Abstract
Aims
We tested the hypothesis that epicardial adipose tissue (EAT) quantification improves the prediction of the presence of obstructive coronary artery disease (CAD) in patients presenting with acute chest pain to the emergency department.
Methods and results
Within this prospective observational cohort study, we included 657 consecutive patients (mean age 58.06 ± 18.04 years, 53% male) presenting to the emergency department with acute chest pain suggestive of acute coronary syndrome between December 2018 and August 2020. Patients with ST-elevation myocardial infarction, haemodynamic instability, or known CAD were excluded. As part of the initial workup, we performed bedside echocardiography for quantification of EAT thickness by a dedicated study physician, blinded to all patient characteristics. Treating physicians remained unaware of the results of the EAT assessment. The primary endpoint was defined as the presence of obstructive CAD, as detected in subsequent invasive coronary angiography. Patients reaching the primary endpoint had significantly more EAT than patients without obstructive CAD (7.90 ± 2.56 mm vs. 3.96 ± 1.91 mm, P < 0.0001). In a multivariable regression analysis, a 1 mm increase in EAT thickness was associated with a nearby two-fold increased odds of the presence of obstructive CAD [1.87 (1.64–2.12), P < 0.0001]. Adding EAT to a multivariable model of the GRACE score, cardiac biomarkers and traditional risk factors significantly improved the area under the receiver operating characteristic curve (0.759–0.901, P < 0.0001).
Conclusion
Epicardial adipose tissue strongly and independently predicts the presence of obstructive CAD in patients presenting with acute chest pain to the emergency department. Our results suggest that the assessment of EAT may improve diagnostic algorithms of patients with acute chest pain.
Keywords: Epicardial adipose tissue, Acute coronary syndrome, Echocardiography, Myocardial infarction, Coronary artery disease
Graphical Abstract
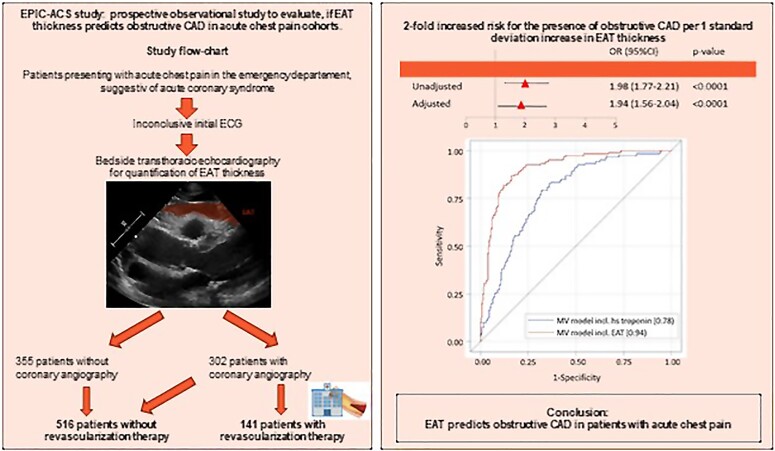
Graphical abstract.
Introduction
Diagnostic evaluation of patients with acute chest pain in the emergency department is challenging, and initial assessment of electrocardiogram (ECG) and biomarker testing is inconclusive in a majority of patients.1 This leads to the need for additional testing to facilitate a safe triage of patients.1–3 For the rule out of coronary artery disease (CAD) and assurance of patients’ safety, non-invasive and invasive procedures are frequently performed even among low-risk patients with chest pain.4,5 Current guidelines suggest the use of computed tomography (CT) coronary angiography (CTA) and non-invasive stress testing for clinical decision-making.6 This approach takes high demands on resources and expertise and causes increased downstream testing.5,7 Even in specialized centres, CTA and stress testing, utilizing dedicated accelerated diagnostic protocols, are provided only during weekday daytime hours, limiting their applicability in patients with acute chest pain.8 While transthoracic echocardiography is routinely available in chest pain units, current guidelines suggest its utilization only with a level C recommendation, acknowledging the need for dedicated clinical trials, evaluating echocardiographic measures to improve established diagnostic algorithms.
Epicardial adipose tissue (EAT) surrounds the heart and the coronary vessels and can reliably be quantified by bedside transthoracic echocardiography.9 As metabolically active visceral adipose tissue, EAT releases protective and pro-inflammatory/pro-fibrotic cytokines, chemokines, and adipokines to the surrounding tissues.10,11 Increasing amount of EAT is linked to augmented inflammatory activity,12,13 leading to coronary atherosclerosis development and progression.14–18 While cross-sectional and longitudinal studies document the strong association of EAT with myocardial infarction,19,20 clinical studies, specifically designed to determine how the assessment of EAT can affect patient management, are lacking. We initiated the Epicardial adipose tissue thickness PredIcts obstructive Coronary artery disease in Acute Coronary Syndrome patients (EPIC-ACS) study to test the hypothesis that echocardiography-derived EAT thickness is associated with the presence of obstructive CAD, requiring coronary revascularization therapy, in patients presenting with acute chest pain and may therefore improve the prediction of underlying CAD in addition to established clinical algorithms.
Methods
The EPIC-ACS trial is a prospective single-centre observational study to investigate whether quantification of EAT thickness via transthoracic echocardiography enables improved prediction of obstructive CAD in patients presenting with acute chest pain to the emergency department (NCT03787797).
Study sample
We included consecutive patients presenting to the emergency department of the University Hospital Essen with acute chest pain suggestive of acute coronary syndrome. Patients presenting during regular office hours between December 2018 and August 2020 were included. Exclusion criteria were defined as ST-elevation myocardial infarction, haemodynamic instability, known obstructive CAD prior to presentation, prior revascularization therapy, age < 18 years, pregnancy, or inability/unwillingness to provide informed consent. The study was approved by the institutional ethical committee (18-8198-BO). All patients provided written informed consent.
