Abstract
BACKGROUND
Preimplantation genetic testing (PGT) of embryos developed in vitro requires a biopsy for obtaining cellular samples for the analysis. Signs of cell injury have been described in association with this procedure. Thus, the consequences of the biopsy on obstetric and neonatal outcomes have been the subject of some quantitative analyses, although the reliability of data pooling may be limited by important issues in the various reports.
OBJECTIVE AND RATIONALE
The present review identifies evidence for whether pregnancies conceived after embryo biopsy are associated with a higher risk of adverse obstetric, neonatal, and long-term outcomes. Available evidence has been summarized considering manipulation at various stages of embryo development.
SEARCH METHODS
We used the scoping review methodology. Searches of article databases were performed with keywords pertaining to the embryo biopsy technique and obstetric, neonatal, and postnatal outcomes. Studies in which embryos were biopsied at different stages (i.e. both at the cleavage and blastocyst stages) were excluded. We included data on fresh and frozen embryo transfers. The final sample of 31 documents was subjected to qualitative thematic analysis.
OUTCOMES
Sound evidence is lacking to fully address the issues on the potential obstetric, neonatal or long-term consequences of embryo biopsy. For polar body biopsy, the literature is too scant to draw any conclusion. Some data, although limited and controversial, suggest a possible association of embryo biopsy at the cleavage stage with an increased risk of low birthweight and small for gestational age neonates compared to babies derived from non-biopsied embryos. An increase in preterm deliveries and birth defects in cases of trophectoderm biopsy was suggested. For both biopsy methods (at the cleavage and blastocyst stages), an increased risk for hypertensive disorders of pregnancy was found. However, these findings may be explained by confounders such as other embryo manipulation procedures or by intrinsic patient or population characteristics.
WIDER IMPLICATIONS
Since there is inadequate evidence to assess obstetric, neonatal, and long-term health outcomes following embryo biopsy, an invasive PGT strategy should be developed with a cautious approach. A non-invasive approach, based on the analysis of embryo cell-free DNA, needs to be pursued to overcome the potential limitations of embryo biopsy.
Keywords: preimplantation genetic testing, embryo biopsy, maternal outcomes, neonatal outcomes, follow-up, trophectoderm biopsy, blastocyst, scoping review
Graphical abstract
No conclusive evidence for an adverse effect of PGT on outcomes. The amber symbol represents weak and/or a controversial body of safety evidence for which further research is required. The red symbol warns that no evidence of safety exists. PGT, preimplantation genetic testing.
Introduction
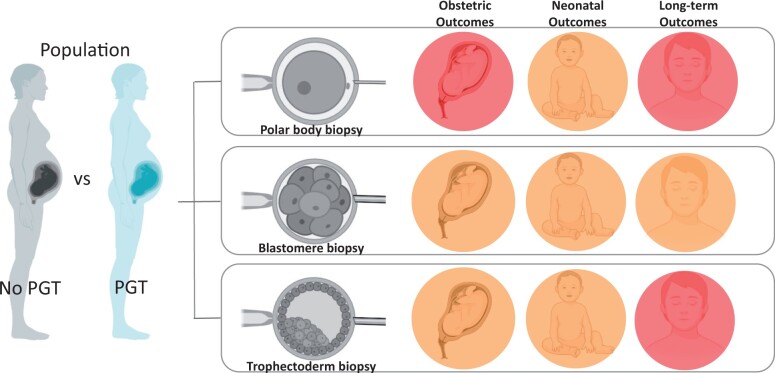
The rapid diffusion of medically assisted reproduction (MAR) in the past three decades has been favoured by the development of technologically sophisticated procedures (Waynforth, 2018). There is, however, growing concern that some MAR-related technologies or processes may increase the risk of obstetric, perinatal, and congenital anomalies. In this context, concern over increased adverse outcomes has been raised for babies born after the collection of samples needed for preimplantation genetic testing (PGT). PGT aims to detect genetic disorders and aneuploidies in in vitro-conceived embryos to avoid the transfer of a chromosomally abnormal or affected conceptus. Three different invasive strategies have been proposed over the years to collect cells to be analysed: polar body (PB) biopsy; blastomere biopsy; and blastocyst stage biopsy (Cimadomo et al., 2020). Recently, data from Europe collected by the ESHRE PGT Consortium showed that cleavage stage biopsy is the most widely used approach for monogenic/single-gene defects (PGT-M) and/or for chromosomal structural rearrangements (PGT-SR) (78% and 67%, respectively). Conversely, in the case of PGT for aneuploidies (PGT-A), the majority of biopsies are performed at the blastocyst stage (87%) (van Montfoort et al., 2021).
Rationale
The aims here were to collect and analyse existing data to provide a review of the short- and long-term risks of embryo biopsy in prenatal or postnatal human development and health. To achieve this goal, we have summarized the available evidence on the obstetric, neonatal, and long-term outcomes of conceptions after MAR and a biopsy at different embryonic stages compared to pregnancies obtained from non-biopsied embryos.
To correctly assess these outcomes, it is vital to consider some critical aspects. This fits well within the framework of a scoping review that should identify gaps and biases in the literature.
Potential damaging effects of embryo biopsy
The mammalian embryo has been shown in a wide range of species to be able to regulate the loss of cells without compromising or affecting post-implantation development and the birth of normal-sized offspring. In 1968, Gardner and Edwards were able to sex rabbit embryos using a sex-specific chromatin pattern in blastocyst biopsies before their transfer to the uterus. Preimplantation testing of embryos is also used routinely in animal husbandry to produce animals of the preferred sex (Braude et al., 2002). However, in humans, the safety of the procedure still presents a concern. PB biopsy on metaphase II oocytes and/or zygotes is potentially less invasive than in any other stage of preimplantation development, since it involves the removal of the ‘waste products’ of meiosis. However, it is a demanding and time-consuming procedure, and it only provides maternal genetic information, not considering parental or mitotic division abnormalities (Greco et al., 2020).
Embryo biopsy at the cleavage stage requires the collection of blastomeres at an early stage through an invasive procedure (Mastenbroek et al., 2007; Alteri et al., 2019). Interestingly, blastomere loss at early stages of development as a consequence of cryopreservation has been reported to be associated with a lower number of cells in resultant blastocysts (Archer et al., 2003). However, even if the removal of one or two cells at the eight-cell stage reduces the inner cell mass (ICM) of the blastocyst (Hardy et al., 1990), embryos with fragmented or damaged blastomeres are routinely transferred, resulting in live births.
The blastocyst biopsy, characterized by the removal of 5–10 trophectoderm (TE) cells without affecting the ICM lineage, has been shown to be safer than blastomere biopsy and, consequently, it is gradually replacing both PB and cleavage-stage biopsy approaches (van Montfoort et al., 2021). However, the real impact of TE biopsy is still unclear. The only randomized controlled trial (RCT) designed to investigate the safety of the TE biopsy procedure, performed by removing a maximum of five cells, demonstrated the safety of this procedure in terms of implantation potential and live birth rate (Scott et al., 2013).
Based on the results of the study by Munné et al. (2019), a mathematical model supported the idea that the indiscriminate application of PGT-A leads to a loss of potentially competent embryos, possibly a result of biopsy-related embryo damage (Pagliardini et al., 2020). Notably, it is possible that the deleterious effects of blastocyst biopsy may not manifest until later in pregnancy. He et al. (2019) analysed the neonatal outcomes of babies born after TE biopsy, dividing them into two groups according to the number of biopsied cells (˂10 and ≥10 biopsied cells); the frequency of neonatal macrosomia and the sex ratio were different between the two groups. The limitations of the study are the small sample size and the borderline statistical significance, but this finding might be worth further investigation to understand the effects of different numbers of biopsied cells.
Since during the biopsy a sample of 5–10 cells is removed from the TE, which ultimately gives rise to the placenta, it is plausible that its disruption might result in abnormalities in placentation. In line with this, some studies reported an association between TE biopsy and abnormal placentation conditions, such as pre-eclampsia and an increased risk of preterm birth (Zhang et al., 2019; Li et al., 2021). Furthermore, serum β-hCG levels on day 12 after the transfer of biopsied blastocysts were found to be statistically significantly lower than the levels of β-hCG after frozen embryo transfer (FET) without blastocyst biopsy (Lu et al., 2020). Since maternal β-hCG is produced by villous syncytiotrophoblast cells as pregnancy progresses, TE biopsy might affect the secretion of β-hCG. Similarly, the β-hCG concentration secreted by a biopsied blastocyst in the culture medium seems to be inversely proportional to the number of TE cells removed (Dokras et al., 1991). In disagreement with this observation, Wu et al. (2021) recently showed no impact of TE biopsy on serum β-hCG levels 14 days after embryo transfer.
