Abstract
Introduction:
As the first step in the HIV care continuum, timely diagnosis is central to reducing transmission of the virus and ending the HIV epidemic. Studies have shown that distance from a testing site is essential for ease of access to services and educational material. This study demonstrates how location-allocation analysis can be used to improve allocation of HIV testing services utilizing existing publicly available data from 2015–2019 on HIV prevalence, testing site location, and factors related to HIV for Philadelphia, Pennsylvania.
Methods:
The ArcGIS Location-Allocation analytic tool was used to calculate locations for HIV testing sites using a method that minimizes the distance between demand point locations and service facilities. ZIP code level demand was initially specified based on percentage of late HIV diagnoses, and in a sensitivity analysis, based on a composite of multiple factors. Travel time and distance from demand to facilities determined the facility location-allocation. This analysis was conducted from 2021–2022.
Results:
Compared to the 37 facilities located in 20 (43%) Philadelphia ZIP codes, the model proposed re-allocating testing facilities to 37 (79%) ZIP codes using percent late diagnoses to define demand. On average, this would reduce distance to the facilities by 65% and travel time to the facilities by 56%. Results using the sensitivity analysis were similar.
Conclusions:
A wider distribution of HIV testing services across the city of Philadelphia may reduce distance and travel time to facilities, improve accessibility of testing, and in turn, increase the percentage of people with knowledge of their status.
INTRODUCTION
For decades, the HIV epidemic has shown how certain individuals (and communities) may lack convenient access to diagnostic and treatment services.1–3 This lack of agency or access may be driven by potentially stigmatizing behavior (such as same sex sexual behavior and injection drug use), historic marginalization (often among Black and Latinx individuals as well as those living in impoverished areas), or a combination of these factors.4,5 The CDC recently estimated that 1 in 7 Americans living with HIV are unaware of their status.6 Accordingly, the Ending the HIV Epidemic in the U.S. (EHE) initiative, developed in 2019 by the DHHS, states that a top priority is to increase the percentage of people with knowledge of their HIV status to 95% by 2030, by diagnosing all people with HIV as early as possible.7 This necessitates adequate testing facilities in locations accessible to individuals who may be living with HIV but unaware, or at risk of becoming infected. Studies have shown that convenience of and distance from testing site location is essential for ease of access to both services and educational material.8–10 While a comprehensive approach to eliminating disparities in access to HIV care is needed, reducing physical barriers through improvement of testing and treatment site locations is an important first step.
This paper seeks to demonstrate and evaluate how location-allocation analysis may be used to address resource allocation problems for HIV prevention and treatment. Location-allocation models are used to identify candidate locations for services in a geographic area, accounting for the spatial distribution of demand for those services in that area. Demand can be conceptualized as the number of individuals in an area that require certain services, such as HIV testing. The location-allocation model determines locations of service facilities based on these demand points and according to a set of criteria or constraints, such as travel time or distance to a facility. Geodemographic groups may be weighted according to the extent to which individuals within a certain group are in need of services.11 With the integration of location-allocation models into GIS software such as ArcGIS (Esri, Redlands, California), the use of these analytic tools is becoming more accessible and thus more common in public health.12–14
As a model to guide planning, this study used location-allocation methods to determine if and how location of current publicly funded, non-mobile HIV testing sites in Philadelphia, Pennsylvania could be improved utilizing existing publicly available data on HIV prevalence, testing site location, and factors related to HIV. Philadelphia is a prototypical urban location with high rates of HIV and widespread need for testing and treatment services. Philadelphia County, which is coextensive to the city of Philadelphia, is a priority jurisdiction for phase one of the EHE initiative.7 Selection of existing HIV test sites in Philadelphia is informed from a combination of historic surveillance data, community partnerships, consideration of priority groups (e.g., people who inject drugs, men who have sex with men), and ability to apply to and meet funding requirements (T. Nassau, personal communication, Philadelphia Department of Public Health, 2022). This study sought to contrast the data-driven model against these existing locations.
