Abstract
Purpose:
To compare the diagnostic performance of biparametric magnetic resonance imaging (bpMRI) versus multiparametric MRI (mpMRI) for the staging of well-differentiated endometrioid endometrial cancer (EC) in potential candidates for fertility-sparing management.
Methods:
This multi-center retrospective study included 48 potential candidates for fertility-sparing management (age <46 years, grade 1 endometroid EC) but who did not wish to undergo fertility-sparing management and thus underwent definitive surgery. Two readers (R1, R2) independently reviewed bpMRI (T1, T2, and diffusion-weighted imaging) and mpMRI (bpMRI and dynamic contrast enhanced imaging, DCE) during two separate sessions which were one month apart for the presence of myometrial invasion (MI), cervical stromal involvement (CSI), malignant adnexal disease (mAD), and pelvic lymphadenopathy (pLNM). Each reader also recorded maximum tumor diameter, tumor volume, and tumor-to-uterine volume ratio (TVR) on T2-weighted imaging. The diagnostic performance of bpMRI and mpMRI was determined for each reader with surgical pathology serving as a gold standard.
Results:
The area under the receiver operating curve (AUC) for bpMRI versus mpMRI was 0.76/0.78 (R1/R2) versus 0.84/0.83 for MI, 0.79/0.76 versus 0.99/0.80 for CSI, 0.84/0.84 versus 0.84/0.80 for mAD, and 0.82/0.82 for pLMN. The sensitivity and specificity of MRI for detecting tumor spread beyond the endometrium was 71%/77% and 71%/65% for bpMRI (R1/R2) vs. 84%/90% and 71%/65% for mpMRI (R1/R2), respectively. The AUC of maximum tumor diameter, tumor volume, and TVR for MI was 0.71/0.61, 0.73/0.75, and 0.75/0.77 for R1/R2, respectively.
Conclusion:
MRI had moderate diagnostic performance across potential candidates for fertility-sparing treatment of EC. mpMRI outperformed bpMRI for detecting EC spreading beyond the endometrium.
Keywords: Endometrial Cancer, fertility preservation, magnetic resonance imaging, patient selection
INTRODUCTION
Endometrial cancer (EC) is the most common gynecologic malignancy among women in developed countries [1,2]. Although EC is primarily diagnosed among postmenopausal women, up to ten percent of patients with EC are diagnosed during their childbearing years [3–5,2]. The standard treatment for EC consists of hysterectomy and bilateral salpingo-oophorectomy [6,2,7], leading to a permanent loss of reproductive potential. However, fertility preservation may be important to young women with EC who desire future children and/or the hormonal benefits of ovarian preservation in order to avoid premature menopause. Since premenopausal women often present with well-differentiated early stage endometrioid adenocarcinoma and have favorable prognosis [8,9,2], conservative fertility-sparing management with high dose progestins may be considered for carefully selected and counseled patients desiring future fertility [2,5].
The typical eligibility criteria for fertility-sparing treatment are: reproductive age (typically 45 years or less), International Federation of Gynecology and Obstetrics (FIGO) grade 1 endometrioid adenocarcinoma or premalignant conditions, absence of contraindications to medical therapy or pregnancy, and imaging demonstrating endometrium-confined disease [6,2,10]. Additional recommendations include the exclusion of significant family history of endometrial and/or colorectal cancer and the testing for defects in DNA mismatch repair genes [11]. Patients are also counseled that fertility-sparing management of early-stage EC represents an alternative strategy to standard-of-care surgery.
Meticulous patient selection includes pretreatment assessment of disease extent to exclude extrauterine disease; definitive surgical management is the treatment of choice when extrauterine disease is present. Most clinical practice guidelines recommend pretreatment magnetic resonance imaging (MRI) to ensure that the tumor is confined to the endometrium [2,12,6,10]. Nevertheless, data about the value of MRI for the initial staging of EC in women of childbearing age is limited [13,14]. Two studies evaluated the detection of myometrial invasion (MI), but neither reported on cervical stromal involvement (CSI), pelvic lymphadenopathy (pLNM), or malignant adnexal disease (mAD), despite the increased risk of ovarian involvement in younger patients with EC [15,16]. The MRI appearance of uterine anatomy varies according to menopausal status [17], which may influence the diagnostic performance of MRI [14]. Further, there is still uncertainty as to whether intravenous contrast should be administered as a part of pretreatment MRI. The European Society of Medical Oncology guidelines provide no guidance about this question [18]. The National Comprehensive Cancer Network and the European Society of Urogenital Radiology guidelines recommend pelvic MRI with intravenous contrast, based largely on the consensus between experts rather than side-by-side comparison of bpMRI to mpMRI in potential candidates for fertility preservation [19,11].
Thus, the aim of our study was to compare the diagnostic performance of biparametric MRI (bpMRI) [i.e. unenhanced MRI with diffusion-weighted imaging (DWI)] versus multiparametric MRI (mpMRI) [i.e. unenhanced MRI with DWI and dynamic contrast-enhanced imaging (DCE)] for staging of well-differentiated EC in potential candidates for fertility-sparing management. Surgical-pathologic staging served as the reference standard.
MATERIALS AND METHODS
Patient population
Patients in this multi-center retrospective study were sourced from three institutions. The study was approved by the institutional review board at each institution and the requirement for informed consent was waived.
The eligibility criteria were as follows: (1) patient age < 46 years, (2) lack of prior hormonal therapy, (3) FIGO grade 1 endometrioid adenocarcinoma diagnosed pathologically by biopsy, (4) definitive surgery from January 2010 to August 2017, and (5) pretreatment MRI including DWI and DCE within three months of surgery. Two patients were excluded because MR image quality was limited by motion or metal susceptibility artifact. Three institutions (Memorial Sloan Kettering Cancer Center, Kyoto University Hospital, and Tottori University Hospital) contributed to the final study population.
Clinical reports of final surgical pathology (rendered by board-certified pathologists at each institution) served as the reference standard for MI, CSI, and, if surgically evaluated, mAD and pLNM. If salpingo-oophorectomy or lymph node evaluation was not performed (n = 4/ n = 2, respectively), mAD and/or pLNM were considered absent.
MRI protocol
All MRI scans were obtained in the supine position using 1.5 or 3.0 Tesla scanners (various vendors). An anti-peristaltic agent was administered intramuscularly prior to imaging at two of three institutions (** and **). Each study included axial T1-weighted imaging (T1WI); axial, sagittal, and oblique T2-weighted imaging (T2WI); axial and/or sagittal DWI; and axial or sagittal pre-contrast and DCE images. Oblique T2WI was oriented perpendicular to the endometrial cavity. DCE was obtained using a three-dimensional gradient echo volume acquisition technique after intravenous administration of 0.1 or 0.2 mmol/kg gadolinium chelate contrast medium at a rate of 2.0 or 2.5 mL/sec (Magnevist®, Berlex Laboratories, Montville, USA; Gadovist®, Bayer, Leverkusen, Germany; ProHance®, Bracco, Milano, Italy; or Omniscan®, Daiichi Sankyo, Tokyo, Japan). MRI protocols from each institution are summarized in Supplemental Table 1. MRI scans that were performed at outside institutions (n = 6) either met or exceeded the above quality standards.
