Abstract
Background
Biologics have proven efficacy for patients with severe asthma but there is lack of consensus on defining response. We systematically reviewed and appraised methodologically developed, defined and evaluated definitions of non-response and response to biologics for severe asthma.
Methods
We searched four bibliographic databases from inception to 15 March 2021. Two reviewers screened references, extracted data, and assessed methodological quality of development, measurement properties of outcome measures and definitions of response based on COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN). A modified GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach and narrative synthesis were undertaken.
Results
13 studies reported three composite outcome measures, three asthma symptoms measures, one asthma control measure and one quality of life measure. Only four measures were developed with patient input; none were composite measures. Studies utilised 17 definitions of response: 10 out of 17 (58.8%) were based on minimal clinically important difference (MCID) or minimal important difference (MID) and 16 out of 17 (94.1%) had high-quality evidence. Results were limited by poor methodology for the development process and incomplete reporting of psychometric properties. Most measures rated “very low” to “low” for quality of measurement properties and none met all quality standards.
Conclusions
This is the first review to synthesise evidence about definitions of response to biologics for severe asthma. While high-quality definitions are available, most are MCIDs or MIDs, which may be insufficient to justify continuation of biologics in terms of cost-effectiveness. There remains an unmet need for universally accepted, patient-centred, composite definitions to aid clinical decision making and comparability of responses to biologics.
Short abstract
There are no patient-centred composite measures of response to biologics for severe asthma. Single outcome measures are available but do not meet quality standards. A composite measure is required that is developed with patients. https://bit.ly/3FOJcXY
Introduction
According to the European Respiratory Society/American Thoracic Society (ERS/ATS) guidelines, severe asthma is defined as asthma requiring treatment based on Global Initiative for Asthma (GINA) steps 4–5 for the previous year or oral corticosteroids for ≥50% of the previous year either to prevent the disease becoming uncontrolled or disease which remains uncontrolled despite this therapy [1]. Even though severe asthma only affects 5–10% of the total population with asthma [1], it represents a significant socioeconomic [2–6], psychological [7, 8] and treatment [9] burden, and is also be associated with risk of mortality [10, 11].
Over the past decades, new biological drugs have demonstrated a positive impact on the lives of many patients with severe asthma by reducing the frequency of exacerbations and dose of oral corticosteroids, and by improving lung function [12–15]. Recently, in addition to total IgE, blood eosinophil counts and fractional exhaled nitric oxide (FENO) have been suggested as a guide to initiate anti-IgE treatment in adolescents and adults [16]. Furthermore, blood eosinophil counts have been used to select patients for anti-interleukin (IL)-5 in adults [16], and FENO/blood eosinophil counts for dupilumab in adolescents and adults [17]. Several studies have described the characteristics of patients who started biologics [18, 19] and the characteristics of responders to treatment [20–23]. It has been shown that some patients reached a “super-response” [24] or “partial response” [25], whereas others experienced a “non-response” [24] or even deterioration [26] of clinical and patient-reported outcome measures (PROMs).
Although many studies have measured responses to different biologics, there are no universally accepted criteria for what constitutes response, and the absence of guidance on criteria is reported as a high-priority research gap in both children and adults [27, 28]. Evidence about responder definitions is critical for understanding the effectiveness of treatment for patients [29], clinicians and regulatory bodies, such as the European Medicines Agency [30] and the Food and Drug Administration [31]. Minimal clinically important difference (MCID) [32] and minimal important difference (MID) [33] are often used for assessing responses; these are defined as the smallest relevant within-person change or group differences between treatments, respectively. According to the Food and Drug Administration report, it is useful to report intra-subject responses based on an a priori responder definition [31]. In November 2016, an ERS Task Force reached a consensus on a traffic-light system to classify patients as non-responders, intermediate responders or super-responders [34]. The Task Force suggested that patients need to be on biological treatment for at least 4 months before an initial assessment of response can be determined [34]. However, this proposal has neither been validated nor further developed.
Given the unmet need to use consistent definitions of response for paediatric and adult patients, we aimed to 1) synthesise evidence about definitions of non-response and response to biological therapy used in patients with severe asthma, 2) assess the quality of the evidence for these definitions, and 3) evaluate the development, measurement properties and quality of outcome measures as supporting evidence for the included definitions. We chose to restrict our systematic review to studies where definitions were methodologically developed, defined and evaluated. Comprehensive assessment of response in clinical practice and trials using prespecified consensus criteria should provide useful guidance for clinical decision making, allow comparison across studies, eliminate unnecessary treatment in patients with inadequate response and ensure that the high cost associated with biological therapies [35] is justified [36].
Methods
This was a systematic review conducted by the 3TR (Taxonomy, Treatment, Targets and Remission) [37] Respiratory Work Package members and external collaborators including academic clinicians, regulatory, patient and pharmaceutical representatives from across Europe. The study is registered at PROSPERO with identifier number CRD42021211249. Our aim was to look at response in severe asthma, but in anticipation that the evidence base would be limited, we initially included studies of all severities of asthma. However, given that there is evidence for definitions of response to biological therapy for severe asthma, the protocol was revised to restrict the systematic review to studies of severe asthma. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist has been used to structure this article (supplementary appendix 1) [38]. The methods are briefly described here. Details are available in the supplementary material.
Search strategy
Four databases were searched (Embase (OVID), MEDLINE (OVID), CINAHL (EBSCOhost, Cumulative Index to Nursing and Allied Health Literature) and ISI Web of Science (Thomson Web of Knowledge)) using a search strategy developed on Embase (OVID) and then adapted for other databases (supplementary appendix 2). In summary, the search strategy was designed to identify papers focused on “asthma” AND “a biological therapy” AND “response/treatment outcome/minimal important difference”. Databases were searched from inception to 15 March 2021. Additional references were searched through the references cited by the identified studies, systematic reviews, reviews, guidelines or highlighted by experts in the field.
Inclusion criteria
Studies were eligible for inclusion if they met the following criteria. 1) Population: children/adolescents (6 –17 years) and/or adults (≥18 years) with a diagnosis of severe asthma. 2) Intervention: any biological therapy which was investigated and/or currently used for severe asthma. 3) Comparator: any comparator, including placebo or no comparator. 4) Outcomes: any definitions of non-response and response to biological therapy for severe asthma which were methodologically developed, defined and evaluated. Sole or a composite of clinical, patient-reported, biological and/or imaging outcome measures were eligible for inclusion. Additional evidence about these outcome measures including development (undertaken in studies of any severity of asthma) and validation (conducted in studies with biologics for severe asthma) was included. 5) Study types: randomised controlled trials, cross-sectional studies, controlled before-and-after studies, non-randomised controlled studies, case–control studies in humans, cohort studies and consecutive case series (with a minimum of 10 participants) published as full-text articles and letters published in English were eligible for inclusion. Additional evidence about development and validation of outcome measures was considered from qualitative and validation studies.
Exclusion criteria
The following were excluded from the analysis: systematic reviews and meta-analyses, narrative reviews, discussion papers, editorials, commentaries, case reports, animal studies, conference abstracts, studies not available in full form, studies published in a language other than English, unpublished material and non-asthma studies (e.g. viral bronchiolitis or viral-associated wheeze). Studies were also excluded if they only used outcome measures and definitions of response to assess treatment effectiveness or efficacy.
Study selection
All references were pooled and de-duplicated in Endnote version X9 (Thomson Reuters, Philadelphia, PA, USA) and subsequently uploaded to Rayyan (https://rayyan.qcri.org), where any remaining duplicates were removed. Titles, abstracts and full texts were screened independently by two reviewers (E.K. and A.R.) according to the predefined selection criteria and categorised as included, excluded or unsure. Any disagreements were resolved through discussion with a third reviewer (G.R.).
Data extraction, risk of bias assessment, quality and synthesis of the results
Data extraction was based on the COnsensus-based Standards for the selection of Measurement INstruments (COSMIN) guideline for outcome measures [39]. Definitions of the measurement properties provided by COSMIN are provided in supplementary table S1 and criteria for good measurement properties (GMPs) are provided in supplementary table S2.
