Abstract
Objectives
Cognitive dysfunction (CD) is a common manifestation of SLE that can have detrimental consequences for those affected. To date, no treatments have been approved for SLE-CD. This study aims to assess the association of azathioprine (AZA) and mycophenolate (MMF) use with SLE-CD, given that these medications have demonstrated neuroprotective qualities in prior studies.
Methods
Consecutive adult SLE patients presenting to a single healthcare center were considered for participation. The ACR neuropsychological battery for SLE was administered to consenting patients at 0, 6 and 12 months. Scores were compared with age- and sex-matched controls. Primary outcome was CD, defined as a z-score ≤−1.5 in two or more cognitive domains. Mixed-effects logistic regression models were constructed to estimate the odds of CD with respect to AZA and MMF use.
Results
A total of 300 participants representing 676 patient visits completed the study; 114 (38%) met criteria for CD at baseline. The cumulative AZA dose (g/kg) was associated with reduced odds of CD [odds ratio (OR) 0.76 (95% CI 0.58, 0.98), P = 0.04]. Years of AZA treatment was also associated with reduced odds of CD [OR 0.72 (95% CI 0.54, 0.97), P = 0.03]. MMF use was not associated with CD.
Conclusion
AZA use was associated with significantly lower odds of SLE-CD, while MMF use was not. Additional studies are warranted to further investigate the relationship of AZA and SLE-CD.
Keywords: SLE, neuropsychiatric SLE, AZA, mycophenolate, cognition
Rheumatology key messages.
Cognitive dysfunction (CD) is a common complication of SLE and is associated with worse outcomes.
Our study is the first to identify an association of azathioprine use with lower odds of SLE-CD.
Prospective studies are needed to further explore the relationship of azathioprine use and SLE-CD.
Introduction
Cognitive dysfunction (CD) is one of 19 neurological and psychiatric syndromes of SLE (NPSLE) described by the ACR [1]. CD is a very common manifestation of SLE, affecting an estimated 40% of patients [2]. SLE-CD is known to be associated with an overall worse prognosis [3, 4], significantly lower health-related quality of life [5, 6] and deleterious effects on employment [7, 8]. Although care must be taken to exclude non-SLE diagnoses when determining the etiology of CD in an SLE patient, the high prevalence of SLE-CD is not fully explained by other comorbid conditions, including depression [9, 10]. To date, no treatments have been approved for SLE-CD, although a call for trials has recently been published [11] and efforts are under way to examine centrally acting angiotensin inhibitors as a potential treatment, with a theoretical mechanism of reduced microglial activation and associated dendritic loss in relevant regions [12]. Currently available evidence for treatment of SLE-CD is very limited, consisting of case series, open-label studies and one post hoc analysis from the Aspreva Lupus Management Study [13]. There are no published human studies, to our knowledge, that uniquely address the treatment of SLE-CD; instead, available literature groups multiple NPSLE manifestations into a single analysis.
CD is most frequently defined as a significant deficit (z-score ≤−1.5) in at least two cognitive domains that include learning and memory, language, attention, executive function, motor function and visual–spatial function [14, 15]. Any area of the CNS may be affected by SLE and subsequently patients with SLE show heterogeneity in affected cognitive domains [16–18]. Deficits are determined through standardized cognitive testing administered by a psychometrist. To obtain z-scores, raw test scores are compared with age-matched controls, and in some circumstances, patients are also matched based on sex or education. The ACR has developed a comprehensive cognitive battery validated for SLE patients that is described elsewhere [19]. Despite the existence of a validated cognitive battery, the high prevalence and significant burden of SLE-CD, this condition is markedly underdiagnosed. The reason for this is likely related to the substantial cost and time required to complete comprehensive cognitive testing, as well as a lack of robust evidence for treatment. Given the time and cost barriers, efforts are currently under way to validate screening tests for SLE-CD [18, 20].
The understanding of the pathophysiology of SLE-CD has improved substantially over the past 2 decades, although it remains imperfectly defined [21]. In addition to numerous lupus-specific mechanisms, microglial activation is now described as an important contributing factor in the pathogenesis of SLE-CD and other neuroinflammatory disorders [21]. Microglia are resident immune cells of the CNS that serve phagocytic and antigen-presenting roles and can be activated to take on various ‘pro-inflammatory’ or ‘neuro-protective’ phenotypes [22]. Multiple murine studies have demonstrated that microglial inhibition can attenuate CD [23–25]. Interestingly, microglia have been shown to remain activated in a pro-inflammatory state for at least 6 months following a CNS insult due to a detrimental feedback loop, which can contribute to progressive neurotoxicity beyond the initial insult [26]. While speculative, persistent microglial activation following a lupus flare could be a contributing factor to explain the disconnect between SLE disease activity and SLE-CD. Consequently we hypothesize that the use of immunosuppressant medications, which have been shown to reduce pro-inflammatory microglial activation and demonstrate limited neurotoxicity, may be associated with lower odds of SLE-CD. Two such medications include MMF and AZA.
MMF inhibits inosine monophosphate dehydrogenase, which halts B and T cell proliferation, strongly inhibits microglial secretion of TNF-α and IL-1β, inhibiting microglial activation and proliferation [27, 28]. It is not known to cause CNS toxicity [29]. Further, MMF was shown to attenuate neuronal damage after excitotoxic injury [30], and its use has been associated with complete or partial response for various NPSLE manifestations in multiple observational studies as well as a post hoc analysis [13]. Similarly, AZA is not known to cause neurotoxicity [29, 31] and has been shown to be potentially beneficial for multiple NPSLE manifestations in observational data [13]. 6-mercaptopurine, an active metabolite of AZA, has been shown to reduce microglial secretion of TNF-α [32] (known to perpetuate microglial activation and neuron damage). Given that AZA and MMF are not known to be neurotoxic and may reduce microglial activation and proliferation, we hypothesize that the use of each of these medications may be associated with reduced odds of SLE-CD.
Methods
Design
This is a longitudinal study that analyzed prospectively collected data. Measurements were completed at baseline, 6 months and 12 months. Assuming a baseline event rate of 40% [2], we presumed that participants in the treatment and control groups may have event rates of 20% and 40%, respectively, for an absolute difference in event rate of 20%. Based on a sample size of at least 47 participants in each group (at least 94 participants total), we computed >80% power to detect such an effect.
Participants
All consenting adult SLE patients attending the University of Toronto/University Health Network Lupus Program between July 2016 and March 2020 were considered for this study. Inclusion criteria were fulfilment of the revised ACR SLE classification criterion [33], English language proficiency (a requirement due to the nature of the neuropsychological tests), age ≥18 years and ability to provide informed consent. Exclusion criteria were as follows: history of irreversible CNS damage, developmental delay or dementia or physical or mental disability preventing full participation in the study. All procedures were performed in accordance with the ethical standards of the Helsinki Declaration. This project was approved by both the University Health Network Research Ethics Board (protocols 15-9582 and 11-0397) and the Albert Einstein College of Medicine Institutional Review Board (protocol 2019-10861). All participants provided written informed consent prior to participation in this study.
