Abstract
Study Objectives:
Giftedness is a multidimensional condition. It is increasingly put forward that gifted children (GC) could be a population at high risk for sleep problems. The current study investigated GC and typically developing children for their habitual sleep, night-to-night sleep variability, and parental reports of child sleep.
Methods:
The sample consisted of 62 GC (31 girls; mean age = 9.63 ± 1.71 years) and 62 typically developing children (31 girls; mean age = 9.68 ± 1.68 years). Groups were age and sex matched. Giftedness was identified using Renzulli’s 3-factor definition of giftedness. Sleep duration, quality, and night-to-night variability were assessed using actigraphy. Parents were asked to complete the short-form version of the Children’s Sleep Habits Questionnaire to report on their child’s sleep. Groups were compared with independent sample t-tests and chi-square analyses.
Results:
GC displayed lower sleep efficiencies, more wake time after sleep onset, and more night-to-night sleep variability than typically developing children. GC were found to experience less social jetlag compared to typically developing children, and they also showed more clinically significant sleep problems as reported by parents.
Conclusions:
Sleep maintenance and stability tend to be challenged in GC. While there is growing evidence that greater sleep variability is associated with poorer physical and emotional health, studies have yet to examine these associations in GC specifically to get a better understanding of giftedness. Overall, there is a need for research focused on both predictors and consequences of sleep patterns and sleep variability in GC.
Citation:
Bastien L, Théoret R, Bernier A, Godbout R. Habitual sleep and intraindividual variability of sleep in gifted children: an actigraphy study. J Clin Sleep Med. 2023;19(5):925–934.
Keywords: sleep/wake patterns, night-to-night sleep variability, social jetlag, giftedness
BRIEF SUMMARY
Current Knowledge/Study Rationale: It has been reported since the 1970s that gifted children have sleep difficulties. Yet, only a handful of studies have investigated sleep in gifted children and the few that did relied on a narrow and outdated definition of giftedness (ie, intelligence quotient cut-off scores).
Study Impact: We used a multidimensional approach to identify giftedness. Objective measures of sleep (actigraphy) revealed that compared to typically developing children, sleep maintenance and stability were impaired in gifted children and parental reports identified more clinically significant sleep problems. Given the association between sleep and well-being, these results point toward the need to include sleep in the clinical assessment of gifted children, searching beyond sleep duration by including awakenings and night-to-night sleep variability.
INTRODUCTION
Giftedness is a multidimensional condition characterized by 3 interconnected spheres that equally contribute to this trait.1 The first sphere refers to above-average ability, that is, high levels of performance in areas such as abstract thinking, reasoning, and memory as well as the capacity to apply various combinations of these abilities to one or more specialized areas of knowledge (eg, math, biology) or performance (eg, arts, sports). The second sphere includes high levels of task commitment, namely a refined or focused form of motivation that is brought to bear on a particular problem (task) or specific performance area. Finally, giftedness encompasses high levels of creativity, characterized by the realization of original productions, creation of innovative solutions to solve problems, openness to experience, and willingness to take risks in thought and action.
Gifted children (GC) are thought to be challenged by an atypical, asynchronous pattern of development. For instance, GC are characterized by superior intellectual development compared to peers, while socioemotional development corresponds to age norms. Different social and emotional needs arise from the asynchronous development profile of GC.2 Indeed, GC are frequently referred to pediatric clinics for socioemotional problems and/or school difficulties.3,4 Emotional and behavioral problems as well as relationship difficulties with peers and family have been found to be more frequent in GC compared to typically developing children (TDC).5,6 Empirical data also suggest that GC are less adapted to their environment and society.7 In addition, many GC experience school failures, and they are 3 times more likely to drop out of school due to lack of interest, boredom, and frustration generated by the slow learning pace of regular classes.4 Hence, to maximize the chances that GC develop their full intellectual and socioemotional potential, it is essential to better understand the unique set of characteristics and factors that make these children at risk for adjustment difficulties.
Given its role in development and cognition,8 sleep may be an especially promising factor to investigate in this regard. Indeed, it is increasingly well documented that different features of sleep, such as its duration, quality, variability, and timing are associated with psychological symptoms (eg, anxiety, depression, social problems, externalizing behavior problems), cognitive functioning [eg, sustained attention, working memory, overall intelligence quotient (IQ)], academic achievement, and physical health.8,9 Therefore, if GC have more sleep difficulties than their peers, this could partly explain why they are at higher risk for maladjustment.
Only a handful of studies have investigated sleep in GC, and the few that did relied on a narrow and outdated definition of giftedness (ie, the IQ score cut-off approach), making it extremely difficult to determine the true extent of sleep difficulties in this population. Using parental reports, studies have shown that 33–50% of children with high IQ (≥ 160) require less sleep than TDC.4,10 Regarding sleep quality, Loureiro et al6 found that 84% of children with high intelligence (IQ ≥ 125) were experiencing parent-reported sleep problems (ie, difficulties falling asleep, nocturnal awakenings, nightmares) compared to 23.3% of TDC (IQ < 125). Revol et al11 reported that children with high IQ (≥ 130) often complained about their sleep (35% vs 9% of TDC), including difficulties falling asleep, night awakenings, and short sleep duration. Guignard-Perret et al12 reported that 52% of parents of children with high intelligence (IQ ≥ 130) reported sleep complaints in their child, mainly insomnia, compared to 12% of control parents. Only 3 studies have examined sleep in children with high IQs using objective sleep measures such as polysomnography (Busby and Pivik13: IQ ≥ 129, n = 6; Guignard-Perret et al12: IQ ≥ 130, n = 33; Grubar14: IQ ≥ 137, n = 5). Results will not be discussed further, as they were sparse, contradictory, and based on small samples.
The studies cited above are consistent with the hypothesis that giftedness could be linked to sleep disturbances. However, in all these previous studies, giftedness was solely defined on the basis of IQ tests. As the field of giftedness evolves, theorists now acknowledge that giftedness is multidimensional and consider as outdated the traditional early notions that intellectual giftedness can be equated with a high score on 1 assessment, as indexed by IQ measurement. Rather, multiple selection criteria for giftedness are recommended.15 Only 1 recent publication16 investigated sleep in GC using a multidimensional approach (3-ring conception of giftedness1: intellectual ability, task commitment, creativity). Based on parental reports of sleep, being in the GC group increased by 4.67 times the risk of having sleep problems compared to the TDC group. Moreover, the GC fell asleep faster while their sleep duration was shorter and more variable from night to night.
