Abstract
Background
Infectious exacerbations are crucial events that dictate the natural course of COPD patients. Pneumococcal vaccination has been shown to decrease incidence of community-acquired pneumonia in COPD patients. There is a paucity of data on outcomes of hospitalisation in pneumococcal-vaccinated COPD patients in comparison with unvaccinated subjects. The objectives of the present study were to evaluate the difference in hospitalisation outcomes in pneumococcal-vaccinated versus -unvaccinated COPD subjects hospitalised with acute exacerbation.
Methods
This was a prospective analytical study on 120 subjects hospitalised with acute COPD exacerbation. 60 patients with prior pneumococcal vaccination and 60 unvaccinated patients were recruited. Outcomes of hospitalisation such as mortality rate, need for assisted ventilation, length of hospital stay, need for intensive care unit (ICU) care and length of ICU stay were collected and compared between two groups with appropriate statistical tools.
Results
60% of unvaccinated patients (36 out of 60) required assisted ventilation, whereas only 43.3% of vaccinated subjects (26 out of 60) needed assisted ventilation (p-value of 0.04). Most of the secondary outcomes were better in the vaccinated group. The mean±SD length of ICU stay in the vaccinated group was 0.67±1.11 days compared to 1.77±1.89 days in the unvaccinated group. The mean±SD length of hospital stay was 4.50±1.64 days and 5.47±2.03 days in the vaccinated and unvaccinated group, respectively (p-value of 0.005).
Conclusions
COPD patients who have received prior pneumococcal vaccination have better outcomes when they are hospitalised for an acute exacerbation. Pneumococcal vaccination may be recommended for all patients with COPD who are at risk of hospitalisation with acute exacerbation.
Short abstract
Prior pneumococcal vaccination improves hospitalisation outcomes in COPD patients admitted with an acute exacerbation https://bit.ly/3XhX4Qr
Introduction
COPD is a condition characterised by persistent airflow limitation that is usually progressive and is associated with an enhanced inflammatory response in the airways and lungs to noxious particles and gases [1]. The burden of COPD in India is huge. Of the total global disability adjusted life-years (DALYs) due to chronic respiratory diseases in 2016, 32.0% occurred in India. The contribution of chronic respiratory diseases to the total DALYs in India increased from 4.5% (95% CI 4.0–4.9) in 1990 to 6.4% (95% CI 5.8–7.0) in 2016 [2]. COPD and asthma were responsible for 75.6% and 20.0% of the chronic respiratory disease DALYs, respectively, in India in 2016 [2].
The goals of COPD management include providing symptom relief, preserving lung function, ensuring acceptable quality of life, etc. Exacerbations are acute events that punctuate the natural course of COPD and are associated with worsening symptoms, escalation of pulmonary and systemic inflammation, worsening functional status and mortality [3, 4]. Exacerbations are increasingly recognised to be crucial events that can potentially dictate the natural course and longevity of COPD patients [5]. Prevention of future exacerbations can be seen as a key strategy in COPD management. The crucial role of bacterial and viral infections as exacerbation triggers has been recognised for decades. Susceptibility to infections poses a major threat in leading to exacerbations of COPD and translates to accelerated decline in pulmonary function [3]. Patients with COPD are at high risk of community-acquired pneumonia (CAP), which imposes a substantial disease burden. Streptococcus pneumoniae is the leading cause of CAP in India [6]. In Europe, the incidence of CAP was found to be >20-fold higher in persons with COPD (22.4 per 1000 person-years) than in the general population (1.07−1.2 per 1000 person-years) with the incidence dramatically higher in persons with severe COPD (forced expiratory volume in 1 s (FEV1) <50% predicted) [7].
Pneumococcal vaccination can substantially reduce the mortality and morbidity risks associated with pneumococcal diseases and is recommended for patients at high risk [8]. Humoral response is important to help eradicate the bacteria during primary infection, although these responses are not as robust during primary infection. Anticapsular polysaccharide antibodies are believed to represent the single most important protective mechanism against invasive disease. In fact, antibodies to pneumococcal polysaccharides were the basis of serum therapy in which passively transferred, serotype-specific antipneumococcal serum was shown to reduce mortality from pneumococcal pneumonia by half [9].