Qualitative assessment
Each MRI examination was independently evaluated by board-certified radiologists. One reader (**, R1) reviewed pretreatment MRI scans for all patients in the cohort (n = 48). Three additional readers (**, **, and **; designated as composite R2) evaluated cases from their respective institutions (n = 18, n = 17, n = 13, respectively). All readers had ten or more years of experience in gynecologic oncologic imaging. The readers were aware of EC diagnosis, but they were blinded to all other clinical information. All readers interpreted each MRI examination in two separate sessions spaced a month apart. During the first session, only bpMRI (T1WI, T2WI, and DWI) images were evaluated; during the second session, mpMRI (T1WI, T2WI, DWI, and DCE) images were reviewed.
Each reader recorded his or her impression regarding the presence of (a) MI, (b) CSI, (c) mAD, and (d) pLNM using a Likert scale with scores ranging from 1 to 5 (1, definitely absent; 2, probably absent; 3, equivocal; 4, probably present; 5, definitely present). In keeping with previously published criteria, MI/CSI was defined as the disruption or irregularity of the normally smooth interface between the junctional zone/inner cervical stroma and the tumor on T2WI, DWI, and DCE [20,13,21,12]. For the adnexa, only suspected mAD was recorded; dermoid cyst, endometrial cyst, functional cyst, fibroma, and hydrosalpinx were marked as benign and excluded from further analysis as these do not affect EC management. The following standard size criteria were used to diagnose pLNM: (1) short axis diameter ≥ 10 mm or (2) short axis diameter ≥ 8 mm if rounded or asymmetric in shape (compared with the opposite side) on T2WI [22]. EC spread beyond the endometrium was considered present if any of the following imaging features were scored as 4 or 5: MI, CSI, pLNM, or mAD.
Quantitative assessment
Each reader measured the maximum tumor diameter, tumor volume, and tumor-to-uterine volume ratio (TVR) [23] on T2WI, using the sagittal, axial, or oblique plane (Figure 1). Readers manually segmented each tumor and body of the uterus on every slice. Similar to the qualitative assessment, portions of subserosal and/or intramural leiomyomas extending beyond the outer contour of uterine body were excluded. No adjustments were made for adenomyosis [23]. All volumetric measurements were performed using AquariusNet (Terarecon, Foster City, USA) or EV insite (PSP, Tokyo, Japan). In four patients, tumors were not visible on MRI; thus, tumor diameter, tumor volume, and TVR were recorded as equal to zero.
Fig. 1-.
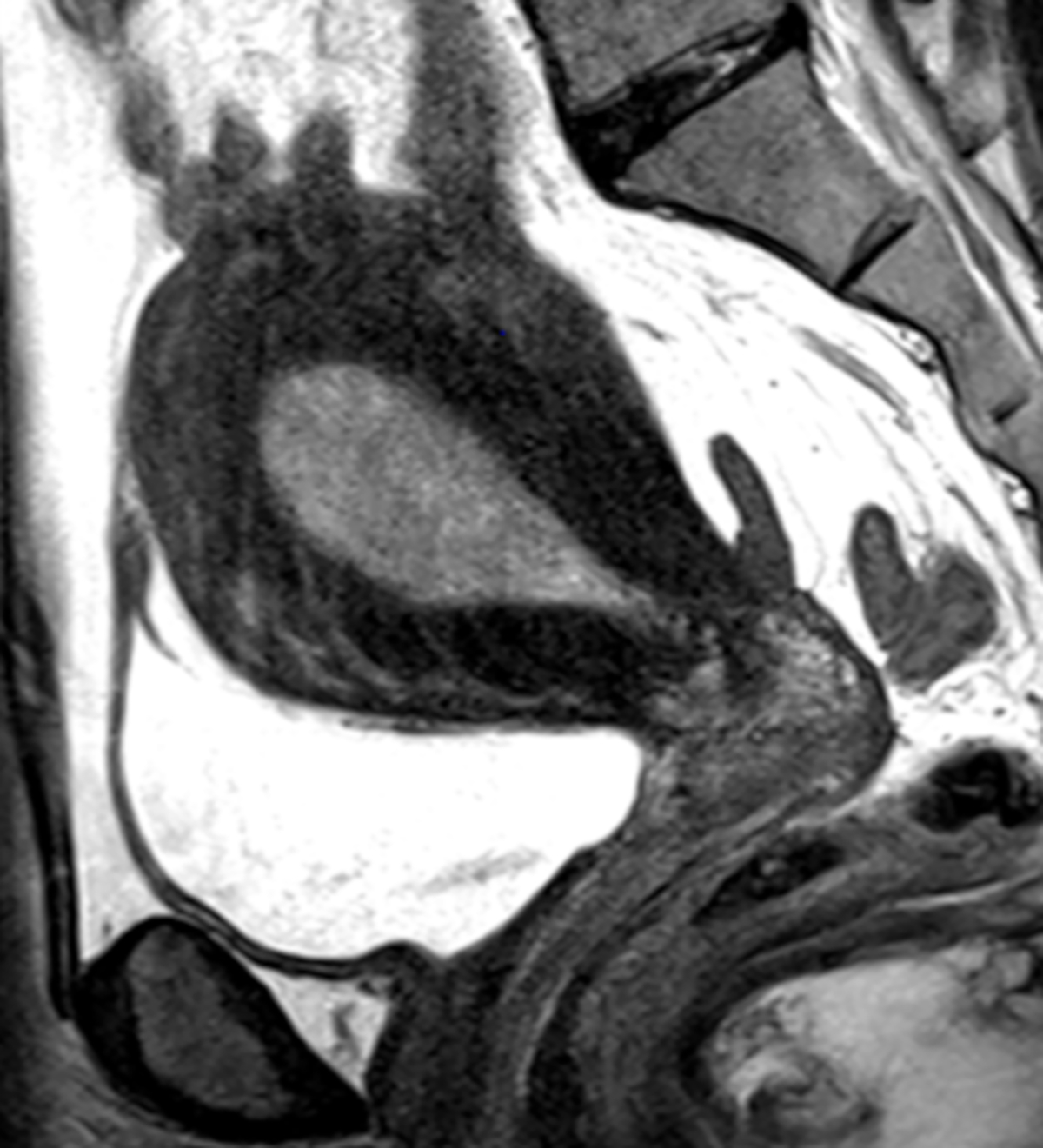
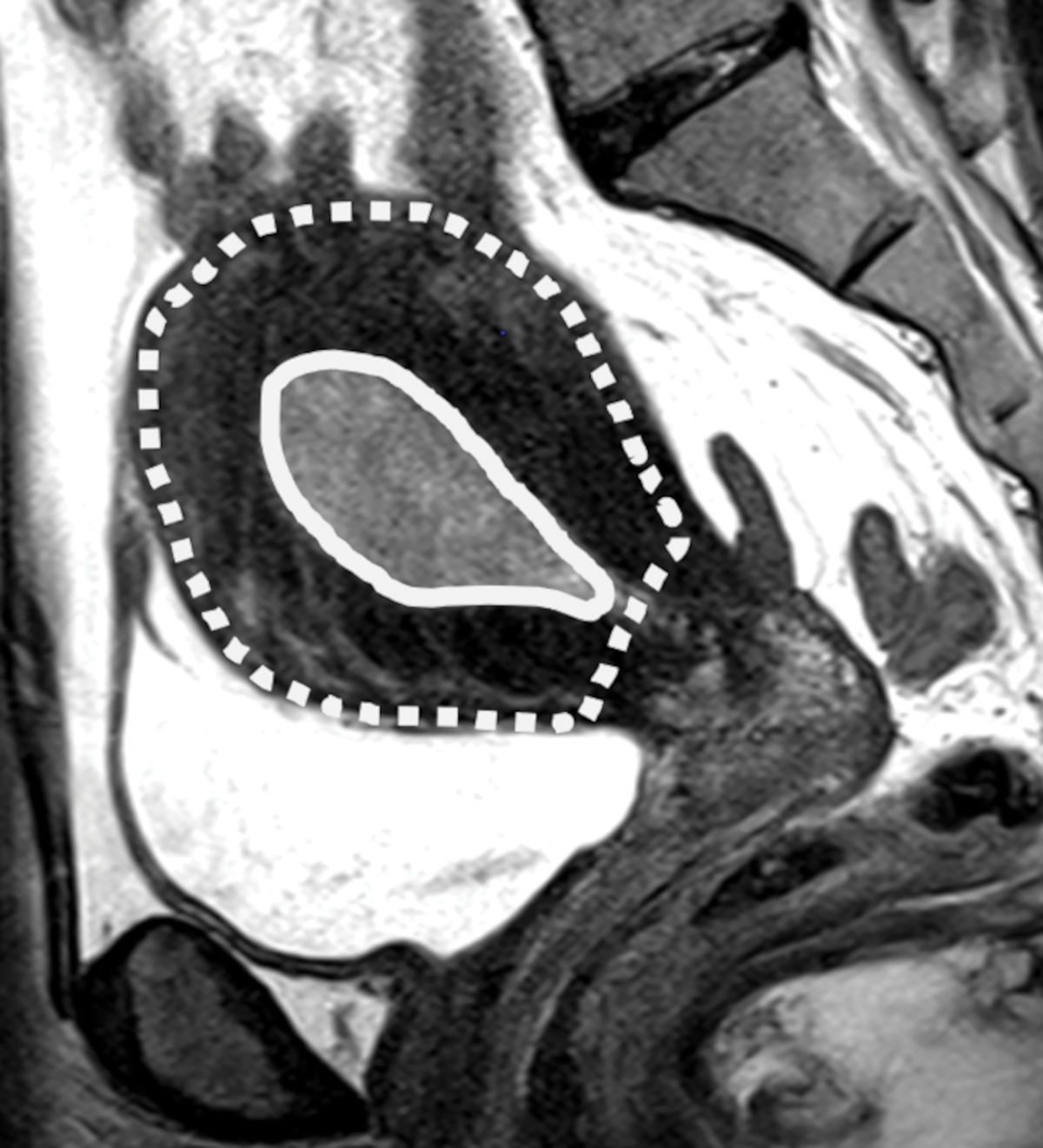
(A) Sagittal T2-weighted image shows dilated endometrial cavity with biopsy proven endometrial cancer. (B) After manual segmentations of tumor (solid line) and the outer contour of the uterine corpus (dotted line) on each T2-weighted image, their volumes were calculated as the sum of cross-sectional volumes. Tumor volume ratio (TVR) was defined as the ratio between the tumor volume and the volume of uterine corpus. In this case, TVR was 0.11 for Reader 1 and 0.10 for Reader 2. Final surgical histology revealed superficial myometrial invasion.
Statistical analysis
