Abstract
Background
Except for public health case reports, the incidence of Zika virus (ZIKV), chikungunya virus (CHIKV), and dengue virus (DENV) infection are not available to assess the potential blood transfusion safety threat in Brazil.
Methods
Pools of 6 donation samples (MP6) left over from human immunodeficiency virus, hepatitis B virus, and hepatitis C virus nucleic acid testing were combined to create MP18 pools (3 MP6 pools). Samples were tested using the Grifols triplex ZIKV, CHIKV, and DENV real-time transcription mediated amplification assay to estimate prevalence of RNAemia and incidence, and to compare these results to case reports in São Paulo, Belo Horizonte, Recife, and Rio de Janeiro, from April 2016 through June 2019.
Results
ZIKV, CHIKV, and DENV RNAemia were found from donors who donated without overt symptoms of infection that would have led to deferral. The highest RNAemic donation prevalence was 1.2% (95% CI, .8%–1.9%) for DENV in Belo Horizonte in May 2019. Arbovirus infections varied by location and time of year, and were not always aligned with annual arbovirus outbreak seasons in different regions of the country.
Conclusions
Testing donations for arboviruses in Brazil can contribute to public health. Transfusion recipients were likely exposed to ZIKV, CHIKV, and DENV viremic blood components during the study period.
Keywords: arboviruses, Zika virus, chikungunya virus, dengue virus, blood donors, transfusion medicine, nucleic acid testing
Small pools of blood donor samples were tested for 3 arboviruses from April 2016 through June 2019. Identified infections varied by location and time of year in different regions of Brazil. Transfusion recipients were likely exposed to viremic blood components.
Arbovirus infections are a major public health concern in Brazil and other tropical/subtropical countries [1]. The potential transmission of arboviruses by transfusion became an important blood safety topic following significant transfusion transmission of West Nile virus in United States (US) epidemics beginning in 2002 [2, 3]. Other arboviruses have not been demonstrated to be as readily transmitted by transfusion. Even so, there are questions on the extent to which the risk of transfusion transmission may be present in different countries. Brazil has the largest number of dengue virus (DENV) cases reported annually in the Americas [4–6]. Virtually all of these infections are assumed to be mosquito-acquired. Chikungunya virus (CHIKV) was introduced into the Americas in 2014 and spread extensively in the Caribbean Islands and Central America in 2015 [7]. Large CHIKV epidemics were expected to occur in Brazil in the following year, but instead an epidemic of a then little-known virus occurred: Zika virus (ZIKV). ZIKV was previously considered to cause mild illness [8–11] with fever, maculopapular rash, conjunctivitis, and arthralgia [12], and so did not attract sustained interest from the scientific and medical communities. Unfortunately, with ZIKV emerging in new areas and affecting high-density, naive populations, it became clear that it could cause severe neurological complications, including Guillain-Barré syndrome (GBS), as first documented during the French Polynesian outbreak [13], and congenital syndromes following intrauterine transmission [14–16].
Subsequently, Brazil has experienced outbreaks of all 4 serotypes of DENV, CHIKV, and ZIKV. The overall population-level exposure to both ZIKV and CHIKV is not of the scale seen on Caribbean Islands, where 25%–40% of the population may have been exposed during the course of 1- or 2-year major outbreaks [17, 18]. Thus, the Brazilian population, including blood donors and therefore the transfusion recipient population, continues to be at risk for CHIKV, ZIKV, and DENV outbreaks. While ZIKV was later documented to have been introduced in Brazil during 2014, it was recognized only in 2015 [19]. ZIKV became a mandatory reportable disease in 2016. Because of cross-reactivity of the serological assays used in the diagnosis of ZIKV and DENV [20], it is possible that in 2015–2016 many reported DENV cases were ZIKV infections. In 2017, CHIKV epidemics occurred in the northeast region of the country. ZIKV case reports decreased rapidly in 2017 and 2018, and there are still substantial proportions of persons naive for ZIKV and CHIKV infections in the populations of many states, including São Paulo and Minas Gerais.
Except for surveillance based on clinical case diagnosis, current data on the incidence of ZIKV, CHIKV, and DENV are not available to assess the potential blood safety threat in Brazil. A transcription-mediated amplification (TMA) nucleic acid testing (NAT) triplex assay with high sensitivity that can detect and discriminate between the 3 agents has been developed in the US. This assay works by simultaneously amplifying unique sequences of RNA for each of these arboviruses. The RNA detection periods of this assay when performed in small pool (minipool) formats of different donations are assumed to approximate the durations of infectious viremia [21]. The objective of this study is to determine the prevalence of ZIKV, CHIKV, and DENV RNAemia in donations and the incidence of these infections in donors at 4 Brazilian blood centers participating in the National Heart, Lung, and Blood Institute of the National Institutes of Health Recipient Epidemiology and Donor Evaluation Study III (REDS-III), and to evaluate whether these data can supplement case surveillance for these arboviruses. We conducted minipool NAT testing of stored donation samples from blood donations collected at blood centers in São Paulo, Belo Horizonte, Recife, and Rio de Janeiro, Brazil. This allowed us to estimate the prevalence of RNAemia and the incidence of asymptomatic infection in donors based on published estimates of the duration of viremia for each virus, and to compare donor incidence to public health case reports in the 4 cities.
METHODS
Study Setting and Sample Acquisition
The participating blood centers were Fundação Pró-Sangue (FPS) in São Paulo, Hemominas in Belo Horizonte, Hemorio in Rio de Janeiro, and Hemope in Recife. Except for Hemope, located in the northeast, the other 3 centers are in the highest-population-density southeast region of Brazil. This study was approved by the Brazilian National Research Ethics Commission, the local ethics committees at each participating center, the Institutional Review Board (IRB) of the University of California, San Francisco in the US, the IRB of record for Vitalant Research Institute, and the IRB of the data coordinating center (Research Triangle Institute).
