Abstract
STUDY QUESTION
What is the risk of miscarriage among pregnant women who received any of the COVID-19 vaccines?
SUMMARY ANSWER
There is no evidence that COVID-19 vaccines are associated with an increased risk of miscarriage.
WHAT IS KNOWN ALREADY
In response to the COVID-19 pandemic, the mass roll-out of vaccines helped to boost herd immunity and reduced hospital admissions, morbidity, and mortality. Still, many were concerned about the safety of vaccines for pregnancy, which may have limited their uptake among pregnant women and those planning a pregnancy.
STUDY DESIGN, SIZE, DURATION
For this systematic review and meta-analysis, we searched MEDLINE, EMBASE, and Cochrane CENTRAL from inception until June 2022 using a combination of keywords and MeSH terms.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We included observational and interventional studies that enrolled pregnant women and evaluated any of the available COVID-19 vaccines compared to placebo or no vaccination. We primarily reported on miscarriage in addition to ongoing pregnancy and/or live birth.
MAIN RESULTS AND THE ROLE OF CHANCE
We included data from 21 studies (5 randomized trials and 16 observational studies) reporting on 149 685 women. The pooled rate of miscarriage among women who received a COVID-19 vaccine was 9% (n = 14 749/123 185, 95% CI 0.05–0.14). Compared to those who received a placebo or no vaccination, women who received a COVID-19 vaccine did not have a higher risk of miscarriage (risk ratio (RR) 1.07, 95% CI 0.89–1.28, I2 35.8%) and had comparable rates for ongoing pregnancy or live birth (RR 1.00, 95% CI 0.97–1.03, I2 10.72%).
LIMITATIONS, REASONS FOR CAUTION
Our analysis was limited to observational evidence with varied reporting, high heterogeneity and risk of bias across included studies, which may limit the generalizability and confidence in our findings.
WIDER IMPLICATIONS OF THE FINDINGS
COVID-19 vaccines are not associated with an increase in the risk of miscarriage or reduced rates of ongoing pregnancy or live birth among women of reproductive age. The current evidence remains limited and larger population studies are needed to further evaluate the effectiveness and safety of COVID-19 vaccination in pregnancy.
STUDY FUNDING/COMPETING INTEREST(S)
No direct funding was provided to support this work. M.P.R. was funded by the Medical Research Council Centre for Reproductive Health Grant No: MR/N022556/1. B.H.A.W. hold a personal development award from the National Institute of Health Research in the UK. All authors declare no conflict of interest.
REGISTRATION NUMBER
CRD42021289098.
Keywords: COVID-19 vaccination, vaccine safety, miscarriage, pregnancy loss, live birth
Introduction
The last 2 years saw the mass rollout of multi-national vaccination campaigns for the SARS-CoV-2 (COVID-19) virus with the hope of attenuating its devastating effect on society and restoring normality (de Gier et al., 2021; Lopez Bernal et al., 2021). The rapid development and rollout of these vaccines raised concerns about their short- and long-term health side effects leading to vaccine hesitancy among pregnant women and those planning a pregnancy (Egloff et al., 2022; Kiefer et al., 2022). However, to date, most studies and regulatory bodies support their safety and effectiveness (American College of Obstetricians and Gynecologists, 2022; Royal College of Obstetricians & Gynaecologists, 2022; UK Health Security Agency, 2022). Most early studies evaluating the efficacy of COVID-19 vaccines excluded pregnant women, which limited evidence synthesis on the safety of vaccines in pregnancy (Polack et al., 2020; Baden et al., 2021; Madhi et al., 2021; Sadoff et al., 2021). The majority of health authorities currently support the safety of COVID-19 vaccination in pregnant women (American College of Obstetricians and Gynecologists, 2022; Royal College of Obstetricians & Gynaecologists, 2022) to reduce the risk of poor pregnancy outcomes observed in unvaccinated women with COVID-19 infection (Stock et al., 2022).
Some authors have raised concerns about the potential cross-reactivity of SARS-CoV-2 spike protein antibodies following mRNA vaccination with human syncytin-1 protein in trophoblastic tissue (Ciapponi et al., 2021; Mattar et al., 2021; Shanes et al., 2021; Schaler and Wingfield, 2022). Autoreactive antibodies against syncytin-1 were presumed to cause placental damage and early pregnancy loss due to the potential homology with the SARS-CoV-2 spike protein. However, further characterization of the SARS-CoV-2 spike protein structure and amino acid sequencing showed low homology with syncytin-1, disproving claims of cross-reactivity, and potential damage to placental tissue (Gong et al., 2005; Kloc et al., 2021; Prasad et al., 2021). Given the increased risk of morbidity and mortality among pregnant women with COVID-19, it is critical to maximize prevention efforts by encouraging vaccine uptake and promoting its safety during pregnancy (Royal College of Obstetricians & Gynaecologists, 2021). We performed a systematic review and meta-analysis of the available literature to evaluate the rates of miscarriage and live birth among women who received a COVID-19 vaccination.
Materials and methods
We performed a systematic review and meta-analysis using a prospectively registered protocol (CRD42021289098) and reported our findings as per PRISMA guidelines (Page et al., 2021).
Search strategy
We searched MEDLINE, EMBASE, and Cochrane CENTRAL until June 2022 using a combination of keyword and MeSH terms for studies of any design that compared the risk of miscarriage and other pregnancy outcomes between vaccinated and non-vaccinated pregnant women (Supplementary Data File S1).
Study selection and inclusion process
Relevant studies were screened in duplicate (M.P.R. and J.J.T.). Studies of any design that reported on miscarriage and other pregnancy outcomes in women who received any COVID-19 vaccine with or without a control cohort (placebo or no vaccine) were included. We excluded animal studies, those reporting on non-clinical outcomes in human participants, review articles and case reports. Data submitted to health regulators for evaluation of vaccine effectiveness and safety were also included if they were made publicly available ahead of peer review.