Pneumococcal vaccines can be broadly divided into the polysaccharide vaccines, which are antibodies to the capsular polysaccharides, and the conjugate vaccines. Since the immunogenicity of polysaccharides is traditionally low, conjugation improves the immunogenicity and immune memory. In 1983, the 23-valent polysaccharide vaccine (PPSV23) was approved for use in the United States for adults and children >2 years of age. This new formulation was developed after worldwide surveillance showed a high frequency of pneumococcal bacteraemia and meningitis by serotypes not covered in the previous 14-valent vaccine [10]. Use of PPSV23 has repeatedly been shown to provide a significant reduction in the rates of invasive pneumococcal disease but sadly, without impact on overall mortality or on overall rates of pneumonia [11]. Conjugate vaccines have been marketed as the 7-valent, 10-valent, 13-valent and 20-valent vaccines (PCV7, 10, 13 or 20). The impetus to administer conjugate vaccine in the elderly population was the robust immunogenicity data in this population. This fact, in conjunction with clinical trial results in immunocompromised populations, was the foundation for the recent substantial shift in adult immunisation practice towards conjugate vaccine. This strategy appears to be well supported by the compelling results observed in the CAPiTA study [12], with 45% efficacy in prevention of CAP and 75% efficacy in prevention of invasive pneumococcal disease.
Pneumococcal vaccination affords protection predominantly against invasive infections like bacteraemia, meningitis and pneumonia. Despite clear-cut recommendations by the Centre for Disease Control, adherence to pneumococcal vaccine among adult subjects has been traditionally low. Published Indian studies reveal that the uptake of pneumococcal vaccine remains low within the general public, elderly population and those with medical comorbidities [13, 14].
Most of the studies, reviews and meta-analyses on the efficacy of pneumococcal vaccination have looked at pneumococcal pneumonias or invasive pneumococcal diseases as the outcome measure [15, 16]. While these may be the best outcomes to look at from a vaccine efficacy point of view, clinically relevant outcomes for COPD patients would be exacerbation rates. A randomised, non-placebo controlled trial, however, showed decreased exacerbation rate in COPD patients after administration of a pneumococcal polyvalent vaccine [17].
Prior pneumococcal vaccination with a polysaccharide vaccine has been associated with a 40–70% reduction in risk of in-hospital death in a large cohort of hospitalised individuals with CAP [18]. Pneumococcal vaccine reduces bacteraemic pneumococcal disease in vaccinated subjects. Pneumococcal bacteraemia may translate to elevated mortality despite an adequate host immune response and appropriate antibiotic therapy because of the release of cell wall components by killed bacteria, which results in an inflammatory cascade causing death in experimental models of pneumococcal disease [19]. Mechanistically, prevention of such a “cytokine storm” would be consistent with the improved outcomes observed in this study. This mechanism might be extrapolated to patients hospitalised with acute exacerbations of COPD and translate to improved outcomes, although there is a lack of evidence in this regard. Further, pneumococcal-vaccinated subjects demonstrate better immunity to pneumococcal infections as well as related organisms in experimental mice (including influenza virus) via complex overlapping mechanisms, which may be postulated to contribute to improved survival and related outcomes in hospitalised COPD patients [20].
There are a lack of data on outcomes of hospitalisation in pneumococcal-vaccinated COPD patients versus -unvaccinated subjects. Further, there is a paucity of Indian data on the benefits of pneumococcal vaccination in COPD patients. The present study was undertaken to explore these aspects in COPD patients.
Objectives
The present study was carried out with the following objectives:
Primary objectives: to evaluate the difference in mortality rate and need for assisted ventilation in pneumococcal-vaccinated (with PCV 13) versus -unvaccinated COPD subjects who were hospitalised with acute exacerbation of COPD.
Secondary objectives: to evaluate the differences in other relevant clinical outcomes (occurrence of respiratory failure, length of hospital stay, need for intensive care unit (ICU) care, length of ICU stay) in pneumococcal-vaccinated (with PCV 13) versus -unvaccinated COPD subjects hospitalised with acute exacerbation of COPD.
Materials and methods
This was a prospective analytical study conducted in the department of Pulmonary Medicine, Rajagiri Hospital, Aluva, a tertiary care institution in Central Kerala, India. The study period spanned from September 2019 to September 2021, which included a 12-month recruitment period from September 2019 to September 2020 and a further follow-up period of 1 year after recruitment of the last study subject. The study population comprised patients hospitalised with an acute exacerbation of COPD within the study period.