In public blood centers in Brazil, all donations are tested for human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) using NAT in a minipool format consisting of 6 pooled samples (MP6). Starting in April 2016, residual pooled plasma samples of 0.3–0.4 mL from MP6 NAT for HIV, HCV, and HBV were combined to create MP18 pools (3 MP6 samples per pool) of between 0.9 and 1.2 mL of plasma. Sampling and MP18 creation were done on a weekly basis. Thus, in situations at the end of the week where the number of available MP6 samples was insufficient, MP12s were created or the original MP6 samples were stored. Following MP18 creation, the study samples were frozen and shipped to the US. About 400 donations per week per site, which corresponds to approximately 67 MP6 samples/week or approximately 23 MP18 samples/week, were obtained for retrospective testing.
Laboratory Testing
Samples were tested on the Panther platform using the Grifols ZIKV, CHIKV, and DENV real-time TMA research use–only assay (Grifols Diagnostic Solutions, San Diego, California). This triplex assay can simultaneously amplify and discriminate ZIKV, CHIKV, and DENV RNA on the Panther platform and reports qualitative results, so we could not directly quantitate RNAemia. ZIKV RNA detection by the Grifols Procleix Zika Virus (a US Food and Drug Administration licensed and Conformite Europeenne [CE]–marked singleplex assay with a 95% LOD 12 copies/mL; 50% LOD 3 copies/mL) and this triplex assay show similar, high sensitivity [22, 23]. The 95% detection probabilities for DENV RNA using the CE-marked singleplex Grifols Procleix Dengue Virus assay in copies/mL and associated 95% fiducial limits are 21.04 (16.75–28.35) for DENV-1, 25.95 (20.19–36.16) for DENV-2, 18.81 (14.49–26.80) for DENV-3, and 28.95 (22.14–41.14) for DENV-4 [24]. Unpublished data show a similar performance for the CE-marked DENV singleplex assay and this triplex assay (personal communication, K. Gui). Information on analytical sensitivity for CHIKV is not available.
At Grifols in San Diego or Vitalant Research Institute in San Francisco, MP18 samples were thawed, and additional plasma (previously screened using the same assay to establish it contained no detectable ZIKV, CHIKV, or DENV RNA) was added to create a total volume of at least 1.2 mL in each tube. Testing was focused on 3 different calendar periods: April 2016–June 2017, November 2017–June 2018, and November 2018–June 2019. For the first period, we conducted extended testing for a consecutive 15-month period for all sites. In addition, for Hemorio all months of 2018 were tested, except some June 2018 samples that were lost in a fire at the center and could not be included.
Reactive pools could not be resolved into the individual reactive donations because only already pooled samples were available for testing. As part of standard HIV, HCV, and HBV MP testing, FPS in São Paulo included donations from the city of São Paulo and 3 adjacent cities, Guarulhos, Osasco, and Barueri. Hemominas, Hemorio, and Hemope included only samples from the city where the blood centers are located (Belo Horizonte, Rio de Janeiro, and Recife, respectively).
We compare donation testing results to the number of clinical case notifications per month for ZIKV, CHIKV, and DENV infections at the city and state level, collected in case report systems, obtained through the national Notifiable Diseases Information System, also known as SINAN, available on the DATASUS website from the Ministry of Health (http://www2.datasus.gov.br/). Case notification data in SINAN are collected at health facilities through epidemiological disease surveillance reporting forms. To calculate incidence of each arbovirus per 100 000 inhabitants, we obtained population estimates of the blood center catchment area from the Brazilian Institute of Geography and Statistics (https://www.ibge.gov.br/cidades-e-estados/).
Statistical Methods
We estimated monthly proportions of donations reactive for ZIKV, CHIKV, and DENV RNA (prevalence of RNAemia) using the Firth method as described by Hepworth and Biggerstaff for proportions from pooled testing that accounts for variable pool sizes, since small numbers of MP6 and MP12 were tested in addition to MP18 [25, 26]. We reported Wilson score confidence intervals (CIs). We assumed that a reactive pool represents at least 1 reactive donation and did not adjust estimates for the sensitivity or specificity of the assay.
To estimate incidence in the donor population, we used the following estimator, derived from a simple dynamic model of short-duration infections:
Where is the incidence estimate, p the prevalence of RNAemia, and d the duration of RNAemia. We used RNAemia duration estimates of 9.9 days (95% CI, 6.8–21.6) for ZIKV [27], 5.1 days (95% CI, 4.1–6.0) for CHIKV [17], and 9.1 days (95% CI, 4.4–13.9) for DENV [28]. Confidence intervals on incidence rates were obtained from parametric bootstrapping by sampling prevalence and durations from truncated normal distributions centered on our estimates and the published duration estimates, respectively, and standard deviations derived from the uncertainty around those values and truncated to prevent impossible values from being drawn. For comparability, monthly incidence was expressed as infections per 100 person-months (PM). We further computed monthly reported case rates (number of cases reported per 100 000 population).
The number of RNAemic blood components released for transfusion was estimated by multiplying RNAemic donation proportions for each blood center and month by the number of donations collected during that month and by the average number of blood components produced from each donation in Brazil. For the latter number we used 2.3, the average number of red blood cells, platelets, and plasma units produced in the country from each whole blood donation in 2018 reported by the Brazilian Health Regulatory Agency [29].
RESULTS
Overall 23 552 minipool samples, comprised of 215 376 donations, were tested from a total population of 994 370 donations collected at the 4 blood centers during the time periods from which samples were tested. Of these, 56 010 tested donations were collected at FPS (São Paulo), 49 374 at Hemominas (Belo Horizonte), 52 476 at Hemope (Recife), and 57 516 at Hemorio (Rio de Janeiro). Supplementary Table 1 provides information on samples tested by month for each blood center.