Epicardial adipose tissue quantification
As part of the study protocol, all patients underwent focused bedside echocardiography evaluation by a dedicated study physician, blinded to the patient’s anamnesis and clinical presentation, ECG and laboratory results, and prior coronary angiography. Two-dimensional transthoracic echocardiography was performed using standard echocardiography systems without the use of specific applications (Philips CX 50 or Philips Sparq system, Philips Healthcare, Best, the Netherlands). Epicardial adipose tissue was defined as the space between the outer wall of the myocardium and the visceral layer of the pericardium.21 Three independent measurements of EAT thickness were performed perpendicular to the free wall of the right ventricle at end-systole in parasternal long- and short-axis views, and the mean of these measurements was calculated. Treating physicians remained unaware of the results of the EAT measurement. For evaluation of reproducibility and quality assurance of EAT measurements, a second assessment of EAT thickness was performed offline at the cardiovascular imaging laboratory of the West German Heart and Vascular Center as a core lab in a subset of 264 patients, using a dedicated software programme (Philips QLab, Philips Healthcare, Amsterdam, the Netherlands). The physician at the core lab was blinded to the initial EAT measurement as well as all patient characteristics. Interobserver reliability was evaluated in these 264 patients and demonstrated very good reproducibility [intraclass correlation coefficient: 0.83 (0.79–0.87), P < 0.0001].
Endpoint definition
The presence of obstructive CAD, defined as the detection of obstructive CAD requiring coronary revascularization therapy (percutaneous coronary intervention/stent implantation or coronary bypass operation) within 90 days after initial presentation, was considered the primary endpoint. The indication for invasive coronary angiography was at the discretion of the treating physicians. Likewise, revascularization therapy was performed according to the discretion of treating interventional cardiologists. Decisions were based on angiographic findings, intravascular ultrasound (Philips IntraSight, Best, the Netherlands), optical coherence tomography (Infinity OCT system, Medtronic, Dublin, Ireland), and/or functional measurements (instantaneous wave-free ratio, fractional flow reserve, Philips IntraSight, Best, the Netherlands).
Covariate assessment
At study inclusion, age and sex were assessed. The patient’s height, body weight, heart rate, and systolic and diastolic blood pressure were measured in a standardized fashion. Body mass index (BMI) was calculated as weight divided by the square of height. Standardized questionnaires assessed smoking status, known hypercholesterinaemia, diabetes, family history of premature CAD, and symptoms (duration of chest discomfort, Killip class). Complementary prior medication of aspirin, P2Y12 antagonist, antihypertensive, lipid-lowering, and antidiabetic treatment was recorded. Serial laboratory results of cardiac markers (troponin, creatinine kinase [CK], myoglobin, and NT-proBNP) as well as baseline creatinine were recorded. At the initiation of the study, a contemporary high-sensitive troponin I assay was available at our site (Siemens Advia Centaur, Erlangen, Germany), which was replaced by a high-sensitive troponin I assay after the inclusion of the first 147 patients (Siemens Atellica, Erlangen, Germany) in clinical routine. After the availability of the high-sensitive troponin assay, both troponin I assays were measured for all baseline and serial assessments in all patients of the present study. For the primary analysis, the contemporary high-sensitive troponin assay was used. Sensitivity analysis was performed in the subgroup of patients with available high-sensitive troponin I, including the more sensitive assay in multivariable models. Total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol as well as triglycerides were assessed using standardized enzymatic methods. Electrocardiogram was evaluated for the presence of ST-segment elevation/depression (≥0.5 mm) or left bundle branch block (LBBB) by a dedicated study physician. Based on the assessed characteristics, the Global Registry of Acute Coronary Events (GRACE) and thrombolysis in myocardial infarction (TIMI) risk scores were calculated.22,23 Non-ST-segment elevation myocardial infarction (NSTEMI) was diagnosed according to the European Society of Cardiology guidelines based on symptoms and a dynamic elevation of cardiac troponin above the 99th percentile of healthy individuals.24
Statistical analysis
Continuous variables are reported as mean ± standard deviation (SD) or median and interquartile range (IQR). Discrete variables are given in frequency and percentiles. Baseline characteristics are specified for the overall cohort as well as stratified by patients with and without the need for coronary revascularization. Continuous variables were compared using a two-sided t-test or Mann–Whitney U test and discrete variables using a χ2 test. Univariate and multivariable logistic regression analyses were performed for the association of EAT thickness with the primary endpoint. For logistic regression analysis, the following models were used: (i) unadjusted; (ii) age, sex, and BMI adjusted; (iii) multivariable adjusted for traditional cardiovascular risk factors (age, sex, BMI, family history of premature CAD, smoking status, hypercholesterolaemia, diabetes, and systolic blood pressure); (iv) Model 3 and cardiac biomarkers (troponin, CK, and myoglobin); (v) the GRACE score; (vi) Model 5 and sex, BMI, hypercholesterolaemia, family history of premature CAD, diabetes, and smoking; and (vii) Model 6 + CK and myoglobin. In sensitivity analysis, the TIMI risk score instead of the GRACE score was used. Here, Model 6 ancillary contained systolic blood pressure, as this variable is not included in the TIMI risk score. Further sensitivity analyses were performed in the subgroup of patients with available hs-troponin as well as using core lab–based EAT quantification. We performed a subgroup analysis, comparing the association of EAT thickness with obstructive CAD, stratified by target vessel for coronary intervention. Due to the limited number of cases with left circumflex stenosis, only univariate logistic regression analysis was possible. In addition, we performed univariate and multivariate regression analyses (Model 1 for univariate analysis and Model 3 for multivariate analysis) to compare EAT thickness in patients without CAD vs. single-vessel disease vs. multivessel disease. For all regression analyses, effect sizes are expressed as odds ratios and 95% confidence interval (CI) per 1 mm increase in EAT thickness. Subgroup analyses were performed stratifying by age groups (≥ vs. <60 years), sex, BMI groups (<25, 25–<30, and ≥ 30 kg/m²), and the presence/absence of traditional cardiovascular risk factors (hypertension, hypercholesterolaemia, diabetes, smoking, and family history of premature CAD), adjusting for variables of Model 3. Receiver operating characteristic (ROC) analysis was performed, and the area under the curve (AUC) was assessed, evaluating the predictive value of EAT in addition to variables contained in Model 7. Youden’s J index was assessed to establish a threshold for the prediction of obstructive CAD. The association of this threshold with obstructive CAD was then tested and validated using unadjusted and adjusted logistic regression analyses in Models 1–3. Again, sensitivity analysis was performed, using a model with the TIMI risk score instead of the GRACE score and when using the subgroup of patients with available high-sensitive troponin assays. All analyses were performed using SAS software (Version 9.4, SAS Institute Inc.). A P-value of <0.05 indicated statistical significance.