Receiver operating characteristic (ROC) curve analyses were performed for each reader as well as imaging feature (scored from 1 to 5) and an area under the curve (AUC) was computed. The standard error of the AUC was calculated using the DeLong method [24]. An AUC of > 0.90 was considered to indicate excellent diagnostic accuracy; 0.81–0.90, very good; 0.71–0.80, good; 0.61–0.70, moderate; 0.51–0.60, poor; and ≤ 0.50, test not useful [25]. The ROC curves for bpMRI and mpMRI were compared using the DeLong method [24], based on the evaluations of R1/composite R2. A p-value of less than 0.05 was considered to be statistically significant.
Next, all Likert scores were dichotomized with scores of 1 through 3 indicating endometrium-confined disease and scores of 4 or 5 indicating tumor spread beyond the endometrium. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for detecting MI, CSI, mAD, pLNM, and EC spread beyond the endometrium were determined for each reader on bpMRI and mpMRI, respectively. Inter-rater agreement between R1 and composite R2 was assessed with the linearly weighted κ statistic. Values > 0.80 were considered excellent; 0.61–0.80, good; 0.41–0.60 moderate; 0.21–0.40 fair; and ≤ 0.20, poor [26].
The ROC curves were constructed to evaluate the diagnostic performance of quantitative measures such as maximum tumor diameter, tumor volume, and TVR for detecting MI. The optimal cut-off value for each measure and for each reader was computed by analyzing these ROC curves. Inter-reader agreement about these quantitative measures was assessed with the concordance correlation coefficient [27], and interpreted as follows: > 0.99, almost perfect; > 0.95 to ≤ 0.99, substantial; > 0.90 to ≤ 0.95 moderate; and ≤ 0.90, poor [28].
All statistical analyses were performed using Medcalc® version 18.5 (MedCalc Softwear, Ostend, Belgium).
RESULTS
Patients
The study population assessed 48 patients (median age, 38.5 years; range, 28–45 years). The number of patients included from each institution were as follows: **, 18 patients; **, 17 patients; and **, 13 patients. The median time interval between pretreatment MRI and surgery was 38.5 days (range: 1–91 days). Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics based on surgical pathology
| Variable | Data (n = 48) | Percentage |
|---|---|---|
|
| ||
| Age (y), range (median) | 28–45 (38.5) | |
| FIGO stage | ||
| IA | 38 | 79.2% |
| IB | 2 | 4.2% |
| II | 2 | 4.2% |
| IIIA | 3 | 6.2% |
| IIIC2 | 3 | 6.2% |
| Any myometrial invasion | ||
| No | 24 | 50.0% |
| Yes | 24 | 50.0% |
| Cervical stromal involvement | ||
| No | 45 | 93.8% |
| Yes | 3 | 6.2% |
| Malignant adnexal disease | ||
| No | 35 | 72.9% |
| Yes | 13 | 27.1% |
| Pelvic lymph node metastasis | ||
| No | 45 | 93.8% |
| Yes | 3 | 6.2% |
| Spread beyond endometrium | ||
| No | 17 | 35.4% |
| Yes | 31 | 64.6% |
FIGO = the International Federation of Gynecology and Obstetrics
Qualitative and Quantitative Analysis of MI
Twenty-four of 48 patients (50%) had pathologically confirmed MI. mpMRI had a higher AUC compared to bpMRI (R1, AUC 0.84 vs 0.76; R2, AUC 0.83 vs 0.78, Figure 2A); however, these differences did not reach statistical significance (R1/R2, p = 0.09/0.17). mpMRI had higher sensitivity compared to bpMRI (R1, 88% vs 63%; R2, 96% vs 79%), while specificity was the same (R1, 71% vs 71%; R2, 63% vs 63%) (Table 2).
Fig. 2-.