The confounding effect of infertility status
Many studies have evaluated the effects of embryo biopsy without distinction among PGT-M, PGT-SR, and PGT-A. Although the technical procedures performed by embryologists are identical, the indications are different, leading to significant heterogeneity in the characteristics of the patients involved. PGT-M is generally applied for fertile individuals. Conversely, PGT-A is used for infertile individuals undergoing MAR to optimize the efficiency of MAR cycles. Similarly, PGT-SR is offered to individuals to avoid a pregnancy with a chromosomally unbalanced product of the translocation and, hence, to reduce the risk of miscarriage. Infertility per se (rather than MAR treatments) is related to adverse obstetric and perinatal outcomes (Thomson et al., 2005; DoPierala et al., 2016), implying a higher risk after biopsies for PGT-A compared to PGT-M. This represents an important source of bias affecting the studies. Two recent systematic reviews and meta-analyses have evaluated obstetric and neonatal outcomes from PGT pregnancies considering pooled data derived from PGT-M, PGT-SR, and PGT-A cases and comparing them to pregnancies derived from natural conceptions or fresh and FET ART cycles without embryo biopsy (Hou et al., 2021; Zheng et al., 2021). Overall, PGT pregnancies were shown to be associated with lower rates of low birthweight (LBW), very low birthweight (VLBW), and very preterm delivery (VPTD) compared to those obtained without embryo biopsy and with increased risks of LBW, preterm delivery (PTD), and hypertensive disorders of pregnancy (HDP) compared to spontaneous conceptions (Hou et al., 2021; Zheng et al., 2021). It is likely that these findings can be simply attributed to the pooling of very different PGT populations in the analysis.
The confounding effect of embryo freezing
Analysis of biopsy samples using the most recent analytical technologies requires time. Therefore, manipulated embryos, particularly at the blastocyst stage, are often vitrified after biopsy and warmed before transfer, allowing the analysis to be completed. However, freezing has been reported to potentially influence obstetric and neonatal outcomes. Recent evidence supports a higher risk of pre-eclampsia in pregnancies after FET compared to fresh cycles (Roque et al., 2019; Wei et al., 2019). Atypical placentation with a reduced uterine artery pulsatility index from 6 to 37 weeks has been reported in singleton pregnancies conceived after the transfer of frozen blastocysts (Cavoretto et al., 2020). Moreover, FETs have been associated with greater foetal crown-rump length at 6–14 weeks and greater foetal growth (with a lower risk of small for gestational age (SGA) and greater estimated foetal weight or birthweight) (Cavoretto et al., 2021a,b).
An RCT demonstrated a higher risk of obstetric complications after frozen vs fresh embryo transfers in women with PCOS (Zhang et al., 2018). Specifically, women with PCOS showed an increased risk of large for gestational age (LGA) babies in singleton pregnancy and a higher risk of pre-eclampsia in twin pregnancies after FET (Zhang et al., 2018). Moreover, placentas from FET pregnancies showed a higher rate of anatomic and vascular pathology than those from fresh transfers, with a significantly higher risk of marginal cord insertion, accessory lobes, foetal vascular malperfusion characteristics with cord anomalies, and subchorionic thrombi after adjustment for potential confounding variables (Sacha et al., 2020). The extent to which TE biopsy might exacerbate the possible impact of embryo cryopreservation on obstetric and neonatal complications remains to be established.
Objectives
The research questions guiding this exploratory review were:
What are the obstetric, neonatal, and long-term outcomes after transfers of biopsied embryos?
What knowledge gaps should be bridged with future research to help ensure safe treatment for patients?
Methods
Protocol and registration
We followed the five stages of the updated scoping review protocol developed by Arksey and O’Malley (2005), Colquhoun et al. (2014), Levac et al. (2010), Peters et al. (2015), and Tricco et al. (2016). This scoping review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis-scoping review extension (PRISMA-ScR) (Tricco et al., 2018). The rationale, eligibility criteria, and study selection were defined a priori. The scoping review protocol was recorded a priori and is available at OSF registries (Registration doi:10.17605/OSF. IO/26X49, https://osf.io/registries).
Eligibility criteria
The intervention group consisted of pregnancies following the transfer of biopsied embryos compared with pregnancies following the transfer of non-biopsied embryos. We included reported data about manipulated embryos with PB, blastomere, and TE, but not morula, biopsy. Studies in which embryos were biopsied at different stages (i.e. at the cleavage and blastocyst stages) were excluded. We included data about both fresh embryo transfers and FETs. All study designs were considered, with the exception of case reports and case series. We excluded studies focused on psychosocial outcomes. This search was limited to articles in English.
Information sources and search
The bibliographical PubMed, Embase, and Cochrane Library databases were searched for studies including combinations of the following terms, alone or combined with Boolean operators: ‘embryo biops*’, ‘blastomere biops*’, ‘blastocyst biops*’, ‘polar body biops*’, ‘trophectoderm biops*’, ‘preimplantation genetic’, ‘longterm’, ‘natal’, ‘obstetric’, and ‘children’. The scoping review included all studies published until April 2021.
Selection of sources of evidence
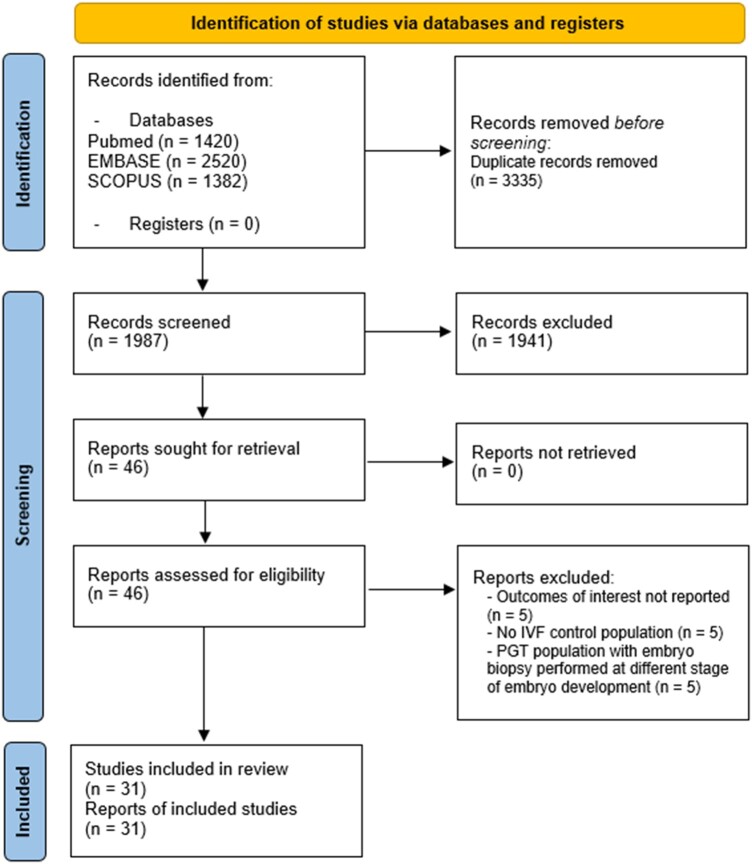
The authors did not use strict inclusion and exclusion criteria (Arksey and O’Malley, 2005). Two authors (A.A. and G.C.C.) independently examined the potentially relevant studies by checking the titles, abstracts, and full texts of the chosen studies using the same inclusion criteria, as suggested by research methodology guidelines (Arksey and O’Malley, 2005). The authors resolved disagreements on study selection and data extraction by discussion with other reviewers (G.G. and M.P.) if needed. When authors identified reviews, the references that potentially met the inclusion criteria were selected and checked for missed studies. The PRISMA flow chart depicts the study selection process, and the entire screening process is shown in Fig. 1.
Figure 1.
PRISMA flow diagram of article screening for the scoping review. PGT, preimplantation genetic testing; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis.
Data-charting process
A data-charting form was jointly developed by two reviewers (A.A. and M.P.) to determine which sensitive variables to extract. Three of the co-authors (A.A., G.C.C., and G.G.) independently extracted and charted data from each study and discussed differences until they reached consensus. The reviewers continuously updated the data-charting form and discussed the results with other reviewers when in doubt.
The selected clinical outcomes are defined as follows. PTD is defined as any delivery resulting in a vital neonate occurring before the completion of 37 weeks of pregnancy (Blencowe et al., 2012). Gestational hypertension is hypertension arising de novo after 20 weeks of gestation in the absence of proteinuria and without biochemical or haematological abnormalities or foetal growth restriction (Brown et al., 2018). Pre-eclampsia is defined as de novo hypertension after 20 weeks of gestation associated with proteinuria and/or evidence of maternal organ dysfunction or foetal growth restriction (Brown et al., 2018). Gestational diabetes is defined as glucose intolerance with hyperglycaemia that begins or is first diagnosed in pregnancy (Saravanan, 2020). Abnormal placentation is defined as a composite outcome measure including several placental disorders, such as placental abruption, placenta accreta spectrum, and placenta or vasa previa. Since heterogeneity was observed among different studies, further details about specific placental abnormalities may be found in each individual paper (Jauniaux et al., 2019). LBW is defined as weight at birth <2500 g, and VLBW is defined as weight at birth <1500 g (World Health Organization, 2014). SGA is defined as an estimated foetal weight or birthweight below the 10th percentile, and LGA is defined as foetal weight or birthweight above the 90th percentile, according to the standard population reference (Nicolaides et al., 2018). Birth defects can be defined as structural or functional abnormalities that are present from birth. Long-term outcomes were defined according to heterogeneous definitions found in each individual study and were related mainly to arterial blood pressure, anthropometric measures, and neurological, psychomotor, and cognitive development.