Results
Overall, 657 patients (mean age 58.06 ± 18.04 years, 53% male) were included in our study. From the total cohort, 302 patients (46.0%) underwent coronary angiography, of whom obstructive CAD requiring revascularization therapy was detected in 141 subjects (21.5%, see Supplementary material online, Figure S1). Median time to coronary angiography was 1 day (0–40 days; mean time to coronary angiography 5.83 ± 13.72 days). A total of 127 (90.1%) underwent percutaneous coronary intervention/stent, and 14 (9.9%) patients underwent coronary artery bypass grafting. Patients receiving coronary revascularization therapy were older, were more frequently male, had higher levels of cardiac biomarkers, and were more frequently diagnosed as NSTEMI. In addition, a diagnosis of hypercholesterolaemia accompanied by pre-existing lipid-lowering therapy was more frequent in patients with obstructive CAD. Furthermore, prior aspirin, antidiabetic, and antihypertensive therapy was more frequent in patients meeting the primary endpoint. Average EAT thickness was 4.8 ± 2.62 mm. Comparing patients with and without the presence of obstructive CAD, we observed significantly higher EAT thickness in patients requiring coronary revascularization therapy (7.9 ± 2.56 mm vs. 3.96 ± 1.91 mm, see Supplementary material online, Figure S2). Detailed patient characteristics are depicted in Table 1.
Table 1.
Baseline characteristics
| Overall cohort (n = 657) | No obstructive CAD (n = 516) | Obstructive CAD (n = 141) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 58.06 ± 18.04 | 55.62 ± 18.57 | 67.0 ± 12.38 | <0.0001 |
| Male | 349 (53.12) | 259 (50.19) | 90 (63.83) | 0.0040 |
| Laboratory parameters | ||||
| Troponin initial (ng/L) | 6.0 (6.0–19.0) | 6.0 (6.0–11.0) | 19.0 (6.0–215.0) | <0.0001 |
| Troponin initial (ng/L) positive | 129 (19.6) | 72 (14.0) | 57 (40.4) | <0.0001 |
| CK (U/L) | 105.0 (67.0–153.0) | 102.5 (67.0–148.0) | 117.0 (85.0–173.0) | 0.0237 |
| Myoglobin (µg/dL) | 54.0 (37.0–82.0) | 49.0 (35.0–76.0) | 76.0 (46.0–109.0) | <0.0001 |
| Total cholesterol (mg/dL) | 180.69 ± 51.32 | 181.3 ± 48.72 | 179.8 ± 54.97 | 0.7995 |
| LDL-C (mg/dL) | 121.48 ± 47.69 | 121.6 ± 46.33 | 121.3 ± 49.58 | 0.9602 |
| HDL-C (mg/dL) | 48.09 ± 17.02 | 50.36 ± 16.97 | 45.1 ± 16.69 | 0.0095 |
| Triglycerides (mg/dL) | 123 (95–186) | 119.0 (89.0–182.0) | 129.0 (103.0–188.0) | 0.0924 |
| Creatinine (mg/dL) | 0.96 (0.83–1.1) | 0.95 (0.82–1.09) | 0.98 (0.85–1.14) | 0.1277 |
| Cardiovascular risk factors | ||||
| BMI (kg/m²) | 27.3 ± 5.1 | 27.3 (26.8–27.7) | 27.6 (26.9–28.4) | 0.3996 |
| Current smoking | 175 (26.64) | 132 (25.58) | 43 (30.5) | 0.1869 |
| Diabetes | 102 (15.53) | 70 (13.57) | 32 (22.7) | 0.0080 |
| Family history of CAD | 136 (20.7) | 101 (19.57) | 35 (24.82) | 0.1728 |
| Hypercholesterolaemia | 190 (28.96) | 126 (24.47) | 64 (45.39) | <0.0001 |
| Clinical presentation | ||||
| NSTEMI | 144 (21.9) | 82 (15.9) | 62 (44.0) | <0.001 |
| Systolic blood pressure (mmHg) | 135.62 ± 19.27 | 134.7 ± 19.39 | 139.1 ± 18.44 | 0.0140 |
| Diastolic blood pressure (mmHg) | 81.26 ± 13.91 | 81.36 ± 13.98 | 80.89 ± 13.67 | 0.7191 |
| Killip class I | 628 (95.59) | 498 (96.51) | 130 (92.2) | 0.0271 |
| Killip class II | 29 (4.41) | 18 (3.49) | 11 (7.8) | |
| Killip class III | 0 (0) | 0 (0) | 0 (0) | |
| Complementary prior medication | ||||
| Aspirin | 93 (14.16) | 65 (12.6) | 28 (19.86) | 0.0284 |
| P2Y12 antagonist | 13 (1.98) | 8 (1.55) | 5 (3.55) | 0.1316 |
| Antidiabetic | 75 (11.42) | 50 (9.69) | 25 (17.73) | 0.0078 |
| Antihypertensive | 363 (55.25) | 266 (51.55) | 97 (68.79) | 0.0003 |
| Lipid-lowering | 117 (17.81) | 73 (14.15) | 44 (31.21) | <0.0001 |
| ECG | ||||
| LBBB | 18 (2.74) | 15 (2.91) | 3 (2.13) | 0.6132 |
| ST-segment derivation | 11 (1.67) | 6 (1.16) | 5 (3.55) | 0.051 |
Values are mean ± SD, median (IQR), or n (%).
