Abstract
The interaction of the fimbriae of Haemophilus influenzae type b (Hib) with two heparin-binding extracellular matrix proteins, human fibronectin (Fn) and heparin-binding growth-associated molecule (HB-GAM) from mouse, were studied. The fimbriated Hib strain 770235 fim+, as well as the recombinant strain E. coli HB101(pMH140), which expressed Hib fimbriae, adhered strongly to Fn and HB-GAM immobilized on glass. Purified Hib fimbriae bound to Fn and HB-GAM, and within the Fn molecule, the binding was localized to the N-terminal 30,000-molecular-weight (30K) and 40K fragments, which contain heparin-binding domains I and II, respectively. Fimbrial binding to Fn, HB-GAM, and the 30K and the 40K fragments was inhibited by high concentrations of heparin. The results show that fimbriae of Hib interact with heparin-binding extracellular matrix proteins. The nonfimbriated Hib strain 770235 fim− exhibited a low level of adherence to Fn but did not react with HB-GAM, indicating that Hib strains also possess a fimbria-independent mechanism to interact with Fn.
Haemophilus influenzae is a normal inhabitant of the human nasopharynx and causes both localized and invasive infections in humans. Unencapsulated H. influenzae strains are responsible for mild infections, such as otitis media, sinusitis, and bronchitis. Encapsulated H. influenzae type b (Hib) strains cause more severe diseases, such as meningitis, septicemia, and arthritis (33). Young children especially are sensitive to infection by Hib strains. The type b capsule is thought to contribute to the pathogenesis of Hib strains by inhibiting neutrophil phagocytosis and resisting complement-mediated bactericidal activity; these properties enhance the bloodstream survival of Hib bacteria (reviewed in reference 32).
The fimbriae of Hib strains mediate bacterial adhesion to oropharyngeal epithelial cells (37, 39) and promote Hib colonization in organ culture models (5, 7, 12). In addition, fimbriated Hib isolates show efficient adhesion to mammalian extracellular matrices (ECMs) (43), which may promote the invasion of Hib strains into the circulation. The expression of the Hib fimbriae is subject to reversible phase variation (6, 40), and the fimbrial expression can change rapidly during infection. During natural infection, Hib isolates colonizing the nasopharynx are fimbriated, while in the circulation, Hib isolates do not express fimbriae. These observations suggest that expression of fimbriae is beneficial for Hib during the initial stages of infection but is not needed at later stages of infection when the bacteria have reached the circulation. Nonfimbriate strains also exhibit adhesiveness to epithelial cells (reviewed in reference 7) and the ECM (43), and it is apparent that structures other than fimbriae also support adhesion of Hib and other isolates of H. influenzae.
The Hib fimbrial-gene cluster consists of five genes (hifA to hifE) which are involved in fimbria biogenesis (14, 28, 39, 41). HifA is the major fimbrial subunit, and HifB and HifC function as a periplasmic chaperone and an outer membrane usher, which are needed in the assembly and cell wall anchoring of the filament. hifD and hifE encode putative minor fimbrial subunits. The Hib fimbriae recognize sialic acid-containing lactosylceramide derivatives on human oropharyngeal epithelial cells (37) and on human erythrocytes carrying the AnWj antigen (35). The identity of the adhesin molecule in the Hib fimbria has remained open; evidence in favor of the major component, HifA, as well as the minor components has been presented (15, 16, 42).
Fibronectin is a well-characterized, multifunctional adhesive protein present in ECMs and in a soluble form in plasma. Fibronectin is involved in many important functions of cells and tissues, such as cell adhesion, spreading and migration, and tissue development and differentiation, as well as blood clot stabilization and wound healing (3, 25, 48). Fibronectin is a dimer of two nearly identical polypeptide chains that are assembled into a series of continuous structural and functional domains (19). Using proteolytic fragments of fibronectin, several domains with different binding activities have been mapped within the molecule (3, 48) (Fig. 1). The major cell-binding site, containing a crucial Arg-Gly-Asp sequence, is located within the 120-kDa fragment in the central part of the fibronectin molecule. The collagen-binding activity is located within the 40- to 45-kDa domain near the amino terminus, and the amino-terminal domain (30 kDa) contains multiple ligand-binding activities with affinities to, e.g., heparin and fibrin. Fibronectin contains a second heparin-binding domain in the carboxyl-terminal part of the molecule. Both domains interact with heparin and heparan sulfate proteoglycans. Heparin-binding activity has been shown to enhance cell adhesion to fibronectin (25, 48).
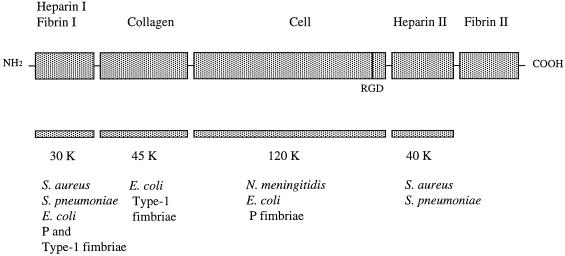
FIG. 1.