Data extraction
Data extraction was performed in triplicate (M.P.R., J.J.T., and S.C.M.) using a piloted electronic data collection tool with the following characteristics collected: study publication year and journal, inclusion–exclusion criteria, type of intervention and comparison evaluated, characteristics of the included study population and the evaluated COVID-19 vaccine, and all relevant clinical outcomes.
Outcome measures
We reported on the following pregnancy outcomes: miscarriage (defined as spontaneous loss of a pregnancy before 24 weeks gestation), live birth (defined as the birth of a live child after 24 weeks gestation), and ongoing pregnancy (defined as a viable pregnancy after 12 weeks gestation).
Risk of bias assessment
Two reviewers (M.P.R. and J.J.T.) assessed the risk of bias and applicability of included studies independently using The Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool (Sterne et al., 2016). We evaluated the risk of bias in the included studies compared to a target randomized trial that evaluated the risk of miscarriage, live birth, and ongoing pregnancy in women of reproductive age who received a COVID-19 vaccine compared to placebo or no vaccine. As most of the included studies were cohorts or interrupted time series that followed up on women who received a COVID-19 vaccine, we assessed the risk of bias due to confounding, participant selection, classification of intervention, deviation from the intended intervention, missing data, outcome measurement, and selection of reported results. We then generated an overall risk of bias assessment for each study. Studies were deemed to be low risk of bias if they were assessed as low risk in all domains, moderate risk of bias if they were assessed as low or moderate risk of bias in any domain, serious risk of bias if they were assessed as serious risk of bias in at least one domain, but not at critical risk of bias in any domain, or critical risk of bias if one or more domains was assessed as critical.
Statistical analysis
We pooled data to evaluate the overall rate of miscarriage and live birth/ongoing pregnancy across all women who received a COVID-19 vaccine and generated a pooled risk ratio (RR) compared to women who were not vaccinated. We reported on the pooled event rate using risk with 95% CIs. For our comparative meta-analysis, we reported on dichotomous outcomes using summary RR with 95% CI and on continuous outcomes using weighted mean difference with 95% CI. We used a random effect meta-analysis and applied a restricted maximum likelihood model. Study heterogeneity among included trials was assessed using the I2 statistic. We also assessed the publication bias and small study effect using a funnel plot for each pairwise comparison and performed Egger’s test to assess its statistical significance. We planned a sensitivity meta-regression and subgroup analyses to investigate potential effect modifiers where relevant. All statistical analyses were conducted in Stata V13 (StataCorp, TX, USA) and Open Meta-analyst software (Brown University, Providence, RI, USA).
Results
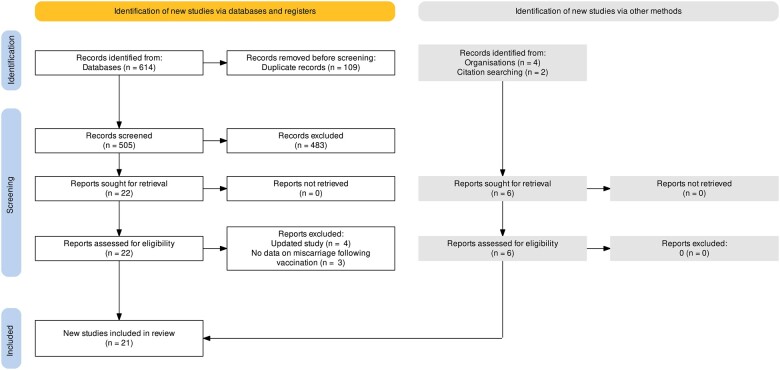
We screened 505 potentially relevant citations, assessed 28 in full and included 21 studies: 5 randomized control trials (RCTs) (United States Food and Drug Administration, 2020a,b, 2021a,b; Hillson et al., 2021) and 16 observational studies (Bleicher et al., 2021; Bookstein Peretz et al., 2021; Kachikis et al., 2021; Kharbanda et al., 2021; Magnus et al., 2021; Nabila Arfah and Murizah, 2021; Qiao et al., 2021; Trostle et al., 2021; Zauche et al., 2021; Aharon et al., 2022; Avraham et al., 2022; Citu et al., 2022; Favre et al., 2022; Huang et al., 2022; Moro et al., 2022; Wang et al., 2022). All together the studies reported on pregnancy outcomes in 149 685 women (Table I and Supplementary Table SI). Two studies reported on the same population (Aharon et al., 2021, 2022), while an additional two studies reported on the same data registry (Shimabukuro et al., 2021; Moro et al., 2022) (Fig. 1). All of the RCTs in this review excluded pregnant women at the time of recruitment but reported on those who became pregnant during the trial.
Table I.
Characteristics of included studies that evaluated the risk of miscarriage and rates of ongoing pregnancy/live birth among pregnant women who received a COVID-19 vaccine.