Risk of bias of individual studies was assessed using the COSMIN checklist for PROMs [40, 41] and composite outcome measures (COSMIN risk of bias for non-patient-reported outcomes) [42]. Risk of bias for each measurement property in the validation studies was rated as very good, adequate, doubtful or inadequate. The certainty of evidence was assessed using the modified GRADE (Grading of Recommendations, Assessment, Development and Evaluation) approach [39, 41, 43]. Data extraction, risk of bias assessment and modified GRADE were completed independently by two reviewers (E.K. and A.R.). Any discrepancies were resolved by discussion or by a third reviewer (G.R.). A descriptive synopsis with summary data tables was produced and results were summarised using narrative synthesis. Detailed methods are provided in supplementary appendix 3. The results were reviewed and discussed within the Core Outcome Measures for Severe Asthma (COMSA) initiative [44] that included a multidisciplinary, European group of academic clinicians, regulatory, patient and pharmaceutical representatives. The group aimed to select the core outcome measure sets for paediatric and adult severe asthma.
Results
Description of studies
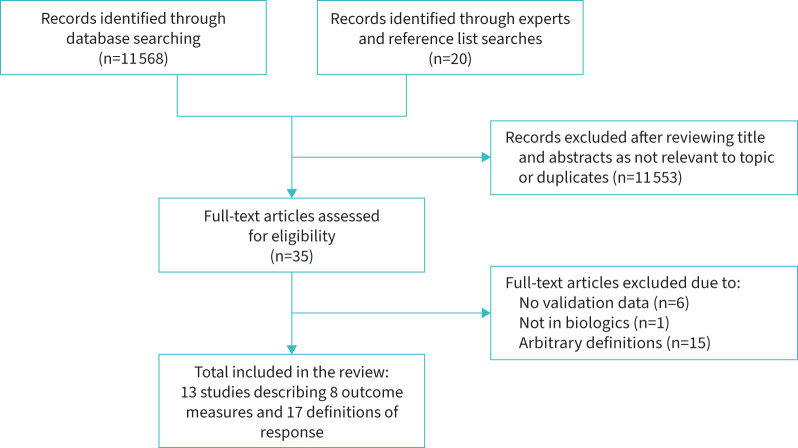
Our search strategy identified a total of 11 588 papers; 11 553 articles were excluded after title and abstract screening. The full text of 35 papers was assessed for eligibility, including 20 articles identified through review of citations. 13 papers were included in the systematic review, of which three were about development of the outcome measures [45–47], five were validation papers [48–52], and five reported development and validation data in the same paper [53–57] (figure 1).
FIGURE 1.
PRISMA diagram demonstrating study selection.
Development and quality of definitions of non-response and response
The approach to development of definitions and their characteristics are shown in tables 1 and 2. Definitions were developed for three composite asthma outcome measures [52–54], three asthma symptom outcome measures [50, 51], one asthma control outcome measure [56] and one quality of life (QoL) measure [49]. The following methods of development were used: consensus [54, 56], anchor-based [49–52] and distribution-based [53] methods. 10 definitions measured response based on MCID [49, 50, 52] or MID [51, 53] and seven [51, 56] based on responder/non-responder levels. Omalizumab [49, 52, 53, 56], brodalumab [51], benralizumab [49, 54], reslizumab [49, 54] and mepolizumab [49, 50, 54] were predominantly used in these studies. Response was evaluated at different time-points, including as early as 4 weeks [49] and up to 60 weeks [52]. Most definitions were developed for adults [49–51, 53, 54], while three were for adolescents [50, 52, 53] and one was for children [52] with severe asthma. Quality of evidence for definitions of response was rated as “high” for all except “moderate” for the Asthma Severity Scoring System (ASSESS) [53] due to a lower number of patients taking biologics.
TABLE 1.
Characteristics of included studies
| Study, year [ref.] | Scale | Study design | Patients (n) | Age (years) | Patient characteristics | Asthma severity (severe %) | Definition of asthma | Biological therapy |
| Composite outcome measures | ||||||||
| Fitzpatrick, 2020 [53] | ASSESS | Post-hoc analysis of 2 RCTs | 562 | 44±0.7 | Female 64.1%; FEV1 74.2±0.9% predicted |
Mild to severe (58.4%) | Modified ERS/ATS | Omalizumab (n=43) |
| Krouse, 2017 [52]# | CASI | Post-hoc analysis of RCT | 419 | 10.8 (IQR 8–14) | Female 42.2%; FEV1 92.0% predicted |
Mild to severe (54.0%) | NAEPP | Omalizumab (n=208) |
| Perez de Llano, 2021 [54] | FEOS | NR | 14 | NR | NR | Severe (100.0%) | GINA step 5, ERS/ATS | Reslizumab (n=6), mepolizumab (n=5), benralizumab (n=3) |
| Asthma symptom outcome measures | ||||||||
| Shen, 2021 [50] | ASUI | Post-hoc analysis of RCT | 497 | 51.0±13.6 | Female 59.2%; FEV1 58.8±15.7% predicted |
Severe eosinophilic (100.0%) | ERS/ATS | Mepolizumab (n=269) |
| Shen, 2021 [50] | ASI | Post-hoc analysis of RCT | 497 | 51.0±13.6 | Female 59.2%; FEV1 58.8±15.7% predicted |
Severe eosinophilic (100.0%) | ERS/ATS | Mepolizumab (n=269) |
| Globe, 2019 [51] | ASD | Post-hoc analysis of RCT | 417 | 47.3±13.6 | Female 59.0% | Moderate-severe | Doctor-diagnosed | Brodalumab (n=283) |
| Asthma control outcome measures | ||||||||
| Lloyd, 2007 [56] | GETE | Post-hoc analysis of 3 RCTs | 1380 | 12–76¶ | NR | Moderate-severe | GINA, ATS, NHLBI | Omalizumab+ |
| Asthma quality of life outcome measures | ||||||||
| Masoli, 2021 [49] | SAQ | Longitudinal cohort | 110 | 49.0 | Female 69.0%; FEV1 67.0% predicted |
Severe (100.0%) | ERS/ATS | Omalizumab (n=16), mepolizumab (n=26), benralizumab (n=62), reslizumab (n=2) |
ACT: Asthma Control Test; ATS: American Thoracic Society; ASSESS: Asthma Severity Scoring System; ASUI: Asthma Symptom Utility Index; ASI: Asthma Symptom Index; ASD: Asthma Symptom Diary; CASI: Composite Asthma Severity Index; ERS: European Respiratory Society; FEOS: FEV1, exacerbations, oral corticosteroids, symptoms score; FEV1: forced expiratory volume in 1 s; GETE: Global Evaluation of Treatment Effectiveness; GINA: Global Initiative for Asthma; IQR: interquartile range; NHLBI: National Heart, Lung, and Blood Institute; NAEPP: National Asthma Education and Prevention Program; NR: not reported; RCT: randomised controlled trial; SAQ: Severe Asthma Questionnaire. #: definition was developed in mild to severe asthma and then evaluated in patients taking biological therapy; ¶: inclusion criteria are reported as the mean age of the participants is unclear; +: n=1380 patients from the randomised, placebo-controlled, double-blind studies were included in the analysis.
TABLE 2.