CAD, coronary artery disease; CK, creatinine kinase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NSTEMI, non-ST-elevation myocardial infarction; ECG, electrocardiogram; LBBB, left bundle branch block.
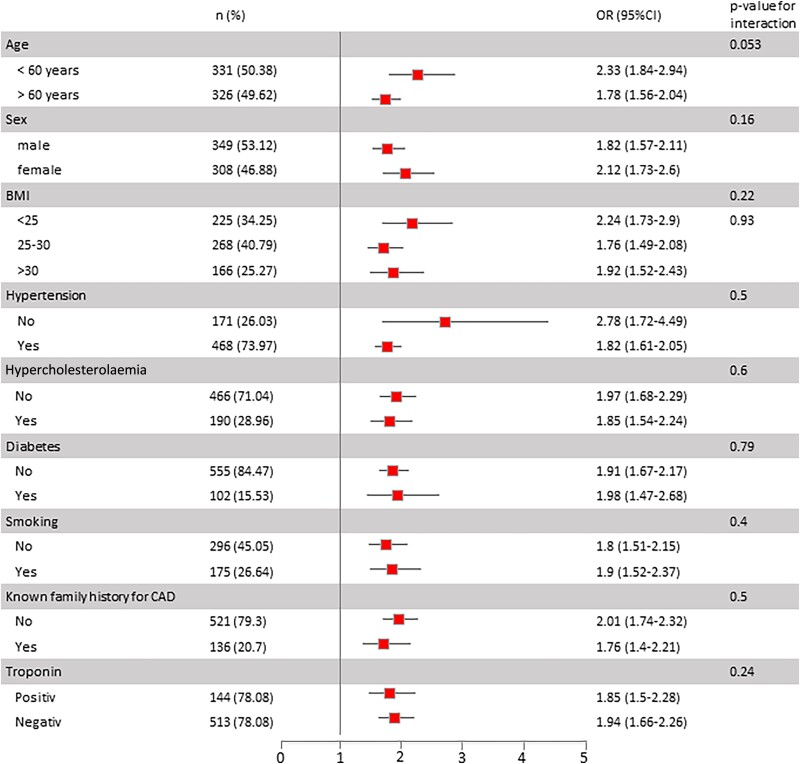
Table 2 shows the association of EAT with the presence of obstructive CAD, requiring revascularization therapy. In unadjusted regression analysis, we observed a two-fold increased odds of obstructive CAD per 1 mm increase in EAT thickness. Effect sizes remained stable and highly significant when controlling for age, sex, and BMI as well as when ancillary controlling for traditional cardiovascular risk factors and cardiac biomarkers. When ancillary controlling for the GRACE score as a clinically established score for disease prognosis in patients with acute chest pain, we observed stable effect sizes. Further adjusting for traditional risk factors and cardiac biomarkers did not influence the association. In sensitivity analysis, the TIMI risk score was used instead of the GRACE score in multivariable regression analyses, which did not affect the association of EAT with the presence of obstructive CAD (see Supplementary material online, Table S1). In the subgroup of patients with available high-sensitive troponin levels (n = 510), effect sizes remained unaltered when contemporary values from high-sensitive assays were replaced by values from high-sensitive assays in multivariable models. Likewise, using core lab–based EAT thickness assessment led to a similar association of EAT with the primary endpoint in multivariable analyses (see Supplementary material online, Table S1). Comparing the association of EAT thickness with obstructive CAD, stratified by target vessel for coronary intervention, we found a significant association for all three vessels with slightly higher effect sizes for the association of EAT thickness with LAD stenoses, however, with overlapping CIs (see Supplementary material online, Table S2). When comparing EAT thickness in patients without CAD vs. single-vessel disease vs. multivessel disease, we observed a stepwise increase in EAT thickness (4.44 ± 2.13 CAD, 7.1 ± 2.14 for single vessel, and 8.29 ± 2.65 for multivessel; P < 0.0001 for single vs. no, P < 0.0001 for multivessel vs. no). Furthermore, we observed significantly higher effect sizes for the association of EAT with obstructive CAD in patients with multivessel disease as compared with single-vessel disease (see Supplementary material online, Table S3). Figure 1 depicts the predefined subgroup analyses for the association of EAT thickness with obstructive CAD. We observed slightly higher effect sizes for younger patients, females, patients with BMI < 25 kg/m², and patients without known hypertension or elevated blood pressure. Importantly, no significant interaction of traditional risk factors or elevated troponin levels was observed.
Table 2.
Univariate and multivariable logistic regression analyses for the association of EAT thickness with the presence of obstructive CAD
| OR (95% CI) | P-value | |
|---|---|---|
| Univariate | 1.979 (1.771–2.212) | <0.0001 |
| Adjusted for age, sex, and BMI | 1.938 (1.723–2.179) | <0.0001 |
| Adjusted for age, sex, BMI, family history of CAD, smoking, hypercholesterolaemia, diabetes, and systolic blood pressure | 1.914 (1.700–2.156) | <0.0001 |
| Adjusted for age, sex, BMI, family history of CAD, smoking, hypercholesterolaemia, diabetes, systolic blood pressure, troponin, CK, and myoglobin | 1.866 (1.644–2.118) | <0.0001 |
| Adjusted for the GRACE risk score | 1.933 (1.723–2.168) | <0.0001 |
| Adjusted for the GRACE risk score, BMI, hypercholesterolaemia, family history of CAD, diabetes, and smoking | 1.927 (1.713–2.168) | <0.0001 |
| Adjusted for the GRACE risk score, BMI, hypercholesterolaemia, family history of CAD, diabetes, smoking, CK, and myoglobin | 1.938 (1.710–2.197) | <0.0001 |
BMI, body mass index; GRACE, Global Registry of Acute Coronary Events; CAD, coronary artery disease; CK, creatinine kinase; OR, odds ratio.