Schematic model of fibronectin and its major domains (modified from a previously published figure [48], with the permission of the publisher). The fibronectin fragments (30K, 45K, 120K, and 40K) used in this study and recognition sites for bacteria or fimbriae are indicated below (1, 4, 11, 29, 30, 38, 46, 47).
The ECM and its components are recognized by a number of invasive bacterial pathogens, including gram-negative as well as gram-positive species (reviewed in references 18 and 44). Adherence to matrix components has been found to enhance bacterial migration from the primary infection site into the circulation or secondary infection loci (24, 31), as well as to enhance bacterial colonization of damaged tissue sites, such as wounds (reviewed in reference 44). The four major meningitis-associated bacterial species, H. influenzae, Neisseria meningitidis, Streptococcus pneumoniae, and Escherichia coli, bind fibronectin (4, 26, 38, 43), which raises the possibility that interaction with an ECM protein has a role in the infectious process in bacterial meningitis. In particular, fimbriated and nonfimbriated clinical isolates of H. influenzae exhibit a broad spectrum of interactions with glycosylated as well as collagenous proteins of the ECM (43).
The bacteria-fibronectin interactions are based on either protein-protein or protein-carbohydrate interaction and are targeted to different domains of the fibronectin molecule. The binding sites for staphylococci, streptococci, and P-fimbriate and type-1-fimbriate E. coli are located within the N-terminal domain of fibronectin, which lacks carbohydrate (1, 11, 29, 30, 46, 47) (Fig. 1). In contrast, binding to fibronectin by the S fimbriae of meningitis-associated E. coli is based on recognition of the sialyl oligosaccharide chains in fibronectin (26). Staphylococcus aureus and S. pneumoniae also bind to the C-terminal heparin-binding domain of fibronectin (1, 11, 38), and P-fimbriate E. coli also binds to the 120- to 140-kDa carboxyl-terminal fragment of the fibronectin molecule (46), which is also recognized by N. meningitidis (4). In this study, we characterize more closely the interaction of Hib fimbriae with fibronectin and show that the Hib fimbriae also interact with the heparin-binding growth-associated molecule (HB-GAM), an ECM protein of developing nervous tissue (22). HB-GAM binds the heparan sulfate side chains of N-syndecan, a cell surface proteoglycan, and its interaction with heparinlike molecules of the neuron surface promotes the formation of neuronal connections (20, 21, 23). In addition to the nervous system, HB-GAM occurs in the basement membranes of several nonneuronal organs (17).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Fimbriated Hib strain 770235 fim+ and the nonfimbriated variant 770235 fim− were cultured overnight at 37°C on brain heart infusion agar plates supplemented with 1% IsoVitalex (BBL Microbiology Systems, Cockeysville, Md.) and 40 mg of hemin (Sigma, St. Louis, Mo.)/liter in a humid atmosphere containing 5% CO2 (2, 34, 35). For adhesion tests, bacteria from the agar were inoculated into brain heart infusion broth supplemented as described above. After overnight cultivation, the bacteria were collected by centrifugation (1,000 × g; 10 min) and washed twice with phosphate-buffered saline (PBS; pH 7.1). The recombinant E. coli strain HB101(pMH140), carrying the Hib fimbrial gene cluster, and the nonfimbriate control strain HB101(pEMBL8) were cultured overnight at 37°C on Luria agar plates supplemented with 100 μg of ampicillin/ml (39, 41, 42). Before use in adhesion tests, the Hib fimbrial expression on bacteria was confirmed by hemagglutination and agglutination tests with Hib fimbria-specific antibodies (36). The recombinant strains were also tested by mannoside-sensitive yeast cell agglutination (8) to confirm lack of expression of E. coli type 1 fimbriae.
Adherence tests.
Bacterial adherence to glass coated with ECM proteins was tested as described earlier (43, 45). Glass slides were coated with human plasma fibronectin (Collaborative Biomedical Products, Bedford, Mass.), recombinant HB-GAM protein (20), and bovine serum albumin (BSA; Sigma Chemical Co., St. Louis, Mo.) at a concentration of 4 μg/ml. Bacteria were used at a concentration of 5 × 107 to 1 × 1010/ml. For inhibition studies, the bacteria were first incubated either with low-molecular-weight heparin (Sigma) or with chondroitin sulfate C (Sigma) for 40 min at room temperature. Before the adhesion tests, some fibronectin-coated wells were treated with Vibrio cholerae neuraminidase (26) (Boehringer Mannheim, Mannheim, Germany) (50 mU/ml in Dulbecco's PBS [Nord Cell, Skärholmen, Sweden]) for 2 h at 37°C or with 100 mM sodium periodate (45) in 100 mM sodium acetate buffer (pH 5.5) for 2 h at 37°C or overnight at 4°C. The control wells were treated with buffer alone. Adherent bacteria were visualized in an Olympus Optical Co. (Hamburg, Germany) microscope equipped with a charge-coupled device camera (catalog no. 4912-5000; Cohu, San Diego, Calif.) after being stained with methylene blue, and the images were digitized with an LG-3 (Scion, Frederick, Md.) scientific frame grabber and Macintosh 7100 80-MHz computer using the public domain NIH Image version 1.55 program as described earlier (43). The number of bacteria in 20 randomly chosen microscopic fields of 1.6 × 104 μm2 were determined by density slicing. Statistical comparisons were carried out using Student's t test.
