Abstract
Background
The incidence of atrial fibrillation (AF) associated with anticancer drugs in cancer patients remains incompletely defined.
Objectives
The primary outcome was the annualized incidence rate of AF reporting associated with exposure to 1 of 19 anticancer drugs used as monotherapy in clinical trials. The authors also report the annualized incidence rate of AF reported in the placebo arms of these trials.
Methods
The authors systematically searched ClinicalTrials.gov for phase 2 and 3 cancer trials studying 19 different anticancer drugs of interest used as monotherapy, up to September 18, 2020. The authors performed a random-effects meta-analysis to compute summary AF annualized incidence rate with its 95% CI using log transformation and inverse variance weighting.
Results
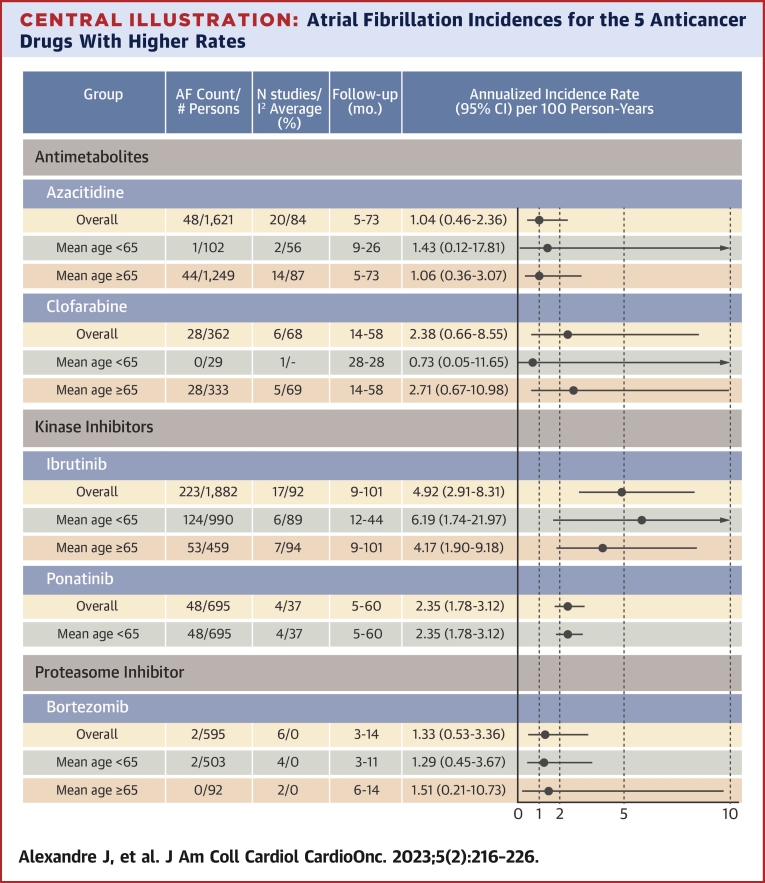
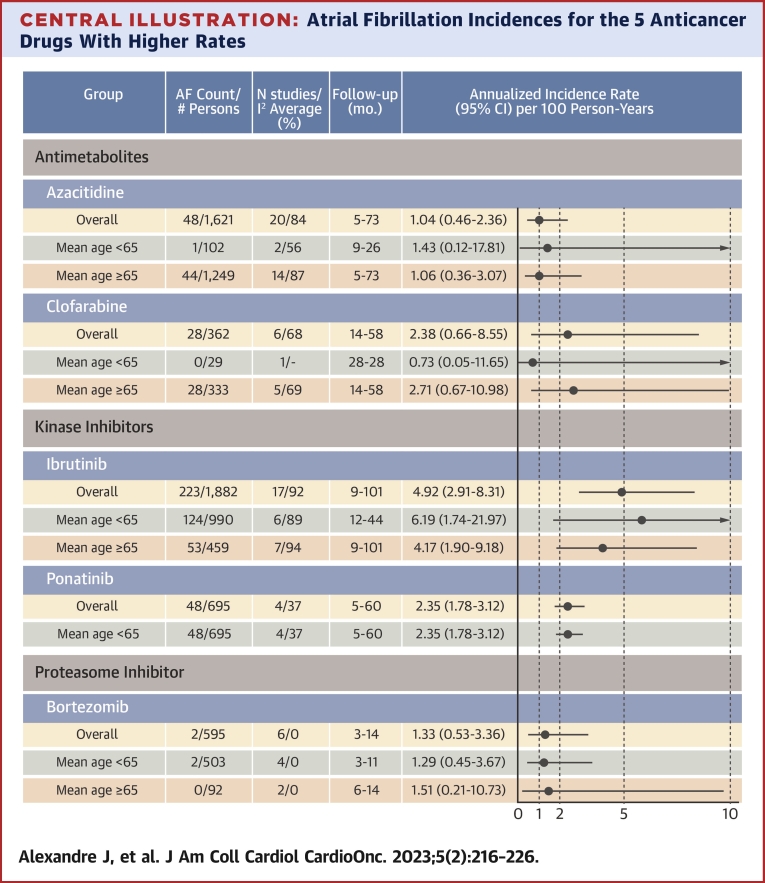
A total of 191 clinical trials (47.1% were randomized) of 16 anticancer drugs across 26,604 patients were included. Incidence rates could be calculated for 15 drugs administered singly as monotherapy. Summary annualized incidence rates of AF reporting associated with exposure to 1 of the 15 anticancer drugs used as monotherapy were derived; these ranged from 0.26 to 4.92 per 100 person-years. The 3 highest annualized incidence rates of AF reporting were found for ibrutinib 4.92 (95% CI: 2.91-8.31), clofarabine 2.38 (95% CI: 0.66-8.55), and ponatinib 2.35 (95% CI: 1.78-3.12) per 100 person-years. Summary annualized incidence rate of AF reporting in the placebo arms was 0.25 per 100 person-years (95% CI: 0.10-0.65).
