa Barbara US) administered at 1000 mg orally 30 minutes after breakfast and supper, furazolidone (LiuYe biotechnology Corporation Shandong China) administered at 100 mg orally 30 minutes after breakfast and supper, and bismuth potassium citrate (LiZhu Pharmaceutical Corporation Jiangsu China) administered at 0.6 g orally 30 minutes before breakfast and supper.
The patients in the vonoprazan triple therapy group were administered with vonoprazan (20 mg orally) 30 minutes after breakfast and supper, amoxicillin (1000 mg orally) 30 minutes after breakfast and supper, and bismuth potassium citrate (0.6 g orally) 30 minutes before breakfast and supper.
The patients of the vonoprazan quadruple therapy group were orally administered with 20 mg vonoprazan, 1000 mg amoxicillin, and 100 mg furazolidone 30 minutes after breakfast and supper, while they were also orally administered with 0.6 g bismuth potassium citrate orally 30 minutes before breakfast and supper.
All patients were treated for 14 days and subsequently were followed up for an additional 4 weeks. They were assessed with the 14C urea breath test following 1 month of drug withdrawal. The treatment duration and antimicrobial dosages were determined according to the H. pylori guidelines published in 2019.
Observation Indicators
These were divided into main and secondary indicators for observation. The main indicator used was the H. pylori eradication rate, whereas the negative 14C urea breath test was considered a successful eradication of H. pylori. The secondary indicator included the adverse effect rate, such as nausea, vomiting, diarrhea, abdominal distention, and constipation.
Statistical Analysis
Statistical Package for the Social Sciences 20.0 software (IBM Corp.; Armonk, NY, USA) was used for statistical analysis. The chi-square test was conducted to measure the H. pylori eradication rate and the adverse effect rate. P < .05 was considered to indicate a significant difference.
Results
Baseline Characteristics
All patients were continuously treated for 14 days. The patients were followed up and accepted the 14C urea breath test after 1 month of drug withdrawal. No significant differences were noted in the gender, age, height, and body mass among the 3 groups of patients (Table 1).
Table 1.
Baseline Characteristics of 3 Groups of Patients
| General Information | Traditional Quadruple Therapy Group | Vonoprazan Triple Therapy Group | Vonoprazan Quadruple Therapy Group |
|---|---|---|---|
| Male | 25 | 23 | 22 |
| Female | 15 | 17 | 18 |
| Age (year) | 50.82 ± 4.83 | 52.14 ± 5.37 | 51.36 ± 3.94 |
| Height (cm) | 171 ± 5.89 | 170 ± 6.73 | 172 ± 7.68 |
| Body mass index (kg/m2) | 23.2 ± 4.1 | 22.8 ± 2.6 | 23.5 ± 3.5 |
Eradication of H. pylori Infection
All patients accepted the 14C urea breath test after 1 month of drug withdrawal. The eradication rate among the 3 groups was 95%, 97.5%, and 80.0%, respectively, while the eradication rate was significantly higher in both the vonoprazan triple therapy and the vonoprazan quadruple therapy groups than that noted in the control group (Table 2 and Figure 1).
Table 2.
Comparison of H. pylori Eradication Rate of the 3 Groups
| H. pylori-Negative Cases | H. pylori-Positive Cases | H. pylori Eradication Rate (%) | |
|---|---|---|---|
| Traditional quadruple therapy group | 32 | 8 | 80 |
| Vonoprazan triple therapy group | 38 | 2 | 95 |
| Vonoprazan quadruple therapy group | 39 | 1 | 97.5 |
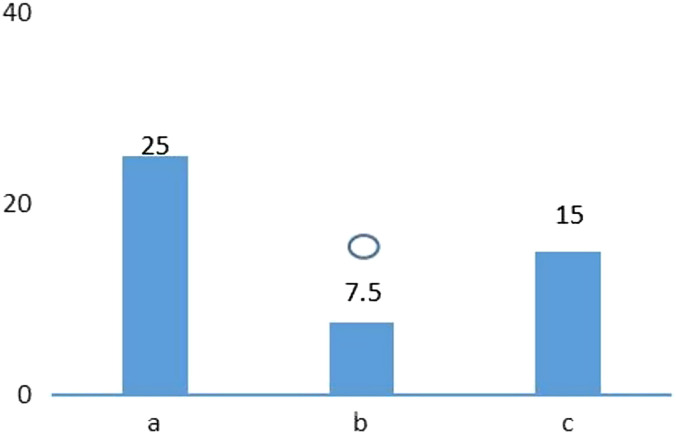
Figure 1.