Testing procedures
A psychometrist administered a comprehensive neuropsychological battery (NB) to assess the cognitive functioning of each participant. On the same day as cognitive testing, demographic and clinical information, Beck Depression Inventory (BDI) [34] scores and Beck Anxiety Inventory (BAI) [35] scores were obtained for each participant. Neuropsychological battery scores were compared with normative, standardized scores stratified based on age and sex.
The NB used in this study is a replication of the ACR neuropsychological battery for SLE that is described elsewhere and measures each major cognitive domain (learning and memory, language, attention, executive function, motor function and visual–spatial function) [19]. A minor change was made to the protocol: the Hopkins Verbal Learning Test–Revised (HVLT-R) [36] replaced the California Verbal Learning Test (CVLT), given that the HVLT-R is shorter and is not meaningfully different from the CVLT. Full details regarding the neuropsychological battery are listed in Supplementary Table S1, available at Rheumatology online.
Outcome measures and predictors
CD was defined as a binary variable with a z-score ≤−1.5 in two or more cognitive domains and comprised the primary outcome for this study. As outlined above, the number of tests performed in each cognitive domain varies (see Supplementary Table S1, available at Rheumatology online). For cognitive domains that were examined with more than a single test, two or more abnormal tests were required to determine CD in that domain. Given the varying probability of participants having an impairment depending on the number of tests performed in a particular domain, we examined an alternate definition of the number of tests required for impairment in each cognitive domain. Further details regarding this sensitivity analysis are available in Supplementary Table S2, available at Rheumatology online.
Primary predictors of this study were total cumulative doses of AZA and MMF (in g/kg); each was treated as a time-varying covariate. The mycophenolate predictor included both mycophenolate sodium and MMF and was recorded as the equivalent MMF dose. Secondary predictors were also treated as time-varying covariates and included active use of AZA and MMF for at least 6 months prior to the study visit and cognitive assessment, defined as binary variables, as well as the duration of AZA and MMF treatment (total number of years). Continuous variables were favored as primary predictors given the relatively small sample size and loss of power that may occur with dichotomization. Covariates were chosen for consideration based on a known association with CD and/or a suspected confounding relationship with predictors [34, 35, 37–46]. A detailed outline of all covariates is listed in Table 1.
Table 1.
Covariate selection
| Covariate |
|---|
| SLEDAI-2000 Glucocorticoid scorea |
| SLICC–ACR Damage Index scorea,d |
| History of any neuropsychiatric systemic lupus manifestations (excluding cognitive dysfunction)b |
| History of lupus nephritisb |
| Presence of additional risk factors not captured elsewhere (hypertension, obesity, and/or active smoker)b |
| aPL antibody positivityb,e |
| Active use of an additional immunomodulator: |
| Antimalarialb |
| Belimumabb |
| Calcineurin inhibitorb |
| Cyclophosphamideb |
| Methotrexateb |
| Rituximabb |
| Prior use of an immunomodulator: |
| Antimalarialb |
| Azathioprineb |
| Calcineurin inhibitorb |
| Cyclophosphamideb |
| Mycophenolateb |
| Methotrexateb |
| Rituximabb |
| BDI scorea |
| BAI scorea |
| Age, yearsa |
| Sex (male vs female)b |
| Ethnicity (Black, Caucasian, Chinese, other)c |
| Employment status (employed or full-time student vs other)b |
| Marital status (married or common-law partner vs other)b |
| Education level (completion of a college or university degree vs not)b |
Recorded as a continuous variable.
Recorded as a binary variable.
Recorded as a categorical variable.
Cumulative damage since the onset of lupus: history of retinopathy, optic atrophy, cataract, major psychosis, cognitive dysfunction, stroke, seizures, neuropathy, transverse myelitis, chronic kidney disease or end-stage kidney disease, heavy proteinuria ≥3.5 g/day, pulmonary hypertension, pulmonary fibrosis, shrinking lung syndrome, pleural fibrosis, coronary artery disease, cardiomyopathy, pericarditis, peripheral vascular disease, venous thrombosis, mesenteric insufficiency, chronic peritonitis, gastrointestinal stricture, pancreatic insufficiency, muscle atrophy, erosive arthritis, osteoporosis, avascular necrosis, scarring alopecia, skin ulcerations, gonadal failure, malignancy, diabetes.
Persistently positive lupus anticoagulant testing and/or anti-β2-glycoprotein 1 IgG or IgM and/or anticardiolipin IgG or IgM above laboratory-specific upper limit of normal, at baseline and again after ≥12 weeks.
Statistical analyses
Statistical analyses were completed with Stata version 16.1 (StataCorp, College Station, TX, USA). Two-sided P-values were considered statistically significant at <0.05. Data were inspected to identify any missing and non-plausible values. Missing values were identified in a minority of cases, addressed through mean imputation (or median, where appropriate) and are outlined in Fig. 1. Cognitive battery scores were not imputed. Descriptive statistics were recorded as number and percent for categorical variables, mean (s.d.) for normally distributed continuous variables and median and interquartile range (IQR) for skewed variables. Overall baseline participant characteristics were documented, as well as baseline participant characteristics grouped by baseline CD status and by AZA and MMF use. Differences in baseline characteristics between groups were determined through a chi-squared test, Mann–Whitney U test, Fisher’s exact test or t-test, where appropriate.
Figure 1.
Missing values (total N=676). SLEDAI-2K: SLEDAI 2000; SLEDAI-2KG: SLEDAI 2000 Glucocorticoid; RCFT: Rey Complex Figure Test; COWAT: Controlled Oral Word Association Test; WAIS-III: Wechsler Adult Intelligence Scale, Third Edition; HVLT-R: Hopkins Verbal Learning Test–Revised. *Component of the cognitive battery
Mixed effects logistic regression models to account for the longitudinal nature of the data were constructed to evaluate primary and secondary outcomes using the vector of three follow-up time points. An unstructured covariance pattern was assumed. Prior to model building, simple regression analyses were completed for predictors with respect to each outcome to assess unadjusted relationships at baseline. Cross-sectional analyses were completed for each time point through multivariable regression and then longitudinal trends were explored through visual inspection of scatter, spaghetti, mean trend and variogram plots. Mixed effects logistic regression models were constructed with an a priori model based on clinical relevance. Linearity assumption was assessed for continuous variables through inspection of Lowess plots. All first-order interactions were investigated and the Lemeshow goodness of fit test was used to assess model fit. Propensity score matched data are available in Supplementary Table S3, available at Rheumatology online. These data were not used in the primary data analysis given the limited number of participants and the loss of sensitivity with the use of dichotomized data.