| Study | Design | Countries | Funding source | Covid-19 vaccine | Vaccine doses | Inclusion criteria | Numbers analysed (n=) | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Aharon (2022) | Cohort | USA | Not stated | Pfizer, Moderna | 2 | Women undergoing fertility treatment who were vaccinated at least 14 days prior to starting medication for ovarian stimulation or a frozen-thawed embryo transfer cycle | 2153 | Moderate |
| Avraham (2022) | Cohort | Israel | No external funding | Pfizer | 2 | Women 20–42 years old undergoing IVF treatment cycles at a single centre | 400 | Moderate |
| Bleicher (2021) | Cohort | USA | Not stated | Pfizer | ≥1 | Being pregnant at enrolment and valid questionnaire | 326 | Serious |
| Bookstein Peretz (2021) | Case-control | Israel | Not stated | Pfizer | 2 | Pregnant women between 2 and 40 weeks’ gestation who completed two doses of vaccine | 57 | Serious |
| Citu (2022) | Cohort | Romania | No external funding | Pfizer, Moderna | ≥1 | Women aged >18 years who were vaccinated during the first trimester of pregnancy | 3094 | Moderate |
| Favre (2022) | Cohort | Switzerland | Swiss Federal Office of Public Health and the CHUV Foundation | Pfizer, Moderna | ≥1 | Pregnant women with at least one injection between 1 week before last menstrual period to end of pregnancy | 228 | Moderate |
| FDA—Janssen (2021) | RCT | Brazil, Chile, Argentine, Colombia, Peru, Mexica, USA, South Africa | Janssen Research and Development | Janssen | 1 | Adults 18–59 years of age and 60 years of age or older, respectively, who were in good or stable health and did not have coexisting conditions that have been associated with an increased risk of severe COVID-19 | 8 | Low |
| FDA—Moderna (2020) | RCT | USA | Biomedical Advanced Research and Development Authority and the National Institute of Allergy and Infectious Diseases | Moderna | 2 | 18 years old and had no known history of SARS-CoV-2 infection and whose locations or circumstances put them at appreciable risk of acquiring SARS-CoV-2 infection or who were at high risk for severe disease (or both) | 13 | Low |
| FDA—Moderna (Booster) (2021) | RCT | USA | Not stated | Moderna booster | 2 + 1 | Individuals 65 years of age and older, individuals 18 through 64 years of age at high risk of severe COVID-19, and individuals 18 through 64 years of age whose recent institutional or occupational exposure to SARS-CoV-2 puts them at high risk of serious complications of COVID-19 including severe COVID-19 | 1 | Low |
| FDA—Pfizer (2020) | RCT | USA, Brazil, Argentina, Turkey South Africa, Germany | BioNTech and Pfizer | Pfizer | 2 | Adults 16 years of age or older who were healthy or had stable chronic medical conditions, including but not limited to human immunodeficiency virus (HIV), hepatitis B virus or hepatitis C virus infection | 23 | Low |
| Hillson (2021) | RCT | UK, Brazil, South Africa | UK Research and Innovation, National Institutes of Health Research (NIHR), The Coalition for Epidemic Preparedness Innovations, the Bill & Melinda Gates Foundation, the Lemann Foundation, Rede D’Or, the Brava and Telles Foundation, NIHR Oxford Biomedical Research Centre, Thames Valley and South Midland's NIHR Clinical Research Network, and AstraZeneca. | AstraZeneca | 2 | Women enrolled on a RCT, who were thought not to be pregnant but found to be pregnant, and this occurred in four ongoing Phase 1, Phase 2, and Phase 3 clinical trials | 67 | Low |
| Huang (2022) | Cohort | China | National Natural Science Foundation of China, Key Research and Development Program of Jiangxi Province | Sinopharm, Sinovac | 2 | Women undergoing a fresh IVF cycle who had received at least two vaccine doses at least 3 weeks apart | 2185 | Moderate |
| Kachikis (2021) | Cohort | USA | National Institute of Child Health and Human Development | Pfizer, Moderna, Janssen | 2 | Women who were pregnant, lactating, or planning pregnancy at the time of COVID-19 vaccination | 6244 | Serious |
| Kharbanda (2021) | Cohort | USA | Centre for Disease Control and Prevention | Pfizer, Moderna, Janssen | ≥1 | Women with ongoing pregnancies between 6- and 19-weeks’ gestation | 105 446 | Moderate |
| Magnus (2021) | Case-control | Norway | Research Council of Norway | Pfizer, Moderna, AstraZeneca | ≥1 | Women who had miscarriage before 14 weeks of gestation or primary care–based confirmation of ongoing pregnancy in the first trimester | 18 477 | Low |
| Moro (2022) | Cohort | USA | No funding received | Pfizer, Moderna, Janssen | ≥1 | Pregnant women who received COVID-19 vaccine and reported an adverse events to VAERS by using a pregnancy-status question in the form | 3462 | Moderate |
| Nabila Arfah (2021) | Cohort | Malaysia | Not stated | mRNA COVID-19 vaccine | ≥1 | Pregnant women after receiving a mRNA COVID-19 vaccine | 45 | Serious |
| Qiao (2021) | Cohort | Brazil | Sinovac Life Sciences | Sinovac, Janssen, AstraZeneca, Pfizer | ≥1 | Pregnant or postpartum women who reported vaccine-related adverse effects to adverse events following immunization surveillance information system | 3333 | Moderate |
| Trostle (2021) | Cohort | USA | Not stated | Pfizer, Moderna | ≥1 | Pregnant women who received at least one dose of an mRNA COVID-19 vaccination during pregnancy | 424 | Moderate |
| Wang (2022) | Cohort | China | National Natural Science Foundation of China | Inactivated COVID-19 vaccine | 2 | Participants who had completed gamete retrieval and embryo cryopreservation prior to vaccination with two doses of inactivated COVID-19 vaccine followed by a frozen-thaw embryo transfer cycle | 1496 | Moderate |
| Zauche (2021) | Cohort | USA | Not stated | Pfizer, Moderna | ≥1 | Singleton pregnancy who had received at least one dose of an mRNA Covid-19 vaccine either before conception or before 20 weeks of gestation and who did not have a pregnancy loss before 6 weeks of gestation | 2203 | Moderate |
Figure 1.
Study screening and inclusion process for systematic review evaluating the risk of miscarriage and ongoing pregnancy live birth among pregnancy women who received COVID-19 vaccine.
Six vaccines were used in included studies, including Pfizer-BioNTech BNT162b2 mRNA, Moderna mRNA-1273 SARS-CoV-2, Janssen Ad26.COV2.S, AstraZeneca ChAdOx1 nCoV-19, Sinopharm BBIBP-CorV, and Sinovac-CoronaVac. Ten studies reported on pregnancy outcomes following at least one vaccine dose, eight studies reported pregnancy outcomes following two doses and one study reported outcomes after a third booster dose (Table I).