Figure 1.
Forrest plot for subgroup analysis for the association of epicardial adipose tissue thickness with the presence of obstructive coronary artery disease. CAD, coronary artery disease; EAT, epicardial adipose tissue.
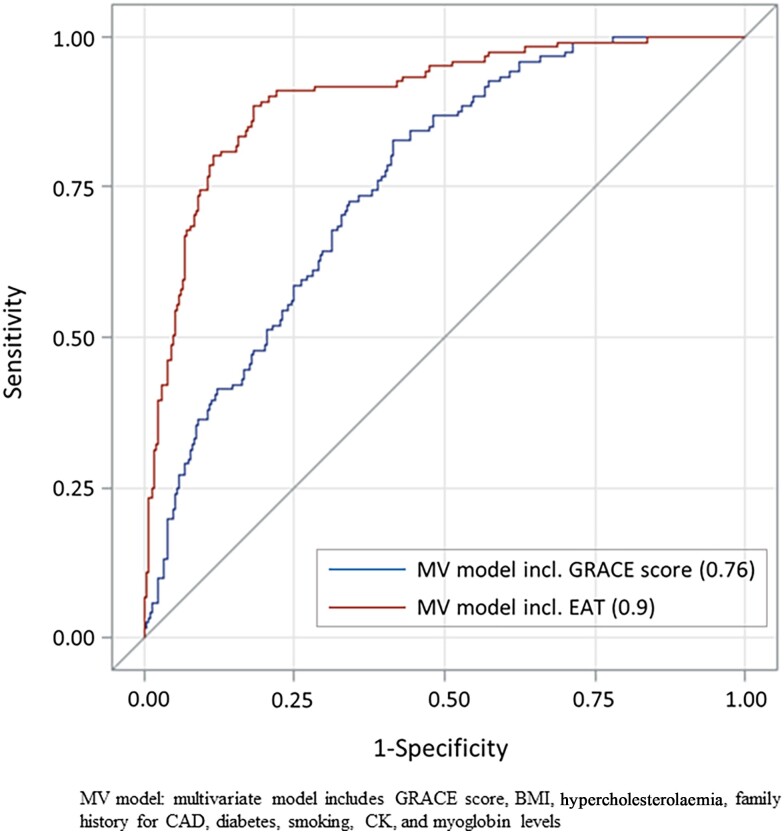
Figure 2 shows the ROC for the improvement of the AUC for EAT in addition to a multivariable model (including the GRACE score, BMI, hypercholesterolaemia, family history of CAD, diabetes, smoking, CK, and myoglobin levels), demonstrating a significant improvement in the prediction of obstructive CAD (AUC 0.759–0.901, P < 0.0001). Similar results were observed for a multivariable model containing the TIMI score instead of the GRACE score (AUC 0.777–0.911, P < 0.0001, see Supplementary material online, Figure S3) as well as when including high-sensitive troponin instead of contemporary high-sensitive troponin to the model (AUC 0.778–0.935, P < 0.0001, see Supplementary material online, Figure S4). Based on the ROC analysis and Youden’s J index, we defined an EAT thickness of 5.5 mm as the best threshold to predict obstructive CAD. In univariate regression analysis, patients with an EAT thickness above this threshold had a 44% increased risk of obstructive CAD as compared with patients with EAT below this threshold [odds ratio (OR) 1.44 (95% CI 1.2–1.73, P < 0.0001]. Effect sizes remained stable in a multivariate model [1.47 (1.21–1.78) P < 0.001].
Figure 2.
Receiver operating characteristic curve, demonstrating an improved prediction of the presence of obstructive coronary artery disease by epicardial adipose tissue thickness in addition to a multivariable model containing traditional risk factors and the GRACE score. CAD, coronary artery disease; EAT, epicardial adipose tissue.
Discussion
We here demonstrate that EAT is a strong predictor of the presence of obstructive CAD, requiring revascularization therapy, in patients presenting with chest pain to the emergency department independent of traditional cardiovascular risk factors and cardiac biomarkers. Effect sizes were similar in patients with troponin-positive and troponin-negative acute chest pain independent of sex, age groups, and the GRACE or TIMI score. Our results suggest that in haemodynamically stable patients with acute chest pain but with inconclusive initial ECG, routine echocardiography imaging of the heart for quantification of EAT could allow for improved detection of patients with underlying CAD. We determined thresholds of EAT thickness, which served as a predictor of obstructive CAD. EAT thickness above the defined thresholds was associated with a nearly 50% increased risk of obstructive CAD. These data suggest that the utilization of EAT thickness of 5.5 mm for patients presenting with acute chest pain can serve as a novel echocardiography-derived parameter, qualifying for the detection of patients with obstructive CAD in an emergency setting.