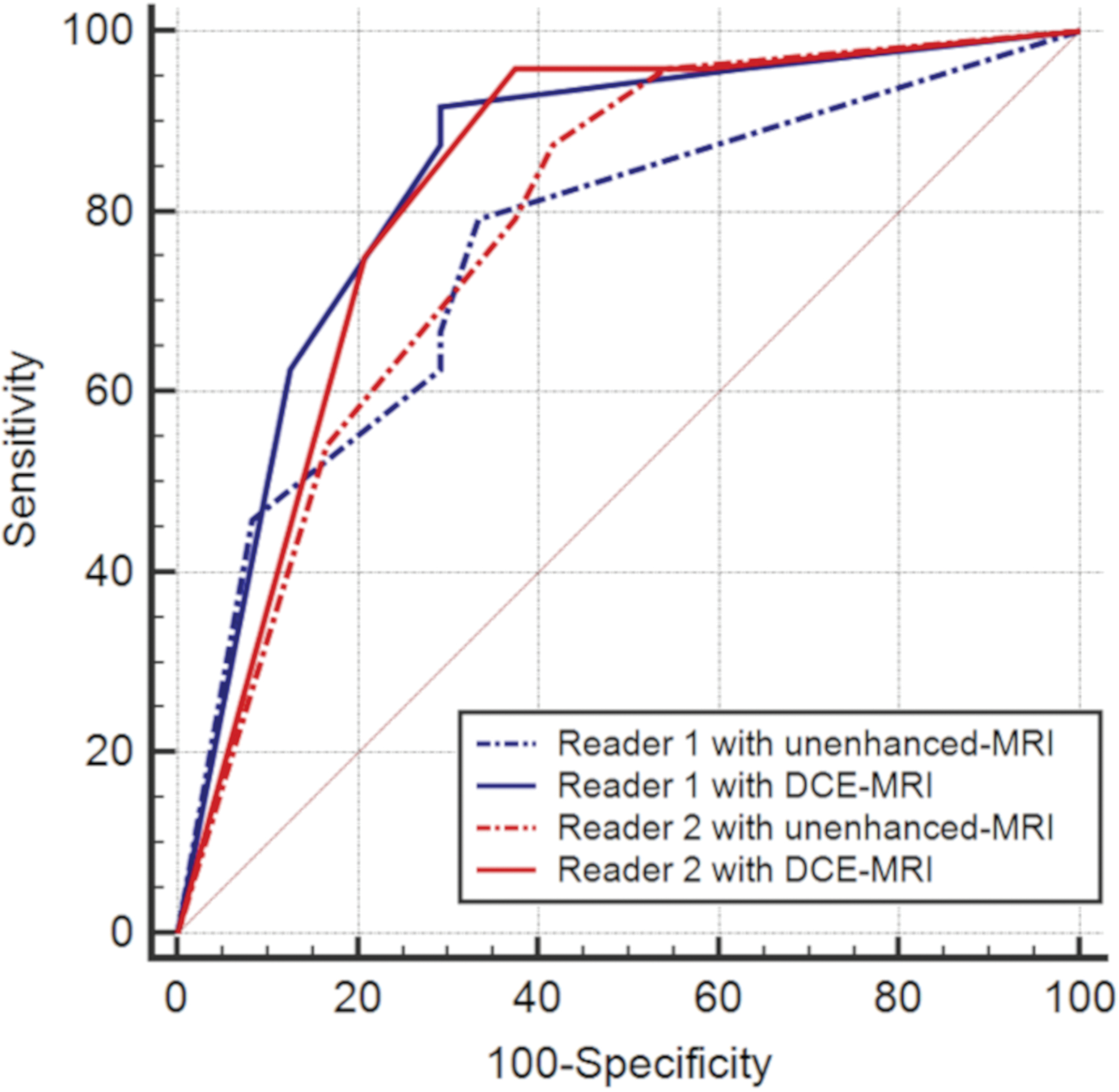
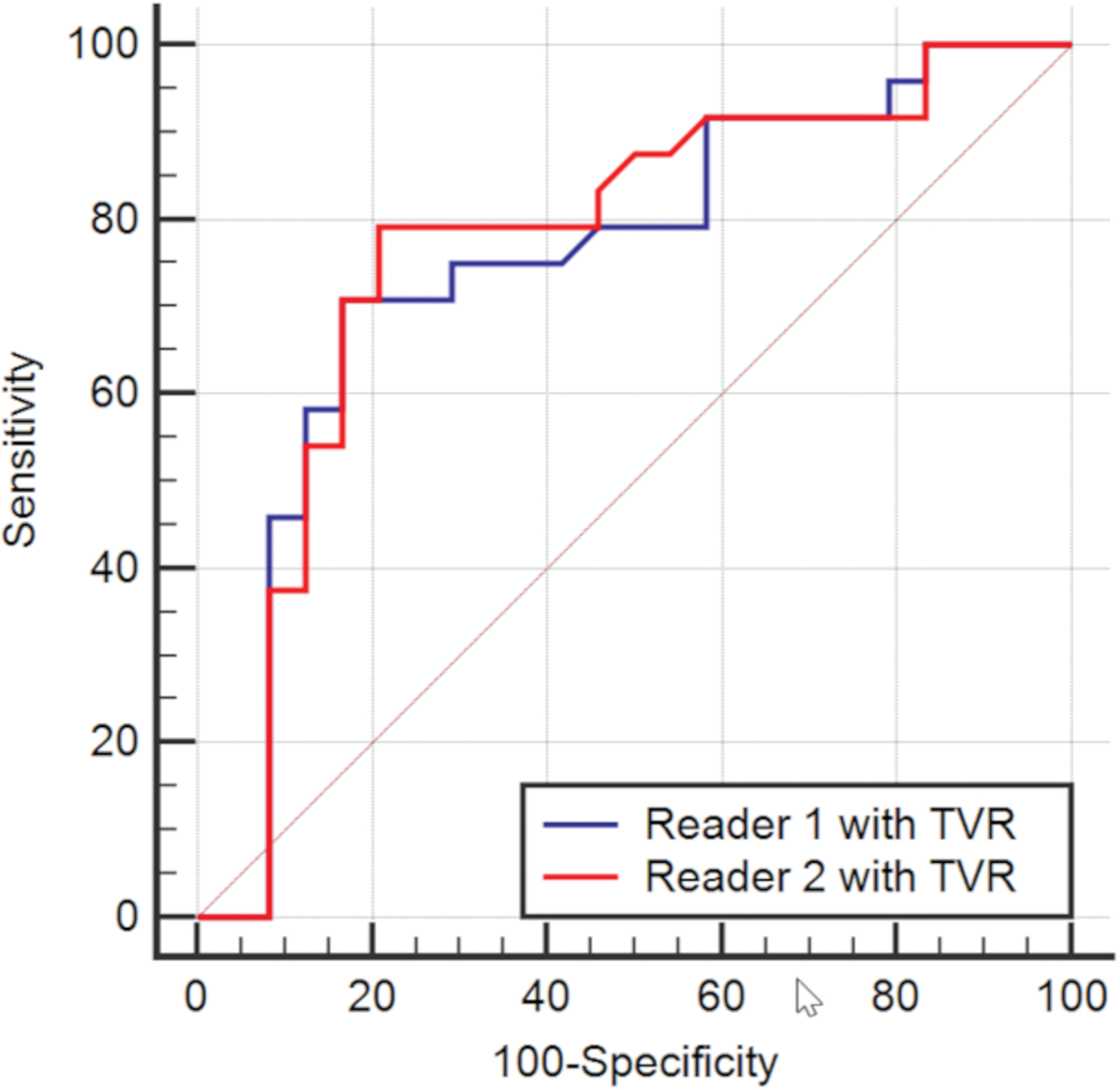
(A) Receiver operating characteristic (ROC) curves of image evaluations of myometrial invasion. The four curves represent the diagnostic performance of biparametric MRI (bpMRI) or multiparametric MRI (mpMRI) separately for each reader. (B) ROC curves of tumor-to-uterine volume ratio (TVR) on T2-weighted images for each reader.