Conclusions
AF reporting is not a rare event associated with anticancer drugs in clinical trials. A systematic and standardized AF detection should be considered in oncological trials, particularly those studying anticancer drugs associated with high AF rates. (Incidence of atrial fibrillation associated with anticancer drugs exposure in monotherapy, A safety meta-analysis of phase 2 and 3 clinical trials; CRD42020223710)
Key Words: anticancer drugs, atrial fibrillation, cancer, safety meta-analysis
Central Illustration
Atrial fibrillation (AF) affects over 33 million individuals worldwide,1 and AF prevalence in the United States is estimated to rise from ∼5.2 million in 2010 to 12.1 million in 2030.2 During the first 90 days after cancer diagnosis, patients with active cancer are more likely to have incident AF than those without cancer.3 During follow-up, cancer patients have an approximately 47% higher risk of AF compared with those without cancer.4 The risk of AF is increased by many factors such as systemic inflammation, immune dysregulation, electrolyte fluctuations, impaired oxygenation, electrolyte or endocrine abnormalities, and higher cancer stages at diagnosis.5, 6, 7 Most of these AF risk factors are also modulated by anticancer drugs, either directly or indirectly (adiposity from steroid use and hypertension from kinase or VEGF inhibitor use),5,8 and therefore, anticancer drugs may result in an increased risk of incident AF in cancer patients.9,10 Recently, incident AF occurring within 30 days after breast cancer diagnosis, and not AF before cancer diagnosis, was associated with a significant increase in both all-cause (adjusted HR [aHR]: 2.15; 95% CI: 1.32-3.48) and cardiovascular (aHR: 3.00; 95% CI: 1.28-7.00) mortality at year 1.7
In oncological trials with a special attention to reporting cardiovascular adverse events (CVAEs), AF reporting represents 4.8% of all CVAEs.11 Unfortunately, most oncological trials only identify and report AF in severe cases requiring immediate medical attention.12 Because 87% of AF patients will never experience any AF-related symptoms, and AF episodes are usually short-lasting, an absence of continuous and rigorous rhythm monitoring likely leads to a significant underestimation of AF incidence in oncological trials.13 AF in cancer patients is becoming a major issue in cardio-oncology, and its incidence is expected to increase in the next years, as the number of living Americans with a history of cancer is anticipated to rise up to 22.1 million persons in 2030.14
Recently, our group highlighted significant associations between AF reporting and 19 anticancer drugs (abiraterone, aldesleukin, azacitidine, bortezomib, cisplatin, clofarabine, dacarbazine, daunorubicin, docetaxel, ibrutinib, idarubicin, ipilimumab, lenalidomide, midostaurin, nilotinib, obinutuzumab, pomalidomide, ponatinib, and rituximab) in the World Health Organization spontaneous reporting pharmacovigilance database VigiBase,15 however, the incidence of AF associated with these therapies remains unknown.16 The aim of this study was to estimate the annualized incidence rate of AF reporting associated with exposure to 1 of 19 anticancer drugs used as monotherapy in clinical trials, using a safety meta-analysis of cancer clinical trials.
Methods
Registration
The study protocol was prospectively registered to the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020223710). No ethics committee approval and subject informed consent were sought because this was a retrospective analysis of publicly available data. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist is available in Supplemental Table 1.17
Data source
ClinicalTrials.gov is the largest clinical trial registry website, holding registrations from over 353,000 trials from 209 countries since 1997. ClinicalTrials.gov provides easy access to data on publicly and privately supported clinical studies on a wide range of diseases and conditions. It contains information about publicly and privately funded clinical trials, the purpose of each experimental drug, subject eligibility criteria to participate in the clinical trial, the location of clinical trial sites being used for a study, and a point of contact for patients interested in enrolling in the trial. The 2007 U.S. Food and Drug Administration (FDA) Amendments Act required clinical trials of all FDA-approved drugs and biologics to post results at ClinicalTrials.gov within 1 year of trial completion.18 It was previously showed that trial results, particularly safety results, were more comprehensively reported at ClinicalTrials.gov than in journal publications.19,20 The ClinicalTrials.gov registry thus provides a unique opportunity to study safety outcomes. The extraction and analysis of the ClinicalTrials.gov results database was previously described and validated to estimate the risk and incidence of adverse events (AEs).21,22 Results are generally submitted to the website by the responsible party (sponsor or principal investigator) soon after the completion of the study, and results can be updated over time. Once submitted, the results go through a quality assurance process and are reviewed by a ClinicalTrials.gov staff member who focuses on internal consistency and logic.
In the ClinicalTrials.gov results database, all AEs are classified according to the Cancer Therapy Evaluation Program Adverse Event Reporting System definition (CTEP-AERS, U.S. National Institutes of Health).
Search strategy
For each of the 19 anticancer drugs of interest in monotherapy,15 we searched ClinicalTrials.gov up to September 18, 2020, for cancer trials with safety results posted by using the anticancer drug name as the sole domain including both the specific drug name and all drug synonyms and alternative names. Terms related to each of the 19 anticancer drugs are listed in Supplemental Table 2.
Study selection and data extraction
Data extraction was performed by 2 reviewers (L.B. and J.A.) independently. A third reviewer (C.D.) was consulted in case of disagreements. Phase 2 and 3 cancer trials with available safety results (including AF), available follow-up duration, and having at least 1 arm studying the exposure of 1 of the 19 anticancer drugs in monotherapy in adult cancer patients were eligible for inclusion. Studies in which patients were not allocated to any monotherapy arm of 1 of the 19 anticancer drugs of interest were excluded. Case reports or case series, case-control (nested) studies, and observational studies (retrospective or prospective) were excluded. Because AF reporting was presumed to be a rare AE, studies with arms that accrued fewer than 20 patients were not retained to provide relevant data to compute AF reporting summary incidence. All available AF cases classified according to the CTEP-AERS in eligible trials reported on the ClinicalTrials.gov website were extracted. Additional data from eligible studies were collected, including median age (years), intervention model, maximum follow-up time frame (months), year of the study, and the type of the control drug (placebo or other anticancer drug) in presence of control groups. Among the trials included in our safety meta-analysis, we also extracted all AF cases in control arms treated by placebo or a monotherapy anticancer drug not belonging to the list of the 19 anticancer drugs of interest. Among the phase 2 and 3 clinical studies eligible for the meta-analysis, we searched for studies having a placebo control arm, and we extracted AF cases reported in these placebo arms.