Due to the anatomical proximity, EAT and the coronary vasculature are connected by paracrine and vasocrine signalling pathways.25 Epicardial adipose tissue is referred to a protective role in secreting anti-inflammatory, anti-atherogenic molecules and nutritive fatty acids.25,26 Intrinsic and extrinsic factors as well as the amount of EAT are inducing a shift of the protective role of EAT to a more pro-inflammatory and pro-atherogenic secretion.10,11 These pathophysiological properties define the molecular effect of EAT on the myocardium and the coronary arteries, which lead to the development and progression of atherosclerosis.16,17 Therefore, a link of EAT with coronary plaque burden, high-risk plaque characteristics, and myocardial ischaemia was shown.27–30
Different imaging technologies for quantification of EAT are described for scientific and clinical approaches. Computed tomography and magnetic resonance imaging (MRI) are considered the gold standard for the measurement of three-dimensional EAT volume.31,32 However, quantification of EAT via CT and MRI requires complex and time-consuming processes, which are currently reserved centres with dedicated expertise.33 In an emergency setting, there is the need for a routinely available, easily accessible, and time-efficient imaging modality. Echocardiography allows for reliable quantification of EAT thickness, is readily available, and can routinely be performed in patients presenting with acute chest pain.6,34 Our finding supports previous results that echocardiographic assessment of EAT thickness allows for reliable stratification of patients, identifying those with increased probability of CAD.35
In the current guidelines for the management of patients with acute coronary syndrome without persistent ST-segment elevations, non-invasive imaging modalities gain importance.6 Computed tomography enables the exclusion of significant CAD stenosis, non-invasive stress testing provides signs of myocardial ischaemia via detection of wall motion abnormalities, and cardiac MRI displays perfusion as well as wall motion abnormalities.6,36,37
Transthoracic echocardiography allows for the evaluation of left ventricular wall motion defects and is able to identify high-risk patients for acute coronary syndrome.38 Additionally, transthoracic echocardiography differentiates alternative pathologies as the cause of chest pain, such as aortic valve stenosis or signs of pulmonary embolism. Thereby, transthoracic echocardiography provides multiple diagnostic information consolidating the indication for an invasive strategy or refuting where not necessary.34 While transthoracic echocardiography is routinely available in chest pain units, we specifically aimed to test the hypothesis that echocardiography-derived EAT thickness is associated with the presence of obstructive CAD in patients presenting with acute chest pain and may therefore improve the prediction of underlying CAD in addition to established clinical algorithms. The present prospective observational study was designed not only to describe the association of EAT and obstructive CAD but to evaluate whether bedside echocardiographic assessment of EAT can improve the prediction using echocardiography-derived EAT measurements in an emergency setting. While further studies on larger cohorts are needed to confirm our results, the present study indicates that echocardiography-derived assessment of EAT serves as an additional marker for CAD prediction in patients presenting with acute chest pain, supporting the routine utilization of echocardiography in chest pain units.
Timing of invasive strategy is based in particular on conditions, classifying patients as very high risk (i.e. haemodynamic instability, cardiogenic shock, life-threatening arrhythmia, etc.) and high risk (established NSTEMI diagnosis, resuscitated cardiac arrest, etc.).6 In addition, established risk scores like the GRACE and TIMI risk scores are accounted for in clinical routine and allow therapeutic decision-making based on the patient’s mortality risk and risk of ischaemic events.22,23 As the initial evaluation of ECG and biomarker testing is inconclusive in the majority of patients, many patients undergo additional non-invasive or even invasive testing for further assessment. Adding quantification of EAT thickness measured via transthoracic echocardiography as a reliable predictor of the presence of obstructive CAD may alter patient management with regard to the pre-test probability of CAD as the cause of chest pain. In a subgroup analysis, comparing the association of EAT thickness with obstructive CAD, stratified by target vessel for coronary intervention, we found a relevant link, irrespective of target vessel, with slightly higher effect sizes for the left anterior descending (LAD). In this observational study, we included an all-comers cohort of patients presenting with chest pain. As indicated by our results, this cohort represents a heterogeneous group with an overall relatively low prevalence of obstructive CAD. Further analyses on high-risk cohorts are needed to confirm our results.
Limitations
The present study was designed as a single-centre observational study. Additional research is warranted to confirm our results in a multicentre setting and to demonstrate that EAT-based alteration of clinical decision-making leads to improved patient management. The decision for coronary angiography was made at the discretion of treating physicians. Only 302 patients (46%) underwent coronary angiography, which represents an important limitation, as obstructive CAD could not definitely be ruled out in the remaining cohort. However, in a subgroup analysis including only these 302 patients, we observed identical results. As a consequence, definite exclusion of CAD was not possible for all patients discharged without further testing. To address the possibility that potential inadequate discharges with the need for later revascularization therapy may bias the results, we performed a follow-up of 90 days, assessing any late revascularizations. The presence of obstructive CAD was defined at the discretion of treating experienced interventional cardiologists. Additional imaging modalities like intravascular ultrasound or optical coherence tomography as well as assessment of lesion haemodynamics via fractional flow reserve/instantaneous wave-free ratio would have further complemented the diagnostic evaluation of these lesions; however, they were not mandatory according to the study protocol. We performed sensitivity analysis in the subgroup of patients undergoing coronary angiography, providing similar effect sizes as for the overall cohort. Dedicated study personnel, blinded to the patient’s clinical presentation and clinical workup results, performed echocardiography in a bedside manner. We cannot rule out that the clinical impression may have influenced the assessment. To address this concern, we performed a core lab–based blinded assessment of echocardiography images for EAT measurement, showing a high correlation with the bedside measures and a similar association with the presence of obstructive CAD. Also, we used transthoracic echocardiography–derived EAT thickness due to its ease of use and broad accessibility over three-dimensional assessment using CT or MRI as the gold standard. However, a recent meta-analysis demonstrated comparable differences in EAT measures in patients with vs. without myocardial infarction independent of the used imaging modality.35 As part of the study protocol, only a focused echocardiographic evaluation was performed. Therefore, we do not have any information on the relationship of EAT with concomitant valvular disease, which may have been the underlying cause of the symptoms of some patients. Further studies are needed to specifically address the value of transthoracic echocardiography in the emergency department as well as the link between EAT and valvular heart disease in acute chest pain patients. Lastly, our results are based on a predominantly Caucasian cohort; hence, generalization to other ethnic groups remains uncertain.
Conclusions
Epicardial adipose tissue strongly and independently predicts the presence of obstructive CAD, requiring revascularization therapy, in patients presenting with acute chest pain to the emergency department. Our results suggest that routine echocardiographic assessment with quantification of EAT may improve diagnostic algorithms of patients with acute chest pain.
Supplementary Material
Contributor Information
Stefanie Jehn, The West German Heart and Vascular Center Essen, Department of Cardiology and Vascular Medicine, University Hospital Essen, Hufelandstr. 55, 45147 Essen, Germany.