Quality of included studies and risk of publication bias
Overall, the quality of the included studies was considered to have low to moderate risk of bias while four studies were considered to have a serious risk of bias (Supplementary Fig. S1). All included studies were assessed as having missing information on adherence to the vaccine administration schedule, not allowing accurate assessment of the risk of bias for deviations from the intended intervention. Six of the included studies had an overall low risk of bias (6/21, 29%), half showed a moderate risk (11/21, 52%), and 4 showed a high risk of bias (4/21, 19%) mainly due to participant selection and measurement and outcomes reporting. Outcome reporting was poor overall with only two studies offering a clear outcome definitions for miscarriage and ongoing pregnancy (Aharon et al., 2021; Hillson et al., 2021). Our funnel plot suggested no major variation across included studies with a non-significant Egger’s test at P = 0.81 (Supplementary Fig. S2).
Pregnancy outcomes
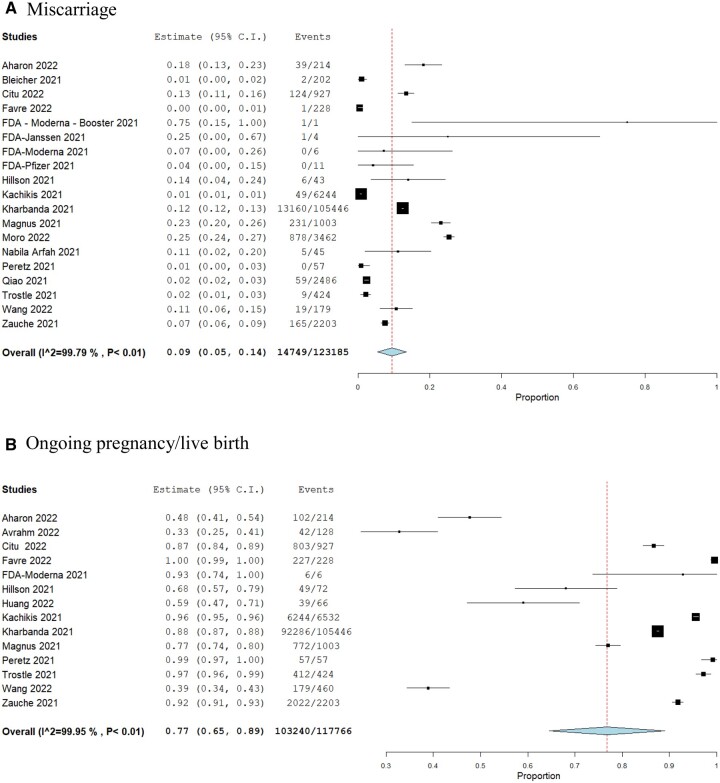
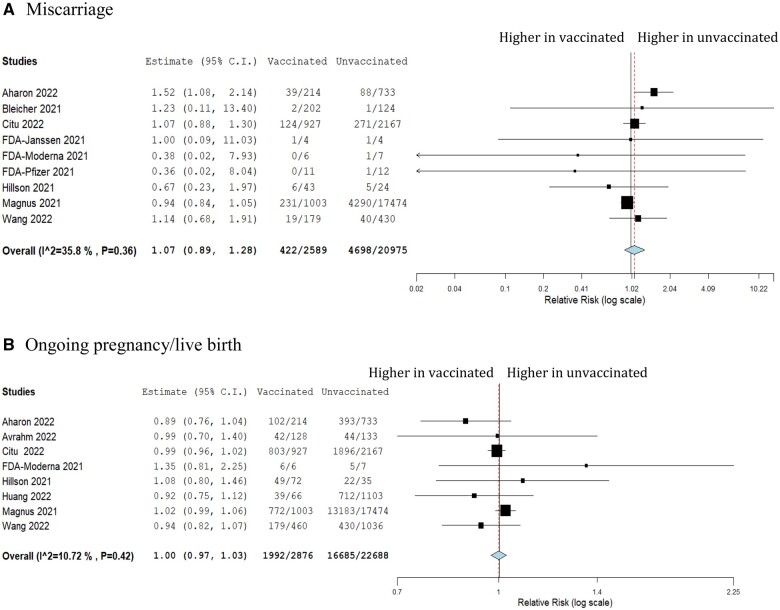
We pooled the overall miscarriage rate across all included studies among women who received any COVID-19 vaccine which was 9% (n = 18 studies, 14 749/123 185, 95% CI 0.05–0.14) (Fig. 2). We then compared the risk of miscarriage among those who received any COVID-19 vaccine to those who did not, which suggested no significant difference between the two groups (RR 1.07, 95% CI 0.89–1.28, I2 35.8%) (Fig. 3).
Figure 2.
Pooled event rate of miscarriage and ongoing pregnancy/live birth among pregnancy women who received the COVID-19 vaccinations. (A) Miscarriage. (B) Ongoing pregnancy/live birth.
Figure 3.
Forest plot showing the risk ratio of miscarriage and ongoing pregnancy/live birth among pregnancy women who received COVID-19 vaccination compared to unvaccinated women. (A) Miscarriage. (B) Ongoing pregnancy/live birth.
The overall proportion of women with ongoing pregnancies or live birth among those who were vaccinated was consistent with the reported population levels at 77% (n = 14 studies, 103 240/117 766, 95% CI 0.65–0.89) (Fig. 2). Compared to the unvaccinated group, women who received the COVID-19 vaccines had similar rates of ongoing pregnancies or live birth (RR 1.00, 95% CI 0.97–1.03, I2 10.7%) (Fig. 3).
Discussion
We identified 21 studies reporting miscarriage or live birth/ongoing pregnancy outcomes among 149 685 women. Our results demonstrate no apparent increase in the risk of miscarriage among pregnant women who received the COVID-19 vaccines, which was consistent with the rate of miscarriage in the general population before the pandemic (Quenby et al., 2021). Compared to unvaccinated women, those who received the vaccine had a slightly higher risk of miscarriage, though this was not statistically significant. This trend could be explained by several confounders, such as population socio-economics, baseline risk factors (e.g. recurrent pregnancy loss), co-morbidities, and access to healthcare services, which were observed in cohort studies evaluating third-trimester pregnancy outcomes among vaccinated women (Fell et al., 2022; Magnus et al., 2022). There was no significant difference in the RR of live birth or ongoing pregnancy among women who received COVID-19 vaccination compared to those who did not receive a vaccine.
