Abstract
Background
Patients with cancer have an increased risk for arterial thromboembolism (ATE). Scant data exist about the impact of cancer-specific genomic alterations on the risk for ATE.
Objectives
The aim of this study was to determine whether individual solid tumor somatic genomic alterations influence the incidence of ATE.
Methods
A retrospective cohort study was conducted using tumor genetic alteration data from adults with solid cancers who underwent Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets testing between 2014 and 2016. The primary outcome, ATE, was defined as myocardial infarction, coronary revascularization, ischemic stroke, peripheral arterial occlusion, or limb revascularization and identified through systematic electronic medical record assessments. Patients were followed from date of tissue-matched blood control accession to first ATE event or death, for up to 1 year. Cause-specific Cox proportional hazards regression was used to determine HRs of ATE for individual genes adjusted for pertinent clinical covariates.
Results
Among 11,871 eligible patients, 74% had metastatic disease, and there were 160 ATE events. A significantly increased risk for ATE independent of tumor type was noted for the KRAS oncogene (HR: 1.98; 95% CI: 1.34-2.94; multiplicity-adjusted P = 0.015) and the STK11 tumor suppressor gene (HR: 2.51; 95% CI: 1.44-4.38; multiplicity-adjusted P = 0.015).
Conclusions
In a large genomic tumor-profiling registry of patients with solid cancers, alterations in KRAS and STK11 were associated with an increased risk for ATE independent of cancer type. Further investigation is needed to elucidate the mechanism by which these mutations contribute to ATE in this high-risk population.
Key Words: arterial thromboembolism, cancer, ischemic stroke, myocardial infarction, tumor genomic alteration
Central Illustration
Patients with cancer are at significant risk for thromboembolism. Arterial thromboembolism (ATE), which includes myocardial infarction and stroke, occurs with an incidence of 1.1% to 4.7% within 6 months of cancer diagnosis.1, 2, 3, 4, 5 ATE in patients with cancer not only leads to hospitalizations and delays in cancer treatment but is also associated with a 3-fold increased risk for mortality.3, 4, 5 The risk for ATE starts to increase 5 months prior to cancer diagnosis and attenuates 1 year after diagnosis.4,6 Increased baseline ATE risk in this population is due partially to shared risk factors between cancer and cardiovascular disease, such as advanced age, obesity, and tobacco smoking. The mechanism for ATE in this patient population is likely multifactorial and related to cancer therapies, the hypercoagulable state of malignancy, and tumor-specific factors.7 Known cancer-related risk factors for ATE include advanced cancer stage, vasculotoxic chemotherapies (antimetabolite chemotherapies, alkylating agents, anti–vascular endothelial growth factor antibodies, tyrosine kinase inhibitors, proteasome inhibitors, immune checkpoint inhibitors, and radiation) and type of cancer (with the highest risk in lung, gastric, kidney, and pancreatic cancer).3,4,8,9 There is evidence that cancer cells contribute directly to the creation of a prothrombotic state by expressing tissue factor, which results in thrombin production and platelet activation.10, 11, 12, 13, 14 Cancer cell–derived extracellular vesicles and neutrophil extracellular traps (NETs) also appear to be implicated in the pathophysiology of cancer-associated ischemic stroke.15, 16, 17
We hypothesized that these ATE-predisposing cancers share tumor-specific genomic alterations that contribute to this increased risk for arterial thrombotic events. To evaluate this hypothesis, we used available data from the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) program. Since 2014, this custom hybridization capture-based next-generation sequencing assay has been used at Memorial Sloan Kettering Cancer Center (MSKCC) to comprehensively profile patient tumors at the molecular level, with the capacity to detect somatic alterations in more than 300 genes at a minimum depth of coverage of 91×.18 To date, more than 55,000 patient tumors have been profiled by MSK-IMPACT.19 We sought to retrospectively analyze solid tumor MSK-IMPACT data to assess whether individual tumoral somatic genomic alterations influence ATE risk.
Methods
Patient cohort
The cohort selection process has been described elsewhere.20 Briefly, adult patients were included if they were enrolled in the MSK-IMPACT program between 2014 and 2016. Patients were excluded if they had histories of myocardial infarction, coronary revascularization, symptomatic stroke, peripheral arterial occlusion, or limb revascularization prior to cohort entry. Individuals with incomplete baseline data were excluded. Data were obtained following approval of this project by the MSKCC Institutional Review Board. Incident ATE, the primary study endpoint, was defined as any instance of myocardial infarction, coronary revascularization, peripheral arterial occlusion, limb revascularization, or ischemic stroke, including an interval covert stroke identified through surveillance brain imaging. Coronary events were adjudicated in accordance with the fourth universal definition of myocardial infarction.21 The MSKCC tumor registry contributed information about individual patient tumors; tumor type was simplified and limited to 1 of 15 categories. Basic demographic information was obtained from the clinical information systems. ATE events were detected using the Clinical Event Detection and Recording System (CEDARS), a natural language processing (NLP)–based electronic medical record document-processing pipeline.22 Briefly, using the spaCy or UDPipe NLP library, electronic medical record clinical notes were processed to obtain sentence boundaries and negation annotation. Keyword filters (Supplemental Tables 1 to 3) were applied, and selected sentences were presented to reviewers using a custom interface. Sentences potentially indicating ATE events were reviewed by a first-line adjudicator. All detected events were reviewed by a second-line disease specialist (neurologist or cardiologist). A random sample (n = 300) from the cohort at large was audited for accuracy via manual chart reviews. In this subset, 41 individuals had at least 1 ATE event detected by manual review, compared with 39 patients using CEDARS. Both missed events had occurred before cohort entry. Metastatic status was determined by merging information from the MSKCC tumor registry and MSK-IMPACT sample type. Patients not determined to have metastatic disease in this manner were then assessed using CEDARS to maximize sensitivity.
