Abstract
The majority of patients with systemic lupus erythematosus (SLE) have cutaneous manifestations at some point in their disease. The skin findings in lupus erythematosus are classified as lupus-specific or lupus-nonspecific based on histopathologic findings. Lupus-specific skin diseases include chronic cutaneous lupus erythematosus, subacute cutaneous lupus erythematosus, and acute cutaneous lupus erythematosus. There are subsets of skin lesions within each group and the likelihood of associated SLE varies amongst them. Lupus nonspecific lesions are more common in patients with SLE and tend to coincide with active systemic disease. Lupus nonspecific lesions may be seen as a feature of another disease process, including other connective tissue diseases. It is important for the rheumatologist to be familiar with the spectrum of cutaneous disease in lupus erythematosus to help prognosticate the likelihood of systemic disease and ensure patients receive timely dermatologic care with the goal of controlling disease activity to prevent damage.
Keywords: Systemic lupus erythematosus, skin, autoimmune
Introduction
Lupus erythematous (LE) is a complex autoimmune disease entity with heterogeneous cutaneous and systemic manifestations that can evolve over the course of disease. The skin is the second most frequently affected organ system in systemic lupus erythematosus (SLE), with cutaneous manifestations occurring in 70–85% of individuals over the course of disease and as a presenting symptom in up to 25% of patients.1 In the 1960s before autoimmune serology became generally available, skin changes were said to be the second most common presenting clinical manifestation of SLE. 2 Skin disease carries a significant burden in terms of psychosocial well-being and medical costs. Patients with cutaneous lupus erythematosus (CLE) have similar or worse emotional components of quality of life than patients with hypertension, congestive heart failure, and type 2 diabetes.3 Population based studies in the US and Europe report an incidence of CLE of 3–4 per 100,000, with a prevalence of 70 per 100,000, while the incidence of discoid lupus erythematosus (DLE) is estimated at 0.8–3.7 per 100,000.4–9 These numbers are comparable to recent incidence and prevalence rates for SLE in the United States.10
Classification of SLE and CLE
A brief review of the classification criteria of SLE and CLE is included to frame the discussion of cutaneous involvement in SLE. Importantly, these criteria are designed for research purposes and not intended to diagnose individual patients.
Four of the eleven criteria in the 1997 American College of Rheumatology (ACR) diagnostic criteria for SLE are cutaneous features of disease, including malar rash, discoid rash, photosensitivity, and oral ulcers.11,12 Based on these criteria patients can be classified as having SLE with only skin manifestations which are not exclusive for SLE (photosensitivity is a typical feature of dermatomyositis), therefore these diagnostic criteria may skew diagnosis and fail to distinguish CLE from SLE.13 The 2019 European League Against Rheumatism (EULAR)/ACR classification criteria for SLE include positive ANA followed by additive weighted criteria in 7 clinical and 3 immunologic domains; patients accumulating ≥10 points are classified as SLE. Mucocutaneous is one of the 7 clinical realms, and includes alopecia (2 points), oral ulcers (2 points), subacute cutaneous lupus erythematous (SCLE) OR DLE(4 points), and acute cutaneous lupus erythematous (ACLE) (6 points).14 In one study, requiring ANA positivity as per the EULAR/ACR criteria excluded 7.5% of CLE patients previously diagnosed with SLE, some of whom had internal organ involvement including cytopenias, proteinuria, and/or inflammatory arthritis.15 The Systemic Lupus Collaborating Clinics (SLICC) criteria classify a patient as having SLE if they have biopsy-proven lupus nephritis with positive ANA or anti-dsDNA antibodies or at least 4 out of 17 criteria including at least one immunologic criterion and one clinical criterion.16 Four of the clinical criteria are mucocutaneous in nature including ACLE, chronic cutaneous lupus erythematosus (CCLE), oral ulcers, and nonscarring alopecia.16
No universally accepted classification criteria exists for CLE. Skin lesions in patients with SLE are divided according to the most-widely used criteria suitable for rheumatologists in everyday clinical practice proposed by Gilliam and Sontheimer, which divides CLE into LE-specific and LE-nonspecific skin conditions.17,18 Other classification criteria, such as the “Duesseldorf Classification”, have been developed but have not gained universal acceptance.19 LE-specific skin conditions include CCLE, SCLE, and ACLE as well as their various subtypes (Figure 1). While lupus erythematosus tumidus (LET) is considered by some a form of CCLE, it is recognized by the “Duesseldorf classification” and European S2k guidelines as a fourth primary subset of CLE known as intermittent CLE (ICLE).20,21 These LE-specific conditions have distinct clinical morphologies, but similar histopathologic features on routine hematoxylin and eosin staining. These histologic features include lichenoid interface dermatitis with basal layer vacuolization, apoptotic keratinocytes, periadnexal and perivascular mononuclear cell infiltrate, epidermal atrophy, and basement membrane thickening (Figure 2). 15 In DLE, there is a tendency for more hyperkeratosis, follicular plugging, and thickening of the basement membrane relative to ACLE or SCLE. However, not all of these features are found in all forms of LE-specific variants, and they can be found in conditions other than CLE. Interface dermatitis, which consists of liquefactive degeneration of the epidermal basal layers, is not typically associated with LET or lupus profundus but is often seen in dermatomyositis.22,23 A biopsy is recommended to confirm the diagnosis of CLE, as there are a variety of other diseases that mimic the variants of CLE (Figure 3).
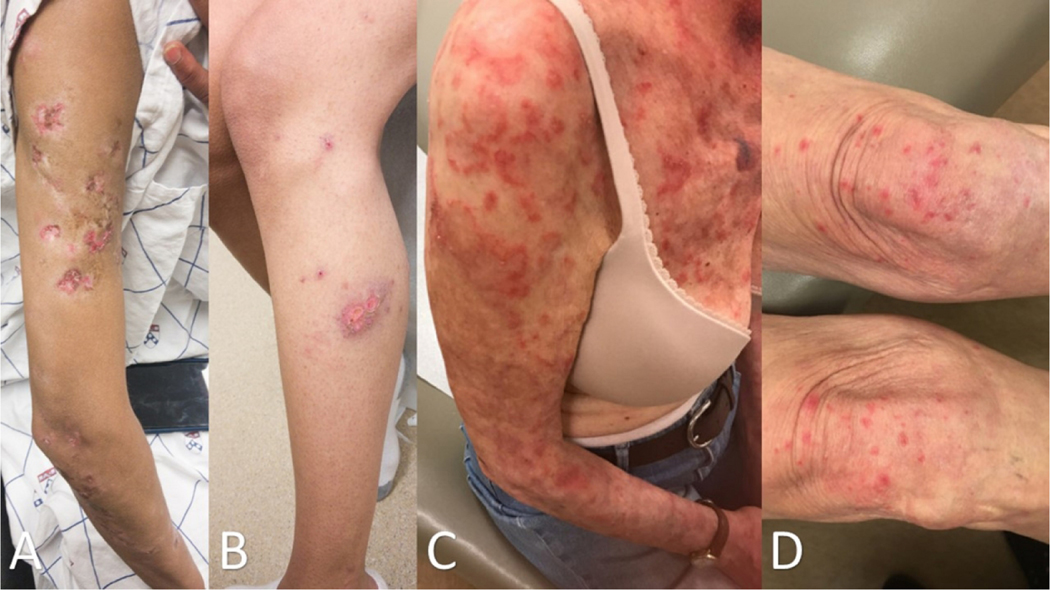
Figure 1:
Typical CLE lesions: (A) Active DLE lesions with erythema and scale are shown along with areas of damage, i.e., dyspigmentation and scarring, from prior active lesions. (B) Erythematous DLE lesions are shown on the leg. (C) Annular SCLE lesions are seen on the arm and chest as well as (D) the legs.