Inclusion criteria
Patients with a previous diagnosis of COPD as per the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.
Patients hospitalised to the study centre with an acute exacerbation of COPD within the study period.
Both criteria needed to be met to qualify for inclusion in the study.
Exclusion criteria:
COPD patients whose previous pneumococcal vaccination status was unclear after personal interrogation and cross-checking of previous medical records.
Patients with overlapping chronic lung diseases like asthma, interstitial lung diseases, bronchiectasis (unrelated to COPD status). etc.
Patients with immunocompromised states including active HIV infection, active haematological or solid organ malignancies, primary immunodeficiencies, current usage of regular systemic steroids or other immunosuppressive agents, etc.
Patients who opted out of active management (e.g. care in the ICU, assisted ventilation, etc.) during their hospitalisation for acute exacerbation of COPD.
The presence of any one criterion led to exclusion from the study.
Sample size
Being a single centre study extending for a limited time period and in the absence of previous similar studies, an arbitrary sample size of 120 was fixed with 60 patients needing to be recruited into the pneumococcal-vaccinated as well as -unvaccinated group. Patients who had previously been administered the 13-valent pneumococcal conjugate vaccine were recruited into the vaccinated group.
Data collection and follow-up
The patients were interrogated immediately on hospitalisation and a detailed medical history including the vaccination status was obtained. Specific attention was paid to baseline demographic factors, medical comorbidities, stage and category of COPD, severity of exacerbation, etc. Focused physical examination was performed and findings were recorded. Results of investigations performed at hospitalisation (arterial blood gas analysis, chest radiograph findings and blood investigations, etc.) were noted. Patients were managed as per standard management guidelines for acute exacerbation of COPD. Attention was paid to site of admission (respiratory ward versus ICU), need for supplemental oxygen, assisted ventilation, etc. Subjects were followed up on a daily basis until discharge from hospital or death. Clinical course and follow-up tests, etc. were ascertained.
The outcome measures focused on included mortality, length of hospitalisation, occurrence of respiratory failure, need for assisted ventilation, need for ICU care, duration of ICU care and duration of mechanical ventilation. Comparisons were made between the vaccinated and unvaccinated subjects with regard to outcomes.
Statistical analysis
Data were entered using a Microsoft Excel spreadsheet and statistical analysis was done using SPSS software 25 version. The qualitative data were expressed as proportions or percentage and the quantitative data as mean±sd. Association between qualitative data was done using Chi-squared test and for quantitative data was done using unpaired t-test. A p-value <0.05 was considered as significant.
Ethical approval
The study protocol was approved by the institutional ethical committee (letter no. RAJH/SRC/DNB0026). It was observed that the study does not pose any ethical issues to the patient.
Results
Demographic characteristics
A total of 120 patients hospitalised with acute exacerbation of COPD were recruited into our study, 60 of whom were previously pneumococcal-vaccinated and 60 were unvaccinated. The mean±SD age of the study population was 72.43±8.764 years. The youngest patient was aged 49 years and the oldest patient was 92 years. Most of the patients (73.3%, 88 patients) belonged to the 61–80 years age group; 91 patients were male (75.8%) and 29 female (24.2%). The mean age of vaccinated patients was 4 years greater and the proportion of female patients was much higher in the vaccinated group compared to the unvaccinated group. Table 1 shows the age distribution of the study patients.
TABLE 1.
Age distribution of the study patients
| Age years | Vaccinated group | Unvaccinated group |
| 45–60 years | 3 (2.5) | 6 (5.0) |
| 61–80 years | 42 (35) | 46 (76.7) |
| >80 years | 15 (12.5) | 8 (13.3) |
| Total | 60 (50.0) | 60 (50) |
Data are presented as n (%).
Baseline characteristics
92 patients belonged to GOLD stage D, 21 belonged to GOLD stage C and seven belonged to stage B. 113 out of 120 (94.2%) patients had >2 exacerbation episodes in the previous year. The mean number of annual exacerbations in the study group was 3.17±1.16. The mean number of annual hospitalisations in the study subjects was 1.25±0.963. Systemic hypertension, diabetes mellitus and ischaemic heart disease were common medical comorbidities in the study patients, being present in 57.5%, 36.6% and 42.5% of patients respectively.