METHODS
Geographic Region
Philadelphia is the sixth-most populous city in the U.S. and the most populous city and the center of economic activity in Pennsylvania. According to 2020 data, Philadelphia had a population of 1.6 million, spanned over 134.1 square miles, and was comprised of 49 populated ZIP codes (Appendix Figure 1). Each ZIP code across Philadelphia had an average of 1,410 (SD=837.2) persons living with HIV (PLWH) per 100,000 population in 2019. Among PLWH, an average of 59% (SD=8.8%) were virally suppressed (viral load <200 copies/mL) citywide. From 2015–2019, an average of 190 (SD=105.4) new cases of HIV per 100,000 population were reported, with an average of 17% (SD=7.1%) identified as late diagnoses (diagnosed with stage 3 (AIDS) within 3 months of initial diagnosis) across the Philadelphia ZIP codes. ZIP code was the unit of analysis for this study due to availability of data, and is commonly used as a neighborhood definition in neighborhoods and health research,15 including in Philadelphia.16
Study Data
Publicly available ZIP code level data was obtained on the epidemiology of HIV and general demography. Epidemiologic HIV data including prevalence, incidence, late diagnoses, linkage to care, receipt of care, and viral suppression were downloaded from AIDSVu, for the years 2015–2019, the most recent year recorded.17 Data were available for all but 3 ZIP codes (19109, 19112, and 19113), which were suppressed due to small numerators or denominators. The number and locations of existing HIV testing sites were acquired from the City of Philadelphia Government18 and the number and locations of existing healthcare facilities were acquired from the Pennsylvania Department of Health.19 Demographic information including population, income, occupation, race, ethnicity, and age, was downloaded from the 2018 American Community Survey20 using the tidycensus (v1.0; Walker & Herman, 2021) package for R (R Foundation for Statistical Computing, Vienna, Austria), and geographic area shapefiles were downloaded from the U.S. Census Bureau.21
Location-Allocation Analysis Step 1: Demand Specification
The ArcGIS Location-Allocation analytic tool (v2.82021) uses heuristic procedures to identify locations for services based on location-specific demand. Within this analysis, facilities are the HIV testing sites and demand points are locations that represent the people requiring the services provided by the facilities. To target individuals with the greatest need for HIV testing services within their ZIP code, demand should reflect areas with more individuals living with HIV unaware of their status. The demand specification selected used a proxy for this hidden population: the percent of persons diagnosed with late HIV, determined by the number of individuals aged 13 years and older who were diagnosed with HIV during the previous 5 years and were diagnosed with AIDS within 3 months of initial diagnoses, divided by the number of individuals aged 13 years and older who were newly diagnosed with HIV in that given 5-year time-period. This may be interpreted as the proportion of individuals living in each ZIP code that, prior to diagnosis, had been living with HIV, and thus in need of testing (and treatment) services.
Recognizing that demand may not only be related to late diagnosis but other observed and unobserved features, a sensitivity analysis was conducted that utilized a demand specification consisting of a composite variable, created using a latent profile analysis via the tidyLPA package (Rosenberg, Beymer, Anderson, Van Lissa, & Schmidt; 2018) for R. A detailed description of the latent profile analysis and the composite demand variable is included in the Appendix.
Location-Allocation Analysis Step 2: Determining Quantity and Locations of Demand Points
Population-weighted ZIP code centroids were used to represent locations of HIV testing site demand,22,23 and demand weight was calculated by imputing the number of Philadelphia residents living with HIV unaware of their status in each ZIP code. The Philadelphia Department of Public Health estimated this number to be 1,700 individuals citywide in a 2020 report.24 Without knowing their specific locations, a proportion of these 1,700 individuals were assigned to each Philadelphia-area population-weighted ZIP code centroid, proportional to the percentage of late HIV diagnoses arising from this ZIP code out of all citywide diagnoses.17 This value would then represent the weight of demand for that ZIP code. For example, if a given ZIP code represented 10% of late diagnoses citywide, this ZIP code centroid received a weight of 170.
Location-Allocation Analysis Step 3: Allocation of Proposed Facilities
Proposed facility locations were allowed to fall anywhere within the ZIP codes, with candidate locations created using the ArcGIS fishnet tool. Travel time and distance from demand points to facilities were used separately to determine appropriate facility location-allocation, and a cutoff of 15 minutes travel time was used as a maximum distance an individual would likely travel to a facility.25 The proposed number of facilities was set to 37, which is equal to the number of existing HIV testing site locations reported on the City of Philadelphia’s website.18 Indicators from the model were compared to the existing HIV testing site locations to quantify the differences resulting from the facility reallocations. Additional methodological details are available in the Appendix.
RESULTS
Location-Allocation Analysis Using Percent Late Diagnoses Demand Variable
Results from the location-allocation analysis, including the locations of the existing and proposed facilities, and choropleth shading to illustrate demand, are depicted in Figure 1. Existing HIV testing facilities (n=37) are in 20 of 49 (43%) Philadelphia ZIP codes. The model proposed re-allocating these facilities to 37 of 49 (79%) ZIP codes when using percent late diagnoses as a demand weight. On average, the existing facilities were 1.59 (SD=1.03) miles away from a given demand point, whereas the proposed facilities would be 0.56 (SD=0.47) miles away (Table 1, Figure 2). This corresponds to a 65% reduction in distance to a proposed facility compared to an existing facility. Similarly, the existing facilities were on average 5.33 (SD=2.45) minutes away by driving compared to the proposed facilities, which would be 2.33 (SD=1.51) minutes away by driving. This corresponds to a 56% reduction in travel time to a proposed facility compared to an existing facility.
Figure 1.