Anja Roggel, The West German Heart and Vascular Center Essen, Department of Cardiology and Vascular Medicine, University Hospital Essen, Hufelandstr. 55, 45147 Essen, Germany.
Iryna Dykun, The West German Heart and Vascular Center Essen, Department of Cardiology and Vascular Medicine, University Hospital Essen, Hufelandstr. 55, 45147 Essen, Germany.
Bastian Balcer, The West German Heart and Vascular Center Essen, Department of Cardiology and Vascular Medicine, University Hospital Essen, Hufelandstr. 55, 45147 Essen, Germany.
Fadi Al-Rashid, The West German Heart and Vascular Center Essen, Department of Cardiology and Vascular Medicine, University Hospital Essen, Hufelandstr. 55, 45147 Essen, Germany.
Matthias Totzeck, The West German Heart and Vascular Center Essen, Department of Cardiology and Vascular Medicine, University Hospital Essen, Hufelandstr. 55, 45147 Essen, Germany.
Joachim Risse, Center of Emergency Medicine, University Hospital Essen, Hufelandstr. 55, 45147 Essen, Germany.
Clemens Kill, Center of Emergency Medicine, University Hospital Essen, Hufelandstr. 55, 45147 Essen, Germany.
Tienush Rassaf, The West German Heart and Vascular Center Essen, Department of Cardiology and Vascular Medicine, University Hospital Essen, Hufelandstr. 55, 45147 Essen, Germany.
Amir A Mahabadi, The West German Heart and Vascular Center Essen, Department of Cardiology and Vascular Medicine, University Hospital Essen, Hufelandstr. 55, 45147 Essen, Germany.
Lead author biography
 Stefanie Jehn studied medicine at the University Duisburg-Essen. After graduating in 2018, she started her training in internal medicine and cardiology at the University Hospital Essen in the Department of Cardiology and Vascular Medicine. For the present study, she was supported by the Junior Clinician Scientist Program by the University Medicine Essen Academy (UMEA), allowing her to combine her clinical training and clinical research.
Stefanie Jehn studied medicine at the University Duisburg-Essen. After graduating in 2018, she started her training in internal medicine and cardiology at the University Hospital Essen in the Department of Cardiology and Vascular Medicine. For the present study, she was supported by the Junior Clinician Scientist Program by the University Medicine Essen Academy (UMEA), allowing her to combine her clinical training and clinical research.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
S.J. was supported as a Junior Clinician Scientist within the University Medicine Essen Academy (UMEA) funded by the Faculty of Medicine of the University of Duisburg-Essen. I.D. was supported by the German Research Foundation (DY149/2).
Consent
All patients provided written informed consent.
References
- 1. Puymirat E, Simon T, Cayla G, Cottin Y, Elbaz M, Coste P, Lemesle G, Motreff P, Popovic B, Khalife K, Labèque J-N, Perret T, Le Ray C, Orion L, Jouve B, Blanchard D, Peycher P, Silvain J, Steg PG, Goldstein P, Guéret P, Belle L, Aissaoui N, Ferrières J, Schiele F, Danchin N. Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017;136:1908–1919. [DOI] [PubMed] [Google Scholar]
- 2. Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 2012;366:1393–1403. [DOI] [PubMed] [Google Scholar]
- 3. Shah ASV, Anand A, Strachan FE, Ferry AV, Lee KK, Chapman AR, Sandeman D, Stables CL, Adamson PD, Andrews JPM, Anwar MS, Hung J, Moss AJ, O'Brien R, Berry C, Findlay I, Walker S, Cruickshank A, Reid A, Gray A, Collinson PO, Apple FS, McAllister DA, Maguire D, Fox KAA, Newby DE, Tuck C, Harkess R, Parker RA, Keerie C, Weir CJ, Mills NL, Marshall L, Stewart SD, Fujisawa T, Vallejos CA, Tsanas A, Hautvast M, McPherson J, McKinlay L, Malo J, Fischbacher CM, Croal BL, Leslie SJ, Walker A, Wackett T, Armstrong R, Stirling L, MacDonald C, Sadat I, Finlay F, Charles H, Linksted P, Young S, Alexander B, Duncan C. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet 2018;392:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smulders MW, Kietselaer B, Wildberger JE, Dagnelie PC, Brunner-La Rocca HP, Mingels AMA, van Cauteren YJM, Theunissen RALJ, Post MJ, Schalla S, van Kuijk SMJ, Das M, Kim RJ, Crijns HJGM, Bekkers SCAM.. Initial imaging-guided strategy versus routine care in patients with non-ST-segment elevation myocardial infarction. J Am Coll Cardiol 2019;74:2466–2477. [DOI] [PubMed] [Google Scholar]
- 5. Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 2012;367:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM, Kastrati A, Mamas MA, Aboyans V, Angiolillo DJ, Bueno H, Bugiardini R, Byrne RA, Castelletti S, Chieffo A, Cornelissen V, Crea F, Delgado V, Drexel H, Gierlotka M, Halvorsen S, Haugaa KH, Jankowska EA, Katus HA, Kinnaird T, Kluin J, Kunadian V, Landmesser U, Leclercq C, Lettino M, Meinila L, Mylotte D, Ndrepepa G, Omerovic E, Pedretti RFE, Petersen SE, Petronio AS, Pontone G, Popescu BA, Potpara T, Ray KK, Luciano F, Richter DJ, Shlyakhto E, Simpson IA, Sousa-Uva M, Storey RF, Touyz RM, Valgimigli M, Vranckx P, Yeh RW, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 7. Becker MC, Galla JM, Nissen SE. Left main trunk coronary artery dissection as a consequence of inaccurate coronary computed tomographic angiography. Arch Intern Med 2011;171:698–701. [DOI] [PubMed] [Google Scholar]
- 8. Redberg RF. Coronary CT angiography for acute chest pain. N Engl J Med 2012;367:375–376. [DOI] [PubMed] [Google Scholar]
- 9. Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr 2009;22:1311–1319. quiz 417-8. [DOI] [PubMed] [Google Scholar]