Definitions of non-response and response to biological therapy for severe asthma and their quality of evidence
| Study, year [ref.] | Scale | Patient input in scale development | Time-point from baseline | Method of development of definition of response | Definition of response | Range of scores | GRADE |
| Composite outcome measures | |||||||
| Fitzpatrick, 2020 [53] | ASSESS | No | 12 months | Distribution-based method | MID 2 points | 0–20 points (higher=worse) | ⊕⊕⊕○A |
| Krouse, 2017 [52]# | CASI | No | 60 weeks | Anchor-based method | MCID 1 point | 0–18 points (higher=worse) | ⊕⊕⊕⊕ |
| Perez de Llano, 2021 [54] | FEOS | No | NR | Delphi exercise, conjoint analysis | Response defined according to different thresholds for each outcome measure with respect to baseline; response ranges from 0 (worsening) to 100 (best) | 0–100 points (higher=better) | ⊕⊕⊕⊕ |
| Asthma symptom outcome measures | |||||||
| Shen, 2021 [50] | ASUI | Yes | 12 weeks | Anchor-based method | MCID 0.07 to 0.11 | 0–1 points (higher=better) | ⊕⊕⊕⊕ |
| Shen, 2021 [50] | ASI | Yes | 12 weeks | Anchor-based method | MCID −0.42 to −0.26 | 0–3 points (higher=worse) | ⊕⊕⊕⊕ |
| Globe, 2019 [51] | ASD¶ | Yes | 12, 24 weeks | MID (change −0.5 to −1.0 ACQ); responder (change ≤ −1.0 ACQ) | Reported for 12 and 24 weeks: Mean 7-day score: MID −0.35 and −0.35; responder −0.54 and −0.68 7-day symptomatic days: MID −1.75 and −1.98; responder −2.34 and −3.22 Minimal symptomatic days-1: MID 1.97 and 2.16; responder 2.43 and 3.23 Minimal symptomatic days-2: MID 1.02 and 1.36; responder 2.31 and 2.56 |
0–4 points (higher=worse) | ⊕⊕⊕⊕ |
| Asthma control outcome measure | |||||||
| Lloyd, 2007 [56] | GETE | No | 28 weeks | Physician consensus | Responder (complete control; marked improvement of asthma); non-responder (discernible, but limited improvement in asthma, no appreciable change in asthma; worsening of asthma) | 0–5 points (higher=better) | ⊕⊕⊕⊕ |
| Asthma quality of life outcome measure | |||||||
| Masoli, 2021 [49] | SAQ | Yes | 4, 8, 12 weeks | Anchor-based method | MCID (SAQ) 0.5 points; MCID (SAQ-global) 11 points | SAQ: 1–7 points; SAQ-global: 0–100 points (higher=better) | ⊕⊕⊕⊕ |
ACQ: Asthma Control Questionnaire; ASSESS: Asthma Severity Scoring System; ASUI: Asthma Symptom Utility Index; ASI: Asthma Symptom Index; ASD: Asthma Symptom Diary; CASI: Composite Asthma Severity Index; FEOS: forced expiratory volume in 1 s, exacerbations, oral corticosteroids, symptoms score; GETE: Global Evaluation of Treatment Effectiveness; GRADE: Grading of Recommendations, Assessment, Development and Evaluation; MCID: Minimal Clinically Important Difference; MID: Minimal Important Difference; NR: not reported; SAQ: Severe Asthma Questionnaire. #: definition was developed in mild to severe using anchor-based method and then evaluated in biologicals (MID was changed to MCID by the review team); ¶: symptomatic days (defined as mean of the 10 ASD daily symptom items ≥1, otherwise non-symptomatic day), minimal symptom days-1 (defined as mean of the 10 ASD daily symptom items ≤1 and no single symptom item score>1, otherwise non-minimal symptom day-1) and minimal symptom days-2 (defined as no single ASD daily symptom item). Certainty of evidence was assessed using the GRADE approach [39, 41, 43]. The reason for downgrading was: A: indirectness.
Development and content validity of the outcome measures
An overview of the developmental process and its quality are shown in table 2 and supplementary table S3. The developmental process was predominantly rated as “sufficient”, while quality of evidence was mainly “very low” to “low”, but “moderate” for the Severe Asthma Questionnaire (SAQ) [46, 55]. Three composite outcome measures were developed by physicians, including FEOS (forced expiratory volume in 1 s (FEV1), exacerbations, oral corticosteroids, symptoms score) [54] for adults and ASSESS [53] which was adapted from the Composite Asthma Symptom Index (CASI) [57] for adolescents/adults and children with asthma, respectively. The Global Evaluation of Treatment Effectiveness (GETE) [56] scale was also developed by physicians. Only four outcomes were developed with patient input, including the SAQ [46, 55], Asthma Symptom Diary (ASD) [45], Asthma Symptom Utility Index (ASUI) [50] and Asthma Symptom Index (ASI) [47], which was adapted from the ASUI by excluding questions about medication side-effects. A summary of key instrument characteristics and feasibility is provided table 3 and supplementary table S4.
TABLE 3.
Summary of the characteristics of the outcome measures
| Recall period | Outcome measure content | |||||||||
| ACT | Asthma control | Albuterol day/night | Asthma symptoms | Exacerbations | Asthma medications | mOCS | FEV1 | Quality of life | ||
| ASSESS [53 ] | Current (FEV1, asthma medications); 4 weeks (ACT); 6 months (exacerbations) | X | X | X | X | |||||
| CASI [57] | Current (FEV1, asthma medications); 2 weeks (symptoms, albuterol use); 2 months (exacerbations) | X | X | X | X | X | ||||
| FEOS [54] | Baseline to current (FEV1 and mOCS); 4 weeks (ACT); 12 months (severe exacerbations) | X | X | X | X | |||||
| ASUI [50] | 2 weeks | X | ||||||||
| ASI [50] | 2 weeks | X | ||||||||
| ASD [45] | Current (morning and evening) | X | ||||||||
| GETE [56] | Baseline to current | X | ||||||||
| SAQ [46] | 2 weeks | X | ||||||||
ACT: Asthma Control Test; ASSESS: Asthma Severity Scoring System; ASUI: Asthma Symptom Utility Index; ASI: Asthma Symptom Index; ASD: Asthma Symptom Diary; CASI: Composite Asthma Severity Index; GETE: Global Evaluation of Treatment Effectiveness; FEOS: FEV1, exacerbations, oral corticosteroids, symptoms score; FEV1: forced expiratory volume in 1 s; mOCS: maintenance oral corticosteroids; SAQ: Severe Asthma Questionnaire. The ASUI and ASI measure frequency and severity of asthma symptoms (cough, wheeze, shortness of breath and night-time awakening), while the ASD measures morning and evening symptoms separately (wheeze, shortness of breath, cough, chest tightness, night-time awakening or impairment of daily activities). The GETE measures effectiveness of biological treatment based on physician and patient view separately.
Risk of bias and quality of evidence for validation studies of outcome measures
Validation data including risk of bias are shown in supplementary tables S5–S7 and methodological quality of the outcome measures rated against criteria for GMPs is presented in table 4. Overall, almost all outcome measures had “inadequate” risk of bias due to lack of involvement of patients in the development, many measurement properties not being reported and none of the studies reporting cross-cultural validity including measurement invariance.
TABLE 4.