In previous works on sleep and giftedness, sleep was mostly assessed using subjective measures, which are known to show poor correspondence with objective sleep measures, likely due to parental biases and the fact that parents may not be aware of their child’s awakenings.17–20 Moreover, objective sleep measures can generate more details on sleep, including its stability and internal structure. Thus, it is relevant to study the sleep/wake patterns of GC using an objective sleep measure and a multidimensional approach to the identification of giftedness.
While sleep duration and sleep quality are the most commonly investigated sleep variables, research increasingly demonstrates that intraindividual variability (IIV) of sleep/wake patterns (ie, night-to-night changes in the same individual’s sleep) may have unique implications for adjustment.21,22 One particular form of sleep IIV among children is the difference between school and nonschool days, with typically earlier bedtimes and shorter sleep duration on nonschool days.9,23,24 This specific form of IIV is referred to as social jetlag. Considering that sleep IIV is gaining recognition as a relevant factor to consider for the promotion of a healthy lifestyle, it is becoming an important variable to include when examining sleep/wake patterns.
The current study aimed to investigate sleep/wake patterns and sleep IIV in a rigorously identified sample of GC, compared with age-matched and sex-matched TDC controls. To address the limitations of previous studies, we used actigraphy as the objective measure of sleep and a multidimensional approach to the identification of giftedness rather than the IQ unidimensional approach. To compare results of the present study with past literature, parental reports on the sleep of their children were also administered. We predicted that GC would have shorter sleep duration and poorer sleep quality than TDC. We also expected higher rates of parental sleep complaints in the GC group compared to the TDC group. Due to the lack of previous studies investigating sleep IIV in GC, no a priori hypotheses were formulated, and the analyses were thus exploratory.
METHODS
Participants
We recruited 62 GC (31 girls and 31 boys: mean age = 9.63 ± 1.71 years) through advertisements on social medias related to giftedness. Giftedness was identified by a clinical neuropsychologist based on the most research-supported and clinically used model (3-ring conception of giftedness1). Inclusion criteria included (1) a minimal total IQ score of 120; (2) parental reports of high levels of task commitment (ie, perseverance, hard work, endurance, and involvement in particular domains); and (3) parental reports of high levels of creativity (ie, flexibility, fluency, originality of thought, sensitivity to stimulation, openness to experiences, and a willingness to take risks). The presence of a medical or psychiatric condition at the time of the study was an exclusion criterion, as confirmed through a semistructured interview with parents. GC were mostly White (67.7%), generally spoke French at home (82.3%), and most (77.4%) had siblings.
The TDC group consisted of 62 children (31 girls and 31 boys: mean age = 9.68 ± 1.68 years) matched for age and sex with the GC group (see Table 1). The TDC sample was drawn from a TDC actigraphy databank. The matching procedure was done on an individual basis, blind to children’s sleep outcomes, ensuring that each GC had a sex-matched and age-matched TDC counterpart. TDC were mostly White (72.6%), generally spoke French at home (80.6%), and almost all (93.5%) had siblings.
Table 1.
Sample characteristics of typically developing children and participants with giftedness.
| TDC (n = 62) | Gifted (n = 62) | t / X2 | df | P a | |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 9.68 ± 1.68 | 9.63 ± 1.71 | −0.178 | 122 | .886 |
| Sex, n | |||||
| Female | 31 | 31 | .000 | 122 | 1.000 |
| Male | 31 | 31 |
Results from independent samples t-test and chi-square test. df = degrees of freedom, TDC = typically developing children.
Parent-completed child sleep diaries were closely examined as part of the data analysis process and abnormalities were investigated; when parents mentioned the presence of a sleep disorder on the diary, or did so spontaneously, the child was excluded from the analyses. Moreover, parents of GC completed a house questionnaire used by one of the authors (RG) at the Sleep Clinic for screening sleep disorders. None of the GC presented behaviors compatible with insomnia, restless legs syndrome, obstructive sleep apnea, or circadian rhythm sleep disorders.
Procedure
In both groups, once parent consent and child assent were obtained, children were asked to follow their typical sleep schedule for up to 1 week while wearing an actigraph on their nondominant wrist. Sleep diaries were completed each night and morning by parents to support the scoring of actigraphy data. The diary provided information about the child’s sleep bedtime, wake time, and any event that might have disturbed the sleep period (eg, illness, medication, visitors at home). To reduce potential confounds, children’s sleep in both groups was examined during the regular academic year. Parents were also asked to complete the short-form version of the Children’s Sleep Habits Questionnaire to report on their child’s sleep.25 Ethical approval was obtained from the Research Ethics Committees of the CIUSSS du Nord-de-l’Île-de-Montréal (#18–22P; GC group) and Université de Montréal (CERFAS-2012-13-004-D; TDC group). All participants received financial compensation for their involvement in this study.
Instruments
Actigraphy was recorded over 7 consecutive nights for the GC group and 3 to 7 consecutive nights for the TDC group. More specifically, the length of recording in the TDC varied according to age: 3 nights of actigraphy for children aged from 6 to 7 years old (n = 10) to maximize compliance and reduce family burden, and 7 nights in children aged 8 years and above. Although Acebo et al26 reported that 5 nights of actigraphy recordings can generate reliable measures, a closer inspection of their data reveals that acceptable levels of reliability are obtained with 3 nights of assessment. We also decided to include participants with 3 nights of actigraphy in line with prior actigraphy research with young children.27
Actigraphy
Actigraphy consists of a small wireless watch-like device and evaluates sleep noninvasively from motor activity through an accelerometer that continuously records child movements. Based on the premise that sleep is accompanied by minimal physical activity, motor data are converted into estimates of sleep (below a predetermined activity threshold) and wake (above a predetermined activity threshold) periods (see Meltzer et al28 for more details on how sleep/wake cycles are estimated from activity data).