Risk of bias and quality assessment
Two authors (P.-M.M. and J.A.) evaluated the risk of bias in individual studies using the Pharmacoepidemiological Research on Outcomes of Therapeutics by a European Consortium (PROTECT) checklist tool specially designed to assess bias in safety meta-analyses.23 A final summary risk of bias assessment was asked for the whole study, and judgments could be low, high, or unclear. Bias domains that could not be completed owing to insufficient data were classified as “unclear risk.” In case of disagreements, a third author (C.D.) was consulted. Publication bias was assessed graphically by constructing a funnel plot based on the standard error of logit transformed proportion against the logit transformed proportion.
Outcomes
The primary outcome was the annualized incidence rate of AF reporting associated with exposure to 1 of 19 anticancer drugs15 used as monotherapy in clinical trials. We also report the annualized incidence rate of AF reported in the placebo arms of these trials. In addition, we performed post hoc subgroup analyses according to cancer site. Because September 2009 was the mandatory date of AE registration in ClinicalTrials.gov, we performed a post hoc subgroup analysis based on this date.
Statistical analysis
We conducted a random-effects meta-analysis to compute the summary annualized incidence rate with its 95% CI. The summary annualized incidence rate of AF reporting per 100 observations associated with anticancer drugs was computed using the log transformation and inverse variance weighting. The person–time estimator was defined as the mean/median time frame multiplied by the number of patients, at study level, because it was assumed the follow-up for AF was identical to the study follow-up in most cases. We assessed between-study heterogeneity using the inconsistency index I2, t2, and Cochran's Q test. Substantial between-study heterogeneity was defined by an I2 value >50%, and significant heterogeneity was defined by a P value <0.10 for Cochran's Q test. The t2 statistic was used to measure the variance of true effect sizes across studies.24 Time frames were expressed as mean/median [range]. Post hoc subgroup analyses on cancer site and the mandatory date of AE registration in ClinicalTrials.gov were performed using Z-test. Data management and meta-analysis of proportions and incidence rates were performed with R v4.2.1 (R Core Team) and the R package meta and presented as forest plots.25 A 2-sided P value <0·05 was considered statistically significant.
Results
Description of included studies
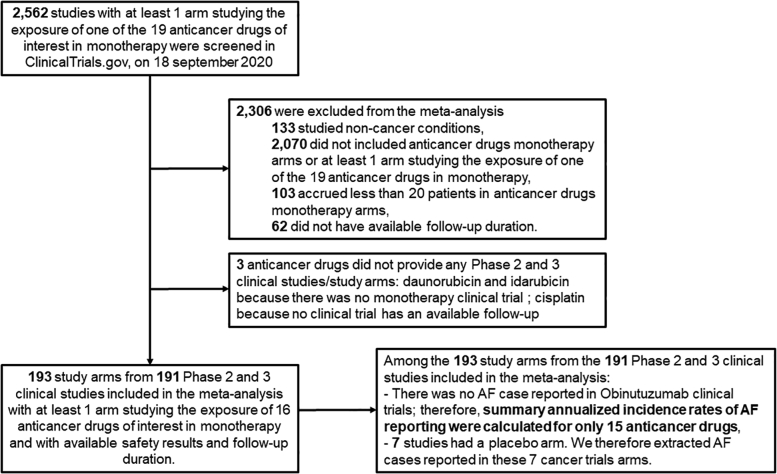
The flow diagram of study selection is presented in Figure 1. Of 2,562 studies screened, 191 (7.4%, 26,604 patients) were included in the main analysis after application of selection criteria, of which 2 were eligible for 2 anticancer drugs (Supplemental Tables 3 and 4). Cancer sites in the included trials are presented in Figure 2. Hematologic malignancies were over-represented compared with solid malignancies. Among the 191 included studies, 90 were randomized controlled trials (90/191, or 47.1%). Across studies, patients’ mean age ranged from 7.5 to 78.0 years, and the proportion of women ranged from 0% to 100%. Follow-up duration ranged from 2.7 to 138 months (Table 1). Among the 191 included studies, we identified 7 placebo arms (Supplemental Table 5) including 3 different types of malignancies (4 myelodysplastic syndrome or myelofibrosis arms, 2 genitourinary [prostate] cancer arms, and 1 melanoma arm).
Figure 1.
Study Flow Diagram
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of systematic review and meta-analysis in ClinicalTrials.gov registries up to September 18, 2020. AF = atrial fibrillation.
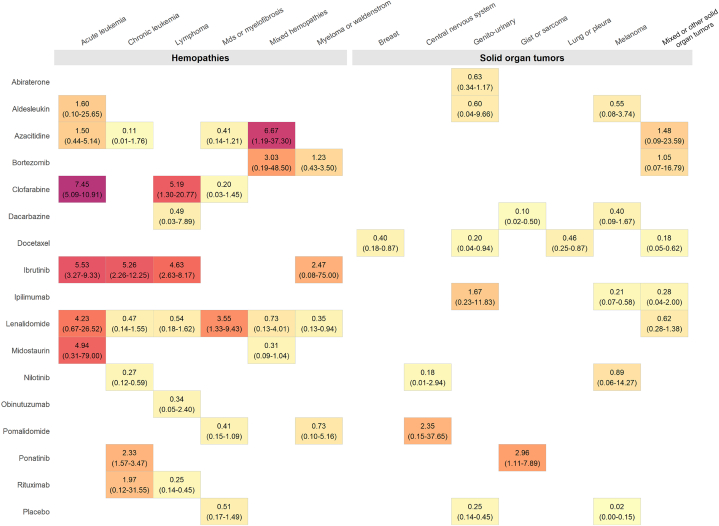
Figure 2.