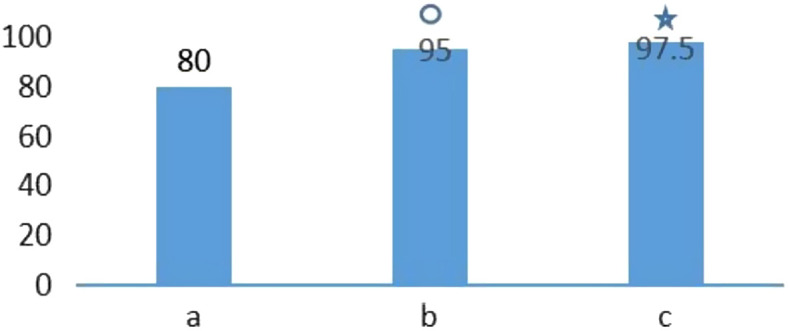
Comparison of the H. pylori eradication rate of the 3 groups. (A) Traditional quadruple therapy group; (B) vonoprazan triple therapy group; (C) vonoprazan quadruple therapy group. Compared with the traditional quadruple therapy group P < .05;  compared with the traditional quadruple therapy group P <.05.
compared with the traditional quadruple therapy group P <.05.
Adverse Effects
Specific adverse effects were observed in the 3 groups, such as nausea, vomiting, diarrhea, abdominal distention, and constipation. The adverse effect rates noted for the 3 groups were 25%, 7.5%, and 15%, respectively. The adverse effect rate was significantly lower in the vonoprazan triple therapy group than that of the control group (Table 3 and Figure 2).
Table 3.
Comparison of Adverse Effects of the 3 Groups
| Traditional Quadruple Therapy Group | Vonoprazan Triple Therapy Group | Vonoprazan Quadruple Therapy Group | |
|---|---|---|---|
| Nausea | 3 | 2 | 1 |
| Vomiting | 1 | 0 | 1 |
| Diarrhea | 1 | 0 | 1 |
| Abdominal distention | 4 | 1 | 2 |
| Constipation | 1 | 0 | 1 |
| Adverse effect rate (%) | 25 | 7.5 | 15 |
Figure 2.
Comparison of adverse effects of the 3 groups. (A) Traditional quadruple therapy group; (B) vonoprazan triple therapy group; (C) vonoprazan quadruple therapy group.  Compared with traditional quadruple therapy group P < .05.
Compared with traditional quadruple therapy group P < .05.
Comparison between vonoprazan triple therapy and vonoprazan quadruple therapy groups.
Both groups exhibited a high eradication rate, and no significant differences were noted in the eradication rate of the vonoprazan triple therapy and vonoprazan quadruple therapy groups. The adverse effect rate was slightly lower in the vonoprazan triple therapy group than that noted in the vonoprazan quadruple therapy group. However, no significant differences were noted.
Discussion
H. pylori is a gram-negative bacterium, which resides in the stomach and duodenum. The infection rate of H. pylori is very high, notably in developing countries. In China, the infection rate in adults is 58.07%.27,28 H. pylori infection is not only the cause of peptic ulcers but it is also associated with atrophic gastritis, gastric cancer, and gastro-esophageal reflux disease.7,29-31 Due to its high infection rate and close association with the development of digestive diseases, it is considered a research hotspot.
According to the fifth national guideline for H. pylori infection, peptic ulcer and mucosa associated lymphoid tissue (MALT) lymphoma are the indicators of H. pylori eradication. The eradication of this microorganism is the first line of prevention of gastric cancer.32 The guideline recommends conventional quadruple therapy as the empirical way for the eradication of H. pylori. In the present study, conventional quadruple therapy included the following compounds and doses: esomeprazole (20 mg twice a day) before a meal, bismuth potassium citrate (0.6 g twice a day) before a meal, amoxicillin (1.0 g twice a day) after a meal, and furazolidone (100 mg twice a day) after a meal. Following the increase in drug resistance, the eradication rate of conventional therapy was decreased. In the present study, the eradication rate was only 80% for the control group. The eradication rate of the control group was slightly higher than the result obtained by Murakami et al.33 This is possibly attributed to the number of days of treatment being 14 instead of 7. Furthermore, bismuth was added to the control group.
Vonoprazan is a new proton pump inhibitor, which provides sustained acid inhibitory effect, and is approved for the treatment of gastro-esophageal reflux disease. During the process of the eradication of H. pylori, the pH level in the stomach has to be increased to fully exert the effects of the antibiotics. H. pylori is more susceptible to antimicrobial agents when it restores its replicative capability at a pH level higher than 6.12 In the present study, the H. pylori eradication efficacy of vonoprazan and esomeprazole was compared. Vonoprazan triple therapy and vonoprazan quadruple therapy could significantly increase the eradication rate of H. pylori, suggesting that the increase in the pH levels in the stomach by using vonoprazan could achieve optimal eradication rate.