Evaluation of outcome measures against good measurement properties (GMPs) and their quality of evidence
| ASSESS [53] | CASI [57]# | FEOS [54] | ASUI [47, 50] | ASI [50] | ASD [45, 51] | GETE [56]¶ | SAQ [46, 48, 49, 55]+ | |||||||||
| Rating | GRADE | Rating | GRADE | Rating | GRADE | Rating | GRADE | Rating | GRADE | Rating | GRADE | Rating | GRADE | Rating | GRADE | |
| Relevance | + | ⊕○○○A,B,C | + | ⊕○○○A,C | + | ⊕○○○A,C | ± | ⊕⊕○○A,C | ± | ⊕⊕○○A,C | ± | ⊕⊕○○A,C | + | ⊕○○○A,B,C | + | ⊕⊕⊕○A |
| Comprehensiveness | + | ⊕○○○A,B,C | − | ⊕○○○A,C | ± | ⊕○○○A,B,C | ± | ⊕○○○A,B,C | - | ⊕○○○A,B,C | + | ⊕⊕○○A,C | − | ⊕○○○A,B,C | + | ⊕⊕⊕○A |
| Comprehensibility | + | ⊕○○○A,B,C | ± | ⊕○○○A,C | + | ⊕○○○A,C | + | ⊕○○○A,B,C | + | ⊕○○○A,B,C | + | ⊕⊕○○A,C | + | ⊕○○○A,B,C | + | ⊕⊕⊕○A |
| Reliability | + | ⊕○○○A,C | ? | ? | + | ⊕⊕⊕○A | + | ⊕⊕⊕○A | ? | ? | +ƒ | ⊕⊕○○A,C | ||||
| Construct validity§ | + | ⊕⊕○○A,C | ? | ? | + | ⊕⊕○○A | + | ⊕⊕○○A | ? | + | ⊕⊕⊕⊕ | +ƒ | ⊕⊕○○A,C | |||
| Responsiveness | + | ⊕○○○A,C | − | ⊕⊕⊕⊕ | ? | + | ⊕⊕○○A | + | ⊕⊕○○A | + | ⊕⊕○○A | ? | +ƒ | ⊕⊕○○A,C | ||
GMPs for each measurement property were rated based on the COSMIN criteria [39, 41] as either sufficient (+), insufficient (−), indeterminate (?) or inconsistent (±, for development criteria only). Empty cells or indeterminate ratings indicate that the measurement property was not investigated or there is insufficient information. Structural validity, internal consistency, measurement error and cross-cultural validity are not shown in the table for all outcome measures due to the same reasons. For construct validity and responsiveness, the review team formulated a priori hypotheses about the expected relationships between an outcome measure and comparator instruments. Overall, ≥75% of the pooled results for the measurement property were expected to meet the criteria in order to be classified as a sufficient rating [39]. ASUI: Asthma Symptom Utility Index; ASI: Asthma Symptom Index; ASSESS: Asthma Severity Scoring System; ASD: asthma symptom diary; CASI: Composite Asthma Severity Index; FEOS: forced expiratory volume in 1 s, exacerbations, oral corticosteroids, symptoms score; GRADE: Grading of Recommendations, Assessment, Development and Evaluation; GETE: Global Evaluation of Treatment Effectiveness; SAQ: Severe Asthma Questionnaire. #: only external validation data were used for analysis as it was performed in a study with biologics; ¶: physician and patient version of the GETE were graded similarly (assessment of the development was based on reviewer rating only); +: the SAQ is based on a formative model; therefore, there was no need to assess structural validity and internal consistency. §: as there is no gold standard in asthma, data about criterion validity were combined with construct validity; ƒ: ratings apply to SAQ subscales (My Life, My Mind, My Body) and SAQ-global. Certainty of evidence was assessed using the modified GRADE approach as “high”, “moderate”, “low” or “very low” [39, 41, 43]. The reasons for downgrading were: A: risk of bias; B: inconsistency; C: indirectness.
The GETE [56] scale has patient and physician versions which demonstrated high quality of evidence for construct validity, although there was a positive skew towards “complete control of asthma” and “marked improvement of asthma” possibly due to the ceiling effect. The CASI [57] showed insufficient responsiveness but “high” quality of evidence. Sufficient measurement properties were rated for ASSESS, including test–retest reliability, construct validity and responsiveness to change, while the quality was mostly “very low”. The ASUI [50] and ASI [50] performed similarly and showed sufficient rating against GMP criteria and “low” to “high” quality. The SAQ [48, 49, 55] again showed sufficient properties and “very low” to “moderate” quality of evidence. Only responsiveness to change was evaluated for the ASD [51] as assessment of other measurement properties was not performed in patients taking biologics for severe asthma. The FEOS [54] scale only contains data about inter-rater agreement which was not possible to assess based on the COSMIN methodology.
Discussion
This study aimed to review the literature on definitions of response and non-response to biological therapy for severe asthma. To the best of our knowledge, the current systematic review is the first to synthesise methodologically developed, defined and evidenced definitions. We identified eight outcome measures: three composite outcome measures, three measuring asthma symptoms, one measuring asthma control and one measuring QoL. Studies utilised a variety of definitions of response criteria, mostly using MCIDs or MIDs where available and measured at different time-points for different biologics. Only GETE [56] defined a non-response, while FEOS [54] is a scale ranging from 0 to 100 (best), with no established cut-off for non-responders.
One of the aims of the review was to assess the development and measurement properties of the identified outcome measures. Results were limited by “very low” to “low” quality of evidence for the development process, except for the SAQ [46, 55], and incomplete reporting of measurement properties for all outcome measures. Based on the COSMIN guideline, none of the outcome measures met all the quality standards. Only four outcome measures were developed with patient input, even though this is considered as a vital step in ensuring that the instrument is meaningful for patients. Responsiveness to change was rated as “low” to “very low”, while definitions of response had “high” quality except for ASSESS [53].
Evaluation of therapeutic response in asthma has received increased attention with the introduction of biological treatments to improve disease treatment and precision management [58]. More than 70% of patients achieved good or excellent response to omalizumab based on GETE [59]; however, this relies on a single global measure to reflect the heterogeneous response to biological treatment. Thus, GETE does not discriminate the different effects of a treatment on different response areas, such as QoL, exacerbations, maintenance corticosteroid use and lung function. Two asthma symptoms questionnaires (ASUI and ASI [50]) were designed to assess cost-effectiveness of treatment, while the ASD [51] is a symptom diary and might impose too much burden on participants of biological therapy trials. The SAQ [55], which was developed with patient input, showed the best quality of evidence and was selected in the COMSA [44, 60].
Several composite outcome measures were identified. Neither the CASI [57] nor ASSESS [53] include a QoL domain, and the CASI [57] does not assess maintenance oral corticosteroid use, even though reduction in oral corticosteroid use and improvement of QoL have been shown to be the best indicators of response to treatment for patients with severe asthma [61]. The 2-point MID for ASSESS showed good specificity but poor sensitivity and the authors suggested that it should be interpreted with caution until more data are available [53]. The FEOS tool to quantify response [54] was developed for adults with severe asthma using novel methodology, but patients were not involved in the selection of outcome measures and it may not also represent the perspectives of international stakeholders. Unlike the COMSA initiative [44], the validity of the included outcome measures for severe asthma was not assessed and exclusion of aspects such as QoL may not represent a patient-centred approach.
This systematic review did not identify any studies which validated definitions of response to biological therapy using clinical outcome measures in patients with severe asthma. Some data are available from the consensus statements, e.g. the MID for FEV1 is 0.20 L [13] or 10% improvement [62] and for FENO a reduction of ≥20% for values over 50 ppb (or ≥10 ppb for values lower than 50 ppb) should be used to indicate response to anti-inflammatory therapy [63]. While a published composite definition of exacerbation has been developed and validated in patients with severe asthma taking benralizumab, no MCID data are available yet [64].
Most outcome measures identified in the systematic review utilised MCIDs or MIDs to assess response, but we do not regard these definitions as interchangeable, e.g. in one paper the term MID was used when it would seem to be more appropriate to use MCID [52]. An improvement that patients might recognise as equivalent to the MCID with an inhaled asthma therapy may potentially be rated as less than the MCID in the context of high-cost biologics administered by injection [35, 36]. Also, to be regarded as cost-effective a biological therapy will demand a greater magnitude of response than a less expensive asthma therapy. A further critical variable may be the duration of response, given the case reports of secondary loss of response [65], i.e. the loss of response during the treatment over time despite an initial primary response [66, 67].
The concept of “super-responders” to biological treatment has emerged recently [24, 68]. In order to standardise the definition, a modified Delphi exercise among healthcare professionals has been conducted but there is a need to understand patient perspectives [69]. The rate of super-responders in patients prescribed anti-IL-5 depending on criteria ranges from 14% to 28% [24, 68, 70], forming a small but important group. Super-response should be the ultimate goal of treatment. However, patients who fail to achieve such a level of improvement may still benefit from biological therapies. Nevertheless, consideration should be given in such cases as to whether a different biologic may be more beneficial. Evaluation of a complete response, as in haematological disorders [71, 72], should be explored further in severe asthma even though only a very small percentage of patients experience remission [73].
Unfortunately, some patients with severe asthma do not respond to biological therapy and may even deteriorate. Differences in treatment response may be multifactorial, reflecting medicinal and/or subject variables including mechanisms of action, target, dose and interval of the biological drug or heterogeneity of asthma phenotypes [74]. For example, non-response might reflect differences in the pharmacokinetics of biological drugs; indeed, monitoring plasma monoclonal antibody levels appears useful in various chronic diseases [75–77].
