Abstract
Objective
To evaluate the long-term efficacy and safety of brodalumab, a fully human anti–interleukin-17 receptor A monoclonal antibody, in patients with axial spondyloarthritis (axSpA).
Methods
Patients receiving subcutaneous brodalumab 210 mg during the 16-week double-blind period of this multicentre, phase 3 study conducted across Japan, Korea and Taiwan continued the same during the 52-week open-label extension, whereas patients receiving placebo switched to brodalumab 210 mg at week 16. Efficacy [Assessment of SpondyloArthritis International Society (ASAS) 40 and ASAS 20 response rates; change from baseline in AS Disease Activity Score using CRP (ASDAS-CRP)] and safety were evaluated.
Results
Overall, 145 patients (brodalumab, n = 77; placebo, n = 68) received brodalumab during the open-label extension. ASAS 40 response rates (95% CI) of 56.3% (44.7%, 67.3%) and 57.4% (44.1%, 70.0%) were achieved in the brodalumab and placebo groups, respectively, at week 68. ASAS 20 response rates (95% CI) achieved at week 68 in both treatment groups were similar [brodalumab, 71.3% (60.0%, 80.8%); placebo, 78.7% (66.3%, 88.1%)]. The least squares mean change (95% CI) in ASDAS-CRP at week 68 suggested a clinically important improvement (change, ≥1.1) in both treatment groups [brodalumab, –1.528 (–1.737, –1.319); placebo, –1.586 (–1.815, –1.357)]. The exposure-adjusted event rates (per 100 patient-years) for treatment-emergent adverse events (TEAEs) and drug-related TEAEs were 255.9 and 147.9, respectively; nasopharyngitis (35.6) and upper respiratory tract infection (14.7) were the most common TEAEs.
Conclusions
Brodalumab demonstrated sustained efficacy and a consistent safety profile in patients with axSpA over 68 weeks.
Study registration
ClinicalTrials.gov, https://clinicaltrials.gov, NCT02985983
Keywords: AS, brodalumab, IL-17 receptor A, non-radiographic axial spondyloarthritis
Rheumatology key messages.
This phase 3 study reports the first long-term results of brodalumab in patients with axSpA. Brodalumab, an interleukin-17 receptor A blocker, demonstrated long-term (68 weeks) sustained efficacy and a consistent safety profile in patients with axSpA.
Brodalumab represents a potential treatment option in patients with axSpA.
Introduction
Activation of IL-17 signalling pathways is believed to have an underlying role in the pathogenesis of several autoimmune and inflammatory diseases. IL-17 receptor A (IL-17RA) is the common receptor subunit involved in the intracellular signalling of the IL-17 cytokines A, F, A/F heterodimer and E [1, 2]. Therefore, IL-17RA blockade represents a novel mechanism of action of inhibiting inflammation and clinical symptoms associated with autoimmune and inflammatory diseases such as psoriasis and PsA [1]. Brodalumab, a fully human anti–IL-17RA monoclonal antibody with a broader IL-17 blocking action [1], has demonstrated favourable responses in non-Japanese and Japanese phase 2 studies in patients with PsA, confirming the involvement of IL-17 pathways in PsA pathogenesis [2, 3].
Axial spondyloarthritis (axSpA), a chronic inflammatory arthritis affecting the axial skeleton, and PsA share common pathophysiology and symptoms. Therefore, this phase 3 study was conducted to evaluate the efficacy and safety of brodalumab in patients with axSpA [4]. In an interim analysis of the study, brodalumab demonstrated a favourable benefit-risk profile over 16 weeks, with a significant difference [19.7%; 95% CI, (5.3%, 34.1%); P = 0.018] observed in ASAS 40 response rates vs placebo [5]. Here, we report the 68-week long-term efficacy and safety results from this study.
Methods
Study design
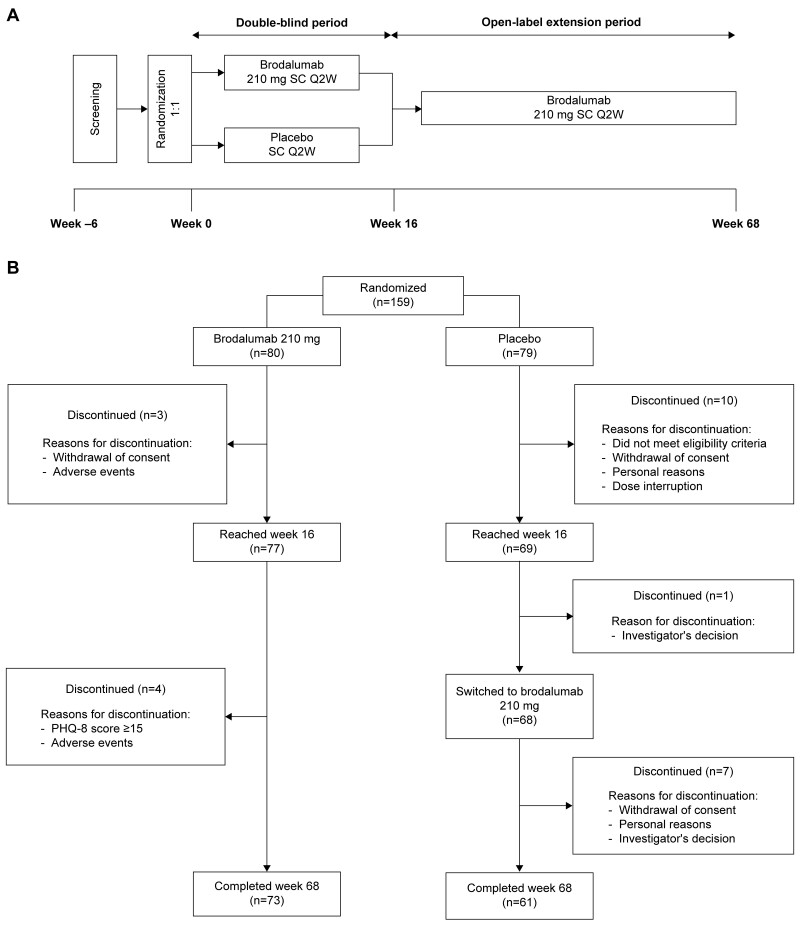
This was a multicentre, randomized, placebo-controlled, phase 3 study with a 16-week double-blind period and a 52-week open-label extension period (Fig. 1A) conducted in patients with axSpA across Japan, Korea and Taiwan. The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the institutional review board or independent ethics committee at each study site (Supplementary Table S1, available at Rheumatology online). All study participants provided written voluntary informed consent. The study is registered at ClinicalTrials.gov (NCT02985983).