MSK-IMPACT sequencing
The MSK-IMPACT assay was described elsewhere.18 After obtaining informed consent, DNA was extracted from the solid tumor and a peripheral blood sample; those 2 samples are not necessarily collected the same day. The first version of the solid MSK-IMPACT panel included 341 genes, comprising both oncogenes and tumor suppressor genes. Subsequent versions comprised 410, 468, and more recently 505 genes. We chose to limit this study to the first panel, to maximize the number of patients included in the final analysis. The mean coverage for tumor samples overall was 753×, and the minimum depth of coverage was 91×. Sequencing data were processed to identify somatic alterations. Germline alterations were not used for this project. These genomic data are available on the cBioPortal for Cancer Genomics.23,24 Even though MSK-IMPACT data include detailed information about mutations, copy number alterations, and fusions, we simplified the results to a binary state indicating whether any given oncogene or tumor suppressor gene was altered. We retained only definite or potential driver alterations. Fusions were curated on the basis of the potential oncogenic effect of each partner, and only the partner(s) having a potential driver effect was noted as altered.
Statistical analysis
The primary endpoint of time to ATE was defined as the time from accession to ATE development in a 1-year period following accession. Accession was defined as the date of blood sample receipt for MSK-IMPACT testing, corresponding to the approximate time a patient consented to testing. Cause-specific Cox proportional hazards regression estimated associations between select somatic mutations and the risk for developing an ATE event. Death was treated as a competing risk, and for the cause-specific model, patients were censored at the time of death. The analysis included the 25 most commonly altered genes in addition to CDKN2B, which was included because of prior knowledge of an association with the risk for venous thromboembolism.20 A separate model was built for each mutation; all models were adjusted for cytotoxic chemotherapy in the year prior to accession, age, and presence of metastatic disease as assessed at accession. Additionally, all models were stratified on the basis of tumor type, and because some tumor samples were previously banked, the years from the procedure to accession: [0], (0-0.25], (0.25-1], (1-5], and (5,+]. Left truncation was used for the subset of procedures that occurred after accession but before the end of the 1-year period. Estimated HRs are reported along with their 95% CIs. The proportional hazards assumption was assessed for all models by visualizing, for each factor, the scaled Schoenfeld residuals over time, along with a score test evaluating a time-varying interaction.25 The Benjamini-Hochberg method was used to adjust P values for false discovery. For identified mutations, the associations were also evaluated using the subdistribution Fine and Gray approach as an alternative measure, and cumulative incidence function estimated the incidence of ATE; both analyses treated death as a competing risk. Summary statistics are presented as median (IQR) or as a total number (percentage) among individual with nonmissing values. The predetermined statistical significance cutoff for the purpose of this analysis was 0.10. R version 3.6.1 was used for all analyses. For original data, please contact the corresponding author.
Results
Patient characteristics and incidence of ATE
From 2014 to 2016, a total of 14,223 adult patients with solid tumor malignancies had MSK-IMPACT genomic sequencing and met initial inclusion criteria for this retrospective study. There were 1,044 patients excluded from cohort entry because of pre-existing ATE (prior ischemic stroke, myocardial infarction, coronary revascularization, peripheral arterial occlusion, or limb revascularization), and 1,308 were excluded because of missing dates or ATE events occurring between time of MSK-IMPACT tissue sampling and blood sample accession. The number of patients per group of time (years) elapsed between procedure and accession was as follows: [0], n = 2,676; (0,0.25], n = 4,444; (0.25,1], n = 2,008; (1,5], n = 2,192; and (5, +], n = 551. Among the 11,871 patients included in the final analysis, the median age was 61 years (IQR: 51-69 years), and 45% were men (Table 1). Most patients in this cohort (n = 8,733 [74% of individuals]) had metastatic disease. The prevalence of individual cancer types included in the MSK-IMPACT cohort was generally reflective of the prevalence of cancer types within the larger U.S. population of patients with cancer; however, as described previously, breast cancer and prostate cancer, which are 2 of the most common cancer types, were under-represented, likely because fewer patients with these tumor types underwent extended genomic testing during the observed time period or possibly because those patients were referred less frequently to the institution.20
Table 1.