Overall, assessing the non-response and response after several months of treatment with biologics facilitates cost control by reducing the duration of ineffective therapy, and should enable better quality of care and patient experience by prescribing alternative treatments including switching to another biological if appropriate [78]. The latter is especially important given the rapidly increasing number of therapeutic options for patients with severe asthma [1, 16].
Strengths and limitations
This systematic review was conducted by a diverse group of academic clinicians, patient representatives, and regulatory and pharmaceutical representatives. This was a strength because it meant that definitions were considered on clinical and patient-centred grounds. A comprehensive search was conducted in four databases and provides a summary of the robust research. Rigorous methods were used including risk of bias assessment and GMPs based on COSMIN followed by the modified GRADE approach to rate the certainty of the evidence. Using transparent and validated COSMIN [39–41] methodology helped to standardise the quality assessment of outcome measures and reduce bias. Many studies were excluded as they used arbitrary definitions of response; only methodologically developed definitions and validated outcome measures were considered for inclusion in the systematic review. Lastly, all studies used data from a large number of paediatric and adult patients with severe asthma who were treated with a variety of biological therapies such as omalizumab, brodalumab, benralizumab, reslizumab and mepolizumab.
Nevertheless, we recognise several limitations. First, only studies published in English were included; however, we screened studies included in the guidelines, previous systematic reviews, references of identified articles and reviews, which made it highly unlikely that relevant studies were missed. Second, the search was conducted in 2021 as part of the development of the COMSA which was published in 2023 [44]. Third, we only searched the literature related to biological therapies and did not look at the evidence from response to non-biological asthma therapies. Biologics have different mechanisms of action, administration approaches, costs and potential adverse effects. Therefore, response criteria could differ with different patient views on what counts as a beneficial response given these considerations. However, it may be possible to also learn from the response to other therapies such as to oral and inhaled corticosteroids in severe asthma. Fourth, definitions of therapeutic response were assessed at different time-points, which might make it difficult to come to definitive conclusions about non-responders and responders. Moreover, COSMIN suggest using the lowest score counts method to assess measurement properties, meaning that having higher quality scores on some items of the checklist was not considered and only the “worst score” was reported. Lastly, it was not possible to run a meta-analysis due to low number of studies per outcome measure and only narrative synthesis was undertaken.
Policy implications and next steps
This systematic review aimed to inform clinicians, regulators and policy makers about the gaps and highlight heterogeneity of the definitions used. Even though the Asthma Control Questionnaire/Test and Asthma Quality of Life Questionnaire are widely used in phase 3 trials of asthma biologics and in clinical practice, definitions of response including MCID or MID have never been specifically assessed in biologics. Further research should aim to explore the identified definitions as primary and secondary outcomes in clinical trials including phase 2 and 3 efficacy studies and assess the MCID/MID of well-validated questionnaires in biological trials. There is also a need to methodologically develop patient-centred definitions of non-response and response to biological therapy for severe asthma for individual PROMs and clinical as well as a composite outcome measures. For example, based on COSMIN methodology for assessing the content validity of PROMs [41], patients should be asked about their relevance, comprehensiveness and comprehensibility. Engagement of patients is a crucial aspect of the development of outcome measures to meet their needs and preferences as well as to inform health decisions [79, 80].
Given the aforementioned, we are planning to develop definitions of non-response and response to biological therapies for paediatric and adult severe asthma trials and clinical practice based on the COMSA selected among key stakeholder groups, including patients with severe asthma [44]. We aim to standardise the definitions, which will allow better tailoring of individual treatment and be used in future clinical trials for documenting therapeutic response. Furthermore, looking at multiple dimensions of asthma such as exacerbations, QoL, asthma control and lung function in one single patient-centred composite would help to determine the correct sample size for future clinical trials, assist regulators in determining whether a new biological therapy is effective and identify predictors of treatment response. Use of such definitions will also help in better understanding the applicability of novel biomarkers such as volatile organic compounds [81], peripheral blood gene expression [82, 83] and serum periostin [84] in the prediction and monitoring of response, which have been shown to be promising in biological treatment for severe asthma.
Conclusions
This systematic review is the first to evaluate the quality of evidence for definitions of response to biological therapy for severe asthma and measurement properties of associated outcome measures. There are several high-quality definitions available for use that are mostly based on MIDs or MCIDs, which might not be sufficient to justify continuation of biological therapy on cost-effectiveness criteria. Even though composite outcome measures are available and able to capture the multidimensional nature of severe asthma, none were developed with patient input and all lack a QoL component. Quality of evidence for the development and validation of the outcome measures was rated predominantly “low” and “very low”, and none met all the methodological quality standards, highlighting an urgent unmet need. Therefore, the forthcoming 3TR project will aim to develop the definitions of non-response and response based on COMSA [44] with involvement of patient representatives and other key stakeholders. Future research will be needed to pilot these definitions in biological trials, and to address practical implications for policy makers, research and clinical practice. Knowing how to evaluate response to biologics using universally acceptable criteria would help in assessing the effectiveness of novel therapies, and improve clinical decision making and the care of patients with severe asthma.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00444-2022.SUPPLEMENT (477.7KB, pdf)
Acknowledgements
We would like to acknowledge the support of 3TR in funding the development of this systematic review. We would like to thank Paula Sands (Research Engagement Librarian, University of Southampton, Southampton, UK) for her assistance in optimising the search strategy.
Provenance: Submitted article, peer reviewed.
This study is registered at PROSPERO with identifier number CRD42021211249.
Disclaimer: The content of this publication reflects only the authors’ views and the Joint Undertaking is not responsible for any use that may be made of the information it contains.
Author contributions: E. Khaleva developed a protocol and a search strategy, and G. Roberts and A. Rattu reviewed this. E. Khaleva and A. Rattu performed abstract screening, data extraction and COSMIN evaluation. E. Khaleva synthesised the evidence and wrote the first draft of the manuscript. All authors critically reviewed the manuscript and approved the final version prior to submission.