Two models of actigraphs, both manufactured by Philips Respironics, were used: the Actiwatch-64 (GC group) and the Actiwatch-2 (TDC group). A Respironics report showed that these 2 actigraph models can be used interchangeably to compute sleep statistics when used with the Actiware software algorithms.29 Both models show satisfactory concordance with polysomnography in school-age children.28 Actigraphy data was computed into 30-second epochs. Trained graduate research assistants scored all actigraphy data using the manufacturer’s scoring algorithm set at the low sensitivity threshold (80 activity counts per epoch). This sensitivity threshold shows satisfactory sensitivity and accuracy when compared to polysomnographic data and higher specificity than the medium and high thresholds.30 In addition, the low threshold is well suited to account for children’s enhanced motor activity during sleep.31 Sleep onset and sleep offset were defined manually by the assistants, based on visual examination of the actogram guided by the bed and rise times indicated on the sleep diary. Each actogram was scored by 1 assistant. The assistants were aware of group membership but blind to this study’s objectives and hypotheses.
Sleep variables were (1) sleep onset latency (time elapsed between the parent’s indication of lights out on the sleep diary and the actigraphy-assessed sleep onset time); (2) wake time after sleep onset (minutes spent awake between sleep onset time and final awakening); (3) total sleep time (time spent sleeping between sleep onset time and final awakening); (4) sleep efficiency (percentage of time spent asleep between sleep onset time and the final awakening); and (5) sleep midpoint (clock time halfway between bedtime and final awakening). Individual means and standard deviations, as a measure of night-to-night sleep variability,22 were separately calculated for school nights (Sunday–Thursday nights), weekend nights (Friday and Saturday nights), and the whole week of assessment. Social jetlag, operationalized as the difference between sleep midpoint on weekdays and the weekend, was also computed.
Among GC, 45 children had 7 nights of valid sleep data, 10 had 6 nights, 3 had 5 nights, 2 had 4 nights and 2 had 3 nights (M = 6.52, SD = 0.97). Among TDC, 32 children had 7 nights of valid data, 12 had 6 nights, 7 had 5 nights and 10 had 3 nights (M = 5.92, SD = 1.46). Participants whose actigraphy recordings did not include at least 2 school nights and 2 weekend nights were excluded from the sleep IIV analyses.21,22 Therefore, IIV analyses were performed on 55 gifted and 36 control participants. Although groups were no longer perfectly age and sex matched for these analyses, they remained equivalent (P > .45), and all variables were still normally distributed.
Parental reports
Parents were asked to complete the short-form version of the Children’s Sleep Habits Questionnaire, a 23-item questionnaire assessing sleep behavior.25 It comprises subscales responsive to behavioral interventions, namely bedtime resistance, sleep onset latency, sleep duration, sleep anxiety, night awakenings, and daytime sleepiness. The modified short-form version demonstrates excellent psychometric properties.25 Items are rated on a 3-point scale: “usually” (5–7 times/week = 3), “sometimes” (2–4 times/week = 2), and “rarely” (0–1 time/week = 1), with total scores ranging from 23 to 69. A total score ≥ 30 discriminates children with and without sleep disorders with acceptable sensitivity and specificity.25
Statistical analyses
All variables showed satisfactory variability and normal distributions adequate for parametric statistical analyses. Group differences in sample characteristics were explored using t tests for independent samples and chi-square analyses. Independent samples t tests were also used to compare means and IIV of each sleep variable for school nights, weekend nights, and the whole week of recording. Finally, the difference in sleep midpoint between school nights and weekend nights (social jetlag) in GC was compared to that in TDC using independent samples t tests. The significance of alpha value was set at .05. Cohen’s d was calculated as a measure of effect size (d = MTDC−MGC/SDpooled), considering d = 0.2 as small, d = 0.5 as moderate, and d = 0.8 as large effect sizes.32 Power analysis (GPower, version 3.1.9.2) revealed that to detect a medium effect (80% probability) as significant at the .05 P level, a sample of 64 participants in each group would have been required. Our actual sample size (62 GC and 62 TDC) yielded a 79% probability of detecting a medium effect size at the .05 P level.
RESULTS
Basic sleep parameters
Table 2 shows there were no significant group differences on sleep latency for school nights, weekend nights, or for the whole week of recording. Wake time after sleep onset was significantly higher in the GC group compared to the TDC group on school nights and for the whole week, with a comparable trend (P = .054, Cohen’s d = 0.38) during weekends. Total sleep time was not different between groups on school nights, weekend nights, or the whole week. Sleep efficiency was lower in the GC group compared to the TDC group for the whole week, with a comparable trend on school nights (P = .052, Cohen’s d = 0.36), whereas no significant differences were observed on weekend nights. Bedtime did not differ between groups, but GC woke up significantly later than TDC on school days, and sleep midpoint was marginally later in GC on school nights compared to TDC (P = .061, Cohen’s d = 0.34).
Table 2.
Basic sleep parameters measured with actigraphy based on one week of recording in typically developing and gifted children.
| TDC (n = 62) | Gifted (n = 62) | t | df | P | Cohen’s d | |
|---|---|---|---|---|---|---|
| mean ± SD | mean ± SD | |||||
| SOL (min) | ||||||
| School nights | 28 ± 16 | 32 ± 22 | −1.219 | 117 | .225 | 0.22 |
| Weekendsa | 27 ± 23 | 35 ± 25 | 1.688 | 99 | .095 | 0.32 |
| Total | 27 ± 15 | 32 ± 22 | 1.307 | 117 | .194 | 0.23 |
| WASO (min) | ||||||
| School night | 31.94 ± 13 | 39.12 ± 18 | −2.557 | 121 | .012 | 0.46 |
| Weekendsa | 30.84 ± 12 | 36.52 ± 18 | 1.951 | 109 | .054 | 0.38 |
| Total | 31.65 ± 12 | 39.11 ± 18 | 2.748 | 121 | .007 | 0.50 |
| TST (min) | ||||||
| School nights | 523.47 ± 34 | 523.54 ± 35 | −0.012 | 121 | .991 | 0.00 |
| Weekendsa | 520.62 ± 52 | 515.83 ± 50 | −0.496 | 109 | .621 | 0.09 |
| Total | 523.34 ± 32 | 523.59 ± 34 | 0.042 | 121 | .966 | 0.01 |
| SE (%) | ||||||
| School nights | 93.87 ± 2 | 92.92 ± 3 | 1.966 | 121 | .052 | 0.36 |
| Weekends1 | 94.06 ± 2 | 93.23 ± 3 | −1.623 | 109 | .107 | 0.32 |
| Total | 93.92 ± 2 | 92.92 ± 3 | −2.149 | 121 | .034 | 0.39 |
| Bedtime (hh:mm ± min) | ||||||
| School nights | 21:04 ± 35 | 21:15 ± 62 | −1.294 | 122 | .198 | 0.23 |
| Weekendsa | 21:56 ± 59 | 21:46 ± 66 | 0.826 | 110 | .411 | 0.25 |
| Total | 21:15 ± 37 | 21:26 ± 62 | −0.054 | 122 | .957 | 0.01 |
| Wake-up time (hh:mm ± min) | ||||||
| School nights | 6:22 ± 30 | 6:41 ± 56 | −2.285 | 122 | .024 | 0.42 |
| Weekendsa | 7:10 ± 53 | 7:02 ± 73 | 0.713 | 110 | .477 | 0.14 |
| Total | 6:33 ± 29 | 6:41 ± 56 | −0.972 | 122 | .333 | 0.14 |
| MP (hh:mm ± min) | ||||||
| School nights | 01:43 ± 29 | 01:58 ± 56 | −1.895 | 122 | .061 | 0.34 |
| Weekendsa | 02:33 ± 50 | 02:24 ± 67 | −0.763 | 110 | .447 | 0.15 |
| Total | 1:54 ± 29 | 1:58 ± 56 | 0.503 | 122 | .616 | 0.09 |
The table shows mean ± SD results from independent samples t-tests and Cohen’s d as a measure of effect size. aWeekend actigraphy data were missing for 10 participants in the typically developing group and 2 participants in the gifted group, leaving 52 control and 60 gifted participants for these analyses. df = degrees of freedom, MP = midpoint, ie, halfway between bedtime and final awakening, SE = sleep efficiency, SOL = sleep onset latency, TDC = typically developing children, TST = total sleep time, WASO = wake after sleep onset.