Results
Participant selection
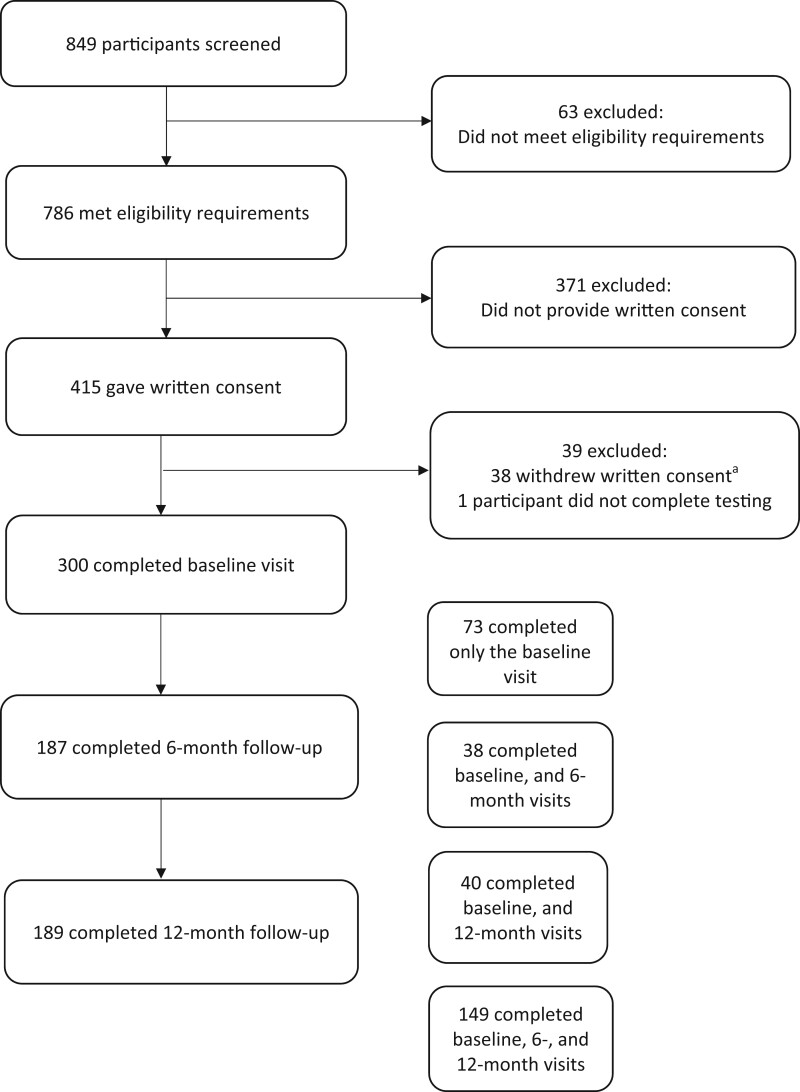
Participant selection processes are outlined in Fig. 2. A total of 849 participants were screened for this study and 300 participants completed the baseline assessment, 187 completed the 6 month and 189 completed the 12 month assessment.
Figure 2.
Process of participant selection. aReasons for withdrawal of consent: too busy/unable to dedicate time to the study (n=16), no longer wanting to participate (n=16), perceived the study to be too long (n=5) and other reasons (n=1)
Participant characteristics
Overall participant characteristics, as well as characteristics by cognitive status are outlined in Table 2. The majority of participants were female [267/300 (89.0%)], Caucasian [162/300 (54.2%)] and had previously completed a college or university degree[(240/300 (80.0%)]. The mean age was 41.1 years (s.d. 12.1) and the mean SLEDAI-2K score [37] was 3.3 (s.d. 3.8). Cognitive dysfunction (z-score ≤−1.5 in two or more cognitive domains) was observed in 114/300 participants (38.0%) at baseline, 54/187 (28.9%) at 6 months and 64/189 (33.9%) at 12 months. A total of 43 of 300 participants (14.3%) were prescribed AZA based on physician judgement to treat SLE at baseline, with a median cumulative dose of 2.3 g/kg (IQR 0.6–4.9) and a median duration of treatment of 4.9 years (IQR 1.6–12.0). A total of 96 of 300 participants (32.0%) were prescribed MMF at baseline, with a median cumulative dose of 37.6 g/kg (IQR 14.5–78.6) and a median duration of treatment of 4.5 years (IQR 1.7–8.6).
Table 2.
Baseline characteristics of participants by cognitive function
| Characteristics | Total (n = 300) | Non-CDa [n = 186 (62.0%)] | CDa [n = 114 (38.0%)] | P-value |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Female | 267 (89.0) | 168 (90.33) | 99 (86.8) | 0.35 |
| Male | 33 (11.0) | 18 (9.7) | 15 (13.2) | |
| Age, years, mean (s.d.) | 41.1 (12.1) | 41.3 (11.7) | 40.7 (12.7) | 0.49 |
| Ethnicity, n (%) | ||||
| Black | 59 (19.7) | 24 (12.9) | 35 (30.7) | <0.01 |
| White | 162 (54.2) | 114 (61.3) | 48 (42.%) | <0.01 |
| Chinese | 33 (11.0) | 20 (10.8) | 13 (11.4) | 0.86 |
| Other | 46 (15.3) | 28 (15.1) | 18 (15.8) | 0.86 |
| Education level (highest achieved), n (%) | ||||
| ≤grade 12 | 60 (20.0) | 31 (16.7) | 29 (25.4) | |
| College or university degreeb | 240 (80.0) | 155 (83.3) | 85 (74.6) | 0.07 |
| Employment Status, n (%) | ||||
| Employed or student | 195 (65.0) | 129 (69.4) | 66 (57.9) | 0.04 |
| Other | 105 (35.0) | 57 (30.7) | 48 (42.1) | |
| Marital status, n (%) | ||||
| Married or common law | 119 (39.9) | 81 (43.8) | 38 (33.9) | 0.10 |
| Other | 179 (60.0) | 105 (56.5) | 74 (66.1) | |
| SLE manifestations, n (%) | ||||
| aPL positivityc | 46 (15.3) | 29 (15.6) | 17 (14.9) | 0.87 |
| Nephritis | 91 (30.3) | 53 (28.5) | 38 (33.3) | 0.69 |
| Mucocutaneous | 172 (57.5) | 105 (56.8) | 67 (58.8) | 0.73 |
| Musculoskeletal | 112 (37.5) | 63 (34.1) | 49 (43.0) | 0.12 |
| Other NPSLE manifestationd | 75 (25.0) | 44 (23.7) | 31 (27.2) | 0.49 |
| Serositis | 26 (8.7) | 18 (9.7) | 8 (7.0) | 0.42 |
| Additional CD risk factorse, n (%) | ||||
| Hypertension | 124 (41.3) | 79 (42.5) | 45 (39.4) | 0.61 |
| Obesity | 97 (32.3) | 57 (30.7) | 40 (35.1) | 0.43 |
| Smoker | 18 (6.0) | 11 (5.9) | 7 (6.1) | 0.94 |
| Disease duration, years, median (IQR) | 12.4 (6.0–21.6) | 14.4 (6.5–22.2) | 11.6 (3.9–19.1) | 0.06 |
| SDI score | 1.0 (0.0–2.0) | 0.0 (0.0–1.1) | 1.0 (0.0–2.0) | |
| Median (IQR) | 1.0 (1.4) | 1.0 (1.4) | 1.1 (1.5) | |
| Mean (s.d.) | 0.35 | |||
| SLEDAI-2K score | ||||
| Median (IQR) | 2.0 (0.0–4.0) | 2.0 (0.0–4.0) | 2.0 (0.0–5.0) | |
| Mean (s.d.) | 3.3 (3.8) | 3.0 (3.4) | 3.7 (4.4) | 0.45 |
| SLEDAI-2KG score | ||||
| Median (IQR) | 3.1 (0.9–6.2) | 3.0 (0.9–6.0) | 4.0 (1.0–6.9) | |