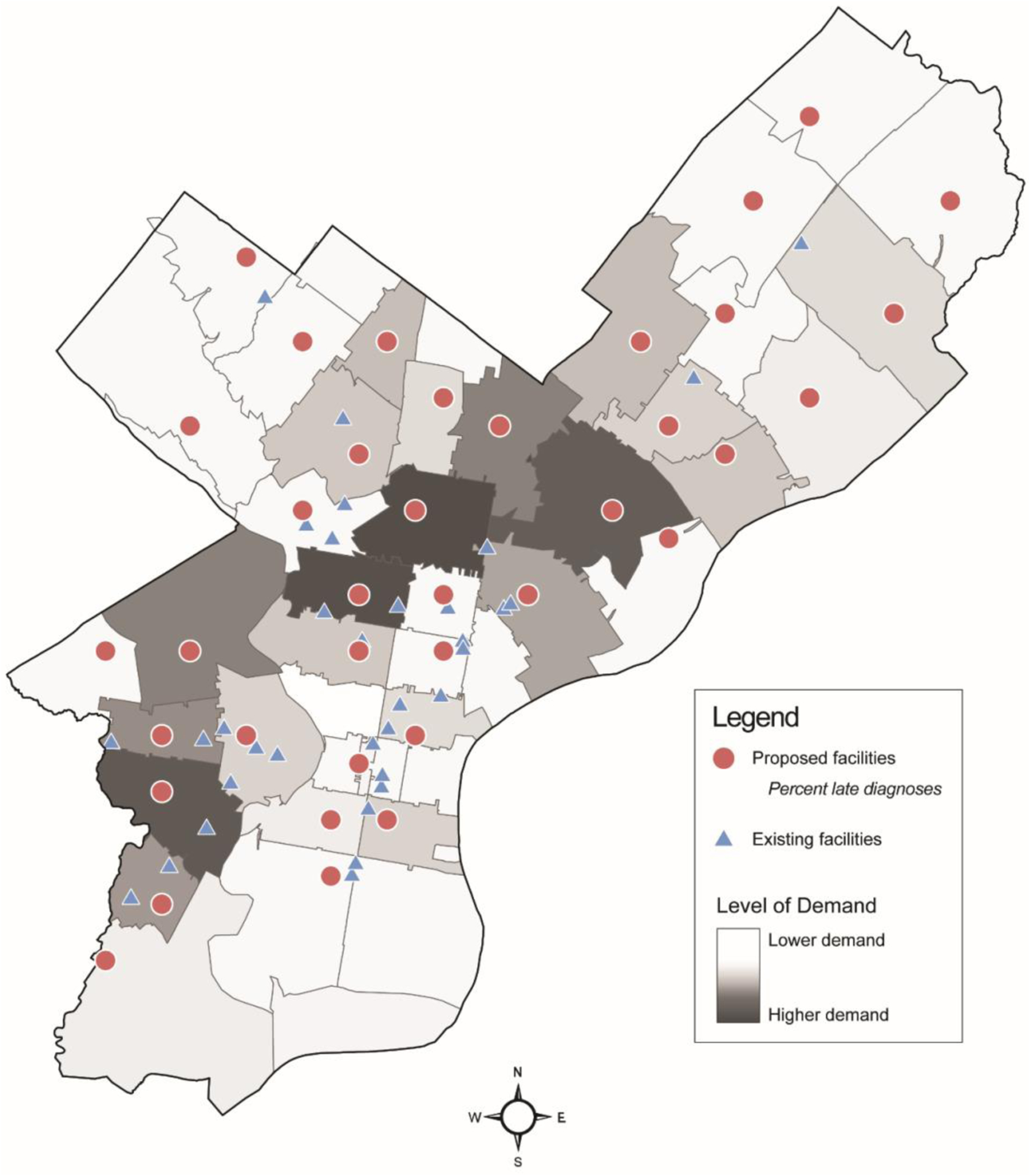
Existing and proposed HIV screening site locations in Philadelphia, Pennsylvania, based on a location-allocation analysis using percent late diagnoses to specify demand.
Table 1.
Descriptive Statistics Describing the 3 Location-Allocation Analyses of Existing and Proposed HIV Testing Facilities
| Variable | Existing facilities | Proposed facilities: percent late diagnoses | Proposed facilities: composite variablea |
|---|---|---|---|
| ZIP codes with at least 1 facility, n (%) | 20 (43) | 37 (79) | 37 (79) |
| Distance to a facility, miles, mean (SD) | 1.59 (1.03) | 0.56 (0.47) | 0.52 (0.41) |
| Time to a facility, minutes, mean (SD) | 5.33 (2.45) | 2.33 (1.51) | 2.16 (1.52) |
Note: Location-allocation models that proposed new facility locations were based on 2 demand specifications: (1) percent late diagnoses and (2) a composite demand variable.
The composite demand variable was created using a latent profile analysis. Variables used in the latent profile analysis included rates of persons living with HIV, risk of persons newly diagnosed with HIV, percent of persons diagnosed with late HIV, percent of persons virally suppressed, density of existing healthcare facilities, and an area deprivation index.
Figure 2.