Intraindividual variability
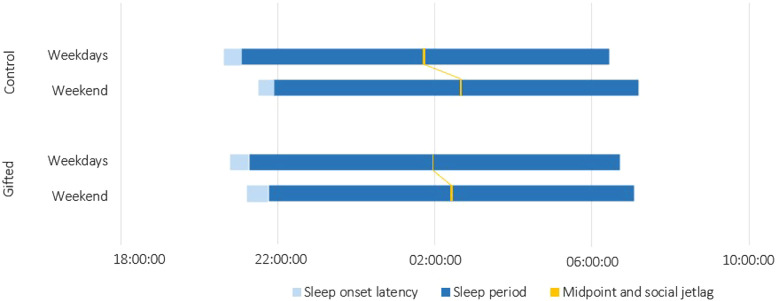
Table 3 shows that sleep onset latency IIV in GC was larger than in TDC on weekend nights, whereas no significant differences were observed on school nights and the whole week. Values for wake time after sleep onset IIV were greater in GC only on school nights and the whole week. There were no significant group differences for total sleep time IIV, although values were marginally higher in GC during school nights (P = .066, Cohen’s d = 0.41). Similarly, there were no significant group differences for sleep efficiency IIV, although values were marginally higher in GC on school nights (P = .087, Cohen’s d = 0.38) and for the whole week (P = .052, Cohen’s d = 0.44). Bedtime IIV was smaller in GC than TDC during the whole week. Wake-up time IIV was larger on school nights in GC but was smaller on weekends, such that the total week showed no significant differences. The sleep midpoint IIV value was lower in GC for the whole week of recording, with a comparable trend for weekend nights (P = .052, Cohen’s d = 0.04). Finally, social jetlag was significantly lower in the GC group compared to the TDC group (Figure 1).
Table 3.
Intraindividual variability and social jetlag measured with actigraphy based on 1 week of recording in typically developing and gifted children.
| TDC (n = 36)a | Gifted (n = 55)a | t | df | P | Cohen’s d | |
|---|---|---|---|---|---|---|
| mean ± SD | mean ± SD | |||||
| SOL (min) | ||||||
| School nights | 12 ± 10 | 14 ± 11 | −1.062 | 84 | .291 | 0.00 |
| Weekendsa | 9 ± 7 | 16 ± 14 | −2.353 | 63 | .022 | 0.63 |
| Total | 14 ± 8 | 16 ± 10 | −1.010 | 84 | .315 | 0.27 |
| WASO (min) | ||||||
| School nights | 6 ± 3 | 8 ± 4 | −2.156 | 88 | .034 | 0.47 |
| Weekendsa | 7 ± 4 | 9 ± 7 | −1.212 | 88 | .229 | 0.28 |
| Total | 7 ± 3 | 9 ± 5 | −2.565 | 88 | .012 | 0.59 |
| TST (min) | ||||||
| School nights | 24 ± 11 | 28 ± 12 | −1.860 | 88 | .066 | 0.41 |
| Weekendsa | 42 ± 36 | 37 ± 27 | 0.751 | 88 | .454 | 0.16 |
| Total | 34 ± 14 | 34 ± 12 | −0.082 | 88 | .935 | 0.02 |
| SE (%) | ||||||
| School nights | 1.04 ± 0.6 | 1.28 ± 0.7 | −1.729 | 88 | .087 | 0.38 |
| Weekendsa | 1.23 ± 0.8 | 1.29 ± 1.1 | −0.316 | 88 | .753 | 0.07 |
| Total | 1.16 ± 0.5 | 1.42 ± 0.7 | −1.967 | 88 | .052 | 0.44 |
| Bedtime (min) | ||||||
| School nights | 22 ± 12 | 20 ± 11 | 0.906 | 89 | .367 | 0.21 |
| Weekendsa | 33 ± 29 | 29 ± 27 | 0.682 | 89 | .497 | 0.15 |
| Total | 38 ± 17 | 31 ± 13 | 2.209 | 89 | .030 | 0.48 |
| Wake-up time (min) | ||||||
| School nights | 16 ± 10 | 24 ± 14 | −2.815 | 89 | .006 | 0.64 |
| Weekendsa | 34 ± 28 | 23 ± 18 | 2.279 | 89 | .025 | 0.46 |
| Total | 35 ± 20 | 30 ± 16 | 1.271 | 89 | .207 | 0.27 |
| MP (min) | ||||||
| School nights | 17 ± 10 | 17 ± 11 | −0.030 | 89 | .291 | 0.00 |
| Weekendsa | 26 ± 22 | 19 ± 15 | 1.971 | 89 | .052 | 0.42 |
| Total | 33 ± 16 | 24 ± 13 | 2.998 | 89 | .004 | 0.63 |
| Social jetlag (min) | 55 ± 40 | 33 ± 29 | 3.343 | 110 | .001 | 0.62 |
The table shows mean ± SD results from independent samples t-tests and Cohen’s d as a measure of effect size. aParticipants whose actigraphy data did not include at least 2 school nights and 2 weekend nights were excluded of the intraindividual variability analyses, leaving 36 control and 55 gifted participants for these analyses. df = degrees of freedom, MP = midpoint, ie, halfway between bedtime and final awakening, SE = sleep efficiency, SOL = sleep onset latency, TDC = typically developing children, TST = total sleep time, WASO = wake after sleep onset.