ELISAs.
Hib fimbrial binding to HB-GAM, fibronectin, and fibronectin fragments was tested in an enzyme-linked immunosorbent assay (ELISA). The Hib fimbriae were purified from the recombinant E. coli strains HB101(pMH140) and HB101(pMH140hifD::kan) by the ammonium sulfate precipitation method (9). A monoclonal antibody specific for the Hib fimbriae was available from previous work (36). Antibody titers for purified Hib fimbriae were determined by ELISA as described earlier (10). Antibody titers, 5.7 and 4.9 for Hib pMH140 and pMH140hifD::kan fimbriae, respectively, are given as the logarithm of the highest dilution of the antiserum giving an absorbance of 0.5 at 405 nm. Polystyrene microplate (Nunc, Roskilde, Denmark) wells were coated with HB-GAM, fibronectin, fibronectin fragments, or BSA (4 μg/ml in PBS) overnight at 4°C. N-terminal 30,000-molecular-weight (30K) (containing the heparin-binding I domain) and 45K (containing the collagen-binding domain) fibronectin fragments were from Sigma. The 120K fragment (containing the “major” cell-binding domain) and the 40K fragment (containing the C-terminal heparin-binding II domain) were from Chemicon International, Inc., Temecula, Calif. After being blocked with 2% BSA, the wells were incubated with Hib fimbriae (1.5 to 100 μg/ml) at room temperature for 2 h. The monoclonal antibody specific for the Hib fimbriae (diluted 1:30,000 [36]) and alkaline phosphatase-conjugated rabbit anti-mouse immunoglobulin G (Dako A/S, Glostrup, Denmark) (diluted 1:500) were used to detect fimbrial binding to target proteins. For inhibition assays, the Hib fimbriae (50 μg/ml) were incubated first either with low-molecular-weight heparin or with chondroitin sulfate for 40 min at room temperature. All ELISAs were repeated two or three times. The results are given as the means from a representative experiment performed with duplicate samples.
Dot blot assays.
Dot blot analysis with digoxigenin-labeled lectins (Glycan differentiation kit; Boehringer Mannheim) were used to detect terminal sialyl structures in fibronectin and fibronectin fragments. Sambucus nigra agglutinin (SNA) recognizes terminal sialyl-α2-6-galactosides, and Maackia amurensis agglutinin (MAA) recognizes sialyl-α2-3-galactosides. The target proteins were immobilized on nitrocellulose membranes (Bio-Rad Laboratories, Richmond, Calif.) at a concentration of 2 μg per dot (26). Lectin staining with SNA and MAA was performed as described by Boehringer.
RESULTS
Bacterial adhesion to fibronectin and HB-GAM.
The fimbriated Hib strain 770235 fim+ adheres strongly to subendothelial ECM as well as to fibronectin and collagens (43). We analyzed the adhesion of Hib strain 770235 fim+ and its isogenic nonfimbriate variant Hib 770235 fim− to human plasma fibronectin and HB-GAM on a glass surface (Fig. 2). Strain 770235 fim+ adhered strongly to fibronectin and less efficiently to HB-GAM (Fig. 2A), whereas no adhesion to the control protein, BSA, was detected. Strain 770235 fim− exhibited adhesiveness to fibronectin but not to HB-GAM or BSA (Fig. 2B); the level of its adhesion to fibronectin was one-fifth of that shown by Hib 770245 fim+.
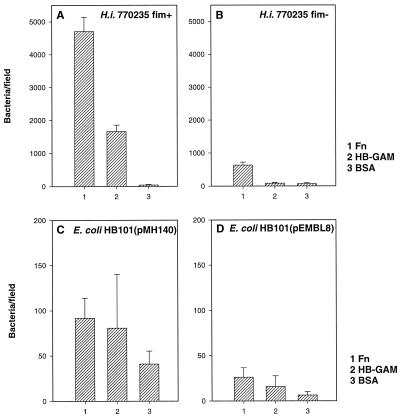
FIG. 2.
(A and B) Adhesion of the fimbriate Hib strain 770235 fim+ and the nonfimbriate strain Hib 770235 fim− to fibronectin (Fn), HB-GAM, and BSA immobilized on glass slides. (C and D) Adhesion of recombinant E. coli HB101(pMH140) expressing Hib fimbriae, and of HB101(pEMBL8) with the vector plasmid alone, to target proteins. Bacteria were tested at concentrations of 109/ml (A and B) and 1010/ml (C and D). The means and standard deviations of adherent bacteria in 20 microscopic fields of 1.6 × 104 μm2 are shown.
