Abstract
STUDY QUESTION
What are the changes in serum concentration of total and cleaved anti-Muüllerian hormone (AMH) molecular forms and of androgens before and throughout pregnancy in women with and without polycystic ovary syndrome (PCOS) in a longitudinal follow-up investigation?
SUMMARY ANSWER
Serum levels of total and cleaved AMH are higher from preconception to the third trimester of pregnancy in women with PCOS as compared to controls, whereas testosterone and androstenedione levels are higher in women with PCOS than in control women before pregnancy and during the second and third trimester of pregnancy.
WHAT IS KNOWN ALREADY
Cross-sectional or partial longitudinal studies have shown higher AMH and androgen levels in pregnant women with PCOS as compared with non-PCOS women. To date, no complete longitudinal dynamic monitoring of the circulating forms of AMH and androgens from pre-conception to the third trimester of pregnancy have compared women with and without PCOS.
STUDY DESIGN, SIZE, DURATION
This systematic prospective quarterly longitudinal monocentric study was a comparative follow-up of 30 women with PCOS and 29 controls before and during pregnancy from April 2019 to July 2022.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women aged 18–43 years with a pre-conception measurement of AMH were included during the first trimester of a singleton pregnancy. The PCOS group was defined according to the Rotterdam diagnostic criteria. The control group patients included in the study had normal ovarian reserves. Circulating total and cleaved AMH, and serum estradiol, LH, and androgen levels were measured during the first, second, and third trimester of pregnancy in all study participants.
MAIN RESULTS AND THE ROLE OF CHANCE
Before pregnancy, patients with PCOS had higher levels of AMH than controls. The total and cleaved AMH forms were significantly higher in women with PCOS than controls from pre-conception to the third trimester of pregnancy (all P < 0.001). Androgens (total testosterone and androstenedione) were higher in women with PCOS than controls from mid-pregnancy onwards.
LIMITATIONS, REASONS FOR CAUTION
Our control population was a population of infertile women with no ovarian problems but most of them had undergone ART treatments to achieve pregnancy.
WIDER IMPLICATIONS OF THE FINDINGS
These results strengthen the hypothesis that gestational hyperandrogenism as well as exposure to elevated AMH levels in utero could be driving forces predisposing female progeny to develop PCOS.
STUDY FUNDING/COMPETING INTEREST(S)
Funding was provided by INSERM, France (grant number U1172) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program, ERC-2016-CoG to P.G. grant agreement n° 725149/REPRODAMH. The authors have nothing to declare.
TRIAL REGISTRATION NUMBER
Keywords: PCOS, AMH, pregnancy, AMH molecular forms, androgens
Introduction
Polycystic ovary syndrome (PCOS) is the most common gyneco-endocrine disorder affecting between 5% and 18% of women worldwide (Teede et al., 2018). It is defined, according to the Rotterdam criteria, by the presence of at least two of the following features: menstrual irregularities, hyperandrogenism, and polycystic-appearing ovaries on ultrasound evaluation (Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group, 2004; Teede et al., 2018). Besides reproductive disorders, women with PCOS frequently have other long-term metabolic health-related repercussions, including obesity, hyperinsulinemia, insulin resistance, and type 2 diabetes (Fauser et al., 2012; Teede et al., 2018; Gomez et al., 2022; Guan et al., 2022; Joham et al., 2022).
Although not yet included among the PCOS diagnostic criteria, serum anti-Muüllerian hormone (AMH) levels are higher in most women with PCOS as compared to control women because they closely reflect the follicle excess (Cook et al., 2002; Pigny et al., 2003). In addition, increased serum AMH levels are associated with the ovulation disorder and the hyperandrogenism of the syndrome, suggesting its role in the disturbed folliculogenesis of PCOS (Homburg and Crawford, 2014; Dewailly et al., 2020). Interestingly, the receptor for AMH, AMHR2, is also expressed outside the ovaries, on a subset of hypothalamic GnRH neurons in mice and humans (Cimino et al., 2016) as well as in the murine (Tata et al., 2018) and human placenta (Novembri et al., 2015). In adult mice, AMH activates GnRH neuron firing and stimulates LH secretion (Cimino et al., 2016; Tata et al., 2018). In addition, exposure to excessive AMH during late gestation in mice causes an inhibition of aromatase gene expression in the placenta, leading to an increase in maternal and, presumably, fetal serum testosterone concentration (Tata et al., 2018). These data are supported by clinical investigations showing that aromatase activity is significantly reduced in the placenta of women with PCOS as compared with controls (Maliqueo et al., 2013). Based on this evidence, it has been proposed that AMH may contribute to the etiology of PCOS through extragonadal actions affecting the brain and the placenta (Cimino et al., 2016; Tata et al., 2018).
AMH is a glycoprotein, secreted by granulosa cells of preantral and small antral follicles, which is synthesized as a 140 kDa homo-dimeric pro-hormone (proAMH) that must undergo proteolytic cleavage to become biologically active (Pepinsky et al., 1988; di Clemente et al., 2010). The cleavage gives rise to two homodimers of 110 kDa (AMHN) and 25 kDa (AMHC), which remain associated in the blood by a non-covalent bond (AMHN,C) (Pepinsky et al., 1988; Nachtigal and Ingraham, 1996) but only the C-terminal portion of the hormone (AMHC) is biologically active, binding its specific AMH receptor, AMHR2. Circulating AMH is thus a mixture of both AMHN,C and proAMH (Pankhurst and McLennan, 2013) and commercial AMH ELISAs, used in daily clinical practice, provide an aggregate measure of two AMH species (total AMH) since they do not distinguish between proAMH and AMHN,C.
Several preclinical investigations have proposed that prenatal hyperandrogenemia as well as exposure to elevated AMH levels in utero could be key factors predisposing female progeny to develop PCOS (Homburg, 2009; Tata et al., 2018; Teede et al., 2018). Moreover, two recent studies report that PCOS-like reproductive and metabolic traits are passed on to third-generation female mouse offspring after prenatal dihydrotestosterone or AMH exposure (Risal et al., 2019; Mimouni et al., 2021). This evidence, together with recent findings that daughters of women with PCOS are five times more likely to be diagnosed with PCOS than those from mothers without PCOS (Risal et al., 2019), suggests an adverse maternal-fetal environment (i.e. high androgens and high AMH) that may act through epigenetic mechanisms in concert with susceptibility variants to produce PCOS phenotypes (Dapas and Dunaif, 2022).
Maternal circulating androgen levels have been documented to be increased in pregnant women with PCOS, however those investigations were either not, or only partially, longitudinal as they did not follow-up the entire evolution of androgen levels from pre-conception to the third trimester of pregnancy (Sir-Petermann et al., 2002; Maliqueo et al., 2013, 2015; Glintborg et al., 2018; Piltonen et al., 2019; Sun et al., 2020).
Cross-sectional or partial longitudinal studies have also shown higher AMH levels in the second trimester of pregnancy and at the time of delivery in women with PCOS compared to control women (Köninger et al., 2018; Tata et al., 2018; Detti et al., 2019; Piltonen et al., 2019; Valdimarsdottir et al., 2019); however, to date, no systematic longitudinal dynamic monitoring of AMH molecular forms have been performed to compare women with and without PCOS before and throughout pregnancy.
To fill this gap in knowledge, we performed the first longitudinal investigation to evaluate the temporal evolution of serum levels of AMH molecular forms (total and cleaved AMH) and of serum androgens in a population of singleton pregnant women with PCOS and to compare these values with those of control women before and during the entire course of pregnancy.
Materials and methods
Population
The patients were recruited at the Jeanne de Flandre Gynecology Hospital (Lille University Hospital) at the first pregnancy ultrasound (between 5 and 10 gestational weeks (GW)) from April 2018 to July 2022. The inclusion criteria for the study were as follows: (i) aged 18–43 years, (ii) having a pre-conceptional infertility assessment in the Gynecology-Endocrinology Department of the Lille University Hospital (with at least a measurement of AMH) before pregnancy, (iii) having a singleton pregnancy obtained spontaneously, after induction of ovulation or after ARTs, and (iv) willingness to be monitored and give birth in the Jeanne de Flandre maternity ward.