Figure 1. Habitual sleep and social jetlag measured with actigraphy based on 1 week of sleep recording in typically developing and gifted children.
Weekend actigraphy data were missing for 10 participants in the control group and two participants in the gifted group, leaving 52 control and 60 gifted participants for the weekend sleep pattern analyses.
Parental sleep reports
Compared to TDC, GC were considered by their parents as having significantly more sleep problems. On individual scales, GC were found to take more than 20 minutes to fall asleep significantly more often, to have more bedtime resistance, and more night awakenings compared to TDC. No differences were found between groups on sleep duration, sleep anxiety, and daytime sleepiness (Table 4).
Table 4.
Parental sleep reports (SF-CSHQ) in typically developing children and participants with giftedness.
| TDC (n = 52) | Gifted (n = 60) | t | df | P | Cohen’s d | |
|---|---|---|---|---|---|---|
| mean ± SD | mean ± SD | |||||
| Bedtime resistance | 6.22 ± 1.86 | 7.37 ± 2.31 | −2.833 | 108 | .006 | 0.43 |
| Sleep onset latency | 1.31 ± 0.64 | 1.73 ± 0.82 | −3.021 | 110 | .003 | 0.57 |
| Sleep duration | 3.81 ± 1.24 | 3.75 ± 1.23 | 0.247 | 110 | .805 | 0.05 |
| Sleep anxiety | 5.18 ± 1.63 | 5.33 ± 1.84 | −0.471 | 109 | .638 | 0.09 |
| Night awakenings | 3.52 ± 0.85 | 3.95 ± 1.40 | −1.935 | 110 | .048 | 0.37 |
| Daytime sleepiness | 9.72 ± 2.32 | 9.63 ± 2.19 | 0.201 | 108 | .841 | 0.04 |
| Total | 27.17 ± 5.22 | 29.55 ± 6.12 | −2.144 | 106 | .034 | 0.42 |
The table shows mean ± SD, results from independent samples t-tests and Cohen’s d as a measure of effect size. df = degrees of freedom, SF-CSHQ = short-form version of the Children’s Sleep Habit Questionnaire, TDC = typically developing children.
DISCUSSION
It is increasingly proposed that GC could be a population at high risk for sleep problems.4 The current study investigated habitual sleep, night-to-night sleep variability, and parental reports of sleep in GC, while considering giftedness as a multidimensional condition. We hypothesized that GC would have shorter sleep durations and poorer sleep quality than TDC. We also predicted higher rates of parent-reported sleep problems in the GC group compared with an age-matched and sex-matched TDC group. The clearest finding to emanate from this study is that GC have lower sleep efficiency, characterized by sleep maintenance problems, and more sleep IIV than TDC. Additionally, we found GC to experience less social jetlag compared to TDC. As expected, GC also showed more clinically significant sleep problems reported by parents.
Sleep quality and intraindividual variability
As previous research using subjective measures showed (eg, Loureiro et al6), parental reports of sleep in GC revealed longer sleep onset latencies compared to TDC. Moreover, actigraphy data indicated that mean sleep onset latency in the GC group extended above 30 minutes, pointing toward possible sleep initiation problems,33 whereas the mean of the TDC group did not. One possibility is that the high level of creativity that characterizes the gifted profile may explain parental reports of their child’s difficulties to fall asleep and why GC exceed, on average, the 30-min threshold indicative of problematic sleep onset. Parents of GC indeed frequently report that their child has difficulty “turning off his/her mind” to fall asleep, especially when in the midst of exciting projects. These children’s intellectual and/or emotional overexcitability would prompt their mind to churn for a while after they go to bed.4 Thus, highly creative children would be at risk of developing difficulties in initiating and maintaining sleep given the cognitive processing, the level of mental activity, and the amount of time that is allocated to planning and executing creative works. In fact, Healey and Runco34 observed that highly creative school-age children report more symptoms of insomnia compared to a control group. As children with giftedness are highly creative by definition according to the 3-ring conception of giftedness,1 creativity may account for the bedtime resistance and difficulty initiating sleep observed among GC.
Whereas the average time it takes GC to fall asleep could be clinically meaningful, actigraphy data indicated no significant differences between groups for sleep onset latency (although the observed difference was in the same direction, with a small effect size). One should bear in mind that GC frequently play quietly (reading, homework completion, game playing) before bedtime.4 States of quiet wakefulness are threats to the reliability of actigraphic measures of sleep latency.35 This might explain why actigraphy data failed to reveal group differences on sleep onset latency while parental reports did. A more accurate estimation of sleep onset latency could require at least 14 days of monitoring.36
In line with past findings, parental reports and actigraphy revealed poorer sleep quality, characterized by more wake time after sleep onset, in GC compared to TDC. While the means of wake time after sleep onset for both groups fell below the cut-off of 41 min indicative of a sleep maintenance problem,33 GC were found to have significantly more wake time after sleep onset compared to TDC. A novel corroborative finding of the present study was that GC showed more variability in wake time after sleep onset compared to TDC. One possible explanation for lower and more variable sleep efficiency in GC is their higher levels of sensitivity to the environment. Indeed, it is generally accepted that GC react overly to stimuli because their intellectual precocity allows them to be more aware as well as to understand and perceive events more sharply than TDC.4 However, the asynchrony between their intellectual and emotional development prevents GC from appropriately regulating their heightened emotional arousal.2 Because they are more easily distracted and have more difficulty disengaging from internal (eg, thoughts) and external (eg, darkness, noise) stimuli, children with higher emotional sensitivity may be especially prone to sleep fragmentation and inconsistent sleep patterns.37,38 Therefore, the emotional reactivity of GC may interfere with their sleep.