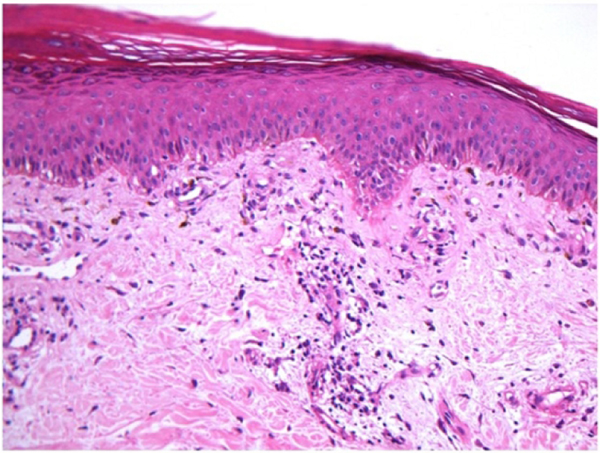
Figure 2:
This biopsy demonstrates the vacuolar interface dermatitis found in most LE specific skin conditions.
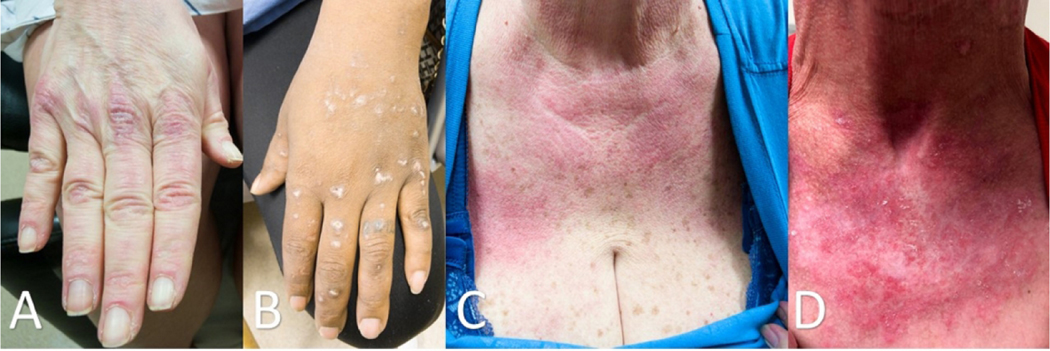
Figure 3:
Careful clinical examination is often required to distinguish CLE from dermatomyositis. (A) Dermatomyositis of the hands often shows confluent erythema of the skin overlying the MCP and ICP joints and the extensor tendons while (B) DLE lesions are less likely to be localized to these areas and can resolve with scarring. Involvement of the v-area of the neck can appear very similar in (C) dermatomyositis and (D) CLE and requires clinical correlation with other areas of involved skin to arrive at the correct diagnosis.
LE Specific Skin Disease
As mentioned above, CLE is divided into the following primary subsets: ACLE, SCLE, CCLE, and also ICLE in certain classification systems (Table 1). It is possible for patients to have more than one form of CLE. A study of 191 patients with CLE showed that 68% had one type, 29% had two types, and 3% had three types.24 A U.S. population-based study showed that 12% of patients with CLE had disease progression to SLE, with a mean time to progression of 8 years.7 The cumulative incidence of SLE among patients with a diagnosis of CLE in the same study was 5% at 5 years, 10% at 10 years, 15% at 15 years, 19% at 20 years, and 23% at 25 years.7 Early recognition of patients with CLE who are at risk for developing SLE is important. Signs of nephropathy, elevated ANA titers, serositis, and arthralgias/arthritis or other new symptoms of systemic disease may suggest transition into SLE and should be closely followed. Patients with localized DLE, hypertrophic LE, lupus erythematosus panniculitis (LEP), and LET are more likely to have skin limited LE; while those with generalized DLE or SCLE often meet ACR criteria for SLE and those with ACLE or LE nonspecific skin lesions are most likely to have systemic disease.25
Table 1.
LE Specific Skin Disease
| CLE Subtype | Clinical Characteristics |
|---|---|
| ACLE | 30–50% patients with SLE. Flares often parallel systemic disease activity. Can see +ANA, +anti-dsDNA, +anti-Smith |
| • Localized | Raised or flat malar rash. Photosensitive, non-scarring, transient. |
| • Generalized | Widespread maculopapular rash above and below the neck. Dorsum of hands sparing MCP and IP joints. Photosensitive, pruritic. |
| • TEN-like | Widespread denudation and blistering on sun exposed areas |
| SCLE | Recurrent course of widespread, highly photosensitive lesions that resolve without scarring though dyspigmentation may occur. 10–15% patients have SLE with arthralgias/myalgias, rare internal organ involvement. Often +ANA, +SSA. 1/3 cases drug induced |
| • Annular | Scaly annular erythematous plaques often merge to polycyclic morphology |
| • Papulosquamous | Resembles psoriasis or eczema |
| • Erythrodermic | Generalized exfoliative erythroderma |
| CCLE | Chronic, recurrent disease course. Rates of SLE vary between subtypes |
| • DLE | Erythematous, sometimes scaly plaques exacerbated by sun-exposure and trauma, progress to dyspigmentation and atrophic scarring. Localized if confined to head and neck. Generalized if extends below neck. |
| • Hypertrophic | Papular lesions on face, extensor surfaces, palms/soles |
| • Mucosal | Erosions and macules on mucosal surfaces |
| • LEP | Indurated subcutaneous nodules or plaques in face, scalp, upper torso, buttocks, proximal extremities. Atrophic scars. |
| • CHLE | Painful violaceous plaques and nodules in cold-exposed areas, may progress to erosions or ulcerations on acral surfaces |
| • LET | Erythematous macules, papules, plaques with smooth surfaces and no scale, sharp raised borders. Very photosensitive. |
Abbreviations: LE, lupus erythematosus; CLE, cutaneous lupus erythematosus; ACLE, acute cutaneous lupus erythematosus; TEN, toxic epidermal necrolysis; SCLE, subacute cutaneous lupus erythematosus; CCLE, chronic cutaneous lupus erythematosus; DLE, discoid lupus erythematosus; LEP, lupus erythematosus panniculitis; CHLE, chilblain lupus erythematosus; LET, lupus erythematosus tumidus
Chronic Cutaneous Lupus Erythematosus
CCLE has several subtypes, including DLE (Figure 1a), LEP, LET, and chilblain lupus.26 CCLE is notable for demonstrating a chronic, recurrent disease course which typically requires long-term treatment with potential for progression to involve internal organs.27,28 DLE is the most common subtype of CCLE representing 50% of cases.28 DLE is considered localized if it involves exclusively the head and neck area while generalized DLE extends below the neck with a predilection for the upper extremity extensor surfaces.28 Generalized DLE is more often associated with SLE, and patients with generalized DLE or progressive localized DLE should be re-evaluated for progressive systemic disease.25 Both localized and generalized DLE consist of erythematous and sometimes scaly plaques in sun-exposed areas which progress to dyspigmentation