Figure 1.
(A) Study design and (B) patient disposition. Q2W: every 2 weeks; PHQ-8: 8-item Patient Health Questionnaire; SC: subcutaneous
Eligibility criteria have been described previously [5]. Eligible patients had active AS or non-radiographic (nr)-axSpA, based on the ASAS classification criteria for axSpA as described previously [5]. During the double-blind period, eligible patients were randomized 1:1 to receive subcutaneous brodalumab 210 mg or placebo at baseline, weeks 1 and 2 and every 2 weeks thereafter. Patients receiving brodalumab during the double-blind period continued the same during the 52-week open-label extension, whereas those receiving placebo switched to brodalumab 210 mg (Fig. 1A).
Assessments
The primary and secondary endpoints at week 16 have been described previously [5]. Assessments of disease activity and quality of life were conducted throughout the 68 weeks of the study. Outcome measures included ASAS 40/20 response rates among patients with axSpA, including patients with AS and nr-axSpA; change from baseline in the Ankylosing Spondylitis Disease Activity Score using CRP (ASDAS-CRP); and disease activity state by improvement in ASDAS-CRP. Additionally, changes in the BASFI, BASDAI, BASMI, AS Quality of Life Questionnaire (ASQoL) score, Short Form-36 Health Survey version 2 (SF-36v2) score, enthesitis count, swollen joint count and Patient Global Assessment (PGA) of spinal pain and axSpA scores were assessed at each visit.
Patients with evidence of spinal inflammation on MRI within 6 weeks before enrolment (patients with AS with additional consent for MRI) underwent MRI assessment at enrolment and at week 16 (only patients with AS) and week 68. The MRI scans were centrally read by two blinded readers and an adjudicator in case of discrepancy between the two readings. The Berlin spine score and Spondyloarthritis Research Consortium of Canada (SPARCC) SI joint score were used for MRI assessment of AS and nr-axSpA, respectively [6].
For safety assessment, patients were followed up for treatment-emergent adverse events (TEAEs) until they recovered to the baseline status or the investigators judged that no further follow-up was necessary based on symptoms, findings and laboratory data, among other parameters. TEAE grades are defined in Supplementary Table S2, available at Rheumatology online. TEAEs identified as risks (neutrophil count decreased, serious infections, serious hypersensitivity) or considered as potential risks (malignancy, IBD, suicide/self-injury-related events) were assessed as ‘TEAEs of interest’ (Supplementary Tables S3 and S4, available at Rheumatology online) [5]. The search list for preferred terms considered were sponsor-defined events of interest (EOI: IBD, colonic abscess, Crohn’s disease, enteritis, large intestinal ulcer perforation, metastatic cutaneous Crohn’s disease and small intestinal ulcer perforation) and Standardized MedDRA Queries (SMQs: gastrointestinal ulceration and ischaemic colitis).
Blood samples for the assessment of antibodies were collected at enrolment; weeks 16, 32, 48 and 68; or at the end of treatment. Anti-brodalumab antibodies were measured by PPD, Inc., using an electrochemiluminescent immunoassay. Positive samples in the immunoassay were further analysed for the presence of neutralizing antibodies using a cell-based bioassay.
Patient and public involvement in research
Patients or the public were not involved in the design or conduct, reporting or dissemination plans of our research.
Statistical analysis
Sample size estimation has been described previously [5]. The point estimate and 95% CI for ASAS 40/20 were calculated for each treatment group and subpopulation.
Missing data at weeks 16 and 68 of binary response variables for ASAS 20/40 were imputed as no response in the treatment group continuing brodalumab. For ASDAS-CRP, if post-dosing data for continuous variables were missing at week 16, the missing value was imputed with the baseline value, that is, change from baseline was treated as 0. For the remaining assessments, an observed case analysis was performed.
TEAEs occurring after the first dose of brodalumab through week 68 were pooled and summarized during the open-label extension period. The percentage of patients who developed anti-brodalumab antibodies was summarized for each time point. Exposure-adjusted event rates (EAERs) were calculated as the total number of TEAEs reported in a given time period of brodalumab exposure divided by the total patient-years (PY) of exposure in that period. The total PY of exposure was calculated as the sum of duration of exposure in days divided by 365.25. Safety events were coded using the Medical Dictionary for Regulatory Activities version 22.0.