Interestingly, nocturnal awakenings were longer and more variable among GC compared to TDC according to actigraphy data and more so on school nights. It may be that GC experience greater stress on school nights due to school challenges specific to the gifted population, such as social fears (feeling less socially skilled than their peers), “maladaptive” perfectionism, underinvestment of teachers who neglect the special needs of GC, or stereotypes and stigma associated with giftedness.39 Greater stress on school nights may also potentiate the occurrence of other problematic behaviors,40 which may make it more difficult for parents of GC to enforce a regular sleep routine, resulting in more sleep IIV and worse sleep quality on school nights.
Sleep duration
Based on parental assessments of sleep, some studies have suggested that GC need less sleep than TDC (eg, Silverman and Kearney10), whereas others have not.41 In the current sample, parental reports and objective assessment of sleep revealed very similar sleep duration in children with and without giftedness. Differences in the literature could likely be explained by sampling bias. Studies that have found shorter sleep duration in GC tend to report on samples of children with a measured IQ of 160 and above. This can introduce significant bias in findings and reduce the generalizability of findings to the broader population of GC. Although more research is needed with objective assessments of sleep among GC as now defined,1 the current results suggest that mean duration may not be a key aspect of sleep that differentiates GC and TDC.
Sleep timing
We found GC to experience less social jetlag compared to TDC. At first glance, this result appears to suggest a better match between GC’s biological clock and morning school schedules, which would be consistent with previous studies investigating diurnal preference in GC. Demirhan et al42 showed, for example, that children with a high IQ (≥ 130) were more morning-oriented compared to TDC (IQ < 130), meaning that they prefer early rising and become sleepy earlier in the evening than TDC. Similarly, Arbabi and colleagues43 found that intelligence was positively related to morningness orientation and that a morning-oriented type was associated with earlier midpoint of sleep and less social jetlag. However, our results revealed that, in fact, GC woke up significantly later than TDC on school mornings, although bedtime and sleep onset latency did not differ significantly from TDC. Furthermore, GC had more wake time after sleep onset and a trend toward a later sleep midpoint. Apart from the fact that such discrepancies in findings across studies may point toward differences between children with multidimensional giftedness and those with unidimensional high intelligence, several possibilities need to be investigated to explain this unexpected sleep timing pattern. One possibility is the impact of being involved in multiple competitions, clubs, or other activities such as sports, theater, art classes, and music lessons.44,45 Anecdotally, we observed this high participation rate in out-of-school and extracurricular school activities in the GC of the current sample, and it was difficult to coordinate data collection with families due to the multiple constraints of their child’s busy schedule. Activities and competition schedules are prone to induce nocturnal awakenings and variability in sleep schedules,46–48 and busy schedules could explain why GC struggle with sleep maintenance and consistency. It is also possible that special schools attended by some GC may have later start times on certain days. Also, the parents of GC might let them sleep in until the last minute on some school mornings (but not all; GC had larger wake-up time IIV on school days) so as to compensate for their high (and variable) levels of nocturnal awakenings. However, these hypotheses are admittedly speculative, and we offer them in the spirit of generating further investigations in this relatively new domain of research.
Strengths and limitations
To our knowledge, this is the first study to compare the sleep of GC and TDC using an objective sleep measure and a multidimensional approach to identify giftedness rather than the unidimensional IQ approach. Moreover, GC were compared to age-matched and sex-matched controls, which rules out several potential confounds and increases statistical power. Also, the size of the sample was relatively large by the standards of the field of giftedness.
Some methodological limitations must also be considered. First, the lack of information on some sociodemographic factors (ie, family socioeconomic status) and children’s sleep environment (ie, shared bedroom) calls for careful interpretation of the results. Second, as giftedness had already been identified by a clinical neuropsychologist, parents’ perception of their child’s sleep was possibly biased, which reaffirms the importance of objective measures of sleep. Third, the short-form version of the Children’s Sleep Habits Questionnaire is a well-validated measure that reflects common clinical symptoms of sleep difficulties, but it does not generate diagnoses of sleep disorders, and neither does actigraphy. Moreover, as actigraphic data were scored manually, possible bias by the actogram analysts cannot be ruled out. Future research should assess sleep using polysomnography to provide detailed information on sleep architecture and reveal sleep disorders that can be identified only with this technology. In addition, polysomnography would help determine the potential clinical implications of the present results and specific clinical recommendations. Finally, the cross-sectional design did not allow for examination of the longitudinal course of sleep/wake patterns in GC. As multiple dynamic factors influence sleep, such as developmental transitions, home environment, parenting styles, or puberty, future longitudinal studies would provide worthwhile information on the course of GC’s sleep patterns and associated risk and protective factors.
CONCLUSIONS
The current study found that sleep maintenance and stability are key assessment items to understand the sleep of children with giftedness. Small group differences on sleep as those found here have been shown to be associated with academic performance, daytime sleepiness, emotional lability, and impulsive behaviors in school-age children.49,50 Because small sleep changes can have profound impacts on school-age children, we suggest that researchers and clinicians assess beyond exclusively classic sleep variables, such as average sleep duration, which mask nocturnal awakenings and night-to-night variability. While there is growing evidence that greater sleep variability is associated with poorer physical and emotional health,21 studies have yet to examine these associations in children with giftedness specifically. Overall, there is a need for research focused on both predictors and consequences of sleep patterns and sleep variability in GC. Our interpretation of the present findings leaned toward a psychological standpoint, but neurophysiological data, including polysomnography and electroencephalogram, could surely provide additional, pertinent elements to further our understanding of sleep patterns in children with giftedness.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed at Université de Montréal and Rivière-des-Prairies Mental Health Hospital. This study was funded by the Canadian Institutes of Health Research, the Social Sciences and Humanities Research Council of Canada, the Natural Sciences and Engineering Research Council of Canada (AB), the Natural Sciences and Engineering Research Council of Canada (RG), an award from the CIUSSS-NIM Research Center (RG) and Fondation Les Petits Trésors (RG). RT was a recipient of a graduate studentship from Fondation Les Petits Trésors. LB was a recipient of a graduate studentship from NSERC. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Marjolaine Chicoine, Claude Gabriel, Élyse Chevrier, Marie-Ève Bélanger, Andrée-Anne Bouvette-Turcot, Christine Gagné, Sarah Hertz, Émilie Tétreault, Marie-Soleil Sirois, Rachel Perrier, Sophie Regueiro, Élizabel Leblanc, Camille Marquis-Brideau, Catherine Cimon-Paquet, and Nadine Marzougui for help with data collection and all children and families who participated in this study.