AF Incidence Rates by Anticancer Drugs and by Cancer Localization
Summary annualized incidence rates of atrial fibrillation (AF) reporting associated with exposure to 1 of 15 anticancer drugs used as monotherapy in clinical trials per 100 observations by anticancer drugs and by cancer localization with ClinicalTrials.gov studies posted up to September 18, 2020 (no AF event was reported for obinutuzumab, and no study was included for anthracyclines and cisplatin). Placebo refers to the summary annualized incidence rates of AF reporting associated with placebo arms of included trials. The color code provides visual information on the summary annualized incidence rate of AF reporting (yellow, the lowest incidences, followed by gold, orange, red, and then purple, the highest incidences). GIST = gastrointestinal stromal tumor; MDS = myelodysplastic syndrome.
Table 1.
Study Details for Each Anticancer Drug
| Anticancer Drug Studied | Number of Studies Screened | Number of Arms Included | Number of Included Randomized Study Arms | Women, % (min-max) | Mean/Median Age, y (min-max) | Mean/Median Follow-Up, mo (min-max) | Total Number of AF Cases | Number of Patients Included | |
|---|---|---|---|---|---|---|---|---|---|
| Alkylating agent | Dacarbazine | 53 | 11 | 10 | 21.4-72.5 | 36-67 | 2-84 | 2 | 1,434 |
| Cisplatin | 496 | 0 | NA | NA | 40-54 | — | NA | NA | |
| Androgen deprivation therapy | Abiraterone | 55 | 14 | 6 | 0 | 63.1-75.1 | 3-60 | 30 | 2,290 |
| Antimetabolites | Clofarabine | 58 | 6 | 1 | 34.5-57.6 | 51-74.2 | 14-58 | 28 | 362 |
| Azacitidine | 115 | 20 | 9 | 0-47.2 | 39.2-75.4 | 5-73 | 48 | 1,621 | |
| Anthracyclines | Daunorubicin | 47 | 0 | NA | NA | NA | 2-84 | NA | NA |
| Idarubicin | 29 | 0 | NA | NA | NA | 9-101 | NA | NA | |
| Kinase inhibitors | Ibrutinib | 42 | 17 | 5 | 23.4-41.9 | 25-73.1 | 9-101 | 223 | 1,882 |
| Nilotinib | 34 | 11 | 3 | 21.1-58.3 | 41.5-69.5 | 12-138 | 5 | 1,817 | |
| Ponatinib | 9 | 4 | 0 | 37.4-47.4 | 43-62 | 5-60 | 48 | 695 | |
| Midostaurin | 9 | 3 | 0 | 34.5-68.2 | 62-65 | 6-103 | 3 | 164 | |
| Immune checkpoint inhibitor | Ipilimumab | 70 | 12 | 10 | 0-100 | 51.3-69.3 | 3-120 | 8 | 2,454 |
| Immunomodulating agents | Aldesleukin | 80 | 4 | 1 | 29.2-39.3 | 48.6-63.9 | 8-46 | 1 | 257 |
| Lenalidomide | 229 | 31 | 6 | 0-76.8 | 36.2-77.8 | 2-94 | 34 | 2,718 | |
| Pomalidomide | 34 | 6 | 2 | 22.4-50.0 | 65-69 | 5-85 | 5 | 420 | |
| Monoclonal antibodies (anti-CD20) | Rituximab | 489 | 18 | 7 | 28.6-67.7 | 38-71 | 6-92 | 4 | 2,609 |
| Obinutuzumab | 30 | 2 | 1 | 38.8-47.7 | 59-59 | 10-27 | NA | 221 | |
| Proteasome inhibitor | Bortezomib | 249 | 6 | 2 | 0-100 | 42-78 | 3-14 | 2 | 595 |
| Taxane | Docetaxel | 434 | 28 | 27 | 0-100 | 52-70.1 | 7-83 | 44 | 7,065 |
| Total | 2,562 | 193a | 90 | NA | NA | NA | 485 | 26,604 |
A total of 193 study arms from 191 studies were included in the meta-analysis. All the values are mean or median (mean/median) and are expressed as min and max (min-max).
AF = atrial fibrillation; RCT = randomized controlled trial; NA = not applicable.
2 studies were eligible for 2 anticancer drugs.
Summary annualized incidence rates of AF reporting
No AF-specific detection strategy was implemented in the included trials, and AF detection was not systematic and only performed using 12-lead electrocardiograms (ECGs) in case of AF-related symptoms. None of the included trials attempted to detect asymptomatic AF. The summary annualized incidence rates of AF reporting associated with anticancer drugs are represented in the Central Illustration, Supplemental Figure 1, and Table 2. Anthracyclines were not administered singly as monotherapy. No AF cases were reported in the obinutuzumab monotherapy groups (AF was not noted among the AEs), and follow-up duration was not available for cisplatin monotherapy groups. Thus, we therefore were able to determine the summary annualized incidence rates of AF reporting for 15 of the 19 anticancer drugs of interest. Overall, 485 AF cases were reported in 26,604 patients. Summary annualized incidence rates of AF reporting of the 15 anticancer drugs of interest ranged from 0.26 to 4.92 per 100 person-years. The top 3 summary annualized incidence rates of AF reporting were found for ibrutinib 4.92 (95% CI: 2.91-8.31), clofarabine 2.38 (95% CI: 0.66-8.55), and ponatinib 2.35 (95% CI: 1.78-3.12) per 100 person-years. The meta-analysis of each of the 15 anticancer drugs is represented by a forest plot in Supplemental Figures 2 to 16.
Central Illustration.
Atrial Fibrillation Incidences for the 5 Anticancer Drugs With Higher Rates
Summary annualized incidence rates and 95% confidence intervals of atrial fibrillation (AF) reporting per 100 observations (overall and for study mean age <65 years and mean age 65 years or more, when available) for the 5 anticancer drugs having the highest annualized incidence rates with ClinicalTrials.gov studies posted up to September 18, 2020.