Results
Patient disposition and baseline patient characteristics
Overall, 84.3% (n = 134) of randomized patients completed the 68-week study: 73/80 (91.3%) in the brodalumab group and 61/79 (77.2%) in the placebo group (Fig. 1B). Of the 146 patients who completed the 16-week double-blind period, 145 (brodalumab, n = 77; placebo, n = 68) entered the open-label extension and received brodalumab; one patient in the placebo group discontinued due to personal reasons. From weeks 16–68, 12 patients (brodalumab, four; placebo, eight) discontinued the study.
For 25 patients who discontinued over the entire study period, the reasons for discontinuation were withdrawal of consent (n = 12), adverse events (n = 4), failure to meet eligibility criteria (n = 3), personal or investigator’s decision (n = 2 each), dose interruption (n = 1) and 8-item Patient Health Questionnaire score (PHQ-8) score ≥15 (n = 1). Baseline characteristics of enrolled patients have been described previously [5].
Efficacy
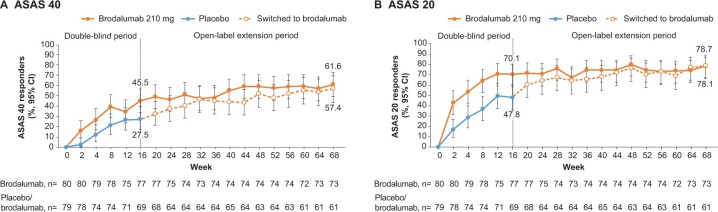
The observed ASAS 40 response rate (95% CI) was 45.5% (34.1%, 57.2%) at week 16 and 61.6% (49.5%, 72.8%) at week 68 in patients who continued brodalumab (Fig. 2A). In patients who switched to brodalumab during the open-label extension, the observed ASAS 40 response rate (95% CI) was 27.5% (17.5%, 39.6%) at week 16 and 57.4% (44.1%, 70.0%) at week 68 (Fig. 2A). The observed ASAS 20 response rate (95% CI) over the open-label extension period was 70.1% (58.6%, 80.0%) at week 16 and 78.1% (66.9%, 86.9%) in patients who continued brodalumab (Fig. 2B). In patients who switched to brodalumab during the open-label extension, the observed ASAS 20 response rate (95% CI) was 47.8% (35.6%, 60.2%) at week 16 and 78.7% (66.3%, 88.1%) at week 68 (Fig. 2B).
Figure 2.
ASAS response in patients with axial spondyloarthritis (observed data). (A) ASAS 40 and (B) ASAS 20. ASAS: Assessment of SpondyloArthritis International Society; CI: confidence interval
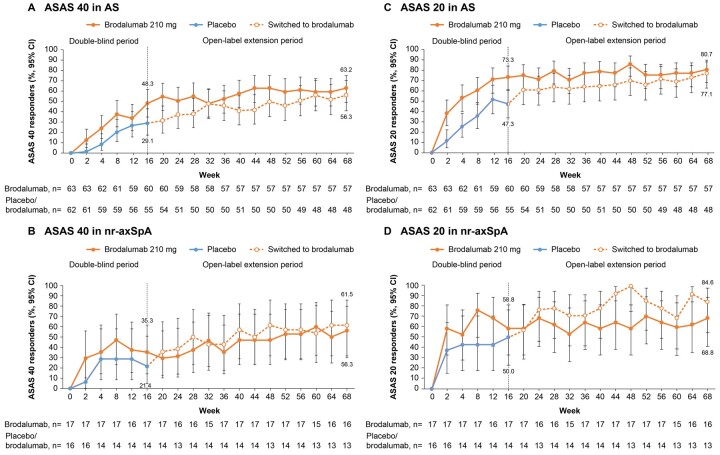
The ASAS 40 response rate (95% CI) by non-responder imputation (NRI) was 43.8% (32.7%, 55.3%; n = 80) at week 16 and 56.3% (44.7%, 67.3%; n = 80) at week 68 in patients who continued brodalumab. The ASAS 20 response rate (95% CI) by NRI was 67.5% (56.1%, 77.6%; n = 80) at week 16 and 71.3% (60.0%, 80.8%; n = 80) at week 68 in patients who continued brodalumab. In subpopulations with AS [brodalumab, 63.2% (49.3%, 75.6%); placebo, 56.3% (41.2%, 70.5%); Fig. 3A] and nr-axSpA [brodalumab, 56.3% (29.9%, 80.2%); placebo, 61.5% (31.6%, 86.1%); Fig. 3B], the observed ASAS 40 response rates (95% CI) at week 68 were comparable in both treatment groups. In post hoc analyses, the observed ASAS 20 response rates (95% CI) at week 68 were comparable in both treatment groups [brodalumab, 80.7% (68.1%, 90.0%); placebo, 77.1% (62.7%, 88.0%)] among patients with AS (Fig. 3C). Among patients with nr-axSpA, the observed ASAS 20 response rate (95% CI) at week 68 was 84.6% (54.6%, 98.1%) in patients who switched to brodalumab and 68.8% (41.3%, 89.0%) in patients who continued brodalumab (Fig. 3D).
Figure 3.