ABBREVIATIONS
- GC
gifted children
- IIV
intraindividual variability
- IQ
intelligence quotient
- TDC
typically developing children
REFERENCES
- 1. Renzulli JS . What makes giftedness? Reexamining a definition . Phi Delta Kappan. 1978. ; 92 ( 8 ): 81 – 88 . [Google Scholar]
- 2. Terrassier JC . [Intellectually precocious children] . Arch Pediatr. 2009. ; 16 ( 12 ): 1603 – 1606 . [DOI] [PubMed] [Google Scholar]
- 3. Guénolé F , Louis J , Creveuil C , Baleyte JM , Montlahuc C , Fourneret P , Revol O . Behavioral profiles of clinically referred children with intellectual giftedness . BioMed Res Int. 2013. ; 2013 ( 2 ): 540153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Webb JT , Amend ER , Beljan P , et al . Misdiagnosis and Dual Diagnoses of Gifted Children and Adults: ADHD, Bipolar, OCD, Asperger’s, Depression, and Other Disorders. 2nd ed. Scottsdale, AZ: : Great Potential Press, Inc; ; 2016. . [Google Scholar]
- 5. Balilashak N , Safavi M , Mahmoudi M . Comparative assessment of mental health of gifted and average students of junior high school . Procedia Soc Behav Sci. 2010. ; 5 : 2027 – 2033 . [Google Scholar]
- 6. Loureiro IS , Lowenthal F , Lefebvre L , Vaivre-Douret L . [ Psychological and psychobiological profiles of highly gifted children] . Enfance. 2010. ; 62 ( 1 ): 27 – 44 . [Google Scholar]
- 7. Yun K , Chung D , Jang B , Kim JH , Jeong J . Mathematically gifted adolescents have deficiencies in social valuation and mentalization . PLoS One. 2011. ; 6 ( 4 ): e18224 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matricciani L , Bin YS , Lallukka T , et al . Past, present, and future: trends in sleep duration and implications for public health . Sleep Health. 2017. ; 3 ( 5 ): 317 – 323 . [DOI] [PubMed] [Google Scholar]
- 9. Sun W , Ling J , Zhu X , Lee TMC , Li SX . Associations of weekday-to-weekend sleep differences with academic performance and health-related outcomes in school-age children and youths . Sleep Med Rev. 2019. ; 46 : 27 – 53 . [DOI] [PubMed] [Google Scholar]
- 10. Silverman LS , Kearney K . Parents of the extraordinarily gifted . Adv Dev. 1989. ; 1 : 41 – 56 . [Google Scholar]
- 11. Revol O , Louis J , Fourneret P . L’enfant précoce: signes particuliers . Neuropsychiatr Enfance Adolesc. 2004. ; 52 ( 3 ): 148 – 153 . [Google Scholar]
- 12. Guignard-Perret A , Thieux M , Guyon A , et al . Sleep of children with high potentialities: a polysomnographic study . J Clin Med. 2020. ; 9 ( 10 ): 3182 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Busby K , Pivik RT . Sleep patterns in children of superior intelligence . J Child Psychol Psychiatry. 1983. ; 24 ( 4 ): 587 – 600 . [DOI] [PubMed] [Google Scholar]
- 14. Grubar JC . Sommeil et efficience mentale: sommeil et précocité intellectuelle . In: La Précocité Intellectuelle: De La Mythologie à La Génétique. 2nd ed. Brussels, Belgium: Mardaga; ; 2000. : 83 – 90 . [Google Scholar]
- 15. Kroesbergen EH , van Hooijdonk M , Van Viersen S , Middel-Lalleman MMN , Reijnders JJW . The psychological well-being of early identified gifted children . Gift Child Q. 2016. ; 60 ( 1 ): 16 – 30 . [Google Scholar]
- 16. Bastien L , Théoret R , Gagnon K , Chicoine M , Godbout R . Sleep characteristics and socio-emotional functioning of gifted children . Behav Sleep Med. 2022. ; 20 ( 5 ): 598 – 609 . [DOI] [PubMed] [Google Scholar]
- 17. Bélanger MÈ , Simard V , Bernier A , Carrier J . Investigating the convergence between actigraphy, maternal sleep diaries, and the child behavior checklist as measures of sleep in toddlers . Front Psychiatry. 2014. ; 5 : 158 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Werner H , Molinari L , Guyer C , Jenni OG . Agreement rates between actigraphy, diary, and questionnaire for children’s sleep patterns . Arch Pediatr Adolesc Med. 2008. ; 162 ( 4 ): 350 – 358 . [DOI] [PubMed] [Google Scholar]
- 19. Rönnlund H , Elovainio M , Virtanen I , Matomäki J , Lapinleimu H . Poor parental sleep and the reported sleep quality of their children . Pediatrics. 2016. ; 137 ( 4 ): e20153425 . [DOI] [PubMed] [Google Scholar]
- 20. Sadeh A . III. Sleep assessment methods . Monogr Soc Res Child Dev. 2015. ; 80 ( 1 ): 33 – 48 . [DOI] [PubMed] [Google Scholar]
- 21. Becker SP , Sidol CA , Van Dyk TR , Epstein JN , Beebe DW . Intraindividual variability of sleep/wake patterns in relation to child and adolescent functioning: a systematic review . Sleep Med Rev. 2017. ; 34 : 94 – 121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bei B , Wiley JF , Trinder J , Manber R . Beyond the mean: a systematic review on the correlates of daily intraindividual variability of sleep/wake patterns . Sleep Med Rev. 2016. ; 28 : 108 – 124 . [DOI] [PubMed] [Google Scholar]
- 23. Bei B , Manber R , Allen NB , Trinder J , Wiley JF . Too long, too short, or too variable? Sleep intraindividual variability and its associations with perceived sleep quality and mood in adolescents during naturalistically unconstrained sleep . Sleep. 2017. ; 40 ( 2 ): zsw067 . [DOI] [PubMed] [Google Scholar]
- 24. Meltzer LJ . Future directions in sleep and developmental psychopathology . J Clin Child Adolesc Psychol. 2017. ; 46 ( 2 ): 295 – 301 . [DOI] [PubMed] [Google Scholar]