Zika Virus
No ZIKV RNAemia was detected in donations collected by FPS or Hemope during the study period (Supplementary Figures 1 and 2), but substantial prevalence of RNAemic donations was detected in Hemominas and Hemorio, with the highest prevalence in 2016 (Table 1). The prevalence of RNAemia during April–October 2016 (period 1) in Hemominas was 88.3/100 000 donations (95% CI, 47.9–162.4) and in Hemorio was 202.6/100 000 donations (95% CI, 139.1–294.9), with lower prevalence in periods 2 and 3, but still >50/100 000 donations in Hemorio during both periods. No ZIKV RNAemic donations were detected at any blood center in November 2018–June 2019 (period 4).
Table 1.
Prevalence of RNAemic Donations by Seasonal Arbovirus Outbreak Period in 4 Blood Centers, Brazil
| Period | Fundação Pró-Sangue, São Paulo | Hemominas, Belo Horizonte | Hemope, Recife | Hemorio, Rio de Janeiro | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZIKV | CHIKV | DENV | ZIKV | CHIKV | DENV | ZIKV | CHIKV | DENV | ZIKV | CHIKV | DENV | |
| Apr 2016–Oct 2016a | 0.0 (.0–26.8) | 7.0 (1.2–39.5) | 28.0 (10.9–71.9) | 88.3 (47.9–162.4) | 0.0 (.0–33.7) | 70.4 (35.7–138.9) | 0.0 (.0–31.8) | 83.0 (45.1–152.8) | 0.0 (.0–31.8) | 202.6 (139.1–294.9) | 111.9 (67.7–184.7) | 14.8 (4.1–53.9) |
| Nov 2016–Jun 2017a | 0.0 (.0–27.7) | 0.0 (.0–27.7) | 0.0 (.0–27.7) | 8.2 (1.4–46.2) | 0.0 (.0–31.3) | 0.0 (.0–31.3) | 0.0 (.0–29.5) | 0.0 (.0–29.5) | 0.0 (.0–29.5) | 94.9 (54.3–165.8) | 7.9 (1.4–44.7) | 0.0 (.0–30.3) |
| Nov 2017–Jun 2018 | 0.0 (.0–27.9) | 0.0 (.0–27.9) | 0.0 (.0–27.9) | 0.0 (.0–31.3) | 0.0 (.0–31.3) | 8.2 (1.4–46.2) | 0.0 (.0–27.5) | 14.4 (3.9–52.3) | 28.8 (11.2–73.9) | 65.3 (33.1–128.7) | 49.1 (22.5–107.0) | 0.0 (.0–31.2) |