Characteristics of Patients
| Overall(N = 11,871) | No ATE Event(n = 11,711) | ATE Event(n = 160) | |
|---|---|---|---|
| Age, y | 61 (51-69) | 61 (51-69) | 66 (55-72) |
| Male | 5,380 (45) | 5,299 (45) | 81 (51) |
| Chemotherapy in prior year | 4,453 (38) | 4,377 (37) | 76 (48) |
| Cancer type | |||
| Othera | 1,916 (16) | 1,891 (16) | 25 (16) |
| Lung | 1,908 (16) | 1,859 (16) | 49 (31) |
| Breast | 1,748 (15) | 1,738 (15) | 10 (6) |
| Colorectal | 1,141 (10) | 1,131 (10) | 10 (6) |
| Gynecologic | 792 (7) | 781 (7) | 11 (7) |
| Prostate adenocarcinoma | 709 (6) | 702 (6) | 7 (4) |
| Pancreatic adenocarcinoma | 508 (4) | 497 (4) | 11 (7) |
| High-grade glioma | 476 (4) | 469 (4) | 7 (4) |
| Soft tissue sarcoma | 463 (4) | 461 (4) | 2 (1) |
| Melanoma | 450 (4) | 445 (4) | 5 (3) |
| Hepatobiliary | 401 (3) | 395 (3) | 6 (4) |
| Bladder | 393 (3) | 389 (3) | 4 (2) |
| Renal | 339 (3) | 336 (3) | 3 (2) |
| Head and neck | 318 (3) | 313 (3) | 5 (3) |
| Esophagogastric | 309 (3) | 304 (3) | 5 (3) |
| Metastatic disease | 8,733 (74) | 8,603 (73) | 130 (81) |
| Tumor mutation burden (mutations/Mb) | 3.9 (2.2-6.9) | 3.9 (2.2-6.9) | 4.9 (3.0-8.9) |
| BMI, kg/m2b | 26.3 (23-30.3) | 26.3 (23-30.3) | 26.6 (22.8-29.6) |
| Diabetes or related medicationc | 1,511 (13) | 1,485 (13) | 26 (17) |
| Hypertension or related medicationc | 4,729 (42) | 4,654 (41) | 75 (49) |
| Hypercholesterolemia or related medicationc | 3,731 (33) | 3,669 (33) | 62 (40) |
| Atrial fibrillationc | 490 (4) | 482 (4) | 8 (5) |
| Heart failurec | 188 (2) | 185 (2) | 3 (2) |
| Anticoagulationd | 999 (9) | 976 (9) | 23 (14) |
| Antiplatelet medicationd | 1,625 (14) | 1,604 (14) | 21 (13) |
| Statind | 2,302 (23) | 2,261 (23) | 41 (31) |
| Beta-blockerd | 1,477 (15) | 1,446 (15) | 31 (23) |
| Smokinge | 5,403 (48) | 5,316 (48) | 87 (58) |
| Prior radiation | 892 (8) | 874 (7) | 18 (11) |
Values are median (IQR) or n (%).
ATE = arterial thromboembolism; BMI = body mass index.
The “other” category comprises all solid tumors not included in any other specific category.
BMI data were missing for 66 individuals.
Information about comorbidities was missing for 502 individuals.
Home medication data were missing or incomplete for 1,886 individuals.
Smoking status was missing for 590 individuals.
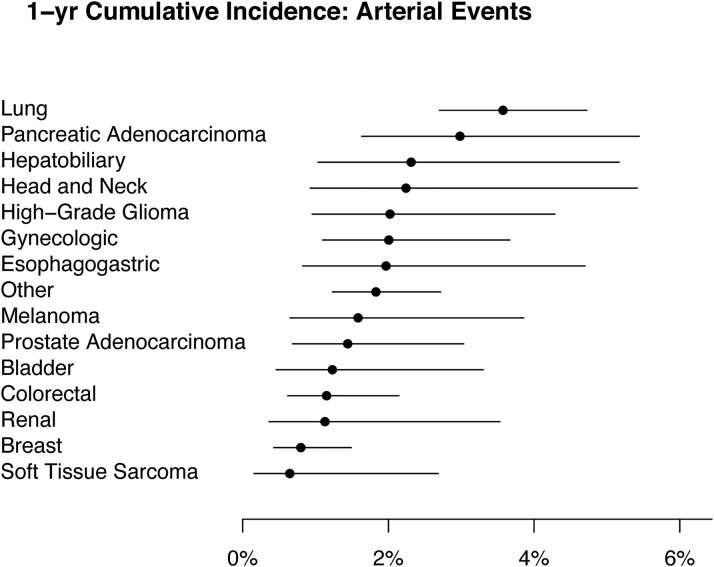
In the first year of observation, a total of 160 ATE events were recorded, including 106 strokes, 1 peripheral embolic event, and 53 myocardial infarction or coronary revascularization events, resulting in a 12-month cumulative incidence of 1.9% (95% CI: 1.6%-2.2%). The highest cumulative incidences were in the subgroups of patients with lung and pancreatic cancer (Figure 1).
Figure 1.
1-Year Cumulative Incidence of Arterial Thromboembolism by Tumor Type
Arterial thromboembolism is defined as a composite of myocardial infarction, coronary revascularization, ischemic stroke, peripheral arterial occlusion, and limb revascularization. The “other” category comprises all solid tumors not included in any other specific category. Death was considered a competing event when estimating the cumulative incidence. Each dot represents the 1-year point estimate, and the corresponding lines are the 95% CIs.
Baseline characteristics associated with an increased risk for ATE
In univariable analysis stratifying for cancer type and time from accession, age was associated with an increased risk for ATE (HR: 1.02 per 1-year higher age; 95% CI: 1.01-1.03; P = 0.001) (Table 2). When assessed individually, traditional cardiovascular risk factors of diabetes, hypertension, hypercholesterolemia, and smoking history were not associated with an increased risk for ATE. However, when these factors were combined into a single risk variable (eg, presence of at least 1 cardiovascular risk factor), they were associated with increased risk for ATE (HR: 1.19; 95% CI: 1.03-1.38; P = 0.019).
Table 2.