Table 2.
Results of image evaluations in detecting myometrial invasion (MI) and spread beyond the endometrium.
| MI | Spread beyond the endometrium | ||||
|---|---|---|---|---|---|
| bpMRI | mpMRI | bpMRI | mpMRI | ||
|
| |||||
| R1 | AUC (SE, 95% CI) | 0.76 (0.07, 0.61–0.87) | 0.84 (0.06, 0.71–0.93) | NA | NA |
| Sensitivity | 63% | 88% | 71% | 84% | |
| Specificity | 71% | 71% | 71% | 71% | |
| PPV | 68% | 75% | 81% | 84% | |
| NPV | 65% | 85% | 57% | 71% | |
| Accuracy | 67% | 79% | 71% | 79% | |
|
| |||||
| R2 | AUC (SE, 95% CI) | 0.78 (0.07, 0.64–0.89) | 0.83 (0.06, 0.69–0.92) | NA | NA |
| Sensitivity | 79% | 96% | 77% | 90% | |
| Specificity | 63% | 63% | 65% | 65% | |
| PPV | 68% | 72% | 80% | 82% | |
| NPV | 75% | 94% | 61% | 79% | |
| Accuracy | 71% | 79% | 73% | 81% | |
AUC = area under the curve, CI = confidence interval, CSI = cervical stromal invasion, MRI = magnetic resonance imaging, bpMRI = biparametric MRI, mpMRI = multiparametric MRI, NA = not applicable, NPV = negative predictive value, PPV = positive predictive value, R1/R2 = Reader 1/Reader 2, SE = standard error
Table 3 shows the results of quantitative assessment (i.e. tumor diameter, tumor volume, and TVR) for detecting MI. TVR had the highest AUC for each reader (R1: AUC 0.75; R2, AUC 0.77, Figure 2B). The optimal cut-off value of TVR for detecting MI was > 0.067 and > 0.070 for R1 and R2, respectively.
Table 3.
Results of quantitative evaluations in detecting myometrial invasion.
| TVR | Tumor volume | Maximum diameter | ||
|---|---|---|---|---|
|
| ||||
| R1 | AUC (SE, 95% CI) | 0.75 (0.07, 0.61–0.87) | 0.73 (0.08, 0.58–0.85) | 0.71 (0.078, 0.56–0.83) |
| Cutoff value | > 0.067 | > 4.75 cm3 | > 4.2 cm | |
| Range (median) | 0–0.70 (0.063) | 0–125cm3 (4.8 cm3) | 0–9.9 cm (3.7 cm) | |
| Sensitivity | 71% | 71% | 63% | |
| Specificity | 83% | 71% | 79% | |
|
| ||||
| R2 | AUC (SE, 95% CI) | 0.77 (0.07, 0.63–0.88) | 0.75 (0.075, 0.60–0.86) | 0.61 (0.083, 0.46–0.75) |
| Cutoff value | > 0.070 | > 5.90 cm3 | > 4.6 cm | |
| Range (median) | 0–0.74 (0.075) | 0–150 cm3 (6.8 cm3) | 0–9.6 cm (3.7 cm) | |
| Sensitivity | 79% | 79% | 42% | |
| Specificity | 79% | 71% | 88% | |
TVR = tumor-to-uterine volume ratio, R1/R2 = Reader 1/Reader 2, AUC = area under the curve, SE = standard error, CI = confidence interval
Qualitative analysis of CSI, mAD, and pLMN
The final surgical pathology demonstrated CSI in 3 of 48 patients (6%), mAD in 13 patients (27%), and pLNM in 3 patients (6%) (Table 1). In 13 patients with mAD, the etiologies were as follows: synchronous or metastatic ovarian cancer (n = 8), isolated fallopian tube micro-metastasis (n = 2), borderline tumor (n = 2), and granulosa cell tumor (n = 1). All patients with pLNM (n = 3) had concurrent para-aortic LNM (stage IIIC2).
For detecting CSI, mpMRI had a higher AUC compared to bpMRI for each reader (R1, AUC 0.99 vs 0.79; R2, AUC 0.80 vs 0.76) (Table 4). mpMRI was also more sensitive for R1. For identifying mAD, the diagnostic performance of mpMRI and bpMRI was similar for each reader (R1, AUC 0.84 for both; R2, AUC 0.84 vs 0.80) (Table 4). No statistically significant differences in AUC were found between mpMRI and bpMRI for detecting either CSI (R1/R2, p = 0.22/0.15) or mAD (R1/R2, p = 0.96/0.63). The sensitivity and specificity for detecting pLMN were 67% and 100% in both readers.
Table 4.
Results of image evaluations in detecting cervical stromal invasion (CSI), malignant adnexal disease (mAD), and pelvic lymph node metastasis (pLMN).
| CSI | mAD | pLMN | ||||
|---|---|---|---|---|---|---|
| bpMRI | mpMRI | bpMRI | mpMRI | bpMRI | ||
|
| ||||||
| R1 | AUC (SE, 95% CI) | 0.79 (0.17, 0.64–0.89) | 0.99 (0.01, 0.91–1.00) | 0.84 (0.07, 0.70–0.93) | 0.84 (0.07, 0.71–0.93) | 0.82 (0.18, 0.69–0.92) |
| Sensitivity | 33% | 100% | 62% | 62% | 67% | |
| Specificity | 96% | 96% | 97% | 97% | 100% | |
| PPV | 33% | 60% | 89% | 89% | 100% | |
| NPV | 96% | 100% | 87% | 87% | 98% | |
| Accuracy | 92% | 96% | 88% | 88% | 98% | |
|
| ||||||
| R2 | AUC (SE, 95% CI) | 0.76 (0.18, 0.62–0.87) | 0.80 (0.17, 0.66–0.90 | 0.84 (0.07, 0.71–0.93) | 0.80 (0.07, 0.66–0.90) | 0.82 (0.18, 0.68–0.92) |
| Sensitivity | 33% | 33% | 62% | 54% | 67% | |
| Specificity | 98% | 98% | 100% | 97% | 100% | |
| PPV | 50% | 50% | 100% | 88% | 100% | |
| NPV | 96% | 96% | 88% | 85% | 98% | |
| Accuracy | 94% | 94% | 90% | 85% | 98% | |
AUC = area under the curve, CI = confidence interval, CSI = cervical stromal involvement, DCE = dynamic contrast enhanced, mAD = malignant adnexal disease, MRI = magnetic resonance imaging, bpMRI = biparametric MRI, mpMRI = multiparametric MRI, NPV = negative predictive value, PPV = positive predictive value, pLMN = pelvic lymph node metastasis, R1/R2 = Reader 1/Reader 2, SE = standard error
Detection of EC spread beyond the endometrium
mpMRI was more sensitive for detecting EC spread beyond the endometrium compared to bpMRI (R1/R2, 84/90% vs 71/77%), while specificity was the same with either approach (R1/R2, 71/65% vs 71/65%) (Table 2). As a result, the accuracy of mpMRI was superior compared to bpMRI (R1/R2, 79/81% vs 71/73%).