| Nov 2018–Jun 2019 | 0.0 (.0–27.2) | 0.0 (.0–27.2) | 49.9 (24.2–102.9) | 0.0 (.0–28.3) | 0.0 (.0–28.3) | 358.2 (269.3–475.6) | 0.0 (.0–28.5) | 0.0 (.0–28.5) | 29.8 (11.6–76.6) | 0.0 (.0–28.6) | 45.0 (20.6–98.1) | 22.5 (7.6–65.9) |
Data are presented as RNAemic donations per 100 000 (95% confidence interval).
Abbreviations: CHIKV, chikungunya virus; DENV, dengue virus; ZIKV, Zika virus.
Testing started in April 2016 and continued for 15 consecutive months. From July 2017, the periods July–October were not tested, since there is normally limited outbreak activity during these months.
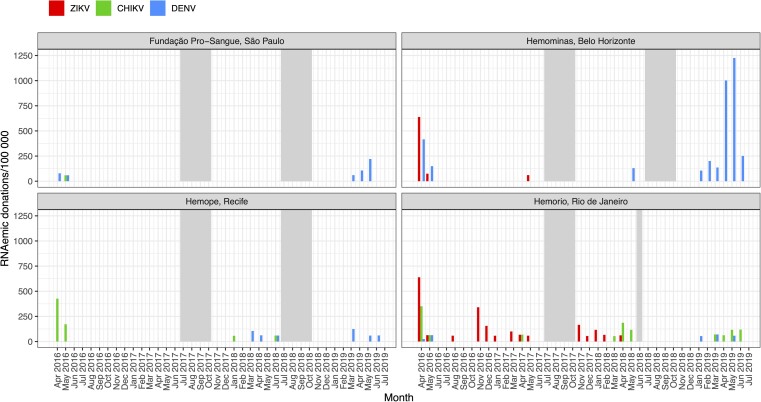
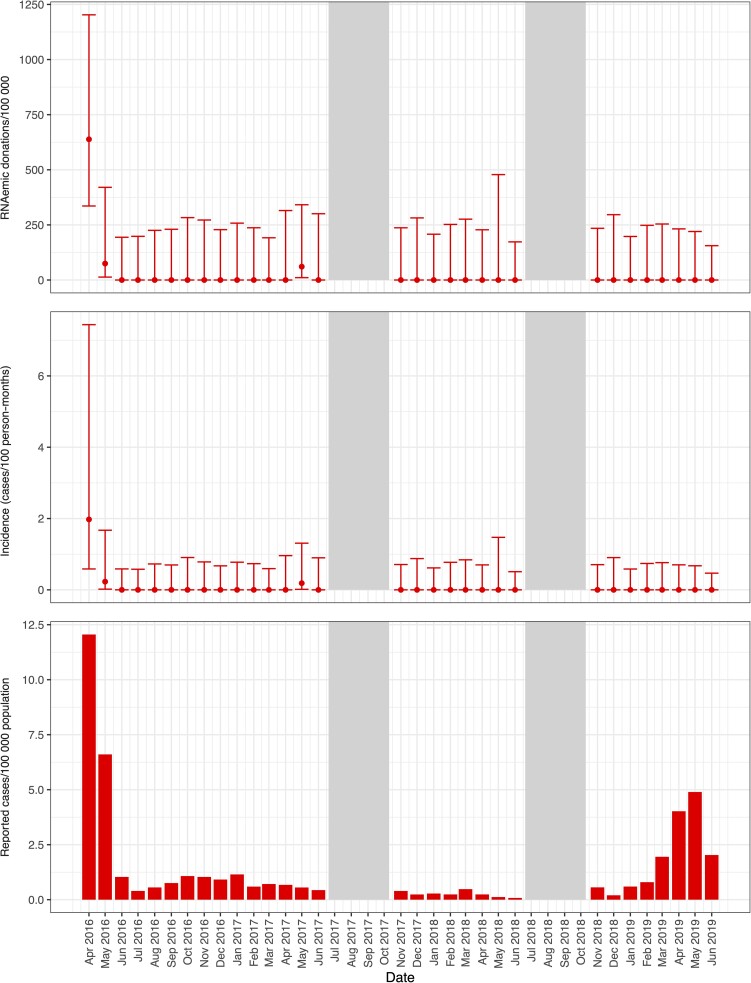
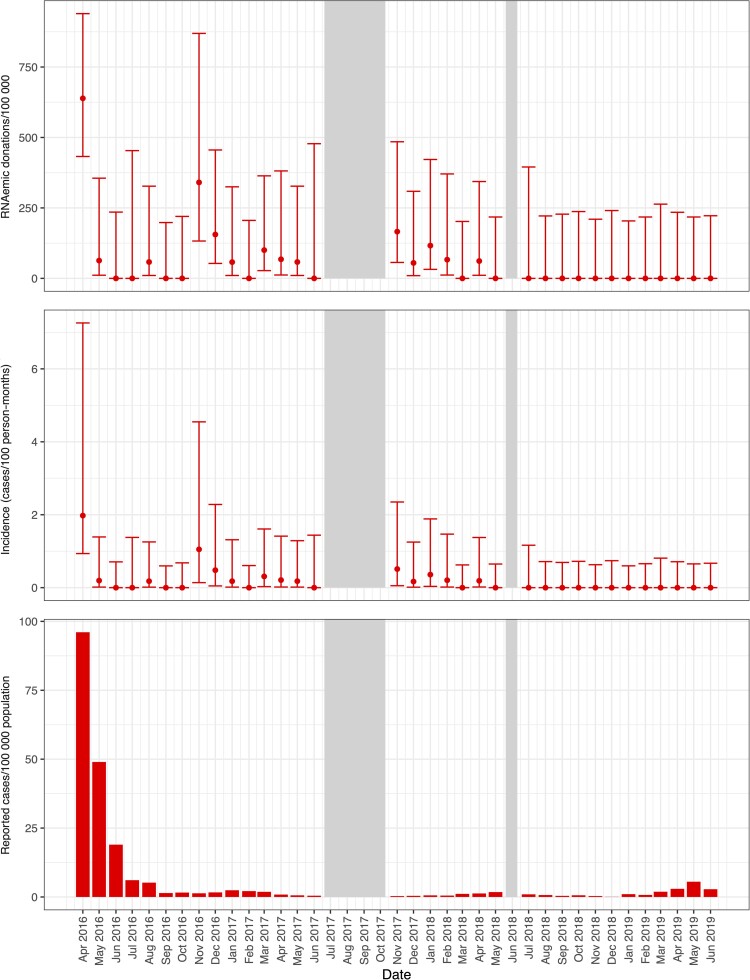
Further specifying the study periods into months, in April 2016, ZIKV RNAemia was detected at prevalence of 638/100 000 donations (95% CI, 336–1203) in Hemominas (Figures 1 and 2) and at 639/100 000 donations (95% CI, 433–939) in Hemorio (Figure 1 and Figure 3). Incidence of RNAemia in Hemominas blood donors during this month was estimated at 2.0 infections/100 PM (95% CI, .6–7.4), and in Hemorio donors at 2.0/100 PM (95% CI, .9–7.3) (Figures 2 and 3). Monthly RNAemic donation prevalence and donor incidence are reported in Supplementary Tables 2 and 3.
Figure 1.
Prevalence of RNAemic donations by month in 4 blood centers. Minipool samples from the months shown in gray were not tested. Abbreviations: CHIKV, chikungunya virus; DENV, dengue virus; ZIKV, Zika virus.
Figure 2.
Prevalence of RNAemic donations and donor incidence rates at Hemominas and reported case rates of Zika virus infections by month in Belo Horizonte. Minipool samples from the months shown in gray were not tested.
Figure 3.
Prevalence of RNAemic and donor incidence rates at Hemorio and reported case rates of Zika virus infections by month in Rio de Janeiro. Minipool samples from the months shown in gray were not tested.