The baseline characteristics, including demography, smoking status, COPD severity, comorbid illness, etc., of the vaccinated and unvaccinated groups are showed in table 2. As previously mentioned, the mean age of vaccinated patients was 4 years greater and the proportion of female patients was much higher in the vaccinated group. The presence of medical comorbidities was much higher in the vaccinated group, and these differences were statistically significant. Fever and multilobar consolidation were seen in a much higher proportion of unvaccinated patients. Among the 60 patients vaccinated with conjugate vaccine (PCV 13), four had a history of receiving PPSV23 also, whereas 54 had not received PPSV23. In contrast, as per the protocol, patients who had not received any pneumococcal vaccine were recruited into the unvaccinated group only.
TABLE 2.
Relationship between baseline characters and vaccination status
| Parameter | Vaccinated group | Unvaccinated group | p-value |
| Age years, mean± sd | 74.43±8.039 | 70.42±9.060 | 0.01# |
| Male:female ratio | 37:23 | 54:6 | 0.002¶ |
| Smoking habit | 25 (41.6) | 25 (41.6) | 0.98¶ |
| Indoor biomass exposure | 18 (30.0) | 18(30.0) | 0.96¶ |
| COPD stage D | 46 (76.7) | 46 (76.7) | 0.19¶ |
| Systemic hypertension | 40 (66.7) | 29 (48.3) | 0.04¶ |
| Diabetes mellitus | 28(46.7) | 16 (26.7) | 0.02¶ |
| Ischaemic heart disease | 32 (53.3) | 19(31.7) | 0.02¶ |
| Chronic kidney disease | 12 (20.0) | 5 (8.3) | 0.06¶ |
| Chronic respiratory failure (before presentation) | 7 (11.6) | 2 (3.3) | 0.08¶ |
| Influenza vaccination in the previous year | 56 (93.3) | 54 (90.0) | 0.94¶ |
|
Respiratory failure
(new onset at presentation) |
33 (50.0) | 40 (66.6) | 0.13¶ |
| Fever at presentation | 13 (21.7) | 38 (63.3) | < 0.001¶ |
| Multilobar consolidation | 4 (6.7) | 36 (60.0) | < 0.001¶ |
Data are presented as n (%) unless otherwise stated. #: independent t-test; ¶: Chi-squared test.
Outcomes
118 out of the 120 patients were discharged from hospital. Only two patients (1.7%) died during the current hospital stay, both of whom belonged to the unvaccinated group. This difference was statistically non-significant. Persisting respiratory failure at discharge was 18.3% in the vaccinated group compared with 28.3% in the unvaccinated group. This difference was statistically non-significant (p-value of 0.13). No additional mortality was seen in either group in the first 30 days after discharge. Three patients from the vaccinated group and four patients from the unvaccinated group needed hospitalisation for another exacerbation in the 30 days following discharge.
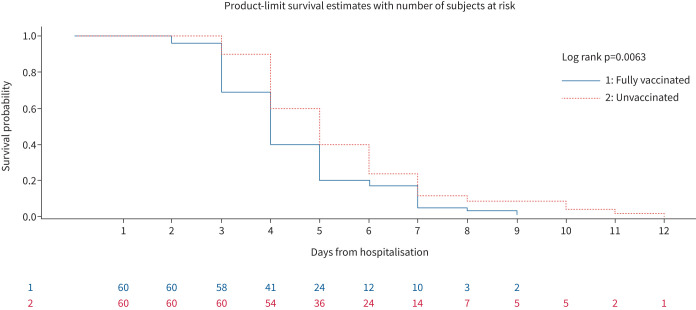
Assisted ventilation was required in 62 (51.7%) patients, 26 of whom were vaccinated and 36 unvaccinated. 60% of unvaccinated patients (36 out of 60) required assisted ventilation whereas only 43.3% of vaccinated subjects (26 out of 60) needed assisted ventilation. This difference was statistically significant with a p-value of 0.04. Figure 1 is a Kaplan–Meier graph which shows the proportion of patients surviving without the need for assisted ventilation during the current admission.
FIGURE 1.
Kaplan–Meier graph showing the probability of surviving without the need for assisted ventilation during the current admission.