Concordance and inter-rater agreement
The inter-rater agreement (weighted κ) on bpMRI/mpMRI were as follows: MI, 0.60/0.59, moderate/moderate; CSI, 0.76/0.40, good/fair; mAD, 0.86/0.85, excellent/excellent; pLNM, 0.41, moderate (Supplemental Table 2). Concordance correlation coefficient for maximum tumor diameter, tumor volume, and TVR ranged from 0.94–0.96 (moderate to substantial correlation), as shown in Supplemental Table 3.
DISCUSSION
In this multi-center retrospective study, we evaluated the diagnostic performance of bpMRI (including DWI) and mpMRI for the initial staging of well-differentiated EC in potential candidates for fertility-sparing management. In our cohort of patients, mpMRI achieved higher AUCs for detecting MI and CSI, compared to bpMRI; however, these differences did not reach statistical significance which may be a result of our relatively small sample size. In addition, mpMRI had higher sensitivity, NPV, and accuracy, with equivalent specificity and PPV for assessing MI and EC spread beyond the endometrium, compared to bpMRI.
To our knowledge, there is limited data about the performance of MRI for evaluating patients prior to fertility-sparing treatment of EC, including the added value of contrast-enhanced imaging. A prior meta-analysis by Andreano et al. found no significant difference in the sensitivity and specificity of DWI-MRI and DCE-MRI for identifying deep MI (≥ 50%) in women of all ages who had EC of various grades [29]. Even less is known about the added value of DWI-MRI and DCE-MRI for detecting any MI, especially in patients of childbearing age with FIGO grade 1 endometrioid EC; and the conclusions vary across published studies [14,13,30–33]. Sakane et al. evaluated 26 premenopausal patients with FIGO grade 1 endometrioid EC (age range: 31–50 years; median: 44 years) and found that the addition of DCE-MRI to T2WI and DWI -MRI did not improve the detection of any MI [14]. The results of their study were limited as 15% of patients received hormonal therapy prior to MRI, altering tumor appearance and potentially influencing EC staging. Lin et al. compared the diagnostic performance of DCE-MRI to that of DWI-MRI in 31 premenopausal patients (age range: 30–59 years) and found that DCE-MRI was superior to DWI-MRI for demonstrating any MI [13].
DCE imaging allows the visualization of enhancement in the subendometrial zone [12]. The disruption of subendometrial enhancement on DCE imaging may facilitate the diagnosis of subtle MI (Supplemental Figure 1). Given that careful patient selection is paramount to confirming patient eligibility for fertility-sparing management, mpMRI may be an optimal imaging approach for the initial staging of EC in women of reproductive age group. Our results support the consensus guidelines from the European Society of Urogenital Radiology which recommend mpMRI for pretreatment evaluation of patients being considered for fertility-sparing management [12].
Recently, Nougaret et al. reported on the value of TVR for detecting deep MI (≥ 50%) in patients with EC [23]. In our study, we computed TVR in women of childbearing age and found that this measure showed both moderate accuracy and inter-reader agreement for detecting any MI (AUC R1/R2, 0.75/0.77). Combined with qualitative analysis, TVR might provide a reproducible and quantitative method for diagnosing MI.
In addition to MI, exclusion of CSI, pLNM, and mAD is also essential to confirm patient eligibility for fertility-sparing management. In fact, several reports have documented increased risk of mAD (primary or metastatic) in women of reproductive age with EC [16,9]. A meta-analysis by Xu et al. evaluated patients of all ages and found that MRI had a pooled sensitivity and specificity of 0.50/0.95 for CSI, 0.65/0.95 for pLMN, and 0.51/0.99 for mAD [34]. Lin et al. evaluated CSI and reported that the AUC of DWI-MRI was superior to that of DCE-MRI, but only for one of two readers [20]. Similar to prior reports in patients of all ages, we showed low to moderate sensitivity and high specificity of MRI (either bpMRI or mpMRI) for identifying CSI, mAD, and pLMN in women of childbearing age. The limited sensitivity of MRI for detecting pLNM is likely explained by the presence of normal-sized metastatic lymph nodes, whereas the limited sensitivity of MRI for demonstrating mAD in part relates to the presence of microscopic tumor deposits (2 of 13 patients (15%) with mAD in our cohort).
There are several limitations to our study. First, only potential candidates for fertility-sparing management were included. We did not include patients who underwent fertility-sparing therapy because these patients are managed medically with high-dose progestins; thus, the surgical-pathological gold standard for the presence of MI, mAD, and pLMN was not available. Second, while one reader, R1, interpreted cases from all 3 institutions, the second reader, R2, was a composite of three readers, with one reader from each respective institution reviewing cases from their home center. This approach was chosen in order to protect patient confidentiality and keep all clinical/imaging data within each institution. Third, MRI protocols differed between the three centers and have evolved over time, reflecting advancements in MR technology; this potentially strengthened our study by mirroring clinical practice. Fourth, a minority of patients did not undergo salpingo-oophorectomy (n = 4) or lymph node dissection (n = 2). This also closely reflects the routine clinical practice of avoiding extra procedures in early-stage EC when intraoperative visual inspection demonstrates no overt abnormality. Finally, our patient cohort was relatively small in size and spanned a number of years in time which is primarily due to relatively low incidence of EC in women under the age of 46 years, i.e. our population of interest.
In conclusion, MRI showed moderate diagnostic performance among potential candidates for fertility-sparing treatment of EC. Our results indicate that mpMRI provides an optimal approach to confirming endometrium-confined disease and, thus, verifying patient eligibility for fertility-sparing management. Our results lend further support to the recommendations of the European Society of Urogenital Radiology to obtain mpMRI (rather than bpMRI) for the evaluation of young women with EC prior to fertility-sparing management [12].
Supplementary Material
Fig. 3-.