and scarring.28 A recent study to develop classification criteria for DLE determined that clinical variables including atrophic scarring, location in the conchal bowl, and preference for the head and neck were most important, with lower points assigned for dyspigmentation, follicular hyperkeratosis and/or plugging, and erythematous to violaceous color.29 Early in disease prior to development of damage it can be difficult to differentiate DLE from SCLE, and SCLE can cause dyspigmentation that can mimic scarring. Careful attention to loss of skin markings including follicular openings is required to establish that scarring is present. Hypertrophic or verrucous DLE is characterized by papular lesions that tend to occur on the face, extensor surfaces, or palms and soles.28 Mucosal DLE typically presents as erosions or macules that can have radiating striae located in the lips, palate, gingiva, or other mucosal surfaces.30 LEP, also known as lupus profundus, presents as indurated subcutaneous nodules or plaques which tend to occur in the face, scalp, upper torso, buttocks, and proximal extremities. These lesions can progress to ulceration or subcutaneous atrophy.30 A biopsy shows lobular panniculitis and it is important to confirm the diagnosis as the differential includes subcutaneous panniculitic-like T-cell lymphoma.25 Approximately 50% of patients with LEP will also have DLE skin lesions visible at the overlying skin surface. As mentioned above, LET is considered by European S2k guidelines to be a fourth primary subset of CLE, ICLE.21 LET lesions tend to occur on the face, neck, upper chest, and shoulders and consist of erythematous macules, papules, and plaques, normally with smooth surfaces and no scale.31 Compared to other variants of CCLE, LET is particularly photosensitive and less likely to be associated with SLE.25 However there is a mucinous form of lupus with a skin biopsy identical to LET that can be seen in patients with SLE.32 Chilblain lupus erythematosus affects cold-exposed areas, particularly the acral surfaces, with painful, violaceous plaques and nodules which may progress to erosions or ulcerations.31 At some point in their disease course, twenty percent of patients with chilblain lupus erythematosus develop features of SLE.33
Subacute Cutaneous Lupus Erythematosus
SCLE is believed to occur in 10–15% of SLE patients.1 Up to 50% of patients with SCLE meet diagnostic criteria for SLE but systemic symptoms are typically arthritis/arthralgias, malaise and myalgias, with internal organ involvement such as renal or nervous system disease occurring in <10%.4,27 70% of patients with SCLE are anti-Ro (SSA) positive and 70–80% are ANA positive.34 Children of women who have SSA or SSB antibodies during pregnancy should be carefully monitored as they are at increased risk of neonatal lupus erythematosus.35 Histologically, SCLE is frequently characterized by a less dense infiltrate than in DLE, but a denser perivascular infiltrate than found in ACLE. Other histologic features include notable atrophy of the epithelium, and more significant vacuolization at the dermal-epidermal junction than in ACLE.28 Dust-like particles representing IgG binding to keratinocytes are a specific, but not sensitive, finding on direct immunofluorescence.36 The two forms of SCLE include the annular and papulosquamous subtypes, both of which are notable for a recurrent course of widespread, highly photosensitive lesions.28 Lesions tend to be distributed symmetrically in sun-exposed regions, though the central face, scalp, and skin below the waist are typically spared.28,31 Lesions usually resolve without scarring though dyspigmentation may occur.31 Some patients exhibit features of both subtypes.31 Annular SCLE presents with scaly annular erythematous plaques, which often merge to form a polycyclic morphology.31 Papulosquamous SCLE can resemble psoriasis or eczema.31 Erythrodermic LE and lupus erythematosus gyrates repens are considered rare variants of SCLE.28 Erythrodermic LE presents with generalized exfoliative erythroderma that may represent a flare of papulosquamous SCLE after sun exposure.28 Only a few cases of lupus erythematosus gyratum repens have been discussed in the literature, and they typically manifest as widely distributed chronic and recurrent figurate erythematous plaques.28 While most cases of SCLE are idiopathic, up to one third of cases are believed to be induced by exposure to drugs. The most common causes of drug-induced SCLE are proton-pump inhibitors (PPIs), antihypertensives (especially thiazide diuretics and calcium channel blockers), anticonvulsants, and antibiotics.37,38 Recently, cases of patients with preexisting SLE who subsequently developed SCLE after exposure to antihypertensives or PPIs have been described.37 It should be noted that SLE patients on systemic corticosteroids are often placed on PPIs prophylactically to prevent gastrointestinal side effects. Adding to the risk of drug-induced SCLE is the fact that there are several over-the-counter forms of PPIs now available to the public in the USA. Particularly relevant to the rheumatologist is the potential for biologic therapies including TNF-α inhibitors, IL-17 inhibitors, IL-12/23 inhibitors and cytotoxic T-lymphocyte associated protein-4 therapy to induce SCLE.39–42 Rowell’s syndrome is an entity that can be associated with SLE, DLE, or SCLE, in which patients develop erythema multiforme-like lesions and have a speckled ANA pattern.25 Sjogren’s syndrome is often a concomitant autoimmune disorder found in patients with SCLE and is more commonly associated with SCLE than other CLE subtypes.43