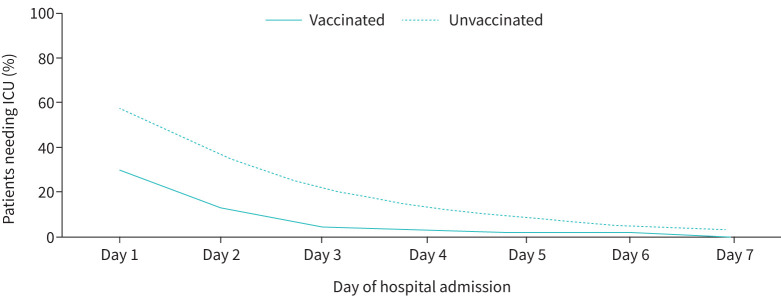
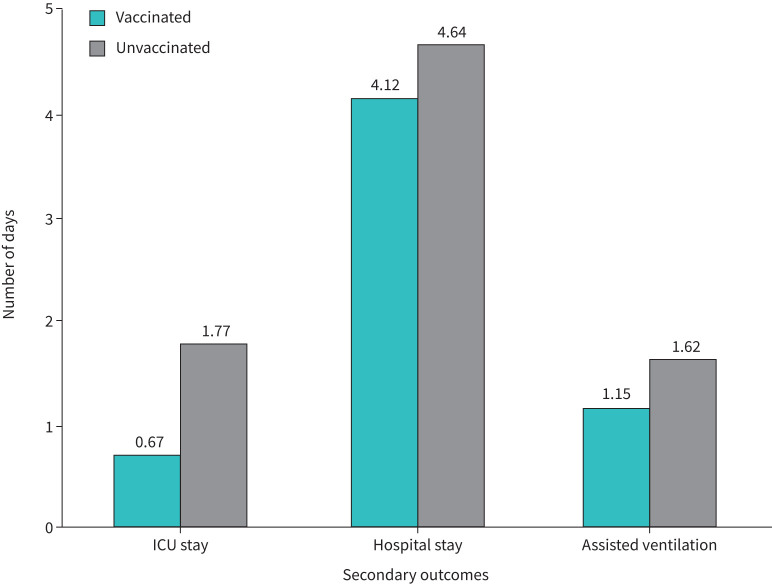
Most of the secondary outcomes were better in the vaccinated group of COPD patients. The place of initial admission was ICU in 53 patients (44.2%). 18 patients (30%) from the vaccinated group needed ICU admission, whereas 35 patients (58.3%) from the unvaccinated group needed ICU admission (p-value of 0.002). The mean length of ICU stay was 1.22±1.22 days in study subjects. Figure 2 shows the percentage of patients needing ICU stay on each day of hospitalisation during the present admission for COPD exacerbation. The length of ICU stay in the vaccinated group was 0.67±1.11 days compared to 1.77±1.89 in the unvaccinated group. The mean number of days of ventilation for those who needed assisted ventilation was 1.47±1.69 days. The mean duration of hospital stay for vaccinated versus unvaccinated groups was 5.39±1.68 days versus 6.06±2.14 days respectively. The mean length of hospital stay was 4.50±1.64 days and 5.47±2.03 days in the vaccinated and unvaccinated groups respectively (p-value of 0.005). The differences in clinically relevant secondary outcomes in vaccinated and unvaccinated subjects are summarised in table 3, and crucial differences are depicted in figure 3.
FIGURE 2.
Percentage of patients needing intensive care unit (ICU) stay on each day of hospitalisation during the present admission for COPD exacerbation.
TABLE 3.
Comparison of clinically relevant outcomes in vaccinated versus unvaccinated subjects
| Parameter | Vaccinated group | Unvaccinated group | p-value |
| In-hospital mortality | 0 (0) | 2 (1.7) | 0.13# |
| Need for assisted ventilation | 26 (43.3) | 36 (60.0) | 0.04# |
| Need for ICU care | 18 (30.0) | 35 (58.3) | 0.002# |
| Length of ICU stay days (n=53) | 0.67±1.11 | 1.77±1.890 | 0.001¶ |
| Length of hospital stay days | 4.12±1.48 | 4.64±1.57 | 0.008¶ |
| Number of days of assisted ventilation (n=62) | 1.15±1.52 | 1.73±1.79 | 0.04¶ |
| Number of patients with respiratory failure at discharge | 11 (18.3) | 17 (28.3) | 0.13¶ |
Data are presented as n (%) or mean±sd. ICU: intensive care unit; ¶: independent t-test; #: Chi-squared test.
FIGURE 3.
Differences in crucial clinical outcomes between vaccinated and unvaccinated patients.