The women were then divided into two groups: the PCOS or control groups.
The group with PCOS was defined according to the modified Rotterdam criteria (2003 then 2011) (Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group, 2004; Dewailly et al., 2011) by at least two of the following three criteria: (i) menstrual cycles irregularities, defined by oligo-spaniomenorrhea or amenorrhea, (ii) clinical or biological hyperandrogenism (total testosterone (TT) ≥0.39 ng/ml and/or androstenedione assay ≥1.75 ng/ml and/or Ferriman and Gallwey score ≥6 or severe acne in at least two areas of the body), and (iii) morphological appearance of polycystic ovaries on ultrasound (PCOM), i.e. ovarian volume >10 cm3 or ovarian surface area >5.5 cm2 or at least 20 follicles of 2–9 mm per ovary (Dewailly et al., 2011), after excluding all other causes of ovulatory disorders or hyperandrogenism, such as hyperprolactinemia, hypogonadism hypogonadotropic, non-congenital adrenal hyperplasia, Cushing syndrome, adrenal, or ovarian tumor.
In the control group, the patients had male or tubal infertility, no cycle disorder, and normal ovarian reserve (FSH <10 IU/l, estradiol (E2) <50 pg/ml, AMH >7, and <35 pmol/l, and an antral follicle count (AFC) >5 and <20 per ovary on D2–D5 of the cycle). These were women without personal ovarian pathology, as their fertility problem was of another origin. This population has also been used as a control for ovarian function for many years and has proven itself in numerous studies (Pigny et al., 2016).
Patients with a multiple pregnancy, pregnancy after oocyte donation, insufficient ovarian reserve (AMH < 7 pmol/l, FSH > 12 IU/l, or AFC < 5 before pregnancy), an isolated PCOM, long-term drug treatment (excluding usual pregnancy supplements), diabetes prior to pregnancy, a history of bariatric surgery, or ovulatory infertility of central or idiopathic origin were excluded.
Study objectives
The main objective of our study was to compare maternal serum AMH levels in the third trimester of pregnancy between women with PCOS and control women.
Our secondary objectives were to compare during pregnancy between PCOS women and control women: (i) the evolution of serum total AMH, (ii) the evolution of the different molecular forms of AMH (proAMH and cleaved forms), and (iii) the evolution of LH, estradiol, dehydroepiandrosterone sulfate (DHEAS), androstenedione, and TT.
A supplementary comparison according to fetal sex was performed. Lastly, we wanted to study the link between the levels of AMH molecular forms and androgen levels at the end of pregnancy.
Investigations
During pregnancy monitoring, in the first (T1: 5–10 GW), second (T2: 18–26 GW), and third trimesters (T3: 30–39 GW), additional blood samples were taken in addition to those of the usual pregnancy check-up. Blood samples were mainly collected at the time of systematic obstetrical ultrasound assessment in the first (T1: 7–10 GW), second (T2: 20–23 GW), and third trimesters (T3: 32–34 GW). Before pregnancy proAMH was measured in bio-banked serum from the pre-conceptional fertility assessment.
Weight and BMI were collected at each time of the follow-up to assess the evolution of the patient’s weight during pregnancy. Information about the delivery route, the sex of the child, and its birth weight were also collected.
Total AMH was assayed by an immunometric method on an Access-Dxi automatic analyzer (Beckman Coulter, USA). FSH, E2, LH, TT, and fasting serum insulin levels (I) were measured by immunoassay using an automatic analyzer (Architect, Abbott Laboratories, USA). Sex hormone-binding globulin (SHBG) and DHEAS were measured by immunoassay using the Immulite analyzer (Siemens, Germany) or the Dxi-analyzer (Beckman Coulter, USA), respectively. Cross-reactivity of hCG in the LH Architect immunoassay varied from 0.01% to 0.03%. Androstenedione levels were determined by LC-MS-MS. Glycemia was performed in the central biochemistry department of the hospital using routine assays on Roche analyzers. Inter-assay coefficients of variation, limits of detection, and reference ranges for each of the assays reported are detailed in Supplementary Table SI.
Finally, proAMH was measured as detailed previously (Peigné et al., 2020). Briefly, a phosphate buffer saline (phosphate-buffered saline (PBS), 0.02M, pH = 6.8, 37°C) was added to a first serum sample of 150 µl (control) and, in another sample, desoxycholate (DOC) (0.23% final concentration) diluted in PBS was added. After incubation for 30 min (in 2 times) at 37°C, the samples were analyzed for the AMH assay by the Access-Dxi automatic analyzer (Beckman Coulter, USA). The control sample treated with PBS gave the result of total AMH while the sample pre-treated with DOC allowed the measurement of proAMH alone. Results are reported as the total AMH or as the subtraction of the proAMH value from the total AMH to obtain the value of cleaved AMH (= total AMH − proAMH).
Sample size calculation
Our first hypothesis was that total AMH is still higher in women with PCOS in the third trimester of pregnancy. In study by Pigny et al. (2006) before pregnancy, the serum AMH level was 72.1 pmol/l (±38.2) in women with PCOS versus 19.9 pmol/l (±5.8) in control women. Because of the difference in the baseline measurement, we expected a large effect also at the end of pregnancy. According to Cohen (Cohen, 1992), a standardized difference (effect size) of 1 is considered large. To demonstrate this effect size with 90% power and 5% first-species risk, it was necessary to include 23 subjects per arm. In order to take into account a loss of follow-up rate of 20%, 29 patients had to be recruited per group (58 patients in total).
Analysis method and strategy
Continuous variables are reported as mean (SD) in the case of normal distribution or median (25th percentile to 75th percentile) otherwise. Categorical variables are reported as frequency (percentage). Normality of distributions was assessed using histograms.
To evaluate the magnitude of differences of patient characteristics before pregnancy between women with PCOS and controls women, absolute standardized differences were calculated; an absolute standardized differences >20% was interpreted as a meaningful imbalance (Cohen, 1962).
Clinical and biological measures during pregnancy were compared between women with PCOS and controls women using linear mixed models including group, trimester and the interaction group * trimester as fixed effect. Those models allowed performing an ANOVA for repeated measures taking into account the correlation between the repeated measures. The choice of the covariance model was based on the Akaike information criterion. Post hoc analysis at each trimester was performed using linear contrasts, after applying a Bonferroni correction for multiple comparisons. Normality of the models’ residuals was checked. If normality of model residuals was not satisfied (except if a logarithmic transformation could be applied), nonparametric analyses were used and physical and biological measures at each trimester were compared using Mann–Whitney U test.
Biological parameters measured before and during pregnancy were compared between women with PCOS and controls women using the same methodology as described above. Pre-specified confounding factors, namely age at the beginning of pregnancy, BMI at the beginning of pregnancy, week of gestation at each sampling time, and type of conception (i.e. spontaneous, ovulation induction (OI) or ARTs) were introduced as fixed effects and adjusted mean and theirs 95% confidence intervals at each time were derived from models.
For the third trimester, the association between total AMH and cleaved AMH and BMI, testosterone total, androstenedione, estradiol, and weight gain (compared to that before pregnancy) was assessed by using the spearman test and correlation coefficient to take into account the non-normality of the variables.
All statistical tests were done at the two-tailed α-level of 0.05 using the SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Ethical and legal considerations
An information note was given to each patient and her written consent was obtained. The trial was conducted in accordance with the approved protocol, Public Health Code, EU GCP, and applicable regulatory requirements. The trial is registered on the ClinicalTrials.gov public database: NCT03483792 and has received the agreement of the Committee for the Protection of Persons (CPP) (2017-A02628-45).
