Abstract
Objective
To assess associations between the extent of fibrotic interstitial lung disease (ILD) and forced vital capacity (FVC) at baseline and change in FVC over 52 weeks in patients with systemic sclerosis-associated ILD (SSc-ILD) in the SENSCIS trial.
Material and methods
We used generalized additive models, which involve few assumptions and allow for interaction between non-linear effects, to assess associations between the extent of fibrotic ILD on high-resolution computed tomography (HRCT), and the interplay of extent of fibrotic ILD on HRCT and FVC % predicted, at baseline and FVC decline over 52 weeks.
Results
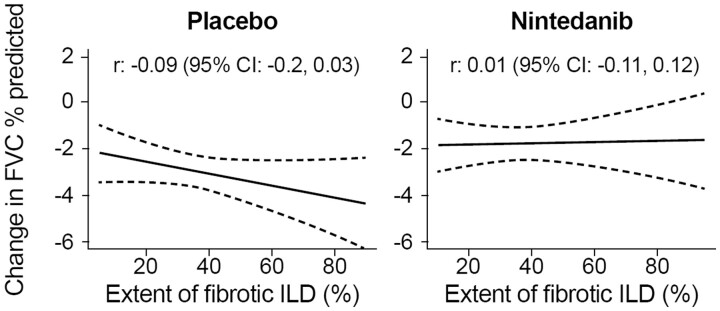
In the placebo group (n = 288), there was weak evidence of a modest association between a greater extent of fibrotic ILD at baseline and a greater decline in FVC % predicted at week 52 [r: –0.09 (95% CI –0.2, 0.03)]. Higher values of both the extent of fibrotic ILD and FVC % predicted at baseline tended to be associated with greater decline in FVC % predicted at week 52. In the nintedanib group (n = 288), there was no evidence of an association between the extent of fibrotic ILD at baseline and decline in FVC % predicted at week 52 [r: 0.01 (95% CI: -0.11, 0.12)] or between the interplay of extent of fibrotic ILD and FVC % predicted at baseline and decline in FVC % predicted at week 52.
Conclusions
Data from the SENSCIS trial suggest that patients with SSc-ILD are at risk of ILD progression and benefit from nintedanib largely irrespective of their extent of fibrotic ILD at baseline.
Study registration
ClinicalTrials.gov, https://clinicaltrials.gov, NCT02597933.
Keywords: autoimmune diseases, pulmonary fibrosis, vital capacity
Rheumatology key messages.
A greater extent of fibrotic SSc-ILD was weakly associated with FVC decline over 52 weeks.
A benefit from nintedanib appeared to exist irrespective of patients’ extent of fibrotic SSc-ILD.
Introduction
SSc is a rare and heterogeneous autoimmune disease characterized by microvascular damage and progressive fibrosis of the skin and internal organs [1]. Interstitial lung disease (ILD) is the leading cause of death in subjects with SSc [2]. Patients with SSc-ILD who have fibrotic ILD of any extent on high-resolution computed tomography (HRCT) have an increased risk of mortality compared with those with no fibrotic ILD [3]. Further, a number of studies have suggested that a greater extent of fibrotic SSc-ILD on HRCT is associated with an increased risk of mortality [3–6]. Lower forced vital capacity (FVC) at baseline [3, 4, 7] or a decline in FVC [3, 8, 9] have also been associated with an increased risk of mortality in patients with SSc-ILD. These observations may not be independent, as a greater extent of fibrotic ILD on HRCT is associated with lower FVC [4, 8, 10].
In the randomized placebo-controlled SENSCIS trial in subjects with SSc-ILD, nintedanib reduced the rate of decline in FVC (mL/year) over 52 weeks by 44% [11] and reduced the proportions of subjects with a decline in FVC of >5% to ≤10% predicted or >10% to ≤15% predicted over 52 weeks [12]. Various approaches have been used to measure the extent of fibrotic SSc-ILD on HRCT and to evaluate the association between the extent of fibrotic SSc-ILD and FVC at baseline and outcomes. In these analyses, we used a flexible regression modelling approach, which allowed for interaction between potentially non-linear effects, to assess associations between the extent of fibrotic ILD on HRCT and FVC % predicted at baseline and change in FVC over 52 weeks in the SENSCIS trial.