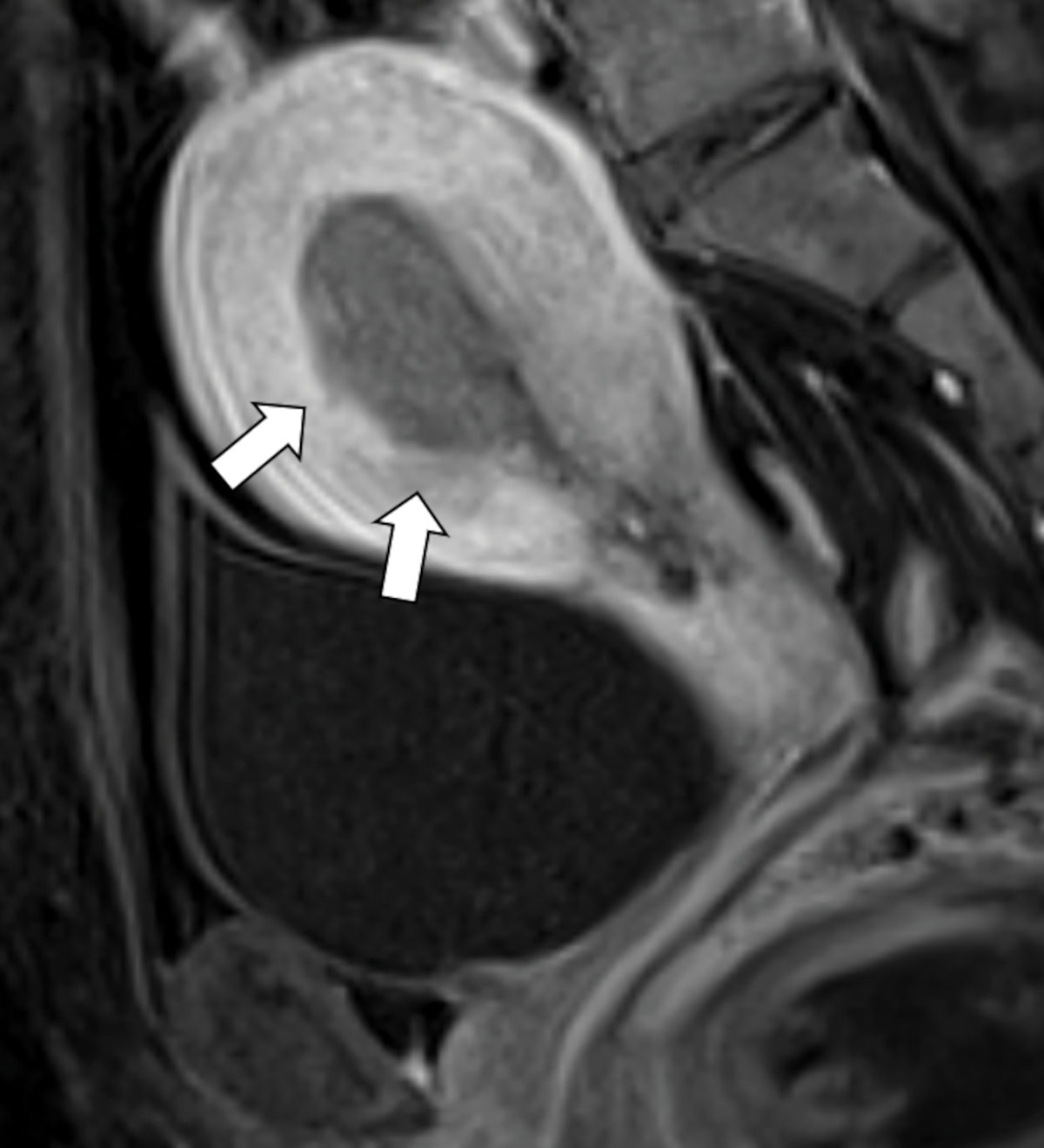
Sagittal diffusion weighted image (DWI, A) and dynamic contrast-enhanced image at 120 seconds (DCE, B) obtained in the same patient as in Figure 1. While myometrial invasion is not visible either on T2 weighted images and DWI (A), DCE shows irregular subendometrial enhancement (B, arrow), indicating shallow myometrial invasion.
Acknowledgements
The authors thank Mrs. Joanne Chin MFA for her editorial assistance with the manuscript.
Funding
HY was supported by Japan Society for the Promotion of Science Overseas Research Fellowship (0690). LY, MJJ, LMM were partially supported by the NIH/NCI Cancer Center Support Grant P30 CA008748. The funding sources above had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Abbreviations
- EC
Endometrial cancer
- FIGO
International Federation of Gynecology and Obstetrics
- MRI
magnetic resonance imaging
- bpMRI
biparametric magnetic resonance imaging
- mpMRI
multiparametric magnetic resonance imaging
- MI
myometrial invasion
- CSI
cervical stromal involvement
- pLNM
pelvic lymphadenopathy
- mAD
malignant adnexal disease
- DWI
diffusion-weighted imaging
- DCE
dynamic contrast-enhanced
- TVR
tumor-to-uterine volume ratio
Footnotes
Compliance with Ethical Standards
Conflicts of interest
LY reports other from Y-mAbs Therapeutics, Inc., outside the submitted work. LMM reports personal fees from JnJ/Ethicon and is an ad hoc speaker for Intuitive Surgical, Inc., outside the submitted work. Other authors have nothing to disclose.
Ethical approval
This retrospective study was approved by the institutional review boards at each institution.
Informed consent
The requirement for informed consent was waived.
Availability of data and material
Anonymized data files may be sent on reasonable request.
Code availability
Not applicable.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA: a cancer journal for clinicians 68 (1):7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Colombo N, Creutzberg C, Amant F, Bosse T, Gonzalez-Martin A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C, Group E-E-EECCW (2016) ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology 27 (1):16–41. doi: 10.1093/annonc/mdv484 [DOI] [PubMed] [Google Scholar]
- 3.Tran BN, Connell PP, Waggoner S, Rotmensch J, Mundt AJ (2000) Characteristics and outcome of endometrial carcinoma patients age 45 years and younger. American journal of clinical oncology 23 (5):476–480 [DOI] [PubMed] [Google Scholar]
- 4.Lee NK, Cheung MK, Shin JY, Husain A, Teng NN, Berek JS, Kapp DS, Osann K, Chan JK (2007) Prognostic factors for uterine cancer in reproductive-aged women. Obstetrics and gynecology 109 (3):655–662. doi: 10.1097/01.AOG.0000255980.88205.15 [DOI] [PubMed] [Google Scholar]
- 5.Carneiro MM, Lamaita RM, Ferreira MC, Silva-Filho AL (2016) Fertility-preservation in endometrial cancer: is it safe? Review of the literature. JBRA assisted reproduction 20 (4):232–239. doi: 10.5935/1518-0557.20160045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebina Y, Katabuchi H, Mikami M, Nagase S, Yaegashi N, Udagawa Y, Kato H, Kubushiro K, Takamatsu K, Ino K, Yoshikawa H (2016) Japan Society of Gynecologic Oncology guidelines 2013 for the treatment of uterine body neoplasms. International journal of clinical oncology 21 (3):419–434. doi: 10.1007/s10147-016-0981-1 [DOI] [PubMed] [Google Scholar]
- 7.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C, Group EGW (2013) Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology 24 Suppl 6:vi33–38. doi: 10.1093/annonc/mdt353 [DOI] [PubMed] [Google Scholar]
- 8.Duska LR, Garrett A, Rueda BR, Haas J, Chang Y, Fuller AF (2001) Endometrial cancer in women 40 years old or younger. Gynecologic oncology 83 (2):388–393. doi: 10.1006/gyno.2001.6434 [DOI] [PubMed] [Google Scholar]
- 9.Evans-Metcalf ER, Brooks SE, Reale FR, Baker SP (1998) Profile of women 45 years of age and younger with endometrial cancer. Obstetrics and gynecology 91 (3):349–354 [DOI] [PubMed] [Google Scholar]
- 10.Group SGOCPECW, Burke WM, Orr J, Leitao M, Salom E, Gehrig P, Olawaiye AB, Brewer M, Boruta D, Herzog TJ, Shahin FA, Society of Gynecologic Oncology Clinical Practice C (2014) Endometrial cancer: a review and current management strategies: part II. Gynecologic oncology 134 (2):393–402. doi: 10.1016/j.ygyno.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network (2021) NCCN Clinical Practice Guidelines in Oncology Uterine Neoplasms NCCN Evidence Blocks.