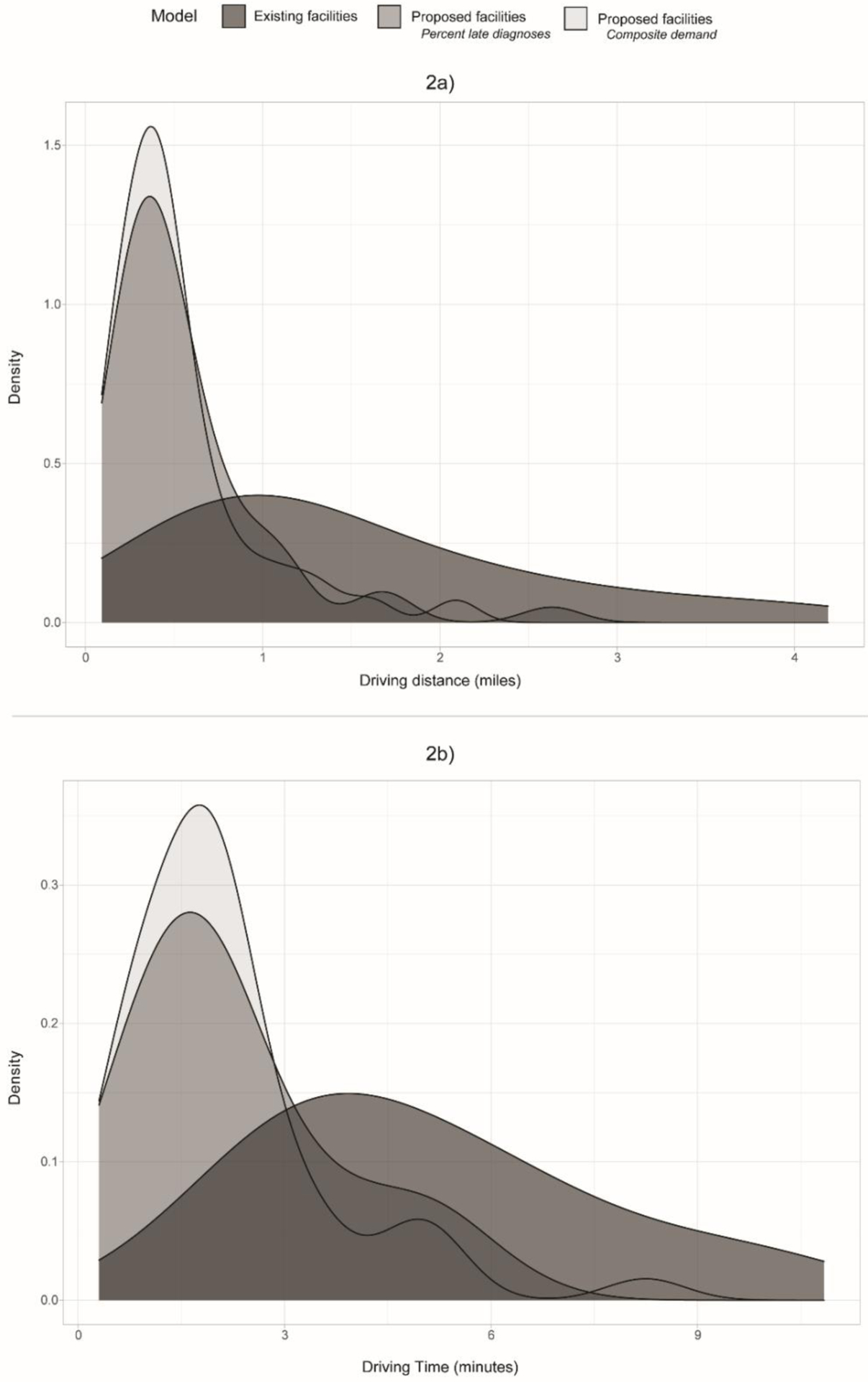
Density plot depicting the distribution of driving distances (2a) and driving times (2b) to a facility for the 3 location-allocation models: (1) for the existing facilities, (2) for the proposed facilities using percentage of late diagnoses for the demand weight, and (3) for the proposed facilities using the latent demand profiles.
The results using the composite demand variable were comparable to using percent late diagnosis. Using the composite demand variable, there was a 67% reduction in distance to a proposed facility and a 59% reduction in travel time to a proposed facility compared to an existing facility (Table 1, Figure 2). Many of the proposed facility locations for this model overlapped with those proposed by the primary analysis (Figure 3).
Figure 3.