Univariable Analysis for Arterial Thromboembolic Events
| HR (95% CI) | P Value | |
|---|---|---|
| Age | 1.02 (1.01-1.03) | 0.001 |
| BMI | 0.98 (0.96-1.01) | 0.15 |
| Diabetes | 1.33 (0.86-2.04) | 0.20 |
| Hypertension | 1.26 (0.92-1.74) | 0.16 |
| Hypercholesterolemia | 1.34 (0.97-1.87) | 0.079 |
| Smoking history | 1.27 (0.9-1.78) | 0.17 |
| Atrial fibrillation | 1.14 (0.56-2.33) | 0.72 |
| Heart failure | 1.29 (0.41-4.11) | 0.66 |
| CVD risk factors combineda | 1.19 (1.03-1.38) | 0.019 |
| Anticoagulation | 0.57 (0.36-0.89) | 0.013 |
| Antiplatelet medication | 0.87 (0.55-1.39) | 0.56 |
| Statin | 1.34 (0.92-1.96) | 0.13 |
| Beta-blocker | 1.72 (1.15-2.59) | 0.009 |
| Vasculotoxic chemotherapy | 1.61 (1.14-2.28) | 0.007 |
| Prior radiation | 1.48 (0.87-2.51) | 0.15 |
| Metastatic disease | 1.77 (1.11-2.82) | 0.017 |
Cox regression estimated the cause-specific HR between the listed variable and the time to arterial thromboembolism. All listed variables were binary except for age and BMI. The HRs for age and BMI represent one-unit increases in age and BMI.
BMI = body mass index; CVD = cardiovascular disease.
A binary variable representing a person’s having any of the following: diabetes, hypertension, hypercholesterolemia, or history of smoking.
Impact of anticoagulation and antiplatelet therapy on risk for ATE
Patients on systemic anticoagulation appeared to have a decreased risk for ATE (HR: 0.57; 95% CI: 0.36-0.89; P = 0.013). Systemic anticoagulation was defined as taking any dose of any anticoagulant agent upon cohort entry, including low–molecular weight heparin, direct oral anticoagulant agents, and warfarin. There was no significant association between antiplatelet therapy and the risk for ATE (HR: 0.87; 95% CI: 0.55-1.39; P = 0.56). Antiplatelet therapy included aspirin and P2Y12 inhibitors.
Impact of cancer and cancer therapy on risk for ATE
In univariable analysis, metastatic disease was associated with an increased risk for ATE (HR: 1.77; 95% CI: 1.11-2.82; P = 0.017). Vasculotoxic chemotherapy was also associated with increased risk for ATE (HR: 1.61; 95% CI: 1.14-2.28; P = 0.007). In our cohort, head, neck, brain, and thorax radiation was not significantly associated with an increased risk for ATE (HR: 1.48; 95% CI: 0.87-2.51; P = 0.15).
Specific somatic alterations in tumor are associated with increased ATE risk
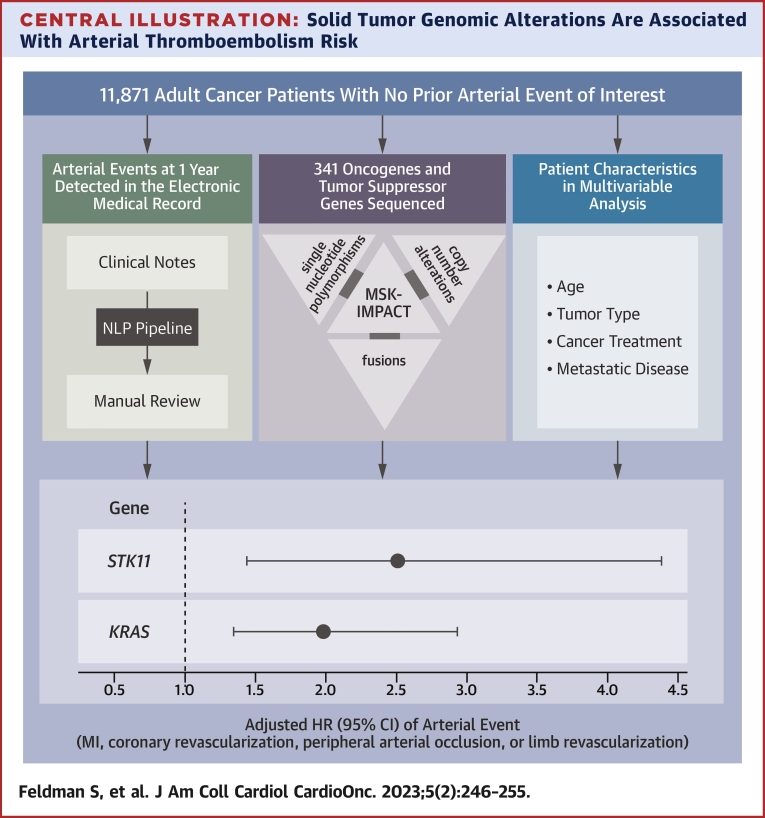
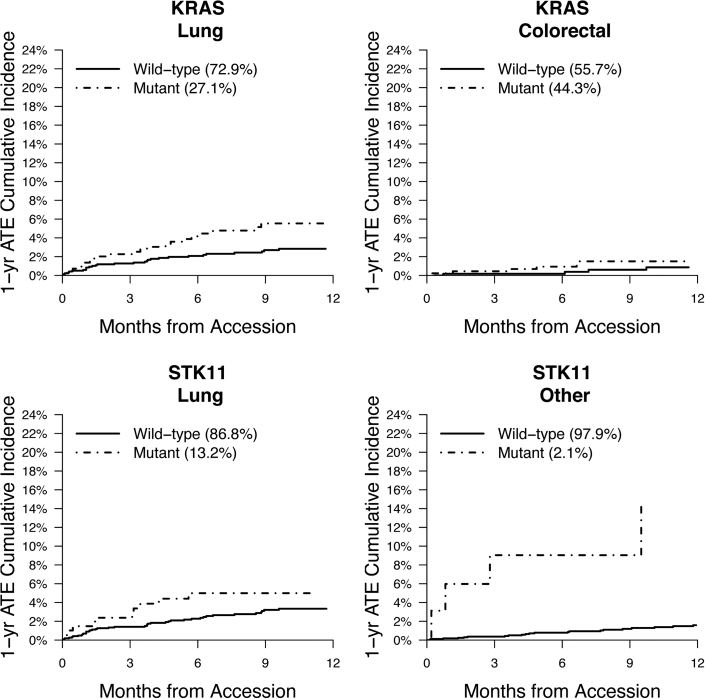
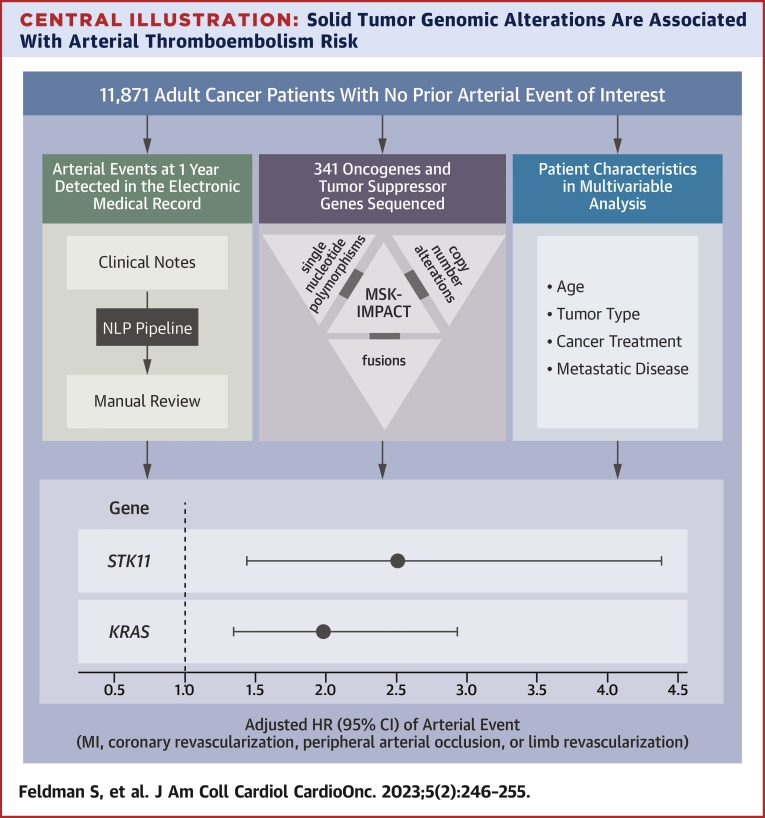