- 10. Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003;108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 11. Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar Sudhesh, McTernan Philip G. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 2006;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, Thomas S, Herdman L, Kotanidis CP, Thomas KE, Griffin BP, Flamm SD, Antonopoulos AS, Shirodaria C, Sabharwal N, Deanfield J, Neubauer S, Hopewell JC, Channon KM, Achenbach S, Antoniades C. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahabadi AA, Rassaf T. Imaging of coronary inflammation for cardiovascular risk prediction. Lancet 2018;392:894–896. [DOI] [PubMed] [Google Scholar]
- 14. Mahabadi AA, Lehmann N, Kälsch H, Robens T, Bauer M, Dykun I, Budde T, Moebus S, Jöckel K-H, Erbel R, Möhlenkamp S. Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: results from the Heinz Nixdorf recall study. JACC Cardiovasc Imaging 2014;7:909–916. [DOI] [PubMed] [Google Scholar]
- 15. Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell Christopher J., Fox Caroline S. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008;117:605–613. [DOI] [PubMed] [Google Scholar]
- 16. Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 2001;103:1057–1063. [DOI] [PubMed] [Google Scholar]
- 17. Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation 2002;105:2893–2898. [DOI] [PubMed] [Google Scholar]
- 18. Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol 2022;19:593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahabadi AA, Berg MH, Lehmann N, Kälsch H, Bauer M, Kara K, Dragano N, Moebus S, Jöckel K-H, Erbel R, Möhlenkamp S. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol 2013;61:1388–1395. [DOI] [PubMed] [Google Scholar]
- 20. Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O'Donnell C. J., Fox C. S., Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J 2009;30:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iacobellis G, di Gioia CR, Cotesta D, Petramala L, Travaglini C, De Santis V, Vitale D., Tritapepe L., Letizia C. Epicardial adipose tissue adiponectin expression is related to intracoronary adiponectin levels. Horm Metab Res. 2009;41:227–31. [DOI] [PubMed] [Google Scholar]
- 22. Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum Á, Flather MD, Fox KAA; GRACE Investigators . A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. Jama 2004;291:2727–2733. [DOI] [PubMed] [Google Scholar]
- 23. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. Jama 2000;284:835–842. [DOI] [PubMed] [Google Scholar]
- 24. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 25. Mancio J, Oikonomou EK, Antoniades C. Perivascular adipose tissue and coronary atherosclerosis. Heart 2018;104:1654–1662. [DOI] [PubMed] [Google Scholar]
- 26. Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res 2008;40:442–445. [DOI] [PubMed] [Google Scholar]
- 27. Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kälsch H, Seibel RM, Erbel R, Möhlenkamp S. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis 2010;211:195–199. [DOI] [PubMed] [Google Scholar]
- 28. Maurovich-Horvat P, Kallianos K, Engel LC, Szymonifka J, Schlett CL, Koenig W, Hoffmann U, Truong QA. Relationship of thoracic fat depots with coronary atherosclerosis and circulating inflammatory biomarkers. Obesity (Silver Spring) 2015;23:1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schlett CL, Ferencik M, Kriegel MF, Bamberg F, Ghoshhajra BB, Joshi SB, Nagurney JT, Fox CS, Truong QA., Hoffmann U. Association of pericardial fat and coronary high-risk lesions as determined by cardiac CT. Atherosclerosis 2012;222:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hell MM, Ding X, Rubeaux M, Slomka P, Gransar H, Terzopoulos D, Hayes S, Marwan M, Achenbach S, Berman DS, Dey D. Epicardial adipose tissue volume but not density is an independent predictor for myocardial ischemia. J Cardiovasc Comput Tomogr 2016;10:141–149. [DOI] [PubMed] [Google Scholar]
- 31. Hendricks S, Rassaf T, Mahabadi AA. Cardiac metabolic implications of fat depot imaging. Curr Cardiovasc Imaging Rep 2020;13:10. [Google Scholar]
- 32. Monti CB, Codari M, De Cecco CN, Secchi F, Sardanelli F, Stillman AE. Novel imaging biomarkers: epicardial adipose tissue evaluation. Br J Radiol 2020;93:20190770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Commandeur F, Goeller M, Betancur J, Cadet S, Doris M, Chen X, Berman DS, Slomka PJ, Tamarappoo BK, Dey D. Deep learning for quantification of epicardial and thoracic adipose tissue from non-contrast CT. IEEE Trans Med Imaging 2018;37:1835–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Breuckmann F, Hochadel M, Voigtländer T, Haude M, Schmitt C, Münzel T, Giannitsis E, Mudra H, Heusch G, Schumacher B, Barth S, Schuler G, Hailer B, Walther D, Senges J. The use of echocardiography in certified chest pain units: results from the German chest pain unit registry. Cardiology 2016;134:75–83. [DOI] [PubMed] [Google Scholar]
- 35. Hendricks S, Dykun I, Balcer B, Totzeck M, Rassaf T, Mahabadi AA. Epicardial adipose tissue is a robust measure of increased risk of myocardial infarction - a meta-analysis on over 6600 patients and rationale for the EPIC-ACS study. Medicine (Baltimore 2021;100:e28060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, Paterson D. I, Syed MA, Aletras AH, Arai AE. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol 2006;47:1427–1432. [DOI] [PubMed] [Google Scholar]
- 37. Lancellotti P, Price S, Edvardsen T, Cosyns B, Neskovic AN, Dulgheru R, Flachskampf FA, Hassager C, Pasquet A, Gargani L, Galderisi M, Cardim N, Haugaa KH, Ancion A, Zamorano J-L, Donal E, Bueno H, Habib G. The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care 2015;4:3–5. [DOI] [PubMed] [Google Scholar]
- 38. Frenkel O, Riguzzi C, Nagdev A. Identification of high-risk patients with acute coronary syndrome using point-of-care echocardiography in the ED. Am J Emerg Med. 2014;32:670–672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.