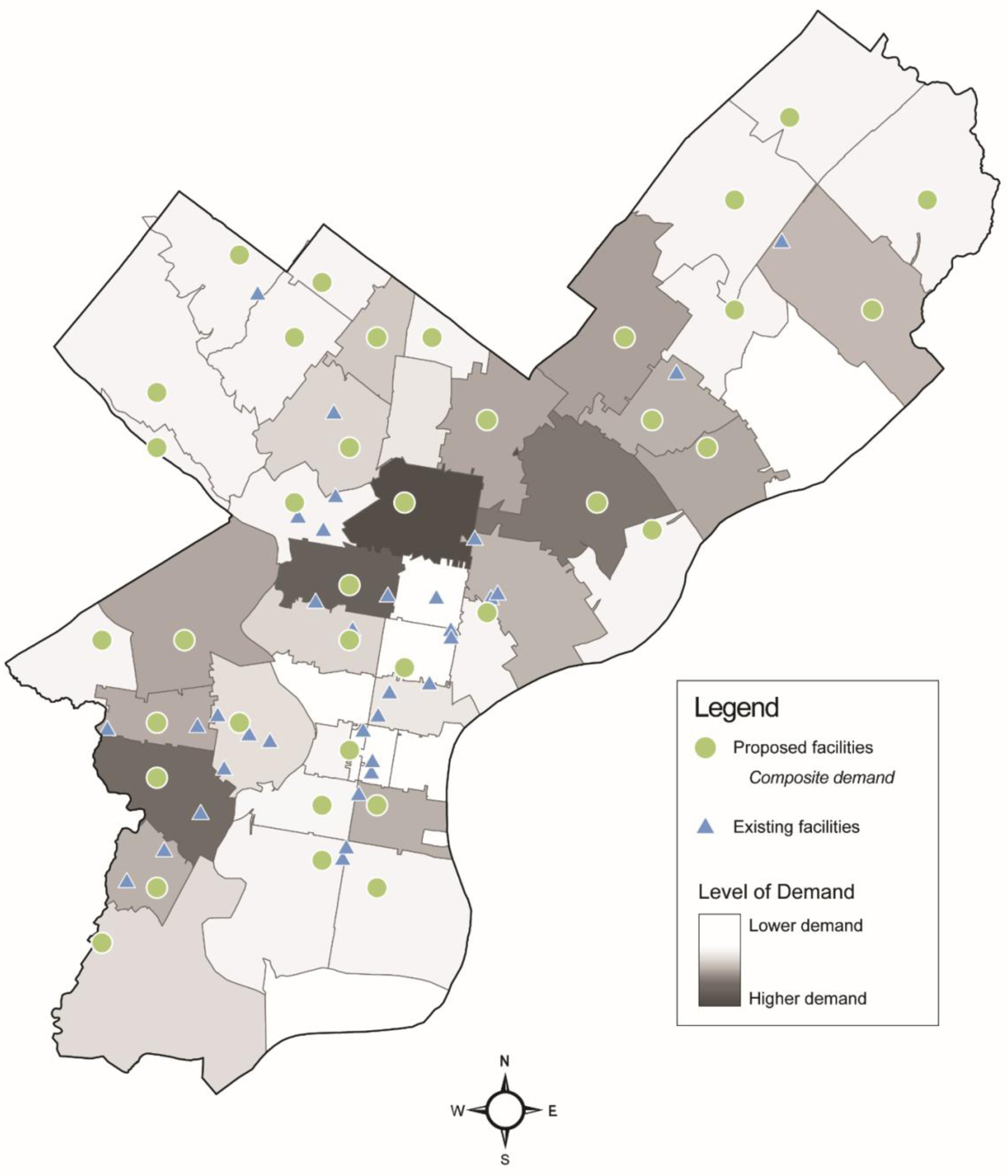
Existing and proposed HIV screening site locations in Philadelphia, Pennsylvania, based on a location-allocation analysis using a composite variable to specify demand.
DISCUSSION
This paper demonstrated the use of location-allocation analysis to refine the locations of HIV testing sites in Philadelphia, Pennsylvania. The results indicate that a wider distribution of testing services across the city of Philadelphia could reduce distance and travel time to testing facilities, although none exceeded the 15-minute threshold. Many of the existing testing sites are in the Center City and West Philadelphia ZIP codes. These areas have increased prevalence of PLWH and incidence of new diagnoses, which are both indicators of a need for testing and treatment services. Many of these locations are near each other yet may serve distinct populations. The models performed for this current analysis suggest that the resources could be distributed to other ZIP codes that are also in need of these services but may be lacking.
Location-allocation approaches are predicated upon accurately quantifying demand, and in this study, demand corresponded to the need for HIV testing services throughout Philadelphia. Accordingly, there are several assumptions needed to properly interpret these results. Under the primary demand specification, percentage of late diagnoses in a ZIP code was used as a proxy for individuals living with HIV unaware of their status. First, this approach allocated testing sites based only on the need of those living with HIV unaware of their status, when in fact, individuals (especially younger ages) who have not been infected but are at risk also require testing services. Second, without knowing the exact location of individuals living with HIV unaware of their status, the locations of the demand points were geopositioned at the population-weighted centroid of the ZIP code. This is assuming that these individuals are living at or close to the most populated location within the ZIP code. If this hidden population lived in less-populated areas of the ZIP code, their distance from a proposed facility could be greater than the average distance calculated by the model. This would be more problematic in larger Philadelphia ZIP codes, where the most populated areas could be up to 7 miles from the least populated areas. However, this method remains more accurate than using geometric centroids, since the goal is to place these points where individuals in need of these services are residing. In addition, this method assumes that an individual will be more likely to get tested if a facility is in proximity to their place of residence. This would not be the case if an individual preferred to test near their work, or if they are uncomfortable testing near their place of residence due to privacy concerns or stigma.26 Finally, it was assumed these individuals were unaware of their HIV status prior to their diagnoses. It is possible that this assumption does not hold for every late HIV diagnosis in Philadelphia, and that some of these individuals were indeed aware of their status prior to their late diagnosis. If this were the case, it would not be appropriate to group these individuals with those who were living with HIV unaware of their status, as their behaviors and attitudes towards HIV may be different. Nonetheless, these individuals would still have a greater need for testing services in their proximity to facilitate earlier diagnoses.