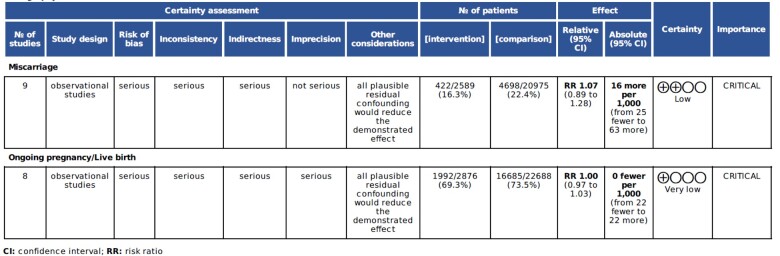
Overall, the certainty in the pooled evidence was low (Fig. 4) due to serious concerns about the consistency, precision and directness of our synthesized effect estimate. Given the high heterogeneity across included studies, our results should be interpreted with caution pending larger well-powered controlled studies.
Figure 4.
GRADE evidence assessment table for the risk of miscarriage and ongoing pregnancy/live birth among pregnancy women who received COVID-10 vaccine.
Strengths and limitations
We present a systematic review that employed a prospectively registered protocol and reported as per established guidelines, therefore offering a comprehensive assessment of the literature on the safety of COVID-19 vaccines in pregnancy. Only about half of the included studies had appropriately matched controls which limited our ability to generate a RR with accurate confidence intervals. Still, we reported narratively on all included studies and generated a weighted average to estimate the overall proportion of miscarriage and ongoing pregnancy or live birth among vaccinated pregnant women.
We included studies from various countries including data from large regulatory randomized trials that were used to licence the use of COVID-19 vaccine in the general population. However, as pregnant women were excluded from these trials at the time of randomization, the evidence included in this review is mainly observational with high level of heterogeneity. Several factors could explain this heterogeneity including variation in study design and patient characteristics, and the high risk of bias across included studies. This limits the generalizability of our meta-analysis and highlights the need for better quality primary studies involving pregnant women.
The majority of the included studies practiced suboptimal and varied outcome reporting which limited our ability to synthesize high-quality evidence, as reflected in our GRADE assessment (Fig. 4). This reduced the certainty of our pooled estimates, especially since other important pregnancy outcomes, e.g. stillbirth and ectopic pregnancy, were not reported.
While we reported a relatively low miscarriage rate (9%) across a large cohort (n = 123 184), our pooled rate offers a limited snapshot assessment over a short period of time and therefore should be interpreted with caution. Clearly, several factors could influence the overall miscarriage rate during the pandemic such as ethnicity, mode of conception, and access to maternity services during lockdown periods (García-Enguídanos et al., 2002).
As most of these studies focused on short snapshot assessment of COVID-19 vaccine safety, the majority reported on the combined outcome of ongoing pregnancy or live birth. Clearly, this outcome does not offer an accurate assessment of long-term reproductive outcomes as not all ongoing pregnancies captured will yield a live birth. Still, we chose to report on this outcome to provide an accurate summary of the current available literature, assess the knowledge gap, and make recommendations to improve the quality of future research.
We planned to perform meta-regression and subgroup analysis to evaluate and adjust for important confounders such as patient characteristics, vaccine types (e.g. mRNA versus vector), and the number of vaccine boosters. However, we were unable to produce these additional analyses due to poor reporting across included studies (Table I). Other important effect modifiers that were also poorly reported included patient age group, method of conception, multiple pregnancy, and the impact across first versus second-trimester miscarriage. Standardized outcome reporting is therefore essential to improve the quality of future evidence synthesis particularly to facilitate patient-level data analyses.
Implications for clinical practice
The COVID-19 pandemic introduced unprecedented challenges with enduring humanitarian and economic crises that are still unfolding (Spinelli and Pellino, 2020). In addition to its high virality, rapid mutations, and lack of curative treatments, a key challenge in controlling the COVID-19 virus was the role of mass media misinformation that often undermined efforts to promote key prevention strategies like mask-wearing, social distancing, and vaccination (Roozenbeek et al., 2020; Loomba et al., 2021).
Generally, concerns about the safety of vaccines in pregnancy could be attributed to the generic immunological and inflammatory response that could impact foetal implantation and embryogenesis (Arora and Lakshmi, 2021; Moodley et al., 2021). However, in the case of COVID-19 mRNA vaccines, there were concerns disseminated on social media platforms claiming higher risk of miscarriage due to the formation of antibodies that could cross the placenta and bind to the spike protein called syncytin-1, a critical protein in the formation of the syncytiotrophoblast layer of the human placenta and embryogenesis (Blake Evans et al., 2021). Several studies have came out since to disprove these claims with no evidence from immunological studies to support such interaction (Moodley et al., 2021). Our findings further support the lack of harmful evidence pending larger, better-quality studies at a population level.
Considering the increased risk of miscarriage and other adverse pregnancy outcomes associated with COVID-19 infection in pregnancy (Stock et al., 2022), vaccines play a vital role to minimize the impact of this disease on pregnant women and their offspring (Arora and Lakshmi, 2021; Moodley et al., 2021). Ideally, the risks of vaccination should be evaluated considering the patient’s current medical health, risk profile for COVID-19 morbidity, and past adverse reactions or febrile illnesses to previous vaccinations. Vaccinations in the first-trimester could pose some risks of high immunogenicity and inflammation from a febrile illness to the foetus; especially in patients who have few or no risk factors for serious morbidity should they contract COVID-19. However, the merits of avoiding COVID-19 vaccination in the first trimester in favour of the pre-conception period or the second trimester remain unclear and further research is needed.