The recommended antibiotics for the eradication of H. pylori in the clinic include the following: amoxicillin, furazolidone, clarithromycin, metronidazole, and levofloxacin.34 Since the resistance rate of clarithromycin, metronidazole, and levofloxacin is very high, these drugs are used less frequently, whereas amoxicillin and furazolidone are used more frequently. Certain side effects, such as nausea, vomiting, diarrhea, and dizziness, are usual, and consequently, many patients quit the treatment schedule. The side effects are related to the duration of eradication of H. pylori using furazolidone as the main antibiotic treatment. In the present study, the side effects of vonoprazan triple therapy and vonoprazan quadruple therapy were examined. The former exhibited reduced side effects than the latter, whereas it had almost the same eradication rate. Therefore, it would be better to select vonoprazan plus bismuth triple therapy as the priority choice for the eradication of H. pylori.
There were some limitations concerning this study. First, this was a single-center study, and there were only 120 patients recruited for study; it would be better if we conducted multi-center study and recruited more patients, and the results would be more accurate. Second, in our study, patients were treated for 14 days, and we only analyzed the results of patients treated for 14 days; it would be better if we added more groups with patients treated for 7 and 10 days.
In conclusion, the present study indicated that the eradication of H. pylori. was decreasing, whereas the adverse effect was increasing. Both vonoprazan triple therapy and vonoprazan quadruple therapy could increase the eradication rate of H. pylori. Vonoprazan triple therapy exhibited reduced side effects and can be applied in the eradication of H. pylori in the clinic.
Footnotes
Ethics Committee Approval: The present study was approved by Ganzhou People’s Hospital Ethics Committee (No: TY-ZKY2011-011-01, July 14, 2021).
Informed Consent: Written consent was obtained from patients.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – J.H., Y.L.; Design – J.H., Y.L.; Supervision – J.H., Y.L.; Resources – Y.L.; Materials – Y.L.; Data Collection and/or Processing – J.H.; Analysis and/or Interpretation – J.H.; Literature Search – Y.L.; Writing Manuscript – J.H.; Critical Review – Y.L.
Acknowledgments: The authors would like to thank the colleagues from the department of gastroenterology in Ganzhou People’s Hospital for providing assistance. The authors thank Ganzhou People’s Hospital for providing the necessary funding.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: Ganzhou People’s Hospital youth fund 1400000017 partially provided funding.
References
- 1. . Li Z, Chen M, Lv N.et al. The consensus of H pylori eradication and gastric cancer prevention in China. Chin Dig J. 2019;39(5):310 316. [Google Scholar]
- 2. . Li C, Zhang Z. The H pylori consensus in Taiwan 2017. J Gastroentolorogy Liver. 2018;27(1):1 12. [Google Scholar]
- 3. . McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362(17):1597 1604. 10.1056/NEJMcp1001110) [DOI] [PubMed] [Google Scholar]
- 4. . Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013;132(6):1272 1276. 10.1002/ijc.27965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. . Dorji D, Dendup T, Malaty HM, Wangchuk K, Yangzom D, Richter JM. Epidemiology of Helicobacter pylori in Bhutan: the role of environment and Geographic location. Helicobacter. 2014;19(1):69 73. 10.1111/hel.12088) [DOI] [PubMed] [Google Scholar]
- 6. . Sugano K, Tack J, Kuipers EJ.et al. Faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353 1367. 10.1136/gutjnl-2015-309252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. . Guevara B, Cogdill AG. Helicobacter pylori: a review of current diagnostic and management strategies. Dig Dis Sci. 2020;65(7):1917 1931. 10.1007/s10620-020-06193-7) [DOI] [PubMed] [Google Scholar]
- 8. . Zou YZ, Qian X, Liu XQ.et al. The effect of antibiotic resistance on Helicobacter pylori eradication efficacy: a systematic review and meta-analysis. Helicobacter. 2020;12:e12714. [DOI] [PubMed] [Google Scholar]
- 9. . Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808 1825. 10.1111/j.1572-0241.2007.01393.x) [DOI] [PubMed] [Google Scholar]
- 10. . Asaka M, Kato M, Takahashi S.et al. Guidelines for the Management of Helicobacter pylori Infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1 20. [DOI] [PubMed] [Google Scholar]