Results
Description of populations before and during pregnancy
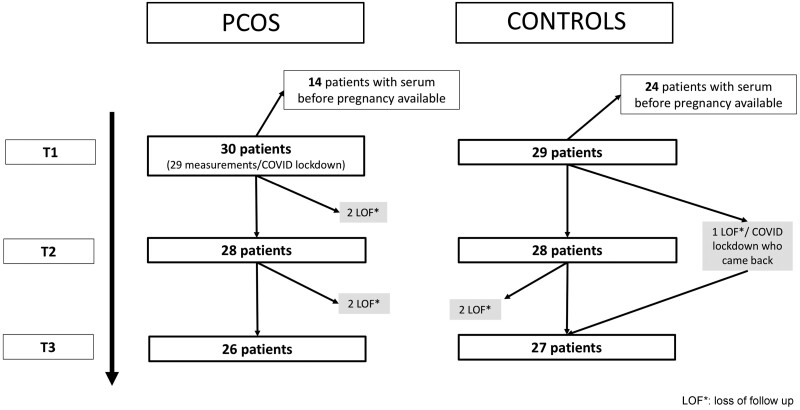
Inclusions began on April 2018 and ended on September 2021, and the follow-up of the women is shown in Fig. 1. The pre-pregnancy description of our PCOS and control populations is detailed in Table I and their description during pregnancy is shown in Table II. The time interval between the pre-pregnancy evaluation and the occurrence of pregnancy ranged from 2 months to 2 years (median 1.05 years).
Figure 1.
Flowchart of the follow-up throughout pregnancy in the PCOS and control groups. PCOS: polycystic ovary syndrome; COVID: due to Coronavirus 19; T1: first trimester of pregnancy; T2: second trimester of pregnancy; T3: third trimester of pregnancy; LOF*: loss of follow-up.
Table I.
Description of the overall population before pregnancy in women with PCOS and controls.
| N | PCOS N = 30 | N | Control N = 29 | ASD (%) | |
|---|---|---|---|---|---|
| Age at initial check-up (years) | 30 | 28.0 (25.0 to 31.0) | 29 | 31.0 (28.0 to 33.0) | 53.08 |
| BMI at initial check-up (kg/m²) | 30 | 24.5 (21.6 to 30.9) | 29 | 24.5 (22.2 to 29.4) | 6.07 |
| Total antral follicular count D2 | 28 | 52.5 (42.0 to 75.0) | 25 | 25.0 (18.0 to 29.0) | 244.49 |
| FSH D2 (IU/l) | 29 | 5.0 (4.4 to 5.8) | 22 | 5.8 (4.6 to 7.0) | 44.86 |
| LH D2 (IU/l) | 28 | 5.7 (3.9 to 8.0) | 17 | 3.4 (2.9 to 4.7) | 95.10 |
| Estradiol D2 (pg/ml) | 29 | 38.0 (31.0 to 52.0) | 22 | 35.5 (29.0 to 50.0) | 18.63 |
| Total AMH (pmol/l) | 30 | 59.0 (42.0 to 85.7) | 29 | 18.4 (15.0 to 23.2) | 320.29 |
| Total testosterone, TT (ng/ml) | 27 | 0.35 (0.27 to 0.46) | 16 | 0.29 (0.23 to 0.37) | 64.52 |
| Androstenedione (ng/ml) | 27 | 1.3 (0.9 to 1.6) | 16 | 1.0 (0.7 to 1.1) | 76.61 |
| DHEAS (µmol/l) | 8 | 5.4 (4.1 to 6.4) | 5 | 4.0 (3.7 to 5.4) | 66.80 |
| 17 OH progesterone (ng/ml) | 27 | 0.6 (0.3 to 0.8) | 16 | 0.4 (0.3 to 0.8) | 22.02 |
| Fasting glycemia (g/l) | 9 | 0.9 (0.8 to 0.9) | 4 | 0.9 (0.8 to 1.0) | NA |
| Fasting insulinemia (mUI/l) | 8 | 13.7 (8.7 to 17.5) | 5 | 7.2 (5.0 to 7.4) | 119.91 |
| Age at the beginning of pregnancy (years) | 30 | 28.0 (27.0 to 33.0) | 29 | 33.0 (28.0 to 35.0) | 53.12 |
| BMI at the beginning of pregnancy (kg/m²) | 28 | 25.0 (22.3 to 29.5) | 29 | 27.4 (21.0 to 31.9) | 6.07 |
| Type of conception | 30 | 29 | NA | ||
| Spontaneous | 5 (16.7) | 2 (6.9) | |||
| Ovulation induction | 16 (53.3) | 0 (0.0) | |||
| ART | 9 (30.0) | 27 (93.1) |
To evaluate the magnitude of differences of patient characteristics before pregnancy between women with PCOS and controls women, absolute standardized differences were calculated; an absolute standardized difference (ASD) >20% (in bold) was interpreted as a meaningful imbalance (i.e. difference). Values are presented as n (percentage) or as median (IQR). IQR: interquartile range; N: number of available observations; NA: not applicable; AMH: anti-Müllerian hormone; PCOS: polycystic ovary syndrome; D2: Day 2 of the menstrual cycle; DHEAS: dehydroepiandrosterone sulfate.
Table II.
Description of the overall population during pregnancy in women with PCOS and controls.
| N | PCOS N = 30 | N | Control N = 29 | P | |
|---|---|---|---|---|---|
| Gestational age at follow-up (GW) | |||||
| T1 | 30 | 8.5 (8.0 to 9.0) | 29 | 9.0 (8.0 to 10.0) | 0.45 |
| T2 | 28 | 22.0 (20.5 to 23.0) | 28 | 22.0 (20.5 to 22.5) | 1.00 |
| T3 | 26 | 34.0 (32.00 to 34.0) | 27 | 33.0 (32.0 to 34.0) | 0.30 |
| Weight (kg) | |||||
| T1 | 28 | 70.5 ± 15.0 | 28 | 74.5 ± 15.2 | 1.00 |
| T2 | 27 | 74.7 ± 14.2 | 25 | 77.1 ± 13.9 | 0.91 |
| T3 | 26 | 79.1 ± 14.0 | 27 | 83.0 ± 14.2 | 0.81 |
| Weight gain (kg) | |||||
| T1 | 28 | 0.7 ± 2.6 | 28 | 1.0 ± 2.3 | 1.00 |
| T2 | 27 | 4.6 ± 3.1 | 25 | 5.1 ± 4.2 | 1.00 |
| T3 | 26 | 8.8 ± 4.3 | 27 | 9.4 ± 6.5 | 1.00 |
| Albumin (g/l) | |||||
| T1 | 24 | 41.0 ± 2.8 | 23 | 38.8 ± 2.6 | 0.012 |
| T2 | 28 | 34.4 ± 2.2 | 28 | 34.4 ± 2.3 | 1.00 |
| T3 | 23 | 31.8 ± 2.2 | 25 | 31.96 ± 2.0 | 1.00 |
| Non-measurable LH (yes) | |||||
| T1 | 29 | 1 (3.4) | 29 | 1 (3.4) | NA |
| T2 | 28 | 18 (64.3) | 28 | 23 (82.1) | 0.46 |
| T3 | 26 | 15 (57.7) | 27 | 18 (66.7) | 1.00 |
| Sex of the fetus | 28 | 28 | 0.58* | ||
| Girl | – | 12 (42.9) | – | 9 (32.1) | – |
| Boy | – | 16 (57.1) | – | 19 (67.9) | – |
Values are presented as median (IQR), as mean ± SD or as n (percentage). P-values were corrected by Bonferonni except for newborn’s gender which has only one occurrence. P-values in bold refer to statistically significant values. IQR: interquartile range; N: number of available observations; P: P-value; NA: not applicable; PCOS: polycystic ovary syndrome; T1: first trimester of pregnancy; T2: second trimester of pregnancy; T3: third trimester of pregnancy; GW: gestational week. *Fisher’s exact text.
At the initial check-up before pregnancy, patients with PCOS were younger and had higher levels than controls for total AMH, LH, TT, androstenedione, fasting insulinemia, and baseline antral follicular count (Table I). For women with PCOS, the phenotypes were mainly A (43.3%) and D (50%).