Conflict of interests: E. Khaleva and A. Rattu declare funding for the present manuscript from the 3TR European Union Innovative Medicines Initiative 2 paid to the university. C. Brightling declares grants from GlaxoSmithKline, AstraZeneca, Novartis, Chiesi, Boehringer Ingelheim, Genentech, Roche, Sanofi, Mologic and 4DPharma, consulting fees from GlaxoSmithKline, AstraZeneca, Novartis, Chiesi, Boehringer Ingelheim, Genentech, Roche, Sanofi, Mologic, 4DPharma and Teva, and support from the 3TR project. A. Bourdin reports being an investigator for clinical trials promoted by AstraZeneca, Chieisi, GlaxoSmithKline, Boehringer Ingelheim, Novartis, Regeneron and Sanofi; having received fees for lectures, attendance of meeting and consultancy from AstraZeneca, Chieisi, GlaxoSmithKline, Boehringer Ingelheim, Novartis, Regeneron and Sanofi; having received research grants from AstraZeneca and Boehringer Ingelheim; and participation on a data safety monitoring or advisory board of AB Science. A. Bossios has received lecture fees from GlaxoSmithKline, AstraZeneca, Teva and Novartis; honoraria for advisory board meetings from GlaxoSmithKline, AstraZeneca, Teva, Novartis and Sanofi; and received support for attending meetings from AstraZeneca and Novartis, all outside the present work; reports being a member of the Steering Committee of SHARP, Secretary of Assembly 5 (Airway Diseases, Asthma, COPD and Chronic Cough) of the European Respiratory Society and Vice-chair of the Nordic Severe Asthma Network (NSAN). K.F. Chung has received honoraria for participating in advisory board meetings of GlaxoSmithKline, AstraZeneca, Roche, Novartis, Merck and Shionogi regarding treatments for asthma, COPD and chronic cough, and has also been renumerated for speaking engagements for Novartis and AstraZeneca. R. Chaudhuri has received lecture fees from GlaxoSmithKline, AstraZeneca, Teva, Chiesi, Sanofi and Novartis; honoraria for advisory board meetings from GlaxoSmithKline, AstraZeneca, Teva, Chiesi and Novartis; sponsorship to attend international scientific meetings from Chiesi, Napp, Sanofi, Boehringer, GlaxoSmithKline and AstraZeneca, and a research grant to her Institute from AstraZeneca for a UK multicentre study. C. Coleman declares funding received to support this work by the European Lung Foundation (ELF) from the European Commission's Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement number 831434 (3TR), and is an employee of the ELF. R. Djukanovic declares funding from European Respiratory Society, Teva, GlaxoSmithKline, Novartis, Sanofi and Chiesi for the SHARP CRC, consulting fees for Synairgen; honorarium for a lecture from GlaxoSmithKline, participation on a data safety monitoring board or advisory board for Kymab (Cambridge) and shares in Synairgen, outside the submitted work. S-E. Dahlen declares funding from 3TR IMI Grant; consulting fees from AstraZeneca, Cayman Co., GlaxoSmithKline, Novartis, Regeneron, Sanofi and Teva; honoraria for lectures from AstraZeneca and Sanofi. A. Exley declares being a minority shareholder in GlaxoSmithKline PLC. L. Fleming declares participation in advisory boards and honoraria for lectures from Sanofi, Respiri UK, AstraZeneca, Novartis and Teva, outside the scope of this publication. All payments were made to her institution. A. Gupta received speaker and advisory board fees from GlaxoSmithKline, Novartis, AstraZeneca and Boehringer Ingelheim. A. Gupta's institution had received research grants from GlaxoSmithKline, Novartis, AstraZeneca and Boehringer Ingelheim. E. Hamelmann declares support from the German Ministry of Education and Research (BMBF) and German Asthma Net (GAN) e.V.; funding for research in severe asthma in children (CHAMP-01GL1742D) and for Severe Asthma Register. G.H. Koppelman reports receiving research grants from the Lung Foundation of the Netherlands, Ubbo Emmius Foundation, H2020 European Union, Teva, GlaxoSmithKline and Vertex, outside this work (money to institution); he reports memberships of advisory boards to GlaxoSmithKline and PURE-IMS, outside this work (money to institution). E. Melen has received consulting fees from AstraZeneca, Chiesi, Novartis and Sanofi outside the submitted work. V. Mahler has no conflict of interest but declares that the views expressed in this review are the personal views of the author and may not be understood or quoted as being made on behalf of or reflecting the position of the respective national competent authority, the European Medicines Agency, or one of its committees or working parties. F. Singer reports being an investigator for clinical trials promoted by Vertex and having received fees for lectures from Vertex and Novartis, outside the submitted work. C. Porsbjerg declares grants, consulting fees and honoraria from AstraZeneca, GlaxoSmithKline, Novartis, Teva, Sanofi, Chiesi and ALK (paid to institution and personal honoraria); participation in the advisory board for AstraZeneca, Novartis, Teva, Sanofi and ALK, outside the submitted work. V. Ramiconi reports grants paid to EFA from Pfizer, Novartis, AstraZeneca, Sanofi, Chiesi Farmaceutici, Regeneron, DBV Technologies, MSD, GlaxoSmithKline, Aimmune, LeoPharma, AbbVie, Boehringer Ingelheim, OM Pharma and Roche; payment for expert testimony from Novartis Global Respiratory Patient Council 2021 and Novartis EPIS Steering Committee to EFA. G. Roberts declares EU IMI funding and consulting fees from AstraZeneca paid to his institution. No other author has any conflict of interest to declare.
Support statement: G. Roberts and E. Khaleva were supported by the NIHR Southampton Biomedical Research Centre. This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement number 831434 (3TR). The JU receives support from the European Union's Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA). The funder had no role in the development of the protocol, conduct or write up of the review or decision to publish. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Chung KF, Wenzel SE, Brozek JL, et al. . International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Safari A, FitzGerald JM, et al. . Economic burden of multimorbidity in patients with severe asthma: a 20-year population-based study. Thorax 2019; 74: 1113–1119. doi: 10.1136/thoraxjnl-2019-213223 [DOI] [PubMed] [Google Scholar]
- 3.Pamuk G, Le Bourgeois M, Abou Taam R, et al. . The economic burden of severe asthma in children: a comprehensive study. J Asthma 2021: 8: 1467–1477. doi: 10.1080/02770903.2020.1802747 [DOI] [PubMed] [Google Scholar]
- 4.Kerkhof M, Tran TN, Soriano JB, et al. . Healthcare resource use and costs of severe, uncontrolled eosinophilic asthma in the UK general population. Thorax 2018; 73: 116–124. doi: 10.1136/thoraxjnl-2017-210531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janson C, Lisspers K, Stallberg B, et al. . Health care resource utilization and cost for asthma patients regularly treated with oral corticosteroids – a Swedish observational cohort study (PACEHR). Respir Res 2018; 19: 168. doi: 10.1186/s12931-018-0855-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Neill S, Sweeney J, Patterson CC, et al. . The cost of treating severe refractory asthma in the UK: an economic analysis from the British Thoracic Society Difficult Asthma Registry. Thorax 2015; 70: 376–378. doi: 10.1136/thoraxjnl-2013-204114 [DOI] [PubMed] [Google Scholar]
- 7.Foster JM, McDonald VM, Guo M, et al. . “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J 2017; 50: 1700765. doi: 10.1183/13993003.00765-2017 [DOI] [PubMed] [Google Scholar]
- 8.Stubbs MA, Clark VL, McDonald VM. Living well with severe asthma. Breathe 2019; 15: e40–e49. doi: 10.1183/20734735.0165-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Efraij K, Johnson KM, Wiebe D, et al. . A systematic review of the adverse events and economic impact associated with oral corticosteroids in asthma. J Asthma 2019; 56: 1334–1346. doi: 10.1080/02770903.2018.1539100 [DOI] [PubMed] [Google Scholar]
- 10.Bourdin A, Molinari N, Vachier I, et al. . Mortality: a neglected outcome in OCS-treated severe asthma. Eur Respir J 2017; 50: 1701486. doi: 10.1183/13993003.01486-2017 [DOI] [PubMed] [Google Scholar]
- 11.Ekstrom M, Nwaru BI, Hasvold P, et al. . Oral corticosteroid use, morbidity and mortality in asthma: a nationwide prospective cohort study in Sweden. Allergy 2019; 74: 2181–2190. doi: 10.1111/all.13874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agache I, Beltran J, Akdis C, et al. . Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines – recommendations on the use of biologicals in severe asthma. Allergy 2020; 75: 1023–1042. doi: 10.1111/all.14221 [DOI] [PubMed] [Google Scholar]
- 13.Agache I, Rocha C, Beltran J, et al. . Efficacy and safety of treatment with biologicals (benralizumab, dupilumab and omalizumab) for severe allergic asthma: a systematic review for the EAACI Guidelines – recommendations on the use of biologicals in severe asthma. Allergy 2020; 75: 1043–1057. doi: 10.1111/all.14235 [DOI] [PubMed] [Google Scholar]
- 14.Agache I, Song Y, Rocha C, et al. . Efficacy and safety of treatment with dupilumab for severe asthma: a systematic review of the EAACI guidelines – recommendations on the use of biologicals in severe asthma. Allergy 2020; 75: 1058–1068. doi: 10.1111/all.14268 [DOI] [PubMed] [Google Scholar]
- 15.Brusselle GG, Koppelman GH. Biologic therapies for severe asthma. N Engl J Med 2022; 386: 157–171. doi: 10.1056/NEJMra2032506 [DOI] [PubMed] [Google Scholar]
- 16.Holguin F, Cardet JC, Chung KF, et al. . Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2020; 55: 1900588. doi: 10.1183/13993003.00588-2019. [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Health and Care Excellence . Dupilumab for treating severe asthma with type 2 inflammation. Technology appraisal guidance TA751. 2021. www.nice.org.uk/guidance/ta751/chapter/1-Recommendations Date last accessed: 9 November 2022.