Data items
First, the authors collected a combination of qualitative or quantitative data from the final list of publications in a ‘data-charting form’ (Arksey and O’Malley, 2005), reporting: study characteristics, including study design and sample size of study groups; embryo biopsy methods; and reported outcome data, including definition of outcomes, key findings, and statistical analysis.
Synthesis of results
To synthesize the range of evidence, the results were classified under three main conceptual categories, defined as ‘obstetric outcomes’, ‘neonatal outcomes’, and ‘long-term outcomes’.
Results
A total of 5322 records were retrieved from the search. After the removal of duplicate records, 1987 records were screened, and 46 were assessed for eligibility, leading to 31 articles being included in the review after exclusions for specific reasons. Figure 1 shows the detailed PRISMA flow diagram.
Obstetric outcomes
Table I describes the main characteristics and findings of studies investigating obstetric complications of PGT pregnancies. As shown, no data are available for these outcomes following PB biopsy.
Table I.
Main characteristics and findings of studies investigating the obstetric and perinatal complications of pregnancies derived from biopsied human embryos, according to the biopsy method.
| Author, year | Study design | Number of children (PGT vs control group) | Study population | Biopsy method | Outcomes |
|---|---|---|---|---|---|
| Montag et al., 2004 | Retrospective cohort study |
|
Biopsied (PGT-A) vs non-biopsied embryos | PB | Birthweight (−) |
|
| |||||
| Liebaers et al., 2010 | Prospective observational cohort study |
|
Biopsied (PGT-M + PGT-SR + PGT-A) vs non-biopsied embryos | BL |
|
| Desmyttere et al., 2012 | Prospective comparative follow-up study |
|
Biopsied (PGT-M + PGT-A) vs non-biopsied embryos | BL |
|
| Hasson et al., 2017 | Retrospective cohort study |
|
Biopsied (PGT-M + PGT-SR) vs non-biopsied embryos | BL |
|
| Bay et al., 2016 | Retrospective cohort study |
|
Biopsied (PGT-M + PGT-SR) vs non-biopsied embryos | BL |
|
| Feldman et al., 2020 | Retrospective cohort study |
|
Biopsied (PGT-M) vs non-biopsied embryos | BL |
|
|
| |||||
| Forman et al., 2012 | Retrospective study |
|
Biopsied (PGT-A) vs non-biopsied embryos | TE | Birth weight (−) |
| Forman et al., 2014 | RCT |
|
Biopsied (PGT-A) vs non-biopsied embryos | TE | Preterm delivery (+) |
| Sacchi et al., 2019 | Prospective observational cohort study |
|
Biopsied (PGT-A) vs non-biopsied embryos | TE |
|
| Zhang et al., 2019 | Prospective observational cohort |
|
Biopsied (PGT-SR + PGT-A) vs non-biopsied embryos | TE |
|
| He et al., 2019 | Retrospective cohort study |
|
Biopsied (PGT) vs non-biopsied embryos | TE |
|
| Lu et al., 2020 | Retrospective cohort study | Singletons: N = 305 vs N = 328 | Biopsied (PGT) vs non-biopsied embryos | TE |
|
| Swanson et al., 2021 | Retrospective cohort study | Singletons: N = 158 vs N = 153 | Biopsied (PGT) vs non-biopsied embryos | TE |
|
| Awadalla et al., 2021 | Retrospective cohort study | Singletons: N = 67 vs N = 78 | Biopsied (PGT) vs non-biopsied embryos | TE |
|
| Sites et al., 2021 | Retrospective cohort study | Singletons: N = 585 vs N = 2191 | Biopsied (PGT-A) vs non-biopsied embryos | TE |
|
| Makhijani et al., 2021 | Retrospective cohort study | Singletons: N = 241 vs N = 515 | Biopsied (PGT-M + PGT-SR + PGT-A) vs non-biopsied embryos | TE |
|
| Riestenberg et al., 2021 | Retrospective cohort study | Singletons: N = 475 vs N = 237 | Biopsied (PGT-A) vs non-biopsied embryos | TE | Abnormal placentation (−) |
| Li et al., 2021 | Retrospective cohort study | Singletons: N = 6244 vs N = 10 002 | Biopsied (PGT-M + PGT-A) vs non-biopsied embryos | TE |
|
“+”, significant difference between the two groups; “−”, no significant difference between the two groups.
BL, blastomere; NICU, neonatal intensive care unit; PB, polar body; PGT, preimplantation genetic testing; PGT-A, preimplantation genetic testing for aneuploidies; PGT-M, preimplantation genetic testing for monogenic disorders; PGT-SR, preimplantation genetic testing for structural rearrangements; RCT, randomized controlled trial; TE, trophectoderm.
Preterm delivery
Polar body (PB) biopsy
No outcome data were available following PB biopsy.
Blastomere biopsy
Three cohort studies described the incidence of PTD defined as a delivery before 37 weeks of gestation (Bay et al., 2016; Hasson et al., 2017; Feldman et al., 2020) and VPTD defined as a delivery before 32 (Feldman et al., 2020) and/or 34 weeks (Hasson et al., 2017; Feldman et al., 2020) in MAR pregnancies with and without a blastomere biopsy, reporting no significant differences between the two groups.
Trophectoderm (TE) biopsy
Nine of the studies investigated the rate of PTD and/or VPTD in pregnancies that occurred following TE biopsy (Forman et al., 2014; He et al., 2019; Sacchi et al., 2019; Zhang et al., 2019; Lu et al., 2020; Awadalla et al., 2021; Li et al., 2021; Makhijani et al., 2021; Sites et al., 2021). In none of these studies, except those by Forman et al. (2014) and Li et al. (2021), a significant difference was observed between the biopsy and non-biopsy groups.
In the RCT on PGT-A by Forman et al. (2014), the PTD risk was significantly higher after the transfer of two untested embryos compared to that after the transfer of a single biopsied euploid embryo (relative risk 2.21, 95% CI 1.04–4.70, P = 0.03). As stated by the authors, differences between groups could likely be attributed to the difference in the risk of a twin pregnancy. Indeed, although not presented, obstetrical and neonatal outcomes between singleton deliveries in the biopsy and non-biopsy groups were reported to be similar. This study will thus not be commented on further in the following paragraphs, as the negative outcomes reported could all be attributed to multiple gestations and not to the technique.
Recently, a large retrospective cohort study based on the Society for Assisted Reproductive Technology Clinical Outcomes Reporting System (SART-CORS) database showed that singletons from the transfer of frozen biopsied embryos (n = 6244) had a slightly increased likelihood of PTD [adjusted odds ratio (aOR) 1.20, 95% CI 1.09–1.33] compared to babies derived from non-biopsied blastocysts (n = 10002) (Li et al., 2021). A sub-analysis comparing cycles with or without biopsy in an infertile population confirmed these findings (aOR 1.23, 95% CI 1.10–1.37) (Li et al., 2021).
Hypertensive disorders of pregnancy
Polar body (PB) biopsy
No outcome data were available following PB biopsy.
Blastomere biopsy
Feldman and coworkers showed an increased risk of HDP, ranging from gestational hypertension to eclampsia, in association with biopsy for PGT-M. This difference persisted even after stratifying for singleton pregnancies (n = 345 in the group after biopsy and n = 422 in the non-biopsy group) (aOR 5.9, 95% CI 1.9–18.2) or twin pregnancies (n = 76 in the group after biopsy and n = 101 in the non-biopsy group) (aOR 3.7, 95% CI 1.1–12.8) (Feldman et al., 2020). A Danish nationwide study failed to show different rates of pre-eclampsia in n = 126 pregnancies after biopsy for PGT-M/PGT-SR compared to n = 30 418 pregnancies derived from non-biopsied embryos (Bay et al., 2016). We observed that both studies combined both fresh embryo transfers and FETs for analysis, providing unclear information on the real impact of the biopsy (Sacha et al., 2020).
Trophectoderm (TE) biopsy
Five of the studies assessed the potential association between TE biopsy and HDP (Zhang et al., 2019; Lu et al., 2020; Makhijani et al., 2021; Sites et al., 2021; Swanson et al., 2021), reporting conflicting data. In all the studies, analyses were adjusted for confounding factors such as maternal age and BMI. Zhang et al. (2019) found a significantly (3-fold) increased risk of pre-eclampsia following fresh embryo transfers or FETs (n = 177) of biopsied embryos compared to those of non-biopsied embryos (n = 180) (aOR 3.02, 95% CI 1.10–8.29, P = 0.02). However, the statistical significance was lost when the analysis was limited to FETs, likely because of the small sample size (n = 134 in the biopsy group vs n = 124 in the non-biopsy group). On the other hand, no difference in gestational hypertension was found between the two groups considered (Zhang et al., 2019).