MSK-IMPACT data were assessed to determine associations between individual genes and ATE risk across solid tumor malignancies. Tumor molecular profiles were analyzed across all cancer types and within subgroups. Mutational frequencies across tumor types (Supplemental Figure 1) revealed rates of somatic alterations similar to those observed in previously published reports.26 TP53 and KRAS alterations had the highest prevalence, present in 43% and 17% of all tumor samples, respectively. Alterations in KRAS (HR: 1.98; 95% CI: 1.34-2.94; multiplicity-adjusted P = 0.015) and STK11 (HR: 2.51; 95% CI: 1.44-4.38; multiplicity-adjusted P = 0.015) were independently associated with a significantly increased risk for ATE independent of tumor type (Table 3). For the alternative Fine and Gray approach, the subdistribution HRs were 1.93 (95% CI: 1.30-2.85) for KRAS and 2.19 (95% CI: 1.23-3.89) for STK11. The unadjusted cumulative incidences of ATE for individuals with vs without somatic mutations in these 2 genes are shown in Figure 2. The effect of a KRAS alteration was consistent between lung and nonlung cancer (lung HR: 1.96; 95% CI: 1.10-3.46; P = 0.021; nonlung HR: 1.95; 95% CI: 1.28-2.97; P = 0.002). Fitting separate gene-specific models adjusting for a different set of predictors (age, sex, smoking, race) yielded similar results: an HR of 1.97 (95% CI: 1.29-3.00) for KRAS compared with 2.65 (95% CI: 1.50-4.71) for STK11.
Table 3.
Multivariable Analysis for Genomic Determinants of Arterial Thromboembolic Events
| Gene | Number Altered | HR (95% CI) | P Value | q Value |
|---|---|---|---|---|
| KRAS | 2,058 | 1.98 (1.34-2.94) | 0.0006 | 0.015 |
| STK11 | 374 | 2.51 (1.44-4.38) | 0.0012 | 0.015 |
Using cause-specific Cox regression, each model includes the individually listed gene and adjusts for chemotherapy in the year prior to accession, age at accession, and metastatic status at accession and stratifies by tumor type and categories of the time from procedure to accession. Multiple comparisons were adjusted for using the Benjamini-Hochberg method (shown as q values). Only genes for which associations had q values <0.05 are shown.
Figure 2.
1-Year Cumulative Incidence of ATE According to Alteration Type
Arterial thromboembolism (ATE) is defined as a composite of myocardial infarction, coronary revascularization, ischemic stroke, peripheral arterial occlusion, and limb revascularization. Death was considered a competing event when estimating the cumulative incidence. Plots are shown for the unadjusted 1-year cumulative incidences of ATE for individuals with vs without somatic alterations in KRAS and STK11. Data are reported for patients with lung cancer, colorectal cancer, and other. The “other” cancer category comprises all solid tumors not included in any other specific category.