At the beginning of pregnancy, patients with PCOS were significantly younger than the controls and their BMIs were not significantly different (Table I).
AMH and its molecular forms throughout pregnancy
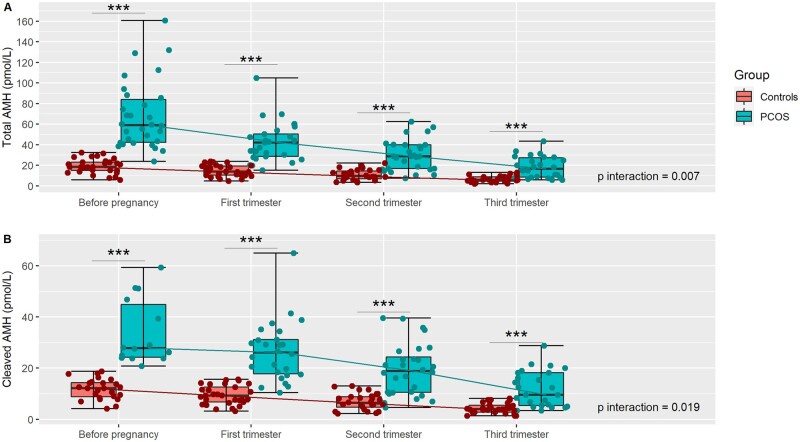
The levels of total AMH and cleaved AMH decreased throughout pregnancy but they remained significantly higher in women with PCOS than in control women before pregnancy and at each trimester (P < 0.001; Table III and Fig. 2). Also, the overall dynamic for each measure was significantly different between the two groups (P interaction = 0.007 and 0.019, respectively).
Table III.
Comparison of the global evolution of the characteristics of the patients between the beginning of the pregnancy and the third trimester.
| N | PCOS N = 30 | N | Control N = 29 | P | P interaction | |
|---|---|---|---|---|---|---|
| Total AMH (pmol/l) | 0.007 | |||||
| T0 | 30 | 35.1 [22.9–53.7]a | 29 | 10.7 [6.8–16.8]a | <0.001a | |
| T1 | 29 | 30.0 [23.5–38.2]a | 29 | 11.2 [8.4–14.9]a | <0.001a | |
| T2 | 28 | 30.8 [23.7–39.9]a | 28 | 12.2 [8.9–16.7]a | <0.001a | |
| T3 | 26 | 28.9 [17.4–47.8]a | 27 | 11.2 [6.7–18.8]a | <0.001a | |
| Cleaved AMH (pmol/l) | 0.019 | |||||
| T0 | 14 | 23.7 [13.8–40.6]a | 24 | 6.8 [3.9–11.9]a | <0.001a | |
| T1 | 26 | 19.0 [13.9–25.8]a | 29 | 7.1 [5.0–10.0]a | <0.001a | |
| T2 | 28 | 18.5 [14.2–24.1]a | 28 | 7.1 [5.2–9.7]a | <0.001a | |
| T3 | 25 | 16.2 [9.4–27.8]a | 27 | 6.1 [3.5–10.4]a | <0.001a | |
| LH (IU/l) | 0.85 | |||||
| T0 | 28 | 1.95 [0.15–24.74]a | 17 | 1.19 [0.09–15.28]a | 0.026 a | |
| T1 | 29 | 0.34 [0.09–1.22]a | 29 | 0.25 [0.07–0.88]a | 0.90a | |
| T2 | 28 | 0.03 [0.01–0.09]a | 28 | 0.01 [0.00–0.06]a | 0.51a | |
| T3 | 26 | 0.08 [0.00–1.33]a | 27 | 0.05 [0.00–0.71]a | 1.00a | |
| Total T, TT (ng/ml) | 0.58 | |||||
| T0 | 27 | 0.43 [0.27–0.67]a | 16 | 0.33 [0.20–0.53]a | 0.10a | |
| T1 | 29 | 0.87 [0.67–1.15]a | 29 | 0.69 [0.51–0.93]a | 0.28a | |
| T2 | 28 | 0.79 [0.64–1.00]a | 28 | 0.53 [0.41–0.70]a | 0.017 a | |
| T3 | 26 | 0.76 [0.47–1.23]a | 27 | 0.50 [0.32–0.81]a | 0.034 a | |
| SHBG (nmol/l) | 0.29 | |||||
| T0 | 8 | 150.5 [−8.1–309.3] | 5 | 163.9 [5.5–322.4] | 1.00 | |
| T1 | 24 | 250.0 [155.3–344.7] | 29 | 289.6 [196.4–382.8] | 0.37 | |
| T2 | 28 | 381.0 [339.3–422.7] | 28 | 381.8 [336.5–427.2] | 1.00 | |
| T3 | 25 | 399.2 [274.7–523.7] | 25 | 416.2 [294.3–538.0] | 1.00 | |
| DHEAS (µmol/l) | <0.001 | |||||
| T0 | 8 | 3.35 [1.43–5.29] | 5 | 2.79 [0.82–4.77] | 1.00 | |
| T1 | 24 | 3.53 [2.21–4.86] | 29 | 3.93 [2.55–5.33] | 1.00 | |
| T2 | 28 | 3.42 [2.71–4.13] | 28 | 3.34 [2.45–4.23] | 1.00 | |
| T3 | 25 | 3.63 [2.25–5.03] | 25 | 3.57 [2.11–5.03] | 1.00 | |
| Androstenedione (ng/ml) | 0.20 | |||||
| T0 | 27 | 1.19 [0.66–2.15]a | 16 | 0.91 [0.49–1.70]a | 0.26a | |
| T1 | 27 | 2.14 [1.52–3.03]a | 28 | 1.94 [1.35–2.79]a | 1.00a | |
| T2 | 28 | 2.13 [1.65–2.77]a | 28 | 1.48 [1.11–1.98]a | 0.054a | |
| T3 | 25 | 2.37 [1.29–4.36]a | 27 | 1.47 [0.81–2.67]a | 0.025 a |
Values are presented as mean [CI 95%] adjusted. Values and P-values were adjusted for pre-specified confounding factors, namely age at the beginning of pregnancy, BMI at the beginning of pregnancy, week of gestation at each sampling time, and conception method (i.e. spontaneous, ovulation induction (OI) or ART). P values for each time were corrected by Bonferroni. P-values in bold refer to statistically significant values. aCalculated after log-transformation. NA: not applicable; P: P-value; N: number of available observations; T0: before pregnancy; T1: first trimester of pregnancy; T2: second trimester of pregnancy; T3: third trimester of pregnancy; AMH: anti-Müllerian hormone; PCOS: polycystic ovary syndrome; Cleaved AMH: (total AMH − proAMH Measurement); SHBG: sex hormone-binding globulin; DHEAS: dehydroepiandrosterone sulfate.
Figure 2.
Individual evolution of molecular forms of AMH throughout pregnancy in women with PCOS and controls. (A) Total AMH (pmol/l); (B) cleaved AMP (pmol/l) = (total AMH − pro AMH). Each plot in red (controls) or in green (PCOS) represent an individual measurement of AMH. For each time of the follow-up, median, Q1, Q3, minimum, and maximum are presented. PCOS: polycystic ovary syndrome; ***P < 0.001; P interaction: comparison of the evolution of hormones levels between women with PCOS and controls.
Estradiol, LH, and androgens levels throughout pregnancy
The estradiol levels increased throughout pregnancy and they were not significantly different between women with PCOS and controls at any time of the follow-up (P = 0.96) (respectively in PCOS and controls groups as mean (CI 95%), at T0: 40.5 pg/ml (34.0–48.3) vs 37.8 pg/ml (31.0–46.2), P = 1.0; at T1: 1406.0 pg/ml (1146.0–1724.1) vs 1381.8 pg/ml (1125.0–1696.1), P = 1.0; at T2: 7778.4 pg/ml (6596.0–9172.1) vs 7500.4 pg/ml (6361.0–8843.1), P = 1.0; at T3: 14 514.0 pg/ml (1227.0–1716.1) vs 13 476.0 pg/ml (1141.0–1591.1), p = 1.0). The overall decreasing trend of the LH levels was not different between the two groups but there was a significant difference before pregnancy with a higher level for women with PCOS (1.95, CI 95% 0.15 to 24.74 vs 1.19, CI 95% 0.09 to 15.28 IU/l; P = 0.026) (Table III). The frequency of non-measurable LH was not different between the women with PCOS and controls (64.3% vs 82.1% in the second trimester; P = 0.46 and 57.7% vs 66.7% in the third trimester; P = 1.00) (Table II).