| Mean (s.d.) | 4.4 (4.7) | 4.1 (4.4) | 5.0 (5.2) | 0.23 |
| Glucocorticoid dose, mg/day | ||||
| Mean (s.d.) | 4.3 (8.1) | 3.9 (7.4) | 5.1 (9.1) | 0.42 |
| Immunosuppressant use, n (%) | ||||
| Antimalarials | 224 (82.4) | 140 (82.8) | 84 (81.6) | 0.79 |
| Azathioprine | 52 (17.3) | 32 (10.7) | 20 (6.7) | 0.03 |
| Belimumab | 14 (4.7) | 10 (5.4) | 4 (3.5) | 0.46 |
| Cyclophosphamide | 2 (0.7) | 2 (1.1) | 0 (0.0) | 0.27 |
| Ciclosporin | 2 (0.7) | 1 (0.5) | 1 (0.9) | 0.73 |
| Glucocorticoids | 132 (48.5) | 80 (47.3) | 52 (50.5) | 0.61 |
| Methotrexate | 25 (8.8) | 13 (7.0) | 12 (10.5) | 0.28 |
| Mycophenolate | 96 (32.0 | 53 (28.5) | 33 (29.0) | 0.93 |
| Rituximab | 14 (4.7%) | 1 (0.5) | 1 (0.9) | 0.73 |
| Immunosuppressive use, g/kg cumulative dose, mean (s.d.) | ||||
| Azathioprine | 0.67 (2.17) | 0.98 (2.66) | 0.39 (1.57) | 0.02 |
| Mycophenolate | 16.32 (35.63) | 14.16 (33.09) | 18.31 (37.83) | 0.18 |
| Prior immunosuppressant use, n (%) | ||||
| Azathioprine | 2 (0.7) | 1 (0.7) | 1 (0.6) | 0.76 |
| Cyclophosphamide | 0 | 0 | 0 | – |
| Ciclosporin | 0 | 0 | 0 | – |
| Methotrexate | 2 (0.7) | 0 | 2 (1.8) | 0.07 |
| Mycophenolate | 23 (7.7) | 11 (7.7) | 12 (7.6) | 0.31 |
| BDIf score, median (IQR) | 14.9 (7.2–18.3) | 13.7 (7.0–16.1) | 14.9 (10.3–24.4) | 0.01 |
| BAIg score, median (IQR) | 15.6 (7.0–20.0) | 15.0 (7.0–18.0) | 15.6 (11.0–23.0) | 0.01 |
Defined as a z-score ≤−1.5 on two or more domains as determined by comprehensive cognitive testing.
Completion of postsecondary schooling including a college (≥2 years) or university (≥4 years) degree.
Positive lupus anticoagulant testing and/or anti-β2-glycoprotein 1 IgG or IgM and/or anti-cardiolipin IgG or IgM above the laboratory-specific upper limit of normal at baseline and again after ≥12 weeks.
Psychosis, seizures, stroke, neuropathy and/or transverse myelitis.
Known risk factors for cognitive dysfunction not captured elsewhere.
BDI score of 0–13 is considered minimal, 14–19 mild, 20–28 moderate and 29–63 severe.
BAI score of 0–7 is considered minimal, 8–15 mild, 16–25 moderate and 26–63 severe.
There was a significantly higher number of Black participants with CD at baseline (35/114 vs 24/186; P < 0.01) and a significantly lower number of Caucasian participants with CD at baseline (48/114 vs 114/186; P < 0.01). Employed/student participants were less likely to have CD (66/114 vs 129/186; P = 0.04). The median BDI and BAI scores were significantly higher in participants with CD [14.9 (IQR 10.3–24.4] vs 13.7 (7.0–16.1) and 15.6 (IQR 11.0–23.0) vs 15.0 (7.0–18.0), respectively]. There were no significant differences in the remaining covariates between the CD and non-CD groups at baseline.
Characteristics by AZA and MMF use are listed in Table 3. There were no significant differences in SLICC/ACR Damage Index (SDI) scores between participants taking AZA vs those not taking AZA [1.2 (s.d. 1.5) vs 1.0 (1.4); P = 0.40] and also for participants taking MMF vs those not taking MMF [1.1 (s.d. 1.4) vs 1.0 (1.5); P = 0.32]. SLEDAI-2K scores were higher for participants taking both AZA [4.3 (s.d. 3.6) vs 3.1 (3.8); P < 0.01] and MMF [4.0 (s.d. 4.2) vs 2.9 (3.5); P = 0.03] compared with those not taking these medications. The glucocorticoid dose was significantly higher in participants taking MMF [7.0 mg/day (s.d. 11.4) vs 3.1 (5.5); P < 0.01]. As anticipated, nephritis was more common in those taking both AZA (25/52 vs 68/248; P < 0.01) and MMF (50/96 vs 43/204; P < 0.01). Hypertension was more common in those prescribed MMF (48/96 vs 76/204; P = 0.04) and smoking was less common (2/96 vs 16/248; P = 0.05). There were significantly fewer participants who self-identified as belonging to ‘other’ ethnicity who were prescribed MMF (7/96 vs 39/204; P = 0.01).
Table 3.