A separate analysis limited to ischemic strokes revealed a similar effect of genomic alterations. The HR for stroke among individuals with vs without KRAS alterations was 2.22 (95% CI: 1.38-3.58), compared with 3.48 (95% CI: 1.87-6.46) among individuals with vs without STK11 alterations. The numbers of patients reaching the other endpoints were not sufficient to perform a dedicated analysis.
Discussion
Summary of findings
This large single-center retrospective study included 11,871 individuals with active malignancy and no histories of ATE (Central Illustration). Over the course of a 12-month observation period, 160 ATE events were observed. Multivariable models for each of the most commonly altered oncogenes and tumor suppressor genes sequenced identified 2 gene loci, KRAS and STK11, for which cancer somatic alterations are associated with an increased risk for ATE event independent of tumor type. These findings are novel and hypothesis generating for future studies to elucidate the mechanism of ATE development and identify targets for prevention.
Central Illustration.
Solid Tumor Genomic Alterations Are Associated With Arterial Thromboembolism Risk
Electronic medical record notes were assessed for 11,871 adult cancer patients from the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) cohort. Notes were processed and searched for keywords indicative of an arterial thromboembolic event, defined as a composite of myocardial infarction, coronary revascularization, ischemic stroke, peripheral arterial occlusion, and limb revascularization. All detected events were confirmed by a subject matter expert. The effect of somatic genomic alterations in select oncogenes and tumor suppressor genes on the risk for arterial thromboembolism was assessed, adjusting for pertinent covariates. Modified from Dunbar et al.20 MI = myocardial infarction; NLP = natural language processing.
Although our approach of using a DNA tumor registry to identify tumor alterations associated with ATE is unique, there have been prior studies investigating ATE in the cancer population. Consistent with these, we found that there is an increased risk for ATE events in patients with older age, metastatic disease, and certain tumor types (lung and pancreatic cancer) and in patients receiving vasculotoxic chemotherapy.1, 2, 3, 4, 5,27 In our cohort, head, neck, brain, and thorax radiation was not associated with an increased risk for ATE (HR: 1.48; 95% CI: 0.87-2.51; P = 0.15). This may be secondary to low statistical power or the fact that the mean and median time to prior radiation in this cohort were 2.3 and 1.17 years (Supplemental Figure 2), whereas radiation-associated atherosclerosis is characterized by a latency period of approximately 5 years.28
Previous work has identified traditional cardiovascular risk factors of hypertension, diabetes, and positive smoking history as associated with an increased risk for ATE, but we did not replicate those findings in the present study, as none of the individual cardiovascular disease risk factors reached statistical significance. Interestingly, patients on systemic anticoagulation were at a significantly decreased risk for ATE (HR: 0.57; 95% CI: 0.36-0.89; P = 0.013). This effect was not reported in a recent Danish cohort in which patients on systemic anticoagulation were at increased risk for ATE (HR: 1.23; 95% CI: 1.11-1.36).5 It is unlikely that systemic anticoagulation itself led to an increased risk for ATE but rather this demonstrates a confounding effect of indication for systemic anticoagulation and overall patient illness severity.
The key finding of this study is that alterations of KRAS and STK11 were associated with a significantly increased risk for ATE. KRAS mutations are known to be prevalent in lung and colorectal cancer and have been identified as conferring an increased risk for venous thromboembolism in these patient populations.20,29, 30, 31, 32 We found a 2-fold risk for ATE events in patients with KRAS alterations, an effect that was preserved when stratifying the analysis by tumor type. Another group identified an association between tumor somatic KRAS alterations and ATE, reported recently in abstract form.33 In this retrospective cohort study of consecutive gastrointestinal and non–small cell lung cancer patients, the OR for ATE in patients with KRAS alterations was 2.46 (95% CI: 1.08-5.60). KRAS is a guanosine triphosphatase signaling protein that regulates proliferation, differentiation, and cell survival. Activating mutations of KRAS up-regulate tissue factor expression on the surface of cancer cells, which in turn activate the coagulation cascade via the extrinsic pathway.34,35 Also, it has been demonstrated previously that tissue factor is found at high concentrations in atherosclerotic plaque, there are higher circulating levels in patients with cardiovascular disease, and there is increased tissue factor activity in patients with unstable angina.36, 37, 38 This hypothesis requires further investigation with dedicated functional studies to better understand the role of this oncogene in the development of thromboembolism.
The second identified gene found to modulate the risk for ATE was STK11, a tumor suppressor gene. It is altered in 3% of all cancers, most frequently in non–small cell lung cancer.39 In a recent study by Dunbar et al20 using the same cohort, STK11 alterations were found to be associated with an increased risk for venous thromboembolism.20 Using data from The Cancer Genome Atlas, STK11 alterations were found to be associated with an increased expression of tissue factor and granulocyte colony-stimulating factor (G-CSF).20 Notably, in the MSK-IMPACT cohort, STK11 alterations were shown to be associated with an increased absolute neutrophil count. On the basis of these findings, it was proposed that G-CSF-mediated NET formation was a potential mechanism responsible for STK11 somatic alterations, leading to an increased risk for venous thromboembolism. In the present study, STK11 alterations were found to be associated with an increased risk for ATE events. To ensure that these findings were not due solely to confounding secondary to an association between cancer type and cardiovascular disease, we evaluated the association of STK11 with the 1-year cumulative incidence of ATE separately in patients with lung cancer and individuals with solid neoplasms not otherwise classified in any of the 14 specific categories (ie, “other” group) and found that the increased risk for ATE was preserved in this group of patients without lung cancer (Figure 2). Given that cancer somatic alterations of STK11 are associated with an increased risk for both venous thromboembolic and ATE events, it is possible that there could be a shared pathway to coagulation activation involving G-CSF-mediated NET formation.