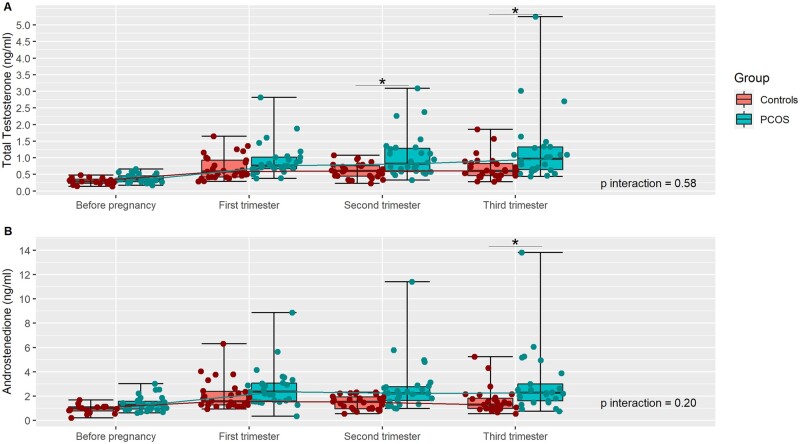
After adjustment, the TT level and the androstenedione level did not have an overall evolution significantly different between women with PCOS and controls (P interaction = 0.58 and 0.20, respectively). However, TT and androstenedione levels were higher in women with PCOS than in control women during the second and third trimester of pregnancy (Table III and Fig. 3). SHBG and albumin levels were not significantly different between the two groups at each time of the follow-up, expect albumin in the first trimester, which was higher in women with PCOS (Tables II and III). DHEAS levels were not significantly different between the two groups at each time of the follow-up, but the overall evolution was found to be significantly different (P < 0.001) (Table III).
Figure 3.
Individual evolution of androgens throughout pregnancy in women with PCOS and controls. (A) Total testosterone (ng/ml); (B) androstenedione (ng/ml). Each plot in red (controls) or in green (PCOS) represent an individual measurement of AMH. For each time of the follow-up, median, Q1, Q3, minimum, and maximum are presented. PCOS: polycystic ovary syndrome; *P < 0.05; P interaction: comparison of the evolution of hormones levels between women with PCOS and controls.
Evolution according to fetal sex and PCOS status
Among the 35 women carrying a boy, there were 16 patients with PCOS and 19 controls. Among the 21 women carrying a girl, there were 12 women with PCOS and 9 controls. Regardless of the fetal sex, AMH, cleaved AMH and androgen levels appeared to remain higher throughout pregnancy in the patients with PCOS (Supplementary Table SII).
Correlations
In the whole population, in the third trimester of pregnancy, only a significant but weak positive correlation between total AMH and androstenedione (r = 0.31; P = 0.023) was found (Table IV).
Table IV.
Spearman coefficient correlations (r) between molecular forms of AMH and hormonal and metabolic parameters in the whole population in the third trimester of pregnancy.
| Total AMH (pmol/l) |
Cleaved AMH (pmol/l) |
|||
|---|---|---|---|---|
| r | P | r | P | |
| BMI (kg/m²) | −0.22 | 0.13 | −0.12 | 0.42 |
| Total testosterone, TT (ng/ml) | 0.24 | 0.086 | 0.20 | 0.15 |
| Androstenedione (ng/ml) | 0.31 | 0.023 | 0.21 | 0.13 |
| Estradiol (pg/ml) | 0.08 | 0.59 | 0.10 | 0.47 |
| LH (IU/l) | 0.08 | 0.56 | 0.08 | 0.59 |
| Weight gain (kg) | −0.04 | 0.79 | 0.03 | 0.84 |
AMH: anti-Müllerian hormone; Cleaved AMH: (total AMH − proAMH Measurement); P: P-value. P-values in bold refer to statistically significant values.
Discussion
In this systematic prospective longitudinal monocentric comparative follow-up investigation of women with PCOS and controls from pre-conception to the third trimester of pregnancy, we have shown that serum levels of AMH are higher in women with PCOS as compared to controls before pregnancy and until the third trimester of gestation. Serum androgen levels are also higher in women with PCOS compared to controls before pregnancy and from the middle of gestation. The major strength of the current study is the inclusion of a pre-pregnancy time point which allows us to appreciate the full magnitude of the pregnancy-related change in circulating AMH.
These findings confirm and extend previous cross-sectional or partial longitudinal studies, showing that AMH levels are higher in patients with PCOS than in controls in the second trimester of pregnancy and at term (Köninger et al., 2018; Tata et al., 2018; Detti et al., 2019; Piltonen et al., 2019; Valdimarsdottir et al., 2019). However, cross-sectional studies of total AMH levels are statistically less powerful than longitudinal studies, due to the large inter-person variation in ovarian reserve. In addition, this is the first study exploring the molecular forms of circulating AMH during pregnancy in women with PCOS. As mentioned in the introduction, commercial AMH immunoassays, used in clinical practice, provide an aggregate measure of two AMH species (total AMH) since they do not distinguish between proAMH and AMHN,C. The goal of this study was thus to evaluate the evolution of total (mix of inactive and bioactive) and cleaved (bioactive) AMH in the circulation of patients before and throughout pregnancy. Our data provide compelling evidence that both total and cleaved AMH levels, remain significantly higher in PCOS women than in controls throughout pregnancy.
It is well known that AMH level decreases during gestation (Lutterodt et al., 2009; Nelson et al., 2010), a physiological situation of ovarian suppression, with a maximal decrease observed in the third trimester of pregnancy, followed by a gradual increase of AMH levels after delivery to return to pre-pregnancy values (Pankhurst et al., 2016, 2021; McCredie et al., 2017). In agreement with previous longitudinal studies in healthy women (Nelson et al., 2010; Köninger et al., 2015; Pankhurst et al., 2016), we observed a progressive decline in circulating AMH during pregnancy in control women. We detected a similar decline in total and cleaved AMH also in the population of women with PCOS included in this study. Mechanistically, it has been proposed (La Marca et al., 2013) that the increase in plasma volume during pregnancy leads to hemodilution of total AMH levels, although this could explain the initial decline in AMH levels but not that observed during late gestation, as the increase in plasma volume is nearly complete by the second trimester of pregnancy (Pivarnik et al., 1994; Faupel-Badger et al., 2007), while AMH levels continue to decline. One likely explanation for the pregnancy-related decline in AMH levels could be found in the significant changes in circulating hormones, such as LH, FSH, progesterone, prolactin, activin, and inhibin, which are also altered during pregnancy and that could impinge on the physiological state of the follicles and/or AMH synthesis. Moreover, inhibition of follicular recruitment due to placental estrogen and progesterone secretion has been postulated (Kuijper et al., 2013).
The sex of the fetuses was recorded in this study, as several research groups have postulated an effect of fetal sex on maternal AMH and androgen levels (Massagué, 2012; Caanen et al., 2016; Piltonen et al., 2019), while others have not found this association (Vanky and Carlsen, 2012; Detti et al., 2019). In our study, no gender-related difference in maternal AMH levels was found. However, these conclusions need to be taken with caution since our sample size was not large enough to prove this point statistically.