ASAS 40 and ASAS 20 responses stratified by disease subpopulation (observed data). (A) ASAS 40 in AS, (B) ASAS 40 in nr-axSpA, (C) ASAS 20 in AS and (D) ASAS 20 in nr-asSpA ASAS: Assessment of SpondyloArthritis International Society; nr-axSpA: non-radiographic axial spondyloarthritis
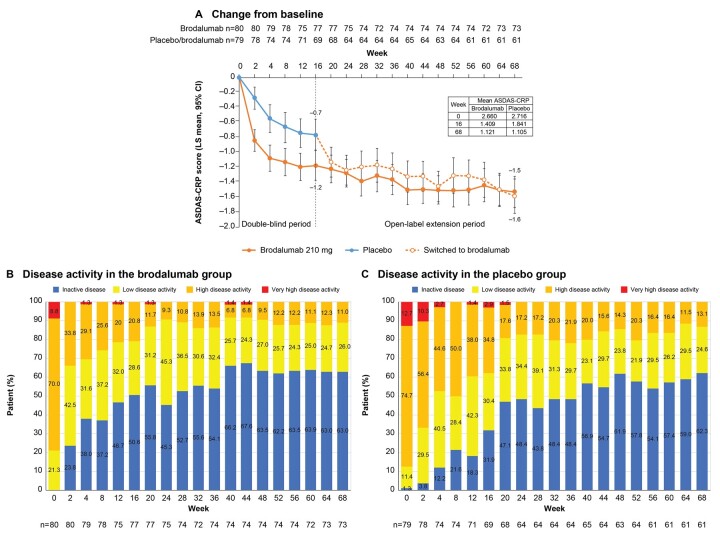
The least squares (LS) mean (95% CI) change in ASDAS-CRP from baseline was comparable at week 68 in both treatment groups [brodalumab, –1.528 (–1.737, –1.319); placebo, –1.586 (–1.815, –1.357); Fig. 4A] and suggested a clinically important improvement (CII; change, ≥1.1). ASDAS-CRP rates of major improvement and CII were similar at week 68 in both treatment groups (Supplementary Table S5, available at Rheumatology online). From weeks 16–68, the proportion of patients with ASDAS-CRP inactive disease increased from 50.6% to 63.0% in the brodalumab group (Fig. 4B) and from 31.9% to 62.3% among patients who switched to brodalumab (Fig. 4C). The mean (s.d.) CRP levels decreased from 1.30 (1.71) mg/dl at baseline to 0.58 (1.54) mg/dl in the brodalumab group and from 1.05 (1.00) mg/dl to 0.64 (1.35) mg/dl among patients who switched to brodalumab (Supplementary Table S6, available at Rheumatology online).
Figure 4.
ASDAS-CRP. (A) Change from baseline, (B) disease activity in the brodalumab group and (C) disease activity in the placebo group. ASDAS-CRP: AS Disease Activity Score using CRP; LS: least squares
The improvement in disease activity was sustained beyond 40 weeks. Improvements from baseline to week 68 in the BASFI, BASDAI, BASMI, ASQoL and SF-36v2 scores; enthesitis count; swollen joint count; and PGA of spinal pain and axSpA were observed in both treatment groups (Supplementary Table S7, available at Rheumatology online).
MRI results
In patients with AS, the change in mean (s.d.) Berlin spine score from baseline [brodalumab (n = 8), 10.8 (2.7); placebo (n = 8), 11.3 (4.9)] to week 16 was –5.6 (4.2) vs –0.3 (3.5) in the brodalumab vs placebo group, respectively (Supplementary Fig. S1, available at Rheumatology online). The improvement in Berlin score was sustained through 68 weeks in patients who continued brodalumab (n = 6), with a mean (s.d.) change of –6.3 (3.2) at week 68. In patients who switched to brodalumab (n = 7), a greater improvement from baseline to week 68 [–4.4 (3.6)] was observed following the switch at week 16. Patients who discontinued before week 68 (brodalumab, n = 2; placebo, n = 1) were excluded.
In patients with nr-axSpA, change from baseline [brodalumab (n = 15), 17.5 (16.2); placebo (n = 13), 17.1 (16.0)] to week 68 in the SPARCC SI joint score in the brodalumab (n = 15) vs placebo (n = 10; three patients discontinued before week 68) group was –10.9 (12.7) vs –6.8 (9.4), respectively (data not shown).
Safety
At least one TEAE was reported in 122/148 (82.4%) patients (Table 1). No TEAEs leading to death were reported throughout the study. Other serious TEAEs were reported in 16 (10.8%) patients, and other significant TEAEs occurred in 20 (13.5%) patients (Table 1). Other serious drug-related TEAEs, reported in five (3.4%) patients during the entire study period, included one case each (0.7%) of herpes zoster oticus, acute myocardial infarction, cellulitis, appendicitis and diverticulitis. Deep venous thrombosis or pulmonary embolism was not reported in any patient.
Table 1.