Similarly, a recent retrospective cohort study comparing n = 241 FETs with TE biopsy and n = 515 cycles without biopsy showed a significantly higher risk of HDP in association with biopsy (aOR 1.94, 95% CI 1.07–3.52) (Makhijani et al., 2021). Stratifying for the severity of hypertensive disorders, the statistical significance was maintained only for non-severe forms. The opposite conclusions were drawn by three studies based on FETs, in which no significant difference in the rate of HDP following the transfer of biopsied or non-biopsied blastocysts was found (Lu et al., 2020; Sites et al., 2021; Swanson et al., 2021).
Abnormal placentation
Polar body (PB) biopsy
No outcome data were available following PB biopsy.
Blastomere biopsy
Only one of the studies assessed the presence of abnormal placentation between pregnancies conceived after blastomere biopsy and those without biopsy, considering both fresh embryo transfers and FETs (Bay et al., 2016). No correlation was observed between the placenta previa rate and blastomere biopsy.
Trophectoderm (TE) biopsy
Four studies found no significant difference in the rate of placental anomalies, such as placenta previa and placenta accreta, between pregnancies derived from biopsied and non-biopsied blastocysts (Zhang et al., 2019; Makhijani et al., 2021; Sites et al., 2021; Swanson et al., 2021). In line with these findings, a recent study did not show significant differences in the rates of foetal and placental anomalies in MAR pregnancies that occurred after biopsy for PGT-A or not (Riestenberg et al., 2021).
Gestational diabetes mellitus
Polar body (PB) biopsy
No outcome data were available following PB biopsy.
Blastomere biopsy
Feldman et al. (2020) found that the rate of gestational diabetes mellitus (GDM) was lower in singleton pregnancies (9.2% vs 15.6%, aOR 0.55, 95% CI 0.3–0.8) associated with embryo biopsy for PGT-M compared to the non-biopsy group. A plausible explanation might be that patients who undergo PGT for monogenic diseases do not suffer from infertility and may have, per se, a lower GDM risk.
Trophectoderm (TE) biopsy
On the other hand, in the four studies in which TE biopsy was performed for PGT cycles without distinction among indications, no significant difference in GDM risk between MAR pregnancies with and without blastocyst biopsy was found (Zhang et al., 2019; Lu et al., 2020; Makhijani et al., 2021; Sites et al., 2021), suggesting that embryo biopsy does not represent a predictor of GDM in pregnancies conceived with MAR.
Neonatal outcomes
The main characteristics and findings of studies investigating the neonatal complications of PGT pregnancies are described in Table I.
Birthweight
Polar body (PB) biopsy
Only a single retrospective study was conducted on this topic (Montag et al., 2004), comparing n = 106 women subjected to embryo biopsy for PGT-A and n = 220 patients whose embryos were not subjected to biopsy in the same period of time. The median birthweight of children in the PB biopsy group was similar to that of children in the control group. No other information was available on neonatal outcomes following PB biopsy.
Blastomere biopsy
Five cohort studies showed that the birthweight of neonates born following an embryo biopsy was comparable to that of neonates conceived without biopsy in both singleton and twin pregnancies (Liebaers et al., 2010; Desmyttere et al., 2012; Bay et al., 2016; Hasson et al., 2017; Feldman et al., 2020). In a sub-analysis of children conceived after biopsy for PGT owing to parental monogenetic disorders, Bay et al. (2016) found that children born after biopsy (n = 58) had a significantly increased risk of LBW (aOR 2.0; 95% CI 1.1–3.9) compared to children conceived without embryo manipulation (n = 14782). Similarly, in multiple pregnancies, the risk of LBW seemed to be higher in the biopsy group (n = 176) than in the non-biopsy group (n = 1323) (OR 1.71; 95% CI 1.21-2.39) (Liebaers et al., 2010). Conversely, according to Desmyttere et al. (2012), fewer multiple births following biopsy for PGT presented an LBW compared to non-biopsy-derived multiple births (16.2% vs 17.8%, respectively, P = 0.005).
Finally, no difference in singletons’ and twins’ VLBW between PGT newborns and an ICSI group was found overall (Liebaers et al., 2010; Desmyttere et al., 2012; Hasson et al., 2017).
Trophectoderm (TE) biopsy
In eight of the studies, the birthweight, and the rates of LBW, and VLBW of singleton infants did not differ between those derived from biopsied or unbiopsied blastocysts, also adjusting for confounding factors (Forman et al., 2012; He et al., 2019; Sacchi et al., 2019; Zhang et al., 2019; Lu et al., 2020; Awadalla et al., 2021; Makhijani et al., 2021; Sites et al., 2021). No statistically significant differences in birthweight were observed between multiple pregnancies with or without TE biopsy (He et al., 2019; Zhang et al., 2019). These data were confirmed by a multivariate analysis of n = 6244 cycles with biopsies for PGT and n = 10 002 non-biopsy cycles in the SART-CORS database showing similar birthweight Z scores in the two groups (Li et al., 2021).
Small and large for gestational age
Polar body (PB) biopsy
No outcome data were available following PB biopsy.
Blastomere biopsy
A retrospective cohort study showed that the SGA rate was higher in the PGT-M singleton group than in the non-biopsy group (12.4% vs 4.5%, respectively, aOR 2.5, 95% CI 1.2–5.0) after adjusting for female age, BMI, and parity (Feldman et al., 2020).
This finding might be correlated with an increased risk of HDP after biopsy for PGT-M compared with pregnancies derived from non-biopsied embryos observed in the same study.
Trophectoderm (TE) biopsy
Two retrospective studies, one of which was based on the SART-CORS database, reported the odds for SGA and LGA newborns and found no difference for FET cycles with or without blastocyst biopsy (Lu et al., 2020; Li et al., 2021).
Neonatal intensive care unit admission
Polar body (PB) biopsy
No outcome data were available following PB biopsy.
Blastomere biopsy
Children born after blastomere biopsy did not seem to have been hospitalized more frequently in their neonatal phase than in controls (Desmyttere et al., 2012; Bay et al., 2016; Hasson et al., 2017; Feldman et al., 2020). In a sub-analysis of a single study, children conceived after embryo biopsy for PGT-M (n = 58) had significantly longer hospital admission (mean difference 30; 95% CI 22–39) than children born from non-biopsied embryos (n = 14782) (Bay et al., 2016).
Trophectoderm (TE) biopsy
Analysis of the admission rate to the neonatal intensive care unit (NICU) after delivery in two studies indicated that children born after blastocyst biopsy were not hospitalized more frequently in their neonatal phase than in controls (Zhang et al., 2019; Makhijani et al., 2021).
Birth defects
Polar body (PB) biopsy
No outcome data were available following PB biopsy.
Blastomere biopsy
Four retrospective studies investigated the major malformation rate of singleton infants following cleavage stage embryo biopsy (Liebaers et al., 2010; Desmyttere et al., 2012; Bay et al., 2016; Hasson et al., 2017). Liebaers et al. (2010) reported a malformation rate of 2.13% after PGT for various indications and of 3.38% for children born from non-biopsied embryos. According to Desmyttere et al. (2012) and Hasson et al. (2017), birth defects were present, respectively, in 2.3% and 3.5% of children born from a biopsied embryo compared to 2.6% and 1.9% of control children derived from a non-biopsied embryo. Bay et al. (2016) described n = 19 children with major malformations derived from n = 149 (12.7%) PGT-M/PGT-SR procedures (Bay et al., 2016). None of these studies showed a significant difference in the percentage of birth defects in children born after embryo biopsy or not.
It is, however, difficult to compare the malformation rates owing to significant heterogeneity in disease definitions and examination methods among the studies.
Trophectoderm (TE) biopsy
A similar birth defect rate was also found between children born after a TE biopsy and controls in three studies (He et al., 2019; Zhang et al., 2019; Makhijani et al., 2021). All three studies included subjects with several indications for PGT (aneuploidy screening, detection of monogenic gene conditions, or structural rearrangements). While two studies considered only vitrified-warmed transfer cycles (He et al., 2019; Makhijani et al., 2021), Zhang et al. (2019) included both fresh and frozen blastocyst transfers in the main analysis. In the Zhang et al. (2019) study, while no statistically significant difference in the overall rate of birth defects was observed, the sub-analysis including only FETs showed a significantly increased likelihood of birth defects in neonates born after biopsy (n = 134) vs no biopsy (n = 124) (aOR 11.90; 95% CI 1.40-100.87). Another study identified a few specific birth defects in children born after embryo biopsy for PGT, including arachnoid cysts, ventricular septal defects, auditory canal malformations, Marfan syndrome, renal cysts, and pyelektasis (Zhang et al., 2019). However, this finding should be interpreted very cautiously owing to the small sample size, wide confidence intervals, and paucity of studies reproducing these results.
Long-term outcomes
Table II shows the main characteristics and findings of studies investigating the long-term outcomes of children born after an embryo biopsy.
Table II.