Table 2.
AF Incidence Rates Associated With 15 Anticancer Drugs Used as Monotherapy in Clinical Trials
| Anticancer Drug | Total Summary Annualized Incidence Rates of AF Reporting, per 100 Person-Years | Summary Annualized Incidence Rates of AF Reporting Before September 2009, per 100 Person-Years | Studies Before September 2009, n | Summary Annualized Incidence Rates of AF Reporting After September 2009, per 100 Person-Years | Studies After September 2009, n | P Value for Subgroups Difference Test |
|---|---|---|---|---|---|---|
| Dacarbazine | 0.30 (0.10-0.90) | 0.14 (0.02-1.17) | 3 | 0.39 (0.11-1.45) | 8 | 0.43 |
| Abiraterone | 0.63 (0.34-1.17) | 0.45 (0.14-1.42) | 4 | 0.75 (0.36-1.57) | 10 | 0.46 |
| Clofarabine | 2.38 (0.66-8.55) | 1.57 (0.32-7.80) | 5 | 7.27 (3.64-14.54) | 1 | 0.085 |
| Azacitidine | 1.04 (0.46-2.36) | 0.36 (0.14-0.97) | 6 | 1.48 (0.55-4.00) | 14 | 0.049 |
| Ibrutinib | 4.92 (2.91-8.31) | 4.92 (2.91-8.31) | 17 | NA | ||
| Nilotinib | 0.29 (0.15-0.58) | 0.32 (0.10-1.05) | 4 | 0.23 (0.08-0.65) | 7 | 0.68 |
| Ponatinib | 2.35 (1.78-3.12) | 2.35 (1.78-3.12) | 4 | NA | ||
| Midostaurin | 0.65 (0.12-3.50) | 0.65 (0.12-3.50) | 3 | NA | ||
| Ipilimumab | 0.26 (0.11-0.63) | 0.17 (0.03-0.92) | 4 | 0.36 (0.14-0.93) | 8 | 0.45 |
| Aldesleukin | 0.73 (0.21-2.52) | 0.53 (0.11-2.64) | 3 | 1.18 (0.17-8.35) | 1 | 0.54 |
| Lenalidomide | 1.03 (0.58-1.81) | 1.04 (0.57-1.92) | 26 | 1.79 (0.33-9.64) | 4 | 0.55 |
| Pomalidomide | 0.52 (0.22-1.23) | 0.76 (0.22-2.62) | 3 | 0.47 (0.11-2.06) | 3 | 0.63 |
| Rituximab | 0.27 (0.15-0.49) | 0.18 (0.05-0.61) | 7 | 0.33 (0.17-0.66) | 11 | 0.39 |
| Bortezomib | 1.33 (0.53-3.36) | 1.29 (0.45-3.68) | 4 | 1.50 (0.21-10.63) | 2 | 0.90 |
| Docetaxel | 0.37 (0.23-0.58) | 0.37 (0.19-0.70) | 13 | 0.37 (0.19-0.71) | 15 | 0.99 |
Values are incidence rate (95% CI), except as noted.
Subgroup analyses are based on publications performed before September 2009 and after September 2009 (no AF event was reported for obinutuzumab, and no study was included for anthracyclines and cisplatin).
Abbreviations as in Table 1.
Summary annualized incidence rate of AF reporting in placebo arms was 0.25 per 100 person-years (95% CI: 0.10-0.65; I2 = 63%; time frame [range]: 69.0 months [18.6-120 months]), including 18 AF cases reported in 1,746 patients (Central Illustration).
Subgroup analysis
Summary annualized incidence rates of AF reporting associated with anticancer drugs of interest with subgroup analyses with regard to cancer site are presented in Figure 2 and in Supplemental Figures 2 to 16. Higher annualized incidence rates were associated with anticancer drugs used in hematologic malignancies and particularly in acute leukemias.
For ibrutinib and ponatinib, none of the included studies was posted before September 2009. For midostaurin, none of the included studies was posted after September 2009. Regarding the 14 anticancer drugs included in the analysis with studies posted both before and after September, 2009, the results are presented in Table 2 and Supplemental Figures 17 to 31.
Quality of evidence
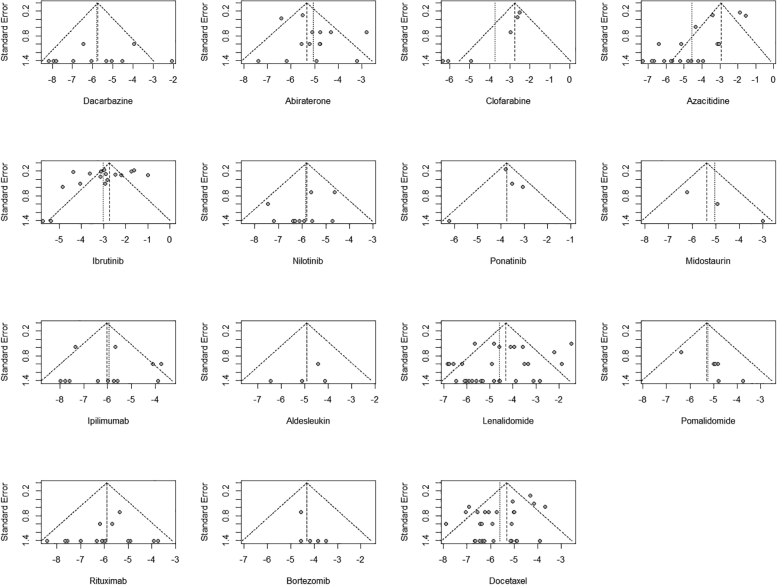
The inverted funnel plot of the primary outcome suggested a clear bias toward unpublished data of studies with higher AF rates for 6 anticancer drugs (clofarabine, azacytidine, ponatinib, lenalidomide, bortezomib, and docetaxel) (Figure 3). Risk of bias assessment of the included studies is presented in Supplemental Table 6 and highlighted that 2 bias domains often could not be completed owing to insufficient data and were therefore qualified as unclear risk (information bias regarding the drug safety outcome and statistical methods excluding methods to control confounding).