- 12.Nougaret S, Horta M, Sala E, Lakhman Y, Thomassin-Naggara I, Kido A, Masselli G, Bharwani N, Sadowski E, Ertmer A, Otero-Garcia M, Kubik-Huch RA, Cunha TM, Rockall A, Forstner R (2019) Endometrial Cancer MRI staging: Updated Guidelines of the European Society of Urogenital Radiology. European radiology 29 (2):792–805. doi: 10.1007/s00330-018-5515-y [DOI] [PubMed] [Google Scholar]
- 13.Lin G, Huang YT, Chao A, Ng KK, Yang LY, Ng SH, Lai CH (2015) Influence of menopausal status on diagnostic accuracy of myometrial invasion in endometrial cancer: diffusion-weighted and dynamic contrast-enhanced MRI at 3 T. Clinical radiology 70 (11):1260–1268. doi: 10.1016/j.crad.2015.06.097 [DOI] [PubMed] [Google Scholar]
- 14.Sakane M, Hori M, Onishi H, Tsuboyama T, Ota T, Tatsumi M, Ueda Y, Kimura T, Kimura T, Tomiyama N (2018) Assessment of Myometrial Invasion in Premenopausal Grade 1 Endometrial Carcinoma: Is Magnetic Resonance Imaging a Reliable Tool in Selecting Patients for Fertility-Preserving Therapy? Journal of computer assisted tomography 42 (3):412–417. doi: 10.1097/RCT.0000000000000689 [DOI] [PubMed] [Google Scholar]
- 15.Navarria I, Usel M, Rapiti E, Neyroud-Caspar I, Pelte MF, Bouchardy C, Petignat P (2009) Young patients with endometrial cancer: how many could be eligible for fertility-sparing treatment? Gynecologic oncology 114 (3):448–451. doi: 10.1016/j.ygyno.2009.05.038 [DOI] [PubMed] [Google Scholar]
- 16.Walsh C, Holschneider C, Hoang Y, Tieu K, Karlan B, Cass I (2005) Coexisting ovarian malignancy in young women with endometrial cancer. Obstetrics and gynecology 106 (4):693–699. doi: 10.1097/01.AOG.0000172423.64995.6f [DOI] [PubMed] [Google Scholar]
- 17.Novellas S, Chassang M, Delotte J, Toullalan O, Chevallier A, Bouaziz J, Chevallier P (2011) MRI characteristics of the uterine junctional zone: from normal to the diagnosis of adenomyosis. AJR American journal of roentgenology 196 (5):1206–1213. doi: 10.2214/AJR.10.4877 [DOI] [PubMed] [Google Scholar]
- 18.Colombo N, Creutzberg C, Amant F, Bosse T, Gonzalez-Martin A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C (2016) ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology 27 (1):16–41. doi: 10.1093/annonc/mdv484 [DOI] [PubMed] [Google Scholar]
- 19.Nougaret S, Horta M, Sala E, Lakhman Y, Thomassin-Naggara I, Kido A, Masselli G, Bharwani N, Sadowski E, Ertmer A, Otero-Garcia M, Kubik-Huch RA, Cunha TM, Rockall A, Forstner R (2018) Endometrial Cancer MRI staging: Updated Guidelines of the European Society of Urogenital Radiology. European radiology. doi: 10.1007/s00330-018-5515-y [DOI] [PubMed] [Google Scholar]
- 20.Lin G, Huang YT, Chao A, Lin YC, Yang LY, Wu RC, Lu HY, Ng SH, Ng KK, Lai CH (2017) Endometrial cancer with cervical stromal invasion: diagnostic accuracy of diffusion-weighted and dynamic contrast enhanced MR imaging at 3T. European radiology 27 (5):1867–1876. doi: 10.1007/s00330-016-4583-0 [DOI] [PubMed] [Google Scholar]
- 21.Nakao Y, Yokoyama M, Hara K, Koyamatsu Y, Yasunaga M, Araki Y, Watanabe Y, Iwasaka T (2006) MR imaging in endometrial carcinoma as a diagnostic tool for the absence of myometrial invasion. Gynecologic oncology 102 (2):343–347. doi: 10.1016/j.ygyno.2005.12.028 [DOI] [PubMed] [Google Scholar]
- 22.Kinkel K, Forstner R, Danza FM, Oleaga L, Cunha TM, Bergman A, Barentsz JO, Balleyguier C, Brkljacic B, Spencer JA, European Society of Urogenital I (2009) Staging of endometrial cancer with MRI: guidelines of the European Society of Urogenital Imaging. European radiology 19 (7):1565–1574. doi: 10.1007/s00330-009-1309-6 [DOI] [PubMed] [Google Scholar]
- 23.Nougaret S, Reinhold C, Alsharif SS, Addley H, Arceneau J, Molinari N, Guiu B, Sala E (2015) Endometrial Cancer: Combined MR Volumetry and Diffusion-weighted Imaging for Assessment of Myometrial and Lymphovascular Invasion and Tumor Grade. Radiology 276 (3):797–808. doi: 10.1148/radiol.15141212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44 (3):837–845 [PubMed] [Google Scholar]
- 25.Simundic AM (2009) Measures of Diagnostic Accuracy: Basic Definitions. Ejifcc 19 (4):203–211 [PMC free article] [PubMed] [Google Scholar]
- 26.McHugh ML (2012) Interrater reliability: the kappa statistic. Biochemia medica 22 (3):276–282 [PMC free article] [PubMed] [Google Scholar]
- 27.Lin LI (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45 (1):255–268 [PubMed] [Google Scholar]
- 28.McBride G (2005) A proposal for strength-of-agreement criteria for Lin. s. Concordance Correlation Coefficient. https://www.medcalc.org/download/pdf/McBride2005.pdf.
- 29.Andreano A, Rechichi G, Rebora P, Sironi S, Valsecchi MG, Galimberti S (2014) MR diffusion imaging for preoperative staging of myometrial invasion in patients with endometrial cancer: a systematic review and meta-analysis. European radiology 24 (6):1327–1338. doi: 10.1007/s00330-014-3139-4 [DOI] [PubMed] [Google Scholar]
- 30.Emlik D, Kiresi D, Ozdemir S, Celik C, Karakose S (2010) Preoperative assessment of myometrial and cervical invasion in endometrial carcinoma: comparison of multi-section dynamic MR imaging using a three dimensional FLASH technique and T2-weighted MR imaging. Journal of medical imaging and radiation oncology 54 (3):202–210. doi: 10.1111/j.1754-9485.2010.02160.x [DOI] [PubMed] [Google Scholar]
- 31.Du L, Li X, Qiu X, Liu X, Wang Y, Yu Y (2016) Application of FLASH-3D dynamic contrast-enhanced imaging for diagnosis of endometrial carcinoma. The British journal of radiology 89 (1066):20160268. doi: 10.1259/bjr.20160268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin G, Ng KK, Chang CJ, Wang JJ, Ho KC, Yen TC, Wu TI, Wang CC, Chen YR, Huang YT, Ng SH, Jung SM, Chang TC, Lai CH (2009) Myometrial invasion in endometrial cancer: diagnostic accuracy of diffusion-weighted 3.0-T MR imaging--initial experience. Radiology 250 (3):784–792. doi: 10.1148/radiol.2503080874 [DOI] [PubMed] [Google Scholar]
- 33.Rockall AG, Meroni R, Sohaib SA, Reynolds K, Alexander-Sefre F, Shepherd JH, Jacobs I, Reznek RH (2007) Evaluation of endometrial carcinoma on magnetic resonance imaging. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society 17 (1):188–196. doi: 10.1111/j.1525-1438.2007.00805.x [DOI] [PubMed] [Google Scholar]
- 34.Xu X, Li N, Chen Y, Ouyang H, Zhao X, Zhou J (2019) Diagnostic efficacy of MRI for pre-operative assessment of ovarian malignancy in endometrial carcinoma: A decision tree analysis. Magnetic resonance imaging 57:285–292. doi: 10.1016/j.mri.2018.12.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