Acute Cutaneous Lupus Erythematosus
ACLE is believed to occur in 30–50% of patients with SLE.1 Systemic involvement is typical and ACLE rashes often flare in parallel with other organ disease activity.31 95% of patients with ACLE have a positive ANA.44 Histologically, ACLE lesions show liquefactive degeneration of the basal layer, an interface dermatitis, with perivascular and periadnexal lymphocytic infiltrate. There are localized and generalized forms of ACLE. The localized form of ACLE is the malar rash, characterized by butterfly-shaped erythema over the cheeks and nasal bridge that tends to spare the nasolabial folds, as opposed to dermatomyositis which typically involves nasolabial folds (Figure 4). The malar rash can be raised or flat and may be associated with a fine scale, and is classically sun-induced, non-scarring, and transient. The less common generalized form of ACLE, sometimes called a “maculopapular rash of lupus” or “photosensitive lupus dermatitis,” occurs above and below the neck and presents as a widespread eruption of macules and papules that is photosensitive and often pruritic. The pattern of involvement on the dorsum of hands can help distinguish generalized ACLE from dermatomyositis; the metacarpophalangeal (MCP) and interphalangeal (IP) joints are normally spared in ACLE.45 Less common presentations of ACLE include involvement of the lips and periorbital edema. Rare cases of toxic epidermal necrolysis (TEN)-like ACLE or hyper-acute CLE have been reported, which encompasses clinical and histological findings of both ACLE and toxic epidermal necrolysis together without an inciting drug or infection.46,47 The majority of patients have a previously confirmed or new diagnosis of SLE or SCLE at the time of TEN-like ACLE.48
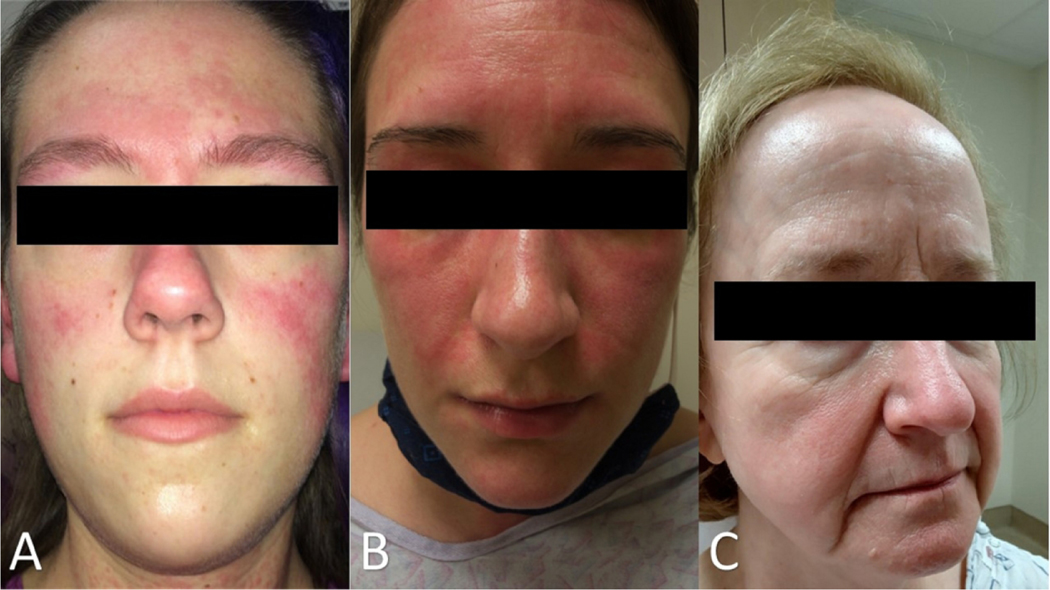
Figure 4:
A “butterfly rash” may be due to a variety of dermatological conditions. (A) The malar rash of ACLE refers to erythema over the nasal bridge and cheeks that spares the nasolabial folds. Erythema of ACLE can be found in other areas of the face, such as the forehead here. (B) Facial erythema in dermatomyositis tends to involve the nasolabial folds. (C) Rosacea can mimic the facial erythema of ACLE but tends to worsen with specific triggers such as alcohol, heat, and spicy foods.
LE Nonspecific Skin Disease
LE-nonspecific skin disease includes skin changes that are frequently associated with LE but are not specific to the disease itself (Table 2). LE-nonspecific skin lesions are common in patients with SLE and often occur during the active phase of disease. Compared to those with LE-specific lesions, those with non-specific lesions tend to have increased SLE disease activity.49 In addition to vascular disease which will be discussed in more depth below, other non-specific cutaneous findings can occur in SLE including sclerodactyly, calcinosis cutis, rheumatoid nodules, urticaria, cutis laxa/anetoderma, acanthosis nigrans, lichen planus, and erythema multiforme. Bullous LE is considered a LE-nonspecific entity. Diagnosis of bullous LE requires an existing SLE diagnosis and patients frequently have increased SLE disease activity. Unlike the lymphocytic inflammation seen in lupus-specific LE, the inflammation in bullous LE is neutrophilic, the blister is subepidermal, and an antibody against type VII collagen is seen in the blood. Skin biopsy shows linear IgG at the dermal-epidermal junction on direct immunofluorescence. Thus, a biopsy for direct immunofluorescence is helpful, and findings are distinguished from epidermolysis bullosa acquisita because of the diagnosis of SLE.50
Table 2.
LE-nonspecific Skin Disease
| Cutaneous vascular disease |
| • Leukocytoclastic Vasculitis |
| ○ Palpable purpura |
| ○ Urticarial vasculitis |
| • Vasculopathy |
| ○ Degos’ disease like lesions |
| ○ Secondary atrophie blanche |
| • Periungual telangiectasias |
| • Livedo reticularis |
| • Thrombophlebitis |
| • Raynaud’s phenomenon |
| • Erythromelalgia |
|
|
| Sclerodactyly |
|
|
| Calcinosis cutis |
|
|
| Rheumatoid nodules |
|
|
| Neutrophilic urticarial dermatosis |
|
|
| Cutis laxa/anetoderma |
|
|
|
|
| Acanthosis nigrans |
|
|
| Papulonodular mucinosis |
|
|
| Lichen planus |
|
|
| Erythema multiforme (Rowell’s syndrome) |
|
|
| Bullous LE |
|
|
| Nonscarring Alopecia |
Abbreviations: LE, lupus erythematosus