Figure 3.
Publication Bias Funnel Plot for the Primary Outcome
Funnel plots are presented for each of the 15 anticancer drugs studied.
Discussion
Using the ClinicalTrials.gov registry, we performed a meta-analysis of 191 studies accruing 26,604 patients, and we determined the summary annualized incidence rates of AF reporting for 15 of 19 anticancer drugs that were previously associated with AF reporting in the world pharmacovigilance database Vigibase.15 Although the ClinicalTrials.gov registry represents to date the most powerful registry to collect data on CVAEs occurring in oncological trials,21 querying only ClinicalTrials.gov may not have included all cancer trials with available AF reporting from other databases. However, to our knowledge, this study is the first largescale analysis documenting annualized incidence rates of AF reporting associated with anticancer drugs used as monotherapy in clinical trials. Importantly, we computed summary AF reporting annualized incidence rate in placebo-treated patients (0.25 per 100 person-years, 95% CI: 0.10-0.65; I2 = 63%; time frame [range]: 69.0 months [18.6-120 months]). Regarding the 15 of 19 anticancer drugs that were previously associated with AF reporting, 3 exhibited annualized incidence AF rates not overlapping with the calculated summary annualized incidence rates of AF reporting in placebo arms: ibrutinib 4.92 (95% CI: 2.91-8.31), clofarabine 2.38 (95% CI: 0.66-8.55), and ponatinib 2.35 (95% CI: 1.78-3.12) per 100 person-years. These results indicate that in oncological trials, AF reporting is not a rare event, even though these results are probably underestimated compared with the “real-life” incidence, as supported by the funnel plots.
In oncological trials reporting CVAEs, AE rates were markedly lower than those observed among real-life populations.11 These findings suggest a global and systemic underreporting and/or underidentification of cardiotoxicity among cancer clinical trial participants, and AF reporting is expected to be particularly affected. Cardiac rhythm abnormalities, particularly subclinical and/or paroxysmal AF, still represent a diagnostic challenge, although they may be frequent in specific populations. In the post-stroke patient population, long-term and continuous cardiac rhythm monitoring with an insertable cardiac monitor detected AF in 12.4% of patients by 12 months, compared with only 2.0% in the control patients without any insertable cardiac monitor.26 Moreover, subclinical AF was associated with a 2.4-fold increase in stroke risk (95% CI: 1.8-3.3; P < 0.001)27 and with 10% to 30% of unexplained stroke in the general population,28 indicating that improvement of cardiac rhythm monitoring and AF detection should be considered in oncological trials, particularly those studying anticancer drugs associated with high AF rates. In our view, trials studying BTK inhibitors, which are associated with an increased risk of AF,5 should consider implementation of a standardized AF detection strategy based, not only on 12-lead ECGs performed only in cases of AF-related symptoms, but also on longer-term ambulatory monitoring or insertable cardiac monitors to detect subclinical AF.29,30 This lack of systematic and standardized rhythm monitoring in oncological trials associated with an underreporting of cardiac AEs11 is also problematic to clearly define which anticancer drugs are significantly associated with AF reporting. In our previous work using the international pharmacovigilance database, we found that 19 anticancer drugs were associated with AF reporting,15 whereas only 3 exhibited an AF reporting annualized incidence rate not overlapping with the calculated AF reporting annualized incidence rates of the placebo arms in the present work. The discrepancy for this is unclear. However, it may be related to underreporting. Although also prone to postmarketing AE underreporting, pharmacovigilance databases are valuable due to the large number of reports available (11,757 vs 551 AF cases in the present study) and the possibility to more easily analyze a combination of several anticancer drugs. In our view, they represent a suitable complementary approach to oncological trial meta-analyses.
We were able to compare annualized incidence rates reported before or after September 2009, the date of the legal obligation to report AEs. The overall trend was toward a higher estimate of the summary annualized incidence rates of AF reporting for studies registered after 2009 than before even though AF detection strategies were similar and based on 12-lead ECGs only. This suggests that AF underreporting was significant for older studies and that the ClinicalTrials.gov requirement was useful in increasing the AF reporting and allows for a more comprehensive approach to the true incidence. Therefore, caution should be exercised in our estimation of the summary annualized incidence rate of AF reporting for midostaurin and clofarabine, which have no studies registered after September 2009. Moreover, although the ClinicalTrials.gov requirement was useful in increasing the AF reporting, CVAEs and especially AF remained underreported AEs mainly due to the lack of systematic cardiovascular monitoring in oncological trials.11
As in the general population,31,32 AF is associated with a significantly increased risk of all-cause mortality, cardiovascular mortality, and cardiovascular events in cancer.7,33 In chronic lymphocytic leukemia patients, using the National Inpatient Sample database, AF was associated with an increased risk of all-cause mortality (6.06%; OR: 1.39; 95% CI: 1.19-1.61), acute coronary syndrome (15.68%; OR: 1.24; 95% CI: 1.12-1.36), acute heart failure (7.50%; OR: 2.16; 95% CI: 1.85-2.52), and stroke (3.09%; OR: 1.94; 95% CI: 1.54-2.44) compared with no-AF patients.33 In a SEER-Medicare analysis, in female patients aged over 66 years with a new primary diagnosis of breast cancer, incident AF occurring within 30 days after breast cancer diagnosis was associated with a significant increase in both all-cause (aHR: 2.15; 95% CI: 1.32-3.48) and cardiovascular (aHR: 3.00; 95% CI: 1.28-7.00) mortality at 1 year compared with the no-AF patients.7 In addition to an increased risk of all-cause and cardiovascular mortality, cancer also increased major bleeding risk in AF patients.34 In a recent observational and retrospective cohort study including 399,344 cancer patients, cancer increased the risk of major bleeding (HR: 1.27; 95% CI: 1.26-1.28) and intracranial hemorrhage (HR: 1.07; 95% CI: 1.05-1.10) during a mean follow-up of 2.0 years, making challenging the question of anticoagulation in AF cancer patients.9,35
Study limitations
We decided to only estimate annualized incidence rates of AF reporting associated with the exposure of anticancer drugs used as monotherapy to evaluate the annualized AF incidence of individual anticancer drugs. We acknowledge that in daily clinical practice, anticancer drugs are often prescribed in combination, and therefore, our results are probably not generalizable to all clinical situations encountered by oncologists and hematologists. However, there are currently a very large number of anticancer drug combinations approved by the FDA and the European Medicines Agency, and it would have been challenging to estimate the summary annualized incidence rates of AF reporting associated with all these combinations with possibly few studies included by combination. We excluded 2,069 studies (80.8%) because patients were treated only with combinations of anticancer drugs; these exclusions may have resulted in a selection bias, thus altering the annualized incidence rates of AF reporting or even precluded estimation for some drugs. For example, we could not estimate the summary annualized incidence rates of AF reporting associated with anthracyclines, cisplatin, and obinutuzumab although these therapies have already been associated with AF.15
The summary annualized incidence rate of AF reporting in placebo arms was pooled from only 3 different types of malignancy and of note, there was a little overlap between the malignancy types leading to the placebo summary result and the malignancy types associated with the 3 anticancer drugs with highest annualized AF incidence. This aspect of the data could limit the generalizability of the summary annualized incidence rate of AF reporting for placebo arms and its comparison with summary annualized incidence rates of AF reporting calculated for the 15 anticancer drugs.