Patients and methods
Trial design
The design of the SENSCIS trial (NCT02597933) has been published and the protocol is publicly available [11]. Briefly, subjects had SSc-ILD with onset of first non-Raynaud symptom in the prior ≤7 years, FVC ≥40% predicted, diffusion capacity of the lung for carbon monoxide (DLco) 30–89% predicted, and fibrotic ILD ≥10% extent on an HRCT scan taken in the last ≤12 months, confirmed by central assessment by an expert radiologist. A recent decline in FVC was not an inclusion criterion. The extent of fibrotic ILD (reticular abnormalities, honeycombing, fibrotic ground glass opacities) was assessed visually in the whole lung to the nearest 5%. Subjects on prednisone ≤10 mg/day (or equivalent) and/or stable therapy with mycophenolate or methotrexate were allowed to participate. Subjects were randomized to receive nintedanib or placebo, stratified by the presence of anti-topoisomerase I antibody (ATA), until the last subject had reached week 52 but for ≤100 weeks. Subjects who discontinued treatment prematurely were asked to attend visits as originally planned. The trial was conducted in accordance with the trial protocol, the principles of the Declaration of Helsinki, and the International Council for Harmonization Guidelines for Good Clinical Practice. The trial was approved by an independent ethics committee or institutional review board at every site. The participating sites are listed in [11]. All subjects provided written informed consent.
Analyses
These post-hoc analyses were conducted in subjects who received ≥1 dose of trial medication. To explore associations between continuous variables, we used generalized additive models, a class of flexible regression models that allows consideration of potentially non-linear (‘spline’) effects of predictor variables and, for change in FVC, also their interactions. We assessed the association between the extent of fibrotic ILD at baseline and decline in FVC over 52 weeks, based on the absolute change from baseline in FVC % predicted and the rate of decline in FVC (mL/year). We assessed the association between the interaction of the extent of fibrotic ILD and FVC % predicted at baseline and FVC decline over 52 weeks, measured as the absolute change from baseline in FVC % predicted, absolute change from baseline in FVC (mL), and rate of decline in FVC (mL/year). We also assessed the association between the extent of fibrotic ILD and FVC % predicted at baseline. Linear associations were evaluated using Pearson correlation coefficients. In analyses of change from baseline in FVC (but not of the rate of decline in FVC), missing FVC values were imputed using a worst observation carried forward approach. These analyses were performed in the nintedanib and placebo groups overall and in subgroups by use of mycophenolate at baseline. Generalized additive models were fit in R, version 1.8–28, using the ‘mgvc’ package (https://cran.r-project.org/src/contrib/Archive/mgcv/). The univariate and bivariate (interaction) smoothing terms were penalized thin plate regression splines. The smoothing parameters were estimated using the restricted maximum likelihood (REML) estimation method.
As a supplementary analysis, we assessed outcomes in subgroups based on thresholds of an extent of fibrotic ILD of 40% and an FVC of 70% predicted at baseline in the overall population. In these subgroups, we analyzed the rate of decline in FVC (mL/year) over 52 weeks; the proportions of subjects who met proposed thresholds for minimal clinically important differences for improved, stable, or worsened FVC based on data from Scleroderma Lung Studies I and II, anchored to the health transition question from the Medical Outcomes Short Form-36 (absolute increase ≥3.0% predicted, absolute increase <3.0% predicted or decrease <3.3% predicted, absolute decrease ≥3.3% predicted) [13] at week 52; and time to absolute decline in FVC ≥10% predicted, or absolute decline in FVC ≥5% to <10% predicted with an absolute decline in DLco ≥15% predicted, or death at week 52. Statistical analyses of these outcomes are described in the Supplementary Material, available at Rheumatology online.
Results
Subjects
A total of 576 subjects received ≥1 dose of trial medication (288 nintedanib, 288 placebo). Their baseline characteristics have been described [11]. Briefly, in the nintedanib and placebo groups, respectively, the mean (s.d.) extent of fibrotic ILD (%) was 36.8 (21.8) and 35.2 (20.7), mean (s.d.) FVC was 2459 (736) and 2541 (816) mL, mean (s.d.) FVC % predicted was 72.4 (16.8) and 72.7 (16.6), and 48.3% and 48.6% of subjects were taking mycophenolate.