- 25. Bonuck KA , Goodlin-Jones BL , Schechter C , Owens J . Modified children’s sleep habits questionnaire for behavioral sleep problems: a validation study . Sleep Health. 2017. ; 3 ( 3 ): 136 – 141 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Acebo C , Sadeh A , Seifer R , et al . Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999. ; 22 ( 1 ): 95 – 103 . [DOI] [PubMed] [Google Scholar]
- 27. Ward TM , Gay C , Anders TF , Alkon A , Lee KA . Sleep and napping patterns in 3-to-5-year old children attending full-day childcare centers . J Pediatr Psychol. 2008. ; 33 ( 6 ): 666 – 672 . [DOI] [PubMed] [Google Scholar]
- 28. Meltzer LJ , Montgomery-Downs HE , Insana SP , Walsh CM . Use of actigraphy for assessment in pediatric sleep research . Sleep Med Rev. 2012. ; 16 ( 5 ): 463 – 475 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Philips Respironics . Equivalence of activity recordings and derived sleep statistics. Published online 2008. . Accessed September 16, 2021. https://www.yumpu.com/en/document/view/48782709/equivalence-of-activity-recordings-and-derived-sleep-actigraphy
- 30. Meltzer LJ , Walsh CM , Traylor J , Westin AML . Direct comparison of two new actigraphs and polysomnography in children and adolescents . Sleep. 2012. ; 35 ( 1 ): 159 – 166 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Koninck J , Lorrain D , Gagnon P . Sleep positions and position shifts in five age groups: an ontogenetic picture . Sleep. 1992. ; 15 ( 2 ): 143 – 149 . [DOI] [PubMed] [Google Scholar]
- 32. Cohen J . Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: : Lawrence Erlbaum; ; 1988. . [Google Scholar]
- 33. Ohayon M , Wickwire EM , Hirshkowitz M , et al . National Sleep Foundation’s sleep quality recommendations: first report . Sleep Health. 2017. ; 3 ( 1 ): 6 – 19 . [DOI] [PubMed] [Google Scholar]
- 34. Healey D , Runco MA . Could creativity be associated with insomnia? Creat Res J. 2006. ; 18 ( 1 ): 39 – 43 . [Google Scholar]
- 35. Van de Water ATM , Holmes A , Hurley DA . Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography--a systematic review . J Sleep Res. 2011. ; 20 ( 1 Pt 2 ): 183 – 200 . [DOI] [PubMed] [Google Scholar]
- 36. Rowe M , McCrae C , Campbell J , et al . Actigraphy in older adults: comparison of means and variability of three different aggregates of measurement . Behav Sleep Med. 2008. ; 6 ( 2 ): 127 – 145 . [DOI] [PubMed] [Google Scholar]
- 37. El-Sheikh M , Buckhalt JA . Vagal regulation and emotional intensity predict children’s sleep problems . Dev Psychobiol. 2005. ; 46 ( 4 ): 307 – 317 . [DOI] [PubMed] [Google Scholar]
- 38. Owens-Stively J , Frank N , Smith A , et al . Child temperament, parenting discipline style, and daytime behavior in childhood sleep disorders . J Dev Behav Pediatr. 1997. ; 18 ( 5 ): 314 – 321 . [DOI] [PubMed] [Google Scholar]
- 39. Pfeiffer SI , Stocking VB . Vulnerabilities of academically gifted students . Spec Serv Schools. 2000. ; 16 ( 1-2 ): 83 – 93 . [Google Scholar]
- 40. Alfano CA , Ginsburg GS , Kingery JN . Sleep-related problems among children and adolescents with anxiety disorders . J Am Acad Child Adolesc Psychiatry. 2007. ; 46 ( 2 ): 224 – 232 . [DOI] [PubMed] [Google Scholar]
- 41. Cook F , Hippmann D , Omerovic E . The sleep and mental health of gifted children: a prospective, longitudinal, community cohort study . Gifted Talent Int. 2020. ; 35 ( 1 ): 16 – 26 . [Google Scholar]
- 42. Demirhan E , Randler C , Beşoluk Ş , Horzum MB . Gifted and non-gifted students’ diurnal preference and the relationship between personality, sleep, and sleep quality . Biol Rhythm Res. 2018. ; 49 ( 1 ): 103 – 117 . [Google Scholar]
- 43. Arbabi T , Vollmer C , Dörfler T , Randler C . The influence of chronotype and intelligence on academic achievement in primary school is mediated by conscientiousness, midpoint of sleep and motivation . Chronobiol Int. 2015. ; 32 ( 3 ): 349 – 357 . [DOI] [PubMed] [Google Scholar]
- 44. Bucknavage LB , Worrell FC . A study of academically talented students’ participation in extracurricular activities . J Second Gift Educ. 2005. ; 16 ( 2-3 ): 74 – 86 . [Google Scholar]
- 45. Olszewski-Kubilius P , Lee SY . The role of participation in in-school and outside-of-school activities in the talent development of gifted students . J Second Gift Educ. 2004. ; 15 ( 3 ): 107 – 123 . [Google Scholar]
- 46. Stutz J , Eiholzer R , Spengler CM . Effects of evening exercise on sleep in healthy participants: a systematic review and meta-analysis . Sports Med. 2019. ; 49 ( 2 ): 269 – 287 . [DOI] [PubMed] [Google Scholar]
- 47. Williams SM , Farmer VL , Taylor BJ , Taylor RW . Do more active children sleep more? A repeated cross-sectional analysis using accelerometry . PLoS One. 2014. ; 9 ( 4 ): e93117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nedelec M , Aloulou A , Duforez F , Meyer T , Dupont G . The variability of sleep among elite athletes . Sports Med Open. 2018. ; 4 ( 1 ): 34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gruber R , Cassoff J , Frenette S , Wiebe S , Carrier J . Impact of sleep extension and restriction on children’s emotional lability and impulsivity . Pediatrics. 2012. ; 130 ( 5 ): e1155 – e1161 . [DOI] [PubMed] [Google Scholar]
- 50. Gruber R , Somerville G , Bergmame L , Fontil L , Paquin S . School-based sleep education program improves sleep and academic performance of school-age children . Sleep Med. 2016. ; 21 : 93 – 100 . [DOI] [PubMed] [Google Scholar]