Cutaneous vascular disease is one subtype of LE-nonspecific skin disease that includes vasculitis, vasculopathy, periungual telangiectasias, livedo reticularis, thrombophlebitis, Raynaud’s phenomenon, and erythromelalgia. Cutaneous vasculitis has been reported in 10–20% of patients with SLE. It is a small-vessel leukocytoclastic vasculitis that manifests as palpable purpura or urticarial vasculitis. Occasionally vessels in the deeper dermis and subcutaneous tissues can be involved, resulting in nodules or ulceration in a polyarteritis nodosa-like presentation. Cutaneous vasculitis is most common with increased SLE activity and is often associated with circulating immune complexes and hypocomplementemia. While vasculitic lesions are due to a primary inflammatory attack on the vessel wall, other vascular skin manifestations associated with SLE are the result of vasculopathy secondary to coagulation abnormalities, including but not limited to antiphospholipid antibody syndrome.51 Sometimes grouped as livedoid vasculopathy, these entities likely represent an inflammatory response due to hypercoagulability. Livedo reticularis, a bluish net-like pattern typically most prominent on the skin of buttocks, legs, and arms, results from reduced arterial blood flow and hypo-oxygenation and is common with cold exposure. Livedo racemosa, with an irregular, ‘broken’ net-like pattern occurs with an underlying focal skin pathology such as thrombi or calcification.52 Other vascular related phenomena that occur in SLE include periungual telangiectasia and erythema in 10–15% of patients. Raynaud’s phenomenon, also common in other connective tissue disease such as scleroderma, dermatomyositis, and mixed connective tissue disease, occurs in many patients with SLE and is characterized by cold-induced blanching followed by livedoid and erythematous color change of fingers and other acral skin. Non-specific changes on nailfold capillaroscopy, including tortuous and dilated capillaries and hemorrhage, are more prevalent in SLE compared to healthy controls.53
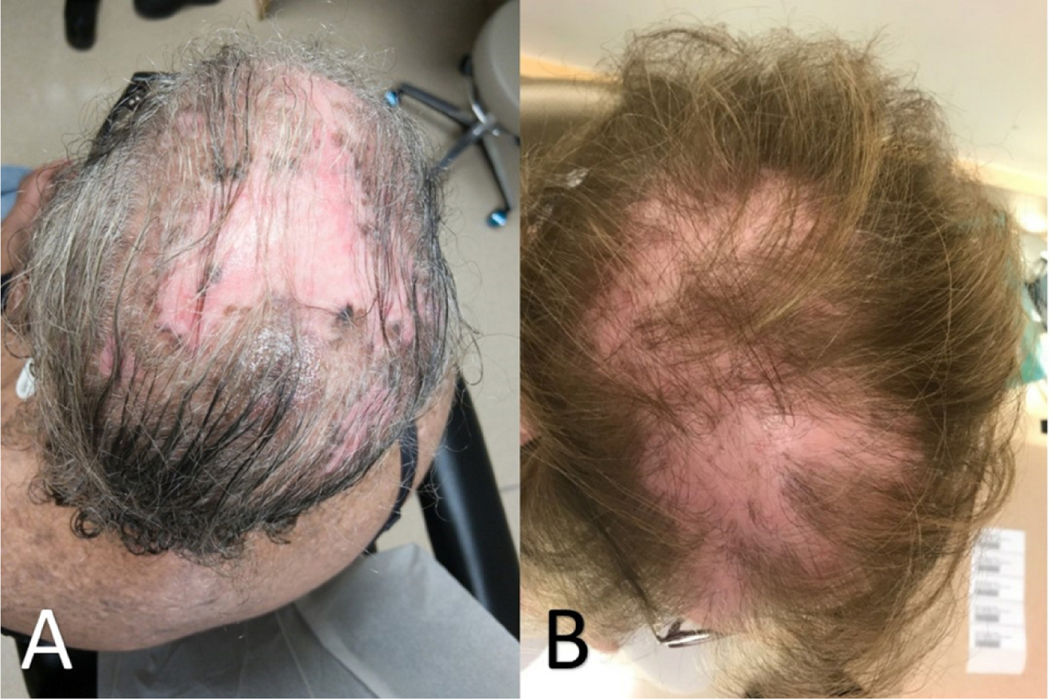
Hair loss is frequent in SLE, occurring in more than half of patients at some point in the disease process (Figure 5).54,55 Nonscarring alopecia, defined as diffuse thinning and fragility of the hair in the absence of other causes, is seen in 40–70% of patients with SLE.16 It is important to rule out other potential causes of non-scarring alopecia before attributing it to SLE. Lupus hair refers to breakage of hair that typically occurs in the frontal scalp. It commonly occurs during disease flares and may be a form of telogen effluvium.
Figure 5:
Alopecia due to (A) CLE can be difficult to distinguish from mimickers such as alopecia due to (B) lichen planopilaris; correlation between clinical and histologic findings may be required to correctly identify the cause of hair loss.
Finally, returning to the cutaneous features included in the 1997 ACR diagnostic criteria for SLE, two of the four criteria that have not yet been discussed are photosensitivity and oral ulcers. Photosensitivity is a phenomenon whereby exposure to ultraviolet light causes skin rash in sun-exposed areas and/or other systemic symptoms of SLE flare-ups. It is a clinical observation and occurs in a variety of other conditions, including dermatomyositis, polymorphous light eruption, photoallergic contact dermatitis, solar urticaria, or porphyrias. Oral or nasopharyngeal ulcers occur in more than 40% of patients with SLE.56,57 Lesions can be painful or painless and while palatal ulcers are the most specific for SLE, ulcers can also occur on buccal mucosa, hard palate, and the vermilion border.
Disease Monitoring
The differentiation between disease activity and damage is important in SLE and CLE, given the chronic nature of disease with periods of flares. The goal in managing SLE and CLE is to prevent and control activity in order to avoid damage, which is frequently irreversible. The Systemic Lupus Erythematous Disease Activity Index (SLEDAI) and SLEDAI-2K, tools used to assess disease activity and guide decisions to increase therapy, use a cut off score of 3–4 to define active disease and includes several cutaneous features. Both versions include alopecia (2 points), oral or nasal mucosal ulcers (2 points), vasculitis including ulceration, gangrene, tender finger nodules, periungual infarction, splinter hemorrhages (8 points); the SLEDAI also includes “new rash”, defined as new onset or recurrence of inflammatory rash (2 points); and the SLEDAI-2K includes “rash”, defined as inflammatory rash (2 points).58,59 The Systemic Lupus International Collaborating Clinics (SLICC) ACR Damage Index, used to assess damage over the course of disease, incorporates scarring chronic alopecia, extensive scarring of panniculum other than scalp and pulp space, and skin ulceration.60 Damage in the SLICC criteria is defined as irreversible change that is not related to active inflammation that has occurred since the onset of disease and has been present for at least 6 months.