Baseline characteristics of participants by AZA and MMF use
| Characteristics | No AZA use (n = 248) | AZA use (n = 52) | P-value | No MMF use (n = 204) | MMF use (n = 96) | P-value |
|---|---|---|---|---|---|---|
| SDI score | ||||||
| Median (IQR) | 1.0 (0.0–1.0) | 1.0 (0.0–2.0) | 0.40 | 0.0 (0.0–1.1) | 1.0 (0.0–2.0) | 0.32 |
| Mean (s.d.) | 1.0 (1.4) | 1.2 (1.5) | 1.0 (1.5) | 1.1 (1.4) | ||
| Disease duration, years, median (IQR) | 12.7 (5.8–21.9) | 11.9 (6.7–16.6) | 0.48 | 13.2 (6.0–22.2) | 11.6 (6.0–19.4) | 0.22 |
| SLEDAI-2K | ||||||
| Median (IQR) | 2.0 (0.0–4.0) | 4.0 (2.0–6.0) | <0.01 | 2.0 (0.0–4.0) | 3.5 (0.0–6.0) | 0.03 |
| Mean (s.d.) | 3.1 (3.8) | 4.3 (3.6) | 2.9 (3.5 ) | 4.0 (4.2) | ||
| SLEDAI-2KG | ||||||
| Median (IQR) | 2.3 (0.0–6.0) | 2.1 (1.5–7.3) | <0.01 | 2.0 (0.0–6.0) | 4.0 (2.0–7.8) | <0.01 |
| Mean (s.d.) | 4.2 (4.9) | 5.5 (3.8) | 3.8 (4.1) | 5.7 (5.7) | ||
| Other immunosuppressant use, n (%) | ||||||
| Antimalarials | 183 (82.1) | 41 (83.7) | 0.79 | 152 (82.6) | 72 (81.8) | 0.87 |
| Belimumab | 11 (4.4) | 3 (5.8) | 0.68 | 10 (4.9) | 4 (4.2) | 0.78 |
| Cyclophosphamide | 2 (0.8) | 0 | 0.52 | 2 (1.0) | 0 | 0.33 |
| Ciclosporin | 2 (0.8) | 0 | 0.52 | 2 (1.0) | 0 | 0.33 |
| Methotrexate | 23 (9.3) | 2 (3.9) | 0.20 | 23 (11.3) | 2 (2.1) | 0.01 |
| Rituximab | 2 (0.8) | 0 | 0.52 | 1 (0.5) | 1 (1.0) | 0.58 |
| Prior immunosuppressant use, n (%) | ||||||
| Azathioprine | – | – | – | 2 (1.0) | 0 | 0.33 |
| Cyclophosphamide | 0 | 0 | – | 0 | 0 | – |
| Ciclosporin | 0 | 0 | – | 0 | 0 | – |
| Methotrexate | 2 (0.8) | 0 | 0.52 | 2 (1.0) | 0 | 0.33 |
| Mycophenolate | 5 (2.0) | 18 (34.6) | <0.01 | – | – | – |
| Glucocorticoid dose, mg/day | ||||||
| Mean (s.d.) | 4.4 (8.5) | 4.2 (5.8) | 0.23 | 3.1 (5.5) | 7.0 (11.4) | <0.01 |
| SLE manifestations, n (%) | ||||||
| Cognitive dysfunction | 137 (55.2) | 20 (38.5) | 0.03 | 102 (50.0) | 55 (57.3) | 0.24 |
| Antiphospholipid positivitya | 34 (13.7) | 12 (23.0) | 0.09 | 28 (13.7) | 18 (18.8) | 0.26 |
| Nephritis | 68 (27.5) | 25 (48.1) | <0.01 | 43 (21.1) | 50 (52.6) | <0.01 |
| Mucocutaneous | 137 (55.5) | 35 (67.3) | 0.12 | 117 (57.4) | 55 (57.9) | 0.93 |
| Musculoskeletal | 90 (36.4) | 22 (42.3) | 0.43 | 76 (37.3) | 36 (37.%) | 0.92 |
| Other NPSLE manifestationsb | 66 (26.6) | 9 (17.3) | 0.16 | 50 (24.5) | 25 (26.0) | 0.78 |
| Serositis | 20 (8.1) | 6 (11.5) | 0.42 | 15 (7.4) | 11 (11.6) | 0.23 |
| Additional CD risk factorsc, n (%) | ||||||
| Hypertension | 100 (40.3) | 24 (46.2) | 0.44 | 76 (37.3) | 48 (50.0) | 0.04 |
| Obesity | 83 (33.5) | 14 (26.9) | 0.36 | 63 (30.9) | 34 (35.4) | 0.43 |
| Smoker | 14 (6.1) | 3 (5.8) | 0.94 | 16 (7.8) | 2 (2.1) | 0.05 |
| Ethnicity, n (%) | ||||||
| Black | 50 (20.2) | 9 (17.3) | 0.64 | 36 (17.6) | 23 (24.0) | 0.20 |
| White | 132 (53.2) | 30 (57.7) | 0.56 | 111 (54.4) | 51 (53.1) | 0.84 |
| Chinese | 27 (10.9) | 6 (11.5) | 0.89 | 18 (8.8) | 15 (15.6) | 0.08 |
| Other | 39 (15.7) | 7 (13.4) | 0.68 | 39 (19.1) | 7 (7.3) | 0.01 |
| Education level (highest achieved), n (%) | ||||||
| ≤grade 12 | 45 (18.1) | 15 (28.8) | 40 (19.6) | 20 (20.8) | ||
| College or university degreed | 203 (81.9) | 37 (71.2) | 0.08 | 164 (80.4) | 76 (79.2) | 0.80 |
Positive lupus anticoagulant testing and/or anti-β2-glycoprotein 1 IgG or IgM and/or anti-cardiolipin IgG or IgM above the laboratory-specific upper limit of normal at baseline and again after ≥12 weeks.
Psychosis, seizures, stroke, neuropathy and/or transverse myelitis.
Known risk factors for cognitive dysfunction not captured elsewhere.
Completion of a college (≥2 years) or university (≥4 years) degree.
SLEDAI-2K: SLEDA Index 2000; SLEDAI-2KG: SLEDAI-2K Glucocorticoid.
Regression analyses
Results of the adjusted mixed effects logistic regression models are outlined in Table 4. There was a 24% decreased odds of CD per 1 g/kg of cumulative AZA [OR 0.76 (95% CI 0.58, 0.98), P = 0.04]. The number of years of treatment with AZA was also significantly associated with decreased odds of CD [OR 0.72 (95% CI 0.54, 0.97), P = 0.03]. The use of AZA vs no use was not statistically significant [OR 0.47 (95% CI 0.12, 1.76), P = 0.26]. White ethnicity [OR 0.10 (95% CI 0.02, 0.49), P = 0.01], Chinese ethnicity [OR 0.08 (95% CI 0.01, 0.89), P = 0.04] and ‘other’ ethnicity [OR 0.13 (95% CI 0.02, 0.70), P = 0.02] were associated with reduced odds of CD in the primary predictor model compared with Black ethnicity. There were no additional statistically significant variables in the model. MMF use was not significantly associated with CD in any model.
Table 4.
Mixed logistic regression models for medication use predicting cognitive dysfunctiona
| Medication | Cumulative dose (g/kg) (n = 676) |
Use for ≥6 months (n = 676) |
Treatment duration (years) (n = 676) |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Azathioprine | 0.76 (0.58, 0.98) | 0.04 | 0.47 (0.12, 1.76) | 0.26 | 0.72 (0.54, 0.97) | 0.03 |
| Mycophenolate | 1.03 (0.93, 1.13) | 0.58 | 0.76 (0.32, 1.79) | 0.53 | 1.12 (0.96, 1.31) | 0.15 |
Defined as a z-score ≤−1.5 on two or more domains as determined by comprehensive cognitive testing.