To relax these assumptions – albeit at the cost of introducing additional complexity – a sensitivity analysis was conducted using a composite of multiple variables to specify demand. The results were similar to the primary demand specification, suggesting that the second latent profile was driving the analysis (Appendix Figure 2). This profile corresponded to below average prevalence of PLWH and incidence of new diagnoses, and the above average percentage of late diagnoses. While it may seem counter-intuitive to allocate testing sites to locations with fewer PLWH and fewer new diagnoses, these results support the theory that not only does being an individual living with HIV unaware of their status increase one’s need for nearby testing services, but also living in a ZIP code with fewer PLWH and fewer new diagnoses could mean that there is less information and fewer resources available on HIV, and potentially more stigma within the community.27,28 Indeed, ZIP codes with higher prevalence of PLWH and new diagnoses should be allocated more treatment facilities: this would be a useful extension of the location-allocation approach demonstrated herein.
Access to healthcare is a fundamental barrier in reducing disparities related to HIV burden in the U.S. As the initial step in the HIV care continuum, testing should be readily accessible to anyone at risk of infection, but more importantly, to those who may be living with HIV unaware of their status. Early detection of HIV infection will allow for faster linkage to care, improved outcomes from viral suppression, and fewer transmissions. Previous studies have examined how testing site locations influence testing behaviors,10,29,30 but none to the authors’ knowledge have empirically evaluated and reallocated existing locations to improve access to testing. This, of course, is not the only example of how inaccessibility of healthcare has led to disparities in disease burden in medically underserved communities. The COVID-19 pandemic has exemplified how differential accessibility of healthcare services, specifically access to testing, not only makes it difficult for infected individuals to receive treatment and ancillary support services but also skews population estimates of the burden of disease.31,32 Across the U.S., poor accessibility has disproportionately impacted communities with a greater proportion of Black and Latinx residents, exacerbating existing inequities. For example, in May 2020, Grigsby-Toussaint and colleagues reported that of the 126 COVID-19 testing sites across New York City, only 7% were located within majority Black ZIP codes, 13% in majority Latinx ZIP codes, and 50% were located within majority White ZIP codes.33 Since 2020, increased availability of at-home COVID-19 tests and telemedicine services has made it easier for all communities, including disproportionately impacted communities (admittedly to a lesser extent) to access testing services.34 This provides an opportunity for HIV services, including at-home testing kits, to follow suit. However, as with COVID-19 testing, at-home rapid HIV tests do not replace the more accurate tests conducted by a healthcare provider, reiterating the need for easily accessible testing facilities.
Limitations
A central limitation to this work is the assumption that accessibility is driven solely by closer testing site location (distance or travel time). While there is an expectation that the reallocation of HIV testing sites will have some impact on getting HIV positive individuals aware of their status, it is far from perfection. Reallocation of testing site locations should be considered in conjunction with other programs to reduce known barriers to testing accessibility such as rapid testing at clinics, self-testing, public health announcements, and educational outreach.35–37 Additionally, any proposed reallocation of HIV service organization should be evaluated using an implementation science framework to ensure equitable access.38 This work is foremost a location-allocation analysis demonstration, and should be taken into consideration alongside other factors (e.g., new infections, facility assets, etc.) when re-allocating facilities in Philadelphia or elsewhere. The goal of this analysis was not necessarily to encourage all 37 facilities to be relocated, but to identify improved locations based on the specifications of demand. As such, many of the existing facilities are already in locations that would be accessible to populations in need of their services. This analysis highlights areas in the county that would likely benefit from the addition of a facility, due to the demand of the population residing in those areas. For example, in the existing Philadelphia facilities, the ZIP codes with residents that travel the furthest to a facility include those in the Northeast (19114, 19149) and Northwest (19144, 19129) regions of the county. Ultimately it is up to the health department to determine the feasibility of facility reallocations or the placement of new facilities in the suggested locations based on community partnerships, priority populations, and funding. Some testing sites exist within permanent health centers that are immobile, however, other locations may be tied to partnerships with clinics or community organizations, many of which exist in various locations across the county.
Limitations to the use of GIS programs to solve location-allocation problems are described in the Appendix.