Discussion
The HOPE COPD study has examined the outcomes of hospitalisation due to COPD exacerbations in pneumococcal-vaccinated versus -unvaccinated patients. 120 patients hospitalised with acute exacerbation of COPD were evaluated in the present study, 60 of whom had received prior pneumococcal vaccination and 60 of whom who had not.
In our study, fever, leukocytosis, multilobar pulmonary infiltrates and need for ICU care were significantly less in the vaccinated population. The occurrence of respiratory failure was higher among unvaccinated subjects, although this difference was not significant statistically. Similar results have been noted by other investigators also [21], where significant differences were observed between groups in age, some symptoms and radiological findings. Although there is no strong association between the radiographic patterns and the causative agents, consolidation is more frequently observed in pneumococcal pneumonia. Accordingly, lower rates of consolidation were observed in vaccinated persons with PCV 13, suggesting that pneumococcal conjugate vaccination may have had a strong preventive effect for pneumococcal pneumonia, or may have resulted in milder forms of radiographic presentation.
In the present study, clinical outcomes were better in the vaccinated group of patients. Prior pneumococcal vaccination decreases the incidence of bacteraemic pneumonia. Our findings are consistent with the observation that polyvalent pneumococcal polysaccharide vaccine reduces bacteraemic pneumococcal disease in adults and might translate to clinically relevant outcomes [22, 23]. Reports of reduction in pneumonia-related mortality among vaccinated individuals in observational studies performed in Sweden and Austria are also available. The vaccinated group had less severe disease as noticed by the decreased incidence of multilobar consolidation. This may be a manifestation of the protective efficacy of PCV 13 and would have translated to lesser ICU need and length of hospital stay. Both cases of mortality reported occurred in the unvaccinated group. However, considering the extremely small mortality number in the study patients, meaningful comparison of outcomes could not be done and the results were not statistically significant.
Fisman et al. [18] looked at 62 918 adults hospitalised with CAP between 1999 and 2003 of whom 7390 (12%) had a record of prior pneumococcal vaccination. Vaccine recipients were less likely to die of any cause during hospitalisation than were individuals with no record of vaccination (adjusted odds ratio (OR) 0.50; 95% CI 0.43–0.59), even after adjustment for the presence of comorbid illnesses, age, smoking and influenza vaccination. Vaccination also lowered the risk of respiratory failure (adjusted OR 0.67; 95% CI 0.59–0.76) and other complications and reduced median length of stay by 2 days, compared with unvaccinated individuals. Owing to the small number of subjects, the mortality rate is low in our study. A Turkish study [24] looked at the outcomes for pneumonia in patients vaccinated with pneumococcal vaccine and influenza vaccine and had contrasting observations. Although there was a trend for lower 30-day mortality and for lower rates of ICU admission, these did not reach statistical significance. A pneumonia severity index score ≥90, CURB-65 score ≥3 and multilobar involvement, but not vaccination status, were identified as independent determinants of ICU admission. The study concluded that prior vaccination does not appear to significantly affect the clinical outcomes.
In another study [25] the mortality rate and ICU admission numbers were lower among vaccinated patients. ICU admission numbers were reduced in the vaccinated group in our study also. A population-based cohort study involving elderly hospitalised patients with pneumonia in Canada demonstrated that PPV23 immunisation was associated with a reduced risk of death or further hospitalisation [26]. Supporting the benefits of pneumococcal vaccination, a prospective cohort study conducted by Vila-Corcoles et al. [27] in Spain found that PPV23 vaccination in the elderly was associated with a considerable reduction of risk of death from pneumonia, although the overall risk of pneumonia or hospitalisation from pneumonia was unaltered. Li et al. [28] in a US-based study, tested the effectiveness of pneumococcal vaccination and influenza vaccination in preventing CAP. They found that prior pneumococcal vaccination alone was not associated with shortened length of admission, reduced risk of inpatient mortality or risk of respiratory complications compared with no vaccination; however being vaccinated for pneumococcal infection and influenza before CAP admission was associated with a 10% reduction in length of admission. The variation in patient characteristics, treatment protocols, duration after vaccination and study protocols would have accounted for the differences in observations observed across different studies. However, the majority of studies suggest a benefit in terms of clinical outcome with the pneumococcal vaccine.