Chikungunya Virus
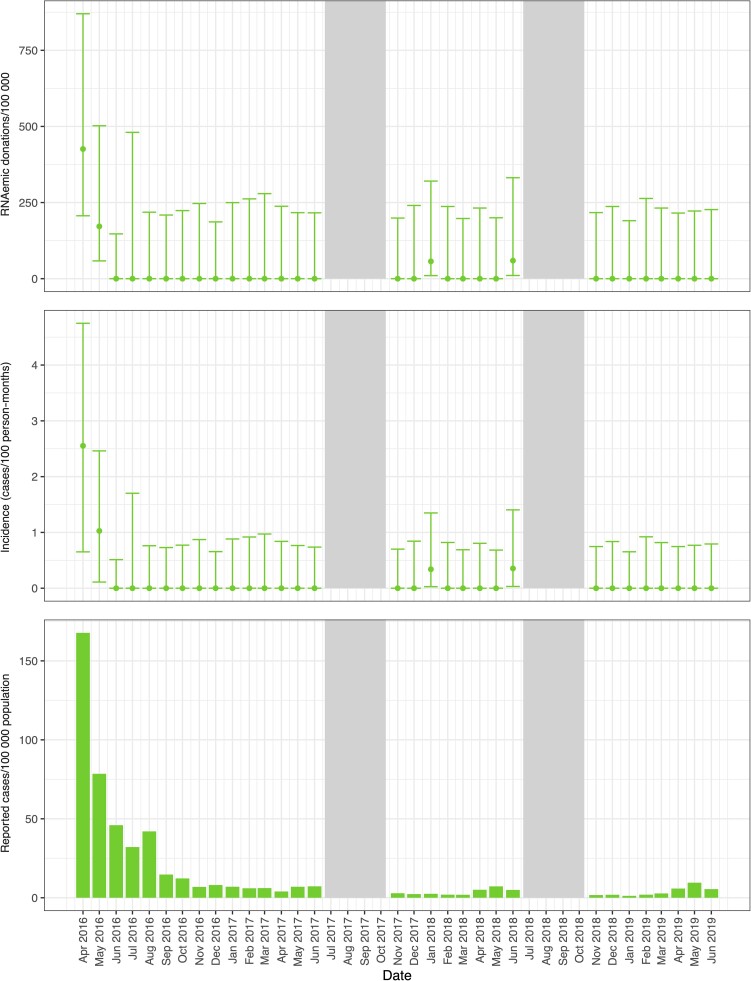
CHIKV RNAemia was detected in samples collected at FPS during period 1 at a prevalence of 7.0/100 000 donations (95% CI, 1.2–39.5; Table 1). At Hemominas no CHIKV RNAemia was detected in any period. Graphical results for FPS and Hemominas summarize these findings (Supplementary Figures 3 and 4). At Hemope, CHIKV RNAemia was detected in periods 1 and 3, at prevalence of 83.0/100 000 donations (95% CI, 45.1–152.8) and 14.4/100 000 donations (95% CI, 3.9–52.3), respectively. At Hemorio, CHIKV RNAemia was documented in all periods, with prevalence ranging from a low of 7.9/100 000 donations (95% CI, 1.4–44.7) in period 2 to high of 111.9/100 000 donations (95% CI, 67.7–184.7) in period 1.
The monthly incidence of CHIKV infection was estimated at 2.6/100 PM (95% CI, .7–4.7) in Hemope donors during April 2016 (Figure 4, Supplementary Table 3). Incidence in Hemorio donors was estimated at 2.1/100 PM (95% CI, 1.0–3.4) in the same month and ranged from 0.4/100 PM to 1.1/100 PM during the sporadic outbreak activity observed in Rio de Janeiro from the months in which samples were tested in the years 2017–2019 (Supplementary Table 3, Supplementary Figure 5).
Figure 4.
Prevalence of RNAemic donations and donor incidence rates at Hemope and reported case rates of chikungunya virus infections by month in Recife. Minipool samples from the months shown in gray were not tested.
Dengue Virus
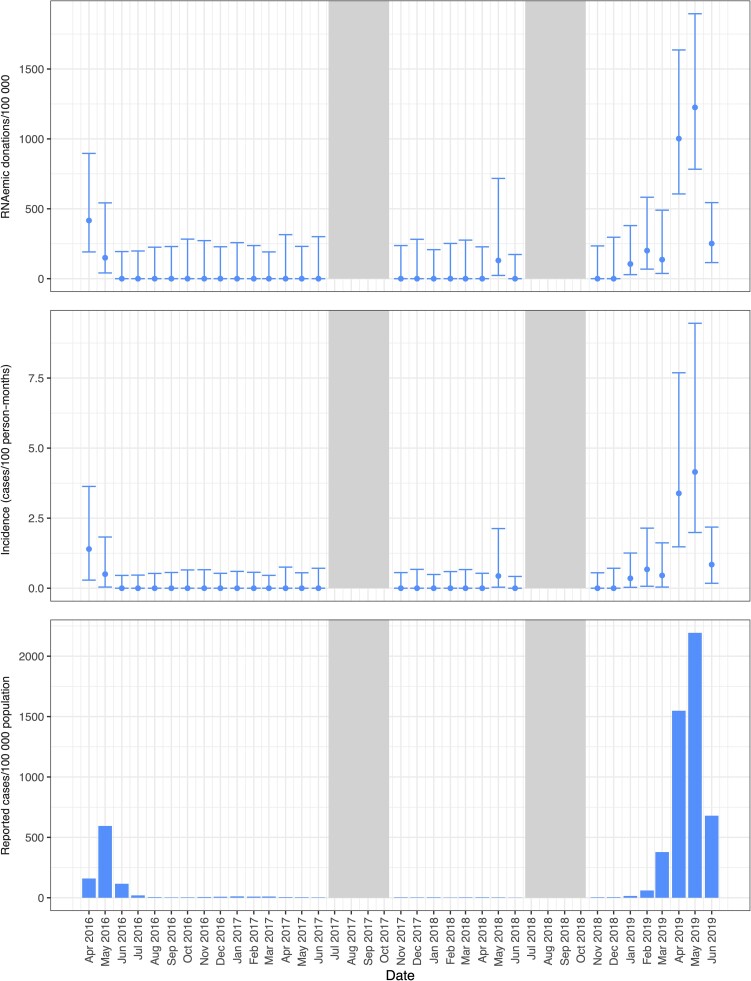
DENV outbreaks were evident based on RNAemia during periods 1 and 4 in all 4 blood centers (Table 1 and Figure 1). The prevalence of DENV RNAemia at FPS was 49.9/100 000 donations (95% CI, 24.2–102.9) during period 4 and at Hemominas were 70.4/100 000 donations (95% CI, 35.7–138.9) and 358.2/100 000 donations (95% CI, 269.3–475.6) during periods 1 and 4, respectively. A very large outbreak was evident in Belo Horizonte in January–June 2019 (period 4), with monthly RNAemia prevalence peaking at 1225/100 000 donations (95% CI, 783.0–1895.3) and donor incidence peaking at 4.1/100 PM (95% CI, 2.0–9.5) during May 2019 (Figure 5, Supplementary Tables 2 and 3). The upper value of each axis in each figure varies to better show the relative rates of donor RNAemia, incidence, and public health case reports within each location rather than across all sites.
Figure 5.
Prevalence of RNAemic donations and donor incidence rates at Hemominas and reported case rates of dengue virus infections by month in Belo Horizonte. Minipool samples from the months shown in gray were not tested.