- 18.Chipps BE, Zeiger RS, Luskin AT, et al. . Baseline asthma burden, comorbidities, and biomarkers in omalizumab-treated patients in PROSPERO. Ann Allergy Asthma Immunol 2017; 119: 524–532. doi: 10.1016/j.anai.2017.09.056 [DOI] [PubMed] [Google Scholar]
- 19.Albers FC, Mullerova H, Gunsoy NB, et al. . Biologic treatment eligibility for real-world patients with severe asthma: the IDEAL study. J Asthma 2018; 55: 152–160. doi: 10.1080/02770903.2017.1322611 [DOI] [PubMed] [Google Scholar]
- 20.Casale TB, Luskin AT, Busse W, et al. . Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO. A prospective real-world study. J Allergy Clin Immunol Practice 2019; 7: 156–164. doi: 10.1016/j.jaip.2018.04.043 [DOI] [PubMed] [Google Scholar]
- 21.Corren J, Garcia Gil E, Griffiths JM, et al. . Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY. Ann Allergy Asthma Immunol 2021; 126: 187–193. doi: 10.1016/j.anai.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 22.Probst M, Gogolka A, Krull M, et al. . In search of clinically relevant parameters to monitor successful omalizumab therapy in allergic asthma. Allergol Select 2018; 2: 49–55. doi: 10.5414/ALX01377E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drick N, Seeliger B, Welte T, et al. . Anti-IL-5 therapy in patients with severe eosinophilic asthma – clinical efficacy and possible criteria for treatment response. BMC Pulm Med 2018; 18: 119. doi: 10.1186/s12890-018-0689-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eger K, Kroes JA, Ten Brinke A, et al. . Long-term therapy response to anti-IL-5 biologics in severe asthma – a real-life evaluation. J Allergy Clin Immunol Pract 2021; 9: 1194–1200. doi: 10.1016/j.jaip.2020.10.010 [DOI] [PubMed] [Google Scholar]
- 25.Abdo M, Watz H, Veith V, et al. . Small airway dysfunction as predictor and marker for clinical response to biological therapy in severe eosinophilic asthma: a longitudinal observational study. Respir Res 2020; 21: 278. doi: 10.1186/s12931-020-01543-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee M, Forero DF, Tran S, et al. . Suboptimal treatment response to anti-IL-5 monoclonal antibodies in severe eosinophilic asthmatics with airway autoimmune phenomena. Eur Respir J 2020; 56: 2000117. doi: 10.1183/13993003.00117-2020 [DOI] [PubMed] [Google Scholar]
- 27.Agache I, Akdis C, Akdis M, et al. . EAACI biologicals guidelines – recommendations for severe asthma. Allergy 2021; 76: 14–44. doi: 10.1111/all.14425 [DOI] [PubMed] [Google Scholar]
- 28.Golebski K, Dankelman LHM, Bjorkander S, et al. . Expert meeting report: towards a joint European roadmap to address the unmet needs and priorities of paediatric asthma patients on biologic therapy. ERJ Open Res 2021; 7: 00381-2021. doi: 10.1183/23120541.00381-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coleman C, Khaleva E, Rattu A, et al. . Narrative review to capture patients’ perceptions and opinions about non-response and response to biological therapy for severe asthma. Eur Respir J 2023; 61: 2200837. doi: 10.1183/13993003.00837-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Medicines Agency . Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products – scientific guideline. 2005. www.ema.europa.eu/en/regulatory-guidance-use-health-related-quality-life-hrql-measures-evaluation-medicinal-products Date last accessed: 26 October 2021.
- 31.Food and Drug Administration . Guidance for industry: patient-reported outcome measures: use in medicinal product development to support labelling claims. 2009. www.fda.gov/media/77832/download Date last accessed: 26 October 2021.
- 32.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989; 10: 407–415. doi: 10.1016/0197-2456(89)90005-6 [DOI] [PubMed] [Google Scholar]
- 33.Revicki D, Hays RD, Cella D, et al. . Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008; 61: 102–109. doi: 10.1016/j.jclinepi.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 34.Buhl R, Humbert M, Bjermer L, et al. . Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J 2017; 49: 1700634. doi: 10.1183/13993003.00634-2017 [DOI] [PubMed] [Google Scholar]
- 35.McQueen RB, Sheehan DN, Whittington MD, et al. . Cost-effectiveness of biological asthma treatments: a systematic review and recommendations for future economic evaluations. Pharmacoeconomics 2018; 36: 957–971. doi: 10.1007/s40273-018-0658-x [DOI] [PubMed] [Google Scholar]
- 36.Anderson WC 3rd, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol 2019; 122: 367–372. doi: 10.1016/j.anai.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 37.3TR . Taxonomy, Treatment, Targets and Remission. 2021. www.3tr-imi.eu Date last accessed: 12 January 2021.
- 38.Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 2021; 18: e1003583. doi: 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prinsen CAC, Mokkink LB, Bouter LM, et al. . COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res 2018; 27: 1147–1157. doi: 10.1007/s11136-018-1798-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mokkink LB, de Vet HCW, Prinsen CAC, et al. . COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual Life Res 2018; 27: 1171–1179. doi: 10.1007/s11136-017-1765-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terwee CB, Prinsen CAC, Chiarotto A, et al. . COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res 2018; 27: 1159–1170. doi: 10.1007/s11136-018-1829-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mokkink LB, Boers M, van der Vleuten CPM, et al. . COSMIN Risk of Bias tool to assess the quality of studies on reliability or measurement error of outcome measurement instruments: a Delphi study. BMC Med Res Methodol 2020; 20: 293. doi: 10.1186/s12874-020-01179-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guyatt G, Oxman AD, Akl EA, et al. . GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–394. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 44.Khaleva E, Rattu A, Brightling C, et al. . Development of Core Outcome Measures sets for paediatric and adult Severe Asthma (COMSA). Eur Respir J 2023; 61: 2200606. doi: 10.1183/13993003.00606-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Globe G, Martin M, Schatz M, et al. . Symptoms and markers of symptom severity in asthma – content validity of the Asthma Symptom Diary. Health Qual Life Outcomes 2015; 13: 21. doi: 10.1186/s12955-015-0217-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyland ME, Lanario JW, Pooler J, et al. . How patient participation was used to develop a questionnaire that is fit for purpose for assessing quality of life in severe asthma. Health Qual Life Outcomes 2018; 16: 24. doi: 10.1186/s12955-018-0851-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Revicki DA, Leidy NK, Brennan-Diemer F, et al. . Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest 1998; 114: 998–1007. doi: 10.1378/chest.114.4.998 [DOI] [PubMed] [Google Scholar]
- 48.Lanario JW, Hyland ME, Menzies-Gow A, et al. . Validation of subscales of the Severe Asthma Questionnaire (SAQ) using exploratory factor analysis (EFA). Health Qual Life Outcomes 2020; 18: 336. doi: 10.1186/s12955-020-01593-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masoli M, Lanario JW, Hyland ME, et al. . The Severe Asthma Questionnaire: sensitivity to change and minimal clinically important difference (MCID). Eur Respir J 2021; 57: 2100300. doi: 10.1183/13993003.00300-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen Q, von Maltzahn R, Nelsen L, et al. . Psychometric properties of the Asthma Symptom Index in patients with severe asthma. J Allergy Clin Immunol Pract 2021; 9: 400–409. doi: 10.1016/j.jaip.2020.08.019 [DOI] [PubMed] [Google Scholar]