To analyze the role of the Hib fimbriae in the observed adherence, we tested the adhesiveness of the recombinant strain E. coli HB101(pMH140) expressing the Hib fimbriae (Fig. 2C). E. coli HB101(pMH140) adhered to fibronectin as well as to HB-GAM. This strain adhered significantly better to fibronectin (P < 0.001) and to HB-GAM (P < 0.01) than to BSA. However, it is notable that the level of adherence was 2 to 5% of that shown by Hib strain 770235 fim+. Strain HB101(pMH140) was also found to be much weaker in hemagglutination than was strain Hib 770235 fim+ (not shown). E. coli strain HB101(pEMBL8) carrying the vector plasmid alone exhibited a significantly (P < 0.001) weaker adhesion than did strain HB101(pMH140) (Fig. 2D).
To analyze the role of terminal sialic acids in the observed bacterial adhesion to fibronectin, we tested the effect of periodate or neuraminidase treatment of fibronectin on bacterial adhesiveness. We observed that periodate or neuraminidase treatment had no effect on the adhesion of Hib strain 770235 fim+ (data not shown).
Binding of purified fimbriae to fibronectin and HB-GAM.
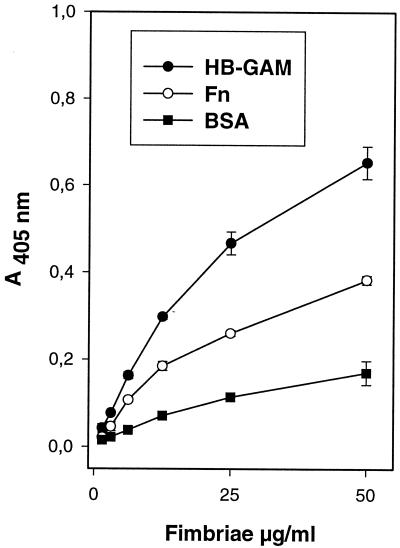
We next tested whether purified Hib fimbriae bind to fibronectin and HB-GAM. The Hib pMH140 fimbriae bound in a dose-dependent manner to HB-GAM and fibronectin (Fig. 3.), and the level of binding was lower with BSA. In contrast to what was detected with fimbriate Hib cells, the level of binding of purified Hib pMH140 fimbriae to HB-GAM protein was higher than the level of binding to fibronectin.
FIG. 3.
Binding of purified Hib pMH140 fimbriae to fibronectin (Fn), HB-GAM, and BSA. The absorbance values are means from a representative experiment performed with duplicate samples. The error bars show the range of absorbance values.
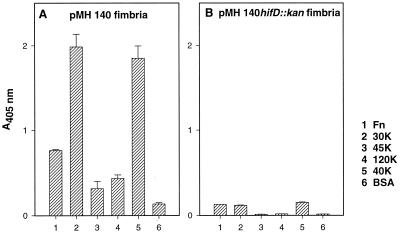
We next tested whether Hib fimbrial binding could be localized to a proteolytic fragment of the fibronectin molecule. Four fibronectin fragments were used as target molecules (Fig. 1) in the ELISA with purified Hib pMH140 fimbriae. The Hib pMH140 fimbriae bound efficiently to the N-terminal 30K and the C-terminal 40K fragments (Fig. 4A, lanes 2 and 5), which contain the heparin-binding domains I and II (Fig. 1). The levels of Hib pMH140 fimbrial binding to these two fibronectin fragments were two to three times higher than the level of binding to the intact fibronectin molecule (Fig. 4A, bar 1). Binding of the Hib pMH140 fimbriae to the fibronectin fragments was also dose dependent (data not shown). The levels of binding to the 45K fragment (Fig. 4A, bar 3) and the 120K fragment (Fig. 4A, bar 4), as well as to BSA (Fig. 4A, bar 6), were low. The mutated pMH140hifD::kan fimbriae showed weak binding to the target proteins (Fig. 4B).
FIG. 4.
Binding of purified pMH140 fimbriae (A) and mutated pMH140hifD::kan fimbriae (B) to fibronectin (Fn), fibronectin fragments (30K, 45K, 120K, and 40K [Fig. 1]), and BSA as measured by ELISA. The absorbance values are means from a representative experiment performed with duplicate samples. The error bars show the range of absorbance values.