CONCLUSIONS
While location-allocation models have a limited history of use in health services research,23,39,40 the availability of easy-to-use implementations should spur additional applications in public health. This paper demonstrated an application of these techniques to reallocate HIV testing facilities in Philadelphia, Pennsylvania. These models can be applied to a multitude of different demand-facility combinations, within many geographic spaces. This analytic tool should be used for quantifying ease of existing access to healthcare facilities and identifying where and how improvements could be made.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the NIH under Award Number K01AI143356 (to NDG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This project was also supported by the Health Resources and Services Administration (HRSA) of the U.S. HHS under grant number 1 UB6HP31689-01-00 “Public Health Training Centers” for $3,699,596. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government.
The authors thank Dr. Kathleen Brady and Dr. Tanner Nassau from the AIDS Activities Coordinating Office of the Philadelphia Department of Public Health.
No financial disclosures were reported by the authors of this paper.
Footnotes
A portion of this work was presented at the Society for Epidemiologic Research’s Annual Meeting, Chicago, IL, June 14-17, 2022.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Campsmith ML, Rhodes PH, Hall HI, Green TA. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J Acquir Immune Defic Syndr. 2010;53(5):619–624. 10.1097/QAI.0b013e3181bf1c45. [DOI] [PubMed] [Google Scholar]
- 2.Ebrahim SH, Anderson JE, Weidle P, Purcell DW. Race/ethnic disparities in HIV testing and knowledge about treatment for HIV/AIDS: United States, 2001. AIDS Patient Care STDS. 2004;18(1):27–33. 10.1089/108729104322740893. [DOI] [PubMed] [Google Scholar]
- 3.Prejean J, Satcher A, Durant T, Hu X, Lee L. Racial/ethnic disparities in diagnoses of HIV/AIDS−−33 states, 2001–2004. MMWR Morb Mortal Wkly Rep. 2006;55(5):121–125. [PubMed] [Google Scholar]
- 4.Arya M, Kumar D, Patel S, Street RL Jr, Giordano TP, Viswanath K. Mitigating HIV health disparities: the promise of mobile health for a patient-initiated solution. Am J Public Health. 2014;104(12):2251–2255. 10.2105/AJPH.2014.302120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford CL, Godette DC, Mulatu MS, Gaines TL. Recent HIV testing prevalence, determinants, and disparities among US older adult respondents to the behavioral risk factor surveillance system. Sex Transm Dis. 2015;42(8):405. 10.1097/OLQ.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Ending the HIV Epidemic: A Plan for America. 2020. https://www.cdc.gov/endhiv/index.html. Accessed September 2021.
- 7.America’s HIV Epidemic Analysis Dashboard (AHEAD). The Six EHE Indicators. Ending the HIV Epidemic. https://ahead.hiv.gov/data/knowledge-of-status. Accessed September 2021.
- 8.Mathews A, Farley S, Conserve DF, et al. “Meet people where they are”: a qualitative study of community barriers and facilitators to HIV testing and HIV self-testing among African Americans in urban and rural areas in North Carolina. BMC Public Health. 2020;20(1):494. 10.1186/s12889-020-08582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Threats M, Boyd DT, Diaz JE, Adebayo OW. Deterrents and motivators of HIV testing among young Black men who have sex with men in North Carolina. AIDS Care. 2021;33(7):943–951. 10.1080/09540121.2020.1852161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leibowitz AA, Taylor SL. Distance to public test sites and HIV testing. Med Care Res Rev. 2007;64(5):568–584. 10.1177/1077558707304634. [DOI] [PubMed] [Google Scholar]
- 11.Brunsdon C, Singleton A. Geocomputation: a practical primer. Sage; 2015. 10.4135/9781473916432. [DOI] [Google Scholar]
- 12.Horst MA, Jammula S, Gross BW, et al. Development of a trauma system and optimal placement of trauma centers using geospatial mapping. J Trauma Acute Care Surg. 2018;84(3):441–448. 10.1097/TA.0000000000001782. [DOI] [PubMed] [Google Scholar]
- 13.Huotari T, Rusanen J, Keistinen T, et al. Effect of centralization on geographic accessibility of maternity hospitals in Finland. BMC Health Serv Res. 2020;20(1):1–9. 10.1186/s12913-020-05222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vora KS, Yasobant S, Sengupta R, De Costa A, Upadhyay A, Mavalankar DV. Options for optimal coverage of free C-section services for poor mothers in Indian state of Gujarat: location allocation analysis using GIS. PloS One. 2015;10(9):e0137122. 10.1371/journal.pone.0137122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decker MJ, Isquick S, Tilley L, et al. Neighborhoods matter. A systematic review of neighborhood characteristics and adolescent reproductive health outcomes. Health Place. 2018;54:178–190. 10.1016/j.healthplace.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Núñez AE. Multilevel and Urban Health Modeling of Risk Factors for Diabetes Mellitus: A New Insight into Public Health and Preventive Medicine. Adv Prev Med. 2014;2014:246049. 10.1155/2014/246049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan PS, Woodyatt C, Koski C, et al. A Data Visualization and Dissemination Resource to Support HIV Prevention and Care at the Local Level: Analysis and Uses of the AIDSVu Public Data Resource. J Med Internet Res. 2020;22(10):e23173. 10.2196/23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.City of Philadelphia. Get Tested for HIV. Updated May 2020. https://www.phila.gov/services/mental-physical-health/sexual-health-and-family-planning/hiv-prevention-testing-and-treatment/get-tested-for-hiv/. Accessed July 2021.