Available COVID-19 vaccines seem to have high immunogenicity and reactogenicity (Gray et al., 2021), often associated with a systemic inflammatory process manifesting with headache, myalgia, chills, and fever (Shimabukuro et al., 2021). Pregnant women receiving COVID-19 vaccines reported a higher incidence of systemic fever after the second dose compared to non-pregnant women (Gray et al., 2021). Fever in early pregnancy and during embryogenesis may be a teratogenic phenomenon and this may increase the risk of miscarriage especially in the first trimester or among those with more severe vaccine side effects (Graham et al., 1998; Dreieret al., 2014). We were unable to explore the optimal timing to provide COVID-19 vaccines in pregnancy and whether such side effects could have a differential impact on first versus second-trimester pregnancies.
As the rate of re-infection with new mutations of the COVID-19 virus is increasing progressively (Jain et al., 2021), there is a need to evaluate the optimal timing to provide COVID-19 vaccines for both de novo and booster immunity. This is particularly relevant to high-risk women planning a pregnancy such as those with chronic disease or those undergoing assisted conception (Han et al., 2022).
Future research
There is a critical need to evaluate the short and long-term safety and effectiveness outcomes of the different COVID-19 vaccines on pregnant women and their offspring. As the use of different COVID-19 vaccines grows (mRNA versus vector vaccines), large prospective cohorts with appropriately matched controls are needed to evaluate the effectiveness and safety of the different COVID-19 vaccination programmes in reducing the reported risks of adverse maternal and neonatal outcomes (Wei et al., 2021).
Several studies have identified binding and neutralizing antibody titres for COVID-19 in infant cord blood and the breast milk of lactating vaccinated women. This could suggest long-lasting protective immunity that might help to reduce the risk of re-infection or severe disease among this vulnerable cohort (Fell et al., 2022; Goldshtein et al., 2022; Magnus et al., 2022). However, more epidemiological and translational studies are needed to evaluate the long-term health outcomes among both mothers and offspring post vaccine exposure.
We encountered a high degree of varied outcome reporting which significantly hindered effective evidence synthesis. Future studies should adopt standardized reporting of core outcomes as per published core sets for miscarriage, fertility and pregnancy to enable more efficient evidence synthesis and reduce research wastage (Smith et al., 2017; Duffy et al., 2020b, 2020a).
Conclusions
COVID-19 vaccines are not associated with an increased risk of miscarriage or decreased rates of ongoing pregnancy or live birth rates among women of reproductive age. The current evidence remains limited and larger population studies are needed to evaluate the effectiveness and safety of COVID-19 vaccines in pregnancy.
Supplementary Material
Contributor Information
Michael P Rimmer, Medical Research Council Centre for Reproductive Health, Institute of Regeneration and Repair, Edinburgh BioQuarter, University of Edinburgh, UK.
Jhia J Teh, Faculty of Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK.
Scott C Mackenzie, Medical Research Council Centre for Reproductive Health, Institute of Regeneration and Repair, Edinburgh BioQuarter, University of Edinburgh, UK.
Bassel H Al Wattar, Beginnings Assisted Conception Unit, Epson and St Helier University Hospitals, London, UK; Comprehensive Clinical Trials Unit, Institute for Clinical Trials and Methodology, University College London, London, UK.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files.
Authors’ roles
M.P.R. and J.J.T. drafted the initial protocol and manuscript, and conducted the search, study selection and initial analysis. S.C.M. contributed to the data extraction and visualization. B.H.A.W. conceived the idea, conducted the final analysis, and drafted the final manuscript. All authors approved the final version of the manuscript.
Funding
No direct funding was provided to support this work. M.P.R. was funded by the Medical Research Council Centre for Reproductive Health Grant No: MR/N022556/1. B.H.A.W. hold a personal development award from the National Institute of Health Research in the UK.
Conflict of interest
All authors declare no conflict of interest.
References
- Aharon D, Canon CM, Hanley WJ, Lee JA, Lederman MA, Stein DE, Copperman AB.. mRNA COVID-19 vaccines do not compromise implantation of euploid embryos. Fertil Steril 2021;116:e77. [Google Scholar]
- Aharon D, Lederman M, Ghofranian A, Hernandez-Nieto C, Canon C, Hanley W, Gounko D, Lee JA, Stein D, Buyuk E. et al. In vitro fertilization and early pregnancy outcomes after coronavirus disease 2019 (COVID-19) vaccination. Obstet Gynecol 2022;139:490–497. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists. COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care. American College of Obstetricians and Gynecologists, 2022. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care. [Google Scholar]
- Arora M, Lakshmi R.. Vaccines—safety in pregnancy. Best Pract Res Clin Obstet Gynaecol 2021;76:23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham S, Kedem A, Zur H, Youngster M, Yaakov O, Yerushalmi GM, Gat I, Gidoni Y, Hochberg A, Baum M. et al. Coronavirus disease 2019 vaccination; and infertility treatment outcomes. Fertil Steril 2022;117:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB. et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake Evans M, Alexander C, Barnard E, Max Ezzati M, Hill MJ, Hoyos LR, Hariton H, Mikhael S, Penzias A. COVID-19 Vaccine and Infertility: Baseless Claims and Unfounded Social Media Panic. 2021. https://www.fertstertdialog.com/posts/covid-19-vaccine-and-infertility-baseless-claims-and-unfounded-social-media-panic (June 2022, date last accessed).