Covariates included in the models: SLEDAI-2KG (37–40) score (incorporates glucocorticoid dose with the SLEDAI-2K), SDI, additional CD risk factors not captured elsewhere (hypertension, obesity, active smoker), persistent aPL antibody positivity (positive lupus anticoagulant testing and/or anti-β2-glycoprotein 1 IgG or IgM and/or anti-cardiolipin IgG or IgM above the laboratory-specific upper limit of normal at baseline and again after ≥12 weeks, recorded as a binary variable), history of lupus nephritis, active use of an additional immunomodulator (antimalarials, belimumab, calcineurin inhibitor, cyclophosphamide, methotrexate, rituximab), prior use of an immunomodulator (AZA, calcineurin inhibitor, cyclophosphamide, methotrexate, MMF), BDI score, BAI score, age (in years), sex (male vs female), ethnicity (Black, Caucasian, Chinese or other), education level (completion of a college or university degree recorded as a binary variable), employment status (employed or full-time student vs other) and marital status (married or common-law partner vs other).
Discussion
This longitudinal analysis examined the association of MMF and AZA use with cognitive function in SLE patients. AZA and MMF were chosen because they are each able to penetrate the CNS, are not known to be neurotoxic and can inhibit activation of microglia, which has been implicated in the development of SLE-CD [11]. Given these qualities, we hypothesize that the use of each of these medications may be associated with lower odds of CD. Consistent with this hypothesis, and despite significantly higher SLE disease activity scores in participants taking AZA compared with those not taking AZA, cumulative AZA dose and increasing duration of AZA treatment were associated with decreased odds of SLE-CD. Continuous predictors were utilized given the small number of participants taking AZA, in order to maximize statistical power. As expected, the use of AZA as a binary predictor was not statistically significant in multivariable analyses, although baseline AZA use was associated with lower odds of CD compared with no AZA use in univariate analyses.
While MMF use has demonstrated therapeutic potential for SLE-CD in some case reports and open-label trials [13], we found no association with cognitive function in our study. Multiple possible explanations exist for this conflicting result, with the most likely being the presence of an indication bias in the setting of a retrospective study (i.e. patients with higher disease severity and renal disease are more likely to be prescribed MMF, and these differences could mask a difference in the odds of CD). Another possible confounder that may have impacted results is the higher dose of daily glucocorticoids prescribed in the MMF group. Prior studies have shown the potential for both improving and worsening of CD with the use of glucocorticoid medications [47, 48]. An additional possibility for the lack of association between MMF and CD is that there could have been insufficient power to reveal a significant result. Our study was powered to detect a 20% difference in the prevalence of CD, although a smaller difference in the prevalence of CD could arguably be clinically meaningful. A further speculative explanation for the discrepancy includes a possible subtle neurotoxic effect, despite MMF being considered a non-neurotoxic medication and showing therapeutic potential for more severe CD and acute NPSLE manifestations. In fact, there are limited reports in the literature tying MMF use with new seizure activity and status epilepticus [49].
The divergent outcomes between AZA and MMF in this study are worth noting, and while theoretical, could possibly highlight differences in the molecular mechanisms of each medication. In particular, MMF reduces de novo purine synthesis mediated by inosine-5′-monophosphate dehydrogenase inhibition, hindering cell replication. Lymphocytes are a relatively specific target of MMF due to their sole reliance on the de novo purine synthesis pathway [50]. In contrast, AZA inhibits both the salvage and de novo purine pathways and produces both cytostatic and cytotoxic effects [50]. This is highly relevant to our study because our working hypothesis is that microglial activation may play a key role in SLE-CD and microglia utilize both de novo and salvage purine pathways. In theory, due to these effects, AZA could possibly result in more potent microglial inhibition compared with MMF. An alternate explanation for the divergent outcomes between AZA and MMF in our study is, of course, that unknown confounders exist between the two groups taking AZA vs MMF, given the observational study design. Supplementary Table S4 (available at Rheumatology online) provides a comparison of documented participant characteristics and outcomes based on AZA and MMF use. The duration of exposure was similar between the MMF and AZA groups. Participants taking AZA had statistically higher SLEDAI-2K scores, were more likely to be taking methotrexate, on average used a lower daily dose of glucocorticoid medications, were more likely to identify with belonging to ‘other’ ethnic minority groups and had slightly lower prevalence of hypertension.
Despite finding a significant outcome for the primary AZA analysis, results should be interpreted with caution given the inherent limitations that exist in this observational study. Specifically, we are only able to account for known confounders of AZA/MMF use and CD. Multiple unknown confounders still exist that may unpredictably influence results and therefore preclude any type of causal conclusions. A prospective, randomized trial would be required to capture unmeasured confounders. Also, for the great majority of patients we did not capture incident CD and therefore the timing of medication use vs development of CD is unclear. Further limitations of this study include a lack of information regarding medication adherence, which can be a common challenge in clinical practice and has the potential to substantially alter the results. Also, this is a single-center study in which all participants were required to speak English due to the nature of the cognitive tests, with the great majority of participants having completed postsecondary education and identifying as White. Further studies are needed to clarify whether results are generalizable to other patient populations. This is especially important when studying cognition, given that White ethnicity is protective against multiple social, economic and educational disparities [44].
Despite these limitations, there are multiple strengths of this study that we would like to highlight. Importantly, to our knowledge, this is the first study to investigate the association of AZA, MMF and SLE-CD. Existing literature, as discussed in the introduction, has included only case series and open-label studies examining multiple NPSLE outcomes simultaneously. Additionally, this study was conducted after the development of an a priori hypothesis that identified a potential mechanism to support the findings. Moreover, despite the retrospective analysis completed for this study, the data were collected prospectively, limiting misclassification and recall biases. Furthermore, the longitudinal design allowed us to account for both intra- and interindividual effects. Also, the use of a cumulative dose of AZA as a primary predictor may support a ‘dose-dependent’ association and, along with the consistency of sensitivity analyses, strengthens a potential relationship between AZA use and cognitive functioning in patients with SLE. Regardless, this project is a preliminary retrospective study and a prospective study is required to accurately determine whether there is a protective effect of AZA with regard to SLE-CD.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
Acknowledgements
We would like to acknowledge the generous donation of our patients’ time and the dedication of the University Health Network clinic staff on the completion of this project. Z.T., J.S., N.A., D.B., R.G., D.E.B., L.R., J.E.W., M.C.T., M.K., S.L., K.B., M.Y.C., J.P.D.M. and M.J.F. were responsible for data acquisition. C.D., Z.T. and C.P. were responsible for the study concept and design. C.D., Z.T., C.P., J.M., M.F., P.K., J.P.D.M. and J.S. were responsible for the analysis and interpretation of data. C.D., Z.T., C.P., J.M., M.F., N.A. and J.S. were responsible for drafting the article. C.D., Z.T., C.P., J.M., M.F., J.S., D.B., R.G., D.E.B., L.R., J.E.W., M.C.T., M.K., S.L., M.Y.C., P.K., K.B. and M.J.F. were responsible for revising the article.