To our knowledge, this is the first systematic report identifying the association between tumor mutations and ATE in patients with solid tumors using a large U.S. Food and Drug Administration–cleared sequencing panel. These findings merit further validation in other cohorts along with functional studies prior to having an impact on potential patient risk stratification and studies of pharmacologic prophylaxis.
Study limitations
Our study has potential limitations related to its inherent retrospective design. We used a dedicated NLP pipeline and manual chart review, but it is possible that ATE events were missed. In this regard, the cumulative incidence of ATE events (1.9%) is relatively lower than what has been previously reported (eg, 6-month cumulative incidence of ATE events of 4.7% in a Surveillance, Epidemiology, and End Results [SEER]–Medicare study by Navi et al,4 12-month cumulative incidence of ATE events of 1.7% in a single-center prospective study in Vienna by Grilz et al,3 6-month cumulative incidence of ATE events of 1.5% in a Danish registry study by Mulder et al5). There are likely several explanations for this discrepancy. First, the observation period in this study corresponds to the time the patient was first enrolled in MSK-IMPACT and followed for the subsequent 12 months, which does not necessarily correspond to the time the patient was first diagnosed with cancer (ie, often a patient will have been diagnosed and have started treatment at a different institution prior to seeking care at MSKCC). This is relevant as patients are at highest risk for cancer-associated ATE events 5 months prior to cancer diagnosis, with risk attenuating 1 year after diagnosis, so many individuals will be outside of this high-risk period by the time they enter the MSK-IMPACT cohort.4,6 Also, the MSK-IMPACT patient cohort is on average younger by a decade than most populations studied in previously mentioned studies. Additionally, the claims-based SEER-Medicare database carries the potential for overcoding, leading to overestimation of events. Notably, SEER-Medicare included select cancers, which although representing about two-thirds of all cancer in the United States, could have influenced the results. Last, the cumulative incidence of ATE events in this study may have been lower because of exclusion of patients with ATE events prior to enrollment or to the use of less strict endpoint definitions by prior studies.
In this study, patients who developed ATE events between tumor sampling and blood control collection were excluded. Had the data structure been set up differently, starting observation at the time of tissue sampling would have been reasonable, and including these early ATE events would have potentially increased power. However, for many patients in this cohort, the consent for MSK-IMPACT testing and matched normal blood collection occurred after tissue collection. Therefore, under these conditions, if patients had ATE events and died after tissue collection but before they could consent and provide matched blood samples, they would not have been included in the dataset. This situation leads to left truncation and had to be accounted for in the analysis, as patients are not “observable” until they consent and provide the matched control blood sample.40
Conclusions
Patients with solid tumor malignancies are at increased risk for ATE, and the mechanisms responsible for this effect remain unclear. Using a large DNA tumor registry, we found that cancer somatic genetic alterations in the KRAS oncogene and the STK11 tumor suppressor gene modulate the risk for cancer-associated ATE. Further epidemiologic and functional studies will be necessary to confirm and further elucidate the role of these alterations in acute thromboembolism formation for this high-risk patient population.
Funding Support and Author Disclosures
This research was supported by a National Cancer Institute Cancer Center Support Grant (P30 CA008748). Dr Willeit was supported by a Dr. Johannes and Hertha Tuba Grant. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: ATE is an important cause of mortality in patients with solid cancer. Tumor somatic alterations in KRAS and STK11 were associated with an increased risk for arterial events. This effect was independent of cancer type and other cardiovascular disease risk factors.
TRANSLATIONAL OUTLOOK: A better understanding of the genomic determinants of cancer-associated ATE could contribute to improved risk stratification and primary prophylaxis.
Acknowledgments
The authors thank Omar Nahhas, MD, and the Marie-Josee and Henry R. Kravis Center for Molecular Oncology.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Zöller B., Ji J., Sundquist J., Sundquist K. Risk of coronary heart disease in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012;48:121–128. doi: 10.1016/j.ejca.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Zöller B., Ji J., Sundquist J., Sundquist K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer. 2012;48:1875–1883. doi: 10.1016/j.ejca.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Grilz E., Königsbrügge O., Posch F., et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. 2018;103:1549–1556. doi: 10.3324/haematol.2018.192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navi B.B., Reiner A.S., Kamel H., et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulder F.I., Horváth–Puhó E., Es Nv, et al. Arterial thromboembolism in cancer patients. J Am Coll Cardiol CardioOnc. 2021;3:205–218. [Google Scholar]