The Cutaneous Lupus Erythematous Disease Area and Severity Index (CLASI) is a validated instrument that has separate scores to measure activity and damage of CLE. Activity scores are based on the extent of erythema, scale/hypertrophy, mucous membrane involvement, acute hair loss, and non-scarring alopecia. Damage scores are based on dyspigmentation and scarring, including scarring alopecia.61,62 Since the CLASI was developed and validated it has been used in two-thirds of clinical studies and trials of CLE outcomes.64 Additional validated activity and damage scores for CLE have been developed. The revised CLASI (RCLASI) includes adjustments to the original CLASI such as new parameters like edema/infiltration and subcutaneous nodule/plaque.63 A working core outcome set for CLE trials was recently developed to guide future clinical and outcomes research in CLE, recommending the CLASI as a primary endpoint and the Cutaneous Lupus Activity IGA (CLA-IGA) as a secondary endpoint for CLE physician reported outcome measures.64
Treatment
As previously mentioned, the goal in management of cutaneous manifestations of lupus is to prevent and treat skin activity to minimize damage. A treatment algorithm for CLE has been put forth in the European S2k guidelines.65 An essential component to managing cutaneous disease in SLE is prevention, with aggressive sun-protective measures including protective clothing, avoiding exposure during peak sunlight hours, and daily use of SPF 70 or higher broad spectrum UVA/UVB sunscreens. Vitamin D supplementation should be considered in all patients, especially when serum levels are below normal range. Patients who use tobacco should be counseled on smoking cessation, as it has been identified as a risk factor for widespread CLE, can increase disease severity and can decrease the efficacy of antimalarial therapy.66,67
Topical and intralesional corticosteroids can be used in limited cutaneous disease or as adjunctive therapy along with systemic agents. As with systemic steroid use, the goal is to use the least potent formula for the shortest amount of time to lower the risk of local complications such as steroid atrophy and telangiectasia. An initial regimen of a medium-strength (class III) topical corticosteroid such a triamcinolone acetonide 0.1% applied daily to lesional skin can be tried, especially on areas off the face; if this does not provide sufficient relief a more potent topical steroid such as clobetasol propionate 0.05% or betamethasone dipropionate 0.05% (class I) should be considered. When class I-III topical corticosteroids are providing clinical benefit in sensitive areas such as the face, one can minimize the chances for developing cutaneous atrophy from longer-term therapy by rotating the topical corticosteroid every 2 weeks with a topical calcineurin inhibitor such as pimecrolimus cream or tacrolimus ointment. 5 Calcineurin inhibitors are recommended as alternative first-line or second-line topical therapeutic options, especially on the face, on the basis of randomized clinical trials.65
Antimalarials (hydroxychloroquine, chloroquine, and quinacrine) are first line therapies for cutaneous disease in SLE. 75% of patients respond to antimalarials with or without the addition of topical glucocorticoids.68 Disease refractory to antimalarials can be treated with immunosuppressives common in the rheumatologists’ armamentarium including methotrexate (MTX), mycophenolate mofetil (MMF), or azathioprine, although azathioprine is frequently less effective.69 Though the algorithm suggested in the European guidelines lists MTX as second line therapy and MMF as third line, there have been limited controlled trials in treating refractory CLE; therefore, the European guidelines were based on a consensus conference of dermatologists rather than being an evidence-based statement.69 A recent cohort study suggests similar efficacy for MTX and MMF between subtypes of CLE, suggesting that other factors such as side effect profile and comorbid conditions may influence medication selection.70 Thalidomide is only recommended in treatment-refractory CLE with careful monitoring for the development of polyneuropathy, a potential adverse effect.65 Another therapeutic option for patients refractory to antimalarials is lenalidomide, a thalidomide analog with superior adverse effect profile to thalidomide.71 Dapsone can be effective in the treatment of bullous LE, LEP, and in some cases of SCLE and DLE; use requires close monitoring for hematologic toxicities and the drug should not be used with patients who have a glucose-6-phosphate dehydrogenase deficiency. Three case series showed significant improvement in CLASI activity scores with belimumab.72–74 ACLE can respond favorably to rituximab, however no beneficial effects and some exacerbations or new-onset disease have been seen in patients with DLE or SCLE.75–77 Anifrolumab, a drug recently approved by the FDA for SLE, was shown in a phase 3 trial to be superior to placebo in improving skin disease measures in patients with at least moderately active skin disease.78 Multiple agents are also under investigation as alternative therapies for CLE. Iberdomide has been shown in a recent phase 2 trial to have beneficial effects on skin disease in patients with SCLE and CCLE, but not ACLE, while BIIB059, a humanized monoclonal antibody targeting BDCA2 on plasmacytoid dendritic cells (pDCs), was shown to improve skin disease activity in patients with CLE in a recent phase 2 trial.79,80 Similarly, VIB7734, a monoclonal antibody that targets pDCs for antibody-dependent cellular cytotoxicity, showed clinically significant improvement in measures of skin disease activity in patients with CLE in a phase 1 trial.81
Conclusion
The spectrum of cutaneous disease in SLE is extremely broad and can occur at any point in the disease. Collaboration between dermatology and rheumatology specialists is essential to properly diagnose and manage affected patients. Skin biopsy is important to differentiate CLE from other skin conditions and must be considered in clinical context to reach a diagnosis. Timely and appropriate therapy to control activity and minimize damage is the goal of treatment.
Key Messages.
Cutaneous manifestations of lupus erythematosus may occur as a separate entity or in association with systemic disease
The likelihood of systemic involvement (SLE) depends on the subtype of cutaneous lupus erythematosus (CLE), with higher rates of SLE in patients with acute cutaneous lupus erythematosus, generalized discoid lupus erythematosus, chilblain lupus erythematosus, and subacute cutaneous lupus erythematosus
A biopsy is recommended to differentiate cutaneous lupus erythematosus from other skin conditions, excepting from dermatomyositis.
Drug-induced CLE is associated with a number of common medication classes used by rheumatologists including proton pump inhibitors, NSAIDs, and TNF inhibitors
Timely and appropriate therapy to control activity and minimize damage is the goal of treatment of CLE.
Photoprotection, smoking cessation, and topical corticosteroids are standard for localized cutaneous disease. Antimalarials are first-line treatment for severe CLE and SLE.
Acknowledgement
Funding sources: United States Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development and Biomedical Laboratory Research and Development) and National Institute of Health [R01AR071653]
Funding Sources:
United States Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development and Biomedical Laboratory Research and Development) and National Institute of Health [R01AR071653]
Footnotes
Conflicts: None
References
- 1.Yell JA, Mbuagbaw J, Burge SM. Cutaneous manifestations of systemic lupus erythematosus. Br J Dermatol 1996;135:355–62. [PubMed] [Google Scholar]
- 2.Dubois EL, Tuffanelli DL. Clinical manifestations of systemic lupus erythematosus: computer analysis of 520 cases. JAMA 1964;190:104–11. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Moghadam-Kia S, Taylor L, et al. Quality of life in cutaneous lupus erythematosus. J Am Acad Dermatol 2011;64:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sontheimer R. The lexicon of cutaneous lupus erythematosus-A review and personal perspective on the nomenclature and classification of the cutaneous manifestations of lupus erythematosus. Lupus 1997;6:84–95. [DOI] [PubMed] [Google Scholar]
- 5.Rothfield N, Sontheimer RD, Bernstein M. Lupus erythematosus: systemic and cutaneous manifestations. Clin Dermatol 2006;24:348–62. [DOI] [PubMed] [Google Scholar]
- 6.Jarukitsopa S, Hoganson DD, Crowson CS, et al. Epidemiology of systemic lupus erythematosus and cutaneous lupus erythematosus in a predominantly white population in the United States. Arthritis Care Res 2015;67:817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durosaro O, Davis MD, Reed KB, Rohlinger AL. Incidence of cutaneous lupus erythematosus, 1965–2005: a population-based study. Arch Dermatol 2009;145:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izmirly P, Buyon J, Belmont HM, et al. Population-based prevalence and incidence estimates of primary discoid lupus erythematosus from the Manhattan Lupus Surveillance Program. Lupus Sci Med 2019. Oct 30 (Epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drenkard C, Parker S, Aspey LD, et al. Racial disparities in the incidence of primary chronic cutaneous lupus erythematosus in the Southeastern US: the Georgia Lupus Registry. Arthritis Care Res 2019;71:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stojan G, Petri M. Epidemiology of systemic lupus erythematosus: an update. Curr Opin Rheumatol 2018;30:144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 12.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 13.Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev 2013;12:444–54. [DOI] [PubMed] [Google Scholar]
- 14.Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarazi M, Gaffney RG, Kushner CJ, Chakka S, Werth VP. Cutaneous lupus erythematosus patients with a negative antinuclear antibody meeting the American College of Rheumatology and/or Systemic Lupus International Collaborating Clinics criteria for systemic lupus erythematosus. Arthritis Care Res 2019;71:1404–9. [DOI] [PubMed] [Google Scholar]
- 16.Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol 1981;4:471–5. [DOI] [PubMed] [Google Scholar]
- 18.Gilliam JN, Sontheimer RD. Skin manifestations of SLE. Clin Rheum Dis 1982;8:207–18. [PubMed] [Google Scholar]
- 19.Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun 2014;48:14–9. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn A, Ruzicka T. Classification of cutaneous lupus erythematosus. Kuhn A, Lehmann P, Ruzicka T, editors. In: Cutaneous lupus erythematosus. Berlin: Springer-Verlag; 2005:53–7. [Google Scholar]
- 21.Worm M, Zidane M, Eisert L, et al. S2k guideline: Diagnosis and management of cutaneous lupus erythematosus–Part 1: Classification, diagnosis, prevention, activity scores. J Dtsch Dermatol Ges 2021;19:1236–47. [DOI] [PubMed] [Google Scholar]
- 22.Bailey EE, Fiorentino DF. Amyopathic dermatomyositis: definitions, diagnosis, and management. Curr Rheumatol Rep 2014;16:465. [DOI] [PubMed] [Google Scholar]
- 23.Yell J, Allen J, Wojnarowska F, Kirtschig G, Burge S. Bullous systemic lupus erythematosus: revised criteria for diagnosis. Br J Dermatol 1995;132:921–8. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe T, Tsuchida T. Classification of lupus erythematosus based upon cutaneous manifestations. Dermatol 1995;190:277–83. [DOI] [PubMed] [Google Scholar]
- 25.Werth VP. Clinical manifestations of cutaneous lupus erythematosus. Autoimmun Rev 2005;4:296–302. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien JC, Chong BF. Not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Invest Dermatol Symp Proc 2017:S69–S74. [DOI] [PubMed] [Google Scholar]
- 27.Lu Q, Long H, Chow S, et al. Guideline for the diagnosis, treatment and long-term management of cutaneous lupus erythematosus. J Autoimmun 2021;123:102707. [DOI] [PubMed] [Google Scholar]
- 28.Herzum A, Gasparini G, Cozzani E, Burlando M, Parodi A. Atypical and Rare Forms of Cutaneous Lupus Erythematosus: The Importance of the Diagnosis for the Best Management of Patients. Dermatol 2021:1–10. [DOI] [PubMed] [Google Scholar]
- 29.Elman SA, Joyce C, Braudis K, et al. Creation and validation of classification criteria for discoid lupus erythematosus. JAMA Dermatol 2020;156:901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper EE, Pisano CE, Shapiro SC. Cutaneous Manifestations of “Lupus”: Systemic Lupus Erythematosus and Beyond. Int J Rheumatol 2021;2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol 2013;27:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stead J, Headley C, Ioffreda M, Kovarik C, Werth V. Coexistence of tumid lupus erythematosus with systemic lupus erythematosus and discoid lupus erythematosus: a report of 2 cases. J Clin Rheumatol 2008;14:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedrich C, Fiebig B, Hauck F, et al. Chilblain lupus erythematosus—a review of literature. Clin Rheumatol 2008;27:949–54. [DOI] [PubMed] [Google Scholar]
- 34.Tebbe B, Mansmann U, Wollina U, et al. Markers in cutaneous lupus erythematosus indicating systemic involvement. A multicenter study on 296 patients. Acta Derm Venereol 1997;77:305–8. [DOI] [PubMed] [Google Scholar]
- 35.Dao KH, Bermas BL. Systemic Lupus Erythematosus Management in Pregnancy. Int J Womens Health 2022;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieboer C, Tak-Diamand Z, Van Leeuwen-Wallau H. Dust-like particles: a specific direct immunofluorescence pattern in sub-acute cutaneous lupus erythematosus. Br J Dermatol 1988;118:725–9. [DOI] [PubMed] [Google Scholar]
- 37.Keyes E, Grinnell M, Vazquez T, Diaz D, Thomas P, Werth VP. Drug-induced subacute cutaneous lupus erythematosus in previously diagnosed systemic lupus erythematosus patients: A case series. JAAD Case Rep 2021;12:18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laurinaviciene R, Sandholdt LH, Bygum A. Drug-induced cutaneous lupus erythematosus: 88 new cases. Eur J Dermatol 2017;27:28–33. [DOI] [PubMed] [Google Scholar]
- 39.Borucki R, Werth VP. Cutaneous lupus erythematosus induced by drugs-novel insights. Expert Rev Clin Pharmacol 2020;13:35–42. [DOI] [PubMed] [Google Scholar]
- 40.Conforti C, Retrosi C, Giuffrida R, et al. Secukinumab-induced subacute cutaneous lupus erythematosus. Dermatol Ther 2020. May 3 (Epub). [DOI] [PubMed] [Google Scholar]
- 41.Tierney E, Kirthi S, Ramsay B, Ahmad K. Ustekinumab-induced subacute cutaneous lupus. JAAD Case Rep 2019;5:271–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarazi M, Aiempanakit K, Werth VP. Subacute cutaneous lupus erythematosus and systemic lupus erythematosus associated with abatacept. JAAD Case Rep 2018;4:698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koskenmies S, Järvinen T, Onkamo P, et al. Clinical and laboratory characteristics of Finnish lupus erythematosus patients with cutaneous manifestations. Lupus 2008;17:337–47. [DOI] [PubMed] [Google Scholar]