Main characteristics and findings of studies investigating the long-term outcomes of children born after embryo biopsy for PGT cycles.
| Author, yeari | Study design | Number of children (PGT vs control group) | Follow-up period | Study population | Biopsy method | Outcomes | |
|---|---|---|---|---|---|---|---|
| Nekkebroeck et al., 2008 | Prospective follow-up study | Singletons: N = 70 vs N = 70 | 2 years | Biopsied (PGT-M + PGT-A) vs non-biopsied embryos | BL | Mental and psychomotor development (−) | |
| Desmyttere et al., 2009a | Prospective follow-up study | Singletons: N = 70 vs N = 70 | 2 years | Biopsied (PGT) vs non-biopsied embryos | BL | Anthropometric outcomes (−) | |
| Desmyttere et al., 2009b | Prospective follow-up study | Singletons + twins: N = 102 vs N = 102 | 2 years | Biopsied (PGT) vs non-biopsied embryos | BL | Anthropometric outcomes (−) | |
| Middelburg et al., 2010 | Prospective multicenter follow-up of an RCT |
|
18 months | Biopsied (PGT-A) vs non-biopsied embryos | BL | Neurological outcomes (+) | |
| Middelburg et al., 2011 | Prospective multicenter follow-up of an RCT |
|
2 years | Biopsied (PGT-A) vs non-biopsied embryos | BL |
|
|
| Schendelaar et al., 2013 | Prospective multicenter follow-up of an RCT |
|
4 years | Biopsied (PGT-A) vs non-biopsied embryos | BL | Neurological outcomes (+ only in twins) Cognitive development (−) |
|
| Beukers et al., 2012 | Prospective multicenter follow-up of an RCT |
|
2 years | Biopsied (PGT-A) vs non-biopsied embryos | BL | Mental and psychomotor development (−) | |
| Beukers et al., 2013 | Prospective multicenter follow-up of an RCT |
|
2 years | Biopsied (PGT-A) vs non-biopsied embryos | BL | Anthropometric outcomes (−) | |
| Seggers et al., 2013 | Prospective multicenter follow-up of an RCT |
|
4 years | Biopsied (PGT-A) vs non-biopsied embryos | BL | Blood pressure and anthropometric outcomes (−) | |
| Winter et al., 2014 | Prospective observational cohort study | Singletons: N = 47 vs N = 49 | 5-6 years | Biopsied (PGT-M) vs non-biopsied embryos | BL | Mental, psychomotor and cognitive development (−) | |
| Belva et al., 2018 | Prospective single-center matched-pair cohort study | Singletons: N = 87 vs N = 87 | 6 years | Biopsied (PGT-M + PGT-SR) vs non-biopsied embryos | BL | Blood pressure and anthropometric outcomes (−) | |
| Kuiper et al., 2018 | Prospective multicenter follow-up of an RCT |
|
9 years | Biopsied (PGT-A) vs non-biopsied embryos | BL |
|
|
| Heijligers et al., 2019 | Prospective observational cohort study |
|
5 years | Biopsied (PGT-M) vs non-biopsied embryos | BL | Blood pressure and anthropometric outcomes (−) | |
“+”, significant difference between the two groups; “−”, no significant difference between the two groups.
BL, blastomere; PGT, preimplantation genetic testing; PGT-A, preimplantation genetic testing for aneuploidies; PGT-M, preimplantation genetic testing for monogenic disorders; PGT-SR, preimplantation genetic testing for structural rearrangements; RCT, randomized controlled trial.
No data are available for the long-term outcome following PB or TE biopsy. All studies examining the long-term outcomes of children born after PGT focused on the effects of biopsy of cleavage stage embryos.
Neurological outcomes
Neurological outcomes have been evaluated in children aged 18 months to 9 years. From the paper by Mastenbroek et al. (2007), four prospective, assessor-blinded follow-up studies were based on children born from women who had or had not undergone embryo biopsy for PGT-A to evaluate neurodevelopmental outcomes at 18 months and 2, 4, and 9 years (Middelburg et al., 2010, 2011; Schendelaar et al., 2013; Kuiper et al., 2018). Dysfunctions in fine motor skills and mildly dysfunctional posture and muscle tone tended to be present significantly more frequently in children up to the age of 18 months after PGT-A, but because of the very small sample size (n = 25 children in the embryo biopsy group compared to n = 31 children in the control group), the results were not conclusive for unfavourable neurological outcomes in association with embryo biopsy for PGT-A (Middelburg et al., 2010). Subsequently, the same authors found a significantly lower neurological optimality score (NOS), a sensitive measure of minor neurological dysfunction, in PGT-A children aged 2 years compared to control children (Middelburg et al., 2011). The same children were evaluated at 4 years of age, and lower fluency scores and NOS scores were found in PGT-A twins but not in singletons (Schendelaar et al., 2013). Since singletons and twins were analysed together in the first 2 years of follow-up (Middelburg et al., 2011), we cannot exclude an important role of twinning in the observation. The negative association between embryo biopsy for PGT-A and the neurological conditions mentioned here did not seem to persist to 9 years. Indeed, at 9 years of age, the same children showed similar NOS scores whether from the group with embryo biopsy for PGT-A or the control group (Kuiper et al., 2018). The population considered was composed only of n = 43 children in the biopsy group and n = 56 children in the control group.
Blood pressure and anthropometric outcomes
As already mentioned, prospective follow-up studies following the RCT performed by Mastenbroek et al. (2007) on PGT-A efficacy focused on the anthropometrics and auxological outcomes of 2-, 4-, and 9-year-old children (Beukers et al., 2013; Seggers et al., 2013; Kuiper et al., 2018). No differences in terms of major and minor anomalies per child were described in 2-year-old children born following or not an embryo biopsy for PGT-A (Beukers et al., 2013). Other studies comparing 2-year-old children born after biopsy for PGT for various indications with matched control children found no differences in major and minor malformation rates, hospital admissions, or surgical interventions (Desmyttere et al., 2009a,b). Children born after biopsy for PGT underwent significantly more complementary investigations (e.g. radiological examinations, measure of pH, isotopic investigations) [χ2(I) = 4.24], but the number of abnormal results was not significantly different between the two groups (Desmyttere et al., 2009a).
In addition, at 4 and 9 years of age, the embryo biopsy did not affect systolic blood pressure, diastolic blood pressure, and anthropometric values as well as weight, height, BMI, total skinfold thickness, and head/waist circumference (Seggers et al., 2013; Kuiper et al., 2018). These findings are in line with those from Belva et al. (2018) and Heijligers et al. (2019), who did not find any difference in anthropometric measures and blood pressure in 6-year-old singletons born after embryo biopsy for PGT-M/PGT-SR and in 5-year-old children born after a biopsy for PGT-M compared to the group without biopsy.
Mental, psychomotor, and cognitive development
Four studies analysed the mental and psychomotor development of children born after embryo biopsy (Nekkebroeck et al., 2008; Middelburg et al., 2011; Beukers et al., 2012; Winter et al., 2014). At 2 years, the mental developmental index and the psychomotor developmental index of MAR children were not associated with biopsy for PGT-A or PGT-M, even after adjusting for sociodemographic confounders (Nekkebroeck et al., 2008; Middelburg et al., 2011; Beukers et al., 2012). These data were also confirmed in 5- to 6-year-old singletons born after biopsy for PGT-M, although the sample size of this latter study was small (n = 47 children in the PGT-M group compared to n = 49 children in the control group) (Winter et al., 2014).
The overall cognitive development of children after embryo biopsy for PGT did not differ from the controls at 2 years of age, from 4 to 6 years of age, and at 9 years of age (Middelburg et al., 2011; Schendelaar et al., 2013; Winter et al., 2014; Kuiper et al., 2018). Although these studies used different tests and some of them included twins and triplets, delayed development was not observed in children born after biopsy for PGT-M or PGT-A.
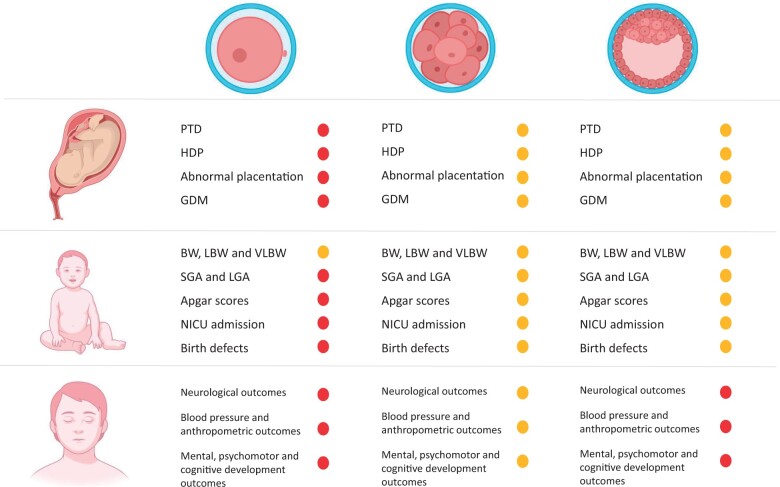
Synthesis of results
The data presented are shown in a graphical summary to best convey the findings revealed in this scoping review and to highlight the gaps in knowledge (Fig. 2). The amber symbol represents weak and/or controversial evidence. The red symbol highlights the absence of safety-related evidence. In summary, there are no safety data for PB biopsy and for the long-term outcomes of TE biopsies, while evidence is controversial for all other groups and outcomes.