Safety results
| TEAE | Brodalumab PY = 163.0 n = 148a |
|
|---|---|---|
| n | % | |
| Any TEAE | 122 | 82.4 |
| Deaths | 0 | 0 |
| Other seriousb TEAE | 16 | 10.8 |
| Other significantc TEAE | 20 | 13.5 |
| Any drug-related TEAE | 96 | 64.9 |
| Deaths | 0 | 0 |
| Other seriousb TEAE | 5 | 3.4 |
| Other significantc TEAE | 17 | 11.5 |
| TEAEs of interestd, n = 148a | n | r |
| Any TEAE of interest | 21 | 12.9 |
| Inflammatory bowel diseasee | 10 | 6.1 |
| Mouth ulceration | 6 | 3.7 |
| Duodenal ulcer | 1 | 0.6 |
| Gastrointestinal pain | 1 | 0.6 |
| Stomatitis | 1 | 0.6 |
| Lip erosion | 1 | 0.6 |
| Crohn’s disease | 0 | 0 |
| Ulcerative colitis | 0 | 0 |
| Neutrophil count decreased | 3 | 1.8 |
| Leucopenia | 2 | 1.2 |
| Neutrophil count decreased | 1 | 0.6 |
| Serious infections | 8 | 4.9 |
| Cellulitis | 2 | 1.2 |
| Appendicitis | 1 | 0.6 |
| Diverticulitis | 1 | 0.6 |
| External ear cellulitis | 1 | 0.6 |
| Pilonidal cyst | 1 | 0.6 |
| Tonsillitis | 1 | 0.6 |
| Herpes zoster oticus | 1 | 0.6 |
| Serious hypersensitivity | 0 | 0 |
| Malignancy | 0 | 0 |
| Suicide/self-injury-related events | 0 | 0 |
n, number of adverse events; r, exposure-adjusted event rate per 100 PY.
Number of patients who received ≥1 dose of brodalumab during the study.
Any serious TEAE other than death.
Any non-serious TEAE leading to withdrawal of the study drug or dose suspension.
TEAEs identified or considered as potential risks were assessed as ‘TEAEs of interest’ and labelled in the following six categories: neutrophil count decreased/serious infections/serious hypersensitivity (identified risks) and malignancy/inflammatory bowel disease/suicide or self-injury-related events (potential risks) (Supplementary Tables S3 and S4, available at Rheumatology online).
Ulcerative colitis or Crohn’s disease (de novo or exacerbation) was not reported in any patient.
PY: patient-years; TEAE: treatment-emergent adverse event.
The EAER (per 100 PY) for TEAEs and drug-related TEAEs was 255.9 and 147.9, respectively, among patients who received ≥1 dose of brodalumab. The EAER (per 100 PY) for grade ≥3 TEAEs was 16; it was the highest for hyperuricaemia (1.8) and cellulitis (1.2) and 0.6 for the remaining grade ≥3 TEAEs. The highest EAERs (per 100 PY) were reported in the system organ classes ‘Infections and infestations’ (90.2), ‘Gastrointestinal disorders’ (28.2), ‘Musculoskeletal and connective tissue disorders’ (24.5) and ‘Investigations’ (22.7). TEAEs by preferred term (PT; Table 2) with the highest EAERs (per 100 PY) were nasopharyngitis (35.6), upper respiratory tract infection (URTI; 14.7) and alanine aminotransferase increased and diarrhoea (4.9 each). Depression, suicidal ideation and suicides were not reported in any patient.
Table 2.
Exposure-adjusted rates (>2 per 100 PY) of TEAEs by PT and other important TEAEs
| TEAEs by PT (>2 per 100 PY) | Brodalumab 210 mg PY = 163.0 n = 148a |
|
|---|---|---|
| n | r | |
| Any | 417 | 255.9 |
| Nasopharyngitis | 58 | 35.6 |
| Upper respiratory tract infection | 24 | 14.7 |
| Alanine aminotransferase increased | 8 | 4.9 |
| Diarrhoea | 8 | 4.9 |
| Aspartate aminotransferase increased | 7 | 4.3 |
| Gastroenteritis | 7 | 4.3 |
| Oropharyngeal pain | 7 | 4.3 |
| Hyperuricaemia | 6 | 3.7 |
| Influenza | 6 | 3.7 |
| Mouth ulceration | 6 | 3.7 |
| Arthralgia | 5 | 3.1 |
| Back pain | 5 | 3.1 |
| Insomnia | 5 | 3.1 |
| Urticaria | 5 | 3.1 |
| Uveitis | 5 | 3.1 |
| Hypertension | 4 | 2.5 |
| Influenza-like illness | 4 | 2.5 |
| Ligament sprain | 4 | 2.5 |
| Liver function test abnormal | 4 | 2.5 |
| Otitis externa | 4 | 2.5 |
| Pharyngitis | 4 | 2.5 |
| Tonsillitis | 4 | 2.5 |
| Other important TEAEsb | n | r |
| Acute myocardial infarction | 1 | 0.6 |
| Tinea pedis | 1 | 0.6 |
| Oral candidiasis | 1 | 0.6 |
| Tinea faciei | 1 | 0.6 |
| Tinea cruris | 1 | 0.6 |
| Depression | 0 | 0 |
| Suicidal ideation | 0 | 0 |
| Suicide | 0 | 0 |
| Deep vein thrombosis/pulmonary embolism | 0 | 0 |
n, number of adverse events; r, exposure-adjusted event rate per 100 PY.
Number of patients who received ≥1 dose of brodalumab during the study.
Other important TEAEs include major adverse cardiovascular events, deep vein thrombosis/pulmonary embolism, candida infection (mucosal, systemic), uveitis and suicide/self-injury-related events.
PT: preferred term; PY: patient-years; TEAE: treatment-emergent adverse event.