The present study is unique in that it has examined the hospitalisation outcomes of COPD patients who were admitted for acute exacerbations, whereas previous studies have looked at patients admitted with CAP. The outcomes of the present study have been unequivocally beneficial in the prior vaccinated subjects.
Limitations of the study
The present study has some limitations. In the absence of a previous study of this sort in hospitalised COPD patients, the sample size has been arbitrarily chosen. All patients in the vaccinated group had previously received the PCV13 vaccine. Some patients in the vaccinated group had received the pneumococcal polysaccharide vaccine also, which might have an impact on the outcomes. However, only four patients in the vaccinated group had received PPSV13, and this small number is unlikely to have an impact on the overall outcome. The vaccinated patients received pneumococcal vaccination at various time points. The level of protection afforded by the polysaccharide vaccine is known to wane over time [29], which may have an impact on patient outcomes. Studies using conjugate vaccine have revealed that sustained immunity against pneumococcal disease is maintained [30]. However, only the vaccination history in the last 5 years was recorded, and the immunity is unlikely to wane in this period. During the course of hospitalisation and in-hospital care for acute exacerbation of COPD, the study investigators were not blinded with regard to vaccination status. Our hospital, though, follows a standardised protocol for management of COPD exacerbations that is unlikely to be biased by the prior pneumococcal vaccination status of the patient. However, the authors perceive that the impact of these limitations on the study outcomes and conclusions is minimal. Given the similar nature of COPD patients hospitalised across various institutions, we feel that the generalisability (external validity) of this study is high. Nevertheless, prospective randomised trials with a larger sample size are encouraged to overcome the limitations of this study.
Conclusions
The HOPE COPD study has attempted to examine the differences in clinically relevant outcomes in pneumococcal-vaccinated versus -unvaccinated COPD patients who are hospitalised with an acute exacerbation. The in-hospital and post-discharge mortality rate among the study subjects was very small and does not permit meaningful conclusions regarding any mortality benefit. COPD patients who have received prior pneumococcal vaccination have better outcomes including a lesser need for ICU admission, a smaller proportion requiring assisted ventilation, a shorter hospital stay, shorter duration of ICU stay, etc. Pneumococcal vaccination is recommended for all patients with COPD who are at risk of hospitalisation with acute exacerbation as it improves hospitalisation outcomes.
Footnotes
Provenance: Submitted article, peer reviewed.
Conflicts of interest: None to declare.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2022. Available from: http://goldcopd.org
- 2.Salvi S, Kumar GA, Dhaliwal RS, et al. India State-Level Disease Burden Initiative CRD Collaborators . The burden of chronic respiratory diseases and their heterogeneity across the states of India: the Global Burden of Disease Study 1990-2016. Lancet Glob Health 2018; 6: e1363-e1374. doi: 10.1016/S2214-109X(18)30409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mannino DM, Watt G, Hole D, et al. The natural history of chronic obstructive pulmonary disease. Eur Respir J 2006; 27: 627–643. doi: 10.1183/09031936.06.00024605 [DOI] [PubMed] [Google Scholar]
- 4.Soler-Cataluna JJ, Martinez-Garcia MA, Roman SP, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60: 925–931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suissa S, Dell'Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012; 67: 957–963. doi: 10.1136/thoraxjnl-2011-201518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal S, Kashyap S, Pal LS, et al. Clinical and bacteriological profile of community acquired pneumonia in Shimla, Himachal Pradesh. Indian J Chest Dis Allied Sci 2004; 46: 17–22. [PubMed] [Google Scholar]
- 7.Müllerova H, Chigbo C, Hagan GW, et al. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med 2012; 106: 1124–1133. doi: 10.1016/j.rmed.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 8.Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 2019; 68: 1069–1075. doi: 10.15585/mmwr.mm6846a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berical AC, Harris D, Dela Cruz CS, et al. Pneumococcal vaccination strategies. An update and perspective. Ann Am Thorac Soc 2016; 13: 933–944. doi: 10.1513/AnnalsATS.201511-778FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins JB, Austrian R, Lee CJ, et al. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis 1983; 148: 1136–1159. doi: 10.1093/infdis/148.6.1136 [DOI] [PubMed] [Google Scholar]