Figure 2.
Schematic representation of the available evidence on obstetric, neonatal, and long-term outcomes after embryo biopsy. Findings following biopsy of the polar body, blastomere, and trophectoderm (left to right, top row) are presented. The amber symbol represents weak and/or a controversial body of evidence, for which further research is required. The red symbol warns that no evidence on safety exists. The illustration was created with BioRender.com. BW, birthweight; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy; LBW, low birthweight; NICU, neonatal intensive care unit; PTD, preterm delivery; VLBW, very low birthweight.
Discussion
Adaptive responses to environmental stimuli during developmental periods in utero or in early life may strongly influence postnatal life (Barker, 1990). Thus, the possibility that embryo biopsy may be associated with potential risks for subsequent offspring development is a concern. In line with this observation, evaluation of the potential adverse maternal and offspring outcomes after biopsy treatment was the main aim of this scoping review. To summarize our findings, this study showed a lack of conclusive evidence in support of increased adverse obstetric, neonatal, and long-term outcomes in conceptions after biopsy for PGT.
In more detail, some findings suggest a possible association between embryo biopsy and an increased risk of LBW and SGA in the case of blastomere biopsy and an increase in PTD and birth defects in the case of TE biopsy. For both biopsy methods (at the cleavage and blastocyst stages), an increased risk for pre-eclampsia and, more generally, to HDP was found. Conversely, no association between embryo biopsy at either the cleavage or blastocyst stage and abnormal placentation, GDM or LGA was detected. For PB biopsy, the literature on this topic is too scant to draw definitive conclusions on a link with adverse maternal and neonatal outcomes (Fig. 2). Concerning long-term outcomes, emerging evidence suggests that children are not negatively affected after embryo biopsy for PGT treatment, but these data were derived from a few studies focusing only on blastomere biopsy. No evidence is available regarding the safety of both PB and TE biopsy in terms of postnatal health (Fig. 2).
Correct interpretation of the available evidence is limited by the extreme heterogeneity in the conditions for embryo freezing and infertility status of the populations evaluated in the various studies. Moreover, some adverse outcomes, such as LBW and adverse neurological outcomes, are associated with multiple pregnancies (Middelburg et al., 2011; Desmyttere et al., 2012). In general, the data regarding potential adverse outcomes associated with invasive PGT are controversial, scarce, and weak.
Similarly, it is difficult to interpret the data derived from the two recently published systematic reviews and meta-analyses on this topic. As already mentioned, in these analyses, the data were pooled without distinguishing the different biopsy techniques (Hou et al., 2021; Zheng et al., 2021). Even if subgroup analyses were performed to reduce heterogeneity among studies and the impact of confounding factors, few studies were included, decreasing the strength and reliability of the evaluation. Zheng and coworkers assessed LBW, PTD, VPTD, VLBW, NICU admission, congenital malformations, gestational age, HDP, placenta previa, and GDM in pregnancies following PGT. The subgroups analysed included PGT-M, PGT-A, fresh transfer, FET, cleavage stage biopsy, and blastocyst biopsy. While the overall meta-analysis found that the risk of some neonatal and maternal complications was modified following PGT, no significant differences could be obtained for any of the outcomes considered in the subgroup analyses (Zheng et al., 2021). Hou et al. (2021) analysed similar outcomes, but in contrast to Zheng et al. (2021), their subgroup analysis comparing FET pregnancies after embryo biopsy or not found some differences between the two groups. A higher rate of intrauterine growth retardation and a lower risk of caesarean section were found to be associated with pregnancies derived from embryo biopsy compared to gestations without biopsy. Overall, because of these relevant limitations and the controversy in the findings, systematic reviews and meta-analyses (Hou et al., 2021; Zheng et al., 2021) on this topic might at present lead to misleading conclusions. A scoping review represents a better strategy when the topic is of a complex or heterogeneous nature and when it is still unclear what other, more specific, questions should be posed and solved to clarify the issue (Munn et al., 2018).
Limitations
Some limitations of the studies reviewed here should be considered. First, the methodology of the invasive biopsy techniques may not be standardized across studies. Thus, an unknown source of heterogeneity is the operator dependency of these procedures. Second, correction for confounders potentially affecting the outcomes (such as maternal age, BMI, comorbidities, parity, presence of infertility and its aetiology, MAR protocols, and use of cryopreservation) was not performed to a large extent. Finally, the majority of the studies were not solid enough in terms of sample size to detect subtle differences in the outcome rates analysed.
Overall, we mapped the body of literature on the topic, but pooling of available evidence was significantly limited owing to the difficulties in removing heterogeneity, biases, confounders, and issues inherent to the study design, potentially leading to unreliable conclusions. Based on the aims of a scoping review, we provided an opportunity to identify key concepts, knowledge gaps to be investigated with future research, and types and sources of evidence to inform practice and research.
Future developments in embryo biopsy
The new challenge in PGT development is the non-invasive approach (niPGT) involving analysis of cell-free DNA obtained from the spent culture media to limit all possible impairments associated with embryo biopsy. The potential advantages of this strategy are wide ranging: the risk of embryo damage is completely averted (Pagliardini et al., 2020); it represents a promising possibility for countries where embryo biopsy is not permitted by law (Feichtinger et al., 2017); and it requires a lower level of expertise of embryologists. While PGT-M from cell-free DNA in spent culture medium does not seem to be sufficiently informative in detecting single-gene disorders, the screening data for aneuploidy are more encouraging (Cimadomo et al., 2020). Not surprisingly, no data on infants born following niPGT are presently available. It is plausible that such a non-invasive approach, with a lower risk of damage to the embryos, could limit potential obstetric, neonatal, and long-term complications in children born after embryo biopsy for PGT.
Conclusion
PGT remains one of the most intensely contested procedures in reproductive medicine. This scoping review has, first, described the confounding factors that limit the reliability of the studies addressing this topic, such as other embryo manipulation procedures or patient characteristics, and, second, suggested a possible association of embryo biopsy at both the cleavage and blastocyst stages with an increased risk of HDP and adverse perinatal outcomes. Long-term adverse effects may be rare but are possible, at least for blastomere biopsy. Third, this study identified gaps in knowledge, including the absence of data on long-term outcomes following TE biopsy or the lack of sufficiently reliable data to be pooled according to the different genetic procedures. Overall, the short- and long-term risks of PGT on prenatal or postnatal health remain uncertain but cannot be excluded.
Contributor Information
Alessandra Alteri, Obstetrics and Gynaecology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy.
Greta Chiara Cermisoni, Obstetrics and Gynaecology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Mirko Pozzoni, Obstetrics and Gynaecology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy.
Gerarda Gaeta, Obstetrics and Gynaecology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy.
Paolo Ivo Cavoretto, Obstetrics and Gynaecology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy; Vita-Salute San Raffaele University, Milan, Italy.
Paola Viganò, Infertility Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy.
Data availability
No new data were generated in support of this research.
Authors’ roles
A.A. and G.C.C. performed the literature search; A.A., G.C.C., M.P., and G.G. extrapolated the data; A.A., G.C.C., M.P., and G.G. wrote the initial article; P.V. revised the draft; and P.I.C. and P.V. contributed to critical revision. All authors approved the final version of the article.