TEAEs of interest, reported in 21 patients, were most commonly categorized as IBD (6.1 per 100 PY) and serious infections (4.9 per 100 PY), and the most common events were mouth ulcerations (3.7 per 100 PY) and cellulitis and leukopoenia (1.2 per 100 PY each) (Table 1). No TEAEs of interest were reported in the categories of malignancy, serious hypersensitivity and suicide/self-injury-related events. The EAER for fungal infections (tinea pedis, oral candidiasis, tinea faciei and tinea cruris in one patient each) was 2.5 per 100 PY, and no cases of ulcerative colitis and Crohn’s disease (de novo or exacerbation) were reported.
At least once during the study, one (0.7%) of 148 patients who received brodalumab was found to be positive for anti-brodalumab-binding antibodies but negative for brodalumab-neutralising antibodies.
Discussion
We present the first long-term results of brodalumab in patients with axSpA from the phase 3 study. The 68-week results demonstrated the sustained efficacy and long-term safety of brodalumab in patients with axSpA, comprising subpopulations of AS and nr-axSpA. The 16-week results of this study had demonstrated significant improvements in ASAS 40 response rates with brodalumab vs placebo in patients with axSpA [5]. IL-17 inhibitors are increasingly gaining acceptance for the treatment of patients with axSpA, with treatment guidelines for axSpA recommending IL-17 inhibitors as third-line treatment options in patients refractory to or with a contraindication to TNF inhibitors. However, only two IL-17 inhibitors are currently approved for the treatment of AS, and evidence is limited, particularly in patients with nr-axSpA [7, 8].
Improvements in ASAS 40/20 response rates were observed beyond 16 weeks among both groups of patients: patients who continued brodalumab and patients who switched to brodalumab from placebo. In both treatment groups, ∼60% and 80% of patients achieved ASAS 40 and ASAS 20 response rates, respectively, at the end of 68 weeks. ASAS 40/20 response rates through 68 weeks in the brodalumab group demonstrated that efficacy was maintained. In the placebo group, the ASAS 40 response rate increased consistently and was comparable to that in the brodalumab group by week 32. Rapid improvements in ASAS 20 response rates were observed as early as week 20 following the switch to brodalumab in the placebo group.
Previous studies in Asian patients with active AS receiving IL-17A inhibitors have shown ASAS responses that were comparable to those achieved with brodalumab in our study. In a pooled subanalysis of data from the phase 3 MEASURE 1 and MEASURE 2 studies, the ASAS 40/20 response rates at 52 weeks were 56.5%/63.0% in 46 Asian patients with active AS receiving continuous secukinumab compared with 59.6%/75.4% in 57 patients with active AS receiving continuous brodalumab in our study [9]. In MEASURE 2-J, the ASAS 40/20 response rates at week 24 were 56.7%/70.0% with secukinumab among 30 Japanese patients with active AS compared with 50.8%/71.2% among 59 Asian patients in our study [10]. Baseline characteristics, such as body mass index (∼25 kg/m2), and proportion of anti-TNF-naive patients were comparable (73%–80%) between the two studies [5, 10].
Although a direct comparison with global studies in patients with active AS receiving IL-17 inhibitors may not be feasible due to differences in study population ethnicity and taking into account that variations in underlying population characteristics could ultimately impact outcomes, ASAS responses achieved across the studies were similar [11–13]. The 68-week ASAS 40/20 response rates (63.2%/80.7%) in patients with active AS in our study are comparable to those achieved over 1 year (66.7%/79.8%) and 5 years (64.5%/77.6%) with secukinumab in the MEASURE 1 global study [11]. Similarly, the 48-week ASAS 40 response rate in patients with active AS with dual IL-17 (A and F) inhibition using continuous bimekizumab in the phase 2 b BE AGILE study was ∼60% and ranged between 50% and 54% among patients who switched after a 12-week placebo treatment [12]. These results are comparable to those in our study after 48 weeks of IL-17RA inhibition (brodalumab, 63.2%; placebo, 50.0%). In a phase 3, randomized, active-controlled clinical study (COAST-V) of ixekizumab, an IL-17A inhibitor, ASAS 40 rates were 50.6%–53.1% in biologic disease-modifying antirheumatic treatment-naive patients with active AS and almost 60% with continuous brodalumab at 52 weeks in our study, which comprised almost 80% of anti-TNF-naive patients [13].
In the randomized, placebo-controlled COAST-X trial, ASAS 40 response rates of 30%–31% were reported at 52 weeks among patients with nr-axSpA receiving ixekizumab compared with those of ∼53% among patients in the continuous brodalumab group reported in our study, suggesting sustained efficacy with brodalumab [14]. However, a direct comparison may not be possible because of differences in the study population and design. Moreover, the sample size for the nr-axSpA subgroup was small in our study.
In our study, ASDAS-CRP improved in both treatment groups over 68 weeks. The LS mean change in ASDAS-CRP at 52 weeks in patients with AS receiving continuous brodalumab in our study (–1.51) was comparable to that reported in a pooled analysis of the MEASURE 1 and MEASURE 2 studies (–1.38) in patients receiving continuous secukinumab [9]. Furthermore, following a switch to brodalumab, the proportion of patients with inactive disease measured by ASDAS-CRP almost doubled in the placebo group, and >62% of patients achieved inactive disease in both treatment groups at week 68 in our study. Improvements were also observed for other efficacy outcomes, including disease activity (BASDAI), spinal mobility (BASMI), physical function (BASFI), patient perception of health status (SF-36v2), signs of inflammation (enthesitis and swollen joint counts), PGA of spinal pain and ASQoL scores.