We did not have access to individual patient data or each included study, and several factors associated with AF development could not be considered in our analysis. Follow-up available in the included studies referred to the follow-up of the studies’ endpoints that were oncological endpoints and not to AF reporting.
Only 1 database (ClinicalTrials.gov registry website) was searched. The reporting of serious AEs in the ClinicalTrials.gov registry website is exhaustive, but most of trials apply a 5% threshold for nonserious AEs, and therefore some nonserious AF may not have been captured in this work, contributing to an underestimation of the calculated incidences. Based on previous work comparing the completeness of trial results posted at ClinicalTrials.gov and in published papers, the authors considered it very unlikely that a large screening in MEDLINE, the Cochrane Central Register, or Web of Science would have resulted in the identification of eligible cancer trials with available AF reporting.19
The summary annualized incidence rates of AF reporting are likely to be underestimated by several factors. The collection of AEs only became mandatory in September 2009 for clinical trials and remains to date underreported. AF is an AE without any standardized or systematic rhythm monitoring, and oncological trials usually only perform 12-lead ECGs in patients with AF-related symptoms. Finally, the PROTECT checklist bias domains that could not be completed owing to insufficient data are those qualified as “unclear risk.”
Conclusions
Although probably underestimated, AF reporting is not a rare AE associated with anticancer drugs in phase 2 and 3 clinical trials. Summary annualized incidence rates of AF reporting associated with exposure to 1 of 15 anticancer drugs used as monotherapy in clinical trials ranged from 0.26 per 4.92 per 100 person-years. The 3 anticancer drugs associated with the highest AF annualized incidence rates were ibrutinib, clofarabine, and ponatinib. Systematic and standardized AF detection should be considered in oncological trials, particularly those studying anticancer drugs associated with high AF rates.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In this safety meta-analysis including 191 phase 2 and 3 clinical trials (n = 26,604), AF reporting was not a rare AE associated with anticancer drugs. Summary annualized incidence rates of AF reporting associated with exposure to 1 of 15 anticancer drugs used as monotherapy in clinical trials ranged from 0.26 to 4.92 per 100 person-years.
TRANSLATIONAL OUTLOOK: Because the detection of arrhythmias is neither systematic nor standardized in oncological trials, the AF reporting annualized incidence rate is likely underestimated. Oncological studies implementing systematic cardiac monitoring are important for fully understanding the risk of AF. This is important given the increased risk of AF-associated morbidity and mortality in cancer.
Funding Support and Author Disclosures
Prof Alexandre has received honoraria for presentations and consulting fees from Bayer, BMS, Pfizer, Amgen, and Bioserenity, outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.Chugh S.S., Havmoeller R., Narayanan K., et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colilla S., Crow A., Petkun W., Singer D.E., Simon T., Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 3.Jakobsen C.B., Lamberts M., Carlson N., et al. Incidence of atrial fibrillation in different major cancer subtypes: a nationwide population-based 12 year follow up study. BMC Cancer. 2019;19:1105. doi: 10.1186/s12885-019-6314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan M., Zhang Z., Tse G., et al. Association of cancer and the risk of developing atrial fibrillation: a systematic review and meta-analysis. Cardiol Res Pract. 2019;2019:8985273. doi: 10.1155/2019/8985273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexandre J., Moslehi J.J., Bersell K.R., Funck-Brentano C., Roden D.M., Salem J.-E. Anticancer drug-induced cardiac rhythm disorders: current knowledge and basic underlying mechanisms. Pharmacol Ther. 2018;189:89–103. doi: 10.1016/j.pharmthera.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Lancellotti P., Marechal P., Donis N., Oury C. Inflammation, cardiovascular disease, and cancer: a common link with far-reaching implications. Eur Heart J. 2019;40(48):3910–3912. doi: 10.1093/eurheartj/ehz645. [DOI] [PubMed] [Google Scholar]
- 7.Guha A., Fradley M.G., Dent S.F., et al. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-Medicare analysis. Eur Heart J. 2022;43(4):300–312. doi: 10.1093/eurheartj/ehab745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020;17:474–502. doi: 10.1038/s41569-020-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fradley M.G., Beckie T.M., Brown S.A., et al. Recognition, prevention, and management of arrhythmias and autonomic disorders in cardio-oncology: a scientific statement from the American Heart Association. Circulation. 2021;144:e41–e55. doi: 10.1161/CIR.0000000000000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farmakis D., Parissis J., Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63:945–953. doi: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Bonsu J.M., Guha A., Charles L., et al. Reporting of cardiovascular events in clinical trials supporting FDA approval of contemporary cancer therapies. J Am Coll Cardiol. 2020;75:620–628. doi: 10.1016/j.jacc.2019.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groarke J.D., Cheng S., Moslehi J. Cancer-drug discovery and cardiovascular surveillance. N Engl J Med. 2013;369:1779–1781. doi: 10.1056/NEJMp1313140. [DOI] [PubMed] [Google Scholar]