Association between extent of fibrotic ILD at baseline and decline in FVC over 52 weeks
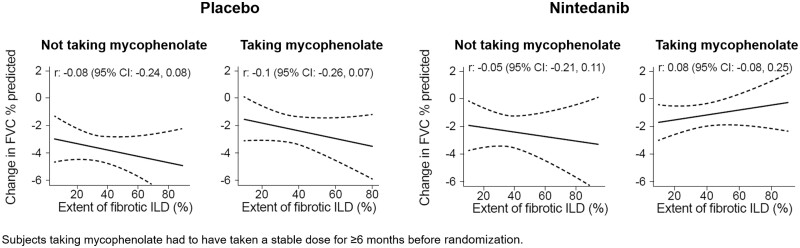
In the placebo group, there was weak evidence of a modest inverse association between the extent of fibrotic ILD at baseline and the decline in FVC % predicted at week 52 in the overall population (Fig. 1) and in subgroups of subjects who were taking and not taking mycophenolate at baseline (Fig. 2). There was no association between the extent of fibrotic ILD at baseline and the rate of decline in FVC (mL/year) over 52 weeks (Supplementary Figs S1 and S2, available at Rheumatology online). In the nintedanib group, there was no evidence of an association between the extent of fibrotic ILD on HRCT at baseline and decline in FVC % predicted at week 52 in the overall population (Fig. 1) or in subgroups of subjects based on use of mycophenolate at baseline (Fig. 2). Similarly, there was no evidence of an association between the extent of fibrotic ILD at baseline and the rate of decline in FVC (mL/year) over 52 weeks (Supplementary Figs S1 and S2, available at Rheumatology online).
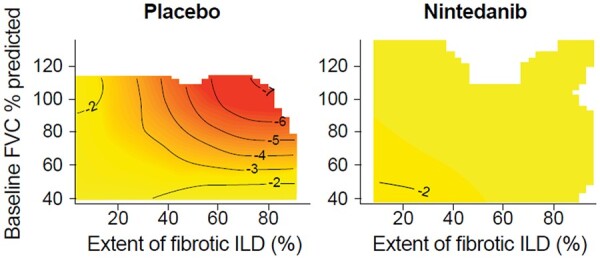
Figure 1.
Associations between extent of fibrotic ILD at baseline and change in FVC. Associations between extent of fibrotic ILD on HRCT at baseline and change in FVC % predicted at week 52. Dashed lines indicate 95% confidence intervals. FVC: forced vital capacity; HRCT: high-resolution CT; ILD: interstitial lung disease
Figure 2.
Associations between extent of fibrotic ILD at baseline and change in FVC in mycophenolate subgroups. Associations between extent of fibrotic ILD on HRCT at baseline and change in FVC % predicted at week 52 in subgroups by mycophenolate use at baseline. Dashed lines indicate 95% CIs. FVC: forced vital capacity; HRCT: high-resolution CT; ILD: interstitial lung disease
Interaction of extent of fibrotic ILD and FVC % predicted at baseline and decline in FVC over 52 weeks
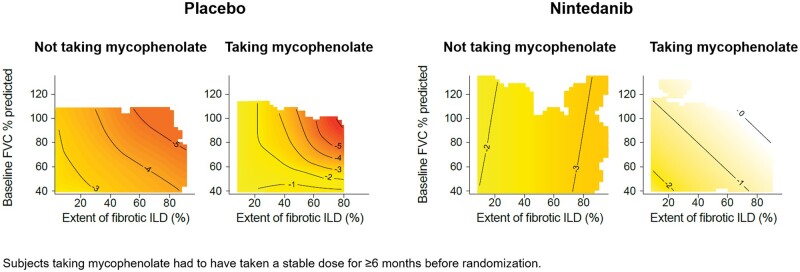
In the placebo group, higher values of both the extent of fibrotic ILD and FVC % predicted at baseline tended to be associated with a greater decline in FVC % predicted (Fig. 3), greater decline in FVC (mL) (Supplementary Fig. S3, available at Rheumatology online) and greater rate of decline in FVC (mL/year) over 52 weeks (Supplementary Fig. S4, available at Rheumatology online). However, these areas were sparsely populated with subjects and none of the bivariate smooth terms in the models reached statistical significance (all P-values >0.15). The association between the interplay of the extent of fibrotic ILD and FVC % predicted at baseline and the decline in FVC % predicted over 52 weeks in the placebo group was more pronounced in subjects taking than not taking mycophenolate at baseline (Fig. 4). Similar patterns were observed in subjects who were and were not taking mycophenolate at baseline for the association between the interplay of the extent of fibrotic ILD and FVC % predicted at baseline and decline in FVC (mL) or rate of decline in FVC (mL/year) over 52 weeks in the placebo group (Supplementary Figs S5 and S6, available at Rheumatology online).