- Bleicher I, Kadour-Peero E, Sagi-Dain L, Sagi S.. Early exploration of COVID-19 vaccination safety and effectiveness during pregnancy: interim descriptive data from a prospective observational study. Vaccine 2021;39:6535–6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, Asraf K, Doolman R, Levin EG, Regev Yochay G. et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol 2021;58:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciapponi A, Bardach A, Mazzoni A, Alconada T, Anderson S, Argento FJ, Ballivian J, Bok K, Comandé D, Erbelding E. et al. Safety of COVID-19 vaccines, their components or their platforms for pregnant women: a rapid review. medRxiv2021.06.03.21258283. [DOI] [PMC free article] [PubMed]
- Citu IM, Citu C, Gorun F, Sas I, Bratosin F, Motoc A, Burlea B, Rosca O, Malita D, Gorun OM.. The risk of spontaneous abortion does not increase following first trimester mRNA COVID-19 vaccination. J Clin Med 2022;11:1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gier B, Andeweg S, Joosten R, Ter Schegget R, Smorenburg N, van de Kassteele J, Hahné SJ, van den Hof S, de Melker HE, Knol MJ.. Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, the Netherlands, February to May 2021. Euro Surveill 2021;26:2100640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JW, Andersen AM, Berg-Beckhoff G.. Systematic review and meta-analyses: fever in pregnancy and health impacts in the offspring. Pediatrics 2014;133:e674–e688. [DOI] [PubMed] [Google Scholar]
- Duffy JMN, AlAhwany H, Bhattacharya S, Collura B, Curtis C, Evers JLH, Farquharson RG, Franik S, Giudice LC, Khalaf Y. et al. Developing a core outcome set for future infertility research: an international consensus development study. Hum Reprod 2020. a;35:2725–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J, Cairns A, Richards-Doran D, van 't Hooft J, Gale C, Brown M, Chappell L, Grobman W, Fitzpatrick R, Karumanchi S. et al. ; International Collaboration to Harmonise Outcomes for Pre-eclampsia (iHOPE). A core outcome set for pre-eclampsia research: an international consensus development study. BJOG 2020b;127:1516–1526. [DOI] [PubMed] [Google Scholar]
- Egloff C, Couffignal C, Cordier AG, Deruelle P, Sibiude J, Anselem O, Benachi A, Luton D, Mandelbrot L, Vauloup-Fellous C. et al. Pregnant women’s perceptions of the COVID-19 vaccine: a French survey. PLoS One 2022;17:e0263512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre G, Maisonneuve E, Pomar L, Winterfeld U, Daire C, Martinez de Tejada B, Delecraz D, Campelo S, Moser M, Todesco-Bernasconi M. et al. COVID-19 mRNA vaccine in pregnancy: Results of the Swiss COVI-PREG registry, an observational prospective cohort study. Lancet Reg Health Eur 2022;18:100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell DB, Dhinsa T, Alton GD, Török E, Dimanlig-Cruz S, Regan AK, Sprague AE, Buchan SA, Kwong JC, Wilson SE. et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA 2022;327:1478–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Enguídanos A, Calle ME, Valero J, Luna S, Domínguez-Rojas V.. Risk factors in miscarriage: a review. Eur J Obstet Gynecol Reprod Biol 2002;102:111–119. [DOI] [PubMed] [Google Scholar]
- Goldshtein I, Steinberg DM, Kuint J, Chodick G, Segal Y, Shapiro Ben David S, Ben-Tov A.. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr 2022;176:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R, Peng X, Kang S, Feng H, Huang J, Zhang W, Lin D, Tien P, Xiao G.. Structural characterization of the fusion core in syncytin, envelope protein of human endogenous retrovirus family W. Biochem Biophys Res Commun 2005;331:1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JM Jr, Edwards MJ, Edwards MJ.. Teratogen update: gestational effects of maternal hyperthermia due to febrile illnesses and resultant patterns of defects in humans. Teratology 1998;58:209–221. [DOI] [PubMed] [Google Scholar]
- Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, Baez AM, Shook LL, Cvrk D, James K. et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol 2021;225:303.e1–303.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han AR, Lee D, Kim SK, Choo CW, Park JC, Lee JR, Choi WJ, Jun JH, Rhee JH, Kim SH; Korean Society for Assisted Reproduction (KOSAR). Effects and safety of COVID-19 vaccination on assisted reproductive technology and pregnancy: a comprehensive review and joint statements of the KSRM, the KSRI, and the KOSAR. Clin Exp Reprod Med 2022;49:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillson K, Clemens SC, Madhi SA, Voysey M, Pollard AJ, Minassian AM; Oxford COVID Vaccine Trial Group. Fertility rates and birth outcomes after ChAdOx1 nCoV-19 (AZD1222) vaccination. Lancet 2021;398:1683–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Xia L, Lin J, Liu B, Zhao Y, Xin C, Ai X, Cao W, Zhang X, Tian L. et al. No effect of inactivated SARS-CoV-2 vaccination on in vitro fertilization outcomes: a propensity score-matched study. J Inflamm Res 2022;15:839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain VK, Iyengar K, Garg R, Vaishya R.. Elucidating reasons of COVID-19 re-infection and its management strategies. Diabetes Metab Syndr 2021;15:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachikis A, Englund JA, Singleton M, Covelli I, Drake AL, Eckert LO.. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open 2021;4:e2121310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda EO, Haapala J, DeSilva M, Vazquez-Benitez G, Vesco KK, Naleway AL, Lipkind HS.. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA 2021;326:1629–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer MK, Mehl R, Costantine MM, Johnson A, Cohen J, Summerfield TL, Landon MB, Rood KM, Venkatesh KK.. Characteristics and perceptions associated with COVID-19 vaccination hesitancy among pregnant and postpartum individuals: a cross-sectional study. BJOG 2022;129:1342–1351. [DOI] [PubMed] [Google Scholar]
- Kloc M, Uosef A, Kubiak JZ, Ghobrial RM.. Exaptation of retroviral syncytin for development of syncytialized placenta, its limited homology to the SARS-CoV-2 spike protein and arguments against disturbing narrative in the context of COVID-19 vaccination. Biology 2021;10:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba S, de Figueiredo A, Piatek SJ, de Graaf K, Larson HJ.. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav 2021;5:337–348. [DOI] [PubMed] [Google Scholar]
- Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, Simmons R, Cottrell S, Roberts R, O’Doherty M. et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ 2021;373:n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, Padayachee SD, Dheda K, Barnabas SL, Bhorat QE. et al. ; Wits-VIDA COVID Group. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021;384:1885–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE.. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med 2021;385:2008–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus MC, Örtqvist AK, Dahlqwist E, Ljung R, Skår F, Oakley L, Macsali F, Pasternak B, Gjessing HK, Håberg SE. et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA 2022;327:1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar CN, Koh W, Seow Y, Hoon S, Venkatesh A, Dashraath P, Lim LM, Ong J, Lee R, Johana N. et al. Addressing anti-syncytin antibody levels, and fertility and breastfeeding concerns, following BNT162B2 COVID-19 mRNA vaccination. medRxiv2021.05.23.21257686.