Our data showing that higher bioactive AMH levels are present in women with PCOS as compared to controls throughout pregnancy strengthen our previous preclinical findings suggesting that prenatal exposure to an AMH-enriched environment could predispose the offspring to develop PCOS in later life, through changes in DNA methylation landmarks and in hypothalamic circuits regulating reproduction and metabolism (Tata et al., 2018; Mimouni et al., 2021). Indeed, we previously demonstrated that AMH has extragonadal functions with AMHR2 being shown to regulate GnRH neuron activity and LH pulsatility, contributing to high LH levels in mice (Cimino et al., 2016). Here, we found a significant increase in LH levels in women with PCOS as compared to controls before pregnancy, further substantiating the neuroendocrine alterations typical of the syndrome (Rebar et al., 1976), but not during pregnancy, when LH levels drop to almost undetectable levels in all women; hence, there is no evidence that AMH induces maternal LH secretion during human pregnancy.
We recently suggested a possible role for AMH in transgenerational PCOS pathogenesis because high gestational AMH levels in mice resulted in a hyperandrogenic and metabolic PCOS-like phenotype in adult female offspring (Tata et al., 2018; Mimouni et al., 2021). Mechanistically, our previous preclinical studies (Tata et al., 2018) demonstrated Amhr2 expression in the placenta and decreased placental aromatase expression in mice exposed to high AMH levels during late pregnancy, resulting in high androgen levels in the dams and in female offspring in adulthood. Importantly, AMHR2 is also expressed in the human placenta (Novembri et al., 2015). Despite the absence of modification in the expression of the aromatase (Maliqueo et al., 2015), its activity has been reported to be decreased in placentas of women with PCOS as compared to controls (Maliqueo et al., 2013). Based on this evidence, we can speculate that the excessive androgen levels in pregnant women with PCOS in the second and third trimesters result from placental aromatase activity inhibition by excessive AMH levels. At the fetus level, this aromatase defect may lead to even greater androgen excess, leading to epigenetic reprogramming, especially during the critical period of the second trimester, as shown by animal studies (Risal et al., 2019; Mimouni et al., 2021).
The hypothesis that higher AMH levels in utero could be at the origin of the maternal hyperandrogenism in women with PCOS is reinforced by previous studies showing a positive correlation between AMH and androgens (TT and androstenedione) in the serum of pregnant women in the second trimester of pregnancy and at term (Piltonen et al., 2019; Valdimarsdottir et al., 2019). In this work, we also found that in our study population (comprising both control and PCOS women), there was a significant but small positive correlation between total AMH and androstenedione, which was detectable during the third trimester of pregnancy (r = 0.31; P = 0.023).
In light of these findings, it is possible that a link between high AMH levels in utero and gestational hyperandrogenism exists in some women, although future studies are warranted to investigate the role of AMH in placental function in humans.
Since the late 1990s, it has been shown that exposure to androgen excess in different animal models consistently replicates a wide range of PCOS symptoms observed in humans; this has provided strong evidence that hyperandrogenism is a major driver in PCOS pathogenesis (Dumesic et al., 1997; Abbott et al., 1998; Stener-Victorin et al., 2020). In agreement with this, previous clinical studies have reported higher circulating levels of androgens in women with PCOS than in control women during pregnancy (Sir-Petermann et al., 2002; Maliqueo et al., 2013, 2015; Glintborg et al., 2018; Piltonen et al., 2019; Sun et al., 2020). However, these studies were either cross-sectional or only partially longitudinal. Moreover, human studies attempting to find evidence of a link between circulating levels of androgens in the child at birth and in the mother with PCOS have provided conflicting results since some cord blood androgen levels from female infants of women with PCOS have been reported to be increased (Barry et al., 2010; Mehrabian and Kelishadi, 2012; Daan et al., 2017; Sun et al., 2020), decreased (Maliqueo et al., 2013; Duan et al., 2020), or unchanged (Hickey et al., 2009; Caanen et al., 2016; Detti et al., 2019; Duan et al., 2020). However, the circulating levels of steroids in children at birth are not very representative of what happens in utero because of the inactive gonadotropic axis of the fetus at birth. The analysis of amniotic fluid during pregnancy is the best way to evaluate steroid secretion in the fetus. No correlation was found between the levels of androgens in the amniotic fluid in the second trimester of pregnancy and the levels of androgens in the cord blood at birth (van de Beek et al., 2004). The only study that measured testosterone in the amniotic fluid of daughters of women with PCOS showed that it was increased compared to control women (Palomba et al., 2012).
To our knowledge, our study is the first longitudinal investigation of the evolution of androgen levels (TT, DHEAS, and androstenedione) in women with and without PCOS from a pre-pregnancy state to the third trimester of pregnancy. We report that women with PCOS have higher levels of TT and androstenedione than control women from mid-gestation.
Our study has some limitations, including the small population sample and the paucity of information regarding the incidence of gestational diabetes which could favor maternal hyperandrogenism, although, during the first trimester of pregnancy, fasting blood glucose did not differ between our two groups. In addition, most women included in the study were women whose pregnancies were the result of ovarian stimulation and/or ART treatments, which could introduce a bias in the interpretation of hormonal measurements. However, this applies for both groups of women who underwent this procedure and we controlled our results as a function of the type of conception (i.e. spontaneous, OI or ART). Lastly, we could not measure serum free testosterone routinely because of the complexity of the method, nor by calculation since the standard calculation formula of free testosterone based on TT, albumin, and SHBG levels is not applicable to pregnant women (Vermeulen et al., 1999).
Another hormone that we could not measure is kisspeptin, because we did not collect our samples specifically for this kind of measurement, which requires a standardized sample collection method to control all preanalytical variables such as collection tube type, processing times, and storage conditions (Ramachandran et al., 2008). Kisspeptin levels have been documented to rise with gestational age during pregnancy, but relatively low circulating levels of kisspeptin have been seen in pregnancies complicated by preeclampsia compared to normal pregnancies, showing an association of low levels of plasma kisspeptin with increased risks of miscarriage (Horikoshi et al., 2003; Jayasena et al., 2014; Matjila et al., 2016). Despite the vast body of literature on PCOS endocrinology, no studies have focused so far on kisspeptin secretion in women with PCOS during pregnancy. Considering the putative pathophysiological implications of kisspeptin in PCOS (Witchel and Tena-Sempere, 2013), it would be thus of interest to assess kisspeptin levels in future longitudinal investigations of pregnant women with PCOS.
To conclude, in this first longitudinal follow-up investigation throughout pregnancy of women with and without PCOS, we found that total AMH and cleaved-bioactive AMH remained higher in women with PCOS than in controls, as did androgen levels from mid-pregnancy onward. More evidence in humans is needed to demonstrate whether AMH has a causative role in modulating steroidogenesis in the placenta of female fetuses that will eventually develop PCOS, but these data strengthen the hypothesis of a potential implication of aberrant hormonal signaling in the womb in the etiology of PCOS.
Supplementary Material
Acknowledgements
We would like to thank Lydie Lombardo, Sylvie Vanoverschele, and all the technical staff of the Department of Biochemistry and Hormonology for their precious help in collecting and processing samples.
Contributor Information
M Peigné, Laboratory of Development and Plasticity of the Neuroendocrine Brain, University of Lille, Inserm, CHU Lille, Lille Neuroscience and Cognition, UMR-S 1172, Lille, France; Department of Reproductive Medicine and Fertility Preservation, AP-HP—Université Sorbonne Paris-Nord, Jean Verdier Hospital, Bondy, France; Department of Medical Gynecology, CHU Lille, Jeanne de Flandre Hospital, Lille, France.
V Simon, Laboratory of Development and Plasticity of the Neuroendocrine Brain, University of Lille, Inserm, CHU Lille, Lille Neuroscience and Cognition, UMR-S 1172, Lille, France; Department of Medical Gynecology, CHU Lille, Jeanne de Flandre Hospital, Lille, France.
P Pigny, Department of Biochemistry and Hormonology, CHU Lille, Centre de Biologie Pathologie, Lille, France.
N E H Mimouni, Laboratory of Development and Plasticity of the Neuroendocrine Brain, University of Lille, Inserm, CHU Lille, Lille Neuroscience and Cognition, UMR-S 1172, Lille, France.
C Martin, Department of Biostatistics, CHU Lille, Lille, France.