Improvements in MRI scores were numerically superior in the brodalumab vs placebo group and continued through 68 weeks; improvements increased following the switch from placebo in patients with AS. A mean improvement in the Berlin spine score of 6.3 was reported at week 68 in patients with AS in our study. In MEASURE 1, improvements in Berlin spine score of 0.3/0.9 were reported with secukinumab 75 mg/150 mg, at week 104 in patients with AS [15]. A mean improvement of 10.9 in the SPARCC SI joint score was reported at week 68 in patients with nr-axSpA in our study. In COAST-X, LS mean improvements of 6.16/4.40 in the SPARCC SI joint score were reported with ixekizumab 2-weekly/4-weekly at week 52 [14]. However, a direct comparison of imaging scores with other studies is not possible due to difference in baseline scores, treatment durations and patient numbers.
The long-term safety profile of brodalumab was consistent with that observed over a short term, and the most common TEAEs were reported in the system organ classes ‘Infections and infestations’ and ‘Gastrointestinal disorders’; no new safety signals were observed [5]. The safety profile of brodalumab was similar to that of other IL-17 inhibitors in previous AS studies, with the most frequently reported TEAEs being URTI and nasopharyngitis [16]. The EAER (per 100 PY) reported in this study (255.9) was similar to that (255.8) reported over 120 weeks with brodalumab in patients with psoriasis [17]. EAERs (per 100 PY) for the TEAEs of interest, IBD and neutrophil count decreased, were 6.1 and 1.8, respectively, and were 4.9 for serious infections, 2.5 for fungal infections and 3.1 for uveitis (TEAE by PT).
Because the association of suicidal ideation and behaviour with brodalumab remains controversial, patients with a history or evidence of suicidal ideation (severity, 4 or 5) or any suicidal behaviour according to the Columbia-Suicide Severity Rating Scale or severe depression (total PHQ-8 score ≥15) were excluded from the study [18, 19]. A 1-year (2017–2018) pharmacovigilance update of brodalumab reported no attempted or completed suicides and serious fungal infections like in this study [20]. The absence of brodalumab-neutralising antibodies over the long term indicates a low rate of immunogenicity and the potential for sustained efficacy with brodalumab, given that anti-drug antibodies have been previously linked to biologic fatigue causing reduced efficacy with biologics in psoriasis [21].
The results from our study have a limited generalizability because of the Asian ethnicity of the study population. Moreover, the open-label design and limited sample size of the subpopulations warrant caution while interpreting results.
In conclusion, the results from this study demonstrated the long-term efficacy and safety of brodalumab in patients with axSpA. Brodalumab treatment resulted in sustained disease control over 68 weeks. The safety profile of brodalumab was consistent throughout the 68-week study period, and no new safety signals were identified. The efficacy of brodalumab was comparable to that of other IL-17 inhibitors, suggesting that IL-17RA blockade is a promising target for the treatment of patients with axSpA and rendering brodalumab as a potential treatment option in patients with axSpA.
Supplementary Material
Acknowledgements
Masayuki Takanuma of Kyowa Kirin Co., Ltd analysed the data. Medical writing support was provided by Dr Deepali Garg, MBBS, PGDHA, of Cactus Life Sciences (part of Cactus Communications) and funded by Kyowa Kirin Co., Ltd These results were presented, in part, at the Asia-Pacific League of Associations for Rheumatology Virtual Congress 2020 (APLAR 2020; October 24–29).
Contributor Information
Tae-Hwan Kim, Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, The Republic of Korea.
Mitsumasa Kishimoto, Department of Nephrology and Rheumatology, Kyorin University School of Medicine, Tokyo, Japan.
James Cheng-Chung Wei, Department of Allergy, Immunology and Rheumatology, Chung Shan Medical University Hospital, Taichung, Taiwan; Institute of Medicine, College of Medicine, Chung Shan Medical University, Taichung, Taiwan; Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan; Department of Medical Research, Taichung Veterans General Hospital, Taichung, Taiwan.
Haeyoun Jeong, Clinical Development Team, Medical Department, Kyowa Kirin Korea Co., Ltd, Seoul, The Republic of Korea.
Akiyo Nozaki, Clinical Development Center, R&D Division, Kyowa Kirin Co., Ltd, Tokyo, Japan.
Shigeto Kobayashi, Department of Internal Medicine and Rheumatology, Juntendo University Koshigaya Hospital, Saitama, Japan.
Supplementary data
Supplementary data are available at Rheumatology online.
Data availability statement
No data are available.
Funding
This study was funded by Kyowa Kirin Co., Ltd.