- 44.Tebbe B, Mansmann U, Wollina U, et al. Markers in cutaneous lupus erythematosus indicating systemic involvement. A multicenter study on 296 patients. Acta Derm Venerol 1997;77:305–8. [DOI] [PubMed] [Google Scholar]
- 45.Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol 2013;27:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marija S, Ivana B, Nina R, et al. Toxic epidermal necrolysis in a child with lupus-associated pancreatitis. Rheumatol Int 2017;37:1221–6. [DOI] [PubMed] [Google Scholar]
- 47.Mir TH, Bhat IA, Jabeen B, Haji MLI. Toxic epidermal necrolysis-like acute cutaneous lupus/acute syndrome of apoptotic pan-epidermolysis. Rheumatol 2021;60:5876–7. [DOI] [PubMed] [Google Scholar]
- 48.Romero LS, Bari O, Forbess CJ, Schneider JA, Cohen PR. Toxic epidermal necrolysis-like acute cutaneous lupus erythematosus: report of a case and review of the literature. Dermatol Online J 2018;24. [PubMed] [Google Scholar]
- 49.Zeĉević R, Vojvodić D, Ristić B, Pavlović M, Stefanović D, Karadaglić D. Skin lesions-an indicator of disease activity in systemic lupus erythematosus? Lupus 2001;10:364–7. [DOI] [PubMed] [Google Scholar]
- 50.Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol 2014;15:517–24. [DOI] [PubMed] [Google Scholar]
- 51.Diógenes MJN, Diógenes PCN, de Morais Carneiro RM, Neto CCR, Duarte FB, Holanda RR. Cutaneous manifestations associated with antiphospholipid antibodies. Int J Dermatol 2004;43:632–7. [DOI] [PubMed] [Google Scholar]
- 52.Obermoser G, Sontheimer RD, Zelger B. Overview of common, rare and atypical manifestations of cutaneous lupus erythematosus and histopathological correlates. Lupus 2010;19:1050–70. [DOI] [PubMed] [Google Scholar]
- 53.Cutolo M, Melsens K, Wijnant S, et al. Nailfold capillaroscopy in systemic lupus erythematosus: a systematic review and critical appraisal. Autoimmun Rev 2018;17:344–52. [DOI] [PubMed] [Google Scholar]
- 54.Moghadam-Kia S, Franks AG. Autoimmune disease and hair loss. Dermatol Clin 2013;31:75–91. [DOI] [PubMed] [Google Scholar]
- 55.Concha JSS, Werth VP. Alopecias in lupus erythematosus. Lupus Sci Med 2018. Oct 25 (Epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urman JD, Lowenstein MB, Abeles M, Weinstein A. Oral mucosal ulceration in systemic lupus erythematosus. Arthritis Rheum 1978;21:58–61. [DOI] [PubMed] [Google Scholar]
- 57.Kudsi M, Nahas LD, Alsawah R, Hamsho A, Omar A. The prevalence of oral mucosal lesions and related factors in systemic lupus erythematosus patients. Arthritis Res Ther 2021;23:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum 1992;35:630–40. [DOI] [PubMed] [Google Scholar]
- 59.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. Journal Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 60.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 61.Albrecht J, Taylor L, Berlin JA, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol 2005;125:889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chong BF, Werth V. Cutaneous Lupus Erythematosus and Dermatomyositis: Utilizing Assessment Tools for Treatment Efficacy. J Invest Dermatol 2022;142:936–43. [DOI] [PubMed] [Google Scholar]
- 63.Kuhn A, Amler S, Beissert S, et al. Revised Cutaneous Lupus Erythematosus Disease Area and Severity Index (RCLASI): a modified outcome instrument for cutaneous lupus erythematosus. Br J Dermatol 2010;163:83–92. [DOI] [PubMed] [Google Scholar]
- 64.Guo LN, Perez-Chada LM, Borucki R, Nambudiri VE, Werth VP, Merola JF. Development of a working core outcome set for cutaneous lupus erythematosus: a practical approach to an urgent unmet need. Lupus Sci Med 2021. Dec 22 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuhn A, Aberer E, Bata-Csörgő Z, et al. S2k guideline for treatment of cutaneous lupus erythematosus–guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol 2017;31:389–404. [DOI] [PubMed] [Google Scholar]
- 66.Kuhn A, Sigges J, Biazar C, et al. Influence of smoking on disease severity and antimalarial therapy in cutaneous lupus erythematosus: analysis of 1002 patients from the EUSCLE database. Br J Dermatol 2014;171:571–9. [DOI] [PubMed] [Google Scholar]
- 67.Bourré-Tessier J, Peschken CA, Bernatsky S, et al. Association of smoking with cutaneous manifestations in systemic lupus erythematosus. Arthritis Care Res 2013;65:1275–80. [DOI] [PubMed] [Google Scholar]
- 68.Callen JP. Management of skin disease in patients with lupus erythematosus. Best Pract Res Clin Rheumatol 2002;16:245–64. [DOI] [PubMed] [Google Scholar]
- 69.Borucki R, Werth VP. Expert Perspective: An Evidence-Based Approach to Refractory Cutaneous Lupus Erythematosus. Arthritis Rheumatol 2020;72:1777–85. [DOI] [PubMed] [Google Scholar]
- 70.Keyes E, Jobanputra A, Feng R, et al. Comparative responsiveness of cutaneous lupus erythematosus patients to methotrexate and mycophenolate mofetil: A cohort study. J Am Acad Dermatol 2021. Sep 16 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuki EFN, Silva CA, Aikawa NE, et al. Thalidomide and lenalidomide for refractory systemic/cutaneous lupus erythematosus treatment: a narrative review of literature for clinical practice. J Clin Rheumatol 2021;27:248–59. [DOI] [PubMed] [Google Scholar]
- 72.Iaccarino L, Bettio S, Reggia R, et al. Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthritis Care Res 2017;69:115–23. [DOI] [PubMed] [Google Scholar]
- 73.Vashisht P, Borghoff K, O’Dell JR, Hearth-Holmes M. Belimumab for the treatment of recalcitrant cutaneous lupus. Lupus 2017;26:857–64. [DOI] [PubMed] [Google Scholar]
- 74.Parodis I, Sjöwall C, Jönsen A, et al. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev 2017;16:343–51. [DOI] [PubMed] [Google Scholar]
- 75.Hofmann S, Leandro M, Morris S, Isenberg D. Effects of rituximab-based B-cell depletion therapy on skin manifestations of lupus erythematosus–report of 17 cases and review of the literature. Lupus 2013;22:932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.da Costa RQ, Aguirre-Alastuey ME, Isenberg DA, Saracino AM. Assessment of response to B-cell depletion using rituximab in cutaneous lupus erythematosus. JAMA Dermatol 2018;154:1432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vital EM, Wittmann M, Edward S, et al. Brief report: responses to rituximab suggest B cell–independent inflammation in cutaneous systemic lupus erythematosus. Arthritis Rheumatol 2015;67:1586–91. [DOI] [PubMed] [Google Scholar]
- 78.Morand EF, Furie R, Tanaka Y, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med 2020;382:211–21. [DOI] [PubMed] [Google Scholar]
- 79.Werth V, Merrill J, Furie R, et al. OP0132 Effect of Iberdomide on cutaneous manifestations in systemic lupus erythematosus: Results of a 24-week, placebo controlled, phase 2 Study. Ann Rheum Dis 2021;80:76–7. [Google Scholar]
- 80.Werth V, Furie R, Romero-Diaz J, et al. OP0193 BIIB059, a humanized monoclonal antibody targeting BDCA2 on plasmacytoid dendritic cells (pDC), shows dose-related efficacy in the phase 2 LILAC study in patients (pts) with active cutaneous lupus erythematosus (CLE). Ann Rheum Dis 2020;79:120–1. [Google Scholar]
- 81.Karnell JL, Wu Y, Mittereder N, et al. Depleting plasmacytoid dendritic cells reduces local type I interferon responses and disease activity in patients with cutaneous lupus. Sci Transl Med 2021. May 26 (Epub). [DOI] [PubMed] [Google Scholar]