- 51.Globe G, Wiklund I, Mattera M, et al. . Evaluating minimal important differences and responder definitions for the asthma symptom diary in patients with moderate to severe asthma. J Patient Rep Outcomes 2019; 3: 22. doi: 10.1186/s41687-019-0109-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krouse RZ, Sorkness CA, Wildfire JJ, et al. . Minimally important differences and risk levels for the Composite Asthma Severity Index. J Allergy Clin Immunol 2017; 139: 1052–1055. doi: 10.1016/j.jaci.2016.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fitzpatrick AM, Szefler SJ, Mauger DT, et al. . Development and initial validation of the Asthma Severity Scoring System (ASSESS). J Allergy Clin Immunol; 145: 127–139. doi: 10.1016/j.jaci.2019.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez de Llano L, Davila I, Martinez-Moragon E, et al. . Development of a tool to measure the clinical response to biologic therapy in uncontrolled severe asthma: the FEV1, exacerbations, oral corticosteroids, symptoms score. J Allergy Clin Immunol Pract 2021; 9: 2725–2731. doi: 10.1016/j.jaip.2021.01.033 [DOI] [PubMed] [Google Scholar]
- 55.Hyland ME, Jones RC, Lanario JW, et al. . The construction and validation of the Severe Asthma Questionnaire. Eur Respir J 2018; 52: 1800618. doi: 10.1183/13993003.00618-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lloyd A, Turk F, Leighton T, et al. . Psychometric evaluation of Global Evaluation of Treatment Effectiveness: a tool to assess patients with moderate-to-severe allergic asthma. J Med Economics 2007; 10: 285–296. doi: 10.3111/13696990701478856 [DOI] [Google Scholar]
- 57.Wildfire JJ, Gergen PJ, Sorkness CA, et al. . Development and validation of the Composite Asthma Severity Index – an outcome measure for use in children and adolescents. J Allergy Clin Immunol 2012; 129: 694–701. doi: 10.1016/j.jaci.2011.12.962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Bona D, Crimi C, D'Uggento AM, et al. . Effectiveness of benralizumab in severe eosinophilic asthma: distinct sub-phenotypes of response identified by cluster analysis. Clin Exp Allergy 2022; 52: 312–323. doi: 10.1111/cea.14026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alhossan A, Lee CS, MacDonald K, et al. . “Real-life” effectiveness studies of omalizumab in adult patients with severe allergic asthma: meta-analysis. J Allergy Clin Immunol Pract 2016; 5: 1362–1370. doi: 10.1016/j.jaip.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 60.Rattu A, Khaleva E, Brightling C, et al. . Identifying and appraising outcome measures for severe asthma: a systematic review. Eur Respir J 2023; 61: 2201231. doi: 10.1183/13993003.01231-2022 [DOI] [PubMed] [Google Scholar]
- 61.Clark VL, Gibson PG, McDonald VM. What matters to people with severe asthma? Exploring add-on asthma medication and outcomes of importance. ERJ Open Res 2021; 7: 00497-2020. doi: 10.1183/23120541.00497-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanojevic S, Kaminsky DA, Miller M, et al. . ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022; 60: 2101499. doi: 10.1183/13993003.01499-2021 [DOI] [PubMed] [Google Scholar]
- 63.Dweik RA, Boggs PB, Erzurum SC, et al. . An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med 2011; 184: 602–615. doi: 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuhlbrigge AL, Bengtsson T, Peterson S, et al. . A novel endpoint for exacerbations in asthma to accelerate clinical development: a post-hoc analysis of randomised controlled trials. Lancet Respir Med 2017; 5: 577–590. doi: 10.1016/S2213-2600(17)30218-7 [DOI] [PubMed] [Google Scholar]
- 65.Cormier M, Chaboillez S, Lemiere C. Secondary loss of response to mepolizumab in severe eosinophilic asthma. J Allergy Clin Immunol Pract 2020; 8: 736–738. doi: 10.1016/j.jaip.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 66.Roda G, Jharap B, Neeraj N, et al. . Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol 2016; 7: e135. doi: 10.1038/ctg.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clark VL, Gibson PG, McDonald VM. The patients’ experience of severe asthma add-on pharmacotherapies: a qualitative descriptive study. J Asthma Allergy 2021; 14: 245–258. doi: 10.2147/JAA.S296147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kavanagh JE, d'Ancona G, Elstad M, et al. . Real-world effectiveness and the characteristics of a “super-responder” to mepolizumab in severe eosinophilic asthma. Chest; 158: 491–500. doi: 10.1016/j.chest.2020.03.042 [DOI] [PubMed] [Google Scholar]
- 69.Upham JW, Le Lievre C, Jackson DJ, et al. . Defining a severe asthma super-responder: findings from a Delphi process. J Allergy Clin Immunol Pract 2021; 9: 3997–4004. doi: 10.1016/j.jaip.2021.06.041 [DOI] [PubMed] [Google Scholar]
- 70.Harvey ES, Langton D, Katelaris C, et al. . Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J 2020; 55: 1902420. doi: 10.1183/13993003.02420-2019 [DOI] [PubMed] [Google Scholar]
- 71.Barosi G, Bordessoule D, Briere J, et al. . Response criteria for myelofibrosis with myeloid metaplasia: results of an initiative of the European Myelofibrosis Network (EUMNET). Blood 2005; 106: 2849–2853. doi: 10.1182/blood-2005-04-1520 [DOI] [PubMed] [Google Scholar]
- 72.Barosi G, Birgegard G, Finazzi G, et al. . Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood 2009; 113: 4829–4833. doi: 10.1182/blood-2008-09-176818 [DOI] [PubMed] [Google Scholar]
- 73.Menzies-Gow A, Bafadhel M, Busse WW, et al. . An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol 2020; 145: 757–765. doi: 10.1016/j.jaci.2019.12.006 [DOI] [PubMed] [Google Scholar]
- 74.Kroes JA, Zielhuis SW, van Roon EN, et al. . Prediction of response to biological treatment with monoclonal antibodies in severe asthma. Biochem Pharmacol 2020; 179: 113978. doi: 10.1016/j.bcp.2020.113978 [DOI] [PubMed] [Google Scholar]
- 75.Warman A, Straathof JW, Derijks LJ. Therapeutic drug monitoring of infliximab in inflammatory bowel disease patients in a teaching hospital setting: results of a prospective cohort study. Eur J Gastroenterol Hepatol 2015; 27: 242–248. doi: 10.1097/MEG.0000000000000279 [DOI] [PubMed] [Google Scholar]
- 76.Haraoui B, Cameron L, Ouellet M, et al. . Anti-infliximab antibodies in patients with rheumatoid arthritis who require higher doses of infliximab to achieve or maintain a clinical response. J Rheumatol 2006; 33: 31–36. [PubMed] [Google Scholar]
- 77.St Clair EW, Wagner CL, Fasanmade AA, et al. . The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002; 46: 1451–1459. doi: 10.1002/art.10302 [DOI] [PubMed] [Google Scholar]
- 78.Magnan A, Bourdin A, Prazma CM, et al. . Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy 2016; 71: 1335–1344. doi: 10.1111/all.12914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tapp H, Derkowski D, Calvert M, et al. . Patient perspectives on engagement in shared decision-making for asthma care. Fam Pract 2017; 34: 353–357. doi: 10.1093/fampra/cmw122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Forsythe LP, Carman KL, Szydlowski V, et al. . Patient engagement in research: early findings from the patient-centered outcomes research institute. Health Aff 2019; 38: 359–367. doi: 10.1377/hlthaff.2018.05067 [DOI] [PubMed] [Google Scholar]
- 81.Brinkman P, Ahmed WM, Gomez C, et al. . Exhaled volatile organic compounds as markers for medication use in asthma. Eur Respir J 2020; 55: 1900544. doi: 10.1183/13993003.00544-2019 [DOI] [PubMed] [Google Scholar]
- 82.Choy DF, Jia G, Abbas AR, et al. . Peripheral blood gene expression predicts clinical benefit from anti-IL-13 in asthma. J Allergy Clin Immunol 2016; 138: 1230–1233. doi: 10.1016/j.jaci.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 83.Upchurch K, Wiest M, Cardenas J, et al. . Whole blood transcriptional variations between responders and non-responders in asthma patients receiving omalizumab. Clin Exp Allergy 2020; 50: 1017–1034. [DOI] [PubMed] [Google Scholar]
- 84.Caminati M, Gatti D, Dama A, et al. . Serum periostin during omalizumab therapy in asthma: a tool for patient selection and treatment evaluation. Ann Allergy Clin Exp Allergy 2017; 119: 460–462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00444-2022.SUPPLEMENT (477.7KB, pdf)