Figure 3.
Change in FVC by extent of fibrotic ILD and FVC at baseline. Contour plots of change in FVC % predicted at week 52 by extent of fibrotic ILD on HRCT and FVC % predicted at baseline. Darker shading indicates greater decline in FVC % predicted at week 52. FVC: forced vital capacity; HRCT: high-resolution CT; ILD: interstitial lung disease
Figure 4.
Change in FVC by extent of fibrotic ILD and FVC at baseline in mycophenolate subgroups. Contour plots of change in FVC % predicted at week 52 by extent of fibrotic ILD on HRCT and FVC % predicted at baseline in subgroups by mycophenolate use at baseline. Darker shading indicates greater decline in FVC % predicted at week 52. FVC: forced vital capacity; HRCT: high-resolution CT; ILD: interstitial lung disease
In the nintedanib group, there was no evidence of an association between the interplay of the extent of fibrotic ILD and FVC % predicted at baseline and decline in FVC % predicted at week 52 (Fig. 3), decline in FVC (mL) at week 52 (Supplementary Fig. S3, available at Rheumatology online) or rate of decline in FVC (mL/year) over 52 weeks (Supplementary Fig. S4, available at Rheumatology online). Similar results were observed in subgroups by use of mycophenolate at baseline (Fig. 4; Supplementary Figs S5 and S6, available at Rheumatology online).
Association between extent of fibrotic ILD and FVC at baseline
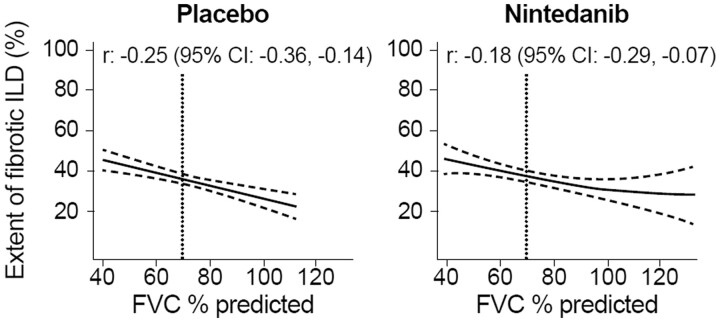
A greater extent of fibrotic ILD at baseline was weakly associated with a lower FVC % predicted at baseline both in the placebo group [r: –0.25 (95% CI: –0.35, –0.14)] and in the nintedanib group [r: –0.18 (95% CI: –0.29, –0.07)] (Fig. 5). An extent of fibrotic ILD of 40% was associated with an FVC of 70% predicted.
Figure 5.