- 13.Diederichsen S.Z., Haugan K.J., Brandes A., et al. Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J Am Coll Cardiol. 2019;74:2771–2781. doi: 10.1016/j.jacc.2019.09.050. [DOI] [PubMed] [Google Scholar]
- 14.Miller K.D., Nogueira L., Mariotto A.B., et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 15.Alexandre J., Salem J.E., Moslehi J., et al. Identification of anticancer drugs associated with atrial fibrillation: analysis of the WHO pharmacovigilance database. Eur Heart J Cardiovasc Pharmacother. 2021;7:312–320. doi: 10.1093/ehjcvp/pvaa037. [DOI] [PubMed] [Google Scholar]
- 16.Rao V.U., Reeves D.J., Chugh A.R., et al. Clinical approach to cardiovascular toxicity of oral antineoplastic agents: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:2693–2716. doi: 10.1016/S0735-1097(21)04048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zorzela L., Loke Y.K., Ioannidis J.P., et al. PRISMAHarms Group PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ. 2016;352:i157. doi: 10.1136/bmj.i157. [DOI] [PubMed] [Google Scholar]
- 18.Guide to FDAAA Reporting Research Results NIH Office of Intramural Research. https://oir.nih.gov/sourcebook/intramural-program-oversight/intramural-data-sharing/guide-fdaaa-reporting-research-results
- 19.Riveros C., Dechartres A., Perrodeau E., et al. Timing and completeness of trial results posted at ClinicalTrials.gov and published in journals. PLoS Med. 2013;10:e1001566. doi: 10.1371/journal.pmed.1001566. discussion e1001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartung D.M., Zarin D.A., Guise J.M., et al. Reporting discrepancies between the ClinicalTrials.gov results database and peer-reviewed publications. Ann Intern Med. 2014;160:477–483. doi: 10.7326/M13-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolladille C., Akroun J., Morice P.M., et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: a safety meta-analysis. Eur Heart J. 2021;42(48):4964–4977. doi: 10.1093/eurheartj/ehab618. [DOI] [PubMed] [Google Scholar]
- 22.Morice P.M., Leary A., Dolladille C., et al. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol. 2021;8(2):e122–e134. doi: 10.1016/S2352-3026(20)30360-4. [DOI] [PubMed] [Google Scholar]
- 23.Faillie J.L., Ferrer P., Gouverneur A., et al. A new risk of bias checklist applicable to randomized trials, observational studies, and systematic reviews was developed and validated to be used for systematic reviews focusing on drug adverse events. J Clin Epidemiol. 2017;86:168–175. doi: 10.1016/j.jclinepi.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Cochrane Handbook for Systematic Reviews of Interventions. Accessed January 20, 2021. https://training.cochrane.org/handbook/current
- 25.Schwarzer G. meta: An R Package for Meta-Analysis. R News. 2007;7(3):40–45. https://cran.rstudio.org/doc/Rnews/Rnews_2007-3.pdf#page=40 [Google Scholar]
- 26.Sanna T., Diener H.C., Passman R.S., et al. CRYSTAL AF Investigators Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan R., Perera T., Elliott A.D., et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39:1407–1415. doi: 10.1093/eurheartj/ehx731. [DOI] [PubMed] [Google Scholar]
- 28.Noseworthy P.A., Kaufman E.S., Chen L.Y., et al. American Heart Association Council on Clinical Cardiology Electrocardiography and Arrhythmias Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology. Council on Cardiovascular and Stroke Nursing; and Stroke Council Subclinical and device-detected atrial fibrillation: pondering the knowledge gap: a scientific statement from the American Heart Association. Circulation. 2019;140:e944–e963. doi: 10.1161/CIR.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diederichsen S.Z., Haugan K.J., Kronborg C., et al. Comprehensive evaluation of rhythm monitoring strategies in screening for atrial fibrillation: insights from patients at risk monitored long term with an implantable loop recorder. Circulation. 2020;141:1510–1522. doi: 10.1161/CIRCULATIONAHA.119.044407. [DOI] [PubMed] [Google Scholar]
- 30.Keramida K., Filippatos G., Farmakis D. Cancer treatment and atrial fibrillation: use of pharmacovigilance databases to detect cardiotoxicity. Eur Heart J Cardiovasc Pharmacother. 2021;7:321–323. doi: 10.1093/ehjcvp/pvaa059. [DOI] [PubMed] [Google Scholar]
- 31.Benjamin E.J., Wolf P.A., D’Agostino R.B., Silbershatz H., Kannel W.B., Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 32.Andersson T., Magnuson A., Bryngelsson I.L., et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case-control study. Eur Heart J. 2013;34:1061–1067. doi: 10.1093/eurheartj/ehs469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ammad Ud Din M., Thakkar S., Patel H., et al. The impact of atrial fibrillation on hospitalization outcomes for patients with chronic lymphocytic leukemia using the National Inpatient Sample Database. Clin Lymphoma Myeloma Leuk. 2022;22(2):98–104. doi: 10.1016/j.clml.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Pastori D., Marang A., Bisson A., et al. Thromboembolism, mortality, and bleeding in 2,435,541 atrial fibrillation patients with and without cancer: a nationwide cohort study. Cancer. 2021;127:2122–2129. doi: 10.1002/cncr.33470. [DOI] [PubMed] [Google Scholar]
- 35.Alexandre J., Cautela J., Ederhy S., et al. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European cardio-oncology guidelines. J Am Heart Assoc. 2020;9:e018403. doi: 10.1161/JAHA.120.018403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.