- 19.Pennsylvania Department of Health. Quality Assurance Facility Directory. https://sais.health.pa.gov/commonpoc/dohqalocatorcommon.asp. Accessed July 2021.
- 20.[dataset] U.S. Census Bureau. Data from: 2014–2018 American Community Survey 5-Year Estimates. 2018.
- 21.U.S. Census Bureau. Data from: 2020 TIGER/Line Shapefiles. 2020.
- 22.Henry KA, Boscoe FP. Estimating the accuracy of geographical imputation. Int J Health Geogr. 2008;7(1):3. 10.1186/1476-072X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman S-u, Smith DK. Use of location-allocation models in health service development planning in developing nations. Eur J Oper Res. 2000;123(3):437–452. 10.1016/S0377-2217(99)00289-1. [DOI] [Google Scholar]
- 24.Philadelphia Department of Public Health. A Community Plan to End the HIV Epidemic in Philadelphia. 2020. December 2020. https://www.phila.gov/media/20201201165516/Ending-the-HIV-Epidemic-in-Philadelphia-A-Community-Plan.pdf. Accessed August 2021.
- 25.Eberhart MG, Share AM, Shpaner M, Brady KA. Comparison of geographic methods to assess travel patterns of persons diagnosed with HIV in Philadelphia: How close is close enough? J Biomed Inform. 2015;53:93–99. 10.1016/j.jbi.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Schnall R, Rojas M, Travers J. Understanding HIV testing behaviors of minority adolescents: a health behavior model analysis. J Assoc Nurses AIDS Care. 2015;26(3):246–258. 10.1016/j.jana.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du H, Chi P, Li X. High HIV prevalence predicts less HIV stigma: a cross-national investigation. AIDS Care. 2018;30(6):714–721. 10.1080/09540121.2017.1401039. [DOI] [PubMed] [Google Scholar]
- 28.Genberg BL, Hlavka Z, Konda KA, et al. A comparison of HIV/AIDS-related stigma in four countries: Negative attitudes and perceived acts of discrimination towards people living with HIV/AIDS. Soc Sci Med. 2009;68(12):2279–2287. 10.1016/j.socscimed.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Zhou F, Hall BJ, et al. Is there a relationship between geographic distance and uptake of HIV testing services? A representative population-based study of Chinese adults in Guangzhou, China. PLoS One. 2017;12(7):e0180801. 10.1371/journal.pone.0180801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eberhart MG, Voytek CD, Hillier A, Metzger DS, Blank MB, Brady KA. Travel Distance to HIV Medical Care: A Geographic Analysis of Weighted Survey Data from the Medical Monitoring Project in Philadelphia, PA. AIDS Behav. 2014;18(4):776–782. 10.1007/s10461-013-0597-7. [DOI] [PubMed] [Google Scholar]
- 31.Griffith GJ, Morris TT, Tudball MJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11(1):5749. 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Röösli E, Rice B, Hernandez-Boussard T. Bias at warp speed: how AI may contribute to the disparities gap in the time of COVID-19. J Am Med Inform Assoc. 2020;28(1):190–192. 10.1093/jamia/ocaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grigsby-Toussaint DS, Shin JC, Jones A. Disparities in the distribution of COVID-19 testing sites in black and Latino areas in new York City. Prev Med. 2021;147:106463. 10.1016/j.ypmed.2021.106463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rader B, Gertz A, Iuliano AD, et al. Use of At-Home COVID-19 Tests-United States, August 23, 2021-March 12, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:489–494. 10.15585/mmwr.mm7113e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton A, Shin S, Taggart T, et al. HIV testing barriers and intervention strategies among men, transgender women, female sex workers and incarcerated persons in the Caribbean: a systematic review. Sex Transm Infect. 2020;96(3):189–196. 10.1136/sextrans-2018-053932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilton A, Thompson N, Choko AT, et al. HIV Self-Testing Uptake and Intervention Strategies Among Men in Sub-Saharan Africa: A Systematic Review. Front Public Health. 2021;9:594298. 10.3389/fpubh.2021.594298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDougall GJ Jr., Dalmida SG, Foster PP, Burrage J. Barriers and Facilitators to HIV Testing Among Women. HIV/AIDS Res Treat. 2016;2016(Se1):S9–s13. 10.17140/HARTOJ-SE-1-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):1–15. 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hakimi SL. Optimum locations of switching centers and the absolute centers and medians of a graph. Oper Res. 1964;12(3):450–459. 10.1287/opre.12.3.450. [DOI] [Google Scholar]
- 40.Rushton G. Use of location-allocation models for improving the geographical accessibility of rural services in developing countries. Int Reg Sci Rev. 1984;9(3):217–240. 10.1177/016001768400900303. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