- 6.Navi B.B., Reiner A.S., Kamel H., et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. 2019;133:781–789. doi: 10.1182/blood-2018-06-860874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuzovic M., Herrmann J., Iliescu C., Marmagkiolis K., Ziaeian B., Yang E.H. Arterial thrombosis in patients with cancer. Curr Treat Options Cardiovasc Med. 2018;20:40. doi: 10.1007/s11936-018-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oren O., Herrmann J. Arterial events in cancer patients-the case of acute coronary thrombosis. J Thorac Dis. 2018;10:S4367–S4385. doi: 10.21037/jtd.2018.12.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drobni Z.D., Alvi R.M., Taron J., et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142:2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muhsin-Sharafaldine M.R., Saunderson S.C., Dunn A.C., Faed J.M., Kleffmann T., McLellan A.D. Procoagulant and immunogenic properties of melanoma exosomes, microvesicles and apoptotic vesicles. Oncotarget. 2016;7:56279–56294. doi: 10.18632/oncotarget.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rak J., Milsom C., May L., Klement P., Yu J. Tissue factor in cancer and angiogenesis: the molecular link between genetic tumor progression, tumor neovascularization, and cancer coagulopathy. Semin Thromb Hemost. 2006;32:54–70. doi: 10.1055/s-2006-933341. [DOI] [PubMed] [Google Scholar]
- 12.Gordon S.G., Hasiba U., Cross B.A., Poole M.A., Falanga A. Cysteine proteinase procoagulant from amnion-chorion. Blood. 1985;66:1261–1265. [PubMed] [Google Scholar]
- 13.Falanga A., Gordon S.G. Isolation and characterization of cancer procoagulant: a cysteine proteinase from malignant tissue. Biochemistry. 1985;24:5558–5567. doi: 10.1021/bi00341a041. [DOI] [PubMed] [Google Scholar]
- 14.Wahrenbrock M., Borsig L., Le D., Varki N., Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest. 2003;112:853–862. doi: 10.1172/JCI18882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang O.Y., Chung J.W., Lee M.J., et al. Cancer cell-derived extracellular vesicles are associated with coagulopathy causing ischemic stroke via tissue factor-independent way: the OASIS-CANCER study. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0159170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung J.W., Cho Y.H., Ahn M.J., et al. Association of cancer cell type and extracellular vesicles with coagulopathy in patients with lung cancer and stroke. Stroke. 2018;49:1282–1285. doi: 10.1161/STROKEAHA.118.020995. [DOI] [PubMed] [Google Scholar]
- 17.Thalin C., Demers M., Blomgren B., et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res. 2016;139:56–64. doi: 10.1016/j.thromres.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng D.T., Mitchell T.N., Zehir A., et al. Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zehir A., Benayed R., Shah R.H., et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunbar A., Bolton K.L., Devlin S.M., et al. Genomic profiling identifies somatic mutations predicting thromboembolic risk in patients with solid tumors. Blood. 2021;137:2103–2113. doi: 10.1182/blood.2020007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 22.Mantha S. CEDARS—Clinical Event Detection and Recording System. https://cedars.io
- 23.Cerami E., Gao J., Dogrusoz U., et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J., Aksoy B.A., Dogrusoz U., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 26.Bailey M.H., Tokheim C., Porta-Pardo E., et al. Comprehensive characterization of cancer driver genes and mutations. Cell. 2018;174:1034–1035. doi: 10.1016/j.cell.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Kim Y.D., Kim C.H. Incidence and risk of various types of arterial thromboembolism in patients with cancer. Mayo Clin Proc. 2021;96:592–600. doi: 10.1016/j.mayocp.2020.05.045. [DOI] [PubMed] [Google Scholar]
- 28.Darby S.C., Ewertz M., McGale P., et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 29.Verso M., Chiari R., Mosca S., et al. Incidence of Ct scan-detected pulmonary embolism in patients with oncogene-addicted, advanced lung adenocarcinoma. Thromb Res. 2015;136:924–927. doi: 10.1016/j.thromres.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Dou F., Li H., Zhu M., et al. Association between oncogenic status and risk of venous thromboembolism in patients with non-small cell lung cancer. Respir Res. 2018;19:88. doi: 10.1186/s12931-018-0791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corrales-Rodriguez L., Soulieres D., Weng X., Tehfe M., Florescu M., Blais N. Mutations in NSCLC and their link with lung cancer-associated thrombosis: a case-control study. Thromb Res. 2014;133:48–51. doi: 10.1016/j.thromres.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 32.Ades S., Kumar S., Alam M., et al. Tumor oncogene (KRAS) status and risk of venous thrombosis in patients with metastatic colorectal cancer. J Thromb Haemost. 2015;13:998–1003. doi: 10.1111/jth.12910. [DOI] [PubMed] [Google Scholar]
- 33.Maharaj S., Bhandari S., Wu X., Rai R., Sharma V. Presented at: International Society on Thrombosis and Haemostasis Virtual Congress; Philadelphia: 2021. Association of KRAS mutation with arterial thromboembolism in advanced lung and gastrointestinal cancer. July 17-21. [Google Scholar]
- 34.Yu J.L., May L., Lhotak V., et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–1741. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 35.Hillen H.F. Thrombosis in cancer patients. Ann Oncol. 2000;11(suppl 3):273–276. doi: 10.1093/annonc/11.suppl_3.273. [DOI] [PubMed] [Google Scholar]
- 36.Marmur J.D., Thiruvikraman S.V., Fyfe B.S., et al. Identification of active tissue factor in human coronary atheroma. Circulation. 1996;94:1226–1232. doi: 10.1161/01.cir.94.6.1226. [DOI] [PubMed] [Google Scholar]
- 37.Wilcox J.N., Smith K.M., Schwartz S.M., Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Misumi K., Ogawa H., Yasue H., et al. Comparison of plasma tissue factor levels in unstable and stable angina pectoris. Am J Cardiol. 1998;81:22–26. doi: 10.1016/s0002-9149(97)00801-1. [DOI] [PubMed] [Google Scholar]
- 39.AACR Project GENIE powering precision medicine through an international consortium. Cancer Discov. 2017;7:818–831. doi: 10.1158/2159-8290.CD-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown S., Lavery J.A., Shen R., et al. Implications of selection bias due to delayed study entry in clinical genomic studies. JAMA Oncol. 2022;8:287–291. doi: 10.1001/jamaoncol.2021.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.