- 11. . Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. 10.1136/bmj.f4587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. . Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5(6):321 331. 10.1038/ncpgasthep1138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. . Kobayashi I, Murakami K, Kato M.et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007;45(12):4006 4010. 10.1128/JCM.00740-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. . Gong EJ, Ahn JY, Kim JM. et al. Genotypic and phenotypic resistance to clarithromycin in Helicobacter pylori Strains. J Clin Med. 2020;9(6):1 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. . Liu WZ, Xie Y, Cheng H.et al. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013;14(5):211 221. 10.1111/1751-2980.12034) [DOI] [PubMed] [Google Scholar]
- 16. . Horie R, Handa O, Ando T.et al. Helicobacter pylori eradication therapy outcome according to clarithromycin susceptibility testing in Japan. Helicobacter. 2020;25(4):e12698. 10.1111/hel.12698) [DOI] [PubMed] [Google Scholar]
- 17. . Bang CS, Lim H, Jeong HM.et al. Amoxicillin or tetracycline in bismuth-containing quadruple therapy as first-line treatment for Helicobacter pylori infection [J]. Gut Microbe. 2020;2:1 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. . Gong YH, Yuan Y. Resistance mechanisms of Helicobacter pylori and its dual target precise therapy. Crit Rev Microbiol. 2018;44(3):371 392. 10.1080/1040841X.2017.1418285) [DOI] [PubMed] [Google Scholar]
- 19. . Lopo I, Libânio D, Pita I, Dinis-Ribeiro M, Pimentel-Nunes P. Helicobacter pylori antibiotic resistance in Portugal: systematic review and meta-analysis. Helicobacter. 2018;23(4):e12493. 10.1111/hel.12493) [DOI] [PubMed] [Google Scholar]
- 20. . Wang YH, Lv ZF, Zhong Y.et al. The internalization of Helicobacterpylori plays a role in the failure of H. pylorieradication. Helicobacter. 2017;22(1):e12324. [DOI] [PubMed] [Google Scholar]
- 21. . Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016;65(5):870 878. 10.1136/gutjnl-2015-311019) [DOI] [PubMed] [Google Scholar]
- 22. . Shin JM, Inatomi N, Munson K.et al. Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438). J Pharmacol Exp Ther. 2011;339(2):412 420. 10.1124/jpet.111.185314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. . Hori Y, Imanishi A, Matsukawa J.et al. 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther. 2010;335(1):231 238. 10.1124/jpet.110.170274) [DOI] [PubMed] [Google Scholar]
- 24. . Matsukawa J, Hori Y, Nishida H, Kajino M, Inatomi N. A comparative study on the modes of action of TAK-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol. 2011;81(9):1145 1151. 10.1016/j.bcp.2011.02.009) [DOI] [PubMed] [Google Scholar]
- 25. . Hori Y, Matsukawa J, Takeuchi T, Nishida H, Kajino M, Inatomi N. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther. 2011;337(3):797 804. 10.1124/jpet.111.179556) [DOI] [PubMed] [Google Scholar]
- 26. . Scott DR, Munson KB, Marcus EA, Lambrecht NW, Sachs G. The binding selectivity of vonoprazan (TAK-438) to the gastric H+, K+ -ATPase. Aliment Pharmacol Ther. 2015;42(11-12):1315 1326. 10.1111/apt.13414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. . Burucoa C, Axon A. Epidemiology of Helicobacter pylori infection. Helicobacter. 2017;22(suppl 1):e12403. 10.1111/hel.12403) [DOI] [PubMed] [Google Scholar]
- 28. . Zhu HM, Li BY, Tang Z.et al. Epidemiological investigation of Helicobacter pylori infection in elderly people in Beijing. World J Clin Cases. 2020;8(11):2173 2180. 10.12998/wjcc.v8.i11.2173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. . Vania C, Toshiro S, Touati E. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2017;22:e12405. [DOI] [PubMed] [Google Scholar]
- 30. . Sameh HE, Ayman E, Hosam GE, Wagdi E, Mohamed AA. Helicobacter pylori, sleeve gastrectomy, and gastroesophageal reflux disease; is there a relation? Obesity Surgery. 2020;30(8):3037 3045. [DOI] [PubMed] [Google Scholar]
- 31. . Yamamichi N, Yamaji Y, Shimamoto T.et al. Inverse Time Trends of Peptic Ulcer and Reflux Esophagitis Show Significant Association with Reduced Prevalence of Helicobacter pylori Infection. Annals Medicine [J]. 2020;22:1 9. 10.1136/gutjnl-2015-311304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. . Gladys MR, Marisol CH, Albert YL. Family history of Gastric Cancer and HelicobacterpyloriTreatment. New England J Med. 2020;82(22):2170 2171. [Google Scholar]
- 33. . Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65(9):1439 1446. 10.1136/gutjnl-2015-311304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. . Luo LS, Huang Y, Liang X, Ji Y, Yu L, Lu H. Susceptibility-guided therapy for Helicobacter pylori-infected penicillin-allergic patients: a prospective clinical trial of first-line and rescue therapies. Helicobacter. 2020;25(4):e12699. 10.1111/hel.12699) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a