Contributor Information
Chrisanna Dobrowolski, Division of Rheumatology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
John McGinley, Department of Neurology, Albert Einstein College of Medicine, Bronx, NY, USA.
Melissa Fazzari, Department of Epidemiology and Population Health and Department of Obstetrics and Gynecology and Women's Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Jiandong Su, University of Toronto Lupus Clinic, Centre for Prognosis Studies in Rheumatic Diseases, Toronto Western Hospital, Toronto, ON, Canada.
Kathleen S Bingham, Centre for Mental Health, University Health Network; Department of Psychiatry, University of Toronto, Toronto, ON, Canada.
Nicole Anderson, University of Toronto Lupus Clinic, Centre for Prognosis Studies in Rheumatic Diseases, Toronto Western Hospital, Toronto, ON, Canada.
Lesley Ruttan, University Health Network-Toronto Rehabilitation Institute, Toronto, ON, Canada.
Dorcas E Beaton, Institute for Work and Health, University of Toronto, Toronto, ON, Canada.
Joan E Wither, University of Toronto Lupus Clinic, Centre for Prognosis Studies in Rheumatic Diseases, Toronto Western Hospital, Toronto, ON, Canada.
Maria Carmela Tartaglia, University of Toronto Krembil Neurosciences Centre, Toronto, ON, Canada.
Mahta Kakvan, University of Toronto Lupus Clinic, Centre for Prognosis Studies in Rheumatic Diseases, Toronto Western Hospital, Toronto, ON, Canada.
Dennisse Bonilla, University of Toronto Lupus Clinic, Centre for Prognosis Studies in Rheumatic Diseases, Toronto Western Hospital, Toronto, ON, Canada.
May Y Choi, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada.
Marvin J Fritzler, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada.
Juan Pablo Diaz Martinez, University of Toronto Lupus Clinic, Centre for Prognosis Studies in Rheumatic Diseases, Toronto Western Hospital, Toronto, ON, Canada.
Patricia Katz, University of California, San Francisco, Novato, CA, USA.
Robin Green, University Health Network-Toronto Rehabilitation Institute, Toronto, ON, Canada.
Chaim Putterman, Department of Microbiology and Immunology, Albert Einstein School of Medicine, Bronx, NY, USA; Division of Rheumatology, Albert Einstein College of Medicine, Bronx, NY, USA; Azrieli School of Medicine, Safed, Israel; Galillee Medical Center, Nahariya, Israel.
Zahi Touma, University of Toronto Lupus Clinic, Centre for Prognosis Studies in Rheumatic Diseases, Toronto Western Hospital, Toronto, ON, Canada.
Data availability statement
The data underlying this article cannot be shared publicly due to concern for the privacy of the individuals who participated in this single-center study. The data will be shared upon reasonable request to the corresponding author.
Funding
This project was funded by the National Institutes of Health/National Center for Advancing Translational Science (Einstein-Montefiore CTSA grant UL1TR002556), the Arthritis Society of Canada, Canadian Institutes of Health Research, Physician’s Services Incorporated, the Province of Ontario Early Research Award, and the Lupus Research Alliance. Z.T. is supported by the Arthritis Society, Young Investigator Award and the Canadian Rheumatology Association–Arthritis Society Clinician Investigator Award and by the Department of Medicine, University of Toronto. The Toronto Lupus Research Program is supported by grants from Lupus Ontario and Schroeder Arthritis Institute.
Disclosure statement: M.C. has received funding from Mitogen Dx. M.F. has received funding from Inova Diagnostics, Werfen International, Alexion Canada and Mitogen Diagnostics. C.P. has received funding from Equllium, Progentec and Kidneycure. Z.T. has received funding from AbbVie, UCB Biopharma, Sarkana Pharma, Janssen and GlaxoSmithKline. All other authors have nothing to declare.
References
- 1. Ainiala H, Hietaharju A, Loukkola J. et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum 2001;45:419–23. [DOI] [PubMed] [Google Scholar]
- 2. Rayes HA, Tani C, Kwan A. et al. What is the prevalence of cognitive impairment in lupus and which instruments are used to measure it? A systematic review and meta-analysis. Semin Arthritis Rheum 2018;48:240–55. [DOI] [PubMed] [Google Scholar]
- 3. Li X, Xiang X, Sun J. et al. Prevalence, outcome and prognostic factors of neuropsychiatric systemic lupus erythematosus: a real world single center study. Mod Rheumatol 2020;30:321–6. [DOI] [PubMed] [Google Scholar]
- 4. Jonsen A, Bengtsson AA, Nived O, Ryberg B, Sturfelt G.. Outcome of neuropsychiatric systemic lupus erythematosus within a defined Swedish population: increased morbidity but low mortality. Rheumatology (Oxford) 2002;41:1308–12. [DOI] [PubMed] [Google Scholar]
- 5. Dobrowolski CG, Ruttan L, Lombardi S. et al. Cognitive impairment and health-related quality of life in a lupus cohort. Arthritis Rheumatol 2018;70(Suppl 10):abstract 782. [Google Scholar]
- 6. Calderon J, Flores P, Aguirre JM. et al. Impact of cognitive impairment, depression, disease activity, and disease damage on quality of life in women with systemic lupus erythematosus. Scand J Rheumatol 2017;46:273–80. [DOI] [PubMed] [Google Scholar]
- 7. Panopalis P, Julian L, Yazdany J. et al. Impact of memory impairment on employment status in persons with systemic lupus erythematosus. Arthritis Rheum 2007;57:1453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Appenzeller S, Cendes F, Costallat LT.. Cognitive impairment and employment status in systemic lupus erythematosus: a prospective longitudinal study. Arthritis Rheum 2009;61:680–7. [DOI] [PubMed] [Google Scholar]
- 9. Bingham KD, Green R, Tartaglia MC. et al. Longitudinal relationships between cognitive domains and DEPRESSION and anxiety symptoms in systemic lupus erythematosus. Semin Arthritis Rheum 2021;51:1186–92. [DOI] [PubMed] [Google Scholar]
- 10. Barraclough M, McKie S, Parker B, Elliott R, Bruce IN.. The effects of disease activity, inflammation, depression and cognitive fatigue on resting state fMRI in systemic lupus erythematosus. Rheumatology (Oxford). 2022;61(SI):SI34–47. [DOI] [PubMed] [Google Scholar]
- 11. Kello N, Anderson E, Diamond B.. Cognitive dysfunction in systemic lupus erythematosus: a case for initiating trials. Arthritis Rheumatol 2019;71:1413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mackay M. Centrally Acting ACE Inhibition in SLE. NCT04486118. https://clinicaltrials.gov/ct2/show/NCT04486118(29 September 2022, date last accessed).