Associations between extent of fibrotic ILD on HRCT and FVC % predicted at baseline. FVC: forced vital capacity; HRCT: high-resolution CT; ILD: interstitial lung disease
Subgroup analyses based on extent of fibrotic ILD and FVC % predicted at baseline
Based on the above finding, we analyzed outcomes in subgroups with an extent of fibrotic ILD on HRCT ≥10 to ≤30% vs an extent of fibrotic ILD on HRCT >40% or an extent of fibrotic ILD on HRCT >30% to ≤40% with FVC <70% predicted. In the placebo group, compared with subjects with an extent of fibrotic ILD ≥10 to ≤30%, the rate of decline in FVC (mL/year) over 52 weeks was numerically greater in subjects with an extent of fibrotic ILD >40% or an extent of fibrotic ILD >30% to ≤40% with FVC <70% predicted (Supplementary Fig. S7, available at Rheumatology online). The proportion of subjects with a decrease in FVC ≥3.3% predicted over 52 weeks was similar between these subgroups (Supplementary Table S1, available at Rheumatology online). The effect of nintedanib vs placebo on reducing the rate of decline in FVC (mL/year) was numerically more pronounced in subjects with an extent of fibrotic ILD >40% or an extent of fibrotic ILD >30% to ≤40% with FVC <70% predicted, but the exploratory interaction P-value did not indicate heterogeneity in the treatment effect between these subgroups (P =0.40) (Supplementary Fig. S7, available at Rheumatology online). The proportion of subjects with a decrease in FVC ≥3.3% predicted over 52 weeks was lower in subjects treated with nintedanib than placebo in both subgroups (Supplementary Table S1, available at Rheumatology online). The proportion of subjects with an absolute decline in FVC ≥10% predicted, or an absolute decline in FVC ≥5% to <10% predicted with absolute decline in DLco ≥15% predicted, or who died over 52 weeks was similar between placebo-treated subjects with an extent of fibrotic ILD ≥10 to ≤30% and those with an extent of fibrotic ILD >40% or extent of fibrotic ILD >30% to ≤40% with FVC <70% predicted, and lower in subjects treated with nintedanib than placebo in both subgroups (Supplementary Table S1, available at Rheumatology online).
Discussion
In patients with SSc-ILD, a greater extent of fibrotic ILD on HRCT, and a lower FVC at baseline, have been associated with a higher rate of ILD progression and mortality [3–7, 14, 15]. These relationships may be considered in terms of thresholds or by considering the extent of fibrotic ILD and FVC as continuous variables. While cut-offs are useful for clinical decision-making, in reality associations are likely to be gradual and not necessarily linear. We used a flexible regression modelling approach to assess associations between the extent of fibrotic ILD and FVC % predicted at baseline and change in FVC over 52 weeks in the SENSCIS trial. This approach offers advantages over linear regression models, as non-linear effects and interactions are considered, giving greater flexibility and involving fewer assumptions [16]. Among subjects who received placebo, we found only weak evidence of a modest association between a greater extent of fibrotic ILD at baseline and a greater decline in FVC over the following 52 weeks. In patients taking both nintedanib and mycophenolate, there was weak evidence of a very modest association between a greater extent of fibrotic ILD on HRCT at baseline and a lower decline in FVC over the following 52 weeks. Analyses of data from Scleroderma Lung Study I based on linear mixed effects modelling [14] and correlation analyses [15], and a proportional hazards analysis of data from the Goh et al. study [4], found stronger associations between the extent of fibrotic SSc-ILD at baseline and the rate of decline in FVC. The differences between the findings of these studies and those of our overall analyses of data from the SENSCIS trial may relate to differences in the patient populations evaluated or to the medications that they were taking, as well as to differences in study design and statistical methodologies. For example, the Goh et al. study [4] enrolled patients with any extent of ILD on HRCT, while patients in the SENSCIS trial had fibrotic ILD of ≥10% extent on HRCT.
Our results suggest that a higher FVC % predicted at baseline was associated with a greater absolute decline in FVC % predicted during the SENSCIS trial. This may reflect that patients who had a higher FVC at baseline had a greater respiratory reserve to lose over the following 52 weeks. Consistent with this, a recent analysis of 826 patients with SSc-ILD in the EUSTAR database found that a greater FVC % predicted at baseline was associated with a higher risk of a decline in FVC >10% to 20% predicted over the next 12 months [17]. However, linear modelling of data from Scleroderma Lung Study I found no association between FVC % predicted at baseline and decline in FVC % predicted over the next 12 months [14]. As observed in previous studies [4, 8, 10, 18], in our study, a greater extent of fibrotic ILD was associated with lower FVC % predicted at baseline, but the correlation was weak.