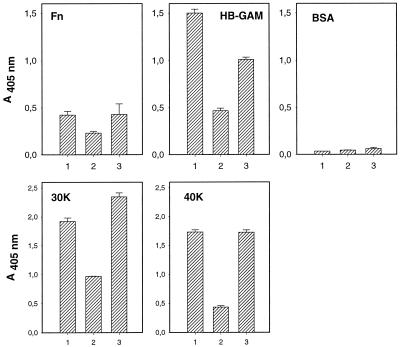
The results described above suggested that the heparin-binding domains in the fibronectin molecule act as binding sites for the Hib fimbriae. We therefore tested the effect of heparin and chondroitin sulfate on fimbrial binding. pMH140 fimbrial binding to fibronectin and to the 30K or the 40K fragment, as well as to HB-GAM, was decreased by 45 to 75% in the presence of 250 μg of heparin/ml (Fig. 5). On the other hand, chondroitin sulfate at the same concentration had a weaker (decreased by 0 to 33%) effect on pMH140 fimbrial binding (Fig. 5).
FIG. 5.
Inhibition of binding of purified Hib pMH140 fimbriae to fibronectin (Fn), HB-GAM, BSA, and 30K and 40K fibronectin fragments. Binding is shown in the presence of PBS (bar 1), low-molecular-weight heparin (250 μg/ml; bar 2), and chondroitin sulfate (250 μg/ml; bar 3). The absorbance values are means from a representative experiment performed with duplicate samples. The error bars show the range of absorbance values.
It has been reported that Hib fimbriae bind to sialic acid-containing receptor molecules on erythrocytes and epithelial cells (35, 37). On the other hand, sialyl oligosaccharide chains do not occur in the terminal 30K and 40K fragments of fibronectin (3, 19) that supported fimbrial binding in this study. We confirmed the lack of terminal sialyl α2-3 and α2-6 galactosides in the 30K and 40K fragments in this study using a lectin differentiation kit (data not shown). On the other hand, terminal sialyl α2-6-galactosides were detected in human plasma fibronectin, fibronectin fragment 45K (collagen-binding domain), and fragment 120K (major cell-binding domain), and terminal sialyl α2-3-galactosides were not found in any of the target proteins (data not shown). Furthermore, the adherence of Hib 770235 fim+ to fibronectin was not decreased after neuraminidase treatment of fibronectin or periodate oxidation of fibronectin carbohydrate (data not shown).
DISCUSSION
Fimbriated and nonfimbriated isolates of H. influenzae exhibit multiple interactions with proteins of the mammalian ECM (43), and our hypothesis is that these interactions contribute to the spread of bacteria through tissue barriers into the circulation and secondary infection sites. We show here that the fimbriae of Hib bind to two heparin-binding ECM proteins, fibronectin and HB-GAM. The wild-type fimbriate Hib strain, as well as the recombinant E. coli strain expressing the Hib fimbriae, showed adhesiveness, and the purified Hib fimbriae bound to both matrix proteins. Within the fibronectin molecule, fimbrial binding was localized to the N-terminal and C-terminal fragments, both of which contain heparin-binding domains. Our previous (43) and present work showed that the nonfimbriated H. influenzae also adheres to fibronectin, which indicated that the Hib isolates also have a fimbria-independent mechanism to interact with fibronectin.
The fimbriae of Hib strain 770235 fim+ efficiently bound to fibronectin. However, the nonfimbriate Hib 770235 fim− also adhered to fibronectin, but at a much lower level than did the fimbriate isolate. This indicates that the Hib isolate 77023 has two mechanism to interact with fibronectin, one fimbria dependent and another that is fimbria independent. Our finding that nonfimbriate, non-type b isolates of H. influenzae expressed fibronectin adherence (43) suggests that the fimbria-independent mechanism(s) is common in H. influenzae. The surface structure mediating this adhesion remains to be identified; it is notable that nonfimbrial adherence factors have been characterized in H. influenzae (6, 27).
The recombinant strain E. coli HB101(pMH140) (Fig. 2C) expressing Hib fimbriae showed a much poorer adhesion to fibronectin than did Hib strain 770235 fim+. The strain was also reduced in its capacity to cause hemagglutination, and it is likely that the poor adhesion by HB101(pMH140) resulted from a low number of fimbriae per bacterium. The finding that pMH140 induced hemagglutination capacity in HB101 indicates that the poor adhesiveness was not due to incorrect assembly of the Hib fimbrae on the E. coli surface.
The contribution of the fimbrial major component, HifA, and the minor components, HifD and HifE, to Hib adherence has remained controversial. van Ham and coworkers (41) analyzed the adhesiveness of recombinant E. coli strains that expressed Hib fimbriae either with or without the minor components and concluded that the minor fimbrial subunits are dispensable for adherence to epithelial cells and that the adhesive domain resides in the major subunit, HifA. Later, McCrea et al. (15, 16) found that anti-HifE antibodies completely block fimbria-specific hemagglutination, which suggests that the minor subunit HifE is involved in Hib fimbria adherence. We found that the fimbriae from the mutated derivative HB101(pMH140hifD::kan) failed to bind to fibronectin, the 30K and 40K fragments, and HB-GAM. This strain carries an antibiotic casette causing a polar termination of transcription and is deficient in both HifD and HifE, and the result thus indicates that HifD and/or HifE is needed for adherence to fibronectin and HB-GAM. Whether both minor components are needed remains to be analyzed. It might be that the Hib fimbriae are analogous in their binding specificity to the P fimbriae of uropathogenic E. coli, which possess two distinct tissue-binding properties, PapG-mediated binding to glycosphingolipid receptors on human uroepithelia (13) and PapE- and PapF-mediated binding to immobilized fibronectin (46, 47). This hypothesis is strongly supported by our finding that terminal sialyl oligosaccharides of fibronectin were not involved in Hib fimbrial binding to fibronectin, although sialyl structures are involved in fimbrial binding to the epithelial cells and erythrocytes (35, 37).