- 13. Papachristos DA, Oon S, Hanly JG, Nikpour M.. Management of inflammatory neurologic and psychiatric manifestations of systemic lupus erythematosus: a systematic review. Semin Arthritis Rheum 2021;51:49–71. [DOI] [PubMed] [Google Scholar]
- 14. Zucchella C, Federico A, Martini A. et al. Neuropsychological testing. Pract Neurol 2018;18:227–37. [DOI] [PubMed] [Google Scholar]
- 15. Guilmette TJ, Sweet JJ, Hebben N. et al. American Academy of Clinical Neuropsychology consensus conference statement on uniform labeling of performance test scores. Clin Neuropsychol 2020;34:437–53. [DOI] [PubMed] [Google Scholar]
- 16. Petri M, Naqibuddin M, Carson KA. et al. Cognitive function in a systemic lupus erythematosus inception cohort. J Rheumatol 2008;35:1776–81. [PubMed] [Google Scholar]
- 17. Touma ZG, Tartaglia C, Ruttan L. et al. Prevalence of cognitive impairment in systemic lupus erythematosus assessed by a comprehensive neuropsychological battery. Arthritis Rheumatol 2017;69(Suppl 10):abstract 1623. [Google Scholar]
- 18. Tayer-Shifman OE, Green R, Beaton DE. et al. Validity evidence for the use of automated neuropsychologic assessment metrics as a screening tool for cognitive impairment in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2020;72:1809–19. [DOI] [PubMed] [Google Scholar]
- 19. Kozora E, Ellison MC, West S.. Reliability and validity of the proposed American College of Rheumatology neuropsychological battery for systemic lupus erythematosus. Arthritis Rheum 2004;51:810–8. [DOI] [PubMed] [Google Scholar]
- 20. Nantes SG, Su J, Dhaliwal A, Colosimo K, Touma Z.. Performance of screening tests for cognitive impairment in systemic lupus erythematosus. J Rheumatol 2017;44:1583–9. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz N, Stock AD, Putterman C.. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol 2019;15:137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kierdorf K, Prinz M.. Factors regulating microglia activation. Front Cell Neurosci 2013;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wadhwa M, Prabhakar A, Ray K. et al. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation 2017;14:222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Acharya MM, Green KN, Allen BD. et al. Elimination of microglia improves cognitive function following cranial irradiation. Sci Rep 2016;6:31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cope EC, LaMarca EA, Monari PK. et al. Microglia play an active role in obesity-associated cognitive decline. J Neurosci 2018;38:8889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DiSabato DJ, Quan N, Godbout JP.. Neuroinflammation: the devil is in the details. J Neurochem 2016;139(Suppl 2):136–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dehghani F, Sayan M, Conrad A. et al. Inhibition of microglial and astrocytic inflammatory responses by the immunosuppressant mycophenolate mofetil. Neuropathol Appl Neurobiol 2010;36:598–611. [DOI] [PubMed] [Google Scholar]
- 28. Liao LX, Song XM, Wang LC. et al. Highly selective inhibition of IMPDH2 provides the basis of antineuroinflammation therapy. Proc Natl Acad Sci USA 2017;114:E5986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anghel D, Tanasescu R, Campeanu A. et al. Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica (Buchar) 2013;8:170–5. [PMC free article] [PubMed] [Google Scholar]
- 30. Dehghani F, Hischebeth GT, Wirjatijasa F. et al. The immunosuppressant mycophenolate mofetil attenuates neuronal damage after excitotoxic injury in hippocampal slice cultures. Eur J Neurosci 2003;18:1061–72. [DOI] [PubMed] [Google Scholar]
- 31. Zhang W, Egashira N, Masuda S.. Recent topics on the mechanisms of immunosuppressive therapy-related neurotoxicities. Int J Mol Sci 2019;20:3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang HY, Chang HF, Tsai MJ, Chen JS, Wang MJ.. 6-Mercaptopurine attenuates tumor necrosis factor-α production in microglia through Nur77-mediated transrepression and PI3K/Akt/mTOR signaling-mediated translational regulation. J Neuroinflammation 2016;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 34. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J.. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–71. [DOI] [PubMed] [Google Scholar]
- 35. Beck AT, Epstein N, Brown G, Steer RA.. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988;56:893–7. [DOI] [PubMed] [Google Scholar]
- 36. Shapiro AM, Benedict RH, Schretlen D, Brandt J.. Construct and concurrent validity of the Hopkins Verbal Learning Test–revised. Clin Neuropsychol 1999;13:348–58. [DOI] [PubMed] [Google Scholar]
- 37. Touma Z, Gladman DD, Zandy M. et al. Identifying a response for the Systemic Lupus Erythematosus Disease Activity Glucocorticoid Index (SLEDAI-2KG). Arthritis Care Res (Hoboken) 2021;73:1243–9. [DOI] [PubMed] [Google Scholar]
- 38. Touma Z, Gladman DD, Su J, Anderson N, Urowitz MB.. A novel lupus activity index accounting for glucocorticoids: SLEDAI-2K glucocorticoid index. Rheumatology (Oxford) 2018;57:1370–6. [DOI] [PubMed] [Google Scholar]
- 39. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH.. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992;35:630–40. [DOI] [PubMed] [Google Scholar]
- 40. Stoll T, Seifert B, Isenberg DA.. SLICC/ACR Damage Index is valid, and renal and pulmonary organ scores are predictors of severe outcome in patients with systemic lupus erythematosus. Br J Rheumatol 1996;35:248–54. [DOI] [PubMed] [Google Scholar]
- 41. Devere R. The cognitive consequences of obesity. Pract Neurol 2018;March/April:22–5. [Google Scholar]
- 42. Swan GE, Lessov-Schlaggar CN.. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev 2007;17:259–73. [DOI] [PubMed] [Google Scholar]
- 43. Yelnik CM, Kozora E, Appenzeller S.. Cognitive disorders and antiphospholipid antibodies. Autoimmun Rev 2016;15:1193–8. [DOI] [PubMed] [Google Scholar]
- 44. Garcia MA, Downer B, Chiu CT. et al. Racial/ethnic and nativity differences in cognitive life expectancies among older adults in the United States. Gerontologist 2019;59:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vance DB, Enah CC, Palmer JJ, Hoenig AK.. The impact of employment on cognition and cognitive reserve: implications across diseases and aging. Nurs Res Rev 2016;6:61–71. [Google Scholar]
- 46. Hakansson K, Rovio S, Helkala EL. et al. Association between mid-life marital status and cognitive function in later life: population based cohort study. BMJ 2009;339:b2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ciriaco M, Ventrice P, Russo G. et al. Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother 2013;4(Suppl 1):S94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bertsias GK, Ioannidis JP, Aringer M. et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis 2010;69:2074–82. [DOI] [PubMed] [Google Scholar]
- 49. Pellerin D, Singh K, Maniatis T, Chalk CH, Green L.. Mycophenolate mofetil-induced status epilepticus. Can J Neurol Sci 2018;45:585–7. [DOI] [PubMed] [Google Scholar]
- 50. Broen JCA, van Laar JM.. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat Rev Rheumatol 2020;16:167–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to concern for the privacy of the individuals who participated in this single-center study. The data will be shared upon reasonable request to the corresponding author.