Clinicians may be more likely to initiate treatment in patients with SSc-ILD who have a greater extent of fibrotic ILD and/or a lower FVC % predicted [19–21] based on a belief that these patients’ ILD is more likely to progress. A post-hoc analysis of data from Scleroderma Lung Study I suggested that patients with more severe reticular changes on HRCT may have a greater response to cyclophosphamide [22]. However, post-hoc analyses of data from the subgroup of patients with early diffuse cutaneous SSc and ILD in the focuSSced trial indicated that the effect of tocilizumab on FVC did not vary by quantitative ILD or lung fibrosis scores at baseline [18]. In our analyses, we found only small, non-significant differences in the effect of nintedanib on the rate of decline in FVC between subjects with differing extents of fibrotic ILD or FVC % predicted at baseline. These findings add to previous analyses of the SENSCIS trial that showed no heterogeneity in the effect of nintedanib on the rate of FVC decline across subgroups based on FVC thresholds of 60%, 70%, 80% and 90% predicted at baseline [23–25]. In the nintedanib group, the findings of our current analyses were similar in subjects who were and were not taking mycophenolate at baseline, consistent with the pre-specified analysis that showed a consistent effect of nintedanib on the rate of FVC decline between these subgroups [26]. Taken together, these findings suggest that baseline characteristics alone, including the extent of fibrotic ILD on HRCT, cannot be used to inform which patients with SSc-ILD should be given nintedanib, as its relative effect is similar across the spectrum of disease severity. We acknowledge that the same degree of ILD progression would likely have more of an impact on functional limitations in patients with more severe disease. However, an argument can also be made for targeting fibrosis in patients with early ILD to slow irreversible loss of lung function in those patients and so improve outcomes, including survival. This argument is strengthened by the unpredictable clinical course of SSc-ILD, which makes it difficult to predict which patients ILD will remain ‘mild’ [3, 17]. In clinical practice, therapeutic decisions need to be made based on more information than markers of disease severity at a given time-point, including evidence of and risk factors for progression, other manifestations of SSc or comorbidities, and patient preferences [19, 21].
Strengths of our analyses include the large sample size, flexible modelling approach, assessment of the extent of fibrotic ILD in the whole lung, based on central review of HRCT scans, and robust measurement of FVC. When interpreting our analyses, it should be borne in mind that the extent of fibrotic ILD on HRCT in the SENSCIS trial was determined using a different methodology to that used in studies such as the Goh et al. study [4] in which the extent of fibrotic ILD was estimated visually to the nearest 5% in five sections and the overall extent computed as the mean of these five scores. This methodology based on assessment of five sections, not including the bases of the lungs, provides a lower estimate for the total extent of fibrotic ILD than the methodology based on assessment of the whole lung that was used in the SENSCIS trial. This is reflected in the observation that an FVC of 70% predicted at baseline reflected an extent of fibrotic ILD of 40% in our analyses but an extent of fibrotic ILD of 20% in the Goh et al. study [4]. Differences in methodology used to quantify fibrotic ILD on HRCT need to be borne in mind in the comparison of subgroup analyses based on data from different studies. In general, comparisons between the current study and the Goh et al. study [4] should be approached with caution, given the differences between the aims, methodologies, durations and patient populations in these studies.
Limitations of our analyses include that the SENSCIS trial was not designed to evaluate associations between the extent of fibrotic ILD on HRCT and FVC % predicted at baseline and change in FVC; that a follow-up period of 52 weeks may be too short to detect such associations; and that our analyses were conducted post-hoc, so our findings should be considered exploratory. Even under the standardized conditions of a clinical trial, measurements of the extent of fibrotic ILD on HRCT and of FVC are subject to error, which may attenuate associations. The use of a single expert radiologist to assess the extent of fibrotic ILD on HRCT meant that inter-reader variability could not be assessed. The exclusion of patients with an FVC <40% predicted or a DLco <30% predicted from the trial may have influenced our findings. The generalizability of our findings beyond patients who met the inclusion criteria for the SENSCIS trial is unknown. Only 19 deaths occurred over the entire SENSCIS trial so we could not evaluate the association between the extent of fibrotic ILD or FVC at baseline and mortality.