- Moodley J, Khaliq OP, Mkhize PZ.. Misrepresentation about vaccines that are scaring women. Afr J Prim Health Care Fam Med 2021;13:e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro PL, Olson CK, Clark E, Marquez P, Strid P, Ellington S, Zhang B, Mba-Jonas A, Alimchandani M, Cragan J. et al. Post-authorization surveillance of adverse events following COVID-19 vaccines in pregnant persons in the vaccine adverse event reporting system (VAERS), December 2020-October 2021. Vaccine 2022;40:3389–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabila Arfah M, Murizah M.. Preliminary findings on inadvertently exposed pregnancies to COVID-19 mRNA vaccine in Kedah Darul Aman. Med J Malaysia 2021;73 (Suppl 3). [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C. et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M, Lin JL, Gu Y, Gupta R, Macary P, Schwarz H.. No crossreactivity of anti-SARS-CoV-2 spike protein antibodies with Syncytin-1. Cell Mol Immunol 2021;18:2566–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Lopes de Abreu A, Dias CZ, Meng X, Ferreira RV, Gonçalves Pereira R, Julian GS, Yin W. Safety profile of COVID-19 vaccines in pregnant and postpartum women in Brazil. medRxiv2021.12.14.21267777.
- Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, Brosens JJ, Brewin J, Ramhorst R, Lucas ES. et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021;397:1658–1667. [DOI] [PubMed] [Google Scholar]
- Roozenbeek J, Schneider CR, Dryhurst S, Kerr J, Freeman ALJ, Recchia G, van der Bles AM, van der Linden S.. Susceptibility to misinformation about COVID-19 around the world. R Soc Open Sci 2020;7:201199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal College of Obstetricians & Gynaecologists. Coronavirus (COVID-19) Infection and Pregnancy. 2022. https://www.rcog.org.uk/guidance/coronavirus-covid-19-pregnancy-and-women-s-health/coronavirus-covid-19-infection-in-pregnancy/ (1 July 2022, date last accessed).
- Royal College of Obstetricians & Gynaecologists. RCOG Supports Calls From NHS to Pregnant Women to Get Vaccinated Against COVID-19. 2021. https://www.rcog.org.uk/news/rcog-supports-calls-from-nhs-to-pregnant-women-to-get-vaccinated-against-covid-19/#:~:text=The%20Royal%20College%20of%20Obstetricians,who%20have%20not%20been%20vaccinated (1 July 2022, date last accessed).
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B. et al. ; ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021;384:2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaler L, Wingfield M.. COVID-19 vaccine—can it affect fertility? Ir J Med Sci 2022;191:2185–2187. [Mismatch [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanes ED, Otero S, Mithal LB, Mupanomunda CA, Miller ES, Goldstein JA.. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol 2021;138:281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT. et al. ; CDC v-safe COVID-19 Pregnancy Registry Team. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med 2021;384:2273–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, Cooper N, Dhillon-Smith R, O'Toole E, Clark TJ, Coomarasamy A.. Core Outcome Sets in Miscarriage Trials (COSMisT) study: a study protocol. BMJ Open 2017;7:e018535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli A, Pellino G.. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg 2020;107:785–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock SJ, Carruthers J, Calvert C, Denny C, Donaghy J, Goulding A, Hopcroft LEM, Hopkins L, McLaughlin T, Pan J. et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med 2022;28:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trostle ME, Limaye MA, Avtushka V, Lighter JL, Penfield CA, Roman AS.. COVID-19 vaccination in pregnancy: early experience from a single institution. Am J Obstet Gynecol MFM 2021;3:100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Health Security Agency. COVID-19 Vaccination: A Guide on Pregnancy and Breastfeeding. Gov.uk, 2022. https://www.gov.uk/government/publications/covid-19-vaccination-women-of-childbearing-age-currently-pregnant-planning-a-pregnancy-or-breastfeeding/covid-19-vaccination-a-guide-on-pregnancy-and-breastfeeding (7 July 2022, date last accessed). [Google Scholar]
- United States Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine (BNT162, PF-07302048). Vaccines and Related Biological Products Advisory Committee Briefing Document. FDA, 2020a. https://www.fda.gov/media/144246/download (March 2022, date last accessed).
- United States Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee December 17, 2020 Meeting Presentation—FDA Review of Efficacy and Safety of Moderna COVID-19 Vaccine Emergency Use Authorization Request. 2020b. https://www.fda.gov/media/144585/download (3 July 2022, date last accessed).
- United States Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting FDA Briefing Document October 14, 2021 EUA Amendment Request for a Booster Dose of the Moderna COVID-19 Vaccine. 2021a. https://www.fda.gov/media/152991/download (3 July 2022, date last accessed).
- United States Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting February 26, 2021 FDA Briefing Document Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. 2021b. https://www.fda.gov/media/146217/download (5 July 2022, date last accessed).
- Wang Y, Ren X, Wang Z, Feng X, Li M, Liu P.. Receipt of inactivated COVID-19 vaccine had no adverse influence on embryo implantation, clinical pregnancy and miscarriage in early pregnancy. Sci China Life Sci 2022;65:2332–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N.. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 2021;193:E540–E548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauche LH, Wallace B, Smoots AN, Olson CK, Oduyebo T, Kim SY, Petersen EE, Ju J, Beauregard J, Wilcox AJ. et al. ; CDC v-safe Covid-19 Pregnancy Registry Team. Receipt of mRNA Covid-19 vaccines and risk of spontaneous abortion. N Engl J Med 2021;385:1533–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Supplementary Information files.