Funding
This study was partially funded by the Italian Ministry of Health—Current Research IRCCS.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Alteri A, Corti L, Sanchez AM, Rabellotti E, Papaleo E, Viganò P.. Assessment of pre-implantation genetic testing for embryo aneuploidies: a SWOT analysis. Clin Genet 2019;95:479–487. [DOI] [PubMed] [Google Scholar]
- Archer J, Gook DA, Edgar DH.. Blastocyst formation and cell numbers in human frozen-thawed embryos following extended culture. Hum Reprod 2003;18:1669–1673. [DOI] [PubMed] [Google Scholar]
- Arksey H, O’Malley L.. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract 2005;8:19–32. [Google Scholar]
- Awadalla MS, Park KE, Latack KR, McGinnis LK, Ahmady A, Paulson RJ.. Influence of trophectoderm biopsy prior to frozen blastocyst transfer on obstetrical outcomes. Reprod Sci 2021;28:3459–3465. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The fetal and infant origins of adult disease. BMJ 1990;301:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay B, Ingerslev HJ, Lemmen JG, Degn B, Rasmussen IA, Kesmodel US.. Preimplantation genetic diagnosis: a national multicenter obstetric and neonatal follow-up study. Fertil Steril 2016;106:1363–1369.e1. [DOI] [PubMed] [Google Scholar]
- Belva F, Roelants M, Kluijfhout S, Winter C, De Schrijver F, Desmyttere S, De Rycke M, Tournaye H, Liebaers I, Bonduelle M.. Body composition and blood pressure in 6-year-old singletons born after pre-implantation genetic testing for monogenic and structural chromosomal aberrations: a matched cohort study. Hum Reprod Open 2018;2018:hoy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukers F, Houtzager BA, Paap MC, Middelburg KJ, Hadders-Algra M, Bos AF, Kok JH; PGS Study Group. Parental psychological distress and anxiety after a successful IVF/ICSI procedure with and without preimplantation genetic screening: follow-up of a randomised controlled trial. Early Hum Dev 2012;88:725–730. [DOI] [PubMed] [Google Scholar]
- Beukers F, van der Heide M, Middelburg KJ, Cobben JM, Mastenbroek S, Breur R, van der Lee JH, Hadders-Algra M, Bos AF, Kok JH; PGS Study Group. Morphologic abnormalities in 2-year-old children born after in vitro fertilization/intracytoplasmic sperm injection with preimplantation genetic screening: follow-up of a randomized controlled trial. Fertil Steril 2013;99:408–413. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard M, Chou D, Moller AB, Narwal R, Adler A, Garcia CV, Rohde S, Say L. et al. National, regional and worldwide estimates of preterm birth. Lancet 2012;379:2162–2172. [DOI] [PubMed] [Google Scholar]
- Braude P, Pickering S, Flinter F, Ogilvie CM.. Preimplantation genetic diagnosis. Nat Rev Genet 2002;3:941–953. [DOI] [PubMed] [Google Scholar]
- Brown M, Magee L, Kenny L, Karumanchi S, McCarthy F, Saito S, Hall D, Warren C, Adoyi S, Ishaku G; International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 2018;72:24–43. [DOI] [PubMed] [Google Scholar]
- Cavoretto PI, Farina A, Gaeta G, Sigismondi C, Spinillo S, Casiero D, Pozzoni M, Vigano P, Papaleo E, Candiani M.. Uterine artery Doppler in singleton pregnancies conceived after in-vitro fertilization or intracytoplasmic sperm injection with fresh vs frozen blastocyst transfer: longitudinal cohort study. Ultrasound Obstet Gynecol 2020;56:603–610. [DOI] [PubMed] [Google Scholar]
- Cavoretto PI, Farina A, Gaeta G, Seidenari A, Pozzoni M, Spinillo S, Morano D, Alteri A, Viganò P, Candiani M.. Greater estimated fetal weight and birth weight in IVF/ICSI pregnancies after thawed as compared with fresh blastocyst transfer: prospective cohort study with novel unified modeling methodology. Ultrasound Obstet Gynecol 2021a;60:76–85. doi: 10.1002/uog.24806. [DOI] [PubMed] [Google Scholar]
- Cavoretto PI, Farina A, Girardelli S, Gaeta G, Spinillo S, Morano D, Amodeo S, Galdini A, Viganò P, Candiani M.. Greater fetal crown-rump length growth with the use of in vitro fertilization or intracytoplasmic sperm injection conceptions after thawed versus fresh blastocyst transfers: secondary analysis of a prospective cohort study. Fertil Steril 2021b;116:147–156. [DOI] [PubMed] [Google Scholar]
- Cimadomo D, Rienzi L, Capalbo A, Rubio C, Innocenti F, García-Pascual CM, Ubaldi FM, Handyside A.. The dawn of the future: 30 years from the first biopsy of a human embryo. The detailed history of an ongoing revolution. Hum Reprod Update 2020;26:453–473. [DOI] [PubMed] [Google Scholar]
- Colquhoun HL, Levac D, O'Brien KK, Straus S, Tricco AC, Perrier L, Kastner M, Moher D.. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol 2014;67:1291–1294. [DOI] [PubMed] [Google Scholar]
- Desmyttere S, Bonduelle M, Nekkebroeck J, Roelants M, Liebaers I, De Schepper J.. Growth and health outcome of 102 2-year-old children conceived after preimplantation genetic diagnosis or screening. Early Hum Dev 2009a;85:755–759. [DOI] [PubMed] [Google Scholar]
- Desmyttere S, De Rycke M, Staessen C, Liebaers I, De Schrijver F, Verpoest W, Haentjens P, Bonduelle M.. Neonatal follow-up of 995 consecutively born children after embryo biopsy for PGD. Hum Reprod 2012;27:288–293. [DOI] [PubMed] [Google Scholar]
- Desmyttere S, De Schepper J, Nekkebroeck J, De Vos A, De Rycke M, Staessen C, Liebaers I, Bonduelle M.. Two-year auxological and medical outcome of singletons born after embryo biopsy applied in preimplantation genetic diagnosis or preimplantation genetic screening. Hum Reprod 2009b;24:470–476. [DOI] [PubMed] [Google Scholar]
- Dokras A, Sargent IL, Gardner RL, Barlow DH.. Human trophectoderm biopsy and secretion of chorionic gonadotrophin. Hum Reprod 1991;6:1453–1459. [DOI] [PubMed] [Google Scholar]
- DoPierala AL, Bhatta S, Raja EA, Bhattacharya S, Bhattacharya S.. Obstetric consequences of subfertility: a retrospective cohort study. BJOG 2016;123:1320–1328. [DOI] [PubMed] [Google Scholar]
- Feichtinger M, Vaccari E, Carli L, Wallner E, Mädel U, Figl K, Palini S, Feichtinger W.. Non-invasive preimplantation genetic screening using array comparative genomic hybridization on spent culture media: a proof-of-concept pilot study. Reprod Biomed Online 2017;34:583–589. [DOI] [PubMed] [Google Scholar]
- Feldman B, Orvieto R, Weisel M, Aizer A, Meyer R, Haas J, Kirshenbaum M.. Obstetric and perinatal outcomes in pregnancies conceived after preimplantation genetic testing for monogenetic diseases. Obstet Gynecol 2020;136:782–791. [DOI] [PubMed] [Google Scholar]
- Forman EJ, Hong KH, Franasiak JM, Scott RT Jr. Obstetrical and neonatal outcomes from the BEST Trial: single embryo transfer with aneuploidy screening improves outcomes after in vitro fertilization without compromising delivery rates. Am J Obstet Gynecol 2014;210:157. [DOI] [PubMed] [Google Scholar]
- Forman EJ, Tao X, Ferry KM, Taylor D, Treff NR, Scott RT Jr. Single embryo transfer with comprehensive chromosome screening results in improved ongoing pregnancy rates and decreased miscarriage rates. Hum Reprod 2012;27:1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco E, Litwicka K, Minasi MG, Cursio E, Greco PF, Barillari P.. Preimplantation genetic testing: where we are today. Int J Mol Sci 2020;21:4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K, Martin KL, Leese HJ, Winston RM, Handyside AH.. Human preimplantation development in vitro is not adversely affected by biopsy at the 8-cell stage. Hum Reprod 1990;5:708–714. [DOI] [PubMed] [Google Scholar]
- Hasson J, Limoni D, Malcov M, Frumkin T, Amir H, Shavit T, Bay B, Many A, Almog B.. Obstetric and neonatal outcomes of pregnancies conceived after preimplantation genetic diagnosis: cohort study and meta-analysis. Reprod Biomed Online 2017;35:208–218. [DOI] [PubMed] [Google Scholar]
- He H, Jing S, Lu CF, Tan YQ, Luo KL, Zhang SP, Gong F, Lu GX, Lin G.. Neonatal outcomes of live births after blastocyst biopsy in preimplantation genetic testing cycles: a follow-up of 1,721 children. Fertil Steril 2019;112:82–88. [DOI] [PubMed] [Google Scholar]
- Heijligers M, Peeters A, van Montfoort A, Nijsten J, Janssen E, Gunnewiek FK, de Rooy R, van Golde R, Coonen E, Meijer-Hoogeveen M. et al. Growth, health, and motor development of 5-year-old children born after preimplantation genetic diagnosis. Fertil Steril 2019;111:1151–1158. [DOI] [PubMed] [Google Scholar]
- Hou W, Shi G, Ma Y, Liu Y, Lu M, Fan X, Sun Y.. Impact of preimplantation genetic testing on obstetric and neonatal outcomes: a systematic review and meta-analysis. Fertil Steril 2021;116:990–1000. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Alfirevic Z, Bhide AG, Belfort MA, Burton GJ, Collins SL, Dornan S, Jurkovic D, Kayem G, Kingdom J. et al. ; Royal College of Obstetricians and Gynaecologists. Placenta praevia and placenta accreta: diagnosis and management: green-top guideline no. 27a. BJOG 2019;126:e1–e48. [DOI] [PubMed] [Google Scholar]
- Kuiper D, Bennema A, la Bastide-van Gemert S, Seggers J, Schendelaar P, Mastenbroek S, Hoek A, Heineman MJ, Roseboom TJ, Kok JH. et al. Developmental outcome of 9-year-old children born after PGS: follow-up of a randomized trial. Hum Reprod 2018;33:147–155. [DOI] [PubMed] [Google Scholar]