In conclusion, using contemporary methodology involving few assumptions, we found weak evidence of a modest inverse association between the extent of fibrotic ILD at baseline and decline in FVC over 52 weeks among patients with SSc-ILD who received placebo in the SENSCIS trial. There were small numerical differences in the effect of nintedanib on slowing the rate of decline in FVC among patients who had differing extents of fibrotic ILD or FVC % predicted at baseline assessed as continuous variables. These findings suggest that patients with SSc-ILD are at risk of progression and benefit from treatment with nintedanib largely irrespective of the severity of their ILD at baseline.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
Acknowledgements
We thank the patients who participated in the SENSCIS trial. We thank David Lynch, National Jewish Health, Denver, USA, for his contribution to the discussion of these data. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment for development of this manuscript. Elizabeth Ng and Wendy Morris of FleishmanHillard, London, UK, provided writing support, which was contracted and funded by BI. BI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Contributor Information
Christopher P Denton, Centre for Rheumatology and Connective Tissue Diseases, University College London Division of Medicine, London, UK.
Nicole S Goh, Respiratory and Sleep Medicine, Austin Health, and Institute for Breathing and Sleep, Melbourne, VIC, Australia.
Stephen M Humphries, Department of Radiology, National Jewish Health, Denver, CO, USA.
Toby M Maher, National Heart and Lung Institute, Imperial College London, London, UK; Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Robert Spiera, Division of Rheumatology, Hospital for Special Surgery, New York, NY, USA.
Anand Devaraj, Department of Radiology, Royal Brompton Hospital and National Heart and Lung Institute, Imperial College London, London, UK.
Lawrence Ho, Center for Interstitial Lung Diseases, University of Washington, Seattle, WA, USA.
Christian Stock, Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim am Rhein, Germany.
Elvira Erhardt, mainanalytics GmbH, Sulzbach, Germany.
Margarida Alves, Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany.
Athol U Wells, National Institute for Health Research Respiratory Biomedical Research Unit, Royal Brompton and Harefield NHS Foundation Trust, and National Heart and Lung Institute, Imperial College London, London, UK.
Data availability statement
Data are available upon reasonable request (see Supplementary Material, available at Rheumatology online).
Funding
The SENSCIS trial was supported by Boehringer Ingelheim (BI).
Disclosure statement: C.P.D. reports grants from Arxx Therapeutics, CSL Behring, GlaxoSmithKline, Inventiva, Servier; consulting fees from Abbvie, Acceleron, Bayer, Boehringer Ingelheim (BI), Corbus, CSL Behring, GlaxoSmithKline, Horizon Therapeutics, Inventiva, Roche, Sanofi; and speaking fees from BI, Corbus, Janssen. N.S.G. reports speaker fees from Novartis. S.M.H. reports grants from BI; the National Heart, Lung, and Blood Institute (NHLBI); Veracyte; Calyx; consulting fees from Lyra Therapeutics and Veracyte; and holds a patent for systems and methods for automatic detection and quantification of pathology using dynamic feature classification assigned to his institution. T.M.M. reports consulting fees from AstraZeneca, Bayer, Blade Therapeutics, BI, Bristol Myers Squibb, Galapagos, Galecto, GlaxoSmithKline, IQVIA, Pliant, Respivant, Roche/Genentech, Theravance, Veracyte; and speaking fees from BI and Roche/Genentech. R.S. reports research support from AstraZeneca, BI, ChemoCentryx, Corbus, Formation Biologics, GlaxoSmithKline, InflaRx, Kadmon, Roche/Genentech; consulting fees from BI, ChemoCentryx, Chemomab, Formation Biologics, GlaxoSmithKline, Janssen, Roche/Genentech, Sanofi; and has participated on Data Safety Monitoring Boards or Advisory Boards for AbbVie, Bristol Myers Squibb. A.D. reports consulting fees from BI, Brainomix, Galapagos, Galecto, Roche, Vicore. L.H. has no disclosures. C.S. and M.A. are employees of BI. E.E. is an employee of mainanalytics GmbH, which was contracted by BI to conduct some of the analyses in this manuscript. A.U.W. reports consulting fees from Blade Therapeutics, BI, Roche and speaking fees from BI and Roche.