- 11.Fine MJ, Smith MA, Carson CA, et al. Efficacy of pneumococcal vaccination in adults: a meta-analysis of randomized controlled trials. Arch Intern Med 1994; 154: 2666–2677. doi: 10.1001/archinte.1994.00420230051007 [DOI] [PubMed] [Google Scholar]
- 12.Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372: 1114–1125. doi: 10.1056/NEJMoa1408544 [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran D, Venkitakrishnan R, Augustine J, et al. Pneumococcal vaccination among adults with respiratory diseases: indications, adherence and roadblocks. J Clin Diagn Res 2018; 12: OC20-OC23. [Google Scholar]
- 14.Gupta AS, Venkitakrishnan R, Augustine J, et al. Pneumococcal vaccination in pulmonary outpatient practice: how far is the adherence and what are the challenges? Eur J Pharmac Med Res 2019; 6: 632–635. [Google Scholar]
- 15.Lee TA, Weaver FM, Weiss KB. Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. J Gen Intern Med 2007; 22: 62–67. doi: 10.1007/s11606-007-0118-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfageme I, Vazquez R, Reyes N, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax 2006; 61: 189–195. doi: 10.1136/thx.2005.043323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohanty T, Patro M, Sahoo J, et al. Effectiveness of pneumococcal vaccine in patients with chronic obstructive pulmonary disease (COPD). Int J Res Med Sci 2018; 6: 3698–3704. doi: 10.18203/2320-6012.ijrms20184433 [DOI] [Google Scholar]
- 18.Fisman DN, Abrutyn E, Spaude KA, et al. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis 2006; 42: 1093–1101. doi: 10.1086/501354 [DOI] [PubMed] [Google Scholar]
- 19.Brown JS, Hussell T, Gilliland SM, et al. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc Natl Acad Sci USA 2002; 99: 16969–16974. doi: 10.1073/pnas.012669199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest 2011; 121: 3657–3665. doi: 10.1172/JCI57762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cetinoglu DE, Uzaslan E, Sayıner A, et al. Pneumococcal and influenza vaccination status of hospitalized adults with community acquired pneumonia and the effects of vaccination on clinical presentation. Hum Vaccines Immunother 2017; 13: 2072–2077. doi: 10.1080/21645515.2017.1339851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson LA, Neuzil KM, Yu O, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348: 1747–1755. doi: 10.1056/NEJMoa022678 [DOI] [PubMed] [Google Scholar]
- 23.Mangtani P, Cutts F, Hall AJ. Efficacy of polysaccharide pneumococcal vaccine in adults in more developed countries: the state of the evidence. Lancet Infect Dis 2003; 3:71–78. doi: 10.1016/S1473-3099(03)00514-0 [DOI] [PubMed] [Google Scholar]
- 24.Candemir I, Turk S, Ergun P, et al. Influenza and pneumonia vaccination rates in patients hospitalized with acute respiratory failure. Hum Vaccin Immunother 2019; 15: 2606–2611. doi: 10.1080/21645515.2019.1613128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mykietiuk A, Carratalà J, Domínguez A, et al. Effect of prior pneumococcal vaccination on clinical outcome of hospitalized adults with community-acquired pneumococcal pneumonia. Eur J Clin Microbiol Infect Dis 2006; 25: 457–462. doi: 10.1007/s10096-006-0161-8 [DOI] [PubMed] [Google Scholar]
- 26.Johnstone J, Marrie TJ, Eurich DT, et al. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med 2007; 167: 1938–1943. doi: 10.1001/archinte.167.18.1938 [DOI] [PubMed] [Google Scholar]
- 27.Vila-Córcoles A, Ochoa-Gondar O, Hospital I, et al. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 2006; 43: 860–868. doi: 10.1086/507340 [DOI] [PubMed] [Google Scholar]
- 28.Li C, Gubbins PO, Chen GJ. Prior pneumococcal and influenza vaccinations and in-hospital outcomes for community-acquired pneumonia in elderly veterans. J Hosp Med 2015; 10: 287–293. doi: 10.1002/jhm.2328 [DOI] [PubMed] [Google Scholar]
- 29.van Westen E, Knol MJ, Wijmenga-Monsuur AJ, et al. Serotype-specific IgG antibody waning after pneumococcal conjugate primary series vaccinations with either the 10-valent or the 13-valent vaccine. Vaccines (Basel) 2018; 6: 82. doi: 10.3390/vaccines6040082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on antipneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013; 31: 3594–3602. doi: 10.1016/j.vaccine.2013.04.084 [DOI] [PubMed] [Google Scholar]