Hib fimbrial binding was localized in two domains of the fibronectin molecule, the amino-terminal 30K and the carboxyl-terminal 40K fragments, which both contain a heparin-binding site also present in HB-GAM. The fimbrial binding was partially abolished in the presence of high concentrations of heparin, and less inhibition was seen with chondroitin sulfate, which is not recognized by the heparin-binding sites in fibronectin or HB-GAM (Fig. 5).
The high concentrations of heparin that were needed to inhibit fimbrial binding to fibronectin and HB-GAM, however, indicate that the inhibition resulted from indirect effects. It could be that heparin binds to a site close to the fimbrial binding sites on the target proteins. The heparin-binding sites in HB-GAM and fibronectin show a low level of identity. Another possibility is that the interaction is based on charge effects, particularly as the sequence of HifE contains regions rich in basic and hydrophobic residues (14, 41). It is also unlikely that the Hib fimbriae or the Hib or E. coli cell surfaces would harbor heparinlike components that would interfere with the binding assays. We also failed to detect any binding of the Hib fimbriae to heparan sulfate proteoglycan and chondroitin sulfate proteoglycan (R. Virkola, unpublished data), indicating that heparan sulfate side chains are not recognized by the Hib fimbriae.
This is the first report of HB-GAM as a target for bacterial adhesion. Although HB-GAM is highly expressed in meninges (23), it is also found in other basement membranes and in developing basement membranes outside the brain (17). Hence, the adhesion property described here may have a pathogenetic function for meningitic bacteria.
ACKNOWLEDGMENTS
This study was supported by the Academy of Finland (grants 29346 and 42103), the Sigrid Jusèlius Foundation, and the University of Helsinki.
We thank Anne Sarén for help in dot blot assays.
REFERENCES
- 1.Bozzini S, Visai L, Pignatti P, Petersen T E, Speziale P. Multiple binding sites in fibronectin and the staphylococcal fibronectin receptor. Eur J Biochem. 1992;207:327–333. doi: 10.1111/j.1432-1033.1992.tb17054.x. [DOI] [PubMed] [Google Scholar]
- 2.Brinton C C, Jr, Carter M J, Derber D B, Kar S, Kramarik J A, To A C-C, Wood S W. Design and development of pilus vaccines for Haemophilus influenzae diseases. Pediatr Infect Dis. 1989;8:554–561. [PubMed] [Google Scholar]
- 3.Carsons S E. The structure and function of fibronectin. In: Carsons S E, editor. Fibronectin in health and disease. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 1–21. [Google Scholar]
- 4.Eberhard T, Virkola R, Korhonen T K, Kronvall G, Ullberg M. Binding to human extracellular matrix by Neisseria meningitidis. Infect Immun. 1998;66:1791–1794. doi: 10.1128/iai.66.4.1791-1794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farley M M, Stephens D S, Kaplan S L, Mason E O., Jr Pilus- and non-pilus-mediated interactions of Haemophilus influenzae type b with human erythrocytes and human nasopharyngeal mucosa. J Infect Dis. 1990;161:274–280. doi: 10.1093/infdis/161.2.274. [DOI] [PubMed] [Google Scholar]
- 6.Gilsdorf J R. Antigenic diversity and gene polymorphisms in Haemophilus influenzae. Infect Immun. 1998;66:5053–5059. doi: 10.1128/iai.66.11.5053-5059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilsdorf J R, McCrea K W, Marrs C F. Role of pili in Haemophilus influenzae adherence and colonization. Infect Immun. 1997;65:2997–3002. doi: 10.1128/iai.65.8.2997-3002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korhonen T K. Yeast cell agglutination by purified enterobacterial pili. FEMS Microbiol Lett. 1979;6:421–425. [Google Scholar]
- 9.Korhonen T K, Nurmiaho-Lassila E-L, Ranta H, Svanborg-Edén C. New method for isolation of immunochemically pure pili from Escherichia coli. Infect Immun. 1980;27:569–575. doi: 10.1128/iai.27.2.569-575.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korhonen T K, Väisänen V, Saxén H, Hultberg H, Svenson S B. P-antigen-recognizing fimbriae from human uropathogenic Escherichia coli strains. Infect Immun. 1982;37:286–291. doi: 10.1128/iai.37.1.286-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuusela P, Vartio T, Vuento M, Myhre E B. Attachment of staphylococci and streptococci on fibronectin, fibronectin fragments, and fibrinogen bound on a solid phase. Infect Immun. 1985;50:77–81. doi: 10.1128/iai.50.1.77-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeb M R, Connor E, Penney D A. A comparison of the adherence of fimbriated and nonfimbriated Haemophilus influenzae type b to human adenoids in organ culture. Infect Immun. 