References
- 1. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elhai M, Meune C, Boubaya M. et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis 2017;76:1897–905. [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann-Vold AM, Fretheim H, Halse AK. et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med 2019;200:1258–66. [DOI] [PubMed] [Google Scholar]
- 4. Goh NS, Desai SR, Veeraraghavan S. et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008;177:1248–54. [DOI] [PubMed] [Google Scholar]
- 5. Moore OA, Goh N, Corte T. et al. Extent of disease on high-resolution computed tomography lung is a predictor of decline and mortality in systemic sclerosis-related interstitial lung disease. Rheumatology 2013;52:155–60. [DOI] [PubMed] [Google Scholar]
- 6. Jhajj A, Gill HP, Hague CJ. et al. Pulmonary physiology is poorly associated with radiological extent of disease in systemic sclerosis-associated interstitial lung disease. Eur Respir J 2019;53:1802182. [DOI] [PubMed] [Google Scholar]
- 7. Nihtyanova SI, Schreiber BE, Ong VH. et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol 2014;66:1625–35. [DOI] [PubMed] [Google Scholar]
- 8. Goh NS, Hoyles RK, Denton CP. et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2017;69:1670–8. [DOI] [PubMed] [Google Scholar]
- 9. Volkmann ER, Tashkin DP, Sim M. et al. Short-term progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Ann Rheum Dis 2019;78:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le Gouellec N, Duhamel A, Perez T. et al. Predictors of lung function test severity and outcome in systemic sclerosis-associated interstitial lung disease. PLoS One 2017;12:e0181692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Distler O, Highland KB, Gahlemann M. et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019;380:2518–28. [DOI] [PubMed] [Google Scholar]
- 12. Maher TM, Mayes MD, Kreuter M. et al. Effect of nintedanib on lung function in patients with systemic sclerosis-associated interstitial lung disease: further analyses of the SENSCIS trial. Arthritis Rheumatol 2021;73:671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kafaja S, Clements PJ, Wilhalme H. et al. Reliability and minimal clinically important differences of forced vital capacity: results from the Scleroderma Lung Studies (SLS-I and SLS-II). Am J Respir Crit Care Med 2018;197:644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khanna D, Tseng CH, Farmani N. et al. Clinical course of lung physiology in patients with scleroderma and interstitial lung disease: analysis of the Scleroderma Lung Study placebo group. Arthritis Rheum 2011;63:3078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khanna D, Nagaraja V, Tseng CH. et al. Predictors of lung function decline in scleroderma-related interstitial lung disease based on high-resolution computed tomography: implications for cohort enrichment in systemic sclerosis-associated interstitial lung disease trials. Arthritis Res Ther 2015;17:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wood SN. Generalized additive models: an introduction with R. 2nd edn. New York: Chapman & Hall/CRC, 2017. [Google Scholar]
- 17. Hoffmann-Vold AM, Allanore Y, Alves M. et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis 2021;80:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roofeh D, Lin CJF, Goldin J. et al. Tocilizumab prevents progression of early systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol 2021a;73:1301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoffmann-Vold AM, Maher TM, Philpot EE. et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheumatol 2020;2:E71–e83. [DOI] [PubMed] [Google Scholar]
- 20. Perelas A, Silver RM, Arrossi AV, Highland KB.. Systemic sclerosis-associated interstitial lung disease. Lancet Respir Med 2020;8:304–20. [DOI] [PubMed] [Google Scholar]
- 21. Roofeh D, Lescoat A, Khanna D.. Treatment for systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol 2021b;33:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roth MD, Tseng CH, Clements PJ. et al. Predicting treatment outcomes and responder subsets in scleroderma-related interstitial lung disease. Arthritis Rheum 2011;63:2797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maher TM, Distler O, Azuma A, Highland KB. et al. Effects of nintedanib in patients with systemic sclerosis-associated ILD (SSc-ILD) and differing FVC at baseline: the SENSCIS trial. Eur Respir J 2019;54:OA3599. [Google Scholar]
- 24. Goh NS, Denton CP, Lynch DA. et al. Effect of nintedanib in patients with limited and extensive systemic sclerosis-associated interstitial lung disease (SSc-ILD): data from the SENSCIS trial. Am J Respir Crit Care Med 2020;201:A1525–A1525. [Google Scholar]
- 25. Wuyts WA, Azuma A, Distler JHW. et al. Effects of nintedanib in patients with SSc-ILD and preserved and highly impaired lung function. Eur Respir J 2020;56:3393. [Google Scholar]
- 26. Highland KB, Distler O, Kuwana M. et al. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med 2021;9:96–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request (see Supplementary Material, available at Rheumatology online).