D Dewailly, Laboratory of Development and Plasticity of the Neuroendocrine Brain, University of Lille, Inserm, CHU Lille, Lille Neuroscience and Cognition, UMR-S 1172, Lille, France; Department of Medical Gynecology, CHU Lille, Jeanne de Flandre Hospital, Lille, France.
S Catteau-Jonard, Laboratory of Development and Plasticity of the Neuroendocrine Brain, University of Lille, Inserm, CHU Lille, Lille Neuroscience and Cognition, UMR-S 1172, Lille, France; Department of Medical Gynecology, CHU Lille, Jeanne de Flandre Hospital, Lille, France.
P Giacobini, Laboratory of Development and Plasticity of the Neuroendocrine Brain, University of Lille, Inserm, CHU Lille, Lille Neuroscience and Cognition, UMR-S 1172, Lille, France.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Authors’ roles
M.P., S.C.-J., D.D., and P.G. designed research; M.P., V.S., and N.E.H.M. performed research; P.P. contributed with analytic tools and analysis; C.M. performed biostatistical analyses; M.P., S.C.-J., and P.G analyzed the data; and M.P., S.C.-J., and P.G. wrote the paper.
Funding
This work was supported by INSERM, France (grant number U1172) and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (ERC-2016-CoG to P.G., Grant Agreement No. 725149/REPRODAMH).
Conflict of interest
The authors have nothing to declare.
References
- Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW.. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab 1998;9:62–67. [DOI] [PubMed] [Google Scholar]
- Barry JA, Kay AR, Navaratnarajah R, Iqbal S, Bamfo JE, David AL, Hines M, Hardiman PJ.. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Inst Obstet Gynaecol 2010;30:444–446. [DOI] [PubMed] [Google Scholar]
- Caanen MR, Kuijper EA, Hompes PG, Kushnir MM, Rockwood AL, Meikle WA, Homburg R, Lambalk CB.. Mass spectrometry methods measured androgen and estrogen concentrations during pregnancy and in newborns of mothers with polycystic ovary syndrome. Eur J Endocrinol 2016;174:25–32. [DOI] [PubMed] [Google Scholar]
- Cimino I, Casoni F, Liu X, Messina A, Parkash J, Jamin SP, Catteau-Jonard S, Collier F, Baroncini M, Dewailly D. et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun 2016;7:10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- Cohen J. The statistical power of abnormal-social psychological research: a review. J Abnorm Soc Psychol 1962;65:145–153. [DOI] [PubMed] [Google Scholar]
- Cook CL, Siow Y, Brenner AG, Fallat ME.. Relationship between serum müllerian-inhibiting substance and other reproductive hormones in untreated women with polycystic ovary syndrome and normal women. Fertil Steril 2002;77:141–146. [DOI] [PubMed] [Google Scholar]
- Daan NMP, Koster MPH, Steegers-Theunissen RP, Eijkemans MJC, Fauser BCJM.. Endocrine and cardiometabolic cord blood characteristics of offspring born to mothers with and without polycystic ovary syndrome. Fertil Steril 2017;107:261–268.e3. [DOI] [PubMed] [Google Scholar]
- Dapas M, Dunaif A.. Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr Rev 2022;43:927–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detti L, Christiansen ME, Francillon L, Ikuwezunma G, Diamond MP, Mari G, Tobiasz AM.. Serum anti-Müllerian hormone (AMH) in mothers with polycystic ovary syndrome (PCOS) and their term fetuses. Syst Biol Reprod Med 2019;65:147–154. [DOI] [PubMed] [Google Scholar]
- Dewailly D, Barbotin A-L, Dumont A, Catteau-Jonard S, Robin G.. Role of anti-Müllerian hormone in the pathogenesis of polycystic ovary syndrome. Front Endocrinol 2020;11:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, Duhamel A, Catteau-Jonard S.. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod 2011;26:3123–3129. [DOI] [PubMed] [Google Scholar]
- di Clemente N, Jamin SP, Lugovskoy A, Carmillo P, Ehrenfels C, Picard J-Y, Whitty A, Josso N, Pepinsky RB, Cate RL.. Processing of anti-mullerian hormone regulates receptor activation by a mechanism distinct from TGF-beta. Mol Endocrinol 2010;24:2193–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C, Pei T, Li Y, Cao Q, Chen H, Fu J.. Androgen levels in the fetal cord blood of children born to women with polycystic ovary syndrome: a meta-analysis. Reprod Biol Endocrinol 2020;18:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Eisner JR, Goy RW.. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril 1997;67:155–163. [DOI] [PubMed] [Google Scholar]
- Faupel-Badger JM, Hsieh C-C, Troisi R, Lagiou P, Potischman N.. Plasma volume expansion in pregnancy: implications for biomarkers in population studies. Cancer Epidemiol Biomark 2007;16:1720–1723. [DOI] [PubMed] [Google Scholar]
- Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JSE. et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 2012;97:28–38.e25. [DOI] [PubMed] [Google Scholar]
- Glintborg D, Jensen RC, Bentsen K, Schmedes AV, Brandslund I, Kyhl HB, Bilenberg N, Andersen MS.. Testosterone levels in third trimester in polycystic ovary syndrome: odense child cohort. J Clin Endocrinol Metab 2018;103:3819–3827. [DOI] [PubMed] [Google Scholar]
- Gomez JMD, VanHise K, Stachenfeld N, Chan JL, Merz NB, Shufelt C.. Subclinical cardiovascular disease and polycystic ovary syndrome. Fertil Steril 2022;117:912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan C, Zahid S, Minhas AS, Ouyang P, Vaught A, Baker VL, Michos ED.. Polycystic ovary syndrome: a “risk-enhancing” factor for cardiovascular disease. Fertil Steril 2022;117:924–935. [DOI] [PubMed] [Google Scholar]
- Hickey M, Sloboda DM, Atkinson HC, Doherty DA, Franks S, Norman RJ, Newnham JP, Hart R.. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab 2009;94:3714–3720. [DOI] [PubMed] [Google Scholar]
- Homburg R, Crawford G.. The role of AMH in anovulation associated with PCOS: a hypothesis. Hum Reprod 2014;29:1117–1121. [DOI] [PubMed] [Google Scholar]
- Homburg R. Androgen circle of polycystic ovary syndrome. Hum Reprod 2009;24:1548–1555. [DOI] [PubMed] [Google Scholar]
- Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, Fujino M.. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab 2003;88:914–919. [DOI] [PubMed] [Google Scholar]
- Jayasena CN, Abbara A, Izzi-Engbeaya C, Comninos AN, Harvey RA, Gonzalez Maffe J, Sarang Z, Ganiyu-Dada Z, Padilha AI, Dhanjal M. et al. Reduced levels of plasma kisspeptin during the antenatal booking visit are associated with increased risk of miscarriage. J Clin Endocrinol Metab 2014;99:E2652–E2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, Boyle J, Teede HJ.. Polycystic ovary syndrome. Lancet Diabetes Endocrinol 2022;10:668–680. [DOI] [PubMed] [Google Scholar]
- Köninger A, Kampmeier A, Schmidt B, Frank M, Strowitzki T, Kimmig R, Gellhaus A, Mach P.. Trends in anti-Müllerian hormone concentrations across different stages of pregnancy in women with polycystic ovary syndrome. Reprod Biomed Online 2018;37:367–374. [DOI] [PubMed] [Google Scholar]
- Köninger A, Schmidt B, Mach P, Damaske D, Nießen S, Kimmig R, Strowitzki T, Gellhaus A.. Anti-Mullerian-hormone during pregnancy and peripartum using the new Beckman Coulter AMH Gen II Assay. Reprod Biol Endocrinol 2015;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper EA, Ket JC, Caanen MR, Lambalk CB.. Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reprod Biomed Online 2013;27:33–63. [DOI] [PubMed] [Google Scholar]
- La Marca A, Grisendi V, Griesinger G.. How much does AMH really vary in normal women? Int J Endocrinol 2013;2013:959487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterodt M, Byskov AG, Skouby SO, Tabor A, Yding Andersen C.. Anti-Müllerian hormone in pregnant women in relation to other hormones, fetal sex and in circulation of second trimester fetuses. Reprod Biomed Online 2009;18:694–699. [DOI] [PubMed] [Google Scholar]
- Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T.. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2013;166:151–155. [DOI] [PubMed] [Google Scholar]
- Maliqueo M, Sundström Poromaa I, Vanky E, Fornes R, Benrick A, Åkerud H, Stridsklev S, Labrie F, Jansson T, Stener-Victorin E.. Placental STAT3 signaling is activated in women with polycystic ovary syndrome. Hum Reprod 2015;30:692–700. [DOI] [PubMed] [Google Scholar]
- Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol 2012;13:616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matjila M, Millar R, van der Spuy Z, Katz A.. Elevated placental expression at the maternal-fetal interface but diminished maternal circulatory kisspeptin in preeclamptic pregnancies. Pregnancy Hypertens 2016;6:79–87. [DOI] [PubMed] [Google Scholar]
- McCredie S, Ledger W, Venetis CA.. Anti-Müllerian hormone kinetics in pregnancy and post-partum: a systematic review. Reprod Biomed Online 2017;34:522–533. [DOI] [PubMed] [Google Scholar]
- Mehrabian F, Kelishadi R.. Comparison of the metabolic parameters and androgen level of umbilical cord blood in newborns of mothers with polycystic ovary syndrome and controls. J Res Med Sci 2012;17:207–211. [PMC free article] [PubMed] [Google Scholar]
- Mimouni NEH, Paiva I, Barbotin A-L, Timzoura FE, Plassard D, Gras SL, Ternier G, Pigny P, Catteau-Jonard S, Simon V. et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab 2021;33:513–530.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtigal MW, Ingraham HA.. Bioactivation of Müllerian inhibiting substance during gonadal development by a kex2/subtilisin-like endoprotease. Proc Natl Acad Sci USA 1996;93:7711–7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Stewart F, Fleming R, Freeman DJ.. Longitudinal assessment of antimüllerian hormone during pregnancy-relationship with maternal adiposity, insulin, and adiponectin. Fertil Steril 2010;93:1356–1358. [DOI] [PubMed] [Google Scholar]
- Novembri R, Funghi L, Voltolini C, Belmonte G, Vannuccini S, Torricelli M, Petraglia F.. Placenta expresses anti-Müllerian hormone and its receptor: sex-related difference in fetal membranes. Placenta 2015;36:731–737. [DOI] [PubMed] [Google Scholar]
- Palomba S, Marotta R, Di Cello A, Russo T, Falbo A, Orio F, Tolino A, Zullo F, Esposito R, La Sala GB. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol (Oxford) 2012;77:898–904. [DOI] [PubMed] [Google Scholar]
- Pankhurst MW, Clark CA, Zarek J, Laskin CA, McLennan IS.. Changes in circulating ProAMH and total AMH during healthy pregnancy and post-partum: a longitudinal study. PLoS One 2016;11:e0162509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst MW, de Kat AC, Jones S, Broekmans FJM, Wheeler BJ.. Serum anti-Müllerian hormone levels in women are unstable in the postpartum period but return to normal within 5 months: a longitudinal study. Endocrine 2021;71:225–232. [DOI] [PubMed] [Google Scholar]
- Pankhurst MW, McLennan IS.. Human blood contains both the uncleaved precursor of anti-Mullerian hormone and a complex of the NH2- and COOH-terminal peptides. Am J Physiol Endocrinol Metab 2013;305:E1241–E1247. [DOI] [PubMed] [Google Scholar]
- Peigné M, Pigny P, Pankhurst MW, Drumez E, Loyens A, Dewailly D, Catteau-Jonard S, Giacobini P.. The proportion of cleaved anti-Müllerian hormone is higher in serum but not follicular fluid of obese women independently of polycystic ovary syndrome. Reprod Biomed Online 2020;41:1112–1121. [DOI] [PubMed] [Google Scholar]
- Pepinsky RB, Sinclair LK, Chow EP, Mattaliano RJ, Manganaro TF, Donahoe PK, Cate RL.. Proteolytic processing of mullerian inhibiting substance produces a transforming growth factor-beta-like fragment. J Biol Chem 1988;263:18961–18964. [PubMed] [Google Scholar]
- Pigny P, Gorisse E, Ghulam A, Robin G, Catteau-Jonard S, Duhamel A, Dewailly D.. Comparative assessment of five serum antimüllerian hormone assays for the diagnosis of polycystic ovary syndrome. Fertil Steril 2016;105:1063–1069.e3. [DOI] [PubMed] [Google Scholar]
- Pigny P, Jonard S, Robert Y, Dewailly D.. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab 2006;91:941–945. [DOI] [PubMed] [Google Scholar]
- Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S, Dewailly D.. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab 2003;88:5957–5962. [DOI] [PubMed] [Google Scholar]
- Piltonen TT, Giacobini P, Edvinsson Å, Hustad S, Lager S, Morin-Papunen L, Tapanainen JS, Sundström-Poromaa I, Arffman RK.. Circulating antimüllerian hormone and steroid hormone levels remain high in pregnant women with polycystic ovary syndrome at term. Fertil Steril 2019;111:588–596.e1. [DOI] [PubMed] [Google Scholar]
- Pivarnik JM, Mauer MB, Ayres NA, Kirshon B, Dildy GA, Cotton DB.. Effects of chronic exercise on blood volume expansion and hematologic indices during pregnancy. Obstet Gynecol 1994;83:265–269. [PubMed] [Google Scholar]
- Ramachandran R, Patterson M, Murphy KG, Dhillo WS, Patel S, Kazarian A, Ghatei MA, Bloom SR.. Preanalytical factors affecting RIA measurement of plasma kisspeptin. Clin Chem 2008;54:615–617. [DOI] [PubMed] [Google Scholar]
- Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F.. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest 1976;57:1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui H-P, Zhao Z, Massart J, Ohlsson C, Lindgren E. et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med 2019;25:1894–1904. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19:41–47. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE.. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod 2002;17:2573–2579. [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Padmanabhan V, Walters KA, Campbell RE, Benrick A, Giacobini P, Dumesic DA, Abbott DH.. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev 2020;41:bnaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Sun B, Qiao S, Feng X, Li Y, Zhang S, Lin Y, Hou L.. Elevated maternal androgen is associated with dysfunctional placenta and lipid disorder in newborns of mothers with polycystic ovary syndrome. Fertil Steril 2020;113:1275–1285.e2. [DOI] [PubMed] [Google Scholar]
- Tata B, Mimouni NEH, Barbotin A-L, Malone SA, Loyens A, Pigny P, Dewailly D, Catteau-Jonard S, Sundström-Poromaa I, Piltonen TT. et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med 2018;24:834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, Andersen M, Azziz R. et al. ; International PCOS Network. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33:1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdimarsdottir R, Valgeirsdottir H, Wikström A-K, Kallak TK, Elenis E, Axelsson O, Ubhayasekhera K, Bergquist J, Piltonen TT, Pigny P. et al. Pregnancy and neonatal complications in women with polycystic ovary syndrome in relation to second-trimester anti-Müllerian hormone levels. Reprod Biomed Online 2019;39:141–148. [DOI] [PubMed] [Google Scholar]
- van de Beek C, Thijssen JHH, Cohen-Kettenis PT, van Goozen SHM, Buitelaar JK. Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormonal exposure? Horm Behav 2004;46:663–669. [DOI] [PubMed] [Google Scholar]
- Vanky E, Carlsen SM.. Androgens and antimüllerian hormone in mothers with polycystic ovary syndrome and their newborns. Fertil Steril 2012;97:509–515.e1. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM.. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672. [DOI] [PubMed] [Google Scholar]
- Witchel SF, Tena-Sempere M.. The Kiss1 system and polycystic ovary syndrome: lessons from physiology and putative pathophysiologic implications. Fertil Steril 2013;100:12–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.