Monthly donor incidence and reported clinical case rates for each infection parallel each other when the outbreak activity is high (Figures 2–5). For larger arbovirus outbreaks, particularly ZIKV in April 2016 in Rio de Janeiro and Belo Horizonte, CHIKV in Recife in April and May 2016, and DENV in Belo Horizonte in April and May in 2019, donor incidence of >1 infection per 100 PM was a reliable indicator of arbovirus activity. The point estimates show that testing of donor samples can identify evidence of wider transmission of ZIKV, CHIKV, or DENV in the blood center donor catchment area at the time clinical case reports are being generated by healthcare centers.
RNAemic Blood Components
With these RNAemic minipool data, we estimate that at least 343 ZIKV RNAemic components, 325 CHIKV RNAemic components, and 675 DENV RNAemic components were donated and assumed to have been released for transfusion during the study periods by the 4 blood centers (Table 2). Estimated numbers of RNAemic donations and components by month are shown in Supplementary Table 4.
Table 2.
Estimated Number of RNAemic Donations and Blood Components Released, by Seasonal Arbovirus Outbreak Period in 4 Blood Centers, Brazil
| Period | Fundação Pró-Sangue, São Paulo |
Hemominas, Belo Horizonte | Hemope, Recife | Hemorio, Rio de Janeiro | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZIKV | CHIKV | DENV | ZIKV | CHIKV | DENV | ZIKV | CHIKV | DENV | ZIKV | CHIKV | DENV | |
| Apr 2016–Oct 2016 | 0 (0) | 6 (12) | 14 (31) | 37 (81) | 0 (0) | 29 (65) | 0 (0) | 56 (122) | 0 (0) | 31 (67) | 18 (40) | 4 (8) |
| Nov 2016–Jun 2017 | 0 (0) | 0 (0) | 0 (0) | 4 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 47 (105) | 4 (9) | 0 (0) |
| Nov 2017–Jun 2018a | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (15) | 0 (0) | 11 (25) | 20 (45) | 34 (76)a | 27 (58)a | 0 (0)a |
| Nov 2018–Jun 2019 | 0 (0) | 0 (0) | 39 (86) | 0 (0) | 0 (0) | 160 (353) | 0 (0) | 0 (0) | 20 (44) | 0 (0) | 27 (59) | 14 (28) |
| Total | 0 (0) | 6 (12) | 53 (117) | 41 (89) | 0 (0) | 196 (433) | 0 (0) | 67 (147) | 40 (89) | 115 (254) | 76 (166) | 18 (36) |
Data are presented as No. of donations (No. of blood components).
Abbreviations: CHIKV, chikungunya virus; DENV, dengue virus; ZIKV, Zika virus.
The majority of samples from Hemorio in June 2018 could not be tested due to a fire in which the samples were destroyed.
DISCUSSION
In this study we tested pooled residual volume plasma samples left over from routine NAT testing for HIV, HCV, and HBV for evidence of ZIKV, CHIKV, and DENV RNAemia at 4 blood centers in Brazil. The results show varying patterns of ZIKV, CHIKV, and DENV in donors who donated to FPS, Hemominas, Hemorio, and Hemope. During arbovirus outbreak seasons in Brazil starting in 2016, ZIKV, CHIKV, and DENV were being transmitted by mosquitoes to donors who donated while they were RNAemic without overt symptoms of infection that would have led to deferral. The circulating arbovirus infections varied by geographic region and study period, and infections in donors were not always aligned with the presumed annual arbovirus outbreak season in different parts of the country.
In particular, ZIKV RNAemic MPs were identified outside of the expected arbovirus outbreak season in Rio de Janeiro in 2017 and early 2018. Thereafter, no ZIKV-reactive MPs were observed in the period of late 2018 to early 2019. While ZIKV is known to be sexually transmitted, the degree to which sexual transmission contributed to the detection of ZIKV RNAemic donations outside of the recognized annual arbovirus outbreak period is unknown. Although there is biological plausibility for sexual transmission, particularly from males to their sexual partners [30], the overall scientific evidence for sexual transmission of ZIKV and other arboviruses remains limited [31, 32]. In the absence of mosquito-borne transmission directly to humans, vertical transmission in Aedes aegypti mosquitoes may be more likely to maintain human exposure to Zika virus than human sexual transmission, which is inefficient from males to females and even more so from females to males. Vertical transmission within mosquitoes has been demonstrated for DENV, CHIKV, and ZIKV and could explain the observed Zika activity in Rio de Janeiro.
The ecological factors that lead to major arbovirus outbreaks remain poorly understood. Our results show these patterns are complex and that even in adjacent states in Brazil (Sao Paulo, Rio de Janeiro, and Minas Gerais) the arboviruses being transmitted to humans by mosquitos are varied. We started our study just after the large ZIKV outbreak occurred in the northeast of Brazil in 2015, including in Pernambuco where Hemope collects blood. The finding of no ZIKV-reactive minipools in Recife in April 2016 was unexpected and shows how quickly arbovirus infections can change. It is noteworthy that we did detect CHIKV RNAemia in donors during this period in Recife. CHIKV has spread throughout Brazil [33], yet large widespread CHIKV outbreaks have not been documented. The ecological factors that can be used to predict large outbreaks need continued investigation [34].
In locations where arbovirus activity was not detected, the upper limit of the 95% CIs provides an indication of the ability of this study to detect infections in donors. The upper limit of the 95% CI is the estimate of how high the infection rate could be given the sample size for each weekly and monthly set of data; that is, any arborvirus activity over that upper CI would likely be detectable given our sample sizes. This provides a way to extrapolate the expected sensitivity of donor surveillance to detect outbreak activity. Expanded testing of donor MP samples would increase the sensitivity to detect infections at lower levels of arbovirus outbreak activity.