- Levac D, Colquhoun H, O'Brien KK.. Scoping studies: advancing the methodology. Implement Sci 2010;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Kort J, Baker VL.. Embryo biopsy and perinatal outcomes of singleton pregnancies: an analysis of 16,246 frozen embryo transfer cycles reported in the Society for Assisted Reproductive Technology Clinical Outcomes Reporting System. Am J Obstet Gynecol 2021;224:500.e1-500. [DOI] [PubMed] [Google Scholar]
- Liebaers I, Desmyttere S, Verpoest W, De Rycke M, Staessen C, Sermon K, Devroey P, Haentjens P, Bonduelle M.. Report on a consecutive series of 581 children born after blastomere biopsy for preimplantation genetic diagnosis. Hum Reprod 2010;25:275–282. [DOI] [PubMed] [Google Scholar]
- Lu MM, Wen YX, Liu YL, Ding CH, Zhou CQ, Xu YW.. Trophectoderm biopsy reduces the level of serum β-human chorionic gonadotropin in early pregnancy. Fertil Steril 2020;114:801–808. [DOI] [PubMed] [Google Scholar]
- Makhijani R, Bartels CB, Godiwala P, Bartolucci A, DiLuigi A, Nulsen J, Grow D, Benadiva C, Engmann L.. Impact of trophectoderm biopsy on obstetric and perinatal outcomes following frozen-thawed embryo transfer cycles. Hum Reprod 2021;36:340–348. [DOI] [PubMed] [Google Scholar]
- Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, Vogel NE, Arts EG, de Vries JW, Bossuyt PM. et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med 2007;357:9–17. [DOI] [PubMed] [Google Scholar]
- Middelburg KJ, Heineman MJ, Haadsma ML, Bos AF, Kok JH, Hadders-Algra M.. Neurological condition of infants born after in vitro fertilization with preimplantation genetic screening. Pediatr Res 2010;67:430–434. [DOI] [PubMed] [Google Scholar]
- Middelburg KJ, van der Heide M, Houtzager B, Jongbloed-Pereboom M, Fidler V, Bos AF, Kok J, Hadders-Algra M; PGS Follow-up Study Group. Mental, psychomotor, neurologic, and behavioral outcomes of 2-year-old children born after preimplantation genetic screening: follow-up of a randomized controlled trial. Fertil Steril 2011;96:165–169. [DOI] [PubMed] [Google Scholar]
- Montag M, van der Ven K, Dorn C, van der Ven H.. Outcome of laser-assisted polar body biopsy and aneuploidy testing. Reprod Biomed Online 2004;9:425–429. [DOI] [PubMed] [Google Scholar]
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E.. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, Silverberg K, Kalista T, Handyside AH, Katz-Jaffe M. et al. ; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril 2019;112:1071–1079.e7. [DOI] [PubMed] [Google Scholar]
- Nekkebroeck J, Bonduelle M, Desmyttere S, Van den Broeck W, Ponjaert-Kristoffersen I.. Mental and psychomotor development of 2-year-old children born after preimplantation genetic diagnosis/screening. Hum Reprod 2008;23:1560–1566. [DOI] [PubMed] [Google Scholar]
- Nicolaides KH, Wright D, Syngelaki A, Wright A, Akolekar R.. Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound Obstet Gynecol 2018;52:44–51. [DOI] [PubMed] [Google Scholar]
- Pagliardini L, Viganò P, Alteri A, Corti L, Somigliana E, Papaleo E.. Shooting STAR: reinterpreting the data from the ‘Single Embryo TrAnsfeR of Euploid Embryo’ randomized clinical trial. Reprod Biomed Online 2020;40:475–478. [DOI] [PubMed] [Google Scholar]
- Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB.. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015;13:141–146. [DOI] [PubMed] [Google Scholar]
- Riestenberg CK, Mok T, Ong JR, Platt LD, Han CS, Quinn MM.. Sonographic abnormalities in pregnancies conceived following IVF with and without preimplantation genetic testing for aneuploidy (PGT-A). J Assist Reprod Genet 2021;38:865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque M, Haahr T, Geber S, Esteves SC, Humaidan P.. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update 2019;25:2–14. [DOI] [PubMed] [Google Scholar]
- Sacchi L, Albani E, Cesana A, Smeraldi A, Parini V, Fabiani M, Poli M, Capalbo A, Levi-Setti PE.. Preimplantation genetic testing for aneuploidy improves clinical, gestational, and neonatal outcomes in advanced maternal age patients without compromising cumulative live-birth rate. J Assist Reprod Genet 2019;36:2493–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacha CR, Harris AL, James K, Basnet K, Freret TS, Yeh J, Kaimal A, Souter I, Roberts DJ.. Placental pathology in live births conceived with in vitro fertilization after fresh and frozen embryo transfer. Am J Obstet Gynecol 2020;222:360.e1–360.e16. [DOI] [PubMed] [Google Scholar]
- Saravanan P; Diabetes in Pregnancy Working Group; Maternal Medicine Clinical Study Group; Royal College of Obstetricians and Gynaecologists, UK. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol 2020;8:793–800. [DOI] [PubMed] [Google Scholar]
- Schendelaar P, Middelburg KJ, Bos AF, Heineman MJ, Kok JH, La Bastide-Van Gemert S, Seggers J, Van den Heuvel ER, Hadders-Algra M.. The effect of preimplantation genetic screening on neurological, cognitive and behavioural development in 4-year-old children: follow-up of a RCT. Hum Reprod 2013;28:1508–1518. [DOI] [PubMed] [Google Scholar]
- Scott RT Jr, Upham KM, Forman EJ, Zhao T, Treff NR.. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril 2013;100:624–630. [DOI] [PubMed] [Google Scholar]
- Seggers J, Haadsma ML, Bastide-van Gemert S, Heineman MJ, Kok JH, Middelburg KJ, Roseboom TJ, Schendelaar P, Van den Heuvel ER, Hadders-Algra M.. Blood pressure and anthropometrics of 4-y-old children born after preimplantation genetic screening: follow-up of a unique, moderately sized, randomized controlled trial. Pediatr Res 2013;74:606–614. [DOI] [PubMed] [Google Scholar]
- Sites CK, Bachilova S, Gopal D, Cabral HJ, Coddington CC, Stern JE.. Embryo biopsy and maternal and neonatal outcomes following cryopreserved-thawed single embryo transfer. Am J Obstet Gynecol 2021;225:285.e1-285–285.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson K, Huang D, Kaing A, Blat C, Rosenstein MG, Mok-Lin E, Gras J, Sperling JD.. Is preimplantation genetic testing associated with increased risk of abnormal placentation after frozen embryo transfer? Am J Perinatol 2021;38:105–110. [DOI] [PubMed] [Google Scholar]
- Thomson F, Shanbhag S, Templeton A, Bhattacharya S.. Obstetric outcome in women with subfertility. BJOG 2005;112:632–637. [DOI] [PubMed] [Google Scholar]
- Tricco AC, Lillie E, Zarin W, O'Brien K, Colquhoun H, Kastner M, Levac D, Ng C, Sharpe JP, Wilson K. et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol 2016;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L. et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–473. [DOI] [PubMed] [Google Scholar]
- van Montfoort A, Carvalho F, Coonen E, Kokkali G, Moutou C, Rubio C, Goossens V, De Rycke M.. ESHRE PGT Consortium data collection XIX-XX: PGT analyses from 2016 to 2017. Hum Reprod Open 2021;2021:hoab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waynforth D. Effects of conception using assisted reproductive technologies on infant health and development: an evolutionary perspective and analysis using UK Millennium Cohort data. Yale J Biol Med 2018;91:225–235. [PMC free article] [PubMed] [Google Scholar]
- Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, Tan J, Liang X, Cao Y, Wang Z. et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet 2019;393:1310–1318. [DOI] [PubMed] [Google Scholar]
- Winter C, Van Acker F, Bonduelle M, Desmyttere S, De Schrijver F, Nekkebroeck J.. Cognitive and psychomotor development of 5- to 6-year-old singletons born after PGD: a prospective case-controlled matched study. Hum Reprod 2014;29:1968–1977. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2014). Global Nutrition Targets 2025: Low Birth Weight Policy Brief. World Health Organization. https://apps.who.int/iris/handle/10665/149020. [Google Scholar]
- Wu Y, Ying Y, Cao M, Liu J, Liu H.. Trophectoderm biopsy of blastocysts for a preimplantation genetic test does not affect serum β-hCG levels in early pregnancy: a study using propensity score matching. J Ovarian Res 2021;14:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wei D, Legro RS, Shi Y, Li J, Zhang L, Hong Y, Sun G, Zhang T, Li W. et al. Obstetric complications after frozen versus fresh embryo transfer in women with polycystic ovary syndrome: results from a randomized trial. Fertil Steril 2018;109:324–329. [DOI] [PubMed] [Google Scholar]
- Zhang WY, von Versen-Höynck F, Kapphahn KI, Fleischmann RR, Zhao Q, Baker VL.. Maternal and neonatal outcomes associated with trophectoderm biopsy. Fertil Steril 2019;112:283–290.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Yang C, Yang S, Sun S, Mu M, Rao M, Zu R, Yan J, Ren B, Yang R. et al. Obstetric and neonatal outcomes of pregnancies resulting from preimplantation genetic testing: a systematic review and meta-analysis. Hum Reprod Update 2021;27:989–1012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated in support of this research.