Disclosure statement: T.-H.K. reports all support from Kyowa Kirin Co., Ltd for the present manuscript. M.K. reports all support from Kyowa Kirin Co., Ltd for the present manuscript; consulting fees from AbbVie, Amgen-Astellas BioPharma, Asahi-Kasei Pharma, Astellas, Ayumi Pharma, Bristol-Myers Squibb, Chugai, Eisai, Eli Lilly, Gilead, Janssen, Kyowa Kirin Co., Ltd, Novartis, Ono Pharma, Tanabe-Mitsubishi and UCB Pharma; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie, Amgen-Astellas BioPharma, Asahi-Kasei Pharma, Astellas, Ayumi Pharma, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Kyowa Kirin, Novartis, Ono Pharma, Pfizer, Tanabe-Mitsubishi, Teijin Pharma and UCB Pharma. J.C.-C.W. reports all support from Kyowa Kirin Co., Ltd for the present manuscript; grants or contracts from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB Pharma; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from TSH Biopharma, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Eisai, Janssen, Novartis, Sanofi-Aventis, UCB Pharma and Pfizer. H.J. is an employee of Kyowa Kirin Korea Co., Ltd. A.N. is an employee of Kyowa Kirin Co., Ltd. S.K. reports all support from Kyowa Kirin Co., Ltd for the present manuscript and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Chugai pharmaceuticals, Eli Lilly Japan K.K. and Gilead Sciences, Inc.
Ethics: The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the institutional review board or independent ethics committee at each study site (Supplementary Table S1, available at Rheumatology online). All study participants provided written voluntary informed consent. The study is registered at ClinicalTrials.gov (NCT02985983). A list of investigators is available as Supplementary Table S8 at Rheumatology online.
References
- 1. Nirula A, Nilsen J, Klekotka P. et al. Effect of IL-17 receptor A blockade with brodalumab in inflammatory diseases. Rheumatology 2016;55:ii43–55. [DOI] [PubMed] [Google Scholar]
- 2. Nakagawa H, Niiro H, Ootaki K.. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci 2016;81:44–52. [DOI] [PubMed] [Google Scholar]
- 3. Mease PJ, Genovese MC, Greenwald MW. et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014;370:2295–306. [DOI] [PubMed] [Google Scholar]
- 4. Raychaudhuri SP, Deodhar A.. The classification and diagnostic criteria of ankylosing spondylitis. J Autoimmun 2014;48–49:128–33. [DOI] [PubMed] [Google Scholar]
- 5. Wei JCC, Kim T-H, Kishimoto M. et al. Efficacy and safety of brodalumab, an anti-IL17RA monoclonal antibody, in patients with axial spondyloarthritis: 16-week results from a randomized, placebo-controlled, phase 3 trial. Ann Rheum Dis 2021;80:1014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carmona L, Sellas A, Rodríguez-Lozano C. et al. Scoring with the Berlin MRI method for assessment of spinal inflammatory activity in patients with ankylosing spondylitis: a calibration exercise among rheumatologists. Clin Exp Rheumatol 2013;31:883–8. [PubMed] [Google Scholar]
- 7. Ward MM, Deodhar A, Gensler LS. et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019;71:1599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tam LS, Wei JC-C, Aggarwal A. et al. 2018 APLAR axial spondyloarthritis treatment recommendations. Int J Rheum Dis 2019;22:340–56. [DOI] [PubMed] [Google Scholar]
- 9. Wei JC, Baeten D, Sieper J. et al. Efficacy and safety of secukinumab in Asian patients with active ankylosing spondylitis: 52-week pooled results from two phase 3 studies. Int J Rheum Dis 2017;20:589–96. [DOI] [PubMed] [Google Scholar]
- 10. Kishimoto M, Taniguchi A, Fujishige A. et al. Efficacy and safety of secukinumab in Japanese patients with active ankylosing spondylitis: 24-week results from an open-label phase 3 study (MEASURE 2-J). Mod Rheumatol 2020;30:132–40. [DOI] [PubMed] [Google Scholar]
- 11. Baraliakos X, Braun J, Deodhar A. et al. Long-term efficacy and safety of secukinumab 150 mg in ankylosing spondylitis: 5-year results from the phase III MEASURE 1 extension study. RMD Open 2019;5:e001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Heijde D, Gensler LS, Deodhar A. et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis 2020;79:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dougados M, Wei JC-C, Landewé R. et al. Efficacy and safety of ixekizumab through 52 weeks in two phase 3, randomised, controlled clinical trials in patients with active radiographic axial spondyloarthritis (COAST-V and COAST-W). Ann Rheum Dis 2020;79:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deodhar A, van der Heijde D, Gensler LS. et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 2020;395:53–64. [DOI] [PubMed] [Google Scholar]
- 15. Braun J, Baraliakos X, Deodhar A. et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology 2019;58:859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yin Y, Wang M, Liu M. et al. Efficacy and safety of IL-17 inhibitors for the treatment of ankylosing spondylitis: a systematic review and meta-analysis. Arthritis Res Ther 2020;22:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Papp K, Menter A, Leonardi C. et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol 2020;183:1037–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hashim PW, Chen T, Lebwohl MG, Marangell LB, Kircik LH.. What lies beneath the face value of a BOX WARNING: a deeper look at brodalumab. J Drugs Dermatol 2018;17:s29–34. [PubMed] [Google Scholar]
- 19. Lebwohl MG, Papp KA, Marangell LB. et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol 2018;78:81–9.e5. [DOI] [PubMed] [Google Scholar]
- 20. Lebwohl M, Leonardi C, Wu JJ. et al. One-year pharmacovigilance update of brodalumab. J Drugs Dermatol 2020;19:807–8. [DOI] [PubMed] [Google Scholar]
- 21. Levin EC, Gupta R, Brown G, Malakouti M, Koo J.. Biologic fatigue in psoriasis. J Dermatolog Treat 2014;25:78–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data are available.