When comparing the minipool testing results to public health clinical case report data, the overlap in the patterns at the same points in time suggest that there may be additional value achieved in surveillance of donors beyond that achieved through symptomatic case reports to public health authorities. While our retrospective study could not provide insights in real time, routine donor surveillance for these infections using multiplex assays would have advantages over case reports. The near real-time monitoring of donors using NAT arbovirus assays would provide information on the specific infection(s) circulating in the local community where blood is donated. This information could help in the clinical diagnoses and care of patients as well as lead to the interdiction of RNAemic blood units to reduce the risk of transfusion transmission. However, the threshold level of arbovirus activity in which donor surveillance could be informative for public health is undefined and would vary by virus and from outbreak to outbreak.
Our study has limitations. First, sampling at 4 blood centers in regions that have been affected by arbovirus activity to varying degrees is not representative of the experience in all regions in Brazil or even within the states where this study was conducted. Second, because samples were obtained from previously pooled specimens which had limited volume and there were no retained individual samples available to us, we could not resolve the reactive pools into the specific donations that tested RNAemic. Consequently, we are unable to conduct analyses of demographic correlates (age, sex, area of residence) of RNAemic donations. Third, detection of RNA in blood samples does not mean the samples had infection-competent viruses that could transmit infections to transfusion recipients; that is, release of RNAemic blood components for transfusion is not a demonstration of transmission of infection to recipients. Fourth, dilutional effects of MP6 with the addition of plasma or pooling of 3 MP6 samples led to an MP testing size of >18 donations per pool. Donations with low-level viremia could be missed in this study.
The results of this study show the utility of minipool testing of donors for arbovirus RNA as part of public health surveillance and provide evidence that blood recipients in Brazil were likely exposed to viremic blood components for each of these arboviruses during the REDS-III MP surveillance study period. Each known and newly emerging arbovirus needs to be studied to identify the risks and clinical consequences of transfusion transmission for recipients. In a highly endemic country for arboviruses, the use of multiplex assays to screen blood donations can identify multiple infectious agents at the same time in the same geographic location. While this study is unable to establish the implications for transfusion recipients, it does provide evidence that RNAemic components are being unintentionally transfused. Previous research has shown a transfusion transmission probability of approximately one-third if DENV RNA is detected in blood donations [35]. Similar data are not available for ZIKV or CHIKV, but probabilities of transfusion transmission are presumed to be lower. Case reports of ZIKV transfusion transmission are documented, but not for CHIKV transfusion transmission. Given the estimated exposure risk in our study, further monitoring of donors and studies to define the implications for patients requiring transfusion are warranted.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank all of the blood donors whose samples were tested as part of this study. We also thank the following research study staff who made this project possible, including the REDS-III Brazil project coordinators Sheila Garcia Mateos and Carol Miranda and the staff at each of the blood centers: Silvia Regina at FPS, Gabriel Pissolati at Hemominas, Ericka Martins de Farias at Hemope, and Jesse Alves da Silva and Elyas Ryan Rufino da Silva at Hemorio. Their dedication and commitment made this study possible.
Financial support. This study was supported by National Heart, Lung, and Blood Institute contracts: Recipient Epidemiology and Donor Evaluation Study (REDS-III) International Brazil (HHSN268201100007I), REDS-III Central Laboratory (HHSN268201100001I), and REDS-III Data Coordinating Center (HHSN268201100006I).
Supplementary Material
Contributor Information
Brian Custer, Vitalant Research Institute, San Francisco, California, USA; Department of Laboratory Medicine, University of California, San Francisco, San Francisco, California, USA.
Eduard Grebe, Vitalant Research Institute, San Francisco, California, USA; Department of Laboratory Medicine, University of California, San Francisco, San Francisco, California, USA; Department of Science and Innovation, National Research Foundation Centre of Excellence in Epidemiological Modelling and Analysis, Stellenbosch University, Stellenbosch, South Africa.
Renata Buccheri, Vitalant Research Institute, San Francisco, California, USA.
Sonia Bakkour, Vitalant Research Institute, San Francisco, California, USA; Department of Laboratory Medicine, University of California, San Francisco, San Francisco, California, USA.
Mars Stone, Vitalant Research Institute, San Francisco, California, USA; Department of Laboratory Medicine, University of California, San Francisco, San Francisco, California, USA.
Ligia Capuani, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Cecilia Alencar, Hospital das Clinicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
Luiz Amorim, Fundação Hemorio, Rio de Janeiro, Brazil.
Paula Loureiro, Fundação Hemope, Recife, Brazil; Faculdade de Medicina da Universidade de Pernambuco, Recife, Brazil.
Anna Barbara Carneiro-Proietti, Fundação Hemominas, Belo Horizonte, Brazil.
Alfredo Mendrone-Junior, Fundação Pró-Sangue, São Paulo, Brazil.
Thelma Gonçalez, Vitalant Research Institute, San Francisco, California, USA.
Kui Gao, Grifols Diagnostics Solutions, San Diego, California, USA.
Kristin W Livezey, Grifols Diagnostics Solutions, San Diego, California, USA.
Jeffrey M Linnen, Grifols Diagnostics Solutions, San Diego, California, USA.
Don Brambilla, Research Triangle Institute International, Rockville, Maryland, USA.
Chris McClure, Research Triangle Institute International, Rockville, Maryland, USA.
Michael P Busch, Vitalant Research Institute, San Francisco, California, USA; Department of Laboratory Medicine, University of California, San Francisco, San Francisco, California, USA.
Ester C Sabino, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.