1988;56:484–489. doi: 10.1128/iai.56.2.484-489.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund B, Lindberg F, Marklund B-I, Normark S. The papG protein is the α-D-galactopyranosyl-(1-4)-β-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci USA. 1987;84:5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCrea K W, Watson W J, Gilsdorf J R, Marrs C F. Identification of hifD and hifE in the pilus gene cluster of Haemophilus influenzae type b strain Eagan. Infect Immun. 1994;62:4922–4928. doi: 10.1128/iai.62.11.4922-4928.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCrea K W, Watson W J, Gilsdorf J R, Marrs C F. Identification of two minor subunits in the pilus of Haemophilus influenzae. J Bacteriol. 1997;179:4227–4231. doi: 10.1128/jb.179.13.4227-4231.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCrea K W, Sauver J L, Marrs C F, Clemans D, Gilsdorf J R. Immunologic and structural relationships of the minor pilus subunits among Haemophilus influenzae isolates. Infect Immun. 1998;66:4788–4796. doi: 10.1128/iai.66.10.4788-4796.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsiadis T A, Salmivirta M, Muramatsu T, Muramatsu H, Rauvala H, Lehtonen E, Jalkanen M, Thesleff I. Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development. Development. 1995;121:37–51. doi: 10.1242/dev.121.1.37. [DOI] [PubMed] [Google Scholar]
- 18.Patti J M, Allen B L, McGavin M J, Höök M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 19.Petersen T E, Skorstengaard K, Vide-Pedersen K. Primary structure of fibronectin. In: Mosher D F, editor. Biology of extracellular matrix: series A. Fibronectin. San Diego, Calif: Academic Press; 1989. pp. 1–24. [Google Scholar]
- 20.Raulo E, Julkunen I, Merenmies J, Pihlaskari R, Rauvala H. Secretion and biological activities of heparin-binding growth-associated molecule: neurite outgrowth-promoting and mitogenic actions of the recombinant and tissue-derived protein. J Biol Chem. 1992;267:11408–11416. [PubMed] [Google Scholar]
- 21.Raulo E, Chernousov M A, Carey D J, Nolo R, Rauvala H. Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule. J Biol Chem. 1994;269:12999–13004. [PubMed] [Google Scholar]
- 22.Rauvala H. An 18-kd heparin-binding protein of developing brain that is distinct from fibroblast growth factors. EMBO J. 1989;8:2933–2941. doi: 10.1002/j.1460-2075.1989.tb08443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauvala H, Vanhala A, Castrén E, Nolo R, Raulo E, Merenmies J, Panula P. Expression of HB-GAM (heparin-binding growth-associated molecules) in the pathways of developing axonal processes in vivo and neurite outgrowth in vitro induced by HB-GAM. Dev Brain Res. 1994;79:157–176. doi: 10.1016/0165-3806(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 24.Roggenkamp A, Neuberger H-R, Flügel A, Schmoll T, Heesemann J. Substitution of two histidine residues in YadA protein of Yersinia enterocolitica abrogates collagen binding, cell adherence and mouse virulence. Mol Microbiol. 1995;16:1207–1219. doi: 10.1111/j.1365-2958.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 25.Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- 26.Sarén A, Virkola R, Hacker J, Korhonen T K. The cellular form of human fibronectin as an adhesion target for the S fimbriae of meningitis-associated Escherichia coli. Infect Immun. 1999;67:2671–2676. doi: 10.1128/iai.67.5.2671-2676.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.St. Geme J W, III, Cutter D, Barenkamp S J. Characterization of the genetic locus encoding Haemophilus influenzae type b surface fibrils. J Bacteriol. 1996;178:6281–6287. doi: 10.1128/jb.178.21.6281-6287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St. Geme J W, III, Pinkner J S, Krasan G P, Heuser J, Bullit E, Smith A L, Hultgren S J. Haemophilus influenzae pili are composite structures assembled via the HifB chaperone. Proc Natl Acad Sci USA. 1996;93:11913–11918. doi: 10.1073/pnas.93.21.11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sukurenko E V, Courtney H S, Abraham S N, Klemm P, Hasty D L. Functional heterogeneity of type 1 fimbriae of Escherichia coli. Infect Immun. 1992;60:4709–4719. doi: 10.1128/iai.60.11.4709-4719.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sukurenko E V, Courtney H S, Ohman D E, Klemm P, Hasty D L. FimH family of type 1 fimbrial adhesins: functional heterogeneity due to minor sequence variations among fimH genes. J Bacteriol. 1994;176:748–755. doi: 10.1128/jb.176.3.748-755.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamm A, Tarkkanen A-M, Korhonen T K, Kuusela P, Toivanen P, Skurnik M. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol Microbiol. 1993;10:995–1011. doi: 10.1111/j.1365-2958.1993.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 32.Tunkel A R, Scheld W M. Pathogenesis and pathophysiology of bacterial meningitis. Clin Microbiol Rev. 1993;6:118–136. doi: 10.1128/cmr.6.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turk D C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1985;18:1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- 34.van Alphen L, Riemens T, Poolman J, Hopman C, Zanen H C. Homogeneity of cell envelope protein subtypes, lipopolysaccharide serotypes, and biotypes among Haemophilus influenzae from patients with meningitis in the Netherlands. J Infect Dis. 1983;148:75–80. doi: 10.1093/infdis/148.1.75. [DOI] [PubMed] [Google Scholar]
- 35.van Alphen L, Poole J, Overbreeke M. The Anton blood group antigen is the erythrocyte receptor for Haemophilus influenzae. FEMS Microbiol Lett. 1986;37:69–71. [Google Scholar]
- 36.van Alphen L, van den Berghe N, Geelen-van den Broek L. Interaction of Haemophilus influenzae with human erythrocytes and oropharyngeal epithelial cells is mediated by a common fimbrial epitope. Infect Immun. 1988;56:1800–1806. doi: 10.1128/iai.56.7.1800-1806.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Alphen L, Geelen-van den Broek L, Blaas L, Van Ham M, Dankert J. Blocking of fimbria-mediated adherence of Haemophilus influenzae by sialyl gangliosides. Infect Immun. 1991;59:4473–4477. doi: 10.1128/iai.59.12.4473-4477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Flier M, Chhun N, Wizemann T M, Min J, McCarthy J B, Tuomanen E I. Adherence of Streptococcus pneumoniae to immobilized fibronectin. Infect Immun. 1995;63:4317–4322. doi: 10.1128/iai.63.11.4317-4322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Ham S M, Mooi F R, Sindhunata M G, Maris W R, van Alphen L. Cloning and expression in Escherichia coli of Haemophilus influenzae fimbrial genes establishes adherence to oropharyngeal epithelial cells. EMBO J. 1989;8:3535–3540. doi: 10.1002/j.1460-2075.1989.tb08519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Ham S M, van Alphen L, Mooi F R, van Putten J P. Phase variation of Haemophilus influenzae fimbriae: transcriptional control of two divergent genes through a variable combined promoter region. Cell. 1993;73:1187–1196. doi: 10.1016/0092-8674(93)90647-9. [DOI] [PubMed] [Google Scholar]
- 41.van Ham S M, van Alphen L, Mooi F R, van Putten J P M. The fimbrial gene cluster of Haemophilus influenzae type b. Mol Microbiol. 1994;13:673–684. doi: 10.1111/j.1365-2958.1994.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 42.van Ham S M, van Alphen L, Mooi F R, van Putten J P M. Contribution of the major and minor subunits to fimbria-mediated adherence of Haemophilus influenzae to human epithelial cells and erythrocytes. Infect Immun. 1995;63:4883–4889. doi: 10.1128/iai.63.12.4883-4889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virkola R, Lähteenmäki K, Eberhard T, Kuusela P, van Alphen L, Ullberg M, Korhonen T K. Interaction of Haemophilus influenzae with the mammalian extracellular matrix. J Infect Dis. 1996;173:1137–1147. doi: 10.1093/infdis/173.5.1137. [DOI] [PubMed] [Google Scholar]
- 44.Westerlund B, Korhonen T K. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 1993;9:687–694. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 45.Westerlund B, Kuusela P, Risteli J, Risteli L, Vartio T, Rauvala H, Virkola R, Korhonen T K. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol Microbiol. 1989;3:329–337. doi: 10.1111/j.1365-2958.1989.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 46.Westerlund B, Kuusela P, Vartio T, Van Die I, Korhonen T K. A novel lectin-independent interaction of P fimbriae of Escherichia coli with immobilized fibronectin. FEBS Lett. 1989;243:199–204. doi: 10.1016/0014-5793(89)80129-2. [DOI] [PubMed] [Google Scholar]
- 47.Westerlund B, Van Die I, Kramer C, Kuusela P, Holthöfer H, Tarkkanen A-M, Virkola R, Riegman N, Bergmans H, Hoekstra W, Korhonen T K. Multifunctional nature of P fimbriae of uropathogenic Escherichia coli: mutations in fsoE and fsoF influence fimbrial binding to renal tubuli and immobilized fibronectin. Mol Microbiol. 1991;5:2965–2975. doi: 10.1111/j.1365-2958.1991.tb01856.x. [DOI] [PubMed] [Google Scholar]
- 48.Yamada K M. Fibronectin domains and receptors. In: Mosher D F, editor. Biology of extracellular matrix: series A. Fibronectin. San Diego, Calif: Academic Press; 1989. pp. 47–121. [Google Scholar]