References
- 1. Levi JE. Emerging infectious agents and blood safety in Latin America. Front Med (Lausanne) 2018; 5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bianco C. Dengue and chikungunya viruses in blood donations: risks to the blood supply? Transfusion 2008; 48:1279–81. [DOI] [PubMed] [Google Scholar]
- 3. Petersen LR, Busch MP. Transfusion-transmitted arboviruses. Vox Sang 2009; 98:495–503. [DOI] [PubMed] [Google Scholar]
- 4. Honorio NA, Nogueira RM, Codeco CT, Carvalho MS, Cruz OG, de Avelar Figueiredo Mafra Magalhães M, et al. Spatial evaluation and modeling of dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl Trop Dis 2009; 3:e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teixeira M, Costa M, Barreto M, Mota E. Dengue and dengue hemorrhagic fever epidemics in Brazil: what research is needed based on trends, surveillance, and control experiences? Cad Saude Publica 2005; 21:1307–15. [DOI] [PubMed] [Google Scholar]
- 6. Vargas J, Ferreira O, Corgozinho P. Tratamento logístico das ocorrências anuais de dengue no Rio de Janeiro (1985–2008) [in Portuguese]. Economia Energia 2009; 12:71. [Google Scholar]
- 7. Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, De Lamballerie X. Chikungunya in the Americas. Lancet 2014; 383:514. [DOI] [PubMed] [Google Scholar]
- 8. Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg 1956; 50:442–8. [PubMed] [Google Scholar]
- 9. Dick GWA. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952; 46:521–34. [DOI] [PubMed] [Google Scholar]
- 10. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–20. [DOI] [PubMed] [Google Scholar]
- 11. Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg 1964; 58:335–8. [PubMed] [Google Scholar]
- 12. Kelser EA. Meet dengue's cousin, Zika. Microbes Infect 2016;18:163–6. [DOI] [PubMed] [Google Scholar]
- 13. Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome–case report, French Polynesia, December 2013. Euro Surveill 2014; 19:20720. [DOI] [PubMed] [Google Scholar]
- 14. de Araujo TVB, Ximenes RAA, Miranda-Filho DB, et al. Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect Dis 2018; 18:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med 2018; 378:985–94. [DOI] [PubMed] [Google Scholar]
- 16. Wilder-Smith A, Wei Y, Araujo TVB, et al. Understanding the relation between Zika virus infection during pregnancy and adverse fetal, infant and child outcomes: a protocol for a systematic review and individual participant data meta-analysis of longitudinal studies of pregnant women and their infants and children. BMJ Open 2019; 9:e026092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simmons G, Bres V, Lu K, et al. High incidence of chikungunya virus and frequency of viremic blood donations during epidemic, Puerto Rico. USA 2014. Emerg Infect Dis 2016; 22:1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quandelacy TM, Healy JM, Greening B, et al. Estimating incidence of infection from diverse data sources: Zika virus in Puerto Rico 2016. PLoS Comput Biol 2021; 17:e1008812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Faria NR, Quick J, Claro IM, et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature 2017; 546:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simmons G, Stone M, Busch MP. Arbovirus diagnostics: from bad to worse due to expanding dengue virus vaccination and Zika virus epidemics. Clin Infect Dis 2018; 66:1181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stone M, Bakkour S, Lanteri MC, et al. ZIKV RNA and IgM persistence in blood compartments and body fluids: a prospective observational study. Lancet Infect Dis 2020; 20:1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grifols Diagnostic Solutions . Procleix Zika virus assay for in vitro diagnostic use. v4.0. Vol. GDSS-IFU-000005. Emeryville, CA: Grifols Diagnostic Solutions, 2020. [Google Scholar]
- 23. Stone M, Lanteri MC, Bakkour S, et al. Relative analytical sensitivity of donor nucleic acid amplification technology screening and diagnostic real-time polymerase chain reaction assays for detection of Zika virus RNA. Transfusion 2017; 57:734–47. [DOI] [PubMed] [Google Scholar]
- 24. Grifols SA . Procleix dengue virus assay [package insert]. 2020. https://www.diagnostic.grifols.com/en/procleix/-assays/product-specifications. Accessed 18 March 2022.
- 25. Hepworth G, Biggerstaff BJ. Bias correction in estimating proportions by pooled testing. J Agric Biol Environ Stat 2017; 22:602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hitt B, Bilder C, Schaarschmidt F, Biggerstaff B, McMahan C, Tebbs J. binGroup2: identification and estimation using group testing. version 1.1.0. Comprehensive R Archive Network, 2021. https://CRAN.R-project.org/package=binGroup2. Accessed 14 November 2021.
- 27. Chevalier MS, Biggerstaff BJ, Basavaraju SV, et al. Use of blood donor screening data to estimate Zika virus incidence, Puerto Rico 2016. Emerg Infect Dis 2017; 23:790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Busch MP, Sabino EC, Brambilla D, et al. Duration of dengue viremia in blood donors and relationships between donor viremia, infection incidence and clinical case reports during a large epidemic. J Infect Dis 2016; 214:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brazilian Health Regulatory Agency (ANVISA) . 7° Boletim de Producao Hemoterapica–Hemoprod 2018 [in Portuguese]. Brasilia: Agência Nacional de Vigilância Sanitária, 2020. [Google Scholar]
- 30. Kurscheidt FA, Mesquita CSS, Damke G, et al. Persistence and clinical relevance of zika virus in the male genital tract. Nat Rev Urol 2019; 16:211–30. [DOI] [PubMed] [Google Scholar]
- 31. Blitvich BJ, Magalhaes T, Laredo-Tiscareno SV, Foy BD. Sexual transmission of arboviruses: a systematic review. Viruses 2020; 12:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Counotte MJ, Kim CR, Wang J, et al. Sexual transmission of Zika virus and other flaviviruses: a living systematic review. PLoS Med 2018; 15:e1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lessa-Aquino C, Trinta KS, Pestana CP, et al. Detection of East/Central/South African genotype chikungunya virus during an outbreak in a southeastern state of Brazil. Epidemiol Infect 2018; 146:2056–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fuller TL, Calvet G, Genaro Estevam C, et al. Behavioral, climatic, and environmental risk factors for Zika and chikungunya virus infections in Rio de Janeiro, Brazil 2015. PLoS One 2017; 12:e0188002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sabino EC, Loureiro P, Lopes ME, et al. Transfusion-transmitted dengue and associated clinical symptoms during the 2012 epidemic in Brazil. J Infect Dis 2016; 213:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.